#TTAGGG
Explore tagged Tumblr posts
Text
La presenza di peptidi può determinare l'età biologica
Alle estremità dei cromosomi fabbriche di molecole anti-età. Sono codificate dai telomeri, utili anche contro i tumori. Le strutture che si trovano all’estremità dei cromosomi, i telomeri, producono due proteine chiave per i tumori e l’invecchiamento: a scoprire questa nuova importante caratteristica dei telomeri è stato un gruppo di ricerca guidato da Jack Griffith e Taghreed Al-Turki, dell’Università del North Carolina, i cui risultati sono stati pubblicati sulla rivista dell’Accademia delle Scienze degli Stati Uniti (Pnas). Scoperti un’ottantina di anni fa i telomeri sono costituiti da brevi sequenze di Dna, in particolare da 6 ‘lettere’ (TTAGGG), ripetute migliaia di volte che hanno la funzione di proteggere le parti terminali del genoma, come una sorta di cappuccio protettivo. Fino a pochi anni fa si riteneva che i telomeri avessero appunto una mera funzione di protezione - scoperta per cui nel 2009 Elizabeth Blackburn insieme a Carol Greider e Jack Szostak ricevettero il premio Nobel per la Medicina - ma alcuni recenti studi hanno dimostrato che queste strane sequenze avrebbero anche un ruolo attivo nei meccanismi di invecchiamento cellulare.

La proteina telomerica VR (sfere verdi) recentemente scoperta si accumula nei nuclei (ovale blu) nelle cellule tumorali dell'osteosarcoma umano colorate in rosso. (Laboratorio Griffith) A tutto questo si aggiungere ora che i telomeri conterrebbero anche le informazioni necessarie alla produzione di 2 proteine chiamate VR (valina-arginina) e GL (glicina-leucina), una delle quali sarebbe molto abbondante in alcuni tipi di cellule tumorali umane. Proteine molto semplici ma che sono in grado di innescare l’attività di altre proteine presenti all’interno delle cellule. "Pensiamo – ha detto Al-Turki – che sia possibile che con l'avanzare dell'età la quantità di VR e GL nel nostro sangue aumenti costantemente. Le due molecole potrebbero essere potenzialmente un nuovo biomarcatore per misurare l'età biologica, differente dall'età cronologica”. “Fino a poco tempo fa si riteneva che i telomeri fossero sequenze genetiche piuttosto ‘noiose’ – ha commentato Fabrizio d'Adda di Fagagna, dell'Istituto Airc di Oncologia Molecolare (Ifom) – le si riteneva infatti delle sequenze ripetitive con una funzione puramente strutturale, ossia per tenere compatti e protetti i cromosomi. Ma quest’immagine sta ora cambiando perché scopriamo gradualmente che hanno un ruolo più attivo del previsto”. A contribuire a comprendere meglio il ruolo dei telomeri erano stati alcuni anni fa anche ricercatori dell’Ifom, che avevano dimostrato il ruolo di queste sequenze genetiche in alcuni dei meccanismi di allarme che determinano l’invecchiamento delle cellule. “Ora si è fatto un ulteriore passo in avanti perché si dimostra che i telomeri portano alla sintesi di proteine molto semplici, peptidi”, – ha aggiunto il ricercatore italiano. Se tutto questo venisse confermato, la presenza nel sangue di alti livelli di questi peptidi potrebbe essere un indicatore dell'insorgenza di alcune forme tumorali e, più in generale, un indicatore affidabile dell’invecchiamento delle cellule del corpo umano. Read the full article
#cromosomi#Dna#glicina-leucina#invecchiamento#molecoleanti-età#Peptidi#proteinatelomerica#TTAGGG#valina-arginina
0 notes
Text


TTAGGG
Twitter
42 notes
·
View notes
Text
O elixir da juventude está dentro de nós....
Telômeros são as "pontas" dos cromossomos que protegem o material genético e modulam a longevidade das células.
Há tempos, os alquimistas acreditavam na existência de um elixir da juventude, que supostamente se encontrava no chamado ouro potável, cujas propriedades permitiriam a cura e a regeneração do organismo, prolongando a vida. Na alquimia, o ouro era considerado um metal puro e perfeito, associado ao sol e à imortalidade.
Acredita-se que o ouro potável, por ser uma forma mais refinada e purificada de ouro, poderia transmitir essas propriedades aos humanos, proporcionando-lhes juventude eterna.
