#Smectite Clays
Explore tagged Tumblr posts
Text
Argiles smectiques, Prévisions de la Taille du Marché Mondial, Classement et Part de Marché des 19 Premières Entreprises
Selon le nouveau rapport d'étude de marché “Rapport sur le marché mondial de Argiles smectiques 2024-2030”, publié par QYResearch, la taille du marché mondial de Argiles smectiques devrait atteindre 2071 millions de dollars d'ici 2030, à un TCAC de 5,2% au cours de la période de prévision.
Figure 1. Taille du marché mondial de Argiles smectiques (en millions de dollars américains), 2019-2030

Selon QYResearch, les principaux fabricants mondiaux de Argiles smectiques comprennent Minerals Technologies (Amcol), Clariant, Imerys (S&B), Bentonite Performance Minerals LLC (BPM), Black Hills Bentonite LLC, Bentonite Company LLC (Russia), Tolsa Group, Wyo-Ben Inc, Ashapura Minechem Limited, Laviosa Minerals SpA, etc. En 2023, les dix premiers acteurs mondiaux détenaient une part d'environ 65,0% en termes de chiffre d'affaires.
Figure 2. Classement et part de marché des 19 premiers acteurs mondiaux de Argiles smectiques (Le classement est basé sur le chiffre d'affaires de 2023, continuellement mis à jour)

À propos de QYResearch
QYResearch a été fondée en 2007 en Californie aux États-Unis. C'est une société de conseil et d'étude de marché de premier plan à l'échelle mondiale. Avec plus de 17 ans d'expérience et une équipe de recherche professionnelle dans différentes villes du monde, QYResearch se concentre sur le conseil en gestion, les services de base de données et de séminaires, le conseil en IPO, la recherche de la chaîne industrielle et la recherche personnalisée. Nous société a pour objectif d’aider nos clients à réussir en leur fournissant un modèle de revenus non linéaire. Nous sommes mondialement reconnus pour notre vaste portefeuille de services, notre bonne citoyenneté d'entreprise et notre fort engagement envers la durabilité. Jusqu'à présent, nous avons coopéré avec plus de 60 000 clients sur les cinq continents. Coopérons et bâtissons ensemble un avenir prometteur et meilleur.
QYResearch est une société de conseil de grande envergure de renommée mondiale. Elle couvre divers segments de marché de la chaîne industrielle de haute technologie, notamment la chaîne industrielle des semi-conducteurs (équipements et pièces de semi-conducteurs, matériaux semi-conducteurs, circuits intégrés, fonderie, emballage et test, dispositifs discrets, capteurs, dispositifs optoélectroniques), la chaîne industrielle photovoltaïque (équipements, cellules, modules, supports de matériaux auxiliaires, onduleurs, terminaux de centrales électriques), la chaîne industrielle des véhicules électriques à énergie nouvelle (batteries et matériaux, pièces automobiles, batteries, moteurs, commande électronique, semi-conducteurs automobiles, etc.), la chaîne industrielle des communications (équipements de système de communication, équipements terminaux, composants électroniques, frontaux RF, modules optiques, 4G/5G/6G, large bande, IoT, économie numérique, IA), la chaîne industrielle des matériaux avancés (matériaux métalliques, polymères, céramiques, nano matériaux, etc.), la chaîne industrielle de fabrication de machines (machines-outils CNC, machines de construction, machines électriques, automatisation 3C, robots industriels, lasers, contrôle industriel, drones), l'alimentation, les boissons et les produits pharmaceutiques, l'équipement médical, l'agriculture, etc.
0 notes
Text
WHERE DID MARS ATMOSPHERE GO??
Blog#441
Wednesday, October 2nd, 2024.
Welcome back,
New research suggests the atmosphere of Mars may be hiding in plain sight, having been absorbed by minerals in the Red Planet's clays. If Mars' envelope of gas did "go to ground" over 3 billion years ago, this could explain how Earth's neighboring planet became so different from our world, potentially losing its capability to host life.
Scientists know that the Red Planet wasn't always the arid and barren landscape that the Mars rovers Perseverance and Curiosity trundle across today.

Both of NASA's rolling robots have uncovered evidence that abundant water flowed over Mars early in its 4.6 billion-year history. But for Mars to have had liquid water, it must also have possessed an atmosphere to stop this water from freezing. The big question for decades has been: where did this atmosphere disappeared?
A team of researchers think that the answer has been under the noses (or the tracks) of Curiosity and Perseverance all this time. In a paper published in Science Advances, they argue that while water was present on the Red Planet, it may have trickled through certain rock types and set off a slow series of reactions that slurped carbon dioxide out of the atmosphere.

This would have then been converted into methane, a form of carbon, and locked up in the clay surface of Mars.
Scientists know that the Red Planet wasn't always the arid and barren landscape that the Mars rovers Perseverance and Curiosity trundle across today. Both of NASA's rolling robots have uncovered evidence that abundant water flowed over Mars early in its 4.6 billion-year history. But for Mars to have had liquid water, it must also have possessed an atmosphere to stop this water from freezing. The big question for decades has been: where did this atmosphere go when disappeared?

A team of researchers think that the answer has been under the noses (or the tracks) of Curiosity and Perseverance all this time. In a paper published in Science Advances, they argue that while water was present on the Red Planet, it may have trickled through certain rock types and set off a slow series of reactions that slurped carbon dioxide out of the atmosphere. This would have then been converted into methane, a form of carbon, and locked up in the clay surface of Mars.

"Based on our findings on Earth, we show that similar processes likely operated on Mars and that copious amounts of atmospheric carbon dioxide could have transformed to methane and been sequestered in clays," team member Oliver Jagoutz, professor of geology at the Massachusetts Institute of Technology’s Department of Earth, Atmospheric and Planetary Sciences (MIT EAPS), said in a statement. "This methane could still be present and maybe even used as an energy source on Mars in the future."
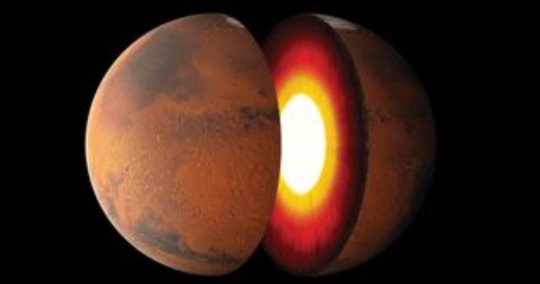
Working within his group at MIT, Jagoutz and colleagues didn't begin their investigation with Mars but with our own planet. The scientists were attempting to determine what geological processes drive the evolution of the hard yet brittle outer shell layer of Earth that encompasses the crust and the upper mantle, and is known as the lithosphere.
The researchers concentrated on a type of surface clay mineral called "smectite," which is very efficient at trapping carbon. Just one grain of smectite is composed of many folds in which carbon can sit and remain for billions of years without being displaced or disturbed.
Originally published on https://www.space.com/
COMING UP!!
(Saturday, October 5th, 2024)
"CAN HUMANS LIVE ON JUPITER'S MOON EUROPA??"
#astronomy#outer space#alternate universe#astrophysics#universe#spacecraft#white universe#space#parallel universe#astrophotography
57 notes
·
View notes
Text







