#Swjal Process
Explore tagged Tumblr posts
Text
The Role of Pneumatic Angle Seated Valves in Enhancing Process Automation
In today’s industrial landscape, automation has become the cornerstone of enhancing productivity, efficiency, and safety. Among the numerous components that contribute to effective automation, pneumatic angle seated valves hold a crucial place. These valves have revolutionized fluid control processes by offering swift, precise operation and ensuring minimal downtime.
Pneumatic angle seated valves operate through compressed air, allowing them to respond rapidly to control signals. This fast actuation is essential for processes where even minor delays can impact production quality. The angled seat design reduces flow resistance, ensuring smooth operation and minimal pressure loss. This feature makes these valves ideal for applications requiring robust performance under high-pressure conditions.

A significant advantage lies in their integration with modern automation systems. Industries today rely on programmable logic controllers (PLCs) and other advanced control technologies to manage complex processes. Pneumatic angle seated valves seamlessly integrate with these systems, ensuring synchronized operation and enhancing overall efficiency.
Another notable feature is their durability. Constructed from corrosion-resistant materials like stainless steel, these valves are built to withstand harsh environments. This makes them particularly suitable for industries such as pharmaceuticals, food and beverage, and chemical processing, where stringent hygiene standards and exposure to aggressive media are common.
Maintenance is another area where these valves shine. Their straightforward design minimizes wear and tear, reducing the frequency of maintenance. When servicing is required, the process is simple, ensuring minimal disruption to operations. This translates into cost savings and improved uptime for industries reliant on continuous processes.
Furthermore, Pneumatic Angle Seated Valves offer exceptional safety features. Their tight sealing capability prevents leaks, ensuring that hazardous substances remain contained. This is especially critical in industries handling volatile chemicals or requiring sterile environments.
By incorporating pneumatic angle seated valves, industries can achieve greater control over their automated processes. Enhanced precision, faster response times, and reduced maintenance requirements make these valves indispensable in modern automated systems. For those looking to improve automation and reliability in their operations, buy Pneumatic Angle Seated Valve from Swjal Process Pvt. Ltd.
#Angle Seated Valve#Pneumatic Angle Seated Valve#Sanitary Valves#Sanitary Valves Manufacturer#Swjal Process#Mumbai#India
0 notes
Text
Fermentation Vessels and Bioreactors: Driving Innovation in Bioprocessing
In the fields of pharmaceuticals, biotechnology, and food production, fermentation vessels and bioreactors play a crucial role in ensuring the growth of microorganisms, cells, and biologically active compounds under controlled conditions. These specialized systems are indispensable in producing vaccines, antibiotics, enzymes, and various biopharmaceutical products, making them the backbone of industrial bioprocessing.
Importance of Fermentation Vessels and Bioreactors
Fermentation vessels and bioreactors provide the ideal environment for cultivating microorganisms and cells essential for producing bio-based products. These vessels offer precise control over key parameters such as temperature, pH, oxygen levels, and agitation, ensuring optimal growth conditions and maximizing product yield.
The ability to maintain a sterile environment is paramount, as contamination can compromise entire production batches. Modern fermentation vessels and bioreactors are designed to minimize risks while enhancing productivity, making them indispensable for industries seeking consistency, scalability, and quality in their processes.
Compliance with Regulatory Standards
In pharmaceutical and biotech applications, adhering to strict regulatory standards is non-negotiable. Compliance with Good Manufacturing Practice (GMP) guidelines, the U.S. Food and Drug Administration (FDA), and other global regulatory bodies is essential to ensure product safety, quality, and efficacy.
Fermentation vessels and bioreactors must meet stringent standards for material selection, surface finish, and sterilization protocols. Regular validation and calibration processes are conducted to guarantee that every step of production aligns with regulatory requirements. Companies investing in compliant systems safeguard not only their products but also their reputations.
Innovation in Manufacturing Processes
Technological advancements have transformed fermentation vessels and bioreactors into highly sophisticated systems capable of automating complex bioprocesses. Innovations such as real-time monitoring, data-driven process control, and advanced sensors enable operators to fine-tune parameters, ensuring consistent performance and minimizing human error.
Additionally, the integration of single-use bioreactors has revolutionized the industry by reducing cross-contamination risks, accelerating production timelines, and lowering operational costs. These innovations empower manufacturers to remain agile in an increasingly competitive market.
Enhancing Productivity with Advanced Technologies
Modern fermentation vessels and bioreactors incorporate advanced technologies to enhance productivity and efficiency. Automation systems optimize processes, reducing downtime and ensuring uniform product quality. Real-time data collection and analysis enable predictive maintenance, minimizing disruptions and extending equipment lifespan.
Moreover, scalable designs allow for seamless transition from laboratory-scale to commercial production, accommodating the growing demands of pharmaceutical and biotech industries. By leveraging these technologies, companies can streamline their operations while maintaining the highest quality standards.
Ensuring Product Safety
Product safety is at the heart of every bioprocess. Fermentation vessels and bioreactors are meticulously designed to maintain sterile conditions throughout production. Features such as clean-in-place (CIP) and sterilize-in-place (SIP) systems ensure thorough cleaning and sterilization between production runs, preventing cross-contamination.
Material selection is equally important, with stainless steel and other non-reactive materials used to ensure product purity. Additionally, advanced filtration and monitoring systems help detect and eliminate potential contaminants, safeguarding both products and consumers.
Fermentation vessels and bioreactors are indispensable for the pharmaceutical, biotech, and food industries, ensuring consistent product quality, regulatory compliance, and enhanced productivity. Advanced technologies and rigorous manufacturing standards have made these systems more reliable and efficient than ever before.
Swjal Process Pvt. Ltd. stands at the forefront of delivering cutting-edge fermentation vessels and bioreactors. As a leading manufacturer in India, Swjal Process Pvt. Ltd. combines innovation, compliance, and expertise to provide robust solutions that empower industries to achieve excellence in bioprocessing.
#industrial vessles#mixing tanks#vessle with agiators#Fermentation vessels and bioreactors#swjal process
0 notes
Text
youtube
EXCLUSIVE INTERIEW HYDERABAD PHARMA PRO & PACK | Industrial Revolution
Freture Techno Pvt. Ltd. and Swjal Process Pvt. Ltd. director Kailas Waghmare gives an exclusive interview at Pharma Pro & Pack, discussing the latest technologies and innovations transforming industrial manufacturing and production. In this insightful conversation, he highlights how Freture Techno is delivering cutting-edge solutions like lined valves and automation products to industries such as Pharma, Oil & Gas, and Chemical Processing. These advancements are making manufacturing processes more efficient, automated, and sustainable. To Know More: Pharma Pro& Pack https://pharmapropack.com/ Sjwal Process https://www.swjal.com/ Freture Techno https://www.freture.com/ Waghmare also touches on the synergy between Freture Techno and Swjal Process Pvt. Ltd., showing how both companies are driving innovation in their respective fields – from purified water systems to precision industrial valves. Get an in-depth understanding of the impact these technologies are having on the industry, and what the future holds for automated and sustainable manufacturing solutions.