Nessa mesma linha de pensamento, alguns filósofos antigos acreditavam em um suposto elixir da longa vida, também conhecido como a água dos filósofos, que traria longevidade aos seus consumidores. Na Bíblia, inclusive, o precioso elixir foi referido como "a água da vida".
Mas histórias a parte, o fato é que nunca ninguém conseguiu produzir até o momento um elixir da longa vida, talvez porque o segredo dessa longevidade esteja muito perto. Dentro de nós!
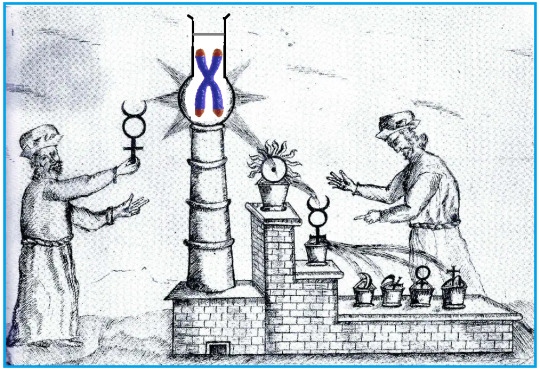
Os telômeros são formados por sequências repetitivas de bases nitrogenadas de DNA (TTAGGG) localizadas nas extremidades dos cromossomos. Essas sequências repetitivas de nucleotídeos atuam como "capas protetoras" do material genético localizado no restante do cromossomo, desempenhando papel importante na estabilidade e segurança dos genes durante a divisão celular (mitose), assim como na "regulação" da longevidade das células.
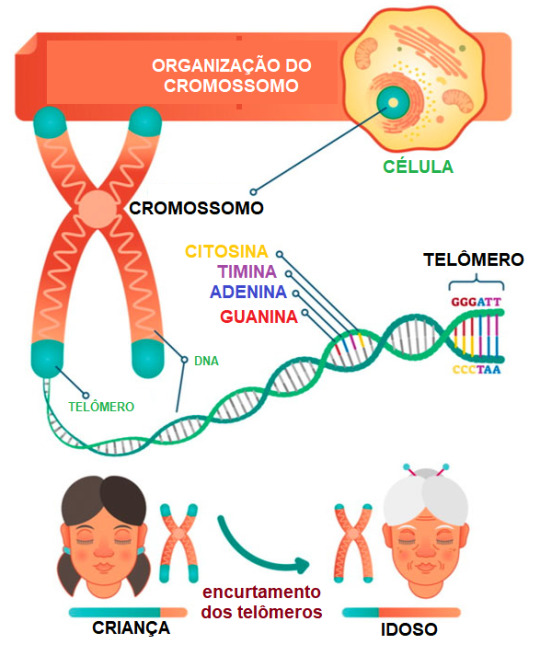
À medida que as células se replicam por mitose ao longo do tempo, os telômeros tendem a encurtar gradualmente. Isso ocorre porque antes da mitose, mais precisamente na fase S da intérfase , a DNA polimerase (enzima responsável pela replicação do DNA), não consegue completar a replicação até o final do cromossomo. Como resultado, as extremidades dos cromossomos são gradualmente perdidas a cada ciclo celular (são perdidos de 50 a 100 nucleotídeos a cada divisão celular).
Quando os telômeros se tornam muito curtos, as células podem entrar em senescência (um estado de parada do crescimento) ou sofrer apoptose (morte celular programada). Estudos científicos demonstraram que o encurtamento dos telômeros está relacionado ao envelhecimento e ao desenvolvimento de doenças associadas à idade.
As células-tronco e certas células do sistema imunológico, no entanto, são dotadas de telomerase, uma enzima que pode "regenerar" os telômeros mantendo a integridade dos cromossomos nessas células. A telomerase adiciona sequências repetitivas de DNA às extremidades dos cromossomos, compensando o encurtamento natural que ocorre durante a replicação do DNA.
A atividade da telomerase é geralmente alta em células-tronco embrionárias, permitindo que elas permaneçam com telômeros longos. Assim, as células-tronco embrionárias preservam a capacidade de se dividirem e se diferenciarem em diferentes tipos de células. Já as células-tronco adultas e principalmente as células permanentes diferenciadas apresentam níveis menores de telomerase quando comparadas às células´ embrionárias.
