#PCR Test Clinic UK
Explore tagged Tumblr posts
Text
3 Benefits of Corporate Covid-19 Testing
Coronavirus is a respiratory disease brought about by SARS-CoV-2. Most patients contaminated with Covid report gentle to direct side effects and recuperate with the Private Pathology Laboratory in the UK. Nonetheless, patients with previous medical conditions like hypertension, disease, and diabetes might encounter unfavorable impacts. In any case, note that anybody can get the infection, become sick or even kick the bucket, no matter what their age.

When can you use Corporate Covid-19 testing?
You ought to get tired or Book a Covid-19 Travel Test from the Clinic of the UK, assuming you experience any side effects of Coronavirus, regardless of whether you have gotten every one of the essential antibodies.
Note that Covid spreads rapidly and effectively, even before seeing any side effects. Thusly, the sooner you know whether you have the infection, the better since you can detach it and lessen the possibility of spreading it to other people.
Once in a while you might test negative for the infection yet feel unwell, demonstrating a bogus negative. In such a situation, you ought to disengage and contact your primary care physician to examine your side effects. Nonetheless, assuming you test positive, you should promptly detach and contact your Private Blood Tests Clinic in the UK to begin the treatment plan.
According to Diagnostic Centre in UK, you ought to do a test following five to seven days on the off chance that you are immunized against the infection. Furthermore, if you're not immunized, you ought to get a test. Given below are the 3 Benefits of Corporate Covid-19 testing:
1. Ensures Successful Detachment
There are various difficulties confronting the battle against the spread of Covid. Luckily, medical services suppliers can utilize fast testing from the Corporate Coronavirus Testing of the UK to analyze whatever number of Coronavirus cases would be prudent before the infection spreads to the more extensive populace.
2. Simple to Utilize
You don't need to be a clinical expert to do a quick Coronavirus test. You can do it with your family utilizing a home Coronavirus test pack.
3. Mitigates Financial Effect
With fast testing, people presented with the infection accept their test results within a couple of hours, implying that they don't need to disengage for a long time or miss various business days as they hold back to accept their experimental outcomes. For more information visit the Private Pathology Laboratory in the UK.
It can be concluded that Patients go through analytic tests from the Diagnostic Centre in the UK to affirm the presence of Coronavirus respiratory sickness. At the point when patients test positive for a fast antigen test, they are disengaged and begin treatment right away.
Also Read: What Is So Fascinating About the Covid-19 Antigen LFT Test?
#Covid-19 Antigen LFT Test UK#Diagnostic Centre in UK#China Covid Travel Test Package UK#Covid Test Clinic in the UK#Urgent PCR Travel Test Clinic UK#RT-PCR Travel Test UK#Best Vitamin Supplement for Men UK#Best Fit to Fly Tests Clinic in UK#Best Multivitamins for Women in UK#Private Ultrasound Scan Clinic UK#Private Pathology Laboratory in UK#Private Blood Tests Clinic UK#Book Covid-19 Travel Test UK#Corporate Coronavirus Testing UK
0 notes
Text
^follow this link^ to access an archive of over 1,000 open-access references to covid studies. Daily updates!
Abstract
BACKGROUND: COVID-19 is associated with acute risk of major adverse cardiac events (MACE), including myocardial infarction, stroke, and mortality (all-cause). However, the duration and underlying determinants of heightened risk of cardiovascular disease and MACE post–COVID-19 are not known.
METHODS: Data from the UK Biobank was used to identify COVID-19 cases (n=10 005) who were positive for polymerase chain reaction (PCR+)-based tests for SARS-CoV-2 infection (n=8062) or received hospital-based International Classification of Diseases version-10 (ICD-10) codes for COVID-19 (n=1943) between February 1, 2020 and December 31, 2020. Population controls (n=217 730) and propensity score—matched controls (n=38 860) were also drawn from the UK Biobank during the same period. Proportional hazard models were used to evaluate COVID-19 for association with long-term (>1000 days) risk of MACE and as a coronary artery disease risk equivalent. Additional analyses examined whether COVID-19 interacted with genetic determinants to affect the risk of MACE and its components.
RESULTS: The risk of MACE was elevated in COVID-19 cases at all levels of severity (HR, 2.09 [95% CI, 1.94–2.25]; P<0.0005) and to a greater extent in cases hospitalized for COVID-19 (HR, 3.85 [95% CI, 3.51–4.24]; P<0.0005). Hospitalization for COVID-19 represented a coronary artery disease risk equivalent since incident MACE risk among cases without history of cardiovascular disease was even higher than that observed in patients with cardiovascular disease without COVID-19 (HR, 1.21 [95% CI, 1.08–1.37]; P<0.005). A significant genetic interaction was observed between the ABO locus and hospitalization for COVID-19 (Pinteraction=0.01), with risk of thrombotic events being increased in subjects with non-O blood types (HR, 1.65 [95% CI, 1.29–2.09]; P=4.8×10−5) to a greater extent than subjects with blood type O (HR, 0.96 [95% CI, 0.66–1.39]; P=0.82).
CONCLUSIONS: Hospitalization for COVID-19 represents a coronary artery disease risk equivalent, with post–acute myocardial infarction and stroke risk particularly heightened in non-O blood types. These results may have important clinical implications and represent, to our knowledge, one of the first examples of a gene-pathogen exposure interaction for thrombotic events.
#mask up#covid#pandemic#covid 19#wear a mask#public health#coronavirus#sars cov 2#still coviding#wear a respirator#long covid#covid conscious#wear a fucking mask#covid is not over
24 notes
·
View notes
Text
Mark your calendar for these health tech conferences in 2024-2025

- By InnoNurse Staff -
Interested in health technology-related events for fall 2024 and 2025? Fierce Healthcare has compiled a list of key conferences, both virtual and in-person, scheduled for the upcoming seasons.
Read more at Fierce Healthcare
///
Other recent news and insights
Lapsi transforms the stethoscope into a health tracking data platform (TechCrunch)
UK: The Department of Health and Social Care set to review clinical risk standards for digital health technologies (Digital Health)
AI-based cancer test determines if chemotherapy is needed (The Financial Express)
New tool enhances microscopic imaging by eliminating motion artifacts (UC Berkeley/Tech Xplore)
Researchers integrate a fast optical coherence tomography system into neurosurgical microscopes (Optica)
AI model achieves clinical-expert-level accuracy in complex medical scans (UCLA/Medical Xpress)
Bioinformatics reveals the hidden prevalence of repeat expansion disorders (Queen Mary University of London/Medical Xpress)
Ultrasound detects 96% of ovarian cancers in postmenopausal women (University of Birmingham)
AI ‘liquid biopsies’ using cell-free DNA and protein biomarkers could improve early ovarian cancer detection (Johns Hopkins Technology Ventures)
Mammograms show potential for detecting heart disease (UC San Diego/Medical Xpress)
IMRT and proton therapy provide similar quality of life and tumor control for prostate cancer patients (American Society for Radiation Oncology/Medical Xpress)
Machine learning enhances MRI video quality (Graz University of Technology/Medical Xpress)
Robotic surgery for colorectal cancer reduces pain and accelerates recovery (Beth Israel Deaconess Medical Center)
Global human brain mapping project releases its first data set (Allen Institute)
AI could speed up PCR tests, aiding faster DNA diagnostics and forensics (Flinders University/Medical Xpress)
AI-powered apps may detect depression through eye snapshots (Stevens Institute of Technology/Medical Xpress)
#events#health tech#digital health#medtech#biotech#health informatics#data science#neuroscience#imaging#radiology#diagnostics#ai#robotics#cancer#lapsi#government#uk
2 notes
·
View notes
Text
Oligonucleotide Synthesis Market Outlook, Size, Growth Factors, and Forecast 2025-2032

Oligonucleotide synthesis market is experiencing rapid expansion, driven by increasing applications in genetic research, diagnostics, therapeutics, and drug discovery. As advancements in synthetic biology and molecular diagnostics continue to evolve, the demand for high-quality oligonucleotides is soaring. According to SkyQuest’s latest report on the Oligonucleotide Synthesis Market, Oligonucleotide Synthesis Market size is poised to grow at a CAGR of 17.4% by 2032, driven by technological innovations and rising research investments.
The oligonucleotide synthesis market plays a crucial role in genomics, molecular biology, and biotechnology. It encompasses the development of short DNA and RNA sequences that serve as essential tools for PCR, gene editing, and targeted therapeutics. With the increasing adoption of oligonucleotides in clinical applications, the market is projected to experience significant expansion in the coming years.
Request a sample of the report here: https://www.skyquestt.com/sample-request/oligonucleotide-synthesis-market
Key Market Drivers Shaping Oligonucleotide Synthesis Growth
Growing Demand for Personalized Medicine
The rise of precision medicine has fueled the demand for custom oligonucleotides. Researchers and pharmaceutical companies are increasingly leveraging oligonucleotides for targeted therapies, particularly in cancer treatment and rare genetic disorders.
Advancements in Gene Editing Technologies
Innovations in CRISPR, RNA interference (RNAi), and antisense oligonucleotides are expanding the scope of oligonucleotide-based therapies. These breakthroughs are transforming genetic research, enabling more precise and effective treatments.
Expanding Applications in Diagnostics
Oligonucleotide probes and primers are widely used in molecular diagnostics, particularly in PCR-based testing, next-generation sequencing (NGS), and microarrays. The increasing prevalence of infectious diseases and genetic disorders has driven the demand for oligonucleotide-based diagnostic solutions.
Increased Investment in Biotechnology Research
Pharmaceutical and biotech companies are investing heavily in oligonucleotide research, aiming to develop novel therapeutics and drug delivery mechanisms. Governments and private organizations are also providing funding to accelerate genetic research.
Speak with an analyst for in-depth market insights: https://www.skyquestt.com/speak-with-analyst/oligonucleotide-synthesis-market
Oligonucleotide Synthesis Market Segmentation:
By Product Type
Synthesized Oligonucleotides – Custom sequences used in research, diagnostics, and therapeutics
Reagents and Consumables – Essential materials for synthesis processes
Equipment – Automated synthesizers and analytical tools for high-throughput oligonucleotide production
By Application
Research & Development – Genomic studies, drug discovery, and synthetic biology
Diagnostics – PCR, NGS, and DNA microarrays
Therapeutics – Antisense oligonucleotides, siRNA, and mRNA-based therapies
By End-User
Biotechnology & Pharmaceutical Companies – Focused on drug development and clinical applications
Academic & Research Institutions – Conducting genomics and molecular biology studies
Contract Research Organizations (CROs) – Supporting large-scale oligonucleotide synthesis and testing
Oligonucleotide Synthesis Market Regional Insights
North America: Leading the Market with Strong Research Infrastructure
The United States and Canada dominate the oligonucleotide synthesis market, driven by strong research capabilities, robust funding, and a high concentration of biotechnology companies. The presence of key industry players and increasing clinical trials further contribute to regional growth.
Europe: Rising Investments in Genetic Research
Countries like Germany, the UK, and France are expanding their biotechnology sectors, investing in advanced gene therapy and diagnostic solutions. The European Union’s support for genomic research is fostering innovation in oligonucleotide applications.
Asia-Pacific: Fastest-Growing Market with Expanding Biotech Industry
China, Japan, and India are witnessing rapid market expansion due to increasing investments in genetic research, a growing pharmaceutical industry, and government support for biotechnological advancements. The demand for oligonucleotide-based diagnostics and therapies is significantly increasing in the region.
Latin America & Middle East: Emerging Markets with High Growth Potential
Countries in Latin America and the Middle East are gradually adopting oligonucleotide synthesis technologies, primarily in medical research and infectious disease diagnostics. Increasing healthcare investments are expected to drive market growth in these regions.
