#Multiple Myeloma Clinical Trial Market
Explore tagged Tumblr posts
Text
Accelerating Research: Key Trends and Insights in Multiple Myeloma Clinical Trial Market
Accelerating research in the multiple myeloma clinical trial market is crucial for improving patient outcomes and advancing our understanding of the disease. Several key trends and insights have emerged in recent years that are shaping the landscape of multiple myeloma clinical trials:
Targeting High-Risk Disease: High-risk multiple myeloma, characterized by genetic abnormalities and poor prognosis, has been a focus of clinical trials. Researchers are investigating novel therapies specifically designed to target high-risk features, such as genetic mutations or cytogenetic abnormalities, in order to improve outcomes for these patients.
Immunotherapy Advancements: Immunotherapy has revolutionized the treatment of multiple myeloma. Chimeric antigen receptor (CAR) T-cell therapy and immune checkpoint inhibitors have demonstrated significant efficacy in clinical trials, leading to their approval for certain patient populations. Ongoing trials explore optimizing immunotherapy combinations, overcoming resistance mechanisms, and extending the benefits to more patients.
Minimal Residual Disease (MRD) Assessment: MRD refers to the small number of cancer cells that may remain in a patient's body after treatment, even when they are undetectable using standard tests. MRD assessment has gained importance in multiple myeloma clinical trials as a measure of treatment response and prognostic indicator. Trials are evaluating MRD-directed treatment strategies to achieve deeper and more durable responses.
Next-Generation Sequencing (NGS): The advent of NGS technologies has enabled comprehensive genomic profiling in multiple myeloma clinical trials. These trials aim to identify genetic alterations, mutations, and molecular subtypes that can guide treatment decisions and facilitate the development of targeted therapies. NGS is also used to monitor disease progression and detect mechanisms of resistance.
Novel Drug Combinations: Combination therapies are a cornerstone of multiple myeloma treatment. Clinical trials are exploring innovative combinations of targeted therapies, immunotherapies, and traditional agents to enhance treatment efficacy and overcome resistance. These trials are driven by preclinical research and a deeper understanding of the complex biology of multiple myeloma.
Real-Time Data Monitoring: The integration of real-time data monitoring and adaptive trial designs is gaining traction in multiple myeloma clinical trials. This approach allows for the modification of trial protocols based on emerging data, enabling faster decision-making and more efficient allocation of resources. Adaptive designs enhance trial flexibility, facilitate patient-centric approaches, and accelerate the drug development process.
Patient-Centric Approaches: Patient-centricity is increasingly emphasized in multiple myeloma clinical trials. This includes incorporating patient-reported outcomes, quality of life assessments, and patient preferences in trial design and treatment decision-making. Patient advocacy groups and patient engagement initiatives play a vital role in shaping research priorities and improving trial participation.
Global Collaboration: Collaborative efforts between researchers, pharmaceutical companies, regulatory authorities, and patient advocacy groups have expanded in the multiple myeloma clinical trial space. These collaborations facilitate the sharing of resources, data, and expertise, leading to more comprehensive and impactful research outcomes.
Real-World Evidence (RWE) Integration: Real-world evidence, derived from observational studies, patient registries, and electronic health records, is increasingly recognized for its value in complementing clinical trial data. RWE provides insights into treatment patterns, long-term outcomes, and comparative effectiveness, contributing to a more holistic understanding of multiple myeloma and informing clinical trial design.
Innovative Trial Designs: To streamline the drug development process, novel trial designs such as basket trials, umbrella trials, and platform trials are being utilized in multiple myeloma research. These designs allow for the evaluation of multiple treatments or patient subgroups simultaneously, reducing costs and accelerating the identification of effective therapies.
For more regional insights into the Multiple Myeloma (Kahler Disease) clinical trials market, download a free report sample
In conclusion, accelerating research in the multiple myeloma clinical trial market is driven by targeting high-risk disease, advancements in immunotherapy, MRD assessment, NGS, novel drug combinations, real-time data monitoring, patient-centric approaches, global collaboration, RWE integration, and innovative trial designs. These trends and insights are shaping the landscape of multiple myeloma research and have the potential to significantly improve patient outcomes in the future.
0 notes
Text
CAR-T Market Size, Target Population, Competitive Landscape, and Market Forecast to 2034
Introduction to CAR-T Cell Therapy
Chimeric Antigen Receptor T-cell (CAR-T) therapy has revolutionized cancer treatment by using genetically modified T cells to target and destroy cancer cells. Its potential to offer durable remissions for patients with otherwise incurable malignancies, particularly hematologic cancers, has driven significant growth and interest in this market. By 2034, the CAR-T therapy market is anticipated to witness robust expansion, driven by technological advancements, expanded indications, and increased global accessibility.
CAR-T Market Size and Growth Projections
The global CAR-T therapy market is projected to grow at a compound annual growth rate (CAGR) exceeding 25% through 2034, reaching multi-billion-dollar valuations. This growth is fueled by the approval and commercialization of novel therapies, expansion into solid tumors, and increased adoption in both developed and emerging markets.
Regions such as North America and Europe currently dominate the market due to established healthcare systems and regulatory frameworks. However, significant growth is anticipated in Asia-Pacific regions, where unmet needs and rising healthcare investments present a lucrative opportunity for market players.
Read more about CAR-T Market @ https://www.delveinsight.com/report-store/car-t-market-forecast
Key factors influencing CAR-T market growth include:
1. Expanding Indications: Initially approved for specific hematologic cancers like acute lymphoblastic leukemia (ALL) and diffuse large B-cell lymphoma (DLBCL), CAR-T therapies are now being developed for solid tumors and autoimmune diseases.
2. Technological Advancements: Innovations in CAR design, such as dual-targeting CARs and allogeneic CAR-T cells, are addressing limitations like relapse and toxicity.
3. Supportive Policy Frameworks: Governments and healthcare agencies are increasing funding and policy support to expand access to CAR-T therapies.
CAR-T Target Population
The primary target population for CAR-T therapies comprises patients with refractory or relapsed hematologic malignancies. These include:
1. Hematologic Cancers: ALL, DLBCL, multiple myeloma (MM), and mantle cell lymphoma (MCL) dominate current indications.
2. Expanding to Solid Tumors: Despite challenges, CAR-T therapies targeting glioblastoma, colorectal cancer, and ovarian cancer are in various stages of clinical development.
3. Non-Cancer Indications: Early research into autoimmune diseases and chronic viral infections signals a broader patient base for CAR-T therapies.
As clinical trials expand the therapeutic scope, the addressable patient population is expected to rise significantly by 2034. This includes previously untreatable conditions and younger patients benefiting from safer, next-generation therapies.
Download sample pages @ https://www.delveinsight.com/sample-request/car-t-market-forecast
CAR-T Market Competitive Landscape
The CAR-T therapy market is highly competitive, with both established pharmaceutical giants and emerging biotechs driving innovation. Key players include:
1. Novartis: The first company to receive FDA approval for a CAR-T therapy (Kymriah) in 2017, Novartis continues to explore label expansions and optimize manufacturing.
2. Gilead Sciences: Through its Kite Pharma subsidiary, Gilead has gained approvals for Yescarta and Tecartus, cementing its position in the lymphoma treatment space.
3. Bristol Myers Squibb: With Breyanzi and Abecma in its portfolio, BMS is a leader in addressing hematologic malignancies.
4. Emerging Players: Companies like Allogene Therapeutics and Cellectis are advancing off-the-shelf, allogeneic CAR-T therapies, aiming to reduce costs and improve scalability.
Key Developments in CAR-T Market Competition:
- Biosimilars and Generics: While still nascent, the emergence of biosimilar CAR-T therapies could create pricing pressure and broaden accessibility.
- Manufacturing Innovations: The transition from autologous to allogeneic therapies is a game-changer, promising reduced costs, faster turnaround times, and broader applicability.