Clay Sketchdump!
Tried to do Clay types, most are finished and some just aren't. None of them have hands on the finished version (per example the Sepiolite group).
Skipped the Medicinal Clay group because I was unsure if it was worth doing. Here's the rundown for those interested:
Clay
This is where Clay would have had more members but it be like dat sometimes. Their job is to model small prototype builings.
Other (Clay Minerals)
Thissss turned out to be messed up and I had to reorganize it.
-Pyrophyllite and Talc make the Pyrophyllite-Talc group (don't look at me, I don't make the name) wich I have finished and did not save the 3 sketches of. Oops.
Pyro/Talc group are more in charge as to keep "the elements" when utilized under control. By elements I mean material, to not create clouds of dust/cremated.
-Sepiolite turned out to have a group but I have the sketches!
Sepiolites are in charge of designing the structure of a fancier building. Falcondoite for aerial, Ferrisepiolite for the ones that need a second evaluation to be re-charged and Loughlinite for pattern-centred buildings.
Ribbon Clay (Attapulgite) was made just for fun, a fancier, sea-centred clay, maybe.
Kaolin
Being more paper-like gems because that totally makes sense. Only 1 missing from the sketches. Instead of paper, they create their own version of paper for signal instructions that can be plastered into other materials/buildings.
Kaolinite makes the most thin version, closer to paper. Dickite makes tube-like shapes, Nacrite makes heavier square shaped ones and Halloysite has the hability to make them shine in the dark (Hidrohalloysite can make it submergible).
Illite
She's literally a mistake, should not be there. It's a Muscovite variety. Shame 😔
Smectite
I have re-made these ones when finishing them because they didn't look to me like they were from the same group + 2 were missing.
They're focused on being more materialistic gems, having the hability to change and purify material for other gems to work with (like Bismuth).
Smectite works with pure-clay material, Beidellite with charcoal, Hectorite with more make-up like material, Montmorillonite with humified material, Nontronite can touch electrified material to work with, Pimelite can work with water conducts, Saponite works with poisonous material (and un-poisons it), Sauconite works with wood, Stevensite is the strongest of all and is also able to work underwater, Swinefordite works with reflective material, Vermiculite works with electric material, Volkonskoite works with iron material and Yakhontovite with rusted material (and can fix it).
Chlorite
The most complex of the clay gang because I already had one designed and I didn't realize dwkfdekgj I had to simplify her design.
They are maintenance workers, analizers and managers. Their hands work like this:

They don't use technology/extra limbs. As clay, they can mold themselves for their task. I wanted to make that clear. Anyway:
-Chlorite, Baileychlore and Clinochlore (or Kämmererite) are the most basic of the group. They focus on analizing building cracks/holes. They can separate their fingers to form wings to better adjust.
-Cookeite and Borocookeite are able to spell fire to cook any small parasite destroying the building.
-Chamosite and Orthochamosite are transport rail fixers as well as testers, since they can use their fingers to mold themselves to have wheels.
-Donbassite can mold and expand their fingers to create tools to put any falling plate into place.
-Franklinfurnaceite is able to create hot clay to fill any hole made by the passing of time.
-Glagolevite is able to levitate and to manipulate liquid obstructing the way.
-Gonyerite is able to create small incinerators to destroy any dust created.
-Nimite is able to fix any scratch on screens.
-Pennantite penalizes any gem if they were the source of any building's destruction (screen broken, cracked wall...). They're also able to manipulate magma to place it (it them becomes solid and can be fixed later).
-Sudoite is able to fix glass and also manipulate liquid to replace glass.
I'm not going too into detail about each gem's personality because you know, this is still a draft and if I'm going to, at least I could share it on indivitual posts for each group. You're free to still ask or to tell me if I missed anyone, or else I think I did many types if not all of Clay Mineral (I'm not counting variations, I want my hand to live).
If you have read this far, thanks!
#steven universe#sketch#su gemsona#su future#su#gemsona#suf#su art#character design#clay compilation
48 notes
·
View notes
Text

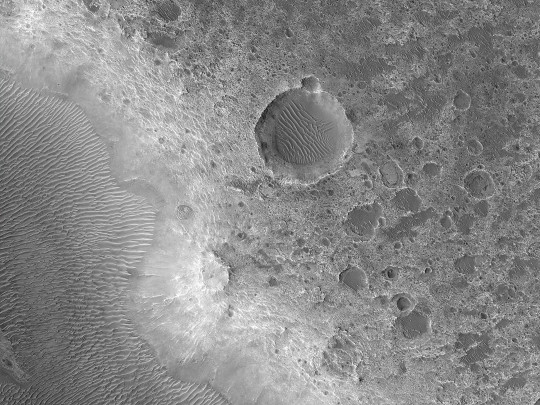
HiPOD: Possible Smectite-Rich Terrain North of Her Desher Vallis
Coverage by the CRISM instrument (also on MRO) of this region of interest hints at a possible outcropping of smectite, which is a clay mineral. This composition is not yet seen elsewhere in the stratigraphy of the area. Color and multispectral analysis will also help us to investigate this geological unit. (Enhanced color cuotut is less than 1 km across; grayscale is less than 5 km.)
ID: ESP_074787_1550 date: 10 July 2022 altitude: 256 km
NASA/JPL-Caltech/UArizona
57 notes
·
View notes
Text
Let's Talk About BENTONITE
There's nothing worse than minding your own business, walking along in the desert when all of a sudden your feet start to feel weighted down. It gets harder and harder to pick up your feet. The mud is sticky and pulling you into the ground. That's bentonite.

Bentonite is type of very soft, plastic clay made primarily of montmorillonite clay.

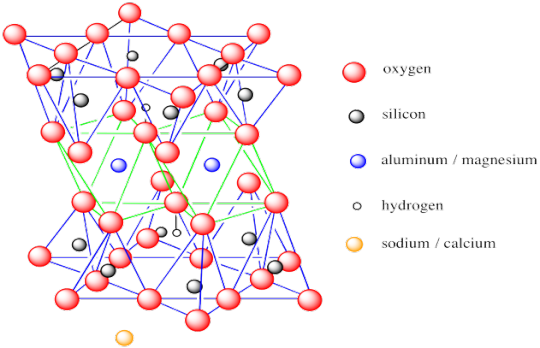
Montmorillonite is a hydrous, aluminum silicate in the smectite group (often you will hear bentonite and smectite used interchangeably).
Bentonite is formed by the alteration of volcanic ash when it comes in contact with water. Clays in general form from the chemical breakdown of feldspars (check out October's posts for more on feldspar!) and when ash gets thrown in the mix something very nasty occurs: swelling clays.
Bentonite increases greatly in volume when water is added...and decreases just as much when the water evaporates or gets absorbed in the groundwater system. This causes the popcorn texture you see in badlands topography.

