#Multiple Myeloma Treatment Market
Explore tagged Tumblr posts
Text
https://360.com.ng/read-blog/32879
8th Nov 2022 Multiple Myeloma Treatment Market SWOT Analysis, Future Growth, Major Key Players, Opportunity and Forecast 2030
As per Multiple Myeloma Research Foundation, multiple myeloma generally occurs in bone marrow particularly located in the pelvic bones, spine, ribs, and the area of the hips and shoulders
0 notes
Text
Multiple Myeloma Market Segmentation Analysis, Prominent Regions, and Forecast to 2032
Multiple Myeloma is a type of blood cancer that affects plasma cells, a form of white blood cell responsible for producing antibodies. These malignant plasma cells accumulate in the bone marrow, hindering the production of healthy blood cells and damaging bones. This condition is often accompanied by symptoms like bone pain, anemia, fatigue, and kidney problems. Though the exact cause of multiple myeloma remains unclear, advancements in treatment options have significantly improved survival rates. With the development of novel therapies such as targeted drugs, immunotherapy, and stem cell transplants, patients are experiencing better outcomes and an enhanced quality of life.
The Multiple Myeloma Market size was estimated at USD 24.01 Billion In 2023 & is estimated to reach USD 59.45 Billion by 2032 and increase at a compound annual growth rate of 10.6% between 2024 and 2032.
Future Scope
The future of multiple myeloma treatment lies in personalized medicine and the use of cutting-edge therapies that target specific molecular and genetic factors of the disease. With ongoing research in immunotherapy, particularly CAR-T cell therapy and bispecific antibodies, the goal is to enhance the body’s immune response to the cancer cells, offering more effective and less toxic treatment options. Precision medicine, which tailors treatment to an individual’s genetic makeup, will continue to play a pivotal role in improving outcomes for multiple myeloma patients. Additionally, combination therapies that integrate multiple drug classes are expected to further advance the standard of care, reducing the risk of relapse and improving long-term remission rates.
Trends
One of the most significant trends in multiple myeloma treatment is the shift towards immunotherapy. This approach, which includes drugs like monoclonal antibodies and CAR-T cell therapy, enhances the immune system’s ability to target and destroy myeloma cells. Another growing trend is the use of minimal residual disease (MRD) testing, which measures the number of cancer cells remaining after treatment. MRD testing allows for more accurate monitoring of disease progression and helps tailor therapy decisions to achieve deeper remission. Additionally, advances in drug development, including the introduction of oral therapies, are making treatment more convenient for patients while maintaining efficacy.
Applications
The primary application of multiple myeloma treatments is to slow disease progression, alleviate symptoms, and improve overall survival rates. Treatments include chemotherapy, stem cell transplantation, immunotherapy, and targeted therapies like proteasome inhibitors and immunomodulatory drugs. These therapies work to reduce the number of cancer cells, manage bone damage, and prevent complications like infections. Supportive care, such as bone-strengthening treatments and pain management, plays a critical role in improving patients' quality of life. Early detection through regular monitoring and genetic testing is also key in optimizing treatment outcomes.
Key Points
Multiple Myeloma is a blood cancer that affects plasma cells and leads to symptoms like bone pain, anemia, and kidney issues.
Future treatments focus on personalized medicine, immunotherapy, and precision medicine tailored to individual genetic factors.
Trends include the rise of immunotherapy, minimal residual disease testing, and the development of more convenient oral therapies.
Treatments aim to slow disease progression, alleviate symptoms, and improve patient survival rates.
Early detection and supportive care are crucial in managing multiple myeloma effectively.
Conclusion
Multiple myeloma treatment has seen remarkable progress in recent years, with new therapies offering hope for improved survival and quality of life. As research continues, the focus on personalized and targeted treatments will drive the next wave of innovation, ensuring better outcomes for patients. With advancements in immunotherapy and precision medicine, the future looks promising for those diagnosed with this challenging condition.
0 notes
Text
Hematologic Malignancies Market Size, Share, Trends, Growth and Competitive Analysis
"Global Hematologic Malignancies Market – Industry Trends and Forecast to 2029
Global Hematologic Malignancies Market, By Type (Leukaemia, Lymphoma, Myeloma), Therapy Type (Chemotherapy, Immunotherapy, Targeted Therapy), Diagnosis (Blood Tests, Biopsy, Imaging Tests, Others), Route of Administration (Oral, Parenteral, Others), Dosage Form (Tablets, Capsules, Injections, Others), End-Users (Hospitals, Specialty Clinics, Homecare, Others), Distribution Channel (Hospital Pharmacy, Retail Pharmacy, Online Pharmacy, Others) – Industry Trends and Forecast to 2029
Access Full 350 Pages PDF Report @
**Segments**
- **Type**: The hematologic malignancies market can be segmented based on the type of malignancy, including leukemia, lymphoma, and multiple myeloma. Leukemia is a cancer of the blood cells, while lymphoma affects the lymphatic system. Multiple myeloma, on the other hand, is a cancer that forms in a type of white blood cell called a plasma cell.
- **Treatment**: Segmentation based on treatment modalities includes chemotherapy, immunotherapy, targeted therapy, stem cell transplant, and others. Chemotherapy is a common treatment for hematologic malignancies that involves the use of drugs to kill cancer cells. Immunotherapy utilizes the body's immune system to fight cancer cells, while targeted therapy focuses on specific molecules involved in cancer growth.
- **End-User**: The market can also be segmented by end-user, such as hospitals, specialty clinics, research institutes, and others. Hospitals are the primary point of care for hematologic malignancies patients, where they receive diagnosis, treatment, and follow-up care. Specialty clinics may offer specialized treatments or clinical trials for these conditions.
**Market Players**
- **Roche**: A leading player in the hematologic malignancies market, Roche offers a range of innovative therapies and diagnostic tools for leukemia, lymphoma, and multiple myeloma. The company's commitment to research and development has resulted in groundbreaking treatments that improve patient outcomes.
- **Johnson & Johnson**: With a focus on cutting-edge oncology therapies, Johnson & Johnson has made significant advancements in the treatment of hematologic malignancies. The company's portfolio includes novel drugs that target specific cancer pathways, providing new options for patients.
- **Novartis**: Known for its expertise in precision medicine, Novartis has developed several targeted therapies for hematologic malignancies. By identifying genetic mutations driving cancer growth, Novartis delivers personalized treatments that are more effective and less toxic for patients.
- **AbbVie**:AbbVie is a key player in the hematologic malignancies market, known for its strong focus on developing innovative therapies for various types of blood cancers. The company's robust pipeline includes potential treatments for leukemia, lymphoma, and multiple myeloma, leveraging cutting-edge technologies and research to address unmet medical needs in this space. AbbVie's commitment to oncology research and development has led to the introduction of novel treatment options that aim to improve patient outcomes and quality of life.
In the competitive landscape of the hematologic malignancies market, AbbVie distinguishes itself through a combination of strategic partnerships, investments in research, and a patient-centric approach to drug development. The company's collaborative efforts with academic institutions, research organizations, and other industry partners have resulted in the acceleration of novel therapeutic solutions for blood cancers. By prioritizing patient needs and engaging in meaningful dialogue with healthcare providers, AbbVie continues to shape the future of hematologic oncology with a focus on personalized medicine and targeted therapies.
AbbVie's portfolio of hematologic malignancy treatments encompasses a diverse range of modalities, including small molecule inhibitors, monoclonal antibodies, and immunotherapies. These innovative therapies target specific pathways and molecular mechanisms involved in the development and progression of blood cancers, offering new hope for patients who may not have responded to conventional treatments. By leveraging its expertise in precision medicine and biomarker-driven approaches, AbbVie continues to advance the field of hematologic oncology with a strong emphasis on tailored treatment regimens that consider individual patient characteristics and disease profiles.