#pharmapro&packHyderabad2024#industrial valves#manufacturers#freture techno#Pure Water System#water for injection#ROEDI water treatment#SWJAL PROCESS PVT LTD#pharmacutical industry#Youtube
0 notes
Text
Ultrafiltration Water System: Advanced Purification for Pharmaceutical Applications
mportance of Ultrafiltration
Ultrafiltration (UF) systems play a crucial role in pharmaceutical and biotechnology industries by ensuring the removal of suspended solids, bacteria, viruses, and endotoxins from water. These systems provide a reliable method for producing high-purity water, essential for maintaining product integrity and complying with stringent regulatory standards.
Critical Applications and Regulatory Standards
Ultrafiltration is widely used in the preparation of purified water, supporting processes such as drug formulation, equipment cleaning, and sterile manufacturing. Compliance with global standards like the United States Pharmacopeia (USP), European Pharmacopoeia (EP), and Indian Pharmacopoeia (IP) is ensured through rigorous quality control measures.
Ultrafiltration Process
The ultrafiltration process involves passing water through semi-permeable membranes with pore sizes ranging from 0.01 to 0.1 microns. This ensures the efficient removal of unwanted particles while preserving the essential qualities of the purified water.
Key Stages in the Process
Pre-treatment: Incoming water is pre-filtered to remove larger particles and reduce membrane fouling.
Membrane Filtration: Water is forced through UF membranes, effectively separating contaminants.
Sanitization and Maintenance: Regular cleaning and sanitization cycles maintain membrane integrity and system performance.
Quality Monitoring: Continuous monitoring ensures compliance with quality parameters like conductivity, pH, and microbial load.
Ensuring Quality and Compliance
High-quality materials such as stainless steel and sanitary-grade components are used to construct UF systems, minimizing contamination risks. Automated control systems enhance reliability by monitoring performance in real time and ensuring consistent water quality.

Regular validation and maintenance protocols ensure ongoing compliance with industry standards, safeguarding product safety and efficacy. Advanced techniques, including automated membrane integrity testing, further enhance system reliability.
Applications in Pharmaceutical and Biotechnology Industries
Ultrafiltration water systems serve various applications, including:
Producing water for injection (WFI) and other high-purity applications.
Supporting aseptic manufacturing environments.
Providing clean-in-place (CIP) and sterilize-in-place (SIP) solutions.
Preparing buffers and media for bioprocessing.
Advancements and Sustainability
Modern UF systems are designed with sustainability in mind, incorporating energy-efficient components and minimizing water wastage. Innovations in membrane technology and automation have led to improved efficiency, reduced downtime, and enhanced process control.
Conclusion: Excellence in Water Purification
In conclusion, ultrafiltration water systems provide a robust solution for achieving high-purity water in pharmaceutical manufacturing. Their advanced filtration capabilities, strict regulatory compliance, and adaptability to diverse applications make them indispensable in ensuring product quality and patient safety. Swjal Process Pvt. Ltd. is a leading Ultrafiltration Water System manufacturer in India.
0 notes
Text
Choosing the Right Chemical Dosing System: Factors, Benefits, and Industry Applications
Selecting the right chemical dosing system is crucial for achieving optimal water treatment results. Several factors influence this choice, including water flow rate, the type of chemicals required, and the desired level of automation. Each application demands a tailored approach to ensure the system aligns with specific treatment objectives.
Key benefits of implementing a chemical dosing system include precise control over chemical use, improved water quality, and reduced operational costs. These systems ensure consistent dosing, preventing issues like overdosing, which can lead to chemical waste, or underdosing, which risks ineffective treatment.
In the pharmaceutical industry, chemical dosing systems maintain the purity of water used in drug manufacturing, while in municipal plants, they ensure safe drinking water by controlling contaminants. Food processing facilities also rely on these systems to meet hygiene standards.
Modern advancements such as smart sensors and IoT integration are enhancing the efficiency and reliability of these systems. With the right chemical dosing system, industries can achieve superior water treatment performance while meeting regulatory requirements and ensuring environmental responsibility.
When considering a chemical dosing system, it is essential to evaluate maintenance requirements and ease of operation. Systems designed with user-friendly interfaces and automated maintenance alerts help streamline daily operations and minimize downtime, ensuring continuous performance.
Cost-effectiveness is another critical factor. While initial investment may vary, the long-term savings achieved through reduced chemical consumption and enhanced equipment lifespan make these systems a smart choice for industries prioritizing operational efficiency.
Finally, collaboration with experienced manufacturers and service providers ensures proper system design, installation, and ongoing support. Such partnerships help industries stay ahead of evolving water treatment challenges, achieving long-term success in water quality management.
#chemical dosing system#chemical dosing#chemical dosing in water treatment#swjal process#water treatment plant manufacturer#pharmacutical industry#biotech industry
0 notes
Text
Ultrapure Water Production: Advanced Technologies and Challenges in Semiconductor Fabrication
Semiconductor fabrication demands an exceptionally high level of water purity to ensure defect-free chip production. Ultrapure water (UPW) is used throughout the manufacturing process for wafer cleaning, etching, and rinsing, requiring the removal of even the smallest impurities at the molecular level. Achieving this level of purity involves advanced water treatment processes that eliminate dissolved solids, organic compounds, and microscopic particulates. This article provides a step-by-step breakdown of UPW production, explores the challenges of maintaining extreme purity, and discusses the latest innovations in real-time monitoring, nanofiltration, and sustainable water management.
Step-by-Step Process of Ultrapure Water Production
Producing ultrapure water for semiconductor manufacturing requires a multi-stage treatment system designed to remove all contaminants, including dissolved ions, bacteria, organic molecules, and nanoparticles.
Pre-Treatment: Raw water undergoes filtration and chemical conditioning to remove large particles, suspended solids, and microbial contaminants.
Reverse Osmosis (RO): A semi-permeable membrane removes up to 99% of dissolved salts, bacteria, and organic compounds, forming the foundation of UPW production.
Deionization (DI): Ion exchange resins eliminate remaining dissolved ions, ensuring extremely low conductivity in the water.
Ultrafiltration (UF): A fine membrane filtration system removes microscopic particles and endotoxins that could compromise wafer processing.
UV Oxidation: High-intensity ultraviolet light is used to break down organic contaminants and disinfect the water by destroying bacteria and viruses.
Degasification: Dissolved gases such as oxygen and carbon dioxide are removed to prevent oxidation and unwanted chemical reactions in semiconductor fabrication.
Final Polishing: A combination of microfiltration and high-efficiency ion exchange further refines the water, ensuring it meets the highest purity standards required for semiconductor manufacturing.