Popcorn texture in Brushy Basin Member, Morrison Formation, near Evil Tree Bonebed. Sauropod rib found in it.
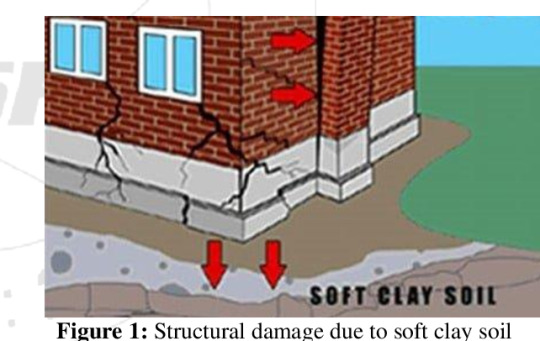
This can also cause structural damage. That is why the building around the Wall at Dinosaur National Monument had to be repaired a few years ago. Basically, the swelling of the bentonite split the foundation.
It also becomes very dangerous to drive on or walk on when it gets wet, becoming a slurry, sticky mess.

This is the main reason why we have to wait for summer to come before we head out to the dig site. We physically cannot get there until the Mancos Shale bentonite is dry enough to drive on.
Bentonite is not all bad though. It has some good uses too.

We actually use it to trap moisture in our skin and hair when we dry out. It's a natural sunblock and relieves burns and itching from bug bites. We even put it in Wendy's Frosty's to give them that smooth texture!

Bentonite is also used in agriculture as a natural pesticide, herbicide, and fungicide in fertilizers.
They also use it to coat drills in the oil industry to keep the drill bit lubricated, as well as coating well walls to keep them from collapsing. If you're a geek, like me, think Avatar, the Last Air Bender and the Fire Nation drill.

Hope you learned a little about bentonite today! Fossilize you later!
63 notes
·
View notes
Text
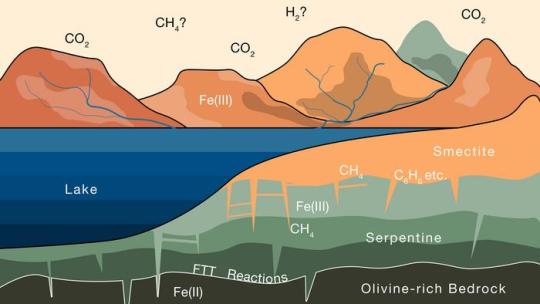
Mars’ missing atmosphere could be hiding in plain sight
Mars wasn’t always the cold desert we see today. There’s increasing evidence that water once flowed on the Red Planet’s surface, billions of years ago. And if there was water, there must also have been a thick atmosphere to keep that water from freezing. But sometime around 3.5 billion years ago, the water dried up, and the air, once heavy with carbon dioxide, dramatically thinned, leaving only the wisp of an atmosphere that clings to the planet today.
Where exactly did Mars’ atmosphere go? This question has been a central mystery of Mars’ 4.6-billion-year history.
For two MIT geologists, the answer may lie in the planet’s clay. In a paper appearing in Science Advances, they propose that much of Mars’ missing atmosphere could be locked up in the planet’s clay-covered crust.
The team makes the case that, while water was present on Mars, the liquid could have trickled through certain rock types and set off a slow chain of reactions that progressively drew carbon dioxide out of the atmosphere and converted it into methane — a form of carbon that could be stored for eons in the planet’s clay surface.
Similar processes occur in some regions on Earth. The researchers used their knowledge of interactions between rocks and gases on Earth and applied that to how similar processes could play out on Mars. They found that, given how much clay is estimated to cover Mars’ surface, the planet’s clay could hold up to 1.7 bar of carbon dioxide, which would be equivalent to around 80 percent of the planet’s initial, early atmosphere.
It’s possible that this sequestered Martian carbon could one day be recovered and converted into propellant to fuel future missions between Mars and Earth, the researchers propose.
“Based on our findings on Earth, we show that similar processes likely operated on Mars, and that copious amounts of atmospheric CO2 could have transformed to methane and been sequestered in clays,” says study author Oliver Jagoutz, professor of geology in MIT’s Department of Earth, Atmospheric and Planetary Sciences (EAPS). “This methane could still be present and maybe even used as an energy source on Mars in the future.”
The study’s lead author is recent EAPS graduate Joshua Murray PhD ’24.
In the folds
Jagoutz’ group at MIT seeks to identify the geologic processes and interactions that drive the evolution of Earth’s lithosphere — the hard and brittle outer layer that includes the crust and upper mantle, where tectonic plates lie.
In 2023, he and Murray focused on a type of surface clay mineral called smectite, which is known to be a highly effective trap for carbon. Within a single grain of smectite are a multitude of folds, within which carbon can sit undisturbed for billions of years. They showed that smectite on Earth was likely a product of tectonic activity, and that, once exposed at the surface, the clay minerals acted to draw down and store enough carbon dioxide from the atmosphere to cool the planet over millions of years.
Soon after the team reported their results, Jagoutz happened to look at a map of the surface of Mars and realized that much of that planet’s surface was covered in the same smectite clays. Could the clays have had a similar carbon-trapping effect on Mars, and if so, how much carbon could the clays hold?
���We know this process happens, and it is well-documented on Earth. And these rocks and clays exist on Mars,” Jagoutz says. “So, we wanted to try and connect the dots.”
“Every nook and cranny”
Unlike on Earth, where smectite is a consequence of continental plates shifting and uplifting to bring rocks from the mantle to the surface, there is no such tectonic activity on Mars. The team looked for ways in which the clays could have formed on Mars, based on what scientists know of the planet’s history and composition.
For instance, some remote measurements of Mars’ surface suggest that at least part of the planet’s crust contains ultramafic igneous rocks, similar to those that produce smectites through weathering on Earth. Other observations reveal geologic patterns similar to terrestrial rivers and tributaries, where water could have flowed and reacted with the underlying rock.
Jagoutz and Murray wondered whether water could have reacted with Mars’ deep ultramafic rocks in a way that would produce the clays that cover the surface today. They developed a simple model of rock chemistry, based on what is known of how igneous rocks interact with their environment on Earth.
They applied this model to Mars, where scientists believe the crust is mostly made up of igneous rock that is rich in the mineral olivine. The team used the model to estimate the changes that olivine-rich rock might undergo, assuming that water existed on the surface for at least a billion years, and the atmosphere was thick with carbon dioxide.
“At this time in Mars’ history, we think CO2 is everywhere, in every nook and cranny, and water percolating through the rocks is full of CO2 too,” Murray says.
Over about a billion years, water trickling through the crust would have slowly reacted with olivine — a mineral that is rich in a reduced form of iron. Oxygen molecules in water would have bound to the iron, releasing hydrogen as a result and forming the red oxidized iron which gives the planet its iconic color. This free hydrogen would then have combined with carbon dioxide in the water, to form methane. As this reaction progressed over time, olivine would have slowly transformed into another type of iron-rich rock known as serpentine, which then continued to react with water to form smectite.
“These smectite clays have so much capacity to store carbon,” Murray says. “So then we used existing knowledge of how these minerals are stored in clays on Earth, and extrapolate to say, if the Martian surface has this much clay in it, how much methane can you store in those clays?”
He and Jagoutz found that if Mars is covered in a layer of smectite that is 1,100 meters deep, this amount of clay could store a huge amount of methane, equivalent to most of the carbon dioxide in the atmosphere that is thought to have disappeared since the planet dried up.
“We find that estimates of global clay volumes on Mars are consistent with a significant fraction of Mars’ initial CO2being sequestered as organic compounds within the clay-rich crust,” Murray says. “In some ways, Mars’ missing atmosphere could be hiding in plain sight.”
IMAGE: This schematic illustrates the progressive alteration of iron-rich rocks on Mars as the rocks interact with water containing CO2 from the atmosphere. Over several billion years, this process could have stored enough CO2 in the clay surface, in the form of methane, to explain most of the CO2 that went missing from the planet’s early atmosphere. Credit Courtesy of Joshua Murray, Oliver Jagoutz, et al
10 notes
·
View notes
Text
Mineral spotlight on: Smectite♡