In addition to its focus on drug development, AbbVie also plays a crucial role in raising awareness about hematologic malignancies and promoting early detection and diagnosis. Through educational initiatives, patient advocacy programs, and community engagement efforts, the company strives to empower patients, caregivers, and healthcare professionals with the knowledge and resources needed to effectively manage blood cancers. By fostering a culture of collaboration and knowledge-sharing, AbbVie contributes to the overall**Global Hematologic Malignancies Market Analysis**
- **Type**: The global hematologic malignancies market, segmented by type, includes leukemia, lymphoma, and multiple myeloma. With advancements in precision medicine and targeted therapies, the market is witnessing a shift towards personalized treatment regimens tailored to the specific type of malignancy, driving growth in the segment.
- **Therapy Type**: The market segmented by therapy type comprises chemotherapy, immunotherapy, and targeted therapy, among others. The rising prevalence of hematologic malignancies and the increasing adoption of novel treatment approaches are driving the demand for innovative therapies, leading to significant market growth in this segment.
- **Diagnosis**: Diagnostic modalities such as blood tests, biopsies, imaging tests, and others play a crucial role in the early detection and management of hematologic malignancies. The emphasis on early diagnosis and personalized medicine is driving the market for diagnostic tools, contributing to the overall growth of the hematologic malignancies market.
- **Route of Administration**: Different routes of administration, including oral, parenteral, and others, offer varied options for delivering hematologic malignancy treatments. The convenience and efficacy of different administration routes influence patient compliance and treatment outcomes, shaping the market dynamics in this segment.
- **Dosage Form**: The market segmented by dosage form includes tablets, capsules, injections, and others. The availability of diverse dosage forms caters to patient preferences and treatment needs, promoting adherence and enhancing the overall therapeutic outcomes in
Key points covered in the report: -
The pivotal aspect considered in the global Hematologic Malignancies Market report consists of the major competitors functioning in the global market.
The report includes profiles of companies with prominent positions in the global market.
The sales, corporate strategies and technical capabilities of key manufacturers are also mentioned in the report.
The driving factors for the growth of the global Hematologic Malignancies Market are thoroughly explained along with in-depth descriptions of the industry end users.
The report also elucidates important application segments of the global market to readers/users.
This report performs a SWOT analysis of the market. In the final section, the report recalls the sentiments and perspectives of industry-prepared and trained experts.
The experts also evaluate the export/import policies that might propel the growth of the Global Hematologic Malignancies Market.
The Global Hematologic Malignancies Market report provides valuable information for policymakers, investors, stakeholders, service providers, producers, suppliers, and organizations operating in the industry and looking to purchase this research document.
TABLE OF CONTENTS
Part 01: Executive Summary
Part 02: Scope of the Report
Part 03: Research Methodology
Part 04: Market Landscape
Part 05: Pipeline Analysis
Part 06: Market Sizing
Part 07: Five Forces Analysis
Part 08: Market Segmentation
Part 09: Customer Landscape
Part 10: Regional Landscape
Part 11: Decision Framework
Part 12: Drivers and Challenges
Part 13: Market Trends
Part 14: Vendor Landscape
Part 15: Vendor Analysis
Part 16: Appendix
Countries Studied:
North America (Argentina, Brazil, Canada, Chile, Colombia, Mexico, Peru, United States, Rest of Americas)
Europe (Austria, Belgium, Denmark, Finland, France, Germany, Italy, Netherlands, Norway, Poland, Russia, Spain, Sweden, Switzerland, United Kingdom, Rest of Europe)
Middle-East and Africa (Egypt, Israel, Qatar, Saudi Arabia, South Africa, United Arab Emirates, Rest of MEA)
Asia-Pacific (Australia, Bangladesh, China, India, Indonesia, Japan, Malaysia, Philippines, Singapore, South Korea, Sri Lanka, Thailand, Taiwan, Rest of Asia-Pacific)
Browse Trending Reports:
Thermal Imaging Cameras Market Baby Food Market Thin Film Encapsulation Market Paper Coating Materials Market Protein Engineering Market Psoriasis Treatment Market Whole Exome Sequencing Market Std Diagnostics Market Medication Delivery Systems Market Lane Keep Assist System Market Liquid Synthetic Rubber Market Mainframe Market Myxoid Round Cell Liposarcoma Drug Market Hematology Analyzer Market Low Differential Pressure Sensor Market Biofuel Enzyme Market Aroma Ingredients Market Coconut Water Market
About Data Bridge Market Research:
Data Bridge set forth itself as an unconventional and neoteric Market research and consulting firm with unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email: [email protected]"

0 notes
Text
Generic Lenalidomide: A Game Changer in Affordable Cancer Treatment
Cancer treatment has come a long way in recent years, and medications like Lenalidomide have played a crucial role in improving the quality of life and survival rates for patients with serious conditions like multiple myeloma and other blood-related cancers. However, with the success of drugs like Lenalidomide comes a hefty price tag that can create significant financial burdens for patients and their families. This is where generic Lenalidomide comes into play, offering an affordable alternative without compromising on quality or efficacy.
In this article, we’ll explore everything you need to know about generic Lenalidomide, its effectiveness, safety, and how it provides a viable solution for patients in need of life-saving treatment without the staggering costs associated with brand-name versions like Revlimid.
What Is Lenalidomide?
Lenalidomide is an immunomodulatory drug (IMiD) that works by altering the body’s immune response, helping to kill cancer cells, reduce inflammation, and prevent tumors from forming new blood vessels. It has gained approval for treating several cancers, including:
Multiple Myeloma: A type of blood cancer that affects plasma cells in the bone marrow.
Myelodysplastic Syndromes (MDS): A group of conditions that cause the body to produce abnormal blood cells.
Mantle Cell Lymphoma (MCL): A rare type of non-Hodgkin lymphoma.
Lenalidomide has become a cornerstone in the treatment of these cancers, helping to extend the lives of thousands of patients worldwide. However, like many advanced cancer therapies, the high cost of the drug has made it difficult for many to afford long-term treatment, especially in countries where healthcare coverage may be limited.
The Need for Generic Lenalidomide
The high cost of brand-name Lenalidomide (Revlimid) has been a significant barrier for patients, with monthly treatments sometimes costing tens of thousands of dollars. For patients undergoing extended therapy, these costs quickly add up, often leaving families struggling to manage the financial burden.
In 2019, the patent for Revlimid began to expire in certain regions, opening the door for generic versions of the drug to enter the market. Generic drugs are just as effective and safe as their brand-name counterparts, but they are available at a fraction of the cost.
Generic Lenalidomide provides the same level of treatment but at a more accessible price, making it a game-changer for cancer patients who otherwise might not be able to afford the medication. The availability of a generic option allows more patients to continue with their prescribed treatment regimens, improving their chances of a better outcome.
How Does Generic Lenalidomide Work?
Generic Lenalidomide works in the same way as the brand-name version by modulating the immune system. The drug performs several functions that help in the fight against cancer:
Immune System Stimulation: It enhances the immune system’s ability to detect and destroy abnormal cancer cells.
Inhibition of Angiogenesis: The drug prevents the growth of new blood vessels that tumors need to expand, essentially starving the cancer cells.
Anti-inflammatory Action: It reduces inflammation, which can be a contributing factor in cancer growth.
Induces Apoptosis: Lenalidomide can induce cancer cells to undergo apoptosis, a form of programmed cell death.