Challenges in UPW Production for Semiconductor Fabs
Despite the effectiveness of these treatment processes, several challenges must be addressed to maintain UPW purity and ensure system efficiency:
Maintaining Extreme Purity Levels: Any deviation in water quality can result in contamination, leading to defects in semiconductor wafers. Real-time monitoring and precise control of UPW systems are essential.
System Reliability and Operational Efficiency: Downtime in UPW systems can halt semiconductor production, requiring highly efficient, redundant purification processes.
Water Wastage and Sustainability: UPW production consumes significant amounts of water, making waste reduction and recycling crucial for both cost-efficiency and environmental responsibility.
Microbial and Particulate Contamination: Even ultra-low levels of bacteria or nanoparticles can interfere with semiconductor fabrication, necessitating advanced filtration and disinfection techniques.
Innovations in UPW Production Technologies
To address these challenges, new technologies are continuously being developed to improve ultrapure water treatment.
Real-Time Monitoring and Smart Sensors: AI-powered monitoring systems detect impurities instantly, allowing for automated adjustments to maintain water quality.
Nanofiltration for Enhanced Purity: This advanced membrane technology provides an additional layer of purification, removing even the smallest contaminants without excessive energy consumption.
Energy-Efficient UPW Systems: New-generation RO and UF membranes require lower pressure, reducing energy use while maintaining superior filtration efficiency.
Sustainable Water Recycling Technologies: Semiconductor manufacturers are investing in water recovery and reuse strategies to minimize waste and optimize resource utilization.
Traditional vs. Modern UPW Treatment Methods
Comparing conventional water purification techniques with modern innovations highlights the evolution of UPW
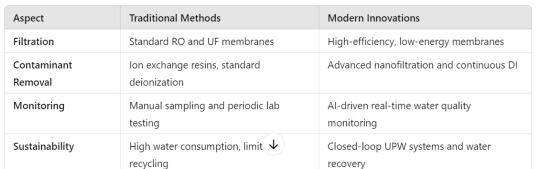
Conclusion
Ultrapure water is a critical component in semiconductor fabrication, requiring precise treatment processes and cutting-edge purification technologies. The continuous evolution of UPW production, driven by real-time monitoring, nanofiltration, and sustainable water management, ensures that semiconductor fabs achieve higher efficiency while minimizing contamination risks. As the demand for advanced semiconductors grows, innovative ultrapure water systems will remain essential in maintaining high production standards and environmental sustainability.
SWJAL PROCESS Pvt. Ltd. offers industry-leading ultra pure water solutions tailored for semiconductor manufacturing, ensuring unmatched purity and reliability in every process.
#high purity water plant#high purity water system#high pure water for semiconductor productionr#pure water solution for critical industry#Swjal process
0 notes
Text
How RO EDI Plants Support Compliance in the Pharmaceutical and Biotech Industries
In the highly regulated pharmaceutical and biotech industries, compliance with water quality standards is non-negotiable. Water is a critical component in drug manufacturing, laboratory testing, and research, and any deviation from regulatory requirements can lead to product recalls, regulatory penalties, and reputational damage. RO EDI Plants (Reverse Osmosis Electrodeionization Plants) play a vital role in ensuring compliance by producing high-purity water that meets stringent regulatory standards. In this article, we explore how RO EDI systems support compliance in the pharmaceutical and biotech sectors.
The Importance of Regulatory Compliance in Pharma and Biotech
Regulatory bodies such as the FDA (Food and Drug Administration), USP (United States Pharmacopeia), EP (European Pharmacopoeia), and WHO (World Health Organization) have established strict guidelines for water quality in pharmaceutical and biotech applications. These guidelines ensure that water used in drug manufacturing, laboratory testing, and research is free from contaminants that could compromise product safety and efficacy.
Key regulatory standards include:
USP <1231>: Specifies requirements for Water for Injection (WFI) and Purified Water.
EP and WHO: Provide guidelines for water quality in pharmaceutical applications.
FDA Regulations: Require validation and documentation of water systems to ensure consistent quality.
Non-compliance with these standards can result in costly penalties, product recalls, and damage to a company’s reputation.
How RO EDI Plants Ensure Compliance
RO EDI Plants are designed to meet the stringent water quality requirements of the pharmaceutical and biotech industries. Here’s how they support compliance:
Production of High-Purity Water:
RO EDI systems combine Reverse Osmosis (RO) and Electrodeionization (EDI) technologies to remove impurities, including ions, organic compounds, and microorganisms.
The result is water with resistivity up to 18.2 MΩ·cm and low TOC (Total Organic Carbon) levels, meeting the requirements for WFI and Purified Water.
Consistent Water Quality:
RO EDI systems provide a continuous supply of high-purity water, ensuring consistent quality for critical applications.
Real-time monitoring and alarm systems alert operators to any deviations, enabling quick corrective actions.
Validation and Documentation:
RO EDI systems are designed to support validation processes, including Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ).
Comprehensive documentation ensures traceability and simplifies regulatory audits.
Chemical-Free Operation:
Unlike traditional ion exchange systems, RO EDI systems do not require chemical regeneration, reducing the risk of contamination and ensuring compliance with environmental regulations.
Key Compliance Features of RO EDI Plants
When selecting an RO EDI Plant for pharmaceutical or biotech applications, look for the following compliance-focused features:
Regulatory-Ready Design: Systems designed to meet USP, EP, and WHO standards.
Material Compliance: Use of materials that meet regulatory requirements for pharmaceutical applications.
Built-In Quality Control: Features such as real-time monitoring, data logging, and alarm systems.
Validation Support: Documentation and protocols to simplify the validation process.
Avoiding Common Compliance Pitfalls
Even with a robust RO EDI system, compliance challenges can arise. Here are some common pitfalls and how to avoid them:
Inadequate Validation:
Ensure that your RO EDI system is fully validated, including IQ, OQ, and PQ.
Work with the manufacturer to obtain comprehensive validation documentation.
Poor Maintenance Practices:
Regularly maintain and service your RO EDI system to ensure consistent performance.
Follow the manufacturer’s maintenance guidelines and schedule.
Lack of Training:
Train operators on the proper use and maintenance of the RO EDI system.
Provide ongoing training to keep staff updated on regulatory requirements and best practices.
Conclusion
Compliance with water quality regulations is a critical aspect of pharmaceutical and biotech operations. RO EDI Plants provide a reliable and efficient solution for producing high-purity water that meets regulatory standards. By investing in an RO EDI system, companies can ensure compliance, protect product quality, and avoid costly penalties.
If you’re looking to enhance your facility’s water purification system, explore our RO EDI solutions designed to support compliance in the pharmaceutical and biotech industries. Contact us today to learn more!
#RO EDI Plant#RO EDI System#commercial reverse osmosis water purification systems#Electrodeionization#pharmacutical industry#biotech industry#swjal process
0 notes
Text
Ensuring Regulatory Compliance in WFI Generation: Best Practices and Innovations
Regulatory compliance is a critical aspect of Water for Injection (WFI) generation in the pharmaceutical industry. Strict guidelines from global regulatory bodies ensure that WFI meets the highest standards of purity and quality. Failure to comply can result in product recalls, financial losses, and reputational damage. This article explores best practices and innovations for ensuring regulatory compliance in WFI generation.