Last Thursday, researchers at MIT published findings that suggest this stylish clay mineral plays a significant role in reducing atmospheric CO2.
An ophiolite is a piece of ocean crust that has been moved onto continental crust by tectonic forces. They contain mafic and ultramafic rock (rocks high in magnesium and iron). And they are a suspect consistently on the scene during global cooling.
Previously, scientists had attributed the creation of ice-house climates during the Paleozoic period to the weathering of these ophiolites.
However, geochemical analysis illustrates that cooling happened through the burial of organic carbon, not weathering. This is where smectite (my beloved) comes in.
Turns out, the weathering of mafic rocks creates smectite clays with very high surface areas. Under a microscope, smectite looks like an accordion.

Carbon gets trapped inside these grooves and removed from the atmosphere! That’s another way the tectonic forces of the geosphere influence global climate.
#earthscience review#research scientist#stem academia#science news#news#science#geoscience#geology#geologist#university#college#climate change#planets#stemblr#dark academia
17 notes
·
View notes
Text
Mars’ missing atmosphere could be hiding in plain sight
New Post has been published on https://thedigitalinsider.com/mars-missing-atmosphere-could-be-hiding-in-plain-sight/
Mars’ missing atmosphere could be hiding in plain sight


Mars wasn’t always the cold desert we see today. There’s increasing evidence that water once flowed on the Red Planet’s surface, billions of years ago. And if there was water, there must also have been a thick atmosphere to keep that water from freezing. But sometime around 3.5 billion years ago, the water dried up, and the air, once heavy with carbon dioxide, dramatically thinned, leaving only the wisp of an atmosphere that clings to the planet today.
Where exactly did Mars’ atmosphere go? This question has been a central mystery of Mars’ 4.6-billion-year history.
For two MIT geologists, the answer may lie in the planet’s clay. In a paper appearing today in Science Advances, they propose that much of Mars’ missing atmosphere could be locked up in the planet’s clay-covered crust.
The team makes the case that, while water was present on Mars, the liquid could have trickled through certain rock types and set off a slow chain of reactions that progressively drew carbon dioxide out of the atmosphere and converted it into methane — a form of carbon that could be stored for eons in the planet’s clay surface.
Similar processes occur in some regions on Earth. The researchers used their knowledge of interactions between rocks and gases on Earth and applied that to how similar processes could play out on Mars. They found that, given how much clay is estimated to cover Mars’ surface, the planet’s clay could hold up to 1.7 bar of carbon dioxide, which would be equivalent to around 80 percent of the planet’s initial, early atmosphere.
It’s possible that this sequestered Martian carbon could one day be recovered and converted into propellant to fuel future missions between Mars and Earth, the researchers propose.
“Based on our findings on Earth, we show that similar processes likely operated on Mars, and that copious amounts of atmospheric CO2 could have transformed to methane and been sequestered in clays,” says study author Oliver Jagoutz, professor of geology in MIT’s Department of Earth, Atmospheric and Planetary Sciences (EAPS). “This methane could still be present and maybe even used as an energy source on Mars in the future.”
The study’s lead author is recent EAPS graduate Joshua Murray PhD ’24.
In the folds
Jagoutz’ group at MIT seeks to identify the geologic processes and interactions that drive the evolution of Earth’s lithosphere — the hard and brittle outer layer that includes the crust and upper mantle, where tectonic plates lie.
In 2023, he and Murray focused on a type of surface clay mineral called smectite, which is known to be a highly effective trap for carbon. Within a single grain of smectite are a multitude of folds, within which carbon can sit undisturbed for billions of years. They showed that smectite on Earth was likely a product of tectonic activity, and that, once exposed at the surface, the clay minerals acted to draw down and store enough carbon dioxide from the atmosphere to cool the planet over millions of years.
Soon after the team reported their results, Jagoutz happened to look at a map of the surface of Mars and realized that much of that planet’s surface was covered in the same smectite clays. Could the clays have had a similar carbon-trapping effect on Mars, and if so, how much carbon could the clays hold?
“We know this process happens, and it is well-documented on Earth. And these rocks and clays exist on Mars,” Jagoutz says. “So, we wanted to try and connect the dots.”
“Every nook and cranny”
Unlike on Earth, where smectite is a consequence of continental plates shifting and uplifting to bring rocks from the mantle to the surface, there is no such tectonic activity on Mars. The team looked for ways in which the clays could have formed on Mars, based on what scientists know of the planet’s history and composition.
For instance, some remote measurements of Mars’ surface suggest that at least part of the planet’s crust contains ultramafic igneous rocks, similar to those that produce smectites through weathering on Earth. Other observations reveal geologic patterns similar to terrestrial rivers and tributaries, where water could have flowed and reacted with the underlying rock.
Jagoutz and Murray wondered whether water could have reacted with Mars’ deep ultramafic rocks in a way that would produce the clays that cover the surface today. They developed a simple model of rock chemistry, based on what is known of how igneous rocks interact with their environment on Earth.
They applied this model to Mars, where scientists believe the crust is mostly made up of igneous rock that is rich in the mineral olivine. The team used the model to estimate the changes that olivine-rich rock might undergo, assuming that water existed on the surface for at least a billion years, and the atmosphere was thick with carbon dioxide.
“At this time in Mars’ history, we think CO2 is everywhere, in every nook and cranny, and water percolating through the rocks is full of CO2 too,” Murray says.
Over about a billion years, water trickling through the crust would have slowly reacted with olivine — a mineral that is rich in a reduced form of iron. Oxygen molecules in water would have bound to the iron, releasing hydrogen as a result and forming the red oxidized iron which gives the planet its iconic color. This free hydrogen would then have combined with carbon dioxide in the water, to form methane. As this reaction progressed over time, olivine would have slowly transformed into another type of iron-rich rock known as serpentine, which then continued to react with water to form smectite.
“These smectite clays have so much capacity to store carbon,” Murray says. “So then we used existing knowledge of how these minerals are stored in clays on Earth, and extrapolate to say, if the Martian surface has this much clay in it, how much methane can you store in those clays?”
He and Jagoutz found that if Mars is covered in a layer of smectite that is 1,100 meters deep, this amount of clay could store a huge amount of methane, equivalent to most of the carbon dioxide in the atmosphere that is thought to have disappeared since the planet dried up.
“We find that estimates of global clay volumes on Mars are consistent with a significant fraction of Mars’ initial CO2 being sequestered as organic compounds within the clay-rich crust,” Murray says. “In some ways, Mars’ missing atmosphere could be hiding in plain sight.”
“Where the CO2 went from an early, thicker atmosphere is a fundamental question in the history of the Mars atmosphere, its climate, and the habitability by microbes,” says Bruce Jakosky, professor emeritus of geology at the University of Colorado and principal investigator on the Mars Atmosphere and Volatile Evolution (MAVEN) mission, which has been orbiting and studying Mars’ upper atmosphere since 2014. Jakosky was not involved with the current study. “Murray and Jagoutz examine the chemical interaction of rocks with the atmosphere as a means of removing CO2. At the high end of our estimates of how much weathering has occurred, this could be a major process in removing CO2 from Mars’ early atmosphere.”
This work was supported, in part, by the National Science Foundation.
#2023#air#atmosphere#author#billion#carbon#Carbon dioxide#chemical#chemistry#climate#CO2#Color#Composition#crust#EAPS#earth#energy#Environment#Evolution#form#Foundation#Fraction#fuel#Full#Fundamental#Future#Geology#Global#highly effective#History
2 notes
·
View notes
Text
Mars’ missing atmosphere could be hiding in plain sight
New Post has been published on https://sunalei.org/news/mars-missing-atmosphere-could-be-hiding-in-plain-sight/
Mars’ missing atmosphere could be hiding in plain sight

