#Potassium Hydroxide Solution
Explore tagged Tumblr posts
Text
Shakti Chemicals - Manufacturer and Exporter of Oil Drilling, Food Grade & Commercial Chemicals
Shakti Chemicals is a company based in Vadodara, Gujarat, India that specializes in the manufacturing and exporting of various chemical products such as:
Ammonium Bisulphite 70% Solution
Ammonium Bisulphite Catalyst
Potassium Hydroxide Solution
Potassium Sulphite (K2SO3)
Potassium Bisulphite (KHSO3)
Ammonium Bisulphite 70% Solution is a clear, colorless liquid that is commonly used in the food industry as a preservative and antioxidant. It can also be used in the production of various chemicals, such as sodium metabisulphite and ammonium thiosulfate.
Potassium Hydroxide Solution is a strong alkali that is commonly used in the production of various chemicals such as detergents, fertilizers, and pharmaceuticals. It is also used in the production of biodiesel.
Potassium Sulphite (K2SO3) and Potassium Bisulphite (KHSO3) are both used as preservatives in the food industry to prevent the growth of bacteria and other microorganisms. They are also used in the production of photographic chemicals, dyes, and pharmaceuticals.
Shakti Chemicals also specializes in the production of Oxygen Scavenger chemicals. Oxygen Scavengers are used in various industries to prevent corrosion and extend the shelf life of products by reducing the amount of oxygen present in a given environment.
Shakti Chemicals offers a range of Oxygen Scavenger products designed to meet the specific needs of different industries. Their products are used in industries such as oil and gas, food and beverage, pharmaceuticals, and water treatment.
The company is committed to providing high-quality products that meet or exceed industry standards. They use advanced manufacturing processes and rigorous quality control measures to ensure that their products are of the highest quality.
Shakti Chemicals also offers excellent customer service and technical support. They work closely with their clients to understand their needs and provide customized solutions to meet their specific requirements. The company has a strong reputation for reliability, quality, and innovation, and is widely recognized as a leader in the Oxygen Scavenger industry.
Overall, Shakti Chemicals specialize in the production of a range of chemical products that have a variety of uses in different industries. To get best quote or more details to buy our chemical products call Mr. Rahul Madan Shimpi (+91-9825043369) or mail us at [email protected].
0 notes
Text

#manganese dioxide#potassium hydroxide#oxygen#alkaline#oxidation#tetrahedral#acidic#paramagnetic#pi-bonding#chemistry#solutions#electrolysis
1 note
·
View note
Text
(the 2 most voted elements will continue onto round 2!)
More info about each element (and propaganda for the ones I like) under the cut. pleeeeeeeeease read some of them at least the one about francium
(disclaimer: these are based off short wikipedia reads and my crumbling high school chemistry knowledge. correct me if I'm wrong about anything.)
HYDROGEN: Hydrogen is the lightest element (consisting of only one proton and one electron). It is also the most abundant element in the universe, it's a gas (at room temperature) and it can explode. It's also quite representative of acids, having the (Arrhenius) definition of an acid straight up saying that it has to dissociate in water to form H+ ions. It's also quite an efficient fuel. Hydrogen is anywhere and Hydrogen is everywhere. If you like explosions, sour beverages, or acid in general, consider voting Hydrogen!
LITHIUM: Lithium, under standard conditions, is by far the least dense metal and the least dense solid element! You may primarily know him from your phone's Lithium-ion batteries. There are Lithium-based drugs used to treat mental illnesses. You can throw a block of lithium in water and it will make a really big explosion. The metal is soft and silvery. I'm running out of things to say about him. If you like batteries vote Lithium? (edit: just realised lithium is used for batteries, and batteries are connected to robotics and engineering. if you like robots and cool mechanical stuff vote lithium!)
SODIUM: You must know him from table salt. That's actually NaCl, his best known involvement. There are many more very important and very commonplace compounds that involve sodium, such as baking soda (NaHCO3) and sodium hydroxide (NaOH) (that's probably the most famous base?). It's also very important to the human body (you shouldn't eat more than 2300mg a day). If you've ever used table salt or baking soda while cooking, consider voting Sodium!
POTASSIUM: Their name was based on the word potash, which was based on an early and easy way of obtaining potassium, from putting ash in a pot, adding water, heating, and evaporating the solution. It's used in a lot of fertilisers because it's an essential plant nutrient. It's also involved in a ton of important compounds: KOH (a strong base), KNO3 (often used as salt bridges in electrochemical cells), K2CrO7 (an oxidising agent often used in organic synthesis), and K2CrO4 (I don't know what this one does). If you have ever eaten food from fertilisers consider voting Potassium!
RUBIDIUM: Rubidium compounds are sometimes used in fireworks to give them a purple color. They've also got a cool name, based on the latin rubidius, for deep red (the color of its emission spectrum). I'll be real, I don't really know much about them beyond that, but that is one cool name. Vote for Rubidium if you like cool names.