These actions make Lenalidomide a powerful tool in cancer therapy, especially in treating blood cancers such as multiple myeloma and MDS. The generic version of the drug maintains these same therapeutic benefits, offering patients a more affordable way to continue their treatment.
The Cost Difference Between Generic and Brand-Name Lenalidomide
The most significant advantage of generic Lenalidomide over brand-name Revlimid is the cost savings. In general, generic drugs are priced much lower than their brand-name counterparts. This is because the manufacturers of generics do not need to invest heavily in research, development, or marketing, as the brand-name drug already did that work. Instead, they can focus on producing a bioequivalent version of the drug, meeting the same strict regulatory standards for safety and effectiveness.
For example, a month’s supply of brand-name Revlimid can cost upwards of $20,000, depending on the dosage and location. In contrast, generic versions of Lenalidomide can be priced 50-80% lower, potentially saving patients thousands of dollars per month. This significant cost reduction makes cancer treatment more accessible to patients who might not have been able to afford it otherwise.
Is Generic Lenalidomide as Effective as Revlimid?
One of the most common concerns patients have about generic medications is whether they are as effective as the brand-name versions. The short answer is yes.
Generic Lenalidomide must meet the same stringent standards set by regulatory bodies like the U.S. Food and Drug Administration (FDA) or the European Medicines Agency (EMA). These organizations require that generic medications be bioequivalent to the original drug, meaning that they must:
Contain the same active ingredient in the same dosage.
Be administered in the same form (e.g., oral capsules).
Provide the same therapeutic benefits.
Bioequivalence ensures that the generic drug works just as well as the brand-name version, with no difference in safety, efficacy, or quality. This means that patients who switch from Revlimid to generic Lenalidomide can expect the same results in their treatment.
How to Access Generic Lenalidomide
With the increasing availability of generic Lenalidomide, patients now have more options to manage the financial aspects of their cancer treatment. There are several ways to access the drug at an affordable price:
1. International Pharmacies
Many patients turn to international pharmacies to access lower-priced generic medications. Countries like Canada and India often offer medications at a fraction of the cost compared to the U.S. Patients should ensure that they are purchasing from reputable, licensed pharmacies to guarantee the quality and safety of their medication.
2. Patient Assistance Programs
Many pharmaceutical companies and non-profit organizations offer patient assistance programs (PAPs) that provide discounts or free medications to eligible patients. These programs can help offset the cost of generic Lenalidomide for patients who meet specific financial criteria.
3. Insurance Coverage
In some cases, health insurance plans may cover the cost of generic Lenalidomide, either fully or partially. Patients should check with their insurance providers to determine what level of coverage is available for generic cancer medications.
Safety and Side Effects of Generic Lenalidomide
Like all medications, generic Lenalidomide can cause side effects. The most common side effects include:
Fatigue
Diarrhea
Rash
Low blood cell counts
Nausea
Patients should consult their healthcare providers for guidance on managing side effects and report any unusual or severe symptoms. It’s essential to follow the prescribed dosage and treatment plan closely to ensure the best possible outcomes.
Conclusion: Why Generic Lenalidomide Is a Smart Choice
The availability of generic Lenalidomide represents a major breakthrough in making cancer treatment more accessible and affordable. Patients now have a cost-effective alternative to the high-priced brand-name Revlimid without sacrificing quality or efficacy. For those battling cancers like multiple myeloma or MDS, generic Lenalidomide offers a lifeline that can make all the difference in maintaining their health and quality of life.
Switching to generic Lenalidomide can significantly reduce the financial burden on patients and their families, allowing them to focus on recovery instead of worrying about exorbitant medication costs. For more information on how to access affordable Lenalidomide, visit Generic Lenalidomide.
This game-changing alternative offers hope and relief to countless cancer patients seeking both effective treatment and financial sustainability.
0 notes
Text
Who is the Manufacturer of Pomabuzz 4 mg Caspsule in India?

Pomalidomide is manufactured in India by several pharmaceutical companies, with prominent manufacturers including Natco Pharma, Cipla, and Dr. Reddy’s Laboratories. Additionally, Chawla Medicos offers its own brand called Pomabuzz 4 mg capsule, which is specially formulated for the treatment of multiple myeloma and other medical conditions. The introduction of Pomabuzz 4 mg capsule under Chawla Medicos’ brand has further enhanced the availability of pomalidomide in the Indian market, contributing to the increased affordability and accessibility of this essential medication for patients in need.
0 notes
Text
0 notes
Text
BMS vs. Janssen: Who Will Prevail in the Race for Multiple Myeloma Treatment Dominance?

The landscape of multiple myeloma treatment has experienced unprecedented growth and innovation in recent years, thanks to advancements in therapeutic options and a deeper understanding of the disease. Among the key players in this evolving market, Bristol Myers Squibb (BMS) and Janssen Pharmaceuticals have emerged as leaders, each vying for a dominant position. This article explores their competitive strategies, product portfolios, and future prospects as they navigate the complexities of the multiple myeloma treatment market throughout this decade.
The Current Landscape of Multiple Myeloma Treatments
Multiple myeloma, characterized by the abnormal growth of plasma cells in the bone marrow, has traditionally been challenging to treat. However, the development of new therapies has significantly improved patient outcomes. The current treatment landscape includes several categories of drugs, including:
Proteasome Inhibitors: These agents, such as bortezomib and carfilzomib, disrupt the degradation of proteins in cancer cells, effectively inducing cell death.
Immunomodulatory Drugs (IMiDs): Drugs like lenalidomide and pomalidomide have shown efficacy in stimulating the immune system to fight cancer cells while simultaneously inhibiting their growth.
Monoclonal Antibodies: Agents such as daratumumab and elotuzumab are designed to specifically target myeloma cells, enhancing the immune response against them.
CAR-T Cell Therapies: Innovative treatments that engineer a patient's T cells to specifically attack cancer cells have gained traction in managing relapsed cases.
Given the increasing prevalence of multiple myeloma, driven by an aging population, the competition among pharmaceutical companies to develop effective therapies has intensified, with BMS and Janssen at the forefront.
Bristol Myers Squibb: Driving Innovation in Oncology
Bristol Myers Squibb has established itself as a formidable force in the oncology space, particularly in multiple myeloma treatment. The company's flagship drug, Revlimid (lenalidomide), remains a standard of care and has significantly improved survival rates for patients. Looking ahead, BMS is dedicated to expanding its treatment offerings through:
Advanced CAR-T Cell Therapies: BMS is pioneering CAR-T therapies, notably Abecma (idecabtagene vicleucel), which targets BCMA (B-cell maturation antigen) and has shown promise in treating patients with relapsed or refractory multiple myeloma.
Innovative Monoclonal Antibodies: The company is actively investigating new monoclonal antibodies to improve treatment outcomes and target various mechanisms of resistance.
Combination Treatment Strategies: BMS is focused on developing combination therapies to enhance efficacy and counteract treatment resistance, positioning itself well for future success.
BMS’s commitment to research and innovation is evident in its collaborations and partnerships that aim to broaden treatment access and improve patient outcomes.
Janssen Pharmaceuticals: A Comprehensive Approach to Treatment
Janssen, a subsidiary of Johnson & Johnson, is a powerful contender in the multiple myeloma treatment arena. The company’s diverse portfolio of therapies has redefined treatment options for patients. Key products include:
Darzalex (daratumumab): This pioneering monoclonal antibody has become a cornerstone therapy for multiple myeloma, providing significant survival benefits and becoming standard practice for many patients.
Ninlaro (ixazomib): An oral proteasome inhibitor that simplifies treatment regimens, enhancing patient adherence and overall satisfaction.