Diversos estudos científicos demonstraram que o encurtamento dos telômeros ao longo do tempo está também indiretamente relacionado ao desenvolvimento de algumas doenças, entre elas:
Doenças cardiovasculares: o encurtamento dos telômeros tem sido associado a um maior risco de doenças cardiovasculares, como doença arterial coronariana, hipertensão arterial e acidente vascular cerebral.
Câncer: telômeros mais curtos estão frequentemente presentes em células cancerígenas. O encurtamento dos telômeros pode levar a instabilidade genômica, o que favorece a ocorrência de mutações que contribuem para o desenvolvimento do câncer.
Doenças pulmonares: pacientes com doenças pulmonares crônicas, como fibrose pulmonar idiopática e doença pulmonar obstrutiva crônica (DPOC), geralmente apresentam telômeros mais curtos em suas células pulmonares.
Doenças neurodegenerativas: há evidências sugerindo que o encurtamento dos telômeros pode estar envolvido em doenças neurodegenerativas, como doença de Alzheimer, doença de Parkinson e esclerose lateral amiotrófica (ELA).
Síndromes genéticas: certas síndromes (raras) estão associadas ao encurtamento dos telômeros, tais a Síndrome de Werner, a Síndrome de disqueratose congênita e a Síndrome de Bloom.
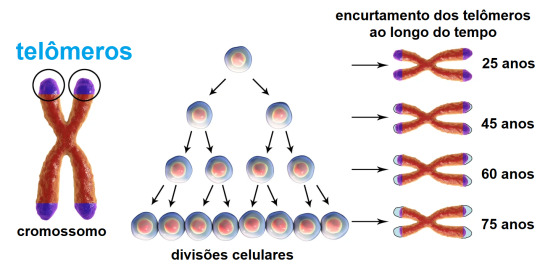
Recentemente, pesquisadores da USP verificaram que animais com carência da enzima telomerase nas células-tronco da medula óssea geram número insuficiente de células de defesa (leucócitos), ou geram células de defesa menos eficazes, além de problemas no fígado. Linhas de pesquisa em outras universidades espalhadas nos Estados Unidos e Europa apontam que a diminuição da atividade da telomerase nas células-tronco da medula óssea pode ter consequências negativas, tais como:
Envelhecimento do sistema hematopoiético: com o encurtamento dos telômeros nas células-tronco da medula óssea ao longo do tempo, as capacidades de autorrenovação e de diferenciação em células sanguíneas diminuem. Isso pode contribuir para o envelhecimento do sistema hematopoiético e para o desenvolvimento de doenças relacionadas à idade, como a anemia.
Aumento do risco de doenças hematológicas: a diminuição da telomerase nas células-tronco da medula óssea pode estar associada a um maior risco de desenvolvimento de certas doenças hematológicas, como a Síndrome Mielodisplásica (SMD) e a leucemia mieloide aguda (LMA).
Disfunção imunológica: a diminuição da atividade da telomerase pode afetar a capacidade das células-tronco da medula óssea de produzir células do sistema imunológico (leucócitos). Isso pode resultar em uma diminuição da resposta imunológica eficiente e em aumento da suscetibilidade a infecções e doenças relacionadas ao sistema imunológico.
No entanto, é importante ressaltar que a relação causal direta entre a diminuição da telomerase nas células-tronco da medula óssea e o desenvolvimento de doenças ainda precisa ser completamente esclarecida.
Pesquisadores da University College de Londres, no Reino Unido, liderados pelo Dr. Alessio Lanna, acenaram com um método preventivo promissor contra o envelhecimento do sistema imunológico. Esses cientistas descobriram que no combate a determinados agentes invasores, determinadas células do sistema imunológico (macrófagos e células dendríticas) podem "transferir telômeros" para os chamados linfócitos T (células de defesa fundamentais) por meio de vesículas membranosas extracelulares. Essa transferência resulta em aumento do comprimento de determinados telômeros dos linfócitos T em até 30 vezes mais que a ação da enzima telomerase.