Read more about the key developments in the CAR-T market: https://www.delveinsight.com/sample-request/car-t-market-forecast
CAR-T Market Challenges and Opportunities
CAR-T Market Challenges:
1. Cost and Accessibility: CAR-T therapies remain prohibitively expensive, limiting access in low- and middle-income countries.
2. Manufacturing Complexities: Autologous therapies require personalized manufacturing, leading to logistical challenges.
3. Safety Concerns: Cytokine release syndrome (CRS) and neurotoxicity are significant adverse effects, necessitating advanced safety protocols.
CAR-T Market Opportunities:
1. Allogeneic Therapies: "Off-the-shelf" CAR-T products offer a scalable, cost-effective alternative to autologous treatments.
2. Global Expansion: Strategic collaborations and regulatory approvals in emerging markets could significantly expand the reach of CAR-T therapies.
3. Combination Therapies: Pairing CAR-T cells with immune checkpoint inhibitors or small-molecule drugs could enhance efficacy and broaden applications.
Request for a sample report @ https://www.delveinsight.com/report-store/car-t-market-forecast
CAR-T Market Forecast to 2034
The CAR-T market is expected to reach unprecedented heights by 2034, with the following key trends shaping its trajectory:
1. Wider Approval Landscape: More than 30 new CAR-T therapies are anticipated to receive regulatory approval for various indications by 2034.
2. Increased Patient Access: Advances in manufacturing and distribution are expected to make CAR-T therapies more affordable and accessible globally.
3. Technological Breakthroughs: Innovations such as CRISPR-edited CAR-T cells and novel targets like BCMA and GPRC5D will expand the scope and efficacy of CAR-T therapies.
North America will likely maintain its leadership position, while Asia-Pacific will emerge as the fastest-growing market due to favorable demographics and government support.
The CAR-T therapy market represents a transformative approach in cancer treatment, with significant growth expected over the next decade. Despite challenges such as cost and manufacturing complexities, the market is poised for expansion, driven by innovation, an expanding patient base, and increased global adoption. By 2034, CAR-T therapies are expected to become a cornerstone of oncology, offering hope to millions of patients worldwide.
For further insights, explore DelveInsight’s comprehensive report on the [CAR-T Market Forecast](https://www.delveinsight.com/report-store/car-t-market-forecast).
0 notes
Text
Hematologic Malignancies Market Size, Share, Trends, Growth and Competitive Analysis
"Global Hematologic Malignancies Market – Industry Trends and Forecast to 2029
Global Hematologic Malignancies Market, By Type (Leukaemia, Lymphoma, Myeloma), Therapy Type (Chemotherapy, Immunotherapy, Targeted Therapy), Diagnosis (Blood Tests, Biopsy, Imaging Tests, Others), Route of Administration (Oral, Parenteral, Others), Dosage Form (Tablets, Capsules, Injections, Others), End-Users (Hospitals, Specialty Clinics, Homecare, Others), Distribution Channel (Hospital Pharmacy, Retail Pharmacy, Online Pharmacy, Others) – Industry Trends and Forecast to 2029
Access Full 350 Pages PDF Report @
**Segments**
- **Type**: The hematologic malignancies market can be segmented based on the type of malignancy, including leukemia, lymphoma, and multiple myeloma. Leukemia is a cancer of the blood cells, while lymphoma affects the lymphatic system. Multiple myeloma, on the other hand, is a cancer that forms in a type of white blood cell called a plasma cell.
- **Treatment**: Segmentation based on treatment modalities includes chemotherapy, immunotherapy, targeted therapy, stem cell transplant, and others. Chemotherapy is a common treatment for hematologic malignancies that involves the use of drugs to kill cancer cells. Immunotherapy utilizes the body's immune system to fight cancer cells, while targeted therapy focuses on specific molecules involved in cancer growth.
- **End-User**: The market can also be segmented by end-user, such as hospitals, specialty clinics, research institutes, and others. Hospitals are the primary point of care for hematologic malignancies patients, where they receive diagnosis, treatment, and follow-up care. Specialty clinics may offer specialized treatments or clinical trials for these conditions.
**Market Players**
- **Roche**: A leading player in the hematologic malignancies market, Roche offers a range of innovative therapies and diagnostic tools for leukemia, lymphoma, and multiple myeloma. The company's commitment to research and development has resulted in groundbreaking treatments that improve patient outcomes.
- **Johnson & Johnson**: With a focus on cutting-edge oncology therapies, Johnson & Johnson has made significant advancements in the treatment of hematologic malignancies. The company's portfolio includes novel drugs that target specific cancer pathways, providing new options for patients.
- **Novartis**: Known for its expertise in precision medicine, Novartis has developed several targeted therapies for hematologic malignancies. By identifying genetic mutations driving cancer growth, Novartis delivers personalized treatments that are more effective and less toxic for patients.
- **AbbVie**:AbbVie is a key player in the hematologic malignancies market, known for its strong focus on developing innovative therapies for various types of blood cancers. The company's robust pipeline includes potential treatments for leukemia, lymphoma, and multiple myeloma, leveraging cutting-edge technologies and research to address unmet medical needs in this space. AbbVie's commitment to oncology research and development has led to the introduction of novel treatment options that aim to improve patient outcomes and quality of life.
In the competitive landscape of the hematologic malignancies market, AbbVie distinguishes itself through a combination of strategic partnerships, investments in research, and a patient-centric approach to drug development. The company's collaborative efforts with academic institutions, research organizations, and other industry partners have resulted in the acceleration of novel therapeutic solutions for blood cancers. By prioritizing patient needs and engaging in meaningful dialogue with healthcare providers, AbbVie continues to shape the future of hematologic oncology with a focus on personalized medicine and targeted therapies.
AbbVie's portfolio of hematologic malignancy treatments encompasses a diverse range of modalities, including small molecule inhibitors, monoclonal antibodies, and immunotherapies. These innovative therapies target specific pathways and molecular mechanisms involved in the development and progression of blood cancers, offering new hope for patients who may not have responded to conventional treatments. By leveraging its expertise in precision medicine and biomarker-driven approaches, AbbVie continues to advance the field of hematologic oncology with a strong emphasis on tailored treatment regimens that consider individual patient characteristics and disease profiles.
In addition to its focus on drug development, AbbVie also plays a crucial role in raising awareness about hematologic malignancies and promoting early detection and diagnosis. Through educational initiatives, patient advocacy programs, and community engagement efforts, the company strives to empower patients, caregivers, and healthcare professionals with the knowledge and resources needed to effectively manage blood cancers. By fostering a culture of collaboration and knowledge-sharing, AbbVie contributes to the overall**Global Hematologic Malignancies Market Analysis**
- **Type**: The global hematologic malignancies market, segmented by type, includes leukemia, lymphoma, and multiple myeloma. With advancements in precision medicine and targeted therapies, the market is witnessing a shift towards personalized treatment regimens tailored to the specific type of malignancy, driving growth in the segment.
- **Therapy Type**: The market segmented by therapy type comprises chemotherapy, immunotherapy, and targeted therapy, among others. The rising prevalence of hematologic malignancies and the increasing adoption of novel treatment approaches are driving the demand for innovative therapies, leading to significant market growth in this segment.
- **Diagnosis**: Diagnostic modalities such as blood tests, biopsies, imaging tests, and others play a crucial role in the early detection and management of hematologic malignancies. The emphasis on early diagnosis and personalized medicine is driving the market for diagnostic tools, contributing to the overall growth of the hematologic malignancies market.
- **Route of Administration**: Different routes of administration, including oral, parenteral, and others, offer varied options for delivering hematologic malignancy treatments. The convenience and efficacy of different administration routes influence patient compliance and treatment outcomes, shaping the market dynamics in this segment.
- **Dosage Form**: The market segmented by dosage form includes tablets, capsules, injections, and others. The availability of diverse dosage forms caters to patient preferences and treatment needs, promoting adherence and enhancing the overall therapeutic outcomes in
Key points covered in the report: -
The pivotal aspect considered in the global Hematologic Malignancies Market report consists of the major competitors functioning in the global market.
The report includes profiles of companies with prominent positions in the global market.