Carvykti (ciltacabtagene autoleucel): A newly approved CAR-T therapy that targets BCMA and offers new hope for patients with limited treatment options.
Janssen's strategic focus on clinical research and real-world evidence ensures that its therapies meet the evolving needs of patients and healthcare providers. The company is committed to exploring combination therapies to optimize treatment outcomes.
Comparative Analysis: Strengths, Weaknesses, and Market Strategies
Pipeline Robustness:
BMS boasts a strong pipeline, particularly in CAR-T cell therapies, positioning it favorably for treating relapsed cases.
Janssen's diverse approach, incorporating monoclonal antibodies and oral therapies, allows it to cater to a broader patient demographic.
Market Penetration and Access:
BMS has made significant strides in securing market access for its therapies, but faces competition from Janssen’s established distribution channels.
Janssen's vast resources enable it to reach a wide patient population, bolstering its competitive advantage.
Clinical Research and Development:
Both companies are heavily invested in clinical trials to expand indications and improve treatment efficacy. Janssen's focus on combination therapies may yield quicker results in enhancing patient outcomes.
Future Outlook: Who Will Lead the Market?
As the decade unfolds, both BMS and Janssen are poised to significantly impact the multiple myeloma treatment landscape. The competition between these two companies will be shaped by several key factors, including:
Advancements in Treatment Paradigms: The introduction of innovative therapies and combination regimens will influence clinician preferences and treatment guidelines.
Patient-Centric Approaches: Companies that effectively communicate the benefits of their therapies and ensure patient access will likely gain a competitive edge.
Regulatory Approvals: Timely approvals for new therapies and indications can drastically alter market dynamics, providing significant competitive advantages.
Conclusion
The rivalry between BMS and Janssen in the multiple myeloma treatment market is intense, with both companies poised to lead through their innovative therapies and extensive pipelines. While BMS focuses on advancing its CAR-T cell offerings, Janssen’s diverse product portfolio positions it well for continued growth. Ultimately, the next decade will be defined by advancements in treatment options, patient accessibility, and the adaptability of these companies to meet the evolving needs of patients and healthcare providers. The competition for dominance in the multiple myeloma treatment market is just beginning, and the outcome will significantly influence the future of cancer care.
#multiple myeloma#multiple myeloma Market#multiple myeloma Forecast#multiple myeloma Companies#multiple myeloma Drugs#multiple myeloma Therapies#multiple myeloma Epidemiology#multiple myeloma Pipeline#multiple myeloma Market Size#multiple myeloma Market Trends
0 notes
Text
Carfilzomib Market Overview and Regional Outlook Study 2024 – 2034
Carfilzomib Market Defination:
TheCarfilzomib Market refers to the economic and clinical landscape surrounding the pharmaceutical drug carfilzomib. Carfilzomib is a proteasome inhibitor used primarily in the treatment of multiple myeloma, a type of cancer affecting plasma cells in bone marrow. It functions by selectively inhibiting the proteasome, a complex protein-degrading machinery essential for cell function and survival. This inhibition leads to the accumulation of proteins within cancer cells, triggering cell death through apoptosis.
Print This Guide for Future Reference:https://wemarketresearch.com/reports/request-free-sample-pdf/carfilzomib-market/1493
Exploring the Carfilzomib Market: Advancements in Multiple Myeloma Treatment
In the realm of oncology, particularly in the treatment landscape of multiple myeloma, carfilzomib has emerged as a cornerstone therapy, offering new hope and improved outcomes for patients. This blog delves into the dynamic carfilzomib market, examining its impact, current trends, challenges, and future prospects.
Understanding Carfilzomib
Carfilzomib is a proteasome inhibitor approved for the treatment of relapsed or refractory multiple myeloma. It works by selectively and irreversibly binding to the 20S proteasome, disrupting protein degradation in cancer cells and inducing apoptosis. Approved by the FDA in 2012, carfilzomib has since been integrated into treatment protocols, often in combination with other agents like lenalidomide and dexamethasone.
Market Dynamics
Current Landscape: The Carfilzomib Market is driven by its efficacy in treating relapsed or refractory multiple myeloma, particularly in patients who have received prior therapies. Its mechanism of action and clinical benefits have positioned it as a valuable option in the treatment algorithm for multiple myeloma.
Treatment Advancements: Clinical studies have demonstrated that carfilzomib-based regimens prolong progression-free survival and overall survival compared to traditional therapies. Its approval marked a significant advancement in the management of multiple myeloma, offering a targeted approach to combating the disease.
Competitive Environment: Within the proteasome inhibitor class, carfilzomib competes with bortezomib and ixazomib, each offering unique profiles in terms of efficacy, safety, and administration convenience. Ongoing research aims to optimize carfilzomib’s use through novel combinations and sequencing strategies to maximize patient benefit.
Clinical Applications
Approved Indications: Carfilzomib is primarily indicated for use in combination with other agents for the treatment of relapsed or refractory multiple myeloma. Clinical trials are also exploring its potential in newly diagnosed patients and maintenance therapy settings, broadening its scope of application.
Future Directions: Research efforts are focused on expanding carfilzomib’s indications and understanding its synergies with emerging therapies such as immunomodulators, monoclonal antibodies, and cellular therapies like CAR-T cells. These endeavors aim to further improve treatment outcomes and offer personalized therapeutic approaches.
Carfilzomib Market Challenges and Opportunities
Challenges: Economic considerations remain a significant challenge in the adoption of carfilzomib, given its high cost as a biologic therapy. Managing treatment-related adverse events, such as cardiovascular complications and hematologic toxicities, also requires vigilant monitoring and proactive management strategies.
Opportunities: Advances in biomarker identification and personalized medicine offer opportunities to tailor carfilzomib-based therapies to individual patient profiles. Moreover, ongoing research into combination therapies and novel formulations aims to enhance efficacy while minimizing adverse effects, thereby improving patient adherence and outcomes.
Patient Impact and Healthcare Considerations
Patient Experience: For patients diagnosed with relapsed or refractory multiple myeloma, carfilzomib represents a crucial treatment option that can potentially extend survival and improve quality of life. Education and support programs play a vital role in helping patients manage treatment-related challenges and adhere to therapy.
Healthcare System Implications: Integrating carfilzomib into clinical practice requires healthcare providers to navigate complex treatment algorithms and ensure appropriate patient monitoring. Collaboration among multidisciplinary teams, including oncologists, hematologists, and supportive care specialists, is essential for optimizing patient care and outcomes.
Regulatory and Market Access
Regulatory Landscape: Regulatory approvals and reimbursement policies influence the accessibility of cCarfilzomib Market in different regions. Streamlining regulatory processes and demonstrating cost-effectiveness through real-world evidence are crucial for enhancing market access and patient affordability.
Market Expansion: As clinical data continues to evolve and new indications are explored, the carfilzomib market is poised for growth. Market expansion strategies should prioritize evidence-based medicine and stakeholder collaboration to drive adoption and improve patient access.
Conclusion
In conclusion, the carfilzomib market represents a significant advancement in the treatment of multiple myeloma, reflecting the transformative impact of targeted therapies in oncology. Its approval and integration into treatment protocols underscore a shift towards personalized medicine and multidisciplinary care approaches that optimize patient outcomes.
While challenges such as economic considerations and treatment-related adverse events persist, ongoing research and collaborative efforts among stakeholders are paving the way for continued innovation and improvement inCarfilzomib-Based Therapies. By addressing these challenges proactively, healthcare providers and pharmaceutical companies can ensure that carfilzomib realizes its full potential in improving the lives of patients battling multiple myeloma.
Stay informed and engaged with the latest developments in the carfilzomib market to contribute to advancements in oncology and patient-centered care.