Buy the full report for comprehensive market analysis: https://www.skyquestt.com/buy-now/oligonucleotide-synthesis-market
Key Players in the Oligonucleotide Synthesis Market
Several major players dominate the oligonucleotide synthesis market, focusing on innovation, product development, and strategic collaborations. Key companies include:
Thermo Fisher Scientific
Agilent Technologies
Merck KGaA
Integrated DNA Technologies (IDT)
LGC Biosearch Technologies
Eurofins Genomics
GenScript Biotech Corporation
TriLink BioTechnologies
These companies are expanding their production capacities and investing in new technologies to meet the rising demand for synthetic oligonucleotides.
Emerging Trends and Technological Innovations
Automated High-Throughput Synthesis
The adoption of automated systems is improving efficiency, scalability, and precision in oligonucleotide production. Advanced synthesis platforms are enabling rapid turnaround times for research and clinical applications.
Expansion of RNA-Based Therapeutics
RNA-based drugs, including mRNA vaccines and RNAi therapies, are gaining significant traction. This trend is expected to drive the demand for oligonucleotide synthesis in pharmaceutical and biotech industries.
Sustainable and Cost-Effective Synthesis Methods
Researchers are developing green synthesis approaches to minimize environmental impact and reduce production costs, making oligonucleotide synthesis more sustainable.
The Future of the Oligonucleotide Synthesis Market
The oligonucleotide synthesis market is on an upward trajectory, driven by advancements in gene editing, diagnostics, and therapeutics. As personalized medicine gains momentum and biotechnology continues to evolve, the demand for high-quality oligonucleotides will continue to rise. Companies investing in automation, innovative research, and sustainable production methods are well-positioned for success in this rapidly growing industry.
For a detailed market analysis and strategic insights, explore the full SkyQuest report: https://www.skyquestt.com/report/oligonucleotide-synthesis-market
#Asia Oligonucleotide Synthesis Market#Europe Oligonucleotide Synthesis Market#Middle East Oligonucleotide Synthesis Market Size#North America Oligonucleotide Synthesis Market
0 notes
Text
Exploring the Allergy Diagnostics Market: Trends, Drivers, and Innovations
The global Allergy Diagnostics Market is experiencing robust growth, fueled by an increased prevalence of allergies, advancements in diagnostic technologies, and heightened awareness among patients and healthcare professionals. With a rising global health burden associated with allergic diseases, allergy diagnostics play a crucial role in accurate detection and management, enabling better treatment outcomes.
Download PDF Brochure
What Are Allergies and Why Are Diagnostics Essential?
Allergies occur when the immune system reacts to substances like pollen, food, or pet dander, triggering symptoms ranging from mild irritations to severe anaphylaxis. Diagnosing allergies involves identifying specific allergens causing adverse reactions, which is vital for developing targeted treatment plans and preventing severe allergic responses.
The Allergy Diagnostics Market encompasses products, technologies, and services used in detecting allergens. This includes skin prick tests, blood tests (specific IgE testing), patch tests, and molecular diagnostic methods. These tools have revolutionized the diagnostic landscape, offering accuracy, reliability, and quicker results.
Key Drivers of the Allergy Diagnostics Market
Rising Allergy Prevalence: Allergies affect approximately 20-30% of the global population, with respiratory, food, and skin allergies being the most common. Factors like urbanization, pollution, and changing dietary habits have contributed to this increase.
Technological Advancements: Modern diagnostic technologies such as ELISA, multiplex assays, and microarray platforms enhance test sensitivity and specificity, making them indispensable tools in laboratories worldwide.
Increased Awareness: Public health campaigns and growing awareness about allergic conditions have led to higher demand for diagnostic services, particularly in developed regions.
Growing Pediatric Population: Children are often more susceptible to allergies, making early diagnostics crucial. This demographic significantly drives market demand.
Government and Private Initiatives: Supportive healthcare policies, funding for allergy research, and reimbursement schemes bolster the market.
Segmentation of the Allergy Diagnostics Market
By Product Type:
Assay Kits: Widely used for in vitro allergy testing, these kits are integral to laboratory workflows.
Instruments: Devices such as immunoassay analyzers, PCR systems, and skin testing instruments.
Consumables: Reagents, probes, and other materials necessary for diagnostics.
By Test Type:
In Vivo Testing: Includes skin prick and intradermal tests.
In Vitro Testing: Blood-based tests like specific IgE and total IgE quantification.
By Allergen Type:
Food Allergens: Milk, eggs, nuts, and seafood.
Inhalant Allergens: Pollen, mold, and pet dander.
Drug Allergens: Penicillin and other antibiotics.
Other Allergens: Insect venom and contact allergens.
By End User:
Hospitals and Clinics: Major centers for allergy testing.
Diagnostic Laboratories: Offer specialized testing services.
Academic and Research Institutes: Focused on allergen research and development.
Regional Insights: Allergy Diagnostics Market
North America: Leading the market due to advanced healthcare infrastructure, high allergy prevalence, and significant R&D investments. The U.S. dominates this region.
Europe: Rising allergy awareness and government initiatives support growth. Countries like Germany and the UK are key players.
Asia-Pacific: Exhibiting the fastest growth due to increasing healthcare access, urbanization, and pollution levels. Emerging economies like China and India drive regional expansion.
Rest of the World: Markets in Latin America and the Middle East are growing steadily, with improving healthcare systems and rising allergy awareness.
Innovations Shaping the Allergy Diagnostics Market
Molecular Diagnostics: Techniques like recombinant allergen testing provide precise results by identifying allergen components at the molecular level.
Point-of-Care Testing: Portable allergy testing devices enable faster diagnostics, particularly beneficial in resource-limited settings.
Artificial Intelligence (AI): AI-powered tools analyze patient data for personalized allergy management and improved diagnostic accuracy.
Next-Generation Sequencing (NGS): Advanced genomic tools are being explored for understanding allergen sensitivity at a genetic level.
Multiplex Testing Platforms: These platforms allow simultaneous detection of multiple allergens, saving time and resources.
Challenges in the Allergy Diagnostics Market
High Costs: Advanced diagnostic tests can be expensive, limiting accessibility in developing regions.
Lack of Standardization: Variability in testing procedures and results across laboratories remains a concern.
Limited Awareness in Emerging Markets: Although awareness is increasing, many regions still lack adequate knowledge and facilities for allergy diagnostics.
The Future of the Allergy Diagnostics Market
The Allergy Diagnostics Market is poised for sustained growth, driven by continuous innovations and the rising global allergy burden. Emerging trends such as wearable diagnostic devices, mobile health applications, and telemedicine integration are expected to redefine the market dynamics. Additionally, increased focus on preventive healthcare and personalized medicine will further enhance diagnostic capabilities.
Conclusion
The Allergy Diagnostics Market is a dynamic and evolving space, addressing critical healthcare needs worldwide. As the prevalence of allergic conditions rises, the demand for accurate, accessible, and advanced diagnostic solutions will continue to grow. Stakeholders in this market must prioritize innovation, affordability, and patient education to ensure widespread adoption and improved healthcare outcomes.
0 notes
Text
Global Compact Thermal Cycler Market Forecast and Strategic Direction Report 2024 - 2031
The global compact thermal cycler market is witnessing significant growth as a result of advancements in biotechnology, pharmaceuticals, and molecular biology. Compact thermal cyclers are essential laboratory instruments used primarily in polymerase chain reaction (PCR) applications, enabling rapid and efficient thermal cycling for DNA amplification. This article explores the market dynamics, key trends, challenges, and future outlook.

Overview of the Compact Thermal Cycler Market
Compact thermal cyclers are designed to optimize the process of temperature cycling in PCR, making them invaluable for research and diagnostic laboratories. Their small footprint and ease of use make them suitable for a variety of applications, from academic research to clinical diagnostics.
The global compact thermal cycler market is poised for substantial growth, driven by advancements in biotechnology, increasing demand for PCR applications, and the shift towards personalized medicine.
Key Features of Compact Thermal Cyclers
Space Efficiency: Compact design allows for easy integration into laboratory settings with limited space.
Rapid Heating and Cooling: Advanced temperature control technology enables faster cycling times, increasing throughput.
User-Friendly Interfaces: Many models come equipped with intuitive touch screens and programmable settings for ease of operation.
Market Dynamics
Drivers of Market Growth
Rising Demand for PCR Applications: The increasing application of PCR in medical diagnostics, genetic testing, and research is driving the demand for thermal cyclers.
Advancements in Biotechnology: Innovations in molecular biology techniques are fueling the need for efficient and precise thermal cycling equipment.
Growing Focus on Personalized Medicine: The shift towards personalized medicine is boosting demand for tools that enable genetic testing and analysis.
Challenges Facing the Market
High Initial Costs: The upfront investment for high-quality compact thermal cyclers can be a barrier for smaller laboratories and startups.
Technological Obsolescence: Rapid advancements in thermal cycling technology may lead to existing models becoming outdated quickly.
Regulatory Challenges: Compliance with stringent regulatory standards in various countries can complicate product development and market entry.
Regional Analysis
North America
North America is a dominant player in the compact thermal cycler market, driven by a strong presence of research institutions and pharmaceutical companies. The United States is particularly significant due to its extensive investment in biotechnology and life sciences.
Europe
Europe is witnessing steady growth in the compact thermal cycler market, with countries like Germany, the UK, and France leading in research and development. The emphasis on innovative healthcare solutions and personalized medicine is supporting market expansion in this region.
Asia-Pacific
The Asia-Pacific region is expected to experience the highest growth in the compact thermal cycler market, driven by rapid industrialization, increasing healthcare investments, and a growing number of research institutions. Countries like China and India are at the forefront of this growth.
Competitive Landscape
Key Players
Thermo Fisher Scientific: A leader in scientific instruments, Thermo Fisher offers a range of compact thermal cyclers known for their reliability and performance.
Bio-Rad Laboratories: Known for innovative laboratory equipment, Bio-Rad provides compact thermal cyclers that cater to various research needs.
Eppendorf AG: A key player in the life sciences market, Eppendorf manufactures compact thermal cyclers that are widely used in laboratories worldwide.
Market Strategies
Product Innovation: Companies are investing in research and development to enhance the capabilities and features of compact thermal cyclers.
Strategic Collaborations: Partnerships with research institutions and healthcare providers are being pursued to expand market reach and enhance product offerings.
Geographic Expansion: Targeting emerging markets in Asia and Latin America to capitalize on the growing demand for molecular biology tools.
Future Outlook
The global compact thermal cycler market is projected to grow significantly in the coming years. As advancements in biotechnology and molecular diagnostics continue, the demand for efficient and compact thermal cyclers will likely increase.
Trends to Watch
Integration of Automation: The rise of automated laboratory workflows will drive the demand for advanced thermal cyclers that can seamlessly integrate into robotic systems.
Eco-Friendly Solutions: Increasing emphasis on sustainability may lead manufacturers to develop more energy-efficient and environmentally friendly thermal cyclers.
Customization Options: Growing demand for tailored solutions that meet specific laboratory requirements will influence product development in the market.
Conclusion
The global compact thermal cycler market is poised for substantial growth, driven by advancements in biotechnology, increasing demand for PCR applications, and the shift towards personalized medicine. By addressing challenges and leveraging emerging opportunities, stakeholders can thrive in this dynamic market. The future of compact thermal cyclers will be characterized by innovation, efficiency, and a commitment to meeting the evolving needs of laboratories and research institutions worldwide.
#Global Compact Thermal Cycler Market Size#Global Compact Thermal Cycler Market Trend#Global Compact Thermal Cycler Market Growth
0 notes
Text
Trends and Projections for the Taq DNA Polymerase Market
The Taq DNA Polymerase Market is witnessing significant growth due to the increasing demand for polymerase chain reaction (PCR) technologies in various fields such as medical diagnostics, biotechnology, and forensic science. As the backbone of PCR, Taq DNA polymerase is indispensable for amplifying DNA sequences, making it a crucial component in research and clinical laboratories worldwide. This article delves into the market size, share, industry trends, and forecasts for the Taq DNA polymerase market through 2032.