CAESIUM: Caesium is used in the definition for a second, meaning that an entire SI unit is based on it! A second can be defined as "the duration of 9,192,631,770 cycles of microwave light absorbed or emitted by the hyperfine transition of caesium-133 atoms in their ground state undisturbed by external fields". It was also discovered from mineral water. Did you know that they had to use 44000 liters of water to find her? If you've ever experienced time or had a conception of it in terms of units, consider voting Caesium!!!
FRANCIUM!!!: Caesium... TWO! It's sad that no one will probably read this far but this is my favourite element in this poll. This element is characterised by instability. Her longest half-life is 22 minutes. Her entire existence was conjoint with Caesium before they discovered that she was her own element. She has never been seen. They literally never confirmed what color she is. She was born in a wet cardboard box all alone. Through the hands of different scientists, she was going to be named after Russia, Virginia, or Moldavia at different points in time. At one point the name catium was proposed (for "cation", since she was believed to be the most electropositive cation), but was rejected because it sounded like a cat element. Which is so fucking sad. We could've had cat element but we ended up with France element. That's right she's also named after France. Just tragic fascinating existence overall. Also isn't it just insane that her half-life is only 22 minutes? Dude, you don't get it, the most of her that's ever existed in one place is a mere 300000 atoms. She's here and she's gone. What the hell.
The charm of Francium can be summarised by the wise words of my good friend Wolfgang Amadeus Mozart:
20 notes
·
View notes
Text
i do find it incredibly funny how i’m the only person in my entire department with absolutely zero chemistry background or lab experience. my supervisor has a phd my coworker is fresh outta college after majoring in environmental chemistry and volunteering with half the organizations we REPORT to and meanwhile i’m just some art school dropout with four years experience working at petco under his belt who just happens to be so insanely autistic about water quality and aquatic animal care that they hired me anyway and are just letting me learn as i go. it rocks. my supervisor asks me how i would do the math to determine how much potassium hydroxide i need to weigh out to make 100mls of 8 normal solution and i have to admit to her that i have no idea what she’s talking about and instead of getting mad at me or frustrated she just shows me where to find the information i need and lets me figure it out and then once she’s sure i know what i’m doing to a level where i’m not going to blow anything up she sets me loose in the lab to apply my newfound knowledge . and i’m getting PAID to do this shit
20 notes
·
View notes
Text
Doing review questions.
Hyperkalemia is a known side effect of ACE inhibitors and angiotensin receptor blockers such as olmesartan. The risk of hyperkalemia is increased with chronic kidney disease, diabetes mellitus, moderately severe to severe heart failure, NSAID use, and older adults. Chlorthalidone and hydrochlorothiazide can cause hypokalemia.
In men who are diagnosed with hypogonadism with symptoms of testosterone deficiency and unequivocally and consistently low serum testosterone concentrations, further evaluation with FSH and LH levels is advised as the initial workup to distinguish between primary and secondary hypogonadism. If secondary hypogonadism is indicated by low or inappropriately normal FSH and LH levels, prolactin and serum iron levels and measurement of total iron binding capacity are recommended to determine secondary causes of hypogonadism, with possible further evaluation to include other pituitary hormone levels and MRI of the pituitary. If primary hypogonadism is found, karyotyping may be indicated for Klinefelter’s syndrome.
Daily use of polyethylene glycol (PEG) solution has been found to be more effective than lactulose, senna, or magnesium hydroxide in head-to-head studies. Evidence does not support the use of fiber supplements in the treatment of functional constipation. No adverse effects were reported with PEG therapy at any dosing regimen. Low-dose regimens of PEG are 0.3 g/kg/day and high-dose regimens are up to 1.0–1.5 g/kg/day. Ref: Tabbers MM, DiLorenzo C, Berger MY, et al: Evaluation and treatment of functional constipation in infants and children: Evidence-based recommendations from ESPGHAN and NASPGHAN. J Pediatr Gastroenterol Nutr 2014;58(2):258-274. 2) Gordon M, MacDonald JK, Parker CE, et al: Osmotic and stimulant laxatives for the management of childhood constipation. Cochrane Database Syst Rev 2016;(8):CD009118. 3) Lauters R, Saguil A: Laxatives for the management of childhood constipation. Am Fam Physician 2017;96(7):433-434
Primary hyperaldosteronism should be suspected as a cause for hypertension if a patient has a spontaneously low potassium level or persistent hypertension despite the use of three or more antihypertensive medications, including a diuretic. This can be evaluated by checking a serum renin activity level and a serum aldosterone concentration and determining the aldosterone/renin ratio. Primary hyperaldosteronism typically presents with a very low serum renin activity level and an elevated serum aldosterone concentration. A 24-hour urine collection for 5-hydroxyindoleacetic acid (5-HIAA) would be used to evaluate for a neuroendocrine tumor, which can present as chronic flushing and diarrhea. Cortisol levels can be checked if Cushing syndrome is suspected. Hypertension can be present in Cushing syndrome, but it is typically associated with other signs such as obesity and an elevated blood glucose level due to insulin resistance.
