#Sigma 1750
Explore tagged Tumblr posts
Text







Heat and Power Plant | Urbex 15 / 2019 | Zone E / Hellveyor | Gallery 1 of 2
#Photographers on Tumblr#Original Photographers#Photography#Nikon#Heat and Power Plant#Power Plant#Conveyor Belt#Heating Plant#Industry#Impressions#Nikon D7200#Sigma 1750#UE#UrbanExploration#Exploration#Urbex#Urbex 2019#UrbexSkalmar#UrbexKind#MaciejSmolenPhotoGraphics
70 notes
·
View notes
Text

Installation 2020.04.27 G2
#Photographers on Tumblr#Original Photographers#Photography#Nikon#Nikon D7200#Sigma 1750#InstallationsAreSigns#MaciejSmolenPhotoGraphics
3 notes
·
View notes
Text
A Phase I Drug Discovery and One-, Two-, and Three-Class of Indoline-2,3-dione Testing for Anticancer and Cytotoxic Activity-JuniperPublishers
Journal of Chemistry-JuniperPublishers
Abstract
Cancer diagnosis begins with a thorough physical examination and a complete medical history. Laboratory studies of blood, urine, and stool can detect abnormalities that may indicate cancer. Normal ranges of Indoline-2,3-dione scaffolds have been studied. We report here one, two, three novels anticancer target compounds 5-Flouro-1-benzyl isatin -2, 3-b-quinoxaline and 5 -Methyl-1-benzyl isatin -2,3-b- quinoxaline and display good quality of molecular arrangement towards cancerous cells.
Go to
Introduction
Cancer has a variety of forms this definition still holds secrete even today and selection removes variation. Cancer comes homologous proteins can differ in their specificities, specific activities, level of expression or some other basic property. However homologous will have some residual similarity in their form and function. Cancer is a biological event has brought some useful innovations in practice to the study of treatment. It was felt there should not be separate drug discovery standard for reduce the dose frequency and reduce adverse effect and shorten time treatment. The natural history of drug discovery could illustrate the complexity in selecting a baseline. Cancer drug discovery is a relatively slow growing chemical agent with an incubation period said to range from a few year to a life time. If any other anatomical symptoms will see during the laboratory trial, it must be treated thoroughly Indoline-2,3-dione scaffolds is multifunctional expressed by drug discovery series of novel 97 compounds were synthesized for anticancer and cytotoxic activity against different 60 cell line cultures [1]. 07 compounds were selected on molecular recognition and screened for anticancer study [2]. Among those is screened compounds RH8, RH 42, RH84, RH89, RH90, RH 94, RH97 found good quality of anticancer activity against most of the cancer cell line with ranges from -77.23 to 55.85% growth. Target compound 5-Flouro-1-benzyl isatin -2, 3-b-quinoxaline (RH-89) display -77.23 to 7.31% growth & another target compound RH 94 display -64.78 to 65.14% growth against most of the leukemic cell lines, while RH-08 has shown very good activity against CCRF-CEM cell line with -31.85% growths and weak anticancer activity against breast cancer cell line with 53.47% growth, but failed to respond against other leukemic cell lines. RH-89 has also shown moderate activity against nonsmall cell-lung cancer (HOP-62) with 34.69% growth. RH-90 has shown good anticancer activity against leukemic cell line SR with 18.70% growth and weak activity against Non small cell lung cancer HOP-92 and NCL-H322M with 57.14 and 57.20% growth respectively. The cytotoxic property [3] of Indoline-2,3-dione scaffolds, among 3-benzohydrazide (RH 06-64) series, RH-22 and 28 were found to be most cytotoxic against L-1210 cell line (9 and 6μg/ml). RH-33 was found to be cytotoxic against CEM/0 cell line (6.9μg/ml). Among 3-isonicotinohydrazide derivatives (RH 65-72), RH-70 was found to have better cytotoxic property against all three types of cell lines (6.1-6.7μg/ml). From 3-quinazolin-4(3H)-one series (RH 74-82), RH-75 was found to be better cytotoxic against Molt4/8 cell line (6.0μg/ml) and RH-77 was found to have better cytotoxicity against L1210 cell line (9μg/ml) remaining compounds RH-79, 80, 82 have shown moderate to weak cytotoxic property. Among 6H-indolo [2,3-b] quinoxaline derivatives (RH 83-96), RH-92 have shown better cytotoxic property against L1210 cell line (7.2μg/ml).