Key Regulatory Requirements for WFI
Different regulatory agencies establish stringent requirements for WFI production to guarantee its safety and effectiveness. The most recognized standards include:
United States Pharmacopeia (USP): Specifies WFI must meet endotoxin limits and be produced through distillation or reverse osmosis (RO) with ultrafiltration.
European Pharmacopoeia (Ph. Eur.): Allows WFI production via distillation and, since 2017, through membrane-based methods like RO and EDI.
World Health Organization (WHO): Mandates strict microbial and chemical quality controls.
Good Manufacturing Practice (GMP) Guidelines: Require continuous monitoring, validation, and risk-based approaches to ensure compliance.

Best Practices for Regulatory Compliance
Pharmaceutical manufacturers must adopt best practices to maintain compliance with evolving regulations.
1. Validation and Qualification
Conduct thorough Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ).
Validate system components such as distillation units, RO membranes, and storage tanks.
Implement re-validation schedules to ensure ongoing compliance.
2. Microbial Control and Monitoring
Use continuous water quality monitoring for microbial and endotoxin levels.
Implement regular biofilm control measures to prevent contamination.
Maintain WFI storage and distribution systems at 80°C or above to inhibit microbial growth.
3. Real-Time Data Logging and Automation
Integrate automated control systems for real-time monitoring of conductivity, TOC, and temperature.
Use 21 CFR Part 11-compliant software for secure electronic record-keeping.
Leverage AI-driven predictive analytics to detect deviations before failures occur.
4. Risk-Based Approach to System Design
Conduct Hazard Analysis and Critical Control Points (HACCP) assessments for risk management.
Design systems with redundant purification stages to prevent contamination.
Implement closed-loop distribution to eliminate external contamination risks.
Innovations in WFI Generation for Enhanced Compliance
Technological advancements continue to enhance regulatory compliance in WFI production.
1. Membrane-Based WFI Production
Reverse osmosis (RO) and electrodeionization (EDI) provide a cost-effective, energy-efficient alternative to distillation.
Ultrafiltration (UF) units ensure bacterial and endotoxin removal, aligning with modern regulatory allowances.
2. Advanced Sanitization Techniques
Ozone sanitization eliminates microbial risks without chemical residues.
UV disinfection systems enhance microbial control in storage and distribution.
Electropolished stainless steel piping minimizes contamination risks and biofilm formation.
3. Cloud-Based Compliance Solutions
Remote monitoring platforms allow regulatory compliance tracking in real-time.
Automated compliance reporting tools ensure efficient audit preparation.
Blockchain-based traceability enhances data integrity and regulatory transparency.
Future-Proofing WFI Compliance
Ensuring regulatory compliance in WFI generation plant requires a proactive approach that integrates validation, automation, and microbial control. By adopting best practices and emerging technologies, pharmaceutical manufacturers can maintain compliance while improving operational efficiency.
0 notes
Text
Bio Kill Plant: An Essential System for Biohazard Waste Treatment
A Bio Kill Plant is a specialized wastewater treatment system designed to neutralize and eliminate biological contaminants, ensuring safe disposal. These systems are critical in industries such as pharmaceuticals, biotechnology, and healthcare, where biohazardous waste is generated. The proper treatment of such waste prevents environmental contamination and ensures compliance with strict regulatory standards. By incorporating advanced biological and chemical treatment processes, Bio Kill Plants play a crucial role in sustainable industrial operations.
Working Principle
The operation of a Bio Kill Plant involves multiple treatment stages to effectively neutralize biohazardous contaminants. Initially, wastewater undergoes biological treatment where microorganisms break down organic matter. This is followed by chemical neutralization, where disinfectants such as chlorine or ozone are introduced to eliminate harmful pathogens. Sterilization techniques, including heat treatment and ultraviolet (UV) disinfection, further ensure the elimination of any remaining biological threats. The final treated water meets environmental discharge norms, making the process safe and compliant.

Key Components & Technologies Used
A Bio Kill Plant integrates various components to ensure efficient and reliable operation. Key elements include:
Bio-reactors: Facilitate biological treatment by breaking down organic contaminants using microbial action.
Chemical Dosing Systems: Control the addition of disinfectants and neutralizing agents to achieve optimal sterilization.
Filtration Units: Remove solid particulates and ensure clarity in the treated effluent.
Control & Automation Systems: Monitor treatment parameters in real-time, ensuring process stability and regulatory compliance. These advanced technologies enhance operational efficiency, reduce manual intervention, and ensure consistent treatment outcomes.
Applications in Various Industries
The Bio Kill Plant is widely used across industries where biological contamination poses a risk. Some key applications include:
Pharmaceuticals & Biotech: Ensuring safe disposal of biohazardous effluents from drug manufacturing and research laboratories.
Hospitals & Healthcare Facilities: Treating medical wastewater contaminated with pathogens, blood, and other hazardous materials.
Food & Beverage Industry: Managing waste generated from microbial fermentation and food processing operations.
Research Laboratories: Handling biohazardous waste from microbiology and genetic engineering experiments. With its versatility, the Bio Kill Plant has become an indispensable solution for industries prioritizing environmental safety and regulatory compliance.
Regulatory Compliance & Safety Standards
To ensure environmental protection, Bio Kill Plants must adhere to strict regulatory guidelines. Compliance with environmental discharge standards, such as those set by the Central Pollution Control Board (CPCB) and the U.S. Environmental Protection Agency (EPA), is mandatory. Industry regulations dictate permissible contamination levels, ensuring that treated effluents are safe for disposal. Additionally, occupational safety standards such as ISO 14001 and Good Manufacturing Practices (GMP) are followed to safeguard workers handling biohazardous waste. Regular audits and monitoring ensure adherence to these safety norms.
Benefits of a Bio Kill Plant
The installation of a Bio Kill Plant offers several advantages, making it an essential system for industrial wastewater treatment. Some key benefits include:
Eco-Friendly Operations: Reduces environmental impact by ensuring safe disposal of biohazardous waste.
Regulatory Compliance: Meets stringent industry standards, avoiding legal penalties and ensuring smooth operations.
Cost Efficiency: Reduces the need for external waste disposal services, lowering operational costs in the long run.
Automation & Reliability: Advanced monitoring and automation ensure consistent treatment quality with minimal human intervention. By integrating a Bio Kill Plant, industries can ensure sustainable wastewater management while maintaining operational efficiency.
Conclusion
A Bio Kill Plant serves as a critical solution for industries dealing with biohazardous waste, ensuring safe and compliant disposal. Its advanced treatment processes, regulatory adherence, and environmental benefits make it a vital investment for pharmaceutical, biotech, and healthcare industries. To implement a reliable and high-performance Bio Kill Plant, partnering with an experienced manufacturer is essential. SWJAL PROCESS Pvt. Ltd. is a leading Bio Kill Plant manufacturer in Mumbai, India, providing cutting-edge solutions tailored to industrial needs. Contact SWJAL PROCESS today for innovative and efficient wastewater treatment solutions.