The sales, corporate strategies and technical capabilities of key manufacturers are also mentioned in the report.
The driving factors for the growth of the global Hematologic Malignancies Market are thoroughly explained along with in-depth descriptions of the industry end users.
The report also elucidates important application segments of the global market to readers/users.
This report performs a SWOT analysis of the market. In the final section, the report recalls the sentiments and perspectives of industry-prepared and trained experts.
The experts also evaluate the export/import policies that might propel the growth of the Global Hematologic Malignancies Market.
The Global Hematologic Malignancies Market report provides valuable information for policymakers, investors, stakeholders, service providers, producers, suppliers, and organizations operating in the industry and looking to purchase this research document.
TABLE OF CONTENTS
Part 01: Executive Summary
Part 02: Scope of the Report
Part 03: Research Methodology
Part 04: Market Landscape
Part 05: Pipeline Analysis
Part 06: Market Sizing
Part 07: Five Forces Analysis
Part 08: Market Segmentation
Part 09: Customer Landscape
Part 10: Regional Landscape
Part 11: Decision Framework
Part 12: Drivers and Challenges
Part 13: Market Trends
Part 14: Vendor Landscape
Part 15: Vendor Analysis
Part 16: Appendix
Countries Studied:
North America (Argentina, Brazil, Canada, Chile, Colombia, Mexico, Peru, United States, Rest of Americas)
Europe (Austria, Belgium, Denmark, Finland, France, Germany, Italy, Netherlands, Norway, Poland, Russia, Spain, Sweden, Switzerland, United Kingdom, Rest of Europe)
Middle-East and Africa (Egypt, Israel, Qatar, Saudi Arabia, South Africa, United Arab Emirates, Rest of MEA)
Asia-Pacific (Australia, Bangladesh, China, India, Indonesia, Japan, Malaysia, Philippines, Singapore, South Korea, Sri Lanka, Thailand, Taiwan, Rest of Asia-Pacific)
Browse Trending Reports:
Thermal Imaging Cameras Market Baby Food Market Thin Film Encapsulation Market Paper Coating Materials Market Protein Engineering Market Psoriasis Treatment Market Whole Exome Sequencing Market Std Diagnostics Market Medication Delivery Systems Market Lane Keep Assist System Market Liquid Synthetic Rubber Market Mainframe Market Myxoid Round Cell Liposarcoma Drug Market Hematology Analyzer Market Low Differential Pressure Sensor Market Biofuel Enzyme Market Aroma Ingredients Market Coconut Water Market
About Data Bridge Market Research:
Data Bridge set forth itself as an unconventional and neoteric Market research and consulting firm with unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email: [email protected]"

0 notes
Text
Carfilzomib Market Overview and Regional Outlook Study 2024 – 2034
Carfilzomib Market Defination:
TheCarfilzomib Market refers to the economic and clinical landscape surrounding the pharmaceutical drug carfilzomib. Carfilzomib is a proteasome inhibitor used primarily in the treatment of multiple myeloma, a type of cancer affecting plasma cells in bone marrow. It functions by selectively inhibiting the proteasome, a complex protein-degrading machinery essential for cell function and survival. This inhibition leads to the accumulation of proteins within cancer cells, triggering cell death through apoptosis.
Print This Guide for Future Reference:https://wemarketresearch.com/reports/request-free-sample-pdf/carfilzomib-market/1493
Exploring the Carfilzomib Market: Advancements in Multiple Myeloma Treatment
In the realm of oncology, particularly in the treatment landscape of multiple myeloma, carfilzomib has emerged as a cornerstone therapy, offering new hope and improved outcomes for patients. This blog delves into the dynamic carfilzomib market, examining its impact, current trends, challenges, and future prospects.
Understanding Carfilzomib
Carfilzomib is a proteasome inhibitor approved for the treatment of relapsed or refractory multiple myeloma. It works by selectively and irreversibly binding to the 20S proteasome, disrupting protein degradation in cancer cells and inducing apoptosis. Approved by the FDA in 2012, carfilzomib has since been integrated into treatment protocols, often in combination with other agents like lenalidomide and dexamethasone.
Market Dynamics
Current Landscape: The Carfilzomib Market is driven by its efficacy in treating relapsed or refractory multiple myeloma, particularly in patients who have received prior therapies. Its mechanism of action and clinical benefits have positioned it as a valuable option in the treatment algorithm for multiple myeloma.
Treatment Advancements: Clinical studies have demonstrated that carfilzomib-based regimens prolong progression-free survival and overall survival compared to traditional therapies. Its approval marked a significant advancement in the management of multiple myeloma, offering a targeted approach to combating the disease.
Competitive Environment: Within the proteasome inhibitor class, carfilzomib competes with bortezomib and ixazomib, each offering unique profiles in terms of efficacy, safety, and administration convenience. Ongoing research aims to optimize carfilzomib’s use through novel combinations and sequencing strategies to maximize patient benefit.
Clinical Applications
Approved Indications: Carfilzomib is primarily indicated for use in combination with other agents for the treatment of relapsed or refractory multiple myeloma. Clinical trials are also exploring its potential in newly diagnosed patients and maintenance therapy settings, broadening its scope of application.
Future Directions: Research efforts are focused on expanding carfilzomib’s indications and understanding its synergies with emerging therapies such as immunomodulators, monoclonal antibodies, and cellular therapies like CAR-T cells. These endeavors aim to further improve treatment outcomes and offer personalized therapeutic approaches.
Carfilzomib Market Challenges and Opportunities
Challenges: Economic considerations remain a significant challenge in the adoption of carfilzomib, given its high cost as a biologic therapy. Managing treatment-related adverse events, such as cardiovascular complications and hematologic toxicities, also requires vigilant monitoring and proactive management strategies.
Opportunities: Advances in biomarker identification and personalized medicine offer opportunities to tailor carfilzomib-based therapies to individual patient profiles. Moreover, ongoing research into combination therapies and novel formulations aims to enhance efficacy while minimizing adverse effects, thereby improving patient adherence and outcomes.
Patient Impact and Healthcare Considerations
Patient Experience: For patients diagnosed with relapsed or refractory multiple myeloma, carfilzomib represents a crucial treatment option that can potentially extend survival and improve quality of life. Education and support programs play a vital role in helping patients manage treatment-related challenges and adhere to therapy.
Healthcare System Implications: Integrating carfilzomib into clinical practice requires healthcare providers to navigate complex treatment algorithms and ensure appropriate patient monitoring. Collaboration among multidisciplinary teams, including oncologists, hematologists, and supportive care specialists, is essential for optimizing patient care and outcomes.
Regulatory and Market Access
Regulatory Landscape: Regulatory approvals and reimbursement policies influence the accessibility of cCarfilzomib Market in different regions. Streamlining regulatory processes and demonstrating cost-effectiveness through real-world evidence are crucial for enhancing market access and patient affordability.
Market Expansion: As clinical data continues to evolve and new indications are explored, the carfilzomib market is poised for growth. Market expansion strategies should prioritize evidence-based medicine and stakeholder collaboration to drive adoption and improve patient access.
Conclusion
In conclusion, the carfilzomib market represents a significant advancement in the treatment of multiple myeloma, reflecting the transformative impact of targeted therapies in oncology. Its approval and integration into treatment protocols underscore a shift towards personalized medicine and multidisciplinary care approaches that optimize patient outcomes.
While challenges such as economic considerations and treatment-related adverse events persist, ongoing research and collaborative efforts among stakeholders are paving the way for continued innovation and improvement inCarfilzomib-Based Therapies. By addressing these challenges proactively, healthcare providers and pharmaceutical companies can ensure that carfilzomib realizes its full potential in improving the lives of patients battling multiple myeloma.
Stay informed and engaged with the latest developments in the carfilzomib market to contribute to advancements in oncology and patient-centered care.