Compounds and Reagents
All chemicals used were procured from Sigma-Aldrich. The purity of chemicals was checked before use and purified. Melting points were recorded by open capillary tube method and uncorrected. Purity of all the synthesized compounds was assessed by thin layer chromatography on silica Gel-G'(100-200 mesh) plates. Structures of compounds were confirmed by FTIR, 1HNMR and Mass spectra. IR spectra were recorded in KBr disk on Nicolate IR-Instrument and reported in cm-1. Mass spectra were recorded on Shimadzu-LCMS 2010 EV-single quadrapole instrument. 1HNMR spectra were recorded on the BRUKER-300 instrument with TMS as the internal standard in CDCl3/DMSO as a solvent. Total 97 derivatives of Indoline-2,3-dione scaffolds have been synthesized by Sandmeyer process methodology using N-methyl morpholine, substituted benzylchlorides, isoniazid, substituted benzoylhydrazide, quinazoline, orthophenylenediamine, halogens and alkyl groups as substituent and compounds were recrystallized by using ethanol-chloroform mixture. Synthesized compounds and melphalan as a standard were also evaluated for their in vitro cytostatic activity against human Molt 4/C8 and CEM T-lymphocytes as well as murine leukemia L1210 and anticancer activity against Leukemia, Non-Small Cell-Lung Cancer, Colon Cancer, and Breast Cancer. Synthesized compounds were screened for anticancer activity at National Cancer Institute, Maryland, USA and Rega Institute, Belgium respectively (Figure 1).
Go to
Result and Discussion (Tables 1 &2).
Chemistry
RH8; MF C15H10N3O2Cl, Yellowish fluffy mass, MW 299.71, MP 280-82,% Yield 49, IR 3226 (-NH), 3044 (-CH, ar.), 1701 (>CO), 1678 (>CO). NMR 14.03 (d, 1H, NH.), 11.28 (s, 1H, NH), 7.88-7.84 (m 2H, ar.), 7.59-7.42 (m, 4H, ar.), 7.25-7.23 (m, 1H, ar.), 6.87-6.84 (m, 1H, ar.). m/s 299 (299.29)
RH 42; MF C22H16N3O2C., Yellowish-fibrous mass, MW 389.83,'22 16 3O Cl MP 170-72, % Yield 81, IR 3363 (-NH), 3196 (-CH, ar.), 3028 (CH, alip.), 1750 (>CO), 1682 (>CO). NMR 14.04 (s, 1H, NH.), 7.97(d, 2H, ar.), 7.82 (s, 1H, ar.), 7.46 (t, 2H, ar.), 7.48 (t, 1H, ar.), 7.257.04 (m, 5H, ar.), 6.76 (d, 1H, ar.), 4.87 (s, 2H, CH2), 2.25 (s, 3H, CH3). m/s 389 (389.42)
RH84: MF C21H14FN3, Reddish Fluffy Mass, MW 327.35,MP 162-13,% Yield 72,IR 3045-3013 (-CH, ar.), 2954-2849 (-CH,
alip.), 1610, (>C=C), 1580 (>C=N).,NMR 8.51 (d, 1H, ar.), 8.348.32 (m, 1H, ar.), 8.16 (d, 1H, ar.), 7.80-7.76 (m, 1H, ar.), 7.737.69 (m, 1H, ar.), 7.63 (t, 1H, ar.), 7.41-7.31(m, 4H, ar.), 7.00 (t, 2H, ar.), 5.69 (s, 2H, -CH2).,
RH89, MF C21H14FN3, Orange Mass, MW 327.35,MP 220-22,% Yield 68, IR 30(56-3004 (CH, ar.), 2904-2849 (CH, alip.), 1618 (>C=C), 1582 (>C=N)., NMR 8.38 (d, 1H, ar.), 8.24-8.19 (m, 2H, ar.), 7.86-7.82 (m, 1H, ar.), 7.79-7.74 (m, 1H, ar.), 7.42 (d, 1H, ar.), 7.39-7.32 (m, 2H, ar.), 7.31-7.28 (m, 4H, ar.), 5.77 (s, 2H, -CH2).
RH90; MF C22H16FN3, Yellowish Mass, MW 341.38,MP 17722 16 380,% Yield 71,IR 3054-3029 (CH, ar.), 2917-2849 (-CH, alip.), 1613 (>C=C), 1585 (>C=N). NMR 8.33 (d, 1H, ar.), 8.18-8.15 (m, 1H, ar.), 7.81-7.77 (m, 1H, ar.), 7.73-7.69 (m,1H, ar.), 7.37-7.29 (m, 3H, ar.), 7.22 (d, 2H, ar.), 7.12 (d, 2H, ar.), 5.68 (s, 2H, -CH2), 2.30 (s, 3H, -CH3).