Psychogenic tremor is characterized by an abrupt onset, spontaneous remission, changing characteristics, and extinction with distraction. Cerebellar tremor is an intention tremor with ipsilateral involvement on the side of the lesion. Neurologic testing will reveal past-pointing on finger-to-nose testing. CT or MRI of the head is the diagnostic test of choice. Parkinsonian tremor is noted at rest, is asymmetric, and decreases with voluntary movement. Bradykinesia, rigidity, and postural instability are generally noted. For atypical presentations a single-photon emission CT or positron emission tomography may help with the diagnosis. One of the treatment options is carbidopa/levodopa. Patients who have essential tremor have symmetric, fine tremors that may involve the hands, wrists, head, voice, or lower extremities. This may improve with ingestion of small amounts of alcohol. There is no specific diagnostic test but the tremor is treated with propranolol or primidone. Enhanced physiologic tremor is a postural tremor of low amplitude exacerbated by medication. There is usually a history of caffeine use or anxiety.
Ref: Crawford P, Zimmerman EE: Tremor: Sorting through the differential diagnosis. Am Fam Physician 2018;97(3):180-186.
I got 100% on the first quiz! :)
3 notes
·
View notes
Text
Dimethyl ether, carbon tetrachloride, sodium thiohydrate, pyridine, hydrogen bromide, barium hydroxide, barium sulfide, phenol, hydrochloric acid, dibromomethane, sodium hydroxide, n-butylene ether, 3-methylpyridine, bromoethane, aluminum trichloride solution, benzene, ethanethiol, octadecyl acetamide, acetonitrile, N N-diisopropylethylamine, hydrogen fluoride [anhydrous], potassium antimony tartrate, n-butylacetate, ethylene oxide, cyclohexane, potassium hydroxide, aluminum trichloride [anhydrous], 2-nitroanisole, 1, 2-dichloropropene, n-butanol, magnesium, O O ≤-diethylthiophosphoyl chloride, phenol solution, N-(phenylethyl-4-piperidine) propionamide citrate, ethyl acetate, 1,4-xylene, 2-aminopropane, isophthaloyl chloride, 2-chlorotoluene, cyclopentene, propionic acid, hydrofluoric acid, 2-butenaldehyde, 2-methylpentane, ethylamine, bromine, coal tar pitch, ethyl formate, ammonia solution [containing ammonia > 10%] 1-aminohydrin, 4-ethoxyphenylamine, diisopropylamine, sodium ethanolate, nitrifying asphalt, hydrazide hydrate [containing hydrazide ≤ 64%], dimethyl sulfate, acetic acid [content > 80%], acetaldehyde, 2-butylketone, aluminum borohydrate, phenylethanolnitrile, 2-chlorobenzoyl chloride, sodium hypochlorite solution [containing available chlorine > 5%], 2-aminophenol, chloroplatinic acid, barium chloride, tert-butylbenzene, tribromide, methyl sulfide, Diphosphate pentasulfide, diethylamine, chlorobenzene, n-butylbenzene, 1,3-xylene, hydrogen peroxide solution [content > 8%], terephthaloyl chloride, red phosphorus, tetramethyl ammonium hydroxide, methanol, propionaldehyde, 2-methoxyphenylamine, bleach powder, triethyl propropionate, 1-bromobutene, cyclohexanone, di-(tert-butylperoxy) phthalate [paste Content ≤ 52%], tetrahydrofuran, trichloroethylene, magnesium aluminum powder, formic acid, sodium ethanol ethanol solution, isopropyl ether, acetic acid solution [10% < content ≤ 80%], 2-methyl-1-propanol, diethyl carbonate, sodium aluminum hydroxide, 2-methylpyridine, n-butylamine, toluene, thiourea, magnesium alloy [flake, banded or striped Containing magnesium > 50%], methyl benzoate, hydrobromide, 4-methylpyridine, iodine monochloride, sodium sulfide, 3-bromo-1-propene, 2-propanol, potassium borohydroxide, triethylamine, ammonia, 4-nitro-2-aminophenol, 1, 2-epichlorohydrin, 1-propanol, cyclopentane chloride, n-propyl acetate, bromoacetic acid, zinc chloride solution, trichloromethane, 1-bromopropane, monoamine [anhydrous], perchloric anhydride acetic anhydride solution, 1-bromopropane Potassium hydroxide solution [content ≥ 30%], boric acid, sodium borohydrate, hydroacetic acid bromide solution, acrylic acid [stable], cyclopentane chloride, ammonium hydrogen sulfate, calcium hydroxide, 2-ethoxyaniline, dimethyl carbonate, sodium nitroso, monomethylamine solution, zinc chloride, hydrogen sulfide, trimethyl acetate, iodine trichloride, nitric acid, sodium hydroxide solution [content ≥ 30%], trimethyl orthoformate, hydrogen chloride [anhydrous], 4-methoxyaniline, sulfur, succinile, acetic anhydride, dipropylamine, methyl acetate, isopropylbenzene, propionyl chloride, ethyl formate, phosphorus pentoxide, formaldehyde solution, nitrogen trifluoride, acetone, ethanol [anhydrous], white phosphorus, 1, 2-xylene, 1, 3-dichloropropene, 1, 1, 1-dichloroethane, N N-diethylethanolamine, sulfuric acid, N, N-dimethyl formamide, methyl mercaptan, 4-chlorotoluene, 1, 2-dichloroethane, dichloromethane, succinyl chloride, 2, 3-dichloropropene, xylene isomer mixture, tartrate nicotine, cyclopentane, petroleum ether, bromocyclopentane Potassium perchlorate, potassium chlorate, aluminum powder, chromic acid, iron chloride, lead nitrate, magnesium powder, nickel chloride, nickel sulfate, perchloroethylene, phosphate, potassium dichromate, sodium dichromate, zinc nitrate
2 notes
·
View notes
Text
for reference of why no one in their right mind would drink Fehling's solution, it has a pH of around 13. That's like 10~15% bleack. Potassium hydroxide has a pKb of .5, it is reactive.