Market Overview
Taq DNA polymerase is a thermostable enzyme extracted from the bacterium Thermus aquaticus, which is capable of withstanding high temperatures required for PCR. This enzyme's ability to replicate DNA sequences efficiently and accurately under thermal cycling conditions has revolutionized molecular biology and genetic research.
Market Size and Share
Taq dna polymerase Market Size was estimated at 1.6 (USD Billion) in 2023. The Taq Dna Polymerase Market Industry is expected to grow from 1.69(USD Billion) in 2024 to 2.53 (USD Billion) by 2032. The taq dna polymerase Market CAGR (growth rate) is expected to be around 5.19% during the forecast period (2024 - 2032).
North America currently holds the largest market share, accounting for over 40% of the global market. This dominance is attributed to the region's well-established biotechnology and pharmaceutical industries, extensive research activities, and the presence of key market players. Europe follows closely, with significant contributions from countries like Germany, the UK, and France. The Asia-Pacific region is expected to witness the fastest growth, driven by increasing investments in biotechnology, rising healthcare expenditure, and growing awareness about molecular diagnostics.
Industry Trends
Technological Advancements: The Taq DNA polymerase market is benefiting from continuous technological innovations. The development of high-fidelity and fast-cycling Taq polymerases has enhanced the efficiency and accuracy of PCR, expanding its applications in various fields.
Rising Demand for Molecular Diagnostics: The increasing prevalence of infectious diseases, genetic disorders, and cancer has fueled the demand for molecular diagnostics. Taq DNA polymerase plays a crucial role in diagnostic assays, including COVID-19 testing, driving market growth.
Expansion of Personalized Medicine: The shift towards personalized medicine, which relies on genetic profiling for tailored treatments, is boosting the demand for Taq DNA polymerase. PCR-based techniques are integral to genetic testing and the development of personalized therapies.
Growing Research and Development Activities: The surge in research and development activities in genomics, proteomics, and biotechnology is propelling the demand for Taq DNA polymerase. Government and private sector investments in research are further stimulating market growth.
Increased Forensic Applications: The application of PCR in forensic science for DNA profiling and criminal investigations is expanding. Taq DNA polymerase is a vital tool in forensic labs, aiding in the accurate identification of individuals from biological samples.
Market Drivers
Advancements in PCR Technology: Innovations such as real-time PCR (qPCR) and digital PCR (dPCR) are driving the adoption of Taq DNA polymerase. These advanced techniques offer higher sensitivity, specificity, and quantification capabilities, broadening the scope of PCR applications.
Growing Biotechnology Industry: The biotechnology industry's rapid growth, coupled with increasing investments in genetic research, is boosting the demand for Taq DNA polymerase. The enzyme is essential for various applications, including cloning, sequencing, and gene expression analysis.
Increasing Prevalence of Genetic Disorders: The rising incidence of genetic disorders and the need for early diagnosis and treatment are driving the demand for PCR-based diagnostic tests. Taq DNA polymerase is a key component in these tests, contributing to market growth.
COVID-19 Pandemic: The COVID-19 pandemic has significantly increased the demand for PCR testing, highlighting the importance of Taq DNA polymerase. The enzyme's critical role in detecting the virus has led to a surge in production and sales.
Challenges
High Cost of Enzyme Production: The production of high-quality Taq DNA polymerase involves complex processes and significant investment, leading to high costs. This can be a barrier for small-scale laboratories and research institutions.
Competition from Alternative Enzymes: The market faces competition from alternative DNA polymerases with improved properties, such as higher fidelity and faster cycling times. The development and adoption of these alternatives can impact the market share of Taq DNA polymerase.
Stringent Regulatory Requirements: The stringent regulatory requirements for the approval and commercialization of diagnostic and research products can pose challenges for market players. Compliance with these regulations can be time-consuming and costly.
Market Forecast (2024-2032)
The Taq DNA polymerase market is poised for substantial growth over the forecast period. Key factors contributing to this growth include:
Expanding Applications in Diagnostics and Research: The ongoing advancements in PCR technology and the expanding applications in diagnostics and research are expected to drive market growth. The enzyme's versatility and reliability make it indispensable in various fields.
Increasing Investments in Biotechnology: The increasing investments in biotechnology and genetic research by governments, private companies, and research institutions will propel the demand for Taq DNA polymerase. The enzyme's role in groundbreaking research and development activities will continue to drive market expansion.
Emerging Markets in Asia-Pacific: The Asia-Pacific region is expected to witness the fastest growth, driven by rising healthcare expenditure, increasing biotechnology investments, and growing awareness about molecular diagnostics. Countries like China, India, and Japan are key markets to watch.
Adoption of Personalized Medicine: The shift towards personalized medicine and the growing demand for genetic testing will boost the market for Taq DNA polymerase. The enzyme's critical role in genetic analysis and the development of personalized therapies will drive its adoption.
Conclusion
The Taq DNA polymerase market is on a robust growth trajectory, driven by technological advancements, increasing demand for molecular diagnostics, and expanding research and development activities. Key players in the industry are focusing on product development, strategic collaborations, and expanding their geographical presence to capitalize on the growing demand for Taq DNA polymerase.
0 notes
Text
In Vitro Diagnostics Market To Reach $101.58 Billion By 2030
The global in vitro diagnostics market size is expected to reach USD 101.58 billion by 2030, according to a new report by Grand View Research, Inc. It is estimated to register a CAGR of 4.4% over the forecast period driven by the increasing geriatric population, COVID-19 pandemic, and technological advancements in diagnostics that are supporting its adoption. Technological advancements in terms of portability, accuracy, and cost-effectiveness are projected to be one of the high-impact rendering drivers. Technological advancements were further accelerated by the launch of COVID-19 IVD diagnostics and enhanced the adoption of instruments and consumables for technologies, such as PCR. Competitors in the market are increasingly adopting agreement and partnership strategies to maintain a constant flow of business for manufacturers & diagnostics for users.
These agreements are also a result of the harsh price containment strategies for government laboratories, which lowers the price in government settings. For instance, in April 2021, the Italian subsidiary of Seegene, Inc. received a USD 108.25 million tenders for public procurement for the supply of extraction reagents, as well as 7.15 million SARS-CoV-2 diagnostic tests. However, it increases the multiparty nature and complexity of the supply chain. The high prevalence of cancer and Cardiovascular Diseases (CVDs) globally is anticipated to drive diagnostic innovation to facilitate early diagnosis and meet the constantly evolving needs of consumers. Novel technologies, such as plasmonic PCR, are anticipated to commercially enter the market during the forecast period, influencing the business of existing products adversely.
Request a free sample copy or view the report summary: In Vitro Diagnostics Market Report
In Vitro Diagnostics Market Report Highlights
Molecular diagnostics is anticipated to grow at the fastest CAGR from 2024 to 2030 owing to the rising adoption and usage rate
Reagents held the largest market share owing to the surge in demand for genetic testing and enhanced availability of technologically advanced diagnostic tests in lower and middle-income countries with unmet clinical needs
The infectious diseases application segment held the largest market share owing to the large volume of testing for infectious diseases globally
North America dominated the global market in 2023 owing to the high demand for novel technologies, a large pool of key players, high prevalence of diseases, and advanced healthcare infrastructure
In Vitro Diagnostics Market Segmentation
Grand View Research has segmented the global in vitro diagnostics (IVD) market report based on product, technology, application, end-use, test location, and region:
IVD Product Outlook (Revenue, USD Million, 2018 - 2030)
Instruments
Reagents
Services
IVD Technology Outlook (Revenue, USD Million, 2018 - 2030)
Immunoassay
Instruments
Reagents
Services
Hematology
Instruments
Reagents
Services
Clinical Chemistry
Instruments
Reagents
Services
Molecular Diagnostics
Instruments
Reagents
Services
Coagulation
Instruments
Reagents
Services
Microbiology
Instruments
Reagents
Services
Others
Instruments
Reagents
Services
IVD Application Outlook (Revenue, USD Million, 2018 - 2030)
Infectious Diseases
Diabetes
Oncology
Cardiology
Nephrology
Autoimmune Diseases
Drug Testing
Others
IVD Test Location Outlook (Revenue, USD Million, 2018 - 2030)
Point of Care
Home-care
Others
IVD End-use Outlook (Revenue, USD Million, 2018 - 2030)
Hospitals
Laboratory
Home-care
Others
IVD Regional Outlook (Revenue, USD Million, 2018 - 2030)
North America
U.S.
Canada
Europe
UK
Germany
France
Spain
Italy
Russia
Denmark
Sweden
Norway
Asia Pacific
Japan
China
India
South Korea
Australia
Thailand
Singapore
Latin America
Brazil
Mexico
Argentina
Middle East and Africa (MEA)
South Africa
Saudi Arabia
UAE
Kuwait
List of Key Players of In Vitro Diagnostics (IVD) Market
Abbott
bioMérieux SA
QuidelOrtho Corporation
Siemens Healthineers AG
Bio-Rad Laboratories, Inc.
Qiagen
Sysmex Corporation
Charles River Laboratories
Quest Diagnostics Incorporated
Agilent Technologies, Inc.
Danaher Corporation
BD
F. Hoffmann-La Roche Ltd.
0 notes
Text
youtube
Business Name: Swift Clinic
Street Address: 7 Elmfield Road
City: Bromley
Zip Code: BR1 1LT
Country: United Kingdom
Business Phone: 020 3814 9355
Business Email: [email protected]
Website: https://www.swiftclinic.co.uk/
Facebook: https://www.facebook.com/swifthealthclinic
LinkedIn: https://www.linkedin.com/company/linkers-travel-health/
Instagram: https://www.instagram.com/swift_clinic/
GMB Site: https://blood-tests-covid-testing-bromley.business.site/
Business Description: Swift Clinic is a UKAS registered Healthcare service Provider. We are listed on GOV UK as a private Covid-19 Travel Testing service provider.We are registered with the Department of Heath & Social Care (DHSC) and working with UKAS to achieve ISO/IEC 15189:2012 We provide both PCR and Antigen tests with travel certificates. Results in as quick as 3 hours. Our healthcare scientists are experienced and qualified experts in their field of work. Swift Clinic is the trading name of Linkers Travel Health based in Bromley. We also cover Lewisham, Crystal Palace, Croydon, Orpington, Sidcup, South East London, East Ham, Stratford and Barking.
Google My Business CID URL: https://www.google.com/maps?cid=3798232879209613395
Business Hours: Sunday Closed Monday 9am-5pm Tuesday 9am-5pm Wednesday 9am-5pm Thursday 9am-5pm Friday 9am-5pm Saturday 9am-5pm
Services: Blood Test, COVID Test
Keywords: Blood testing in Bromley, Prostate Testing, Cholesterol Testing, Pregnancy Testing, Allergy testing, Cholesterol Testing, Diabetes Testing, Erectile Dysfunction Testing, Sexual health testing, STD testing, Fertility testing
Location:
Service Areas:
1 note
·
View note
Text
I have to present a certificate of a negative PCR test before I go and I just had a panic attack at the realisation Im going to have to battle with the UK's health system a final time. The NHS now doesnt provide PCR tests. Yes this is fucking insane but it is what it is. I do not know where I can get a PCR done privately in my town. You can get home PCR tests but I think that doesnt give you a certificate. There are special travel clinics you can go to down south, but I cant afford to travel again. i dont want to leave it to moving day and have to run from the airport to a travel clinic and pray to god I can get a result within 4 hours before I go. Im going to cry lol.