RH 94; MF C22H17N3, Reddish Fluffy Mass, MW 323.39, MP 215-18, % Yield 75, IR 3059-3024 (CH, ar.), 2966-2850 (-CHalip.), 1616 (>C=C), 1578 (>C=N).NMR 8.38-8.36 (m, 2H, ar.), 8.20-8.18 (m, 1H, ar.), 7.83-7.79 (m, 1H, ar.), 7.76-7.72 (m, 1H, ar.), 7.49 (d, 1H, ar.), 7.38-7.29 (m, 6H, ar.), 5.67(s, 2H, -CH2), 2.59 (s, 3H, -CH3).
RH97: MF C21H15FN3, Yellowish Mass, MW 309.36,MP 173-75,% Yield 6(3 IR 3048-3030 (CH, ar.), 2908-2856 (-CH, alip.),1610 (>C=C), 1562(>C=N).NMR 8.32 (d, 2H, ar.), 8.14 (d, 1H, ar.) 7.79-7.75 (m, 1H, ar.), 7.71-7.68 (m, 1H, ar.), 7.45 (d, 1H, ar.), 7.32-7.23 (m, 3H, ar.) 6.98-6.95 (m, 2H, ar.), 5.66, (s, 2H, -CH2), 2.55 (s, 3H, CH3).
RH-22; MF C15H9BrFN3O2, Orange gritty powder, MW 362.15, MP >310, % Yield 57, IR 3229 (-NH), 3056 (-CH, ar.), 1681 (>CO), 1620 (>C=C), 1552 (C=N), 1463 (C-H). NMR
RH28; Yellowish fluffy mass, MW 423.06, MP >310, % Yield 84,IR 3263 (-NH), 3035 (-CH, ar.), 2783 (-CH, alip), 1672 (>CO), 1665 (>C=C), 1529 (C=N), 1467 (C-H).
RH65;MF C21H8N4O2Cl, Yellowish fluffy mass, MW 390.82, MP 197-99, % Yield 76 IR3252 (-NH), 3073 (-CH, ar.), 2918, (CH, alip.), 2850 (CH, alip.) 1691 (>CO). 13.87 (s, 1H, -NHCO), 8.873 (d, 2H, ar.) 7.813 (d, 2H, ar.), 7.765 (s, 1H, ar.), 7.611 (d, 1H, ar.), 7.339 (m, 4H, ar.), 7.05 (d, 1H, ar.), 5.018 (s, 2H,-CH2)
RH 72; MF C14H2N4O2ClF, Orange gritty powder, MW 318.69, MP299-300, % Yield 70, IR 3249 (-NH), 3070 (-CH, ar.), 29312849 (-CH, alip.), 1700-1691 (>CO), 1613 (>C=C), 1520 (C=N), 1472 (C H). NMR13.95 (s, 1H, - NHCO), 8.86 (d, 2H, ar.), 7.80 (d, 2H, ar.), 7.5 (s, 1H, ar.), 7.305 (m, 6H, ar.), 6.9685 (d, 1H, ar.), 4.987 (s, 2H, -CH2 -), 2.296 (s, 3H, -CH3).
*50% inhibitory concentration (IC50) value, expressed in uM
Go to
Conclusion
A comparison of the synthesized and evaluated novel Indoline-2,3-dione scaffolds and its series which showed nanomolar activity against most of the leukemia cell line and other cancers cell line. Taste compounds RH8, RH 42, RH84, RH89, RH90, RH 94, and RH97 possess inhibitory activity. Target compound RH89 and RH 94 displays -77.23 to 7.31% and another target compound - 64.78% to 65.14% growth respectively. All the 97 compounds designed analogs with chemical substitutions at the various position confirmed by analytical model showing better interactive scores respectively Molecular recognition studies in the series removal of alky group from 4'-position of N-benzyl ring causes loss in activity (RH-71) and replacement of benzyl group by -H makes the compound inactive (RH-72), while RH-08 has shown very good activity against CCRF-CEM cell line with -31.85% growths and weak anticancer activity against breast cancer cell line with 53.47% growth, but failed to respond against other leukemic cell lines. RH-89 has also shown moderate activity against non-small cell- lung cancer (HOP-62) with 34.69% growth. RH-90 has shown good anticancer activity against leukemic cell line SR with 18.70% growth and weak activity against Non small cell lung cancer HOP-92 and NCL-H322M with 57.14 and 57.20% growth respectively. The comparison of activity with structure similarity and differences in substitution reveals that compounds with quinoxaline moiety are more active than benzohydrazide moiety (RH-89 & 94> RH-08). Further, 5-fluoro or 5-methyl substituted compounds (RH-89 & 94) were more active than un-substituted analogues (RH-97). It was also observed that any substitution on the 4' position of N-benzyl ring causes loss in activity (RH-90 & 97). Similarly among benzohydrazide series compounds with chlorine substitution at 5th position is active (RH-08), however N-benzylation reduces the activity (RH-42). However role of N-benzyl group has mixed effect on the activity. The cytotoxic results of benzohydrazide series show that RH-22 and RH-28 were most cytotoxic against L-1210 cell line (9 and 6μg/ml). RH- 33 was found to be cytotoxic against CEM/0 cell line (6.9μg/ml). The activity report shows that presence of halogen increases the cytotoxic property. Replacement of halogen from 4' position of benzohydrazide causes loss in activity as in case of RH-06, 25, 