shit i’ve heard chemistry majors say
- *student in a lab coat, cutting in the cafeteria line* YOU DON’T UNDERSTAND I DON’T HAVE A LOT OF TIME MY EXPERIMENT IS GOING TO CATCH FIREEEE
- *loud pop* student, in very calm voice: well that was painful
- lab assistant, seeing me frantically pulling on gloves: oh no. what did you do now
- professor: come on guys, don’t hate on social sciences majors… it’s not their fault they were born this way
- so i was grading your tests last night. i wanted to kill someone.
- you have five minutes until the end of class to finish the test. but i want to go outside for a smoke, so three
- *section of lab report titled “applications of compound”* i heard that a drug cartel used it to dissolve bodies, should i list that?
- “i’m synthesizing this compound in my next lab class, what kind of stuff effects the success rate and yield?” “dunno man, it depends on your karma”
- based on my recent lab assignments, i have come to the conclusion that the professor wants me to get killed
- dude, Fehling’s solution contains glucose, what if it tastes like lemonade? *proceeds to dip finger in and lick it* well that was a disappointment. the potassium hydroxide makes it kinda bitter.
- professor: you’ll understand this concept in your fifth year student: sir, this is a four-year program professor: oh, then never
72K notes
·
View notes
Text
High-Quality Potassium Hydroxide Pellets at Best Price
Are you looking for high quality and efficient potassium hydroxide pellets then Atlas Pellets are trusted manufacturer in India. Atlas Pellets is trusted manufacturer and supplier of premium grade KOH pellets, widely used in industrial, chemical and laboratory applications. their pellets are highly pure then make sure that excellent solubility and efficiency in various processes, including soap production, chemical synthesis and water treatment. They are guarantee to consistence quality and performance with precise composition and controlled manufacturing.

Atlas Pellets are committed to providing potassium hydroxide pellets that compete international standards. Their pellets are carefully processed to maintain stability and they can make sure that their pellets are safe handling and storage. Their high purity KOH pellets are delivers optimal results if you need them for industrial or research purpose. Atlas Pellets are reliable and cost effective solution that compete you needs.
Atlas Pellets are delivers pellets in bulk order and inquiries. What are you waiting for pick up your phone and contact causticpellets.com today. They are receive your call any time.
#sodium hydroxide pellet#atlas pellets industries#sodium hydroxide pellet exporters#best sodium hydroxide pellet#caustic pellet#caustic pellets#atlas pellet#potassium hydroxide pellets#sodium hydroxide pellets#sodium hydroxide pellets exporters
0 notes
Text
The Future of Clean Energy: Unlocking the Potential of Hydrogen Production Electrolysis
With the increasing demand for clean and sustainable energy, hydrogen is emerging as a powerful alternative to fossil fuels. One of the most promising methods for producing hydrogen is hydrogen production electrolysis. This process utilizes electricity to split water into hydrogen and oxygen, offering a zero-emission solution to energy generation. But how exactly does it work, and why is it gaining so much attention? Let’s dive into the details and explore the future of this revolutionary technology.
Understanding Hydrogen Production Electrolysis
Hydrogen production through electrolysis is a process that uses an electric current to break down water molecules (H₂O) into hydrogen (H₂) and oxygen (O₂). The method is appealing because it produces pure hydrogen without emitting greenhouse gases, especially when powered by renewable energy sources like wind and solar.
How Does Electrolysis Work?
Electrolysis takes place in an electrolyzer, which consists of an anode and a cathode separated by an electrolyte. When an electric current is applied:
Water molecules at the anode split into oxygen gas and positively charged hydrogen ions.
The hydrogen ions move through the electrolyte to the cathode, where they combine with electrons to form hydrogen gas.
The result is clean hydrogen that can be used for various applications, including power generation, transportation, and industrial processes.
Types of Hydrogen Production Electrolysis
There are three main types of hydrogen production electrolysis, each with its own advantages:
Alkaline Electrolysis (AEL): This is the most established method, using a liquid alkaline solution (typically potassium hydroxide) as the electrolyte. It is cost-effective and suitable for large-scale hydrogen production.
Proton Exchange Membrane (PEM) Electrolysis: PEM electrolyzers use a solid polymer membrane as the electrolyte. This method offers higher efficiency, faster response times, and compatibility with fluctuating renewable energy sources.