0 notes
Text
Water Treatment Plant Required in Pharmaceutical and Biotech Industry
Importance of Water Treatment in Pharmaceutical and Biotech Industries
Water is a critical component in pharmaceutical and biotech industries, where high purity is essential for manufacturing, formulation, and cleaning processes. Contaminants such as dissolved solids, bacteria, and organic matter must be eliminated to comply with stringent industry standards. Water treatment plants ensure the production of high-purity water, maintaining product integrity and regulatory compliance.
Key Purification Technologies for Pharmaceutical and Biotech Industries
Advanced purification technologies are required to meet the industry's stringent demands. Reverse Osmosis (RO) is commonly used for removing dissolved impurities, while Electrodeionization (EDI) ensures ultra-pure deionized water. Ultrafiltration (UF) plays a crucial role in bacterial and endotoxin removal, while Water for Injection (WFI) systems provide pyrogen-free water for injectable formulations. The integration of these technologies guarantees consistent high-quality water.
Regulatory Compliance and Industry Standards
Regulatory bodies such as the US FDA, WHO, and cGMP enforce strict guidelines for pharmaceutical-grade water. Compliance with these standards is necessary to prevent contamination and ensure patient safety. Water treatment plants must meet validation protocols, including periodic monitoring and documentation, to demonstrate adherence to quality requirements.
Challenges in Pharmaceutical Water Treatment
Several challenges arise in pharmaceutical and biotech water treatment, including microbial contamination, scaling, and system validation. Maintaining water purity across storage and distribution systems requires continuous monitoring and automated control solutions. Failure to address these challenges can lead to compromised drug formulations and regulatory non-compliance.
Benefits of Advanced Water Treatment Systems
High-efficiency water treatment plants offer several benefits, including:
Enhanced Product Quality – Ensuring the purity of raw materials and formulations.
Regulatory Compliance – Meeting global pharmaceutical standards.
Cost Efficiency – Reducing maintenance and operational costs.
Sustainability – Minimizing wastewater generation and energy consumption.
Conclusion
Investing in a reliable water treatment plant is essential for pharmaceutical and biotech companies to maintain high-quality standards and regulatory compliance. Advanced purification technologies such as RO-EDI sytem, and WFI system ensure the highest level of water purity. A well-maintained water treatment system enhances efficiency, minimizes risks, and supports the production of safe and effective pharmaceutical products.
#RO-EDY#WFI System#Water for injection#high purity water solution#swjal process#manufacturer#mumbai#india
0 notes
Text
Pharmaceutical Water for Injection System Plant Manufacturers in India
The pharmaceutical industry relies on the highest standards of water purity, particularly when it comes to Water for Injection (WFI). WFI is an essential component in the production of injectables, parenteral drugs, and other pharmaceutical applications that demand the utmost sterility. In India, a growing number of manufacturers specialize in designing and installing Water for Injection system plants that comply with international regulatory standards such as the United States Pharmacopeia (USP), European Pharmacopoeia (EP), and World Health Organization (WHO) guidelines.
Understanding Water for Injection (WFI)
Water for Injection (WFI) is highly purified water that is free from pyrogens, bacteria, and organic contaminants. It is primarily used for intravenous medications, diluents for injections, and cleaning pharmaceutical equipment. The production of WFI requires stringent treatment processes, including reverse osmosis (RO), ultrafiltration (UF), distillation, and sterilization.
A well-designed WFI system ensures consistent water quality that meets pharmacopeial standards while minimizing contamination risks. The leading pharmaceutical WFI plant manufacturers in India employ cutting-edge technologies to provide cost-effective, reliable, and compliant solutions.
Key Technologies Used in WFI Systems
Multiple Effect Distillation (MED): This method involves a series of distillation columns to remove impurities and pyrogens, ensuring the highest purity level.
Vapor Compression Distillation (VCD): Known for energy efficiency, VCD utilizes vapor recompression technology to produce WFI with minimal water wastage.
Reverse Osmosis (RO) and Ultrafiltration (UF) Combination: A non-distillation method where a combination of reverse osmosis and ultrafiltration is used to generate WFI.
Electrodeionization (EDI): A process that removes ionic contaminants without the need for chemical regeneration, ensuring continuous purified water production.
Key Features of a Pharmaceutical WFI System
Regulatory Compliance: Leading manufacturers design systems that adhere to global standards, including cGMP, USP, and EP guidelines.
High-Purity Output: The latest WFI systems ensure ultra-low conductivity, pyrogen-free water suitable for pharmaceutical applications.
Automated Control Systems: Advanced automation, including PLC and SCADA-based controls, ensures real-time monitoring and validation.
Sterile and Sanitary Design: Made from SS 316L stainless steel with electro-polished interiors, ensuring a contamination-free environment.
Energy Efficiency: Modern WFI systems are designed for low energy consumption and minimal environmental impact.
CIP/SIP Integration: Clean-in-place (CIP) and steam-in-place (SIP) processes maintain hygiene and prevent biofilm formation.
Top Pharmaceutical Water for Injection System Manufacturers in India
India has a robust network of manufacturers providing high-quality WFI systems that cater to the pharmaceutical, biotechnology, and healthcare industries. Here are some of the leading companies:
Swjal Process Pvt. Ltd.
Specializes in customized WFI solutions with a strong focus on automation and compliance.
Offers end-to-end solutions, including design, installation, and validation services.
Expertise in reverse osmosis, electrodeionization, and distillation-based WFI systems.
Hitech Water Solutions
Provides turnkey WFI plants with energy-efficient designs.
Ensures compliance with cGMP and international quality standards.
Offers preventive maintenance and service support.
Pure Water Technologies
Manufactures WFI systems with innovative energy-saving technologies.
Expertise in multiple effect distillation and vapor compression distillation.
Provides pharmaceutical-grade storage and distribution systems.
Pharmatech Process Equipments
Specializes in stainless steel WFI generation and storage solutions.
Offers integrated automation for seamless plant operation.
Compliant with WHO, US FDA, and European standards.
Aqua Purification Systems
Designs and installs complete WFI systems for pharmaceutical manufacturing.
Utilizes advanced purification techniques to meet stringent water quality requirements.
Provides on-site validation and documentation services.

Why Choose Indian WFI System Manufacturers?
India has emerged as a global hub for pharmaceutical equipment manufacturing, offering high-quality solutions at competitive prices. The key advantages of choosing Indian WFI system manufacturers include:
Cost-Effectiveness: Indian manufacturers provide world-class WFI systems at a fraction of the cost compared to Western suppliers.
Advanced Technology: The industry has embraced cutting-edge technologies such as AI-based monitoring and IoT-enabled predictive maintenance.