26, 28 and 31. Among 3-isonicotinohydrazide derivatives RH-70 was found to have better cytotoxic property against all three types of cell lines (6.1-6.7μg/ml). Removal of alky group from 4'-position of N-benzyl ring causes loss in activity (RH-71) and replacement of benzyl group by -H makes the compound inactive (RH-72). It shows that halogen at 5th position and alkyl group at 4' position of N-benzyl ring is essential for activity. From 3-quinazolin- 4(3H)-one series RH-75 was found to be better cytotoxic against Molt4/8 cell line (6.0μg/ml) and RH-77 was found to have better cytotoxicity against L1210 cell line (9μg/ml). Among 6H-indolo [2,3-b]quinoxaline derivatives RH-92 has shown better cytotoxic property against L1210 cell line (7.2 μg/ml) which shows the importance of halogen at 5th position and presence of N-benzyl moiety in the compound. Alteration in the combination leads to loss in activity (RH-84, 87, 88, 89, 90, 91, 93-96). Based on the results without any halogen has either very weak activity or inactive and none of them are close to the standard Malphalan (2.1-3.2μg/ml) [4-11].
Go to
Acknowledgment
This study has been guided under the guidance of Renowned Laboratory Scientist Respected Dr. Ramesh Paranjape, Founder National AIDS Research Institute India. I express my sincere gratitude towards Respected Sir for motivation and being great knowledge source for this work.
To know more about Journal of chemistry,
Click here:
https://juniperpublishers.com/omcij/index.php
To know more abour juniper Publishers,
click here:
https://juniperpublishers.com/index.php
#Juniper Publishers Indexing Sites List#Juniper Publishers#Juniper Publishers group#Juniper Publisher Reviews#JuniperPublishers#inorganic chemistry#organic chemistry#chemistry journal#Open access Journal of chemistry#chemistry
0 notes
Text
Biomed Grid | Features of Reduction of Th1, Th5 Lymphocytes Functions After Intoxication of Various Toxic Chemicals
Depression, Anxiety, Stress and Fear
Toxic chemicals (TCh) may have a different effect on Th1, Th5 lymphocyte function, identifying features of the humoral and cellular immune response, leading to infectious complications and diseases [1-6]. It is interesting to study the effects of TCh (organophosphorus compounds, nitriles, alcohols and chlorinated hydrocarbons), widely used in industry and agriculture, on the activity of Th1, Th5 lymphocytes, which can cause acute poisoning in case of accidents at chemical plants and safety violations [2,3,7]. It should be noted that there is a possibility of using organophosphorus compounds (along with other chemical warfare agents) as chemical warfare agents for terrorist, criminal and military purposes, which can lead to mass destruction of people [2,3,8-10].
Th1 cells, participating in the implementation of the cellular immune response, producing IFN-γ. Th5 lymphocytes (in particular, synthesizing IL-4) are involved in the formation of humoral immune responses, contributing to the production by B lymphocytes (plasma cells) of immunoglobulins of the main classes (IgG1, IgA, IgE and IgD [3,11]. From the ratio Th1, Th5 type lymphocyte activity (their function) may depend on the likelihood of respectively viral or microbial infections [3,4,11,12], also the formation of skin or respiratory hypersensitivity [4,13,14]. Thus, the activity of Th5-lymphocytes is associated with the synthesis of IgE, which provide respiratory manifestation of allergic reactions [2-4,11,14].
Thus, knowing the characteristics of Th1 and Th5 lymphocyte TCh lesions, it is possible not only to predict the risk of development of cellular or humoral immunity, leading to various infectious complications and diseases, but also to make choices that are adequate to the nature of the immune status of the immunomodulators [2-4,7,11,14].
Aim of the study
The aim of the study was to determine the types of dysfunction of Th1, Th5-lymphocytes and their production of cytokines, respectively, IFN-γ and IL-4 in the formation of suppression of humoral and cellular immune reactions in acute intoxication with various toxic chemicals.