Solid Oxide Electrolysis (SOEC): SOEC electrolyzers operate at high temperatures and use ceramic materials as electrolytes. This method has the potential for high efficiency and can utilize waste heat from industrial processes.
Benefits of Hydrogen Production Electrolysis
1. Zero Carbon Emissions
One of the primary advantages of hydrogen production electrolysis is its ability to generate hydrogen without releasing carbon dioxide. When powered by renewable energy, the entire process is 100% clean and sustainable.
2. Energy Storage and Grid Stability
Hydrogen serves as an excellent energy storage medium. Excess electricity from wind or solar power can be converted into hydrogen and stored for later use, helping to balance energy supply and demand.
3. Fuel for the Future
Hydrogen produced via electrolysis can be used in fuel cells to generate electricity for homes, vehicles, and industrial applications. This can significantly reduce dependence on fossil fuels.
4. Versatile Applications
Industries such as transportation, aerospace, and chemical manufacturing are increasingly adopting hydrogen as a clean alternative to traditional fuels and feedstocks.
Challenges and Limitations
Despite its advantages, there are some challenges associated with hydrogen production electrolysis:
1. High Energy Requirements
Electrolysis requires a significant amount of electricity. The sustainability of this process depends on the availability and affordability of renewable energy sources.
2. Infrastructure Development
For hydrogen to be widely adopted, substantial investments are needed to build storage and distribution infrastructure, including pipelines and refueling stations.
3. Production Costs
Currently, electrolysis is more expensive than hydrogen production from fossil fuels (such as steam methane reforming). However, technological advancements and economies of scale are expected to lower costs over time.
The Future of Hydrogen Production Electrolysis
Governments and private companies worldwide are investing heavily in hydrogen technologies. Several key trends indicate a bright future for hydrogen production electrolysis:
1. Advancements in Electrolyzer Efficiency
Ongoing research is focused on improving electrolyzer efficiency and durability, making hydrogen production more cost-effective.
2. Scaling Up Green Hydrogen Projects
Large-scale green hydrogen projects are being developed in Europe, the U.S., and Asia, demonstrating the potential of electrolysis in decarbonizing industries.
3. Integration with Renewable Energy
Hydrogen production electrolysis is becoming a crucial component of the renewable energy transition, enabling efficient energy storage and utilization.
Conclusion
As the world seeks cleaner energy solutions, hydrogen production electrolysis stands out as a key technology in the transition toward sustainability. With zero emissions, versatile applications, and the ability to integrate with renewable energy, it holds immense promise for the future. While challenges remain, continuous advancements and investments are paving the way for a hydrogen-powered world. The next decade could be transformative, bringing us closer to a sustainable energy future driven by clean hydrogen.
0 notes
Text
Understanding Lab Chemicals: Essential Components for Scientific Experiments
Lab chemicals are the cornerstone of scientific research and experimentation. Whether you are working in a school lab, a research facility, or an industrial lab, chemicals are integral to producing results, testing theories, and discovering new things. Lab chemicals come in many forms, and their role in the scientific process cannot be overstated. In this article, we will explore what lab chemicals are, how they are categorized, their importance, and safety tips for working with them.
What Are Lab Chemicals?
Lab chemicals are substances used in scientific laboratories for conducting experiments, testing hypotheses, and carrying out analyses. They can be in the form of solids, liquids, gases, or even powders. These chemicals are essential for research and industrial processes, such as manufacturing pharmaceuticals, creating new materials, conducting biological tests, and analyzing environmental factors.
From high school chemistry classes to advanced research in biochemistry or pharmacology, chemicals serve as the foundation of all scientific work. They are often produced and stored in specific conditions to ensure their effectiveness and safety.
Categories of Lab Chemicals
Lab chemicals can be divided into several categories, depending on their function and properties. Some of the main types include:
Reagents: Reagents are chemicals used in chemical reactions to detect, measure, or produce other substances. These chemicals are critical in a wide variety of experiments. Examples include acids, bases, solvents, and indicators. Reagents are often chosen based on the specific reaction required in an experiment.
Solvents: Solvents are used to dissolve other substances. They play a key role in preparing solutions, cleaning lab equipment, and facilitating reactions. Common solvents include water, ethanol, acetone, and hexane. The choice of solvent depends on the solubility of the substance being dissolved and the nature of the reaction.
Standards and Reference Materials: These are substances with known properties used for calibration and to ensure the accuracy of results. Standard chemicals often serve as a benchmark for measuring other substances in lab experiments. For example, the use of certified reference materials (CRMs) is important in environmental testing and calibration of instruments.
Buffers: Buffers are solutions that resist changes in pH when an acid or base is added. They are crucial for maintaining a stable environment in chemical and biological experiments. For example, biological systems such as enzymes work best within a narrow pH range, so buffer solutions are used to keep that environment consistent.
Chemicals for Synthesis: These chemicals are used in the creation of new compounds. Synthetic chemicals are crucial in industries ranging from pharmaceuticals to agriculture, where new compounds are constantly being developed for use in treatments, fertilizers, or pest control.