5 notes
·
View notes
Text
Additional resources and studies that I have looked at listed below! As I’ve said in a few other posts, I intentionally did not post tons and tons of studies because I knew no one would have time to click through them all and I wanted to keep it short and sweet. However, I do want to provide more resources for people who are inclined to keep reading about this, so here are a few more studies. I may just start doing this every so often, where I post links/results from whatever I’ve been reading. A link to full article is included in the title of each. I don’t really intend to add much of my own commentary here. Just providing things for your own reading and criticism.
A few additional studies and data sets, including the early randomized controlled trials for the mRNA vaccines, are discussed in the original post, and further discussion of mortality associated with vaccination is discussed in this follow up.
1. Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant
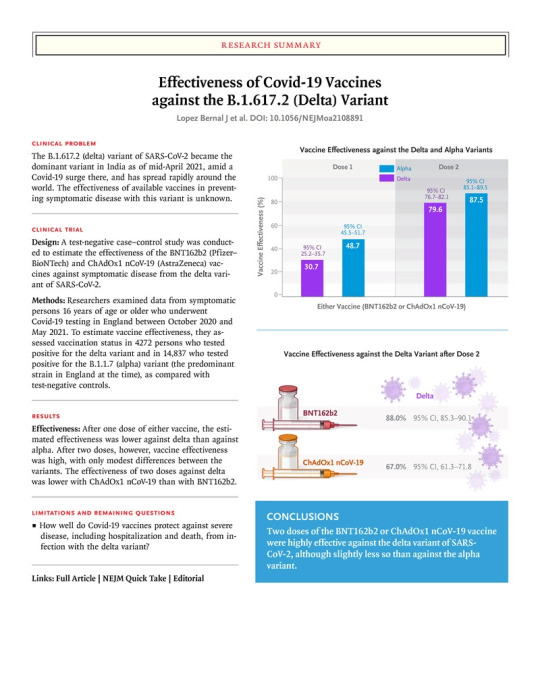
Study defined COVID-19 as a positive PCR test with symptoms. Children under age 16 were excluded, as were people with previous positive testing. A weakness of the study is that it is observational, and you can read about what the authors say regarding this toward the end of the article.
Results: “Effectiveness after one dose of vaccine (BNT162b2 or ChAdOx1 nCoV-19) was notably lower among persons with the delta variant (30.7%; 95% confidence interval [CI], 25.2 to 35.7) than among those with the alpha variant (48.7%; 95% CI, 45.5 to 51.7); the results were similar for both vaccines. With the BNT162b2 vaccine, the effectiveness of two doses was 93.7% (95% CI, 91.6 to 95.3) among persons with the alpha variant and 88.0% (95% CI, 85.3 to 90.1) among those with the delta variant. With the ChAdOx1 nCoV-19 vaccine, the effectiveness of two doses was 74.5% (95% CI, 68.4 to 79.4) among persons with the alpha variant and 67.0% (95% CI, 61.3 to 71.8) among those with the delta variant.”
Authors’ conclusions: “Only modest differences in vaccine effectiveness were noted with the delta variant as compared with the alpha variant after the receipt of two vaccine doses. Absolute differences in vaccine effectiveness were more marked after the receipt of the first dose. This finding would support efforts to maximize vaccine uptake with two doses among vulnerable populations.”
2. BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting
This study out of Israel looked at vaccine effectiveness against a variety of outcomes including infection, symptomatic infection, severe disease, and death using a pretty impressive sample size.
Results: “Each study group included 596,618 persons. Estimated vaccine effectiveness for the study outcomes at days 14 through 20 after the first dose and at 7 or more days after the second dose was as follows: for documented infection, 46% (95% confidence interval [CI], 40 to 51) and 92% (95% CI, 88 to 95); for symptomatic Covid-19, 57% (95% CI, 50 to 63) and 94% (95% CI, 87 to 98); for hospitalization, 74% (95% CI, 56 to 86) and 87% (95% CI, 55 to 100); and for severe disease, 62% (95% CI, 39 to 80) and 92% (95% CI, 75 to 100), respectively. Estimated effectiveness in preventing death from Covid-19 was 72% (95% CI, 19 to 100) for days 14 through 20 after the first dose. Estimated effectiveness in specific subpopulations assessed for documented infection and symptomatic Covid-19 was consistent across age groups, with potentially slightly lower effectiveness in persons with multiple coexisting conditions.”
Authors’ conclusions: “This study in a nationwide mass vaccination setting suggests that the BNT162b2 mRNA vaccine is effective for a wide range of Covid-19-related outcomes, a finding consistent with that of the randomized trial.”
3. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK
This study involved an RCT looking at a viral vector vaccine rather than mRNA vaccine. Overall efficacy appears to be high, but slightly lower than what we are seeing with some other mRNA ones. This study also followed outcomes for those who got COVID-19, and only people in the control arm (i.e., the ones who got a placebo) got seriously ill or died from COVID.
Results: “Between April 23 and Nov 4, 2020, 23 848 participants were enrolled and 11 636 participants (7548 in the UK, 4088 in Brazil) were included in the interim primary efficacy analysis. In participants who received two standard doses, vaccine efficacy was 62·1% (95% CI 41·0–75·7; 27 [0·6%] of 4440 in the ChAdOx1 nCoV-19 group vs71 [1·6%] of 4455 in the control group) and in participants who received a low dose followed by a standard dose, efficacy was 90·0% (67·4–97·0; three [0·2%] of 1367 vs 30 [2·2%] of 1374; pinteraction=0·010). Overall vaccine efficacy across both groups was 70·4% (95·8% CI 54·8–80·6; 30 [0·5%] of 5807 vs 101 [1·7%] of 5829). From 21 days after the first dose, there were ten cases hospitalised for COVID-19, all in the control arm; two were classified as severe COVID-19, including one death. There were 74 341 person-months of safety follow-up (median 3·4 months, IQR 1·3–4·8): 175 severe adverse events occurred in 168 participants, 84 events in the ChAdOx1 nCoV-19 group and 91 in the control group. Three events were classified as possibly related to a vaccine: one in the ChAdOx1 nCoV-19 group, one in the control group, and one in a participant who remains masked to group allocation.“
Authors’ conclusion: “ChAdOx1 nCoV-19 has an acceptable safety profile and has been found to be efficacious against symptomatic COVID-19 in this interim analysis of ongoing clinical trials.”
4. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study
This one is a case control study looking at effectivnes of two different vaccines against a variety of outcomes.
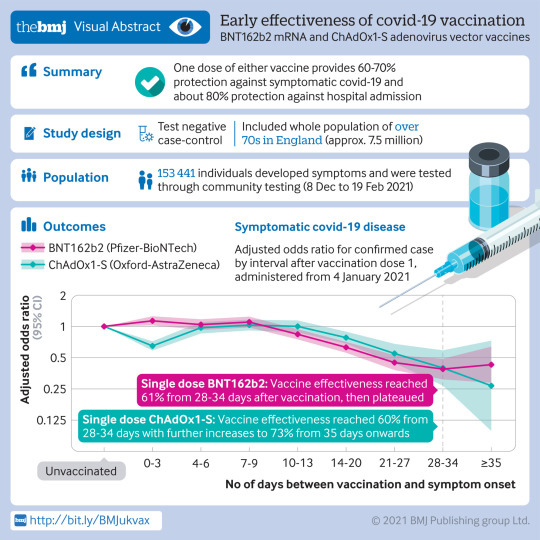
Results Participants aged 80 years and older vaccinated with BNT162b2 before 4 January 2021 had a higher odds of testing positive for covid-19 in the first nine days after vaccination (odds ratio up to 1.48, 95% confidence interval 1.23 to 1.77), indicating that those initially targeted had a higher underlying risk of infection. Vaccine effectiveness was therefore compared with the baseline post-vaccination period. Vaccine effects were noted 10 to 13 days after vaccination, reaching a vaccine effectiveness of 70% (95% confidence interval 59% to 78%), then plateauing. From 14 days after the second dose a vaccination effectiveness of 89% (85% to 93%) was found compared with the increased baseline risk. Participants aged 70 years and older vaccinated from 4 January (when ChAdOx1-S delivery commenced) had a similar underlying risk of covid-19 to unvaccinated individuals. With BNT162b2, vaccine effectiveness reached 61% (51% to 69%) from 28 to 34 days after vaccination, then plateaued. With ChAdOx1-S, effects were seen from 14 to 20 days after vaccination, reaching an effectiveness of 60% (41% to 73%) from 28 to 34 days, increasing to 73% (27% to 90%) from day 35 onwards. On top of the protection against symptomatic disease, a further 43% (33% to 52%) reduced risk of emergency hospital admission and 51% (37% to 62%) reduced risk of death was observed in those who had received one dose of BNT162b2. Participants who had received one dose of ChAdOx1-S had a further 37% (3% to 59%) reduced risk of emergency hospital admission. Follow-up was insufficient to assess the effect of ChAdOx1-S on mortality. Combined with the effect against symptomatic disease, a single dose of either vaccine was about 80% effective at preventing admission to hospital with covid-19 and a single dose of BNT162b2 was 85% effective at preventing death with covid-19.
Authors’ conclusion: Vaccination with either one dose of BNT162b2 or ChAdOx1-S was associated with a significant reduction in symptomatic covid-19 in older adults, and with further protection against severe disease. Both vaccines showed similar effects. Protection was maintained for the duration of follow-up (>6 weeks). A second dose of BNT162b2 was associated with further protection against symptomatic disease. A clear effect of the vaccines against the B.1.1.7 variant was found.
24 notes
·
View notes
Link
1 note
·
View note
Text
Travel testing guide for the UK’s most popular holiday destinations
PCR? Lateral Flow? 48 or 72 hours? Quarantine or no quarantine? Each country has its own Covid-19 travel rules to follow – both before you leave the UK and on arrival. We’ve listed the key requirements for some of the most popular holiday destinations from the UK.
The information in this guide is reviewed and updated regularly. Last updated: 07/09/21.
Please ensure you review the Foreign Office guidance and government and tourist websites for your destination. The arrival information is for entry into England only. If you are travelling to Scotland, Wales or Northern Ireland, please check here as the requirements may be different.
Covid19 health Clinic provides accurate, affordable & reliable private Covid tests to the public at various walk-in locations across the UK. Book online your private Covid test in UK Today!
Jump to Country:
Croatia
France
Germany
Greece
Italy
Malta
Portugal
Spain
USA
Croatia
: Green Watchlist
Who can enter Croatia ?
Croatia is open for travellers from all countries with various entry requirements.
Departure test required ?
Yes – for all travellers aged 12 years and over, regardless of whether they are fully vaccinated or not.
No – for all children under the age of 12 years accompanied by a parent or guardian.
Which departure tests are accepted?
Lateral Flow Antigen Fit to Fly Tests and PCR Fit to Fly Tests.
Testing window :
For PCR: 72 hours before travel.
For Lateral Flow Antigen: 48 hours before travel.
Quarantine on arrival ?
No.
Required arrival documentation ?
A completed Traveller Health Declaration (completed 24 hours before arrival)
Pre-return test required ?
Yes – for everyone aged 11 or over.
Testing window :
Within 3 days before arriving in England.
Which pre-return tests are accepted ?
Take a Return Antigen test abroad with you or find a local testing centre in Croatia.
Arrival tests & quarantine ?
Croatia is on the green watchlist.
Everyone entering England from a green list country does not need to quarantine, but must take a PCR test on arrival day 2. The day of arrival in England = day 0.
More information ? https://www.gov.uk/foreign-travel-advice/croatia
France
: Amber List
Who can enter France ?
All fully vaccinated travellers from the UK. Non-fully vaccinated travellers from the UK must give an essential reason to be allowed to enter France.