Materials and Methods
The experiments were performed on random-bred albino rats of both sexes weighing 180-240 g. TCh (malathion - MT – [O,O-Dimethyl- S- (1,2-dicarbethoxyethyl) dithiophosphate]; methanol - MTh, acetonitrile- AN and trichloromethane -TChM) (Sigma-Aldrich] was administered subcutaneously at a single dose of 0.5 LD . LD50 of MT, MTh, AN and TChM for rats after subcutaneous administration was, respectively, 815,4±28,0, 8050,0±430, 1750±95 and 6010±615 mg/kg. Immunity system indicators were evaluated by generally accepted methods in experimental immunotoxicology and immunology [2,3,11] after administered of TCh (acute intoxication). The control group of rats was administered subcutaneously at a single dose of 0.25% aqueous solution (1,0 ml) of dimethyl sulfoxide (DMSO). TCh was administered subcutaneously in 1.0-1.5 ml of a 0.25% solution of DMSO. TCh was dissolved in DMSO, a 0.25% aqueous solution containing a toxicant was prepared
The function of Th1 lymphocytes was determined by delayed- type hypersensitivity (DTH) reaction. The DTH was studied in animals by weight gain of the hind paw foot in %. The resolving dose of RSBC (5×108) was administered under the aponeurosis of foot of the hind paw on 4 days after immunization, which was performed intraperitoneally almost simultaneously with the administered of TCh. The reaction of DTH was evaluated after 24 h [2,3,5,6].
The function of Th5 lymphocytes was investigated by the number antibody-forming cells (AFC) in the spleen, synthesizing IgG to sheep blood cells (RSBC), in the spleen on 8 days after intraperitoneally immunization 2×108 RSBC almost simultaneously with the administered of TCh) by indirect local hemolysis in the gel [2,3,11].
The concentration of immunoregulatory cytokines IFN-γ (#MBS824935) and IL-4 (# MBS2883072), proinflammatory cytokine IL-6 (# MBS2885203)) [3,15] was examined in rat blood plasma, respectively, on 5 and 8 days after the first injection of TCh using kits (ELISA Kits MyBioSoure) in accordance with the manufacturer’s instructions. Blood for research was taken from the retroorbital venous sinus. The data obtained were processed statistically using the Student’s t-test. Differences between the parameters were considered reliable at p<0.05.
Results
Under the influence of TCh (Table 1) - malathion, methanol, acetonitrile, trichloromethane - there was a decrease in the function of Th1 lymphocytes, assessed by the DTH reaction compared to the control level, respectively, in 2.27; 1.39; 1.86 and 1.53 times (p<0.05). The activity of Th5 lymphocytes after acute intoxication of MT, MTh, AN and TChM on day 8 after immunization of RSBC, estimated by the concentration of IgG (the number of AFC in the spleen), decreased, respectively, by 1.51; 1.95; 1.78 and 1.49 times (p <0.05).
Table 1:
Thus, the parameters characterizing various immune reactions and the associated functions of Th1 and Th5 lymphocytes, under the action of MT decreased in 2.27 and 1.51 times, respectively, and with MTh acute intoxication – in 1.39 and 1.95 times, AN acute intoxication – in 1.86 and 1.78 times, with TChM acute intoxication – in 1.53 and 1.49 times, respectively. This suggests that under the influence of the organophosphorus compound (MT) acute intoxication the greater degree reduces functions of Th1 lymphocytes, MTh causes a reduction of mainly Th5 lymphocytes activity, and AN and TChM acute intoxications the Th1 and Th5 lymphocytes are equally impaired.
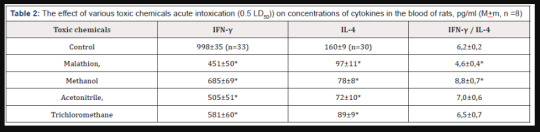
The revealed features of the lesion of various TCh of Th1- and Th5 lymphocytes functions is confirmed by a study of the concentration of cytokines in the blood of rats (Table 2). After acute intoxication of organophosphorus compound (MT), there was decrease of the concentrations of IFN-γ and IL-4, respectively, in 2.21 and 1.65 times (p <0.05), after MTh acute intoxication - in 1.46 and 2.05 times (p <0.05), AN - 1.98 and 2.22 times (p <0.05), after exposure of TChM – in 2.72 and 1.80 times (p <0.05), respectively.