0 notes
Text
Bortezomib Market Estimated to Witness High Growth Owing to Rising Adoption of Proteasome Inhibitors

The bortezomib market is primarily driven by high incidence and prevalence of multiple myeloma across the globe. Bortezomib, which is commonly sold under the brand name Velcade among others, is a proteasome inhibitor primarily used for the treatment of multiple myeloma and mantle cell lymphoma.
The global proteasome inhibitor drug market size is valued at approximately US$ 24.54 million in 2024 and is expected to register a CAGR of 4.7% over the forecast period of 2024-2031. The introduction of novel proteasome inhibitors and their increasing adoption in the treatment of cancer are the major factors anticipated to propel market growth. Key Takeaways
Key players operating in the bortezomib market are Hikma Pharmaceuticals, Pfizer, Meitheal Pharmaceuticals, Novartis International AG, Bristol Myers Squibb, NATCO Pharma, Teva Pharmaceuticals, Dr. Reddy's Laboratories, Gland Pharma, Shilpa Medicare, Qilu Pharmaceutical, Scion Pharmaceuticals, Farmhispania Group, Coresyn, Chem-Stone (Guangzhou), Hubei Honch Pharmaceutical, Vinkem Labs, Icrom, TAPI Teva, and Chengdu Aslee Biopharmaceuticals.
The introduction of generic versions of Bortezomib Market Demand has led to increased adoption and lowered treatment costs. Moreover, ongoing clinical trials evaluating the efficacy of bortezomib in other cancer indications are expected to expand the eligible patient pool. Technological advancements in proteasome inhibitor development focused on overcoming resistance, reducing toxicity, and novel delivery systems are further anticipated to support market growth. Market Drivers
The primary factors driving the growth of the global bortezomib market include rising prevalence of multiple myeloma globally, increasing adoption of proteasome inhibitors in treatment regimens, availability of generic versions, and ongoing clinical research evaluating the efficacy of bortezomib in other cancer indications. Additionally, improving healthcare infrastructure and expenditures in emerging economies will further support the market growth during the forecast period. Current challenges in Bortezomib Market
The Bortezomib Market Size And Trends faces several challenges primarily due to the presence of alternative therapeutic options for treating multiple myeloma (MM). Some of the major challenges include increasing generic competition from drugs like ixazomib and daratumumab which are leading to lower sales of bortezomib drugs. Further, the patents of bortezomib drugs have expired in several regions making them available in generic forms at lower costs. This increasing availability of low-cost generics is a major challenge faced by innovator bortezomib drug companies. Additionally, the adverse side effects associated with bortezomib drugs like neuropathy and thrombocytopenia require close patient monitoring during treatment posing operational challenges. Stringent regulations for drug approval is another regulatory challenge for new market entrants. SWOT Analysis
Strength: Well-established drug with proven efficacy and safety profile in treating MM. It was the first proteasome inhibitor approved and remains a standard of care. Weakness: Patent expiry has led to availability of low-cost generics reducing sales of innovator brands. Further, it causes serious side effects like neuropathy requiring cautious use. Opportunity: Emerging economies with growing cancer burden and healthcare spending present an opportunity. Combination therapies with other anti-MM drugs can boost its use further. Threats: Increasing competition from newer oral proteasome inhibitors and monoclonal antibody based therapies poses pricing and market share threats. Stringent regulations for approval delays market entry of new players.
Geographical regions with high market concentration
In terms of value, North America accounts for the largest share of over 40% of the global bortezomib market led by the US. This is due to established healthcare infrastructure and higher adoption of innovative therapies. Europe is the second major regional market with a value share of over 30% supported by favourable reimbursement policies. The Asia Pacific region is projected to be the fastest growing market during the forecast period due to rising healthcare expenditure, growing cancer incidence and increasing demand for cancer treatments from middle-income countries like China and India. Fastest growing geographical region
The Asia Pacific region is poised to exhibit the highest growth rate during the forecast period in the global bortezomib market. This is attributed to rising disposable incomes, growing awareness about cancer treatments, expansion of healthcare facilities and increasing private sector investment in pharmaceutical research in emerging economies like China and India. Large patient pools undergoing cancer treatment in Asia present lucrative opportunities for bortezomib drug makers looking to tap high future growth potential in this region. Get More Insights On, Bortezomib Market For More Insights Discover the Report In language that Resonates with you French, German, Italian, Russian, Chinese, Korean About Author: Ravina Pandya, Content Writer, has a strong foothold in the market research industry. She specializes in writing well-researched articles from different industries, including food and beverages, information and technology, healthcare, chemical and materials, etc. (https://www.linkedin.com/in/ravina-pandya-1a3984191)
#Bortezomib Market Demand#Bortezomib Market Size#Bortezomib Market Trends#Bortezomib Market Forcast#Bortezomib#Bortezomib Market
0 notes
Text
Global amyloidosis therapeutic treatment market is expected to develop at a compound annual growth rate (CAGR) of 7.60%, from its estimated USD 2251.2 million in 2023 to USD 4352.35 million in 2032.Amyloidosis is a rare and complex group of diseases characterized by the abnormal accumulation of amyloid proteins in tissues and organs. These proteins can lead to severe organ damage and potentially life-threatening complications. The therapeutic treatment market for amyloidosis is evolving rapidly, driven by advancements in medical research, the development of new drugs, and increasing awareness of this challenging condition.
Browse the full report at https://www.credenceresearch.com/report/amyloidosis-therapeutic-treatment-market
Market Overview
The global amyloidosis therapeutic treatment market is experiencing significant growth, fueled by a combination of rising incidence rates, expanding research initiatives, and increasing healthcare expenditures. The market encompasses a range of therapeutic options, including pharmacological treatments, supportive therapies, and emerging novel therapies.
Pharmacological Treatments
Pharmacological treatment options for amyloidosis primarily target the underlying cause of the disease or aim to alleviate symptoms. The market is currently dominated by drugs that target the specific types of amyloidosis:
1. AL Amyloidosis: This form of amyloidosis results from abnormal immunoglobulin light chains and is often associated with multiple myeloma. The treatment approach involves managing the underlying plasma cell disorder. Therapies include proteasome inhibitors such as Bortezomib and Carfilzomib, immunomodulatory drugs like Lenalidomide, and monoclonal antibodies such as Daratumumab. These drugs have shown efficacy in reducing the production of amyloidogenic light chains and improving patient outcomes.
2. ATTR Amyloidosis: Caused by the accumulation of transthyretin protein, ATTR amyloidosis is further classified into hereditary (hATTR) and wild-type (wtATTR) forms. The therapeutic landscape includes: - Tafamidis: This drug stabilizes the transthyretin protein, preventing its misfolding and aggregation. Tafamidis has demonstrated significant benefits in slowing disease progression and improving quality of life for patients with ATTR amyloidosis. - Diflunisal: An older non-steroidal anti-inflammatory drug, Diflunisal has been repurposed for ATTR amyloidosis treatment due to its ability to stabilize transthyretin. - Gene Silencing Therapies: Emerging treatments such as Patisiran and Inotersen use RNA interference and antisense oligonucleotides to reduce the production of transthyretin. These therapies have shown promise in clinical trials and represent a significant advancement in the treatment of ATTR amyloidosis.
Supportive Therapies
Supportive therapies play a crucial role in managing the symptoms and complications of amyloidosis. These include symptomatic management of heart failure, renal impairment, and neuropathy. For instance, diuretics and antihypertensive agents are commonly used to manage cardiac amyloidosis, while dialysis may be required for patients with renal involvement. Pain management and physical therapy are also essential for addressing neuropathic symptoms.
Emerging Therapies and Research
The amyloidosis therapeutic treatment market is witnessing a surge in research and development activities aimed at discovering innovative treatments. Key areas of focus include:
1. Monoclonal Antibodies: Researchers are exploring the use of monoclonal antibodies targeting amyloid deposits directly or modulating the immune system to enhance amyloid clearance.
2. Small Molecules: New small molecules are being developed to disrupt amyloid fibril formation or promote the disaggregation of existing fibrils. These compounds have the potential to offer new treatment options for various forms of amyloidosis.