0 notes
Text
Bortezomib Market Estimated to Witness High Growth Owing to Rising Adoption of Proteasome Inhibitors

The bortezomib market is primarily driven by high incidence and prevalence of multiple myeloma across the globe. Bortezomib, which is commonly sold under the brand name Velcade among others, is a proteasome inhibitor primarily used for the treatment of multiple myeloma and mantle cell lymphoma.
The global proteasome inhibitor drug market size is valued at approximately US$ 24.54 million in 2024 and is expected to register a CAGR of 4.7% over the forecast period of 2024-2031. The introduction of novel proteasome inhibitors and their increasing adoption in the treatment of cancer are the major factors anticipated to propel market growth. Key Takeaways
Key players operating in the bortezomib market are Hikma Pharmaceuticals, Pfizer, Meitheal Pharmaceuticals, Novartis International AG, Bristol Myers Squibb, NATCO Pharma, Teva Pharmaceuticals, Dr. Reddy's Laboratories, Gland Pharma, Shilpa Medicare, Qilu Pharmaceutical, Scion Pharmaceuticals, Farmhispania Group, Coresyn, Chem-Stone (Guangzhou), Hubei Honch Pharmaceutical, Vinkem Labs, Icrom, TAPI Teva, and Chengdu Aslee Biopharmaceuticals.
The introduction of generic versions of Bortezomib Market Demand has led to increased adoption and lowered treatment costs. Moreover, ongoing clinical trials evaluating the efficacy of bortezomib in other cancer indications are expected to expand the eligible patient pool. Technological advancements in proteasome inhibitor development focused on overcoming resistance, reducing toxicity, and novel delivery systems are further anticipated to support market growth. Market Drivers
The primary factors driving the growth of the global bortezomib market include rising prevalence of multiple myeloma globally, increasing adoption of proteasome inhibitors in treatment regimens, availability of generic versions, and ongoing clinical research evaluating the efficacy of bortezomib in other cancer indications. Additionally, improving healthcare infrastructure and expenditures in emerging economies will further support the market growth during the forecast period. Current challenges in Bortezomib Market
The Bortezomib Market Size And Trends faces several challenges primarily due to the presence of alternative therapeutic options for treating multiple myeloma (MM). Some of the major challenges include increasing generic competition from drugs like ixazomib and daratumumab which are leading to lower sales of bortezomib drugs. Further, the patents of bortezomib drugs have expired in several regions making them available in generic forms at lower costs. This increasing availability of low-cost generics is a major challenge faced by innovator bortezomib drug companies. Additionally, the adverse side effects associated with bortezomib drugs like neuropathy and thrombocytopenia require close patient monitoring during treatment posing operational challenges. Stringent regulations for drug approval is another regulatory challenge for new market entrants. SWOT Analysis
Strength: Well-established drug with proven efficacy and safety profile in treating MM. It was the first proteasome inhibitor approved and remains a standard of care. Weakness: Patent expiry has led to availability of low-cost generics reducing sales of innovator brands. Further, it causes serious side effects like neuropathy requiring cautious use. Opportunity: Emerging economies with growing cancer burden and healthcare spending present an opportunity. Combination therapies with other anti-MM drugs can boost its use further. Threats: Increasing competition from newer oral proteasome inhibitors and monoclonal antibody based therapies poses pricing and market share threats. Stringent regulations for approval delays market entry of new players.
Geographical regions with high market concentration
In terms of value, North America accounts for the largest share of over 40% of the global bortezomib market led by the US. This is due to established healthcare infrastructure and higher adoption of innovative therapies. Europe is the second major regional market with a value share of over 30% supported by favourable reimbursement policies. The Asia Pacific region is projected to be the fastest growing market during the forecast period due to rising healthcare expenditure, growing cancer incidence and increasing demand for cancer treatments from middle-income countries like China and India. Fastest growing geographical region
The Asia Pacific region is poised to exhibit the highest growth rate during the forecast period in the global bortezomib market. This is attributed to rising disposable incomes, growing awareness about cancer treatments, expansion of healthcare facilities and increasing private sector investment in pharmaceutical research in emerging economies like China and India. Large patient pools undergoing cancer treatment in Asia present lucrative opportunities for bortezomib drug makers looking to tap high future growth potential in this region. Get More Insights On, Bortezomib Market For More Insights Discover the Report In language that Resonates with you French, German, Italian, Russian, Chinese, Korean About Author: Ravina Pandya, Content Writer, has a strong foothold in the market research industry. She specializes in writing well-researched articles from different industries, including food and beverages, information and technology, healthcare, chemical and materials, etc. (https://www.linkedin.com/in/ravina-pandya-1a3984191)
#Bortezomib Market Demand#Bortezomib Market Size#Bortezomib Market Trends#Bortezomib Market Forcast#Bortezomib#Bortezomib Market
0 notes
Text
Velcade Market Size, Share, Industry Trends, and Forecast 2032
Introduction
Velcade, also known by its generic name bortezomib, is a proteasome inhibitor used primarily in the treatment of multiple myeloma and mantle cell lymphoma. Since its approval, Velcade has significantly impacted the oncology market, offering a promising therapeutic option for patients with these malignancies. The global Velcade market is characterized by its dynamic nature, driven by advancements in oncology research, increasing incidence of hematologic cancers, and a growing focus on personalized medicine.
Market Size and Growth Dynamics
Velcade Market Size was estimated at 1.76 (USD Billion) in 2023. The Velcade Market Industry is expected to grow from 1.83(USD Billion) in 2024 to 2.5 (USD Billion) by 2032. The Velcade Market CAGR (growth rate) is expected to be around 4.01% during the forecast period (2024 - 2032). This growth is fueled by the rising prevalence of multiple myeloma and other related cancers, which are increasingly being diagnosed due to advances in diagnostic technologies. Furthermore, the aging population, which is more susceptible to cancer, is also contributing to the expanding market.
North America holds the largest market share, attributed to the region's advanced healthcare infrastructure, high adoption rates of new therapies, and a strong focus on research and development. Europe follows closely, driven by similar factors. However, the Asia-Pacific region is expected to witness the fastest growth during the forecast period, spurred by increasing healthcare expenditures, improving access to cancer treatments, and rising awareness about hematologic cancers.
Market Share Analysis
Velcade, originally developed by Millennium Pharmaceuticals and marketed by Takeda Oncology, has maintained a dominant position in the proteasome inhibitor segment. Its efficacy, safety profile, and first-mover advantage have contributed to its strong market share. However, with the expiration of key patents, the market has seen the entry of generic versions, which has led to increased competition and a shift in market dynamics.
The introduction of generics has made the treatment more accessible, particularly in developing regions, but it has also put pressure on the market share of branded Velcade. Despite this, the brand continues to hold a significant share due to its established presence and the trust it has garnered among healthcare professionals.
Industry Trends
Several key trends are shaping the Velcade market:
Rise of Combination Therapies: The use of Velcade in combination with other drugs, such as lenalidomide and dexamethasone, is becoming increasingly common. These combination therapies have shown improved efficacy and are becoming a standard of care in multiple myeloma treatment protocols.
Focus on Personalized Medicine: With the growing emphasis on personalized medicine, there is a trend towards tailoring treatments based on individual patient profiles, which includes genetic makeup and disease characteristics. This approach is expected to drive demand for Velcade as part of personalized treatment regimens.
Increased Research and Development: The oncology sector continues to see significant investment in research and development, leading to the discovery of new therapeutic targets and treatment options. While this fosters innovation, it also intensifies competition as new drugs enter the market.