Table 2: The effect of various toxic chemicals acute intoxication (0.5 LD50)) on concentrations of cytokines in the blood of rats, pg/ml (М+m, n =8)
An increase in the IFN-γ/IL-4 ratio characterizes a decrease of the functional activity of Th5 lymphocytes as compared with the function of Th1 cells, and a decrease in this ratio indicates a greater suppression of the activity of Th1 lymphocyte lymphocytes compared to Th5 cells [2,3]. We found that the ratio of IFN-γ / IL-4 in acute intoxication with MT, MTh, AN and TChM was 4.6±0.4 (p<0.05); 8.8±0.7 (p <0.05); 7.0±0.6 and 6.5± 0.7, respectively (control - 6.2±0.2). This confirms the results testifying about the features of lesion of Th1 and Th5 cell damage by various TCh.
Discussion
The main mechanisms of dysregulation of immunogenesis and the function of T and B immunity with organophosphorus compound (MT) acute intoxication are: changes in the redistribution of immunocytes between the organs of the immune system; violation of the cooperation of T-and B-lymphocytes; inhibition T-cell acetylcholinesterase, natural killer cells (NK) acetylcholinesterase and acetylcholinesterase of cells of antibody-dependent cellular cytotoxicity (ADCC); effect on cholinergic receptors of immunocompetent cells of high acetylcholine concentrations; immunosuppressive effect of corticosteroids [2,3,16-18].
The immunotoxic effect under the action of methanol mainly associated with the reduction of IgG production (Th1 and B cells functions), is also associated with the suppression of B lymphocyte function due to a violation of folic acid metabolism, the immunosuppressive effect of corticosteroids, the initiation of immunocyte lipid peroxidation. It should be noted that the immunotoxic effect of methanol is predominantly associated with the action of metabolites of MTh - formaldehyde and formic acid [1,2,19]. Th5 lymphocytes are probably more sensitive to the highly toxic metabolites of biotransformation of methanol formaldehyde and formic acid [1,2].
An important role in the implementation of the immunotoxicity of AN is played not so much by the toxicant as its toxic metabolites, in particular hydrogen cyanide. It is cyanide by inhibiting component a3 of cytochrome-c-oxidase of the system of enzymes of tissue respiration of mitochondria of immunocompetent cells, which determines the main immunotoxic effect of AN. It should be noted that hydrogen cyanide during the metabolism of AN enters the system of tissue respiration of mitochondria of cells of the lymphoid organs within a few hours (up to a day). In addition, AN inhibits of T lymphocytes acetylcholinesterase, NK and ADCC cells acetylcholinesterase (to a lesser extent than organophosphorus compound) [2,20].
In the mechanism of damage to the immune system after TChM acute intoxication, activation of the hypothalamic-pituitary-adrenal system (the action of corticosteroids) [2,3,16,17], initiation of lipid peroxidation and inhibition of acetylcholinesterase T lymphocytes, NK and ADCC cells plays a significant role [2].
Conclusion
Thus, the various toxic chemicals acute intoxication (in equiletal dose 0.5 LD50) can lead to relatively greater damage to Th1 or Th5 lymphocytes, as well as disrupt their function equally. Organophosphorus compound (malathion) acute intoxication in the greater degree reduces function of Th1 lymphocytes and production IFNγ, in comparison with of Th5 lymphocytes function and synthesis of IL-4 by them; the opposite effect is characteristic for methanol (decreases Th5 lymphocytes function and IL-4 production to a greater extent); acetonitrile and trichloromethane acute intoxications invokes a reduction of function of Th1 and Th5 lymphocytes and production of cytokines by them (IFNγ and IL-4) equally.
Read More About this Article:https://biomedgrid.com/fulltext/volume3/features-of-reduction-of-th1-th2-lymphocytes-functions-after-intoxication-of-various-toxic-chemicals.000698.php
For more about: Journals on Biomedical Science :Biomed Grid
#biomedgrid#American Journal of Biomedical Science & Research#Journals on Cancer medicine#Open access clinical and medical journal#American medical journal
0 notes
Photo

astm a53 grade b
Tycoon piping is one of the explorer in providing, assembling of PED affirmed ASTM A53 Grade B ERW Pipes, where it is imperative to keep temperatures over 1750 Degree F so as to keep the development of sigma stage. You can pick suitable pipe with wanted evaluation with best cost in India.