Departure test required ?
No – for fully vaccinated adults.
Yes – for children aged 12 and over travelling with fully vaccinated adults.
Yes – for travellers who are not fully vaccinated but are allowed to enter France for essential reasons.
Which departure tests are accepted?
Lateral Flow Antigen Fit to Fly Tests and PCR Fit to Fly Tests.
Testing window :
48 hours before travel.
Quarantine on arrival ?
No – for fully vaccinated adults and accompanying over 12s.
Yes – 7 days isolation for not fully vaccinated adults and accompanying over 12s.
Required arrival documentation ?
A completed Sworn Statement.
Vaccination Proof: NHS Covid Pass for travellers from England and Wales
NHS letters for travellers from Scotland
Covid certificate for travellers from Northern Ireland
Pre-return test required ?
Yes – for everyone aged 11 or over.
Testing window :
Within 3 days before arriving in England.
Which pre-return tests are accepted ?
Take a Return Antigen test abroad with you or find a local testing centre in France.
Arrival tests & quarantine ?
France is on the amber travel list.
Everyone aged 11 or over entering England from an amber list country who have been fully vaccinated in the UK, US or EU will need to take a PCR test on arrival day 2. Children aged 5-10 will also need to test on day 2. The day of arrival in England = day 0.
Anyone aged 18+ entering England from an amber list country who hasn’t been fully vaccinated in the UK, US or EU will need to quarantine at home for 10 days and take two PCR tests on arrival day 2 and day 8, with the option to reduce the quarantine period by taking an additional PCR test on day 5.
The quarantine requirement has been lifted for all under 18s.
Germany
: Green List
Who can enter Germany ?
Germany is open for Citizens and Residents, Family Members, and Diplomats. UK residents may only enter Germany if they are serving an important role, there is an urgent need to travel, or if they are fully vaccinated.
Departure test required ?
No – for fully vaccinated adults.
No – for travellers who have proof of recovery from Covid-19.
Yes – for travellers who are not fully vaccinated.
N.B. Minors between the ages of 12-17 are only allowed to enter Germany if they have an urgent need or if they have been fully vaccinated.
Which departure tests are accepted?
Lateral Flow Antigen Fit to Fly Tests and PCR Fit to Fly Tests.
Testing window :
For PCR: 72 hours before travel.
For Lateral Flow Antigen: 48 hours before travel.
Quarantine on arrival ?
Yes – for travellers arriving in Germany from a high-risk area (e.g. the UK) have to quarantine for 10 days on entering Germany. Travellers arriving in Germany after having been in a high-risk area can end their home quarantine early if they submit proof of vaccination or proof of recovery via the entry portal. Alternatively, from day 5 they also have the option of doing a “test to release” and submitting proof of a negative test.
Yes – for children under the age of 12 years. 10 days quarantine on arrival but can end early by presenting a vaccination / recovery certificate. Alternatively, quarantine can be ended early with a negative Covid-19 test on the fifth day after entry.
Required arrival documentation ?
A completed Digital Registration on Entry.
Vaccination Proof: NHS vaccination certificate.
Pre-return test required ?
Yes – for everyone aged 11 or over.
Testing window :
Within 3 days before arriving in England.
Which pre-return tests are accepted ?
Take a Return Antigen test abroad with you or find a local testing centre in Germany.
Arrival tests & quarantine ?
Germany is on the green list.
Everyone entering England from a green list country does not need to quarantine but must take a PCR test on arrival day 2. The day of arrival in England = day 0.
Greece
: Amber List
Who can enter Greece ?
Greece is allowing entry for EU and Schengen citizens, and for individuals residing permanently in the US, EU, and Schengen states, and certain other countries.
Departure test required ?
No – for fully vaccinated adults.
No – for travellers who have proof of recovery from Covid-19 (a positive PCR result dated 30-180 days before travel).
Yes – for travellers who are not fully vaccinated.
Yes – for children aged 12 or over.
Which departure tests are accepted?
PCR Fit to Fly Tests only.
Testing window :
72 hours before travel.
Quarantine on arrival ?
No.
N.B. You may have to take a rapid Covid-19 test on arrival in Greece, with a quarantine in a hotel for at least 10 days if positive. You may also have to self-isolate if other passengers on your flight or ferry test positive for Covid-19.
Required arrival documentation ?
A completed Passenger Locator Form.
Vaccination Proof: NHS Covid Pass for travellers from England and Wales
NHS letters for travellers from Scotland
Covid certificate for travellers from Northern Ireland
Pre-return test required ?
Yes – for everyone aged 11 or over.
Testing window :
Within 3 days before arriving in England.
Which pre-return tests are accepted ?
Take a Return Antigen test abroad with you or find a local testing centre in Greece.
Arrival tests & quarantine ?
Greece is on the amber travel list.
Everyone aged 11 or over entering England from an amber list country who have been fully vaccinated in the UK, US or EU will need to take a PCR test on arrival day 2. Children aged 5-10 will also need to test on day 2. The day of arrival in England = day 0.
Anyone aged 18+ entering England from an amber list country who hasn’t been fully vaccinated in the UK, US or EU will need to quarantine at home for 10 days and take two PCR tests on arrival day 2 and day 8, with the option to reduce the quarantine period by taking an additional PCR test on day 5.
The quarantine requirement has been lifted for all under 18s.
Italy
: Amber List
Who can enter Italy ?
Italy is open for travellers arriving from within the EU and Schengen member countries, as well as those arriving from countries defined as safe by the EU.
Departure test required ?
Yes – for all travellers over the age of 6 years.
Which departure tests are accepted?
Lateral Flow Antigen Fit to Fly Tests and PCR Fit to Fly Tests.
Testing window :
48 hours before entry to Italy.
Quarantine on arrival ?
No – for fully vaccinated travellers. Yes – for unvaccinated travellers and those arriving without proof of vaccination and a negative test will be required to self-isolate for 5 days.
N.B. Travellers will need to contact the regional health office (ASL) to inform them where you will be completing the quarantine period as you will be under surveillance. A test will be required at the end of the 5 day period.
Children under the age of 6, from the UK, do not need to test but are not exempt from self-isolation.
Required arrival documentation ?
A completed online digital form.
Pre-return test required ?
Yes – for everyone aged 11 or over.
Testing window :
Within 3 days before arriving in England.
Which pre-return tests are accepted ?
Take a Return Antigen test abroad with you or find a local testing centre in Italy.
Arrival tests & quarantine ?
Italy is on the amber travel list.
Everyone aged 11 or over entering England from an amber list country who have been fully vaccinated in the UK, US or EU will need to take a PCR test on arrival day 2. Children aged 5-10 will also need to test on day 2. The day of arrival in England = day 0.
Anyone aged 18+ entering England from an amber list country who hasn’t been fully vaccinated in the UK, US or EU will need to quarantine at home for 10 days and take two PCR tests on arrival day 2 and day 8, with the option to reduce the quarantine period by taking an additional PCR test on day 5.
The quarantine requirement has been lifted for all under 18s.
Malta
: Green List
Who can enter Malta ?
Anyone aged 12 and over can travel to Malta as long as they hold a certificate of full vaccination for an EMA-approved COVID 19 Vaccine, including EU Digital COVID Certificates, Maltese and NHS (UK) certificates.
Departure test required ?
No – for fully vaccinated adults.
Yes – for children aged 5-11 travelling with fully vaccinated adults.
N.B. Children aged 12-18 will only be able to travel if they have proof of full vaccination.
Which departure tests are accepted?
Lateral Flow Antigen Fit to Fly Tests and PCR Fit to Fly Tests.
Testing window :
For PCR: 72 hours before travel.
For Lateral Flow Antigen: 48 hours before travel.
Quarantine on arrival ?
No.
Required arrival documentation ?
A completed online Passenger Locator Form
Vaccination Proof: NHS Covid Pass for travellers from England and Wales
NHS letters for travellers from Scotland
Covid certificate for travellers from Northern Ireland
Pre-return test required ?
Yes – for everyone aged 11 or over.
Testing window :
Within 3 days before arriving in England.
Which pre-return tests are accepted ?
Take a Return Antigen test abroad with you or find a local testing centre in Malta.
Arrival tests & quarantine ?
Malta is on the green list.
Everyone entering England from a green list country does not need to quarantine but must take a PCR test on arrival day 2. The day of arrival in England = day 0.
Portugal (including the Azores)
: Amber List N.B. Madeira is on the green watchlist. The Azores will move to the green list at 4am on Monday 30th August.
Who can travel to Portugal ?
All travellers from the UK can enter Portugal, however, there are entry restrictions. If you travel to mainland Portugal from the UK, you must quarantine for 14 days unless you can prove you are fully vaccinated with an EU-approved vaccination. Children under 18 years travelling with a fully vaccinated adult do not have to quarantine.
Departure test required ?
No – for fully vaccinated travellers.
Yes – for all travellers who are not fully-vaccinated aged 12 and over.
Which departure tests are accepted?
Lateral Flow Antigen Fit to Fly Tests and PCR Fit to Fly Tests.
Testing window :
For PCR: 72 hours before travel.
For Lateral Flow Antigen: 48 hours before travel.
Quarantine on arrival ?
No – for fully vaccinated travellers.
No – for children under 18 years old, as long as they travel accompanied by their parents, or a guardian who is fully vaccinated.
Yes – for 14 days, for travellers who are not fully vaccinated.
Required arrival documentation ?
All travellers: a completed Passenger Locator Card.
Vaccination Proof: NHS Covid Pass for travellers from England and Wales
NHS letters for travellers from Scotland
Covid certificate for travellers from Northern Ireland 3. For unvaccinated travellers: a completed Isolation Form.
Pre-return test required ?
Yes – for everyone aged 11 or over.
Testing window :
Within 3 days before arriving in England.
Which pre-return tests are accepted ?
Take a Return Antigen test abroad with you or find a local testing centre in Portugal.
Arrival tests & quarantine ?
Portugal is on the amber travel list.
Everyone aged 11 or over entering England from an amber list country who have been fully vaccinated in the UK, US or EU will need to take a PCR test on arrival day 2. Children aged 5-10 will also need to test on day 2. The day of arrival in England = day 0.
Anyone aged 18+ entering England from an amber list country who hasn’t been fully vaccinated in the UK, US or EU will need to quarantine at home for 10 days and take two PCR tests on arrival day 2 and day 8, with the option to reduce the quarantine period by taking an additional PCR test on day 5.
The quarantine requirement has been lifted for all under 18s.
Madeira is on the green watchlist. The Azores are being added to the green list on Monday 30th August. If you arrive before 4am on 30th August from the Azores, please follow the rules above. If arriving after 4am 30th August, follow the rules below.
Everyone entering England from a green watchlist country does not need to quarantine but must take a PCR test on arrival day 2. The day of arrival in England = day 0.
Spain (including the Balearic Islands and the Canary Islands)
: Amber list
Who can travel to Spain ?
Spain is open for travellers arriving from within the EU and Schengen member countries, as well as those arriving from certain other third countries listed in the Annex or for travellers with valid vaccination certificates.
Departure test required ?
No – for fully vaccinated adults.
Yes – for children aged 12 and over travelling with fully vaccinated adults.
Yes – for travellers who are not fully vaccinated.
Which departure tests are accepted?
PCR Fit to Fly Tests only.
Testing window :
72 hours before travel.
Quarantine on arrival ?
No.
Required arrival documentation ?
A completed Health Control Form (completed no more than 48 hours before travelling to Spain by air or sea)
Vaccination Proof: NHS Covid Pass for travellers from England and Wales
NHS letters for travellers from Scotland
Covid certificate for travellers from Northern Ireland
Pre-return test required ?
Yes – for everyone aged 11 or over.
Testing window :
Within 3 days before arriving in England.
Which pre-return tests are accepted ?