Acids and Bases: Acids and bases are fundamental to many chemical processes. Acids like hydrochloric acid and sulfuric acid are used in a variety of reactions, from cleaning metal surfaces to dissolving materials. On the other hand, bases such as sodium hydroxide or potassium hydroxide are used to neutralize acids or alter the pH of a solution.
Specialty Chemicals: This category includes chemicals used in specific fields or for unique applications. For example, pharmaceutical-grade chemicals, chemicals for food testing, or chemicals used in environmental research.
The Importance of Lab Chemicals
Lab chemicals are essential for a wide range of experiments and applications. Without them, the scientific community would be unable to conduct essential research, test hypotheses, or develop new materials. Here are a few key ways in which lab chemicals contribute to science:
Scientific Discovery: Lab chemicals are crucial for testing scientific theories and making groundbreaking discoveries. Researchers use chemicals to observe reactions, test the properties of materials, and understand the underlying mechanisms of biological and chemical processes.
Quality Control: In industries like pharmaceuticals, food production, and environmental testing, lab chemicals are used to ensure products meet safety and quality standards. Proper chemical testing can help detect contaminants or measure the concentration of active ingredients in a product.
Medical Advancements: Pharmaceuticals and medical treatments are developed using lab chemicals. From creating life-saving drugs to understanding disease mechanisms, chemicals play a vital role in improving health outcomes worldwide.
Environmental Protection: Lab chemicals are used to monitor environmental pollution, test water quality, and measure air pollutants. They are instrumental in developing new methods for conserving natural resources and reducing environmental damage.
Safety Considerations When Working with Lab Chemicals
While lab chemicals are powerful tools for scientific progress, they also pose potential risks. Improper handling, storage, or disposal of chemicals can lead to accidents, contamination, or exposure to toxic substances. Therefore, safety is a top priority in any laboratory setting.
Here are some essential safety guidelines when working with lab chemicals:
Wear Protective Gear: Always wear the appropriate personal protective equipment (PPE), such as lab coats, gloves, goggles, and face shields, to protect yourself from chemical splashes and exposure.
Read the Label: Before using any chemical, make sure you read its label, safety data sheet (SDS), and any instructions for use. The SDS provides valuable information about the chemical’s properties, hazards, and how to handle it safely.
Proper Storage: Store chemicals in their proper containers and according to their storage requirements. Some chemicals are reactive and must be kept in specific conditions (e.g., temperature, humidity) to prevent dangerous reactions.
Disposal: Dispose of chemicals properly by following local disposal regulations. Never pour chemicals down the drain unless instructed to do so by safety guidelines. Proper disposal prevents environmental contamination and protects human health.
Ventilation: Ensure proper ventilation in the laboratory, especially when working with volatile or toxic chemicals. Fume hoods and exhaust systems are essential to protect against inhaling harmful fumes.
Conclusion
Lab chemicals are indispensable in scientific research and industrial processes. They are crucial for conducting experiments, analyzing materials, and developing new products. While they offer great benefits, they also require careful handling and respect to ensure safety in the lab. By understanding the types of chemicals and their uses, researchers can optimize their experiments while minimizing risks. Always remember: safety first, and the results will follow!
#scientific discovery#chemicals#laboratory chemical supplier india#lab instruments#lab chemicals#science#lab#scifi#laboratory chemicals#setting up a new lab
0 notes
Text
HOW 2 INVENT SOAP:
INVENT TALLOW. Get some animal fat. Chop it up, add some salt and water, and cook it for a few hours. Scoop the melted liquid fat off the top and filter it through something to get any meaty bits that might still be in there out. When it cools down, it will turn back into solid fat, and you can repeat the process as many times as you want to get more non-fat stuff out.
INVENT POTASH. Fill a container with wood ashes (hardwoods work best). Then pour in rain water. Then let it soak. After a while, collect the liquid, which will now be a horrible caustic alkali solution. If you dry this out, you'll get crystals of potassium hydroxide, but you don't really need to, because the next step is...
MELT THE TALLOW AND PUT SOME POTASH WATER IN IT. This will be stupid hot and there will visibly be chemistry happening. Stir it up good for a while while absolutely not getting it on your hands.
Congratulations, you have now invented soap. Aren't you glad you did this part before you tried the penicillin?
I can understand how "modern person thrown into the past gets by pretending to be a healer/doctor" is as surprisingly common of a trope as it is. I mean I'm fluent enough at bullshitting to be pretty sure I could pull it off to impersonate a doctor in any time pre-1800s. If I have no idea what something is or how to treat it, I could just get the opinion of the other whatever-passes-as-medical-professionals around, but if their suggestions sound like bullshit I'm not doing it. And I'll beat the shit out of anyone suggesting bloodletting or mercury. With my healing stick. I've tied little bells on it, that jingle comically with every smack.
The awesome curative powers of my healing stick come from two separate sources: Placebo, and me using it to beat anyone trying to give my patients mercury.