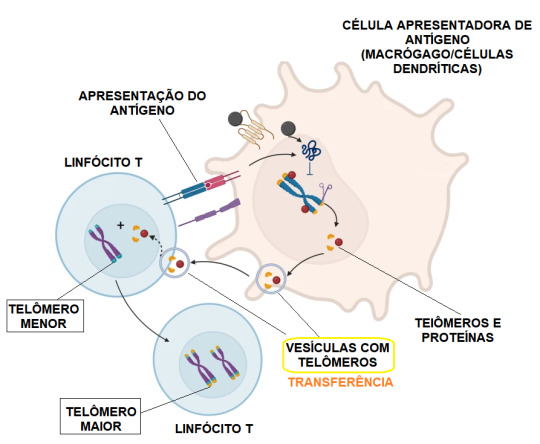
Após a incrível descoberta, a equipe da University College passou a investigar e a purificar as vesículas carregadas de telômeros. A ideia é tentar adicioná-las aos linfócitos T para aumentar a longevidade dessas células em estado natural, independentemente da ocorrência de uma infecção. No decorrer da pesquisa, os cientistas concluíram que as vesículas membranosas carregadas de telômeros poderiam ser administradas com sucesso, por exemplo, quando combinadas com uma vacina, de modo a prolongar a duração da resposta imunológica do imunizante.
Mas será que, em tese, seria possível aplicar para fins preventivos ou regenerativos a enzima telomerase nas células de algum paciente?
Bem, sim!
Mas o fato é que ainda não há relatos de ensaios clínicos que utilizaram essa abordagem, afinal mexer com telomerase e telômeros não é tão simples assim!
A aplicação da telomerase como uma abordagem terapêutica ainda é uma área de pesquisa em andamento. É verdade que teoricamente a aplicação ou a ativação da enzima telomerase poderia prolongar a vida útil das células e retardar o encurtamento dos telômeros, reduzindo os efeitos do envelhecimento e prevenindo certas doenças associadas ao encurtamento dos telômeros.
Só que a utilização indiscriminada da telomerase poderia também resultar em efeitos negativos à saúde, pois sabe-se que algumas células cancerígenas são capazes de ativar mecanismos que preservam os telômeros, permitindo que continuem se dividindo indefinidamente. Esses mecanismos incluem a ativação da enzima telomerase. Nesse caso, adicionar telomerase extra poderia significar "apagar o fogo com gasolina", isto é, estimular a atividade da telomerase em células normais também poderia aumentar o risco do desenvolvimento de tumores.
No entanto, a pesquisa sobre a manipulação da telomerase e da extensão dos telômeros continua em andamento, e várias abordagens estão sendo exploradas para o desenvolvimento de tratamentos potenciais.
Tudo é recente! A descoberta dos telômeros e a relação deles com o envelhecimento ocorreu na década de 1970. Os cientistas Elizabeth Blackburn, Carol Greider e Jack Szostak foram premiados com o Prêmio Nobel de Fisiologia ou Medicina em 2009 pela descoberta da telomerase e do papel dos telômeros na proteção do material genético. Desde então, muitas pesquisas estão sendo financiadas para entender melhor a função dos telômeros e da telomerase.
Embora ainda haja muito a aprender sobre telômeros e telomerase, avanços na tecnologia permitirão melhor entendimento sobre os impactos desses elementos à saúde humana.
O que acontecerá nas relações humanas, o dia em que a ciência mostrar formas seguras e eficazes das células preservarem seus telômeros? Envelheceríamos de forma mais lenta? Nesse caso, o sonho dos alquimistas seria alcançado? O que viria depois?