Regulatory Expertise: Indian manufacturers have extensive experience in meeting global pharmaceutical regulations.
Comprehensive Services: Many companies offer complete solutions, from consultation and design to installation, commissioning, and maintenance.
Scalability: Indian manufacturers cater to a wide range of production capacities, from small-scale biotech firms to large pharmaceutical plants.
Future Trends in WFI System Manufacturing
The pharmaceutical industry is witnessing continuous advancements in WFI system design and operation. Some of the upcoming trends include:
Sustainable Water Management: New systems focus on reducing water wastage and optimizing energy use.
Digital Integration: Smart monitoring solutions using AI and IoT are enhancing efficiency and reliability.
Modular Systems: Compact, pre-validated modular WFI units are gaining popularity for ease of installation.
Non-Distillation Methods: Membrane-based WFI generation is becoming a viable alternative to traditional distillation.
Conclusion
Pharmaceutical Water for Injection (WFI) systems play a crucial role in ensuring the safety and efficacy of injectable drugs. India’s leading manufacturers offer state-of-the-art WFI solutions that comply with global regulatory standards while maintaining cost efficiency and high performance. Companies like Swjal Process Pvt. Ltd. and other top manufacturers are setting new benchmarks in WFI system design, automation, and sustainability. As the pharmaceutical industry continues to grow, the demand for reliable and efficient WFI systems will only increase, making India a key player in global pharmaceutical water treatment solutions.
SWJAL PROCESS Pvt. Ltd. is a leading Pharmaceutical Water for Injection System Plant Manufacturer in Mumbai, India.
#wfi system#water for injection#pharmacutical WFI plant#water treatment process plant manufacturers#manufacturer#swjal process#biotech industry#pharmacutical industry
0 notes
Text
Pure Water Storage and Distribution System: Ensuring Pure Water Availability
In industries such as pharmaceuticals, biotechnology, and healthcare, pure water is a vital resource used across production, cleaning, and testing. Maintaining the purity and availability of water throughout its journey is critical. Water storage and distribution systems are designed to preserve the quality of purified water while ensuring an uninterrupted supply to meet operational demands.
Importance of Pure Water Storage and Distribution Systems
Pure Water treated through advanced purification methods, such as Reverse Osmosis (RO) and Electrodeionization (EDI), must retain its purity until it is used. This is where storage and distribution systems play a key role. These systems prevent recontamination by minimizing exposure to air, controlling microbial growth, and maintaining consistent flow rates. The reliability of these systems directly affects the safety and efficacy of pharmaceutical products, making them indispensable in industries governed by strict regulatory requirements.
Key Features of Pure Water Storage Systems
Pure Water storage systems are designed to maintain the chemical and microbiological stability of purified water. Some critical features include:
Sanitary Construction: Storage tanks are made from corrosion-resistant materials such as stainless steel (SS316L) to meet pharmaceutical-grade standards. The internal surfaces are electropolished to prevent microbial adhesion.
Vent Filters: These are used to protect the stored water from airborne contaminants. Hydrophobic filters allow the escape of gases while preventing particles or microorganisms from entering the tank.
Temperature Control: Thermal jackets or insulation systems are often integrated to maintain water temperature, reducing the risk of microbial proliferation.
Sloped Design: Tanks are designed with sloped bottoms for complete drainage, ensuring no water stagnates, which could lead to contamination.
Efficient Distribution Systems
Pure Water distribution systems are engineered to maintain a consistent supply of high-purity water throughout the facility. The systems are designed with precision to prevent contamination and ensure compliance with stringent quality standards. Key components include:
Loop Piping System: The loop design ensures that water is continuously circulated, preventing stagnation and microbial growth.
Sanitary Valves and Pumps: These components maintain a hygienic environment within the distribution system and are made of materials compatible with purified water.
Automated Control Panels: PLC-based control systems allow real-time monitoring of flow rates, temperature, and pressure, ensuring operational efficiency.
UV Sterilizers: These are installed to eliminate any residual microbial contamination during water circulation.
Benefits of Advanced Pure Water Storage and Distribution Systems
Consistent Water Quality: Advanced systems ensure that the purity of water remains intact, meeting pharmaceutical-grade standards such as USP and cGMP.
Operational Efficiency: Automated monitoring and control systems reduce downtime and the need for manual intervention.
Regulatory Compliance: High-quality materials and advanced features ensure adherence to stringent industry norms.
Cost-Effectiveness: Energy-efficient designs and low maintenance requirements contribute to reduced operational costs.
Applications in the Pharmaceutical Industry
In pharmaceutical manufacturing, Pure water storage and distribution systems are critical for various applications, including:
Preparation of Sterile Solutions: Ensuring the availability of high-purity water for drug formulation.
Equipment Cleaning: Facilitating the cleaning of reactors, pipelines, and storage vessels to prevent cross-contamination.
Analytical Testing: Providing ultrapure water for laboratory testing to ensure accurate results.
Ensuring Compliance with Standards
Pure Water storage and distribution systems are designed to meet stringent guidelines established by regulatory bodies like the US FDA, EMA, and WHO. Features such as online monitoring, documentation capabilities, and validation protocols ensure compliance with Good Manufacturing Practices (GMP). Regular maintenance and qualification processes are essential to uphold these standards.
Innovations in Water Storage and Distribution Systems
Advancements in technology have led to the development of more efficient and sustainable systems. Features like real-time IoT-based monitoring, energy recovery systems, and advanced materials with enhanced corrosion resistance are becoming increasingly popular. These innovations enable industries to maintain high water quality standards while optimizing resource consumption.
Conclusion
Pure Water storage and distribution systems are integral to industries that require high-purity water for critical applications. By ensuring consistent quality, operational efficiency, and compliance with stringent standards, these systems support the production of safe and effective products.
SWJAL PROCESS Pvt. Ltd. is a trusted manufacturer of high-quality water storage and distribution systems in Mumbai, India, delivering reliable solutions tailored to industry-specific requirements.
#Pure Water Storage and Distribution System#storage and distribution plant#RO EDI plant#DM Water Plant#WFI Plant#swjal process
0 notes
Text
DM Water Plant Technology: How They Work
DM water plants use ion exchange technology to remove dissolved minerals and impurities, producing high-purity water. This process is the backbone of 2000 LPH, 1200 LPH, and 600 LPH systems, which cater to various industrial needs.
Ion Exchange Process
Cation Exchange:
Removes positively charged ions (calcium, magnesium) by replacing them with hydrogen ions.
Anion Exchange:
Replaces negatively charged ions (chlorides, sulfates) with hydroxide ions.
Regeneration:
Resins are restored using acid and alkali solutions for repeated use.

Advanced Features
PLC Controls: Automated systems for consistent performance.
Dual-Bed and Mixed-Bed Configurations: Enhanced purity for demanding applications.
Corrosion-Resistant Materials: Ensures longevity and reliability.
Applications of Ion Exchange Technology
Power Generation: Ensures scale-free operations in boilers.