37K notes
·
View notes
Text
What is Electrolysis Hydrogen Production? A Beginner’s Guide
As the world transitions toward cleaner energy solutions, hydrogen has emerged as a promising alternative to traditional fossil fuels. One of the most sustainable methods of producing hydrogen is through electrolysis. But what exactly is electrolysis hydrogen production, and why is it important? This beginner’s guide aims to answer these questions and shed light on its potential to revolutionize the energy landscape.
Understanding Electrolysis Hydrogen Production
Electrolysis is a process that uses electricity to split water ( H₂O ) into its basic components: hydrogen ( H₂ ) and oxygen ( O₂ ). When the electricity used comes from renewable energy sources like wind or solar, the hydrogen produced is often referred to as “green hydrogen” due to its minimal environmental impact.
The Basic Process
Electrolyzer: The device used for electrolysis consists of an anode and a cathode submerged in water, separated by an electrolyte.
Electric Current: A direct current (DC) is passed through the water.
Separation: Hydrogen gas collects at the cathode, while oxygen is released at the anode.
This simple yet effective process is at the heart of electrolysis hydrogen production, offering a clean and efficient way to generate hydrogen for various applications.
Why is Electrolysis Hydrogen Production Important?
1. A Cleaner Energy Source
Hydrogen produced via electrolysis emits no greenhouse gases during its use, making it an excellent substitute for fossil fuels in sectors like transportation, manufacturing, and power generation.
2. Energy Storage
Hydrogen acts as a powerful energy carrier, enabling the storage of excess energy generated by renewable sources. This addresses the intermittency issues of solar and wind power.
3. Industrial Applications
Industries like steel production, ammonia manufacturing, and chemical refining benefit from the high purity hydrogen generated through electrolysis.
Types of Electrolyzers Used in Hydrogen Production
1. Alkaline Electrolyzers
How It Works: Uses a liquid alkaline electrolyte, such as potassium hydroxide, to conduct electricity.
Advantages: Proven technology, cost-effective, and scalable.
Disadvantages: Lower efficiency compared to advanced systems.
2. Proton Exchange Membrane (PEM) Electrolyzers
How It Works: Utilizes a solid polymer electrolyte to separate hydrogen and oxygen.
Advantages: Higher efficiency and compact design.
Disadvantages: More expensive due to the use of precious metals like platinum.
3. Solid Oxide Electrolyzers
How It Works: Operates at high temperatures to split water molecules.
Advantages: Extremely efficient, especially when integrated with industrial heat sources.
Disadvantages: Still under development and requires further cost reductions.
Applications of Hydrogen from Electrolysis
1. Clean Transportation
Hydrogen-powered fuel cells are being increasingly used in vehicles, providing a zero-emission alternative to gasoline and diesel engines.
2. Power Generation
Hydrogen can be burned or used in fuel cells to generate electricity, making it a flexible energy source for both grid and off-grid applications.
3. Industrial Uses
In industries requiring high temperatures, such as steelmaking, hydrogen offers a cleaner fuel alternative to coal.
4. Export Potential
Countries investing in electrolysis hydrogen production can export green hydrogen to nations seeking to decarbonize their energy systems.
Challenges in Electrolysis Hydrogen Production
1. High Energy Requirements
Electrolysis requires significant amounts of electricity, making its efficiency directly dependent on the energy source used.
2. Cost Factors
The cost of electrolyzers and renewable energy infrastructure is still relatively high, which impacts the overall affordability of green hydrogen.
3. Scaling Up
While the technology is promising, scaling up to meet global energy demands requires further innovation and investment.
The Future of Electrolysis Hydrogen Production
The global push toward sustainability has positioned electrolysis hydrogen production as a cornerstone of the energy transition. Governments and organizations worldwide are investing in research, infrastructure, and policies to support the adoption of green hydrogen. With advancements in electrolyzer technology and renewable energy integration, the cost of hydrogen production is expected to decrease, making it more accessible across industries.
Why Choose TryIn Solution for Hydrogen Solutions?
At TryIn Solution, we understand the transformative potential of green hydrogen and are dedicated to supporting businesses in adopting sustainable energy practices. From providing cutting-edge technology to offering expert guidance, we help you harness the benefits of electrolysis hydrogen production effectively and efficiently.
Conclusion
Electrolysis hydrogen production is a game-changing technology that promises to redefine how we produce and use energy. By offering a clean, sustainable, and versatile energy source, it addresses some of the most pressing challenges of our time, from reducing carbon emissions to ensuring energy security. As we move toward a greener future, investing in this innovative solution will be pivotal for industries and governments alike.
Explore how TryIn Solution can help you integrate hydrogen solutions into your operations. Contact us today to learn more!
0 notes
Text
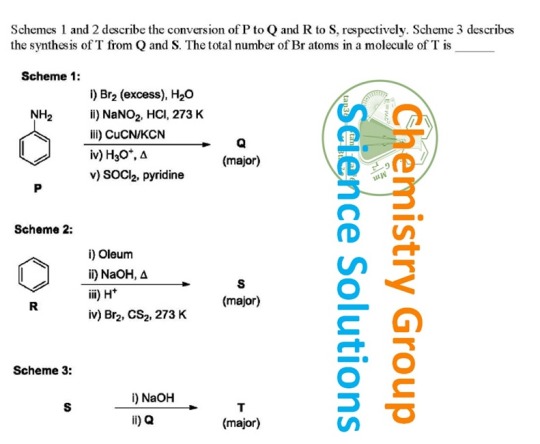
#bromine#hydrochloric acid#potassium cyanide#oleum#sodium hydroxide#pyridine#chemistry#solutions#water
1 note
·
View note
Text
What Are Laboratory Chemicals And Why Are They Essential?