Leia também:
1-https://jornal.usp.br/ciencias/cientistas-descobrem-efeitos-importantes-da-doenca-dos-telomeros-no-sistema-imunologico/ (acesso em 06 de julho de 2023)
2-https://www.scielo.br/j/rbhh/a/rrjrCBxN8KFgtKh8FmccNwG/?lang=pt (acesso em 06 de julho de 2023)
3-https://oglobo.globo.com/saude/medicina/noticia/2022/09/longevidade-descoberto-mecanismo-que-retarda-envelhecimento-do-sistema-imunologico-mostra-estudo-da-nature.ghtml (acesso em 06 de julho de 2023)
4-https://genotipia.com/genetica_medica_news/transferencia-de-telomeros/ (acesso em 06 de julho de 2023)
5-https://www.ucl.ac.uk/news/2022/sep/new-mechanism-extends-life-immune-system?_ga=2.107367938.1640029816.1688673765-1213671299.1688673765 (acesso em 06 de julho de 2023)
6-https://sentcell.life/about/ (acesso em 06 de julho de 2023)
7-https://www.nature.com/articles/s41423-022-00949-z (acesso em 06 de julho de 2023)
8-https://www.ucl.ac.uk/news/2022/sep/new-mechanism-extends-life-immune-system (06 de julho de 2023)
5 notes
·
View notes
Text
Rabbit anti-TRF2 Antibody, Affinity Purified
Rabbit anti-TRF2 Antibody, Affinity Purified Catalog number: B2019929 Lot number: Batch Dependent Expiration Date: Batch dependent Amount: 100 uL Molecular Weight or Concentration: NA Supplied as: Liquid Applications: a molecular tool for various biochemical applications Storage: -20°C Keywords: TRBF2, TRF2, TTAGGG repeat-binding factor 2, telomeric DNA-binding protein, telomeric repeat binding…
0 notes
Text
Why do we need sunscreen? Take you to learn about sunscreen
In recent years, due to the strong emphasis on the importance of sun protection, people's current attitude towards sun protection is still very reassuring compared to ten years ago or earlier. People are paying more and more attention to photoaging and the damage caused by ultraviolet rays to the skin. I believe you have seen a photo of a truck driver's face exposed to sunlight countless times, right? Due to the fact that one side of the face is exposed to the sun all year round while the other side is not, over time, the contrast between the two sides is very obvious. This is often used to remind everyone to pay attention to the best case of sun protection. Europeans and Americans are fond of sunbathing, and correspondingly, the incidence of skin cancer is relatively high. Ultraviolet radiation is closely related to the occurrence of skin cancer. Why sunscreen The length of telomeres at both ends of our chromosomes to some extent determines our human lifespan. Because every time a cell divides, the telomeres at the top of the chromosome shorten once. When the telomeres cannot be further shortened, the cell no longer continues to divide, reaching the commonly believed limit of 100 divisions and beginning to die. Telomeres are abundant tandem repeat sequences located at the end of linear chromosomes. The structure of our human telomeres is (TTAGGG) * n. As the number of cell passages increases, the length of telomeres gradually shortens. At the same time, due to the shorter length of telomeres in tissues of older individuals compared to younger individuals, telomeres are considered as molecular markers of cell aging in biology.

If we receive high-intensity ultraviolet radiation every day, to ensure that DNA is not damaged, telomeres will absorb the energy of ultraviolet radiation, which will also cause telomeres to shorten. Both domestic and foreign studies have shown that due to DNA being the main target of cell damage caused by ultraviolet radiation, natural aging and photoaging can ultimately shorten telomeres through different mechanisms, leading to a series of changes related to aging. Exaggerated, the above are the reasons why we need sunscreen. Fortunately, we have the protection of the atmosphere, which helps us block the vast majority of ultraviolet rays. Let's do the rest ourselves. Let's first get to know ultraviolet radiation. What is ultraviolet radiation Ultraviolet radiation is the light with a wavelength of 10-400nm in sunlight. Roughly speaking, it can be considered that the ultraviolet radiation emitted by the sun includes UVA, UVB, and UVC. UVA has a wavelength of 320-400nm and is a long wave. It has strong penetration and can penetrate most transparent glass and plastics. The long wave ultraviolet rays contained in sunlight, more than 98% of which can penetrate the ozone layer and clouds to reach the Earth's surface. UVA can directly reach the dermis layer of the skin, damaging elastic fibers and collagen fibers, and tanning our skin;
0 notes
Link
0 notes
Text
Let's test something
1 note
·
View note
Conversation
FUCK IT
...TTAGGG...
I'M ENDING IT
2 notes
·
View notes
Text
Going through questions:
After transcription, pre-mRNA (hnRNA, which contains introns and exons) is edited. To become mature mRNA (exons only), pre-mRNA needs to get a 5' methylguanosine cap, have introns removed, have exons spliced together, and get a 3' polyadenine tail. Splicing = removal of introns (non-coding segments). Splicing is done by spliceosomes. Spliceosomes are made of small nuclear ribonucleoproteins (snRNPs). Introns with GU at the 5' splice site and AG at the 3' splice site are removed by spliceosomes. Post-transcriptional modicfication of pre-mRNA: 5' guanosine cap-> 5' cap methylation-> poly A added at 3' end-> introns removed by spliceosomes.