3. Gene Therapy: Advances in gene therapy hold promise for addressing the genetic basis of hereditary amyloidosis. By correcting or replacing faulty genes, these therapies could potentially prevent or cure the disease.
Challenges and Opportunities
Despite the progress in amyloidosis treatment, several challenges remain. The rarity of the disease can lead to difficulties in diagnosis and treatment, and the high cost of innovative therapies can be a barrier to access for many patients. Additionally, the complexity of amyloidosis requires a multidisciplinary approach to manage the diverse manifestations of the disease effectively.
However, the growing investment in research and development, coupled with advancements in personalized medicine, presents significant opportunities for improving patient outcomes. Continued innovation and collaboration among researchers, healthcare providers, and pharmaceutical companies are essential to overcoming these challenges and advancing the treatment landscape for amyloidosis.
Key Players
Prothena Corporation Plc.
Eidos Therapeutics
Pfizer Inc.
SOM Biotech
Corino Therapeutics
Johnson and Johnson Services, Inc.
AstraZeneca Plc.
Alnylam Pharmaceuticals, Inc.
GlaxoSmithKline, Plc.
Others
Segmentation
By Type of Amyloidosis
AL Amyloidosis (Primary Amyloidosis)
ATTR Amyloidosis (Hereditary and Wild-Type)
AA Amyloidosis (Secondary Amyloidosis)
By Treatment Modalities
Chemotherapy
Immunomodulatory Drugs (IMiDs)
Monoclonal Antibodies
TTR Stabilizers
RNA Interference (RNAi) Therapies
Liver Transplantation
Supportive Care
By Disease Severity
Newly Diagnosed Patients
Relapsed or Refractory Disease
Advanced Disease
By Region
North America
The U.S.
Canada
Mexico
Europe
Germany
France
The U.K.
Italy
Spain
Rest of Europe
Asia Pacific
China
Japan
India
South Korea
South-east Asia
Rest of Asia Pacific
Latin America
Brazil
Argentina
Rest of Latin America
Middle East & Africa
GCC Countries
South Africa
Rest of Middle East and Africa
Browse the full report at https://www.credenceresearch.com/report/amyloidosis-therapeutic-treatment-market
About Us:
Credence Research is committed to employee well-being and productivity. Following the COVID-19 pandemic, we have implemented a permanent work-from-home policy for all employees.
Contact:
Credence Research
Please contact us at +91 6232 49 3207
Email: [email protected]
Website: www.credenceresearch.com
0 notes
Text
Globalization and Market Expansion in Multiple Myeloma

The Multiple Myeloma Market size was estimated at USD 24.01 Billion In 2023 & is estimated to reach USD 59.45 Billion by 2032 and increase at a compound annual growth rate of 10.6% between 2024 and 2032.The Multiple Myeloma market is characterized by a dynamic interplay of research, treatment advancements, and patient-centric care initiatives. As one of the most prevalent hematologic cancers, it continuously draws attention from pharmaceutical innovators and healthcare providers alike. Recent years have witnessed a surge in targeted therapies, immunotherapies, and personalized medicine approaches tailored to combatting its complexities. This evolving landscape not only fosters competition among biopharmaceutical companies but also emphasizes the importance of early detection and multidisciplinary treatment strategies. With ongoing clinical trials promising novel therapeutic avenues, the Multiple Myeloma market remains poised for further breakthroughs in extending patient survival and enhancing quality of life.
The Multiple Myeloma Market research study for the term also includes a variety of business opportunities and growth potential. A business plan detailing market risks and constraints as well as the effects of various regulatory regimes is given to executives by the market research. This is carried out to assist companies in reaching their main goals and making better judgments.
Get Sample Copy Of This Report @ https://www.snsinsider.com/sample-request/3490
Market Segmentation
By Type
Chemotherapy
Monoclonal Antibody
Protease Inhibitors
Others
By Disease Type
Smoldering Multiple Myeloma
Active Multiple Myeloma
By End User
Clinics
Hospitals
Others
Regional Outlook
The geographical categories that make up the Multiple Myeloma Market each have their own revenue, market share, sales, and growth rates. Among the important geographical areas covered in the market analysis are Europe, Asia-Pacific, South America, North America, and the Middle East and Africa. Latin America is expected to have a small market share in value, while North America is forecast to maintain its global leadership position and have a significant market share in both volume and value.
COVID-19 Impact Analysis
In the first half of 2020, the COVID-19 virus started to spread over the world, infecting millions of people and forcing major nations to implement work stoppage and foot restrictions. Nearly every area of the economy has suffered, with the exception of medical goods and equipment for life support, including the Multiple Myeloma Market .
Competitive Landscape
The competitive analysis section of the global Multiple Myeloma Market offers details and insights on the participants. Among the details provided are information on competition, a market overview by business status, and revenue projections by region. These businesses use a variety of strategies, such as product launches, partnerships, alliances, technology advancements, and contracts, to boost market income.
Conclusion
The market research is supported by first-hand experience, qualitative and quantitative analysis by industry analysts, and comments from key market players and actors in the value chain. The study investigates parent industry trends, micro and macroeconomic data, governing factors, and market attractiveness on a segment-by-segment basis. The study also illustrates how different market factors might have a qualitative impact on market segmentation based on geography and Multiple Myeloma Market.
About Us
SNS Insider is a market research and insights firm that has won several awards and earned a solid reputation for service and strategy. We are a strategic partner who can assist you in reframing issues and generating answers to the trickiest business difficulties. For greater consumer insight and client experiences, we leverage the power of experience and people.
When you employ our services, you will collaborate with qualified and experienced staff. We believe it is crucial to collaborate with our clients to ensure that each project is customized to meet their demands. Nobody knows your customers or community better than you do. Therefore, our team needs to ask the correct questions that appeal to your audience in order to collect the best information.
Related Reports
Flash Chromatography Market Size
Cystic Fibrosis Market Size
Cancer Biopsy Market Size
Glaucoma Therapeutics Market Size
Genomics Services Market Size
0 notes
Text
Minimal Residual Disease Testing Market Future Trends to Look Out | Bis Research
Minimal Residual Disease Testing is a sophisticated diagnostic technique used primarily in oncology to detect and quantify residual cancer cells that may remain in the body during or after treatment.
The Global Minimal Residual Disease Testing Market is a rapidly growing segment in the healthcare industry, driven by the increasing demand for accurate and sensitive methods to monitor and manage cancer patients.
The Minimal Residual Disease Testing market was valued at $1.67 billion in 2023 and is expected to reach $6.67 billion by 2033, growing at a CAGR of 14.81% between 2023 and 2033.
MRD Testing Overview
Minimal Residual DiseaseTesting refers to the detection and quantification of residual cancer cells that remain in a patient after treatment, which are below the detection threshold of conventional diagnostic methods. MRD testing is particularly significant in hematologic malignancies such as leukemia, lymphoma, and multiple myeloma, where even a small number of remaining cancer cells can lead to relapse.
Have a look at our sample page here !
Market Segmentation
By Technology
By Target Detection
By End Users
By Region
China dominated the Asia-Pacific Minimal Residual Disease Testing Market in 2022 with a share of 36.08%. Although the market is expected to remain in a strong growth phase due to the massively growing number of cancer cases and the rising health-related awareness among people in Asia-Pacific, a significant barrier to the increasing adoption is an uneven economic balance among countries within the region.
Importance of Minimal Residual Disease Testing Market
Assessing Treatment Response
Predicting Relapse
Tailoring Therapy
Key Factors
The Minimal Residual Disease Testing Market has experienced significant growth in recent years, driven by several key factors like
advancements in technology
rising cancer burden,
clinical evidence supporting MRD monitoring
Key Players In the Minimal Residual Disease Testing Market includes
QIAGEN N.V.
Thermo Fisher Scientific Inc.
Sysmex Corporation
Mission Bio
OPKO Health
Bio-Rad Laboratories, Inc
ICON plc
Hoffmann-La Roche
and many others
Techniques used in Minimal Residual Disease Testing
Flow Cytometry - This technique uses fluorescent antibodies to identify cancer-specific markers on the surface of cells.