0 notes
Photo

🎨 Makeup by @Glambyangel on model @heyyimlexx EPIC things happens when collaborating with an AMAZING team in #Cleveland ~~~~~~~~~~~ 📷 Full Creative project idea & photographer @jordan_kage ~~~~~~~~~~~ Lipstick/Lashes : @caincosmetics Brows : Dip brow ebony @anastasiabeverlyhills #Kreativekage #kagegang #applypressure #applyingpressure #clevelandmakeupartist #clevelandphotographer #clevelandmodel #glambyangel #beauty #makeup #motd #caincosmetics #cleveland #216 #makeupforblackwomen #beautiful #gorgeous #nikon #sigma #1750 #d750 #canon #alienbee #paulcbuff #ebonyqueen #ebony #ebony goddess #ink @makeupforblackwomen https://www.instagram.com/p/BotjsbgA6AI/?utm_source=ig_tumblr_share&igshid=1n0kdyxsi6ltl
#cleveland#kreativekage#kagegang#applypressure#applyingpressure#clevelandmakeupartist#clevelandphotographer#clevelandmodel#glambyangel#beauty#makeup#motd#caincosmetics#216#makeupforblackwomen#beautiful#gorgeous#nikon#sigma#1750#d750#canon#alienbee#paulcbuff#ebonyqueen#ebony#ink
0 notes
Text
The MeerKAT Telescope as a Pulsar Facility: System verification and early science results from MeerTime. (arXiv:2005.14366v1 [astro-ph.IM])
We describe system verification tests and early science results from the pulsar processor (PTUSE) developed for the newly-commissioned 64-dish SARAO MeerKAT radio telescope in South Africa. MeerKAT is a high-gain (~2.8 K/Jy) low-system temperature (~18 K at 20cm) radio array that currently operates from 580-1670 MHz and can produce tied-array beams suitable for pulsar observations. This paper presents results from the MeerTime Large Survey Project and commissioning tests with PTUSE. Highlights include observations of the double pulsar J0737-3039A, pulse profiles from 34 millisecond pulsars from a single 2.5h observation of the Globular cluster Terzan 5, the rotation measure of Ter5O, a 420-sigma giant pulse from the Large Magellanic Cloud pulsar PSR J0540-6919, and nulling identified in the slow pulsar PSR J0633-2015. One of the key design specifications for MeerKAT was absolute timing errors of less than 5 ns using their novel precise time system. Our timing of two bright millisecond pulsars confirm that MeerKAT delivers exceptional timing. PSR J2241-5236 exhibits a jitter limit of <4 ns per hour whilst timing of PSR J1909-3744 over almost 11 months yields an rms residual of 66 ns with only 4 min integrations. Our results confirm that the MeerKAT is an exceptional pulsar telescope. The array can be split into four separate sub-arrays to time over 1000 pulsars per day and the future deployment of S-band (1750-3500 MHz) receivers will further enhance its capabilities.
from astro-ph.HE updates on arXiv.org https://ift.tt/2XM0Ggv
0 notes
Link
Fastwell is one of the leaders in supplying, manufacturing of PED approved ASTM A53 Grade B ERW Pipes, where it is important to keep temperatures above 1750 Degree F in order to prevent the formation of sigma phase. You can choose the appropriate pipe with the desired grade with the best price in India
0 notes
Text
MARUTI SUZUKI S-CROSS - DDIS 200 SIGMA 1.3
MARUTI SUZUKI S-CROSS - DDIS 200 SIGMA 1.3
Maruti S Cross has been able to successfully announce its entry in the crossover car segment through the diesel variants. It has able to garner gravely positive and appealing responses from the users. Maruti Suzuki have pretty high expectations for this vehicle and are planning to launch the petrol variants later this year to improve their stronghold into the segment.
Overview
The exteriors of the car look quite inspired by the SX4 model from Maruti. With chrome finished front grille and slightly slanting roof, the vehicle gives a menacing look. Maruti S Cross interiors are far more wonderful and the designers are being increasingly applauded for their success in delivering ample space as well as comfort.
Maximum power: 88.5 bhp @ 4000 rpm and 118 Nm at 3750 rpm
Maximum torque: 200 Nm @ 1750 rpm and 320 Nm @ 1750 rpm
No. of cylinders: 4
The main competitors in this segment are Hyundai Creta and Ford Ecosport. The car belongs to the premium car segment from Maruti Suzuki and therefore is available through booking at Maruti Nexa.
Variant
Presently the vehicle is available in only diesel variants at Nexa showrooms. Maruti Suzuki is expected to launch S Cross petrol later this year. It is one of the most awaited launches of the year in the auto industry. It is expected to cost at around Rs 9 Lakhs for the base model.
The diesel variants are available in five variants. Four of these come with a maximum torque capacity of 200 Nm while one model comes with a more powerful torque capacity of 320 Nm. The 200 Nm models are named as Alpha, Zeta, Sigma and Delta. The base model here is Sigma and is priced at Rs 8.65 Lakhs ex showroom while the top model in 200 Nm variant, Alpha is available at Maruti Suzuki Nexa price of Rs 11.3 Lakhs. The price tag for the 320 Nm Alpha variant is set at Rs 11.35 Lakhs ex showroom.