Mars wasn’t always the cold desert we see today. There’s increasing evidence that water once flowed on the Red Planet’s surface, billions of years ago. And if there was water, there must also have been a thick atmosphere to keep that water from freezing. But sometime around 3.5 billion years ago, the water dried up, and the air, once heavy with carbon dioxide, dramatically thinned, leaving only the wisp of an atmosphere that clings to the planet today.
Where exactly did Mars’ atmosphere go? This question has been a central mystery of Mars’ 4.6-billion-year history.
For two MIT geologists, the answer may lie in the planet’s clay. In a paper appearing today in Science Advances, they propose that much of Mars’ missing atmosphere could be locked up in the planet’s clay-covered crust.
The team makes the case that, while water was present on Mars, the liquid could have trickled through certain rock types and set off a slow chain of reactions that progressively drew carbon dioxide out of the atmosphere and converted it into methane — a form of carbon that could be stored for eons in the planet’s clay surface.
Similar processes occur in some regions on Earth. The researchers used their knowledge of interactions between rocks and gases on Earth and applied that to how similar processes could play out on Mars. They found that, given how much clay is estimated to cover Mars’ surface, the planet’s clay could hold up to 1.7 bar of carbon dioxide, which would be equivalent to around 80 percent of the planet’s initial, early atmosphere.
It’s possible that this sequestered Martian carbon could one day be recovered and converted into propellant to fuel future missions between Mars and Earth, the researchers propose.
“Based on our findings on Earth, we show that similar processes likely operated on Mars, and that copious amounts of atmospheric CO2 could have transformed to methane and been sequestered in clays,” says study author Oliver Jagoutz, professor of geology in MIT’s Department of Earth, Atmospheric and Planetary Sciences (EAPS). “This methane could still be present and maybe even used as an energy source on Mars in the future.”
The study’s lead author is recent EAPS graduate Joshua Murray PhD ’24.
In the folds
Jagoutz’ group at MIT seeks to identify the geologic processes and interactions that drive the evolution of Earth’s lithosphere — the hard and brittle outer layer that includes the crust and upper mantle, where tectonic plates lie.
In 2023, he and Murray focused on a type of surface clay mineral called smectite, which is known to be a highly effective trap for carbon. Within a single grain of smectite are a multitude of folds, within which carbon can sit undisturbed for billions of years. They showed that smectite on Earth was likely a product of tectonic activity, and that, once exposed at the surface, the clay minerals acted to draw down and store enough carbon dioxide from the atmosphere to cool the planet over millions of years.
Soon after the team reported their results, Jagoutz happened to look at a map of the surface of Mars and realized that much of that planet’s surface was covered in the same smectite clays. Could the clays have had a similar carbon-trapping effect on Mars, and if so, how much carbon could the clays hold?
“We know this process happens, and it is well-documented on Earth. And these rocks and clays exist on Mars,” Jagoutz says. “So, we wanted to try and connect the dots.”
“Every nook and cranny”
Unlike on Earth, where smectite is a consequence of continental plates shifting and uplifting to bring rocks from the mantle to the surface, there is no such tectonic activity on Mars. The team looked for ways in which the clays could have formed on Mars, based on what scientists know of the planet’s history and composition.
For instance, some remote measurements of Mars’ surface suggest that at least part of the planet’s crust contains ultramafic igneous rocks, similar to those that produce smectites through weathering on Earth. Other observations reveal geologic patterns similar to terrestrial rivers and tributaries, where water could have flowed and reacted with the underlying rock.
Jagoutz and Murray wondered whether water could have reacted with Mars’ deep ultramafic rocks in a way that would produce the clays that cover the surface today. They developed a simple model of rock chemistry, based on what is known of how igneous rocks interact with their environment on Earth.
They applied this model to Mars, where scientists believe the crust is mostly made up of igneous rock that is rich in the mineral olivine. The team used the model to estimate the changes that olivine-rich rock might undergo, assuming that water existed on the surface for at least a billion years, and the atmosphere was thick with carbon dioxide.
“At this time in Mars’ history, we think CO2 is everywhere, in every nook and cranny, and water percolating through the rocks is full of CO2 too,” Murray says.
Over about a billion years, water trickling through the crust would have slowly reacted with olivine — a mineral that is rich in a reduced form of iron. Oxygen molecules in water would have bound to the iron, releasing hydrogen as a result and forming the red oxidized iron which gives the planet its iconic color. This free hydrogen would then have combined with carbon dioxide in the water, to form methane. As this reaction progressed over time, olivine would have slowly transformed into another type of iron-rich rock known as serpentine, which then continued to react with water to form smectite.
“These smectite clays have so much capacity to store carbon,” Murray says. “So then we used existing knowledge of how these minerals are stored in clays on Earth, and extrapolate to say, if the Martian surface has this much clay in it, how much methane can you store in those clays?”
He and Jagoutz found that if Mars is covered in a layer of smectite that is 1,100 meters deep, this amount of clay could store a huge amount of methane, equivalent to most of the carbon dioxide in the atmosphere that is thought to have disappeared since the planet dried up.
“We find that estimates of global clay volumes on Mars are consistent with a significant fraction of Mars’ initial CO2 being sequestered as organic compounds within the clay-rich crust,” Murray says. “In some ways, Mars’ missing atmosphere could be hiding in plain sight.”
“Where the CO2 went from an early, thicker atmosphere is a fundamental question in the history of the Mars atmosphere, its climate, and the habitability by microbes,” says Bruce Jakosky, professor emeritus of geology at the University of Colorado and principal investigator on the Mars Atmosphere and Volatile Evolution (MAVEN) mission, which has been orbiting and studying Mars’ upper atmosphere since 2014. Jakosky was not involved with the current study. “Murray and Jagoutz examine the chemical interaction of rocks with the atmosphere as a means of removing CO2. At the high end of our estimates of how much weathering has occurred, this could be a major process in removing CO2 from Mars’ early atmosphere.”
This work was supported, in part, by the National Science Foundation.
0 notes
Text
Choline Chloride Market - Forecast(2024 - 2030)
Choline Chloride Market Overview
Choline Chloride Market size is forecast to reach $1.1 billion by 2025, after growing at a CAGR of 5.1% during 2020-2025. Choline chloride is an organic compound. It is used in the animal feed additive. It is mainly related with the metabolism and act as methyl donor. It builds and maintains cell structure and function. It is very essential for growing animal and its feed intake helps in preventing perosis, conditions like fatty liver syndrome, etc. Apart from animal feed additive, Choline Chloride Market also finds application in human nutrition and as a clay stabilizer in oil and gas industry. One of the major factors driving growth in the Choline Chloride Market is increasing consumption of poultry meat and egg.
Report Coverage
The report: “Choline Chloride Market – Forecast (2020-2025)”, by IndustryARC, covers an in-depth analysis of the following segments of the Choline Chloride Market Industry.
By Grade: 50%, 75%, 70%, 60%, and 98%.
By Form: Powder and Liquid.
By End-User Industry: Animal Feed, Oil & Gas, Human Nutrition, Personal Care, Pharmaceuticals and Others.
By Geography: North America, South America, Europe, APAC, and RoW
Request Sample
Key Takeaways
New product developments and increasing investments in research and development of choline chloride are expected to drive the growth of this market.
Among end users, oil & gas segment is projected to lead the Choline Chloride Market during the forecast period.
Asia Pacific dominated the Choline Chloride Market.
By Grade - Segment Analysis
70% segment held a significant share in Choline Chloride Market in 2019. Choline chloride 70% is primarily available in the form of a colorless to amber colored viscous liquid or a tan colored powder with a characteristic odor containing 70% choline chloride active substance by weight. Choline chloride 70% finds widespread adoption in the oil & gas sector as an ideal substitute for potassium chloride owing to its greater performance in clay stabilization, compatibility with drilling muds and safe environmental profile. The product is also widely used as a feed additive for calves, crustaceans, fish, poultry, pigs and pet food pertaining to its ability to prevent fatty liver, improved muscle control and growth performance.