Lab chemicals are important for many scientific experiments, research, and industrial processes. These substances help to carry out accurate tests that form the basics of chemistry, biology, physics, and environmental science. In this blog, we will discuss different types of laboratory chemicals, their uses in scientific work as well as leading laboratory chemicals manufacturers and suppliers in India.
What are Laboratory Chemicals?
Laboratory chemicals are substances used in laboratories for research or experimental purposes such as analysis, synthesis, or for experiment. They can be elements, compounds, or mixtures that serve various roles like reagents for reactions; solvents; indicators, etc.
Common Laboratories Chemicals And Their Uses
Below are some common laboratory chemicals, detailing their specific uses and significance:
Acetic Acid Glacial (1%)
It is a highly concentrated form of acetic acid commonly used as a reagent in chemical reactions at 1% concentration; solvent for different organic syntheses and for pH adjustment of solutions.
Acetocarmine Sulphate (Nucleic Acid)
Acetocarmine Sulphate is primarily used as a stain in microscopy for observing nucleic acids, especially chromosomes during cell division. It Helps enhance the contrast of the structures, making them more visible under microscopes.
Barium Sulphate
Widely employed as a radiopaque agent during medical imaging procedures because it makes certain areas more visible under x-rays than others; also acts as filter material in the plastics industry alongside rubber production and other uses – such filters may remove contaminants from liquids or gasses passing through them based on their pore sizes which allow only desired molecules to pass through while blocking unwanted ones, Analytical chemists often use it when looking at ways of determining sulfate ions within samples.
Carbon Disulphide
Carbon disulfide is a volatile solvent used in chemical synthesis and industrial applications. It is often used for the extraction of oils and fats and in the manufacture of rayon and cellophane
Copper Sulphate
Copper sulfate is widely used in laboratories for various purposes, including as a reagent in Fehling’s solution for testing reducing sugars, as a fungicide, and in the preparation of Bordeaux mixture for agricultural use.
Potassium And Sodium Tartrates
These potassium and sodium tartrates are used in laboratories as reagents in Fehling’s solution and in the food industry as emulsifiers and acidity regulators. They are also involved in the manufacture of some medicinal products.

Potassium Bromide
Infrared spectroscopy samples are commonly prepared using Potassium Bromide. As a reagent in analytical chemistry with applications within the pharmaceutical industry there were also other uses.
Potassium Chloride
It is used For the different chemical reactions at laboratories, it is used as a reagent together with being used to prepare buffer solutions. Also, it serves as an electrolyte replenisher during medical treatments.
The Function Of Chemicals And Laboratory Reagents
To create reactions and study the outcomes, substances are used in the lab commonly known as laboratory reagents or chemicals. They are necessary for qualitative as well as quantitative analysis; this helps scientists understand the composition, properties and behavior of different materials. Each may have its own purpose in an experiment so there can be many types of them.
Common Laboratory Reagents
Laboratory Reagents are compounds or mixtures introduced into systems to elicit a chemical reaction or test if one does occur. Some examples include:
Hydrochloric Acid (HCL)
Sodium Hydroxide (NaOH)
Ethanol (C2H5OH)
Sulphuric Acid (H2SO4)
Silver Nitrate (AgNO3)
These are some of the most commonly used reagents in labs, they could be used for different analytical methods like titrations, precipitations and pH adjustments.
A Chemical reagent is a substance or compound that is added to a reaction to determine whether it occurs or not. For example Fehling’s solution which can detect presence-reducing sugar by heating after mixing with a sample gives positive results indicated by brick red precipitate showing these sugars were present.
Why Choose Science Lab Export?
Lab chemicals and reagents form the backbone for scientific research and industrial processes worldwide. This is because they enable accurate experimentation; new product development as well as understanding nature better. These products therefore cannot be overlooked be it medical imaging, agricultural testing or even synthesis of chemicals during various experiments we do need them always.
It is important to comprehend different types of Lab chemical, if one wants to succeed academically or professionally in this field. If you require high-quality lab chemicals, consider Science Lab export. As a leading manufacturer and supplier of lab chemicals, we provide a wide-range of products to meet our scientific needs.
#laboratory chemicals manufacturers and suppliers in India#laboratory chemicals manufacturers and suppliers#laboratory chemicals manufacturer
0 notes
Text
had a good day at work today btw. supervisor taught us how to make a potassium hydroxide solution 😀 and we got to do bleach titrations again which was sooo fun it’s probably the coolest chemical reaction we get to do (i am partial to the calcium and alkalinity titrations too though. the transition is much more gradual yeah but they make such pretty shades of pink and blue and purple)
4 notes
·
View notes
Text
0 notes