Alternative splicing is when exons of pre-mRNA are spliced together in different ways, which allows for different proteins to be made. E.g., you start off with pre-mRNA containing exon 1, exon 2, exon 3, and exon 4. Alternative splicing allows you to remove and include whichever exons you want in order to make different mRNA strands. So from the pre-mRNA, you could make mRNA that looks like: exon 1--exon 2--exon 3--exon 4 or mRNA that looks like: exon 1--exon 2--exon 4 (removed exon 3). Alternative splicing is basically removing some exons so that you can make different proteins from the same gene.
Some cancer cells can use alternative splicing to avoid detection by the immune system. FasR is a receptor on cells that binds to FasL on cytotoxic T cells. The FasR-FasL interaction leads to programmed cell death (apoptosis). Cancer cells can do alternative splicing to remove the exon that makes the transmembrane part of the FasR, therefore FasR won't be in the cell membrane. If that happens, the cell can avoid apoptosis.
Lol. The Fas receptor (FasR) has "death domains" on the intracellular part of it. When the Fas ligand (FasL) binds to FasR, the death domains cause activation of caspases, which lead to executioner caspases that cause apoptosis. It sounds macabre and funny. I did a question the other day about caspases. They're involved in apoptosis.
Cystathionine synthase deficiency causes homocystinuria; it's autosomal recessive. Pts present with lens dislocation, intellectual disability, marfanoid body habitus (long arms/legs/fingers), and thromboembolism (because homocysteine causes hypercoagulability). Tx: avoid methionine in the diet, supplement cysteine and vitamin B6.
Aminoacyl tRNA sythetase loads the correct amino acid to the terminal 3' OH on the CCA tail of tRNA.
Restriction Fragment Length Polymorphism = RFLP.
Ok, see I was confused about meiosis and the way he talked about in in OnlineMedEd just made me more confused. But this question I just answered makes sense to me. Homologous chromosomes are separated in meiosis I; sister chromatids are separated in meiosis II. Nondisjunction is failure of either homologous chromosomes to separate in meiosis I or failure of sister chromatids to separate in meiosis II. Trisomy = having an extra chromosome; monosomy = losing a chromosome. Nondisjunction-> monosomies and trisomies. In this question, the parents were normal, so they each had 2 bands on RFLP analysis. Their son had trisomy 21, so he had 3 bands on RFLP analysis because he had an extra chromosome 21. I really didn't understand this RFLP analysis, but I got the question right because I remembered from OnlineMedEd that maternal nondisjunction in meiosis I often causes Down syndrome. Anyway, the RFLP analysis showed that the kid had 2 bands from mom and one band from dad, so he got the extra chromosome from his mom. The kid got two different bands from the mom, which means he got each of her homologous chromosomes = nondisjunction in meiosis I (which failed to separate her homologous chromosomes during her egg formation).
Telomerase is the enzyme that prevents shortening of telomeres. Increased telomerase activity occurs cancers. Telomeres at the end of chromosomes have lots of GT repeats.
Endonucleases are needed for nucleotide excision repair, which is the process that fixes DNA that's damaged by UV light (UV light creates pyrimidine dimers in DNA). Without endonuclease and nucleotide excision repair, you get xeroderma pigmentosum. XP is an AR condition where you have impaired nucleotide excision repair, usually due to deficiency in endonuclease.
Never heard of dyskeratosis congenita, but I got the question right because the pt had premature aging due to telomerase deficiency. Telomoerase prevents the ends of the chromosomes from being degraded. Every time you go through cell division, the ends of the chromosomes (telomeres) get shortened. Telomerase adds to the telomeres so that won't happen. Cells that divide frequently need telomerase. The cells include hematopoeitic stem cells, epithelial cells, and lymphocytes. Telomerase is a reverse transcriptase that adds TTAGGG repeats to the telomeres. This protects the chroomosomes from being shortened. Because once the chromosomes are shortened enough, the cells containing them will undergo apoptosis. Without telomerase, pts will have dystrophic nails, bone marrow failure, oral leukoplakia, and pulmonary fibrosis. Cells that don't regenerate don't have telomerase activity--so those cells that don’t regenerate include cardiac cells, CNS cells.