Healthcare: Provides contaminant-free water for critical processes.
Automotive Industry: Supports the production of high-performance components.
Conclusion
Understanding the technology behind DM water plants helps in appreciating their role in industrial operations. Whether it’s a 2000 LPH plant for large industries or a 600 LPH system for smaller setups, ion exchange remains at the core of their efficiency.
#DM water plant 2000 LPH#1200 LPH Commercial DM Water Plant#600 LPH Water#dm water plant#manufacturer#swjal process#mumbai#india
0 notes
Text
Pure Steam Generator: An Essential Component in Sterile Processing
A Pure Steam Generator (PSG) plays a pivotal role in industries requiring sterile and contamination-free processes, particularly in pharmaceuticals, biotechnology, and healthcare. These advanced systems are engineered to produce pure steam, free from contaminants and impurities, ensuring compliance with stringent regulatory standards.
Pure steam is predominantly utilized for sterilizing equipment, pipelines, and vessels, as well as in humidification and cleanroom environments. It is also essential for autoclaving, ensuring that critical manufacturing processes meet international guidelines such as the US FDA, EMA, and GMP standards.
Working Principle of Pure Steam Generators
Pure Steam Generators are designed to generate steam by evaporating purified feedwater. The process begins with deionized or reverse osmosis (RO) water, which is heated using an energy source, typically steam or electricity. The water is vaporized in a controlled environment, and the resulting steam is free from contaminants such as endotoxins, microorganisms, and volatile impurities.
To achieve consistent quality, PSG systems incorporate multiple stages of separation. These stages remove any entrained water droplets, ensuring that only high-purity steam is produced. Advanced models are equipped with state-of-the-art controls for monitoring temperature, pressure, and steam quality in real-time, which ensures operational reliability and process efficiency.

Key Features and Advantages
Pure Steam Generators are designed to meet the unique demands of various industries. Key features include:
High-Purity Steam Production: Ensures sterility and compliance with pharmaceutical-grade requirements.
Energy Efficiency: Optimized design reduces energy consumption, minimizing operational costs.
Corrosion Resistance: High-grade stainless steel construction prevents contamination and enhances durability.
Automation and Monitoring: Integrated control systems enable precise operation, ensuring consistency in steam production.
Compact Design: Space-saving models are ideal for installations in constrained environments.
The use of pure steam generators minimizes the risk of contamination, safeguards product quality, and ensures compliance with health and safety standards.
Applications of Pure Steam Generators
Pure steam generators find widespread application in industries where cleanliness and sterility are critical. Some of the key applications include:
Pharmaceutical Industry: Sterilization of process equipment, pipelines, and manufacturing environments.
Biotechnology: Ensuring aseptic conditions for sensitive biological processes.
Hospitals: Autoclaving surgical instruments and maintaining sterile operating rooms.
Food and Beverage: Sterilization of containers, packaging materials, and processing lines.
Maintenance and Validation
Regular maintenance and validation are essential for ensuring the long-term performance and compliance of pure steam generators. Maintenance tasks include cleaning, inspection of critical components, and replacement of worn-out parts. Validation processes, such as steam quality testing, are conducted to verify compliance with global standards. These measures ensure that the system operates efficiently, delivering pure steam without interruptions.
Industry Standards and Compliance
Manufacturers of Pure Steam Generators adhere to strict standards to ensure quality and reliability. Common standards include ASME-BPE, cGMP, and ISPE guidelines. These standards govern the design, materials of construction, and operational parameters of the systems, ensuring that they meet the specific needs of critical applications.
Conclusion
Pure Steam Generators are indispensable in industries that demand sterility and high-quality steam. Their role in ensuring compliance, safeguarding product integrity, and optimizing operational efficiency cannot be overstated. From advanced automation features to robust designs, these systems address the evolving needs of modern industries.
SWJAL PROCESS Pvt. Ltd. is a leading Pure Steam Generator manufacturer in India.
0 notes
Text
Water for Injection A Critical Component in Pharmaceutical Processes
Water for Injection (WFI) holds a pivotal role in pharmaceutical and healthcare industries. As a highly purified form of water, WFI is indispensable for producing injectables, parenteral solutions, and other critical formulations. Its stringent quality requirements ensure that it remains free from pyrogens, microbes, and contaminants, making it safe for direct introduction into the human body. The meticulous production and storage of WFI system are essential to maintain its integrity and suitability for use in sensitive applications.
Understanding Water for Injection
It is governed by strict standards set by pharmacopeias such as the United States Pharmacopeia (USP), European Pharmacopeia (EP), and Indian Pharmacopeia (IP). These standards outline precise requirements for conductivity, microbial load, and endotoxin levels. WFI must exhibit high purity with conductivity less than 1.3 µS/cm at 25°C and endotoxin levels not exceeding 0.25 EU/mL.
The primary applications of WFI include:
Parenteral Drug Manufacturing: Used as a solvent for drug formulations administered via injection.
Sterile Product Preparation: Serves as a base ingredient in sterile solutions and suspensions.
Cleaning Processes: Used for sterilizing equipment, containers, and closures in aseptic environments.
Methods of WFI Production
Producing Water for Injection involves advanced purification techniques to meet the required standards. Key methods include:
Distillation: Considered the most reliable method, distillation effectively removes impurities, pyrogens, and bacteria. Multi-effect distillation (MED) and vapor compression distillation (VCD) are commonly used systems.
Reverse Osmosis (RO) with Electrodeionization (EDI): An efficient alternative combining RO and EDI to achieve high purity. This method is often complemented with ultrafiltration to ensure endotoxin removal.
Ultrafiltration: Typically employed in conjunction with other methods, ultrafiltration ensures the elimination of pyrogens and endotoxins from water.
The choice of method depends on the scale of production, energy efficiency requirements, and compliance with regulatory norms.
Storage and Distribution of WFI
Maintaining the quality of Water for Injection post-production is as critical as its generation. Specially designed storage and distribution systems are employed to prevent microbial contamination and ensure consistent quality. Key features include:
Sanitary Design: Stainless steel tanks and piping with smooth, polished surfaces minimize microbial growth.
Temperature Control: Storage systems are often maintained at high temperatures (typically 80°C) to inhibit bacterial proliferation.
Circulating Loops: Continuous circulation prevents water stagnation and maintains consistent quality.
Advanced Monitoring: Real-time sensors and automated controls ensure compliance with quality standards.

Regulatory Compliance and Validation
Regulatory bodies mandate rigorous validation of WFI systems to ensure consistent performance. Validation includes:
Design Qualification (DQ): Verification of system design against user requirements.
Installation Qualification (IQ): Confirmation that the system is installed correctly.
Operational Qualification (OQ): Testing to ensure the system operates within specified parameters.
Performance Qualification (PQ): Evaluation of the system’s ability to produce WFI consistently over time.
Routine maintenance, monitoring, and periodic re-validation are also essential to ensure ongoing compliance.