Polymerase Chain Reaction- PCR amplifies cancer-specific genetic sequences, allowing for the detection of one cancer cell among a million normal cells.
Next Generation Sequencing - NGS provides detailed genetic information by sequencing DNA or RNA at high depth, offering unparalleled sensitivity and the ability to identify clonal diversity and mutations.
Digital Droplet PCR- A more recent advancement, ddPCR partitions the sample into thousands of droplets and performs PCR on each droplet individually, providing high sensitivity and precise quantification.
Applications for Minimal Residual Disease Testing Market
Treatment Response Monitoring
Relapse Prediction
Treatment Decision-making
Prognostic Assessment
Clinical Trials and drug development
Minimal Residual Disease Testing Market Dynamics
Market Drivers
Advent of MRD and its Awareness among Consumers
Increasing Incidence of Cancer Cases Demanding MRD
Rise in administration of solid tumors
Expanding Medicare Coverage for MRD
Recent Developments in the Minimal Residual Disease Testing Market
•Quest Diagnostics acquired Haystack Oncology, expanding its oncology portfolio with the inclusion of advanced liquid biopsy technology. This addition aimed to enhance personalized cancer care by offering highly sensitive diagnostic capabilities. Integrated DNA Technologies launched the Archer FUSIONPlex Core Solid Tumor Panel, a pioneering cancer research testing solution that has been enhanced and fine-tuned to include a broader range of single nucleotide variant (SNV) and indel coverage.
Visit our precision medicine page here!
Key Questions Answered
Q What is MRD ?
Minimal residual disease (MRD) testing is a supplementary approach to detect extremely low levels of blood cancer cells and solid tumors following the treatment of conditions such as acute and chronic leukemia, lymphoma, or multiple myeloma. MRD specifically pertains to the minute population of cancer cells that persist in the body despite achieving complete remission (CR) through chemotherapy or stem cell transplantation.
Q What kinds of New Strategies are adopted by the existing market players to strengthen their positions in the Industry ?
The global MRD market is currently witnessing several developments, primarily aimed at introducing new products and services. Major manufacturers of MRD products, along with the service providers, are actively undertaking significant business strategies to translate success in research and development into the commercial clinical setting.
Conclusion
In conclusion, Minimal Residual testing is a powerful tool in modern oncology, offering the potential to significantly improve patient outcomes through more precise and personalized treatment strategies.
0 notes
Text
Oncology Drugs Market Growth, Trends, Size, Share, Demand And Top Growing Companies 2031

In a landscape where the battle against cancer rages on, advancements in healthcare systems, public health measures, and novel pharmaceutical therapies have ushered in a new era of hope. According to the National Cancer Institute, the United States saw an estimated 1,806,590 new cancer cases and approximately 606,520 deaths due to the disease in 2020. However, over the past five decades, cancer survival rates have soared from 50% in 1970 to an impressive 70%, thanks to a trifecta of progress.
For more information: https://www.fairfieldmarketresearch.com/report/oncology-drugs-market
Unprecedented Growth Trajectory: The global oncology therapy sales are forecasted to surpass US$ 300 billion by 2026, with oncology contributing 21.7% to total pharmaceutical sales. Fueling this growth are the top 10 pharmaceutical companies, which have declared oncology as their key focus area, driving multibillion-dollar M&A deals and strategic collaborations. Pfizer's acquisition of Array BioPharma for US$11 billion in 2019 and AbbVie's strategic partnership with Genmab for a bispecific antibody development deal worth US$3 billion are testament to this focus.
Diverse Indications Drive Demand: While oncology represents over 20 different indications, a significant portion of revenue stems from just five of them: breast cancer, multiple myeloma, non-small-cell lung carcinoma (NSCLC), prostate cancer, and non-Hodgkin's lymphoma (NHL), which collectively accounted for approximately 65% of the market in 2020. Moreover, with breast, lung, and colorectal cancers expected to collectively account for ~50% of all new cancer diagnoses by 2026, the demand for innovative therapies continues to surge.
Disruptive Trends Reshape Landscape: Innovation in oncology is accelerating, with disruptive technologies such as cell therapy, RNA therapy, viral vectors, and stem cell therapy gaining traction. Recent approvals of CAR-T cell therapies like Kymriah and Yescarta for acute lymphocytic leukemia (ALL) and diffuse large B-cell lymphoma (DLBCL) respectively signal a new frontier in cancer treatment. Precision medicine is also driving progress, with over 160 oncology biomarkers approved by 2019, paving the way for more targeted and effective therapies.
Impact of COVID-19: Despite remarkable progress, oncology has been among the worst-hit therapeutic areas amid the COVID-19 pandemic. Decreased demand for physician-administered products, disruptions in cancer screenings, and a decline in new clinical trials have posed significant challenges. However, the industry remains resilient, adapting to the evolving landscape and ensuring continued innovation.
Immuno-Oncology Leads the Way: Immuno-oncology sales are expected to soar to ~US$ 95 billion by 2026, with agents and protein kinase inhibitors comprising ~65% of sales. With over 550 active cell- and gene-therapy agents under clinical development, the future of cancer treatment looks promising. Investments in combination studies and the exploration of new mechanisms underscore the industry's commitment to advancing immuno-oncology therapies.Roche and Keytruda: Leading the Charge: In a highly concentrated market where the top 10 companies capture over 75% of the market value, F. Hoffmann-La Roche AG (Roche) and Merck & Co. stand out as leaders. While Roche maintains its global leadership position, Merck's Keytruda is poised to become the world's top-selling oncology
0 notes
Text
T Cell Therapy Market Size was valued at USD 3.8 Billion in 2023 and is expected to reach a market size of USD 49.9 Billion by 2032
The T cell therapy market size was valued at USD 3.8 Billion in 2023 and is expected to reach a market size of USD 49.9 Billion by 2032 at a CAGR of 32.9%.
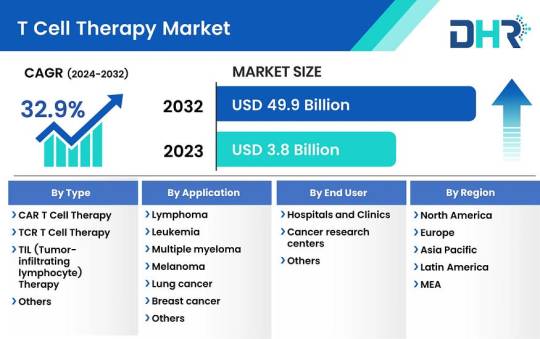
Request Sample Report: https://datahorizzonresearch.com/request-sample-pdf/t-cell-therapy-market-3114
Top Companies are:
· Novartis AG
· Gilead Sciences, Inc.
· Bristol-Myers Squibb Company
· Adaptimmune Therapeutics plc
· Amgen Inc.
· Atara Biotherapeutics, Inc.
· Autolus Therapeutics plc
· bluebird bio, Inc.
· Cellectis S.A.
· Iovance Biotherapeutics, Inc.
· Kite Pharma (a Gilead Company)
· Tmunity Therapeutics, Inc.
Market Segmentations:
By Type-
CAR T cell therapy
TCR T cell therapy
TIL (Tumor-infiltrating lymphocyte) therapy
Others
By Application-
Lymphoma
Leukemia
Multiple myeloma
Melanoma
Lung cancer
Breast cancer
Colorectal cancer
Autoimmune disorders
Infectious diseases
Others
By End User-
Hospitals and Clinics
Cancer research centers
Others
Regional Analysis:
The dominance of the T Cell Therapy market in North America is underpinned by the presence of established biopharmaceutical firms, a robust clinical trial infrastructure, and a favorable regulatory landscape. Among North American countries, the United States stands out as the primary contributor, buoyed by the FDA’s approval of several CAR T cell therapies such as Kymriah, Yescarta, and Abecma for treating hematological malignancies. The U.S. National Library of Medicine’s database reveals an extensive presence of over 1,000 active clinical trials dedicated to evaluating T cell therapies, underscoring the region’s steadfast commitment to research and development in this field.