During translation, the first stop codon on the mRNA causes binding of a release factor. The anticodon is on the tRNA. From Wikipedia:
Dyskeratosis congenita (DKC), also known as Zinsser-Engman-Cole syndrome is a rare progressive congenital disorder with a highly variable phenotype.[3] The entity was classically defined by the triad of abnormal skin pigmentation, nail dystrophy, and leukoplakia of the oral mucosa, but these components do not always occur.[3] DKC is characterized by short telomeres. Some of the manifestations resemble premature aging (similar to progeria). The disease initially mainly affects the skin, but a major consequence is progressive bone marrow failure which occurs in over 80%, causing early mortality.
PKU is AR mutation of the gene that codes for phenylalanine hydroxylase. So you need two bad alleles to have the disease.
Hemophilia A and B are X-linked recessive. Probability confuses me, so even if I remembered that hemophilia is X-linked recessive, I still probably would've gotten this question wrong. Anyway, if a woman's brother has hemophilia and one of the parents is a carrier of hemophilia, that means the woman has a 50% chance of being a carrier. The probability of her passing it on to her son is 50%. The probability of her child being male is 50%. So ultimatley, the probability of her child being affected is the probability of the mom being a carrier x the probability that the mom passes it in to her child x the probability that her child is a male = 0.5 x 0.5 x 0.5 = 1/8.
RNA pol I, II, and III are in eukaryotic cells. RNA pol I makes ribosomal RNA. RNA pol II makes messenger RNA, snRNA, and micro RNA. RNA pol III makes transer RNA and 5S ribosomal RNA. I think I made a mnemonic for this a long time ago. RNA pol I = rRNA, RNA pol II = mRNA, RNA pol III = tRNA. The nucleolus in a cell is basophilic, meaning it stains darkly. rRNA is transcribed in the nucleolus. Cancer cells have a lot of rRNA and their nucleoli are dark-staining.
Remember that DNA is made in the 5' to 3' direction, but read by DNA pol in the 3' to 5' direction.
AAUAAA in the mRNA is the consensus sequence that causes addition of the poly A tail, which protects the mRNA from degradation when it goes into the cytoplasm. So it has a bunch of adenine.
Frameshift mutation = addition or deletion of a number of bases that's not divisible by 3-> altered reading frame and premature stop codon.
#Alternative splicing#genetics#snRNP#splicing#Fas#FasL#FasR#apoptosis#caspase#caspases#homocystinuria#homocysteine#telomere#telomerase#xeroderma pigmentosum#nucleotide excision repair#endonuclease#PKU#hemophilia#inheritance patterns#RNA#poly A tail#consensus sequence#hnRNA
2 notes
·
View notes
Audio
AnyPay のデザイナー石田智子さん(@tomokoishida19)と事業会社で働くデザイナーの歩み方について話を伺いました。様々なタスクを要求されるなか、いかにモノつくりをしていくのか。彼女がデザインマネージャーをしていた頃を振り返りながら、今のデザイナーに必要なスキル、働き方について話し合いました。
石田智子 / Designer|note
paymo (ペイモ) | わりかんを思い出に
AnyPay株式会社
【課題解決型デザイナーを目指そう】アイデアからUIへ、発想をつなぐデザインワークショップ(増枠) - connpass
事業会社で働くデザイナーの悩み
デザインと即興
何をデザインすることが仕事なのか
お金という『価値』の交換
なんとなく安心と思える状態
Hiroko Wada | adtech tokyo official website
真実と安心・納得できることはイコールではない
Factfulness: Ten Reasons We're Wrong About The World - And Why Things Are Better Than You Think
まず作りきることを体験する
デザインをプレゼンするのも仕事の一部
作る時間の尊さ
アイデア創出から作り上げるまでの『間』
デザイナーの責任をもつ
作ることの尊さ
1 note
·
View note
Photo

TRIAL PIECE /// TEST SHOOTING CD&ST /// SHINTARO KUROKAWA @shintarokurokawa PH /// DAISUKE SUGAYA @d.iam HM /// RYO ISHIYAMA,RIE YOSHIZAWA,RISA OKOCHI @jesuis_ryo @jesuis_rie @jesuis.risa MD /// ATOM @metellus.a.nicholas /// Special Thanks STUDIO FABWORK @studiofabwork TEENY LANCH @teeny_ranch TTAGGG TOKYO @ttaggg.tokyo JACKET /// ULU @ INNER /// JUHA @juhatokyo PANTS /// SECOND LAYER @secondlayer_us GLOVE /// NIKE @nikesportswear WATCH /// VINTAGE TIE /// VINTAGE ZIP BOOTS /// FACTOTUM @factotum.official https://www.instagram.com/p/B98KFWPFV1P/?igshid=7xfm198i5vhh
0 notes
Text
Rabbit anti-TRF2 Antibody
Rabbit anti-TRF2 Antibody Catalog number: B2019752 Lot number: Batch Dependent Expiration Date: Batch dependent Amount: 100 uL Molecular Weight or Concentration: NA Supplied as: Liquid Applications: a molecular tool for various biochemical applications Storage: -20°C Keywords: TRBF2, TRF2, TTAGGG repeat-binding factor 2, telomeric DNA-binding protein, telomeric repeat binding protein 2 Grade:…