Significance of WFI in Pharmaceutical Manufacturing
The importance of Water for Injection in pharmaceutical manufacturing cannot be overstated. Its purity and sterility are fundamental to ensuring patient safety and product efficacy. Without adherence to stringent standards, the risks of contamination and adverse reactions increase significantly, compromising the quality and safety of pharmaceutical products.
Pharmaceutical manufacturers must invest in advanced WFI systems that deliver consistent quality while optimizing operational efficiency. The integration of automation and real-time monitoring has further enhanced the reliability and traceability of WFI systems.
Future Trends in WFI Production
As the pharmaceutical industry evolves, innovations in WFI production and management are becoming increasingly critical. Emerging trends include:
Energy-Efficient Systems: New technologies are reducing energy consumption in WFI generation, making processes more sustainable.
Enhanced Automation: Advanced monitoring and control systems improve operational efficiency and regulatory compliance.
Integrated Solutions: Combining WFI production with other water treatment processes for seamless integration and cost efficiency.
The industry’s focus on innovation ensures that WFI production continues to meet the growing demands of pharmaceutical manufacturing.
It remains a cornerstone of pharmaceutical processes, ensuring the highest standards of purity and safety. Its production, storage, and distribution require precision, adherence to stringent norms, and continuous innovation.
Swjal Process Pvt. Ltd. is a leading Water for Injection Plant manufacturer in India, delivering state-of-the-art solutions that align with global standards and support the pharmaceutical industry’s mission of safeguarding health and well-being.
#wfi system#wfi plant#water for injection#pharmacutical industry#high purity water solution#Swjal Process#India
0 notes
Text
Optimizing Pharmaceutical and Biotech Processes with RO and EDI Systems
The pharmaceutical and biotech industries demand precision and reliability in every step of their processes. Among these, water purification stands as a cornerstone for manufacturing, research, and quality control. Reverse Osmosis (RO) and Electrodeionization (EDI) systems have become the gold standard for producing ultrapure water that meets stringent regulatory requirements. This article explores how these technologies enable efficiency, sustainability, and compliance in the industry.
The Need for High-Purity Water
Water plays a critical role in pharmaceutical and biotech operations, serving as a raw material, cleaning agent, and process facilitator. Regulatory frameworks such as the United States Pharmacopeia (USP) and European Pharmacopeia (EP) specify strict standards for water purity, including thresholds for microbial contamination, conductivity, and total organic carbon (TOC). Impure water can compromise drug safety, hinder research outcomes, and lead to costly recalls or regulatory penalties.
RO and EDI systems are tailored to address these challenges, ensuring consistent production of high-quality water suitable for applications ranging from drug formulation to laboratory analysis.
How RO and EDI Systems Work
Reverse Osmosis (RO): RO systems operate by forcing water through a semi-permeable membrane that filters out dissolved salts, organic compounds, and other contaminants. This technology serves as the primary purification step, achieving up to 99% rejection of impurities. Advanced RO systems now incorporate features like anti-scaling agents and energy recovery systems to enhance efficiency and durability.
Electrodeionization (EDI): EDI systems refine the water further, using ion-exchange resins and an electrical current to remove remaining ions. Unlike traditional deionization methods, EDI operates continuously without the need for chemical regenerants, making it both environmentally friendly and cost-effective.
Key Advantages of RO and EDI in Pharma and Biotech
Regulatory Compliance: RO and EDI systems consistently produce water that meets stringent standards for Purified Water, Highly Purified Water, and Water for Injection (WFI). This ensures seamless adherence to regulatory requirements worldwide.
Operational Efficiency: Continuous purification capabilities reduce downtime associated with maintenance and chemical handling, improving overall operational throughput.
Environmental Sustainability: By eliminating the need for harsh chemicals in regeneration, EDI reduces environmental impact. Energy-efficient RO systems further contribute to sustainability goals by optimizing water recovery and reducing power consumption.
Cost Savings: Automation and reduced reliance on consumables lower long-term operational costs, making these systems economically viable for facilities of all sizes.
Adaptability and Scalability: Modular RO and EDI units can be customized to meet the specific needs of small labs or large-scale manufacturing plants, ensuring flexibility in design and deployment.
Applications Across the Industry
RO and EDI systems have versatile applications in the pharmaceutical and biotech sectors:
Drug Manufacturing: High-purity water is essential for preparing solutions, diluents, and APIs. Any contaminants in the water can compromise the stability and efficacy of pharmaceutical products.
Cleaning and Sterilization: Water used for cleaning equipment and sterilizing containers must be free of microbial and ionic impurities to ensure compliance with Good Manufacturing Practices (GMP).
Cell Culture and Bioprocessing: The sensitive nature of cell cultures and bioprocesses demands ultrapure water to avoid adverse reactions that could compromise yields or product quality.
Laboratory and Analytical Testing: Techniques such as HPLC, GC-MS, and spectrophotometry rely on ultrapure water to avoid interference with test results, ensuring accuracy and reproducibility.
Technological Innovations Driving Excellence
The evolving needs of pharmaceutical and biotech industries have spurred significant advancements in RO and EDI systems:
Smart Monitoring Systems: Integration of IoT-enabled sensors allows real-time monitoring of water quality parameters, such as conductivity and TOC. These systems can trigger alerts and provide actionable insights for predictive maintenance.
Enhanced Membrane Performance: New-generation RO membranes offer improved fouling resistance, higher permeability, and extended lifespans, reducing both downtime and operating costs.
Energy Optimization: Advanced energy recovery devices in RO systems reduce power consumption, aligning with the industry's sustainability objectives.
Hybrid Configurations: Combining RO and EDI with complementary technologies like UV disinfection and ozone generation creates multi-barrier systems that deliver exceptional water quality and microbial control.
Case in Point: A Biotech Facility’s Transformation
A biotechnology firm specializing in vaccine production faced challenges with its aging water purification system, which required frequent maintenance and chemical handling. The company replaced its setup with an integrated RO-EDI system. This upgrade delivered remarkable results:
Improved Water Quality: Conductivity levels dropped below 0.1 µS/cm, ensuring compliance with USP standards.
Cost Reduction: Operational costs decreased by 25%, thanks to the elimination of chemical regenerants and reduced energy usage.
Increased Efficiency: Automated controls streamlined operations, allowing for uninterrupted water supply during peak production cycles.
Sustainability Gains: Water recovery rates improved by 15%, and the facility's carbon footprint was significantly reduced.
The Path Forward
As the pharmaceutical and biotech industries continue to evolve, the demand for reliable, efficient, and sustainable water purification systems will grow. RO and EDI technologies are well-positioned to meet these demands, offering a blend of performance, compliance, and environmental responsibility.
For organizations looking to optimize their water systems, adopting advanced RO-EDI solutions can provide a competitive edge. By ensuring water purity, these technologies not only enhance product quality and safety but also support operational excellence and sustainability goals. In a sector where precision is paramount, RO and EDI systems remain indispensable tools for success.
0 notes