Patent Expirations and Generic Competition: The expiration of Velcade’s patents has opened the market to generic competition, leading to reduced prices and increased accessibility. This trend is expected to continue, particularly in cost-sensitive markets.
Expanding Indications: Research is ongoing to explore the potential of Velcade in treating other cancers and conditions beyond multiple myeloma and mantle cell lymphoma. If successful, these efforts could lead to new indications, further driving market growth.
Forecast Through 2032
The Velcade Market CAGR (growth rate) is expected to be around 4.01% during the forecast period (2024 - 2032). Key drivers of this growth include the increasing global cancer burden, advancements in cancer therapy, and the expansion of healthcare infrastructure in emerging markets.
However, challenges such as the rising cost of cancer treatment, the availability of alternative therapies, and regulatory hurdles may pose risks to market expansion. Additionally, the shift towards biosimilars and generics is likely to impact the market dynamics, particularly in terms of pricing and market share distribution.
The forecast period is also expected to witness a greater emphasis on real-world evidence and outcome-based reimbursement models, which could influence the adoption of Velcade and its competitors.
Conclusion
The Velcade market is poised for significant growth in the coming years, driven by a combination of factors including rising cancer incidence, advancements in treatment protocols, and the expanding reach of healthcare services. While challenges such as generic competition and pricing pressures exist, the overall outlook remains positive, with opportunities for growth in emerging markets and through the development of new therapeutic indications. As the oncology landscape continues to evolve, Velcade is expected to remain a key player, contributing to improved outcomes for patients worldwide.
0 notes
Text
Global amyloidosis therapeutic treatment market is expected to develop at a compound annual growth rate (CAGR) of 7.60%, from its estimated USD 2251.2 million in 2023 to USD 4352.35 million in 2032.Amyloidosis is a rare and complex group of diseases characterized by the abnormal accumulation of amyloid proteins in tissues and organs. These proteins can lead to severe organ damage and potentially life-threatening complications. The therapeutic treatment market for amyloidosis is evolving rapidly, driven by advancements in medical research, the development of new drugs, and increasing awareness of this challenging condition.
Browse the full report at https://www.credenceresearch.com/report/amyloidosis-therapeutic-treatment-market
Market Overview
The global amyloidosis therapeutic treatment market is experiencing significant growth, fueled by a combination of rising incidence rates, expanding research initiatives, and increasing healthcare expenditures. The market encompasses a range of therapeutic options, including pharmacological treatments, supportive therapies, and emerging novel therapies.
Pharmacological Treatments
Pharmacological treatment options for amyloidosis primarily target the underlying cause of the disease or aim to alleviate symptoms. The market is currently dominated by drugs that target the specific types of amyloidosis:
1. AL Amyloidosis: This form of amyloidosis results from abnormal immunoglobulin light chains and is often associated with multiple myeloma. The treatment approach involves managing the underlying plasma cell disorder. Therapies include proteasome inhibitors such as Bortezomib and Carfilzomib, immunomodulatory drugs like Lenalidomide, and monoclonal antibodies such as Daratumumab. These drugs have shown efficacy in reducing the production of amyloidogenic light chains and improving patient outcomes.
2. ATTR Amyloidosis: Caused by the accumulation of transthyretin protein, ATTR amyloidosis is further classified into hereditary (hATTR) and wild-type (wtATTR) forms. The therapeutic landscape includes: - Tafamidis: This drug stabilizes the transthyretin protein, preventing its misfolding and aggregation. Tafamidis has demonstrated significant benefits in slowing disease progression and improving quality of life for patients with ATTR amyloidosis. - Diflunisal: An older non-steroidal anti-inflammatory drug, Diflunisal has been repurposed for ATTR amyloidosis treatment due to its ability to stabilize transthyretin. - Gene Silencing Therapies: Emerging treatments such as Patisiran and Inotersen use RNA interference and antisense oligonucleotides to reduce the production of transthyretin. These therapies have shown promise in clinical trials and represent a significant advancement in the treatment of ATTR amyloidosis.
Supportive Therapies
Supportive therapies play a crucial role in managing the symptoms and complications of amyloidosis. These include symptomatic management of heart failure, renal impairment, and neuropathy. For instance, diuretics and antihypertensive agents are commonly used to manage cardiac amyloidosis, while dialysis may be required for patients with renal involvement. Pain management and physical therapy are also essential for addressing neuropathic symptoms.
Emerging Therapies and Research
The amyloidosis therapeutic treatment market is witnessing a surge in research and development activities aimed at discovering innovative treatments. Key areas of focus include:
1. Monoclonal Antibodies: Researchers are exploring the use of monoclonal antibodies targeting amyloid deposits directly or modulating the immune system to enhance amyloid clearance.
2. Small Molecules: New small molecules are being developed to disrupt amyloid fibril formation or promote the disaggregation of existing fibrils. These compounds have the potential to offer new treatment options for various forms of amyloidosis.
3. Gene Therapy: Advances in gene therapy hold promise for addressing the genetic basis of hereditary amyloidosis. By correcting or replacing faulty genes, these therapies could potentially prevent or cure the disease.
Challenges and Opportunities
Despite the progress in amyloidosis treatment, several challenges remain. The rarity of the disease can lead to difficulties in diagnosis and treatment, and the high cost of innovative therapies can be a barrier to access for many patients. Additionally, the complexity of amyloidosis requires a multidisciplinary approach to manage the diverse manifestations of the disease effectively.
However, the growing investment in research and development, coupled with advancements in personalized medicine, presents significant opportunities for improving patient outcomes. Continued innovation and collaboration among researchers, healthcare providers, and pharmaceutical companies are essential to overcoming these challenges and advancing the treatment landscape for amyloidosis.
Key Players
Prothena Corporation Plc.
Eidos Therapeutics
Pfizer Inc.
SOM Biotech
Corino Therapeutics
Johnson and Johnson Services, Inc.
AstraZeneca Plc.
Alnylam Pharmaceuticals, Inc.
GlaxoSmithKline, Plc.
Others
Segmentation
By Type of Amyloidosis
AL Amyloidosis (Primary Amyloidosis)
ATTR Amyloidosis (Hereditary and Wild-Type)
AA Amyloidosis (Secondary Amyloidosis)
By Treatment Modalities
Chemotherapy
Immunomodulatory Drugs (IMiDs)
Monoclonal Antibodies
TTR Stabilizers
RNA Interference (RNAi) Therapies
Liver Transplantation
Supportive Care
By Disease Severity
Newly Diagnosed Patients
Relapsed or Refractory Disease
Advanced Disease
By Region
North America
The U.S.
Canada
Mexico
Europe
Germany
France
The U.K.
Italy
Spain
Rest of Europe
Asia Pacific
China
Japan
India
South Korea
South-east Asia
Rest of Asia Pacific
Latin America
Brazil
Argentina
Rest of Latin America
Middle East & Africa
GCC Countries
South Africa
Rest of Middle East and Africa
Browse the full report at https://www.credenceresearch.com/report/amyloidosis-therapeutic-treatment-market
About Us:
Credence Research is committed to employee well-being and productivity. Following the COVID-19 pandemic, we have implemented a permanent work-from-home policy for all employees.