Inquiry Before Buying
By Form - Segment Analysis
Liquid segment held a significant share in Choline Chloride Market in 2019. Liquid choline chloride is a colorless, odorless, clear substance which is soluble in water, non-corrosive and non-toxic in nature. The product is extensive adopted in the poultry feed industry on account of its ability to enhance growth & performance, nutrient utilization, fat metabolism and improves reproductive ability in animals. The product helps prevent health disorders such as liver enlargement and leg deformity, also called perosis in chicks. Choline supplementation helps enhance weight gain in broilers and plays an essential role in the formation of egg yolk in layer chickens.
By End-User Industry - Segment Analysis
Oil & Gas segment held a significant share in Choline Chloride Market in 2019 growing at a CAGR of XX% during the forecast period. Choline chloride serves as an excellent clay stabilizer which ensures cost effectiveness and enhances production rates in the oil & gas sector. Choline chloride helps tackle various challenges which arise during oil drilling operations such as permeability reduction, fines migration and wellbore instability. The product’s biodegradable nature makes it an environment-friendly substitute for conventional clay stabilizers. Choline chloride is ideal for mixed layer, illite and smectite clay minerals & is highly compatible with conventional fracturing fluids and cross-linkers. Growing energy demand from the domestic & industrial sectors, rising exploration of natural gas resources and technological advancement should drive the oil & gas industry and stimulate the Choline Chloride Market.
Geography- Segment Analysis
Asia Pacific dominated the Choline Chloride Market with a share of more than XX%, followed by North America and Europe. This region has the largest consumer base for choline chloride, specifically China, due to the increased demand for poultry meat. Choline chloride improves the feed conversion rate and enhances weight gain in pigs. Moreover, consumers in the U.S. & Canada increasingly prefer bacon-based breakfast options over pancakes on account of rising trend to experience new products and changing palates. Rising consumer preference for Vietnamese & Korean cuisines and increasing trend of dining at fast-food restaurants is expected to drive bacon consumption and accelerate the Choline Chloride Market sales.
Schedule a Call
Drivers – Choline Chloride Market
High Demand from Animal Feed Industry
Choline chloride is extensively consumed in the end-user segment like animal feed because of the growing demand for egg, high yield meat, etc. It is used as an essential feed additive amongst animal species, particularly chickens. It is also known as vitamin B4 and is an organic compound. Choline chloride is related to metabolism and it acts as a methyl donor. It helps in building and maintaining cell structure and functioning. It is used as a feed additive for growing poultry animals. Its benefits include prevention of perosis, a condition in animals like fatty liver syndrome, etc. In 2019, poultry meat consumption across the globe was about 128 million tons and is forecasted to reach about 135 million tons by 2025. All the aforementioned factors are expected to drive the Choline Chloride Market from the animal feed industry during the forecast period.
Challenges – Choline Chloride Market
Health Effects
Owing to hazardous effects related to human health such as vomiting, diarrhea, gastro intestinal distress and inhalation problems etc. during its preparation, the use of Choline Chloride has been regulated and restrained by health and safety management authorities for end user applications. Furthermore, fluctuation in raw material costs is another key factor which is expected to act as an impediment for the growth of Choline Chloride Market over the forecast period. Moreover, lack of consumer awareness about the nutritional value of choline is expected to slow down the growth of choline chloride.
Buy Now
Market Landscape
Technology launches, acquisitions and R&D activities are key strategies adopted by players in the Choline Chloride Market. In 2019, the market of choline chloride has been consolidated by the top five players accounting for xx% of the share. Major players in the Choline Chloride Market are Balchem Corporation, Taminco, BASF SE, Algry Química, Havay Chemical Company, NB Group Co., Ltd, and Jubilant Life Science among others.
Product Launch/ Partnership
In October 2019, global food & feed ingredient manufacturer Kemin Industries launched a new product range CholiGEM which caters to the health & nutrition requirements of ruminants. This is an innovative feed ingredient which offers a greater concentration of choline chloride as compared to conventional animal feed products. This product launch provides a competitive advantage to Kemin Industries and allows it to meet growing demand for animal feed additives for improved liver function.
In November 2017, global chemicals manufacturer BASF entered into a strategic partnership with Indian pharmaceuticals company Vital Therapeutics. This partnership entails the launch of a new choline chloride product Vitachol which caters to poultry feed manufacturers in India and South Asia.
#Choline Chloride Market#Choline Chloride Market Share#Choline Chloride Market Size#Choline Chloride Market Forecast#Choline Chloride Market Report#Choline Chloride Market Growth
0 notes
Text
Smectite Clay Mineral Market Size, Shares and Opportunity Valuation and Forecast to 2031
The Market report can help clients make business decisions and understand Industry Recent Trends, Share, Size, Growth, Opportunity, and Forecast 2024 to 2031” report by Report Ocean delivers a thorough industry evaluation, covering market trends, competitor analysis, regional insights, and the Recent market developments. Ideal for investors, researchers, consultants, and marketing strategists, it is a valuable resource for those looking to engage in the market. The study emphases on whole estimate of the value chain, technological progresses, prospects, future roadmaps and distributor study.
Get the complete sample, please click:
Key Regions & Countries
North America (United States, Canada and Mexico) Europe (Germany, France, UK, Russia and Italy) Asia-Pacific (China, Japan, Korea, India and Southeast Asia) South America (Brazil, Argentina, Colombia etc.) Middle East and Africa (Saudi Arabia, UAE, Egypt, Nigeria and South Africa).
The Report Covers - -Complete in-depth study of the market and Vital changes in market dynamics. - Detailed considerate of market-particular drivers, Trends, constraints, Restraints, Opportunities and important micro markets. - the report focus on Complete valuation of all prospects and risk. - In depth study of business tactics for growth of the market core players. - Market latest innovations and key procedures. - the Research focus on Vital changes in market aspects, product development, current trends, Competitive Landscape. - Conclusive study about the development conspiracy of market for forthcoming years.
Table Of Content:
Some Major TOC Points:
Chapter 1. Report Overview
Chapter 2. Global Growth Trends
Chapter 3. Market Share by Main Players
Chapter 4. Breakdown Data by Type and Application
Chapter 5. Market by End Users/Application
Chapter 6. COVID-19 Outbreak: Chair Lifts Sales Industry Impact
Chapter 7. Opportunity Analysis in Covid-19 Crisis
Chapter 8. Market Driving Force
To Be Continued…!
Reason To Buy:
Robust study methodology with important analysis including Porter's Five Investigation and SWOT analysis.
Extensive analysis of aggressive commerce regulations and rules of numerous government agencies both internationally and regionally from the report to incorporate a wide picture of this market's potential.
Supplying crucial opportunities for market expansion throughout the forecast period.
Study of a Huge historic Information about market behavior, functionality, and creation from companies.
True and factual statistics consisting of a succinct graphical representations, tables, and statistics of this market in the report.
Contact Us:
Proficient Market Insights
Phone:
US : +1 424 253 0807
UK : +44 203 239 8187
Email:
Web: https://proficientmarketinsights.com/
0 notes
Text
Argiles smectiques, Prévisions de la Taille du Marché Mondial, Classement et Part de Marché des 19 Premières Entreprises
Selon le nouveau rapport d'étude de marché “Rapport sur le marché mondial de Argiles smectiques 2024-2030”, publié par QYResearch, la taille du marché mondial de Argiles smectiques devrait atteindre 2071 millions de dollars d'ici 2030, à un TCAC de 5.2% au cours de la période de prévision.
Figure 1. Taille du marché mondial de Argiles smectiques (en millions de dollars américains), 2019-2030