Key highlights of the report include:
1. The report delivers thorough Market analysis, furnishing valuable insights to guide strategic decision-making.
2. The comprehensive research outlined in the study enhances the depth of your presentations and marketing strategies.
3. By offering crucial insights into key market competitors, the study empowers businesses with a strategic edge.
4. It delivers a precise assessment of evolving market dynamics, ensuring readers stay abreast of the latest industry trends.
5. With meticulous breakdowns of various market niches, the report facilitates informed decision-making processes.
0 notes
Text
Cereblon E3 Ligase Modulators Market: Size, Target Population, Competitive Landscape, and Forecast to 2034
Cereblon E3 ligase modulators (CELMoDs) are a new frontier in targeted therapies, particularly in the treatment of cancers such as multiple myeloma. This emerging drug class is showing immense potential to surpass conventional immunomodulatory drugs (IMiDs), offering greater efficacy and improved safety profiles. The market for these innovative therapeutics is expected to grow significantly, driven by advancements in clinical research, the increasing prevalence of multiple myeloma, and the need for more effective treatment options.
Cereblon E3 Ligase Modulators Market Size and Growth Potential
The global market for CELMoDs is poised for robust growth through 2034. With leading candidates such as iberdomide and mezigdomide in development, companies are focusing on addressing unmet needs in cancer treatment. Iberdomide has demonstrated enhanced potency and binding affinity to cereblon, leading to better degradation of transcription factors like Ikaros and Aiolos, critical in tumor survival. This specificity makes CELMoDs a superior alternative to earlier drugs like Revlimid, whose market share is declining due to patent expirations and generic competition.
By 2034, the CELMoD market is expected to capture a significant portion of the oncology treatment landscape, particularly within hematological malignancies like multiple myeloma. Key factors contributing to this growth include increasing investments in research, strategic collaborations among pharmaceutical companies, and the expansion of the eligible patient population due to the aging global demographic.
Request for sample pages @ https://www.delveinsight.com/report-store/cereblon-e3-ligase-modulators-market-forecast
Cereblon E3 Ligase Modulators Target Population
Multiple myeloma patients represent the primary target population for CELMoDs. This disease predominantly affects older adults, with the majority of diagnoses occurring in individuals aged 65 and above. The increasing incidence of multiple myeloma, coupled with the rising prevalence of relapsed or refractory cases, underscores the need for more effective treatments. CELMoDs are particularly promising for patients who have shown resistance to traditional therapies, including IMiDs and proteasome inhibitors.
Additionally, the potential expansion of CELMoDs into other cancers and autoimmune diseases may further broaden their target population. Preclinical and early-phase studies are exploring the use of CELMoDs in solid tumors and inflammatory conditions, indicating a wider application of this drug class in the future.
Download sample pages @ https://www.delveinsight.com/sample-request/cereblon-e3-ligase-modulators-market-forecast
Cereblon E3 Ligase Modulators Competitive Landscape
The competitive landscape for CELMoDs is shaped by pharmaceutical giants like Bristol-Myers Squibb (BMS), which has pioneered this space with iberdomide and mezigdomide. These compounds are designed to improve upon the efficacy and safety profiles of earlier IMiDs such as Revlimid and Pomalyst. BMS is investing heavily in clinical trials to establish CELMoDs as the new standard of care for multiple myeloma, aiming to replace Revlimid in earlier lines of treatment.
Other companies are also entering the market, recognizing the potential of CELMoDs. Collaboration and competition in this space are expected to accelerate innovation, leading to the development of next-generation ligase modulators with broader applications and fewer adverse effects.
Read more about the market landscape @ https://www.delveinsight.com/sample-request/cereblon-e3-ligase-modulators-market-forecast
Challenges and Opportunities
One of the main challenges facing the CELMoD market is the high cost of development and clinical trials. Additionally, long-term safety data are still being gathered, and regulatory hurdles may slow the entry of these drugs into broader markets. However, the opportunities outweigh these challenges. The unmet need for effective and well-tolerated treatments in multiple myeloma provides a fertile ground for the success of CELMoDs.
The growing understanding of cereblon biology and protein degradation pathways opens doors for the design of more targeted and efficient therapies. Furthermore, the anticipated approval of CELMoDs in major markets like the U.S., Europe, and Asia will likely drive substantial growth over the forecast period.
Cereblon E3 Ligase Modulators Market Forecast to 2034
The CELMoD market is expected to expand significantly, supported by rising demand for advanced treatments and favorable regulatory environments. By 2034, CELMoDs could become a cornerstone of oncology treatment, particularly for hematologic cancers. Pharmaceutical companies are expected to continue investing in this area, with potential breakthroughs leading to the introduction of new compounds and therapeutic strategies.
The Cereblon E3 Ligase Modulators market represents a transformative development in cancer treatment. With their enhanced efficacy, safety profiles, and potential applications beyond multiple myeloma, CELMoDs are set to redefine therapeutic standards. The coming decade will likely witness rapid growth in this market, driven by innovation, strategic collaborations, and an increasing understanding of the underlying science. By 2034, CELMoDs could emerge as a dominant force in targeted cancer therapy, offering hope to patients worldwide.
For more detailed insights, visit [DelveInsight's report on the Cereblon E3 Ligase Modulators Market](https://www.delveinsight.com/report-store/cereblon-e3-ligase-modulators-market-forecast).
0 notes
Text
Global Focus on Improved Outcomes: Global Blood Cancer Treatment market
The Blood Cancer Treatment market. According to a recent analysis, the market size is anticipated to increase from US$ 5,935.9 million in 2023 to US$ 15,735.3 million in 2033. During the projection period, Blood Cancer Treatment sales are expected to grow at a noteworthy Compound Annual Growth Rate (CAGR) of 10.2%.
Blood cancer, encompassing leukemia, non-Hodgkin lymphoma, Hodgkin lymphoma, and multiple myeloma, represents a significant healthcare challenge globally. The projected growth in the Blood Cancer Treatment market reflects the increasing prevalence of these malignancies and the growing demand for effective treatment options.
Discover Industry Secrets Through Your Sample Report: https://www.futuremarketinsights.com/reports/sample/rep-gb-1425
Understanding Blood Cancers: A Complex Disease
Blood cancers are a group of malignancies that affect the blood, bone marrow, or lymphatic system. These cancers disrupt the normal production of blood cells, leading to a variety of health issues. The four main types of blood cancer are:
Leukemia
Hodgkin lymphoma (HL)
Non-Hodgkin lymphoma (NHL)
Multiple myeloma
Key Takeaways:
The global blood cancer treatment market is expected to reach US$15,735.3 million by 2033, reflecting a significant rise from US$5,935.9 million in 2023.
This growth is projected at a robust compound annual growth rate (CAGR) of 10.2% throughout the forecast period.
Increased research and development efforts for novel therapies, coupled with rising cancer awareness initiatives, are key drivers for market expansion.
Combating Blood Cancers: A Focus on Innovation and Accessibility
The global blood cancer treatment market is experiencing significant growth, driven by a multi-pronged approach. Increased investment in research and development by key players is leading to the creation of novel and targeted therapies for various blood cancers, including leukemia, lymphoma, and multiple myeloma.
Competitive Landscape:
Some of the key participants present in the global blood cancer treatment market are:
Novartis Pharmaceuticals
Merck & Co. Inc.
Bristol-Myers Squibb Company
AbbVie Inc.
Johnson & Johnson Pvt. Ltd.
Celgene Corporation
Amgen Inc.
Teva Pharmaceutical Industries Ltd.
Bayer AG
Pfizer Inc.
Takeda Pharmaceutical Co. Ltd.
Attributed to the presence of such a high number of participants, the market is highly competitive. While global players such as Takeda Pharmaceutical Company Limited, AstraZeneca, Bayer AG, and Novartis AG, account for a considerable market size, several regional-level players are also there operating across key growth regions, particularly in North America.