0 notes
Photo

Taiki instagram update: #ttaggg #0nff0
12 notes
·
View notes
Text
High-throughput single-molecule telomere characterization [METHOD]
We have developed a novel method that enables global subtelomere and haplotype-resolved analysis of telomere lengths at the single-molecule level. An in vitro CRISPR/Cas9 #RNA-directed nickase system directs the specific labeling of human (TTAGGG)n DNA tracts in genomes that have also been barcoded using a separate nickase enzyme that recognizes a 7-bp motif genome-wide. High-throughput imaging and analysis of large DNA single molecules from genomes labeled in this fashion using a nanochannel array system permits mapping through subtelomere repeat element (SRE) regions to unique chromosomal DNA while simultaneously measuring the (TTAGGG)n tract length at the end of each large telomere-terminal DNA segment. The methodology also permits subtelomere and haplotype-resolved analyses of SRE organization and variation, providing a window into the population dynamics and potential functions of these complex and structurally variant telomere-adjacent DNA regions. At its current stage of development, the assay can be used to identify and characterize telomere length distributions of 30–35 discrete telomeres simultaneously and accurately. The assay's utility is demonstrated using early versus late passage and senescent human diploid fibroblasts, documenting the anticipated telomere attrition on a global telomere-by-telomere basis as well as identifying subtelomere-specific biases for critically short telomeres. Similarly, we present the first global single-telomere-resolved analyses of two #cancer cell lines. http://bit.ly/2h3BQrb #CSH
0 notes
Text
New Post has been published on Abbkine - Antibodies, proteins, biochemicals, assay kits for life science research
New Post has been published on http://www.abbkine.com/tert-polyclonal-antibody-released/
TERT Polyclonal Antibody Released
Telomerase is a ribonucleoprotein polymerase that maintains telomere ends by addition of the telomere repeat TTAGGG. hTERT (TERT) is the rate-limiting catalytic subunit of telomerase. The enzyme consists of a protein component with reverse transcriptase activity, encoded by this gene, and an RNA component which serves as a template for the telomere repeat. Telomerase expression plays a role in cellular senescence, as it is normally repressed in postnatal somatic cells resulting in progressive shortening of telomeres. Deregulation of telomerase expression in somatic cells may be involved in oncogenesis. Studies in mouse suggest that telomerase also participates in chromosomal repair, since de novo synthesis of telomere repeats may occur at double-stranded breaks.
Abbkine TERT Polyclonal Antibody was affinity-purified from rabbit serum by affinity-chromatography using synthetic peptide from human protein at AA range: 1268-1341. The verified reactivity and applications respectively are human and ELISA, IHC-p, WB. Abbkine suggested the starting dilutions are as follows: WB: 1:500-1:2000, IHC-p: 1:50-1:300, ELISA 1:5000-1:20000.
Supplied as liquid solution at 1 mg/ml, the antibody is stable for one year at -20°C from the date of shipment. For maximum recovery of product, centrifuge the original vial after thawing and prior to removing the cap. Aliquot to avoid repeated freezing and thawing.
1 note
·
View note
Text
New Post has been published on Abbkine - Antibodies, proteins, biochemicals, assay kits for life science research
Interests on http://www.abbkine.com/tert-polyclonal-antibody-released/
TERT Polyclonal Antibody Released
Telomerase is a ribonucleoprotein polymerase that maintains telomere ends by addition of the telomere repeat TTAGGG. hTERT (TERT) is the rate-limiting catalytic subunit of telomerase. The enzyme consists of a protein component with reverse transcriptase activity, encoded by this gene, and an RNA.... Click for moreTERT Polyclonal Antibody Released.
1 note
·
View note