Contact:
Credence Research
Please contact us at +91 6232 49 3207
Email: [email protected]
Website: www.credenceresearch.com
0 notes
Text
8th Nov 2022 Multiple Myeloma Treatment Market SWOT Analysis, Future Growth, Major Key Players, Opportunity and Forecast 2030
As per Multiple Myeloma Research Foundation, multiple myeloma generally occurs in bone marrow particularly located in the pelvic bones, spine, ribs, and the area of the hips and shoulders
0 notes
Text
Globalization and Market Expansion in Multiple Myeloma

The Multiple Myeloma Market size was estimated at USD 24.01 Billion In 2023 & is estimated to reach USD 59.45 Billion by 2032 and increase at a compound annual growth rate of 10.6% between 2024 and 2032.The Multiple Myeloma market is characterized by a dynamic interplay of research, treatment advancements, and patient-centric care initiatives. As one of the most prevalent hematologic cancers, it continuously draws attention from pharmaceutical innovators and healthcare providers alike. Recent years have witnessed a surge in targeted therapies, immunotherapies, and personalized medicine approaches tailored to combatting its complexities. This evolving landscape not only fosters competition among biopharmaceutical companies but also emphasizes the importance of early detection and multidisciplinary treatment strategies. With ongoing clinical trials promising novel therapeutic avenues, the Multiple Myeloma market remains poised for further breakthroughs in extending patient survival and enhancing quality of life.
The Multiple Myeloma Market research study for the term also includes a variety of business opportunities and growth potential. A business plan detailing market risks and constraints as well as the effects of various regulatory regimes is given to executives by the market research. This is carried out to assist companies in reaching their main goals and making better judgments.
Get Sample Copy Of This Report @ https://www.snsinsider.com/sample-request/3490
Market Segmentation
By Type
Chemotherapy
Monoclonal Antibody
Protease Inhibitors
Others
By Disease Type
Smoldering Multiple Myeloma
Active Multiple Myeloma
By End User
Clinics
Hospitals
Others
Regional Outlook
The geographical categories that make up the Multiple Myeloma Market each have their own revenue, market share, sales, and growth rates. Among the important geographical areas covered in the market analysis are Europe, Asia-Pacific, South America, North America, and the Middle East and Africa. Latin America is expected to have a small market share in value, while North America is forecast to maintain its global leadership position and have a significant market share in both volume and value.
COVID-19 Impact Analysis
In the first half of 2020, the COVID-19 virus started to spread over the world, infecting millions of people and forcing major nations to implement work stoppage and foot restrictions. Nearly every area of the economy has suffered, with the exception of medical goods and equipment for life support, including the Multiple Myeloma Market .
Competitive Landscape
The competitive analysis section of the global Multiple Myeloma Market offers details and insights on the participants. Among the details provided are information on competition, a market overview by business status, and revenue projections by region. These businesses use a variety of strategies, such as product launches, partnerships, alliances, technology advancements, and contracts, to boost market income.
Conclusion
The market research is supported by first-hand experience, qualitative and quantitative analysis by industry analysts, and comments from key market players and actors in the value chain. The study investigates parent industry trends, micro and macroeconomic data, governing factors, and market attractiveness on a segment-by-segment basis. The study also illustrates how different market factors might have a qualitative impact on market segmentation based on geography and Multiple Myeloma Market.
About Us
SNS Insider is a market research and insights firm that has won several awards and earned a solid reputation for service and strategy. We are a strategic partner who can assist you in reframing issues and generating answers to the trickiest business difficulties. For greater consumer insight and client experiences, we leverage the power of experience and people.
When you employ our services, you will collaborate with qualified and experienced staff. We believe it is crucial to collaborate with our clients to ensure that each project is customized to meet their demands. Nobody knows your customers or community better than you do. Therefore, our team needs to ask the correct questions that appeal to your audience in order to collect the best information.
Related Reports
Flash Chromatography Market Size
Cystic Fibrosis Market Size
Cancer Biopsy Market Size
Glaucoma Therapeutics Market Size
Genomics Services Market Size
0 notes
Text
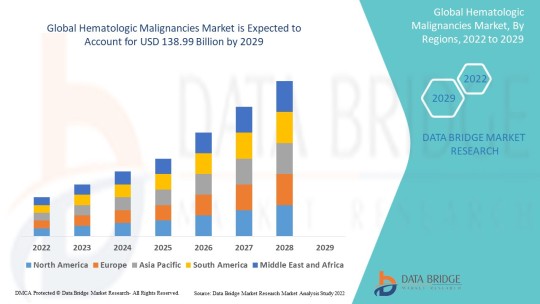
Hematologic Malignancies Market Size, Share, Trends, Growth and Competitive Analysis
"Global Hematologic Malignancies Market – Industry Trends and Forecast to 2029
Global Hematologic Malignancies Market, By Type (Leukaemia, Lymphoma, Myeloma), Therapy Type (Chemotherapy, Immunotherapy, Targeted Therapy), Diagnosis (Blood Tests, Biopsy, Imaging Tests, Others), Route of Administration (Oral, Parenteral, Others), Dosage Form (Tablets, Capsules, Injections, Others), End-Users (Hospitals, Specialty Clinics, Homecare, Others), Distribution Channel (Hospital Pharmacy, Retail Pharmacy, Online Pharmacy, Others) – Industry Trends and Forecast to 2029
Access Full 350 Pages PDF Report @
**Segments**
- Leukemia: Leukemia, a type of hematologic malignancy, is characterized by the rapid production of abnormal white blood cells in the bone marrow, leading to complications in the immune system's function. The leukemia segment is significant in the hematologic malignancies market, with a high prevalence globally. Factors such as genetic predisposition, exposure to radiation, and certain chemotherapy drugs contribute to the development of leukemia.
- Lymphoma: Lymphoma is another key segment in the hematologic malignancies market, affecting the lymphatic system and lymphoid tissues. There are two main types of lymphoma: Hodgkin lymphoma and non-Hodgkin lymphoma. Hodgkin lymphoma is characterized by the presence of Reed-Sternberg cells, while non-Hodgkin lymphoma comprises a diverse group of lymphomas with varying characteristics and prognosis. The lymphoma segment is witnessing advancements in treatment options, including immunotherapy and targeted therapies.
- Myeloma: Multiple myeloma is a type of hematologic malignancy that affects plasma cells in the bone marrow. This segment of the market is characterized by the abnormal production of monoclonal proteins, leading to bone damage, renal complications, and other symptoms. The myeloma segment has seen significant progress in treatment modalities, including proteasome inhibitors, immunomodulatory drugs, and monoclonal antibodies. The market for myeloma therapies continues to expand, with a focus on improving patient outcomes and quality of life.
**Market Players**
- Roche: Roche is a prominent player in the hematologic malignancies market, offering a range of innovative therapies for leukemia, lymphoma, and myeloma. The company's portfolio includes targeted therapies, immunotherapies, and personalized medicine options for patients with hematologic malignancies. Roche invests heavily in research and development to introduce novel treatments and improve existing standards of care for these conditions.
- Novartis: Novartis isIn the competitive landscape of the hematologic malignancies market, Roche and Novartis stand out as key market players with a significant presence and impact on the industry. Roche, a Swiss multinational healthcare company, has established itself as a leader in providing innovative therapies for leukemia, lymphoma, and myeloma. With a diverse portfolio that includes targeted therapies, immunotherapies, and personalized medicine options, Roche continues to drive advancements in treatment options for patients with hematologic malignancies. The company's strong focus on research and development enables it to introduce novel treatments that address unmet medical needs and improve patient outcomes in this complex and challenging disease area.
Novartis, another major player in the hematologic malignancies market, has made substantial contributions to advancing the field of oncology with its portfolio of innovative therapies. The company's commitment to developing cutting-edge treatments for leukemia, lymphoma, and myeloma has made it a key player in the market. Novartis's emphasis on precision medicine and personalized treatment approaches has led to the development of targeted therapies that aim to improve the efficacy and safety profiles of treatments for hematologic malignancies. By investing in research and collaborations with key stakeholders in the healthcare ecosystem, Novartis continues to drive progress in addressing the evolving needs of patients with these complex diseases.