Take a Return Antigen test abroad with you or find a local testing centre in Spain.
Arrival tests & quarantine ?
Spain (including the Balearic Islands and the Canary Islands) is on the amber travel list.
Everyone aged 11 or over entering England from an amber list country who have been fully vaccinated in the UK, US or EU will need to take a PCR test on arrival day 2. Children aged 5-10 will also need to test on day 2. The day of arrival in England = day 0.
Anyone aged 18+ entering England from an amber list country who hasn’t been fully vaccinated in the UK, US or EU will need to quarantine at home for 10 days and take two PCR tests on arrival day 2 and day 8, with the option to reduce the quarantine period by taking an additional PCR test on day 5.
The quarantine requirement has been lifted for all under 18s.
USA
: Amber list
Who can travel to the USA ?
It is not possible for most British nationals to enter the USA if they have been in the UK, Ireland, Schengen zone, and a number of other countries, within the previous 14 days. US citizens and permanent residents of the USA, certain specified close family members, and certain other limited categories of visas holders (such as UN staff and diplomats) are exempt. They will still be able to enter the USA, subject to normal entry requirements.
Departure test required ?
Yes – for all travellers.
Which departure tests are accepted?
The USA requires pre-departure tests to meet the requirements stated by the CDC. Tests can be either antigen tests or PCR tests. Self-tests (e.g. at home test kits) are acceptable if they include a telehealth service affiliated with the manufacturer of the test that provides real-time supervision remotely through an audio and video connection.
Testing window :
No more than 3 days before departure.
Quarantine on arrival ?
No – for fully vaccinated travellers with an FDA-authorised vaccine. Travellers must complete a viral test (e.g. Antigen or PCR test) 3 to 5 days after travel.
No – for those who have recovered from a documented case of Covid-19 within the last 3 months. These individuals should follow all requirements and recommendations for fully vaccinated travellers, with the exception of testing 3 to 5 days after travel.
Yes, for 7 days – for travellers who are not fully vaccinated. Unvaccinated travellers must complete a viral test (e.g. Antigen or PCR test) 3 to 5 days after travel.
Required arrival documentation ?
Consult the US State Department website to determine which documents you will need.
Pre-return test required ?
Yes – for everyone aged 11 or over.
Testing window :
Within 3 days before arriving in England.
Which pre-return tests are accepted ?
Take a Return Antigen test abroad with you or find a local testing centre in the USA.
Arrival tests & quarantine ?
The USA is on the amber travel list.
Everyone aged 11 or over entering England from an amber list country who have been fully vaccinated in the UK, US or EU will need to take a PCR test on arrival day 2. Children aged 5-10 will also need to test on day 2. The day of arrival in England = day 0.
Anyone aged 18+ entering England from an amber list country who hasn’t been fully vaccinated in the UK, US or EU will need to quarantine at home for 10 days and take two PCR tests on arrival day 2 and day 8, with the option to reduce the quarantine period by taking an additional PCR test on day 5.
The quarantine requirement has been lifted for all under 18s.
1 note
·
View note
Text
Neurodiagnostics Market Size, Share, Trends, CAGR Status, Growth Opportunities
Market Highlights
According to MRFR analysis, the Global Neurodiagnostics Market is expected to register a CAGR of 6.88% from 2019 to 2025 and held a value of USD 4,848.48 Million in 2018.
Neurodiagnostic tests which are also known as neurodiagnostics. records and monitors electrical activities of patient’s peripheral nerves, spinal cord, and brain. These tests help physicians to confirm or rule out a neurological disorder or other medical condition. The growth of the global neurodiagnostics market is boosted by factors such as rising number of strategic initiatives by key players such as mergers, joint ventures, acquisitions, partnerships, coupled with the advancements in technology across the globe. Furthermore, many non-profit organizations in various countries are working towards creating awareness regarding neurological diseases. For instance, the World Federation of Neurology (WFN), founded in July 2016, is a membership organization focused on raising awareness about the age and neurological conditions and diseases associated with age.
However, the high cost of neurodiagnostic treatment is likely to restrain the market growth to a certain extent in the coming years.
Market players such as GE Healthcare, Siemens Healthineers, Philips Healthcare, and Hitachi, Ltd., currently dominate the global neurodiagnostics market. The key players are involved in product launches and agreements to strengthen their market positions. For instance, in December 2019, GE Healthcare (US), signed USD 100 million technology partnership agreement with AFFIDEA (Ireland). GE Healthcare will install 200+ new equipment in Affidea’s network of centers across Europe. The deal includes the provision of 60 new MRIs, 50 ultrasound devices, 40 CT scanners and 30 X-rays machines in the next 3 years. It also includes a six-year service contract.
Regional Analysis
The market has been divided, by region, into the Americas, Europe, Asia-Pacific, and the Middle East & Africa. The Americas held maximum share in the base year 2018, owing to the high incidence rates of neurological disorders in countries such as US. According to the American Neurological Association, as of 2016, mealy 100 million Americans were affected by at least one of the neurological diseases. The neurodiagnostics market in the Americas has further been branched into North America and Latin America, with the North American market divided into the US and Canada. The European neurodiagnostics market has been categorized as Western Europe and Eastern Europe. The Western European market has further been classified as Germany, France, the UK, Italy, Spain, and the rest of Western Europe. The neurodiagnostics market in Asia-Pacific has been segmented into Japan, China, India, South Korea, Australia, and the rest of Asia-Pacific. The neurodiagnostics market in this region is anticipated to be the fastest-growing during the assessment period due to the increasing awareness about the diagnostic treatments and favorable reimbursement policies. The neurodiagnostics market in the Middle East & Africa has been divided into the Middle East and Africa.
Segmentation
The Global Neurodiagnostics Market size has been segmented based on Product, Condition, and End User.
The market, based on product type, has been divided into diagnostic imaging systems, clinical diagnostic instruments, and reagents & consumables. The diagnostic imaging systems segment held a major share in 2018 owing to the rising number of diagnostic laboratories an imaging centers in the developing as well as developed countries. The reagents & consumables was the fastest-growing segment in 2018 due to high consumption in various routine techniques. The market, based on diagnostic imaging systems, has been further segment is segmented as, MRI systems, EEG systems, CT scanners, PET scanners, EMG devices, ultrasound imaging systems, MEG devices, angiography systems, and others. The market, based on clinical diagnostic instruments has been further bifurcated into PCR instruments, NGS instruments, sanger sequencers, and others. The market, based on reagents & consumables has been further segmented into media & sera, antibodies, buffers, solvents, enzymes, proteins, & peptides, probes, and other.
The global neurodiagnostics market has been segmented, based on the condition, into neurodegenerative diseases, stroke, epilepsy, headache disorders, sleep disorders, and others. In 2018, stoke segment held the majority share of the market owing to the growing geriatric population. The neurodegenerative diseases segment is anticipated to be the fastest growing during the forecast period due to the high number of Parkinson’s and Alzheimer’s disease population.
The global neurodiagnostics market, based on end user, has been segmented into hospitals & surgical centers, diagnostic laboratories & imaging centers, neurology centers, ambulatory care centers, and research laboratories & academic institutes. The hospitals & surgical centers segment held the maximum share in 2018 owing to its high purchasing power to buy expensive equipment. The neurology centers segment is expected to be the fastest-growing during the assessment period due to the lower cost and short waiting period compared to hospitals.
Browse Full Report Details @ https://www.marketresearchfuture.com/reports/neurodiagnostics-market-8762
Key Players
Some of the key players in the global neurodiagnostic market are GE Healthcare (US), Siemens Healthineers (Germany), Philips Healthcare (Netherlands), Hitachi, Ltd. (Japan), Canon, Inc. (Japan), Lifelines Neuro Company, LLC (US), Thermo Fisher Scientific, Inc. (US), FUJIFILM Holdings Corporation (Japan), Mitsar Co., Ltd. (Russia), Natus Medical Incorporated (US), Hoffman-La Roche AG (Switzerland), and QIAGEN N.V. (Netherlands).
1 note
·
View note
Text
https://www.aier.org/article/an-education-in-viruses-and-public-health-from-michael-yeadon-former-vp-of-pfizer/
➖➖➖Dr. Michael Yeadon is an Allergy & Respiratory Therapeutic Area expert with 23 years in the pharmaceutical industry. He trained as a biochemist and pharmacologist, obtaining his PhD from the University of Surrey (UK) in 1988.
Dr. Yeadon then worked at the Wellcome Research Labs with Salvador Moncada with a research focus on airway hyper-responsiveness and effects of pollutants including ozone and working in drug discovery of 5-LO, COX, PAF, NO and lung inflammation. With colleagues, he was the first to detect exhaled NO in animals and later to induce NOS in lung via allergic triggers.
Joining Pfizer in 1995, he was responsible for the growth and portfolio delivery of the Allergy & Respiratory pipeline within the company. He was responsible for target selection and the progress into humans of new molecules, leading teams of up to 200 staff across all disciplines and won an Achievement Award for productivity in 2008.
Under his leadership the research unit invented oral and inhaled NCEs which delivered multiple positive clinical proofs of concept in asthma, allergic rhinitis and COPD. He led productive collaborations such as with Rigel Pharmaceuticals (SYK inhibitors) and was involved in the licensing of Spiriva and acquisition of the Meridica (inhaler device) company.
Dr. Yeadon has published over 40 original research articles and now consults and partners with a number of biotechnology companies. Before working with Apellis, Dr. Yeadon was VP and Chief Scientific Officer (Allergy & Respiratory Research) with Pfizer.
Below is a transcript of the video above:
My name is Dr Michael Yeadon.
My original training was a first-class honours degree in biochemistry and toxicology. Followed by a research-based PhD into respiratory pharmacology; and after that I’ve worked my entire life, uh, on the research side of the pharmaceutical industry – both big pharma and also biotech. My specific focus has been inflammation, immunology, allergy in the context of respiratory diseases (so the lung, but also the skin). So I would say I’m a kind of a deeply experienced inflammation, immunology, pulmonology kind of research person.
I initially became concerned about, the, our response to the coronavirus pandemic towards the middle or back end of April as early as that. It had become clear that if you look at the number of daily deaths versus the date the pandemic had turned. Really, pleasingly, already the wave was fundamentally over, and we would just watch it fall for a number of months – which is what it did. And so I became very perturbed about increasing restrictions on the behavior and movement of people in my country and I could see no reason for it then and I still don’t.
Government’s response to emergencies is guided by the scientific group who sit together under the Scientific Advisory Group for Emergencies or SAGE. So they should provide scientific advice to the government about what’s appropriate to do. SAGE has got several things wrong, and that has led to advice that’s inappropriate and – uh, not only has had horrible economic effects, but has had continuing medical effects in that people are no longer being treated properly.
SAGE took the view that since SARS-CoV-2 was a new virus that they believed there wouldn’t be any immunity at all in the population. So, I think that’s the first thing. I remember hearing that and I puzzled, because I already knew – because I read the scientific literature that SARS-CoV-2 was 80% similar to another virus you may have heard of called SARS that moved around the world a bit in 2003, and more than that: it’s quite similar, in pieces of it, to common cold-causing coronaviruses.
So, when I heard that there was this coronavirus moving across the world I wasn’t as worried as perhaps other people were, because I figured that since there are four common cold-causing coronaviruses, I figured that quite a lot of the population we’ve been exposed to one of those viruses, and would probably have a perhaps substantial protective immunity. And just to explain why I was so confident everybody knows the story of Edward Jenner and vaccination, and the story of cowpox and smallpox. And that the old story was that milkmaids had very, uh, clear complexions: they never suffered from things like smallpox, that if it didn’t kill you would leave your skin permanently scarred. And the reason that they had the protection was that they were exposed to a more benign, related virus called cowpox.