VariantsEx Showroom PriceBookDDIS 200 Sigma 1.3 8,43,138 Book NowDDIS 200 Delta 1.3 MT 9,16,479 Book NowDDIS 200 Zeta 1.3 MT 9,75,636 Book NowDDIS 200 Alpha 1.3 MT 11,07,546 Book NowDDIS 320 Alpha 1.3 MT 12,74,983
Specification
Model
S-Cross
No
Variant
DDIS 200 Sigma 1.3
Diesel
Anti-Lock Braking System
Yes
22.7
Passenger Airbag
Yes
1598 cc
BHP
118
Yes
Parking Sensors
Yes
Yes
Tyre Type
Tubeless, Radial
5
Transmission Type
Manual
4300 mm
Touch Screen
No
Fuel Type
Diesel
Mileage
22.7
Engine
1598 cc
A/C
Yes
Power Steering
Yes
Seating Capacity
5
Length
4300 mm
Click Here For More Information :-
http://nexapune.com/nexa-new-cars/maruti-suzuki-s-cross
#nexa showrooms in pune#nexa showroom in pune#nexa showroom in pune baner#nexa dealers in pune#nexapune#book maruti scross in pune#maruti scross price
0 notes
Text










Heat and Power Plant | Urbex 15 / 2019 | Zone D / Turbino-Zen Underground | Gallery 1 of 3
#Photographers on Tumblr#Original Photographers#Photography#Nikon#Heat and Power Plant#Power Plant#Industry#Nikon D7200#Sigma 1750#UE#UrbanExploration#Exploration#Urbex#Urbex 2019#UrbexSkalmar#UrbexKind#MaciejSmolenPhotoGraphics
31 notes
·
View notes
Text

Installation 2020.04.27 B
#Photographers on Tumblr#Original Photographers#Photography#Nikon#Nikon D7200#Sigma 1750#InstallationsAreSigns#MaciejSmolenPhotoGraphics
3 notes
·
View notes
Text







Heat and Power Plant | Urbex 15 / 2019 | Zone F / Tiny Kettles Hall | Gallery 1 of 3
#Photographers on Tumblr#Original Photographers#Photography#Nikon#Heat and Power Plant#Power Plant#Heating Plant#Industrial Hall#Industry#Nikon D7200#Sigma 1750#UE#UrbanExploration#Exploration#Urbex#Urbex 2019#UrbexSkalmar#UrbexKind#MaciejSmolenPhotoGraphics
13 notes
·
View notes
Text









Heat and Power Plant | Urbex 15 / 2019 | Zone A / Structure of Ash | Gallery 2 of 5
#Photographers on Tumblr#Original Photographers#Photography#Nikon#Heat and Power Plant#Power Plant#Industrial Hall#Industry#Nikon D7200#Sigma 1750#UE#UrbanExploration#Exploration#Urbex#Urbex 2019#UrbexSkalmar#UrbexKind#MaciejSmolenPhotoGraphics
15 notes
·
View notes
Text






Heat and Power Plant | Urbex 15 / 2019 | Zone B / Short Distance
#Photographers on Tumblr#Original Photographers#Photography#Nikon#Heat and Power Plant#Power Plant#Industry#Nikon D7200#Sigma 1750#UE#UrbanExploration#Exploration#Urbex#Urbex 2019#UrbexSkalmar#UrbexKind#MaciejSmolenPhotoGraphics
11 notes
·
View notes
Text










Heat and Power Plant | Urbex 15 / 2019 | Zone K / Roof | Gallery 1 of 3
#Photographers on Tumblr#Original Photographers#Photography#Nikon#Heat and Power Plant#Power Plant#Roof#Chimney#Industry#Nikon D7200#Sigma 1750#UE#UrbanExploration#Exploration#Urbex#Urbex 2019#UrbexSkalmar#UrbexKind#MaciejSmolenPhotoGraphics
8 notes
·
View notes
Text







Heat and Power Plant | Urbex 15 / 2019 | Zone J / Trans Tower / Top | Gallery 1 of 2
#Photographers on Tumblr#Original Photographers#Photography#Nikon#Heat and Power Plant#Power Plant#Tower#Industry#Impressions#Street Art#Nikon D7200#Sigma 1750#UE#UrbanExploration#Exploration#Urbex#Urbex 2019#UrbexSkalmar#UrbexKind#MaciejSmolenPhotoGraphics
5 notes
·
View notes