Selon QYResearch, les principaux fabricants mondiaux de Argiles smectiques comprennent Minerals Technologies (Amcol), Clariant, Imerys (S&B), Bentonite Performance Minerals LLC (BPM), Black Hills Bentonite LLC, Bentonite Company LLC (Russia), Tolsa Group, Wyo-Ben Inc, Ashapura Minechem Limited, Laviosa Minerals SpA, etc. En 2023, les dix premiers acteurs mondiaux détenaient une part d'environ 65.0% en termes de chiffre d'affaires.
Figure 2. Classement et part de marché des 19 premiers acteurs mondiaux de Argiles smectiques (Le classement est basé sur le chiffre d'affaires de 2023, continuellement mis à jour)

À propos de QYResearch
QYResearch a été fondée en 2007 en Californie aux États-Unis. C'est une société de conseil et d'étude de marché de premier plan à l'échelle mondiale. Avec plus de 17 ans d'expérience et une équipe de recherche professionnelle dans différentes villes du monde, QYResearch se concentre sur le conseil en gestion, les services de base de données et de séminaires, le conseil en IPO, la recherche de la chaîne industrielle et la recherche personnalisée. Nous société a pour objectif d’aider nos clients à réussir en leur fournissant un modèle de revenus non linéaire. Nous sommes mondialement reconnus pour notre vaste portefeuille de services, notre bonne citoyenneté d'entreprise et notre fort engagement envers la durabilité. Jusqu'à présent, nous avons coopéré avec plus de 60 000 clients sur les cinq continents. Coopérons et bâtissons ensemble un avenir prometteur et meilleur.
QYResearch est une société de conseil de grande envergure de renommée mondiale. Elle couvre divers segments de marché de la chaîne industrielle de haute technologie, notamment la chaîne industrielle des semi-conducteurs (équipements et pièces de semi-conducteurs, matériaux semi-conducteurs, circuits intégrés, fonderie, emballage et test, dispositifs discrets, capteurs, dispositifs optoélectroniques), la chaîne industrielle photovoltaïque (équipements, cellules, modules, supports de matériaux auxiliaires, onduleurs, terminaux de centrales électriques), la chaîne industrielle des véhicules électriques à énergie nouvelle (batteries et matériaux, pièces automobiles, batteries, moteurs, commande électronique, semi-conducteurs automobiles, etc.), la chaîne industrielle des communications (équipements de système de communication, équipements terminaux, composants électroniques, frontaux RF, modules optiques, 4G/5G/6G, large bande, IoT, économie numérique, IA), la chaîne industrielle des matériaux avancés (matériaux métalliques, polymères, céramiques, nano matériaux, etc.), la chaîne industrielle de fabrication de machines (machines-outils CNC, machines de construction, machines électriques, automatisation 3C, robots industriels, lasers, contrôle industriel, drones), l'alimentation, les boissons et les produits pharmaceutiques, l'équipement médical, l'agriculture, etc.
0 notes
Text
0 notes
Text
Mysterious Ocean Mineral Could Keep Earth Cool
Geologists from MIT have unearthed a groundbreaking revelation hidden beneath the ocean ‘s depths: the extraordinary potential of smectite, a clay mineral, as a potent ally in combating climate change. Transitioning from microscopic observations to a global impact, this discovery marks a pivotal breakthrough in understanding Earth’s intricate climate mechanisms. UNEARTHING THE CARBON-CAPTURING…