Edward Jenner came up with the idea that if it’s cowpox that saves the fair maid – he reasoned that if he could give another person an exposure to the cowpox, he would be able to protect them from smallpox. Now, he did an experiment that you can’t do now – and he never should have done it – but apocryphally, or really, or maybe you’re ill, we’re not sure. Edward Jenner acquired some of the liquid from a person infected with cowpox. Relatively mild pustules that then go away. And he got some of this and he – he scraped it into the skin of a small boy and a few weeks later, he obtained some liquid from some poor person that was dying of smallpox and infected the boy. And, lo and behold, the boy did not get ill and that gave birth to the whole field of what’s called vaccination. And vax, the vaccine’s “vac.” It comes from “vaccus,” the Latin name for cow. So, we are really familiar with the principle of cross immunization.
I’ve thought quite a lot about, you know, the vulnerable people in in care homes and there’s an awareness that, even though people really careful using PPE and so on, but that’s only going to go so far in a kind of, hot house environment where people are pretty close together in a care home. So the question I’ve had all year is: once one or two people, you know, got the virus in a care home, why wouldn’t almost everyone get infected? And of course the truth is, they didn’t. And one interpretation of that distinction is that a large proportion of people in the care homes had prior immunity.
At this time of year, about 1 in 30 people have a cold, caused by one of these coronaviruses. And just like the protection against smallpox provided by previous exposure to cowpox, so people exposed to having had a cold caused by one of these coronaviruses they’re now immune to SARS-CoV-2. So, 30% of the population was protected before the start. SAGE said it was zero – and I don’t understand how they could possibly have justified that. There’s a second, and equally fatal, unaccountable error that they have made in their model. The percentage of the population that SAGE asserts have been infected to date by the virus is about seven percent. I know that that’s what they believe and you can see it in a document they published in September called “Non-pharmaceutical interventions” and it says sadly more than 90% of the population is still vulnerable.
It’s unbelievably wrong. And I’m just going to explain why: they’ve based their number on the percentage of people in the country who have antibodies in their blood. And only the people who became most ill needed to actually develop and release antibodies around their body. So, it is certainly true that the people who have lots of antibodies were infected. But a very large number of people had milder symptoms, and even more people had none at all. And the best estimates that we can arrive at is that those people either made no antibodies, or so low amounts that they will have faded from now.
A recent publication on the percentage of care home residents who have antibodies to the virus very, very interesting. This time they were using high sensitivity tests for antibodies and they carefully picked out residents that never were PCR-positive: these are people who never got infected. And they found that 65% of them had antibodies to the virus; they never got infected. So I believe there was high prevalence of immunity in that population prior to the virus arriving. Big story in the media, recently, was that the percentage of people with antibodies against the virus in their blood was falling. Now, this was cast as a concern that immunity to SARS-CoV-2 doesn’t last very long. Well, you know, anyone with knowledge of immunity would – would just simply reject that. It’s not the way immunity to virus works – that would be T-cells. So, if the antibodies are falling gradually over time – which they have – from spring to present, the only plausible explanation is that the prevalence of the virus in the population is falling, and that’s why the antibody production gradually subsides.
Less than 40% of the population are susceptible. Even theoretical epidemiologists would tell you that that’s too small a number to support a consolidated and growing outbreak, community immunity, herd immunity. So, SAGE says that we’re not even close, and I’m telling you that the best science, by the best scientists in the world, published in the top peer-reviewed journals, says they’re wrong: that more than 60 of the population are now immune, and it’s simply not possible to have a large and growing pandemic.
Really good news, genuine good news, to hear that there’s data emerging from the vaccine clinical trials, and we are seeing vaccines that raise not just antibodies – but they’re also producing T-cell responses. This is great; back to proper science, proper immunology. That’s how immunity to viruses works. So, my surprise though, and it’s just annoying that when we’re talking about, uh, the percentage of the population that’s still susceptible we only talk about antibodies, like seven percent from SAGE. Why are we not talking about the 50% that have got T-cell immunity?
And so you might be thinking if Mike – and Dr Mike Yeadon is telling you these things… – or how come the pandemic isn’t over? Well, this may come as a surprise to you, but I believe fundamentally it is over. The country has experienced almost a complete cycle now of the virus sweeping through the land, and we are at the end of it. London was –was horribly affected in the spring, and somewhere in early April they were experiencing several hundred deaths per day from people dying with similar symptoms in respiratory failure and, uh, inflammation. And at the moment the number of people dying of SARS-CoV-2 in the capital is less than 10. So it’s down by 98, or something like that. And, the reason it’s down, is because there are now too few people in London susceptible to allow the virus to magnify, to amplify, to get an epidemic. And, and they would have been hit by now, because they were the first place hit in the spring. And I think what we’re seeing now in the Northeast and the Northwest would be the dying embers of the spreading out of this virus. And I’m very sorry that it is still true, that a small number of people are catching it, getting ill, and dying.
So why aren’t the media telling us that the pandemic is over? It’s not over because SAGE says it’s not. So SAGE consists of very many scientists, from a range of disciplines – mathematicians and clinicians – and there are multiple committees. But I found to my surprise – and I’m actually going to use the word – horror, that in the spring, all the way through the spring and summer, SAGE did not have on their committee someone who I would call a card-carrying immunologist; a clinical immunologist. I have to say I think that in the spring and summer SAGE was deficient in the expertise it had. They should have armed themselves, you know, with – around the table all the people required to to understand what was happening, and they didn’t do that. People asked me then, “Well Mike, if it’s, you know, if it’s fundamentally over, why are we still getting hundreds of deaths a day from SARS-CoV-2?” And I’ve thought a lot about this. There is a test that’s performed where people have their noses and tonsils swabbed, and then a test (called a PCR test) is performed on that. And what they’re looking for isn’t the virus – you might think it’s looking for the virus, but it’s not. What they’re looking for is a small piece of genetic sequence; it’s called RNA. Unfortunately, that bit of RNA will be found in people’s tonsils and nose not if they’ve just caught the virus, and they’re about to get ill, or they’re already ill. It’s also going to be found if they were infected previously weeks – or even, sometimes, a small number of months ago. Let me just explain why that is.
If you’ve been infected, and you’ve fought off the virus (which most people do), you’ll have broken, dead bits of virus. These are tiny things smaller than your cells, perhaps spread all the way through your airway, embedded in bits of mucus, maybe inside an airway lining cell. And so over a period of weeks or months you bring up cells that contain broken, dead pieces of the virus that you have conquered and killed. However, the PCR test is not able to detect whether the viral RNA has come from a living virus or a dead one (as I’ve just described). So I think a large proportion of the so-called positives are, in fact, what I call “cold” positives: they’re correctly identifying that there is some viral RNA in the sample – but it’s from a dead virus. It can’t hurt them, they’re not going to get ill, they can’t transmit it to anybody else. So they’re not infectious. So that accounts for a large number of the so-called positive cases. These are people who’ve beaten the virus. Why are we using this test that cannot distinguish between active infection and people who’ve conquered the virus?
This test has never been used in this way – and I’ve worked in this field. It’s not a suitable technique it’s a – it’s the kind of technique you would use for forensic purposes, if you were trying to do a DNA test to establish whether or not a person was at the scene of a crime. You would not be doing these tests by a windy, supermarket car parking; what looks like plastic marquee tents; on picnic tables. It’s not suitable at all – and it definitely shouldn’t be done in the way it’s been done. It’s subject to many mechanical errors, should we say, handling errors. If this was a test being used for legal purposes, for forensic purposes like a DNA identity test, the judge would throw out this evidence; would say it’s not admissible. It produces positives even when there’s no virus there at all. We call that a false positive.
As we’ve increased the number of tests done per day, so we’ve had to recruit less and less experienced laboratory staff – and now we’re using people who’ve never worked professionally in this area. What that does is it increases the frequency of mistakes, and the effect of this is that the false positive rate rises and rises. So, if you had a false positive rate of one percent – which Mr. Matt Hancock [British Secretary of State for Health and Social Care] told us was roughly the number they had in the summer – then if you tested a thousand people that had no virus ten of them would be positive, astonishingly. If the prevalence of the virus was only one in a thousand, that’s 0.1% – as the Office for National Statistics told us it was through the summer – then if you use the PCR test only one of them will be positive and genuinely so. But if the false positive rate is as low as one percent, you’ll also get 10 positives that are false.
Some people did say to me, “Well, there’ll be a higher percentage of people coming forward for testing in the community,” so-called “Pillar 2” testing, because they’ve been instructed only to come if they’ve got symptoms. But I call B.S. on that one. I don’t think that’s true. I know lots of friends and relatives who’ve been told by an employer, “Well, you’ve sat near someone who’s tested positive, and I don’t want you to come back to work until you’ve got a negative test.” I’ve seen information from many towns in the North – certainly Birmingham was one; Manchester was another; Bolton – where councils (and I really think they were trying to be helpful) were out leafleting the people of their cities saying, “We’re going to come round and swab you all because we want to track down this virus.” Now once you start testing people, more or less randomly, instead of [those] having symptoms you get the same amount of virus in the population as the Office of National Statistics found which is, at the time was, one in a thousand. And I’ve just told you Matt Hancock confirmed during the summer they had a false positive rate of about one percent. So that means out of a thousand people 10 would test positive, and it would be a false result, and only one would test positive and it was correct.
This test is monstrously unsuitable for detecting who has live virus in their airway. It’s subject to multiple distortions that are worsening as we get into the winter. As the number of tests done per day increase[s], the number of errors made by these overworked, not very experienced lab staff increase[s]. I think it’s not unreasonable to say a best guess of the false positive rate at the moment – what’s called the operational false positive rate is about five percent. Five percent of 300,000 is 15,000 positives. I think some of those positives are real; I don’t think it’s very many. Now, the problem with this false positive issue [is] it doesn’t just stop it at “cases”: it extends to people who are unwell and go to hospital. So people who go to hospital having tested positive – and it could be a false positive, and I think most of them are at the moment – if you go to hospital and you’ve tested positive previously, or you test positive in hospital, you’ll be counted now as a Covid admission.
Although there are more people in hospital now than a month ago, this is normal for autumn. Regrettably, people catch respiratory viruses and become ill, and some will die. I just don’t believe it’s got anything to do with Covid-19 anymore. There are more people in intensive care beds now than there were a month or so ago. That’s entirely normal as we move through late autumn into the early winter: those beds become used. But there aren’t more people than is normal for the time of year, and we’re not about to run out of capacity, certainly at a national level. But I think you know it is going now: if you should now die, you’ll be counted as a Covid death. But that’s not correct; these are people who might have – have gone to hospital having had a broken leg, for example, but they’ll – three percent of them will still test positive, and they’re not, they haven’t got the virus. It’s a – it’s a false positive, and if they die they’ll be called a Covid death – and they are not. They’ve died of something else.
One of the most troubling things I’ve heard this year was Mr. Johnson telling us about the “Moonshot” testing everybody often, maybe every day, is the way out of this problem. I’m telling you it’s the way to keep us in this problem: that number of tests is orders of magnitude higher than we’re already testing now, and the false positive rate (however low it is) will be far too large to accept. It will produce an enormous number of false positives.
What we should do is stop mass testing. Not only is it an affront to your liberty, it will not help at all: it will be immensely expensive and it will be a pathology all of its own. We’ll be fighting off stupid people – mostly government ministers – I’m sorry to say, who are not numerate, and do not understand statistics. If you test a million people a day with a test that produces one percent false positives, 10 000 people a day will wrongly be told they’ve got the virus. If the prevalence of the virus was say 0.1%, like the Office of National Statistics said it was in summer, then only a tenth of that number, uh, 1,000 would correctly be identified. But you can’t distinguish amongst the 11,000 who have genuinely got the virus and who are false positives. Moonshot, I think, will have a worse false positive rate. It’s not fixable, and it’s not necessary either. The pandemic – having passed through the population not only of, of the UK, but of all of Europe – and probably all of the world quite soon – it won’t return. Why won’t it return? Well, they’ve got T-cell immunity. We know this. It’s been studied by the best cellular immunologists in the world.