Both Roche and Novartis play a vital role in shaping the hematologic malignancies market through their focus on innovation, research, and patient-centric approaches to therapy development. These companies leverage their expertise in oncology and biotechnology to bring forward novel treatment options that have the potential to transform the standard of care for patients with leukemia, lymphoma, and myeloma. In addition to developing new therapies, Roche and Novartis also engage in strategic partnerships, regulatory initiatives, and patient advocacy efforts to drive awareness, access, and affordability of hematologic malignancy treatments on a global scale.
As the landscape of hematologic malignancies continues to evolve with advancements in technology**Segments:**
- Leukemia: Leukemia, a type of hematologic malignancy, is a significant segment in the market due to its high prevalence globally and the complexities associated with the rapid production of abnormal white blood cells. Factors such as genetic predisposition and exposure to certain chemicals play a role in the development of leukemia. The market for leukemia treatments is driven by continuous research and development efforts to improve patient outcomes and quality of life.
- Lymphoma: Lymphoma, affecting the lymphatic system, comprises Hodgkin lymphoma and non-Hodgkin lymphoma. Advancements in treatment options, including immunotherapy and targeted therapies, have significantly impacted the lymphoma market. The focus on personalized medicine and precision therapies is shaping the future of lymphoma treatment, with a strong emphasis on improving therapeutic efficacy and reducing adverse effects for patients.
- Myeloma: Multiple myeloma, characterized by the abnormal production of monoclonal proteins, poses challenges such as bone damage and renal complications. The myeloma segment has witnessed remarkable progress in treatment modalities, with the introduction of novel therapies such as proteasome inhibitors and monoclonal antibodies. The market for myeloma therapies is expanding, driven by the need to address unmet medical needs and enhance patient care.
**Global Hematologic Malignancies Market:** - By Type: Leukemia, Lymphoma, Myeloma - Therapy Type: Chemotherapy, Immunotherapy, Targeted Therapy
Key points covered in the report: -
The pivotal aspect considered in the global Hematologic Malignancies Market report consists of the major competitors functioning in the global market.
The report includes profiles of companies with prominent positions in the global market.
The sales, corporate strategies and technical capabilities of key manufacturers are also mentioned in the report.
The driving factors for the growth of the global Hematologic Malignancies Market are thoroughly explained along with in-depth descriptions of the industry end users.
The report also elucidates important application segments of the global market to readers/users.
This report performs a SWOT analysis of the market. In the final section, the report recalls the sentiments and perspectives of industry-prepared and trained experts.
The experts also evaluate the export/import policies that might propel the growth of the Global Hematologic Malignancies Market.
The Global Hematologic Malignancies Market report provides valuable information for policymakers, investors, stakeholders, service providers, producers, suppliers, and organizations operating in the industry and looking to purchase this research document.
TABLE OF CONTENTS
Part 01: Executive Summary
Part 02: Scope of the Report
Part 03: Research Methodology
Part 04: Market Landscape
Part 05: Pipeline Analysis
Part 06: Market Sizing
Part 07: Five Forces Analysis
Part 08: Market Segmentation
Part 09: Customer Landscape
Part 10: Regional Landscape
Part 11: Decision Framework
Part 12: Drivers and Challenges
Part 13: Market Trends
Part 14: Vendor Landscape
Part 15: Vendor Analysis
Part 16: Appendix
Countries Studied:
North America (Argentina, Brazil, Canada, Chile, Colombia, Mexico, Peru, United States, Rest of Americas)
Europe (Austria, Belgium, Denmark, Finland, France, Germany, Italy, Netherlands, Norway, Poland, Russia, Spain, Sweden, Switzerland, United Kingdom, Rest of Europe)
Middle-East and Africa (Egypt, Israel, Qatar, Saudi Arabia, South Africa, United Arab Emirates, Rest of MEA)
Asia-Pacific (Australia, Bangladesh, China, India, Indonesia, Japan, Malaysia, Philippines, Singapore, South Korea, Sri Lanka, Thailand, Taiwan, Rest of Asia-Pacific)
Browse Trending Reports:
Thermal Imaging Cameras Market Baby Food Market Thin Film Encapsulation Market Paper Coating Materials Market Protein Engineering Market Psoriasis Treatment Market Whole Exome Sequencing Market Std Diagnostics Market Medication Delivery Systems Market Lane Keep Assist System Market Liquid Synthetic Rubber Market Mainframe Market Myxoid Round Cell Liposarcoma Drug Market Hematology Analyzer Market Low Differential Pressure Sensor Market Biofuel Enzyme Market Aroma Ingredients Market Coconut Water Market
About Data Bridge Market Research:
Data Bridge set forth itself as an unconventional and neoteric Market research and consulting firm with unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email: [email protected]"
0 notes
Text
Selecting the Best Pure Turmeric Powder: A Comprehensive Guide
Turmeric is a golden-yellow spice used for enhancing flavour, colour and nutrition to your food. There are several health benefits of turmeric powder as it boosts your immunity, improves your brain function and kidney health, control cholesterol and anxiety. Some studies suggest that it is also used for cancer treatment (pancreatic cancer, prostate cancer and multiple myeloma). Ancient people have been using turmeric for ages as it has several medicinal uses.

Choosing the right quality of turmeric powder is very important these days as a lot of adulterated qualities are available in the market. Irrespective of your uses, it is important that you choose the right pure turmeric powder. Below are some useful guidelines that will help you to choose the right quality of pure turmeric powder.
It is very important to analyse the source of raw materials when it comes to pure turmeric powder procurement. Go for brands who source their raw materials from well-known farms and follow organic practices.
Certifications does play a major role in understanding the quality of turmeric powder. Look for organic certified brands as they ensure that the turmeric powder you are buying is free from chemicals and pesticides. Check the packaging of the band if they have USDA organic, EU Organic or India organic logo.
Identification of pure turmeric powder can be determined by checking the colour and aroma. Pure turmeric powder is golden yellow colour and has a unique earthy aroma whereas inferior quality turmeric powder looks dull and have a mouldy aroma.
Pure turmeric powder can be determined by testing the presence of chemicals present in it. Mix one tablespoon of turmeric powder in a glass of water and dissolve it. If the waters turns pale yellow and the powder settles at the bottom, it is the right quality but if the water turns dark yellow then it is an adulterated quality.
Pure turmeric powder can also be identified by the curcumin percentage which is an active compound present in it. Curcumin in the main compound present in turmeric which has numerous health benefits. Look for the curcumin content embedded on the labels as high content indicates higher quality.
Cross check the packaging of the turmeric powder if it is properly sealed to ensure its freshness and reliability.
Before purchasing your turmeric powder give some time to read the reviews from other customers as their experiences can provide you valuable insights about the effectiveness and quality of the product.
Although price is not the sole factor albeit excessively cheap turmeric powder may be indicative of low quality. You should invest in a product that offers value for money and meets your required quality standards.
If you follow the above tips then you can confidently choose the right and pure turmeric powder that would suffice your requirements.
Although there are several organic turmeric powder manufacturers in India, Veerral Agro Tech/Kisan Agro leads the industry and convers all your needs. Every batch of organic turmeric powder supplied by Veerral Agro Tech/Kisan Agro is carefully manufactured and passes a stringent quality check to ensure highest level of customer satisfaction.
We not just provide you the quality of top notch but also ensure that the prices you get is highly competitive in the market. Seeking for a reliable organic turmeric powder manufacturers in India, visit us at www.viralspices.com and get your needs covered.
#Turmeric Fingers and Powder#Pure Turmeric Powder#how to choose pure turmeric powder#tips to choose pure turmeric powder
0 notes
Text
0 notes