View On WordPress
0 notes
Text
SCIENCE SATURDAY!
All month, I have been teaching y'all bits and pieces about the minerals known as feldspars. They are the most common minerals in earth's crust. Today, we are going to learn some of the basic chemistry behind feldspar crystallization and erosion.
FELDSPAR CHEMISTRY
Feldspars are formed as a precipitate as magmas cool. As a result, there are many different kinds. Below is a phase diagram:

Ignore B, all we care about it the colorful triangle. All right, so we have 3 endmembers: Orthoclase (kspar), Albite (sodium plag), and Anorthite (calcium plag). Then, there are all the minerals in between which have different mixed percentages of sodium, calcium and/or potassium. For example, Bytownite is 70-90% calcium and 30-10% sodium. See why there are so many types?
All right, now magma. Magmas cool at different rates for various reasons I really don't want to go into because I am a paleontologist, not an igneous petrologist and that research I don't feel like doing.

Feldspar structure: feldspars have what is called a "crankshaft" structure. We have a bunch of tetrahedrons linked by shared oxygen molecules and we make these fun hexagons.
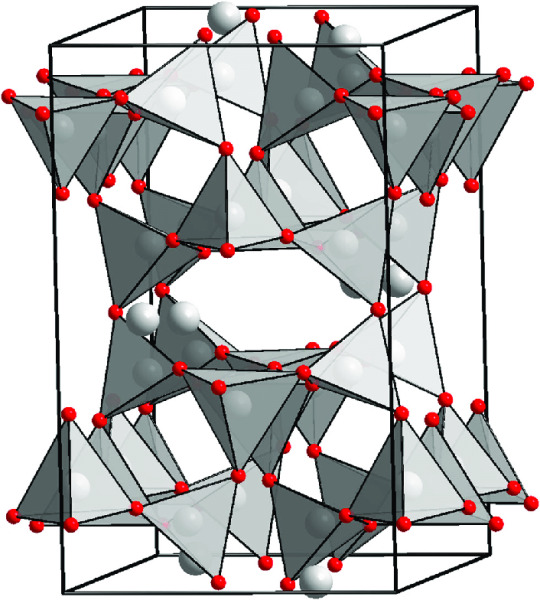
Now, the basic chemical formula is (X)AlSi3O8. What we are essentially seeing is an Al 3+ substituted in for an Si 4+ causing a charge imbalance because 3 does not equal 4. This requires additional cations (called coupled substitutions).
EXAMPLE: Al 3+ and Na+ or K+ OR 2Al 3+ and 1 Ca 2+
Where is the aluminum? That depends on the temperature of our magma! High temperatures make the position more random while low temps make it more ordered.
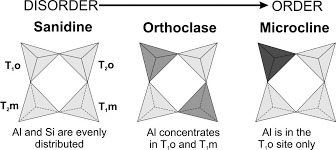
If we look at kspar (geologists are lazy and potassium feldspar is a lot to say) we have a K-Al coupled substitution with three polymorphs controlled by temperature and ordering. If we set up a graph where the y-axis is cooling rate and the x-axis is order, we would see the feldspar Sanidine has the lowest order and the fastest cooling and Microcline has the highest order and the slowest cooling while Orthoclase is somewhere in the middle.
Plagioclase has a complete solid solution between the endmembers Albite and Anorthite as I described earlier. Things to note are temperature (once again) plays an important role. Albite forms in low temp magma (800 degrees Celsius) and Anorthite forms in high temp magmas (1100 degrees Celsius). Yes, I know, 800 is a lot but not as mush as 1100 so deal.
They also contain different amounts of silica (SiO2). Albite is 75% silica while Anorthite only has 50%. Anorthite is also the first felspar mineral to crystallize in cooling magma.
HYDROLYSIS
This is the chemical weathering of feldspars into clays such as illite, kaolinite, and smectite.



(That last one overlooks the dinosaur site I work at).
Due to the high temps that feldspars form at, they are not very stable at the surface. Therefore, they weather extraordinarily easily. Hydrolysis happens when water reacts with feldspar minerals (basic or acidic water works best because IONS). The feldspars are dissolved and then produce new ions in solution (K+, Ca2+, Na+).
Here is an example:
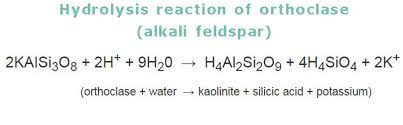
And now you know a little bit about the chemistry of feldspars!
36 notes
·
View notes
Text
Mysterious Ocean Mineral Could Keep Earth Cool
Geologists from MIT have unearthed a groundbreaking revelation hidden beneath the ocean ‘s depths: the extraordinary potential of smectite, a clay mineral, as a potent ally in combating climate change. Transitioning from microscopic observations to a global impact, this discovery marks a pivotal breakthrough in understanding Earth’s intricate climate mechanisms. UNEARTHING THE CARBON-CAPTURING…

View On WordPress
0 notes