Sometimes people will say, “Well, it looks like the immunity is starting to fade.” You’ll sometimes see [statements] like that, and when I saw the first headline like this I remember being really quite confused, because that’s not the way immunology works. Just think about it for a moment. If that was how it worked it could kill you. When you had to fight it off, and if you had successfully done that, it somehow didn’t leave a mark in your body. Well, it does leave a mark on your body. The way you fought it off involved certain pattern recognition receptors, and has left you with – as it were – memory cells that remember what it was they fought off. And if they see that thing again it’s very easy for them to get those cells to work again in minutes or hours, and they will protect you. So the most likely explanation is it’ll last a long time.
So I read a bit more about this so-called tailing off of immunity – and I realized they were talking about antibodies. Just incorrect to – to think that antibodies, and how long they stay up, is a measure of immune protection against viruses. I mean you can tell I’m – I don’t agree with this. It says there have been some classic experiments done on people who have inborn errors in parts of their immune system, and some of them have inborn arrows that means they can’t make antibodies, and guess what: they – they are able to handle respiratory viruses the same as you and me. So, I don’t think it’s harmful to have antibodies, although some people are worried about the potential for amplifying inflammation from antibodies, but – but my view is that they’re – they’re probably neutral, and you definitely should not believe the story that because the antibody falls away you’ve lost immunity. Again, that’s just not the way the human immune system works.
The most likely duration of immunity to a respiratory virus like SARS-CoV-2 is multiple years. Why do I say that? We actually have the data for a virus that swept through parts of the world 17 years ago called SARS, and remember SARS-CoV-2 is 80% similar to SARS, so I think that’s the best comparison that anyone can provide. The evidence is clear. These very clever cellular immunologists studied all the people they could get hold of who had survived SARS 17 years ago. They took a blood sample, and they tested whether they responded or not to the original SARS, and they all did. They all have perfectly normal, robust T-cell memory. They are actually also protected against SARS-CoV-2 because it’s so similar, it’s cross-immunity. So, I would say the best data that exists is that immunity should be robust for at least 17 years. I think it’s entirely possible that it is lifelong. The style of the responses of these people’s T-cells were the same as if you’ve been vaccinated and then you come back years later to see, has that immunity been retained? And so I think the evidence is really strong that the duration of immunity will be multiple years, and possibly lifelong.
There have been but a tiny handful of people who appear to have been infected twice – now they’re very interesting, we need to know who they are and understand them very well, they’ve probably got certain rare immune deficiency syndromes. So I’m not pretending no one ever gets reinfected, but I am pointing out that it’s literally five people (or maybe 50 people), but the World Health Organization estimated some weeks ago that 750 million people have been infected so far by SARS-CoV-2. That means most people are not being reinfected, and I can tell you why that is: it’s normal. It’s what happens with viruses, respiratory viruses. Some people have – have called for “zero Covid” as if it’s some political slogan. And there are some people I’ve heard calling for it almost every day; they’re completely unqualified to tell you anything.
Something that’s really important to know is that SARS-CoV-2 – it’s an unpleasant virus. There’s no question about it, but it’s not what you were told in spring. We were originally told that it would kill perhaps three percent of people it infected – which is horrifying. That’s 30 times worse than flu. We always overestimate the lethality of new infectious diseases when we’re in the eye of the storm. I believe the true infection fatality ratio of Covid-19, the true threat to life is, the same as seasonal flu.
So there’s no reason why you would want to try and drive Covid to zero. It’s a nonsense – that’s just not how biology is. And all the means I have heard, uh, proposed, as ways to get us there are much more damaging and pathological, I would say, than than the virus itself. It’s simply not possible to get rid of every single copy of the Covid-19 virus, and the means to get you there would destroy society. Forget the cost – although it would be huge – it would destroy your liberty, you would need to not go out until you’ve been tested and have your result back. And I have described how the false positive rate would just destroy it from a statistical perspective. I don’t believe it can be done: it’s not scientifically realistic, it’s not medically realistic, and it’s not what we have ever done.
As the virus swept towards the UK in the – in the late winter and early spring I too was concerned, because at the time we were told perhaps three percent might die. So when the Prime Minister called for a lockdown I wasn’t pleased about it, but I understood that we should try this. But it’s important that you understand, that when we look at the profile of the pandemic as it passed through the population, that it was clear that the number of infections every day was falling. We’d passed the peak quite a long time before lockdown started. So we took all that pain, that locked down pain which was multiple weeks – I don’t remember exactly how many multiple weeks – we took it for nothing. If there was a really important effect of lockdown on the number of people who died, or the rate of it, you should at least be able to order them. Like, these people had locked down, and these didn’t – and you cannot. All heavily infected countries’ shapes are the same, whether they had locked down or not. They don’t work. I don’t know why anyone is allowing you, know you, to be pushed into this corner.
I don’t think we entirely know why it is that some countries were hit harder than others, but I have to say I think scientifically the smart money is on a mixture of forces. One would be this cross immunity. Although China had an awful time in Wuhan, in Hubei province, it didn’t spread elsewhere in the country, and I suspect that meant because a lot of them had this cross immunity. And I think nearby countries, in the main, had lots of cross immunity. So that’s one possibility. The other one, though, is in terms of the severity of what did the virus do to a particular population. We’ve seen devastating effects in countries like UK and in Belgium, uh, France, and maybe even in Sweden, and much smaller numbers of deaths in other countries like – like Greece and in Germany. And you might think, “Well, was that was it something that they did?” And I wish it was true, because if it was something we did we could learn from it and do it and it would work in the future. But there’s no evidence whatsoever that it was anything humans did. The passage of this virus through the human population is an entirely natural process that completely ignored our puny efforts to control it.
So there is this theory – I don’t like the name very much – but it’s called “dry tinder.” If people in a country who are vulnerable for to dying in the winter (usually of respiratory viruses), if you have a very mild winter season, like UK did – we had a very mild seasonal flu last year and the year before and so did Sweden – then what happens is there are larger number of very vulnerable people who are even older than usual, and – and I think that’s why we suffered a rather large number of deaths. It was still only 0.06% of the population, equivalent to about four weeks of normal mortality. But countries that had very severe winters recently, and Greece and Germany certainly had very lethal winter flus in the last two years. I think then, they had a smaller population of very vulnerable people, and that is the main reason why they lost fewer people. It’s not to do with locking down, it’s not to do with testing, or tracking, or tracing. I personally don’t think any of those measures have made any difference at all. So Belgium and UK and Sweden were particularly vulnerable, whereas adjacent Nordic countries – I – I get fed up with hearing about this, uh, idea that they locked down and that’s why it saved them and afraid the other Nordic countries had normal flu epidemics the last two or three years. Sweden, like UK, had very mild epidemics: you can just go and look at the number of deaths, it’s sub-normal for UK and Sweden. And now we’ve got a supra-normal, a larger-than-normal, number of deaths from Covid.
Now there may be other reasons, I’m not saying there are not but I think those two main forces – the amount of prior immunity and the so-called “dry tinder,” what vulnerable fraction of the population did you have as a result of seasonal flu being intense or not – I think that accounts for most of it. And it’s – it’s just puberistic and, uh, and – and kind of silly that our government and advisors tell you that doing things that have never worked in the past, like lockdown are going to make any difference to the transfer of respiratory viruses. I don’t believe it for a moment. There’s no scientific evidence behind it and there are much stronger scientific hypotheses that do explain it. You might think that in terms of numbers of deaths – excess deaths – that Covid has produced such a large number that this will be an awful year for excess deaths, but surprisingly not. 2020 is lining up to be about eighth in a list since 1993.
Roughly 620,000 people die every year in this country. They say in life we are also in death – and it’s true, it’s been awful for those who have been personally affected by illness and death, but it’s not particularly unusual in terms of the number of people who’ve died. So one of the things I’ve noticed has happened in – in recent years is that we almost seem to be moving, uh, you know post-science, post-fact as if – as if facts don’t matter. For someone who’s qualified and practiced as a professional scientist for 35 years I think it’s deeply distressing that, I don’t think you should listen to me if I talked about – I don’t know, the design of motorways or something – like, I don’t know anything about motorways or – or how to grow trees better, I don’t know anything about that. But I do know quite a lot about immunology, infection, inflammation, and the way infectious organisms move through a population.
I’ve no other reason for giving this interview other than I really care what happens to my country – and we have to pull ourselves out of this. And I personally believe the way forward is twofold, it’s not difficult. One, we should cease mass testing of the mostly-well in the community immediately – it only provides misleading and grey information, and yet we’re driving policy almost completely based on it. It’s definitely wrong, we should not do it. Use the tests in hospital – I’m not saying don’t test – don’t continue mass testing, and for God’s sake, don’t increase the number of tests. It is a pathology all of its own which must be stamped out by right thinking people. And I’m afraid the people on SAGE, who have provided the modeling, the predictions, the – the measures that should be taken, that their work is so badly, and obviously flawed – lethally incompetent, that you should have no more to do with these people. They should be fired immediately. And the effect of that advice has been to – have cost lots of innocent people their lives from non-Covid causes, they should be dismissed and reconstituted using an appropriate group of skilled individuals – especially avoiding any who might even have the suggestion of a conflict of interest. I think we’re right at the edge of the precipice. I really hope that we can pull back.
1 note
·
View note
Text
ND Labs Ltd Manufacturing Rapid COVID 19 Test Kit for Detecting the Coronavirus in Just Ten Minutes
COVID-19 disease is spreading rapidly and continually across the globe. Different research-based firms are making use of varied screening procedures for screening suspected Coronavirus patients. The RT PCR testing technique is being used on an extensive scale. But the problem with this process is it offers results in three to four hours. Since there has been an unparalleled increase in the number of Covid-19 cases, this technique is not found to help the cause. This is the reason why, we, at ND Labs Ltd recommend going for our COVID 19 test kit in UK called Realy Tech. One of the best things about our test kit is that it offers results in just ten minutes. Not to forget, it can be used very easily, and its portability is just mind-blowing.
Our rapid testing kit can easily be used in hotspot regions of countries for screening both symptomatic and asymptomatic patients. It will be a great idea to buy COVID test kit in UK from ND Labs Ltd as it helps in controlling the spread of the respiratory virus. Our rapid testing kit is a prick-based test kit that does not involve testing for the virus at a laboratory. It can help in detecting the presence of antibodies very quickly. Our kit is based on the IgM antibody wherein the presence of this specific antibody helps in identifying whether the virus has infected the individual recently or earlier.

A noteworthy feature of our COVID 19 testing kit is that it can be used very easily. This kit, is specifically meant for professional use only. Blood sample from the tip of the finger is drawn with the needle and is placed on the test cassette. The cassette features markings for antibodies. Demarcated colours appear for samples testing positive for the antibodies. These colours can effectively be used for differentiating between the negative and the positive results.
Our rapid testing kit is easily available on our site, and it is CE certified as well. One exclusive feature of our test kit is that it helps in speeding up the procedure of screening both symptomatic and asymptomatic individuals. And yes, within the shortest time possible. The most pronounced benefit of our COVID test kit is that it can effortlessly be deployed directly at the healthcare units in clinics and hospitals. The kit can be of profound use in countries where emergencies have reached an advanced level.
Early detection of the novel Coronavirus is useful for the health agencies and the government authorities in halting the spread of the same within communities. So, what are you waiting for? If you are a healthcare professional, then you must go for our COVID 19 testing kit.
1 note
·
View note