Recent Developments
In June 2021, Bayer announced that the company had submitted the supplemental new drug application (sNDA) to the USA Food and Drug Administration (FDA). The company had also applied to marketing authorization application (MAA) to the European Medicines Agency (EMA) for the oncology treatment combination of copanlisib and rituximab in the United States of America.
In 2021, Novartis announced strong data from the analysis of the pivotal Phase II ELARA trial of Kymriah in patients with relapsed or refractory follicular lymphoma, with one-time Kymriah infusion, which showed an analysis of the ELARA trial demonstrated a 66% complete response rate and 86% overall response rate.
In February 2021, Bristol Myers Squibb announced that the company received approval for cancer immunotherapy from the USA Food and Drug Administration (FDA) for certain lymphomas. Further, the FDA approved the therapy as a treatment for adults who have certain types of non-Hodgkin lymphoma.
Key Segments Covered in the Blood Cancer Treatment Market Report:
By Application:
for Biological/Immunotherapy Applications
for Chemotherapy
for Radiation Therapy
for Targeted Therapy
for Stem Cell Transplantation
By End User:
in Hospitals
in Clinics
in Cancer Rehabilitation Centers
By Region:
North America
Latin America
Western Europe
Eastern Europe
Asia Pacific Excluding Japan (APEJ)
Japan
The Middle East & Africa (MEA)
0 notes
Text
Carfilzomib Market Statistics, Segment, Trends and Forecast to 2034

TheCarfilzomib Market: Trends, Opportunities, and Future Outlook
Introduction
In recent years, the pharmaceutical industry has seen significant advancements in the treatment of multiple myeloma, a type of cancer that affects plasma cells in the bone marrow. Among these advancements, Carfilzomib has emerged as a critical player. This proteasome inhibitor, marketed under the brand name Kyprolis, has made substantial impacts in the management of multiple myeloma. In this blog, we will explore the current state of theCarfilzomib market, its growth drivers, opportunities, and future outlook.
Free Sample pdf copy:
What is Carfilzomib?
Carfilzomib is a next-generation proteasome inhibitor used in the treatment of multiple myeloma, especially in patients who have relapsed or are refractory to other therapies. Unlike its predecessor, bortezomib, Carfilzomib is known for its more selective action on the proteasome, potentially leading to fewer side effects and enhanced efficacy. Approved by the FDA in 2012, it has since become an integral part of combination therapies for multiple myeloma.
Carfilzomib Market Landscape
Growth Drivers
Rising Incidence of Multiple Myeloma: The increasing number of multiple myeloma cases globally is a significant driver for the Carfilzomib market. As the global population ages, the prevalence of multiple myeloma is expected to rise, leading to a higher demand for effective treatments.
Advancements in Treatment Protocols: Carfilzomib is often used in combination with other drugs like lenalidomide and dexamethasone, enhancing its effectiveness. This combination therapy approach has shown promising results, making Carfilzomib a preferred choice in advanced treatment regimens.
Increasing Awareness and Diagnosis: Improved diagnostic techniques and greater awareness about multiple myeloma have led to earlier detection and treatment. This trend is likely to boost the demand for Carfilzomib as part of first-line and subsequent lines of treatment.
Ongoing Clinical Trials: Continuous research and clinical trials exploring new indications and combination therapies for Carfilzomib are expanding its potential market. Studies focusing on different stages of multiple myeloma and other cancers could open new avenues for Carfilzomib use.
Carfilzomib Market Challenges
High Cost of Treatment: Carfilzomib is a high-cost drug, and its price can be a barrier to access, particularly in developing countries. The high cost of treatment may limit its market potential and lead to a preference for alternative therapies.
Side Effects and Resistance: Although Carfilzomib is well-tolerated, some patients may experience side effects such as cardiovascular issues or renal complications. Additionally, resistance to the drug can develop, leading to the need for alternative therapies.
Competition from Other Therapies: The multiple myeloma treatment landscape is competitive, with several other proteasome inhibitors and novel therapies in development. This competition can impact Carfilzomib’s market share and pricing strategies.
Market Opportunities
Expanding Indications: Research into expanding the use of Carfilzomib to other types of cancer or earlier stages of multiple myeloma could provide significant market opportunities. Successful clinical trials in these areas could lead to new indications and broaden its market.
Developing Markets: As healthcare infrastructure improves in developing regions, there ispotential for growth in these Carfilzomib Market Strategic partnerships and pricing strategies could enhance Carfilzomib’s reach in these areas.
Combination Therapies: Exploring new combination therapies and optimizing treatment regimens can improve patient outcomes and increase the demand for Carfilzomib. Collaborations with other pharmaceutical companies and research institutions could drive innovation in this space.
Carfilzomib Market Future Outlook
The Carfilzomib market is poised for continued growth driven by advancements in treatment protocols and increasing prevalence of multiple myeloma. However, challenges such as high treatment costs and competition from alternative therapies will require strategic planning and innovation.
The future of Carfilzomib will likely involve ongoing research and development to expand its therapeutic indications and improve patient outcomes. As the market evolves, stakeholders will need to navigate these dynamics to maximize the potential of Carfilzomib in the treatment of multiple myeloma and beyond.
Conclusion
Carfilzomib Market has made asignificant impact in the management of multiple myeloma, and its market prospects remain strong. With ongoing advancements in treatment protocols, increasing awareness, and expanding indications, Carfilzomib is set to continue playing a crucial role in cancer therapy. However, addressing market challenges and seizing emerging opportunities will be essential for stakeholders to fully realize the potential of this innovative drug.
#Carfilzomib Market Share#Carfilzomib Market Demand#Carfilzomib Market Scope#Carfilzomib Market Analysis#Carfilzomib Market Trend
0 notes
Text
Multiple Myeloma Therapeutics Market: Growth and Challenges
In the ever-evolving landscape of healthcare, where the buzzwords are usually as exciting as watching paint dry, the global Multiple Myeloma Therapeutics Market has decided to break the monotony. Buckle up, because we’re about to take you on a thrilling ride through the fascinating world of multiple myeloma treatment, where science meets survival, and the stakes are high.

A Rollercoaster of Growth: From $26.69 Billion to $47.04 Billion!
Hold onto your seats, folks, because we’re talking serious numbers here. The market size for multiple myeloma therapeutics skyrocketed from a humble USD 26.69 billion in 2022 to a whopping USD 47.04 billion in the mystical year of 2031. That’s a CAGR of 6.5%, making Wall Street traders wish they’d invested in plasma cells instead of GameStop.
Why the Sudden Surge? Blame it on Aging and Science!
Wondering why this market is experiencing a growth spurt that could put teenagers to shame? Well, it seems the aging population is playing a key role. As people get older, the likelihood of dancing with the devil called multiple myeloma increases. It’s like the disease is sending out invitations to join the party, and everyone over a certain age is on the guest list.
But hold your horses; it’s not just about growing old gracefully. The real MVP here is science. We’ve gone beyond the basics of knowing multiple myeloma exists; now, we’re delving into its molecular intricacies. It’s like we’ve upgraded from playing checkers to a high-stakes game of chess with cancer cells. The result? Specialized medicines and treatments that are more potent than grandma’s secret chili recipe.
Therapeutic Techniques: The Avengers of Multiple Myeloma
Now, let’s talk about the superheroes fighting the villainous multiple myeloma. Picture this: stem cell transplants, immunomodulatory medications, proteasome inhibitors, chemotherapy, monoclonal antibodies, and supportive therapies — a formidable league of extraordinary treatments. Move over Avengers; the real action is happening in the bone marrow.
Market Snapshot — North America Leading the Charge
If the global multiple myeloma therapeutics market were a race, North America would be leading the pack like it stole the checkered flag. With a healthcare system that’s as intricate as a Shakespearean play and R&D investments that could make Elon Musk blush, North America is the undisputed champion.
But wait, there’s an underdog in this story — the Asia-Pacific region. With better access to healthcare, increased medical investments, and a growing understanding of multiple myeloma, this region is giving North America a run for its money. It’s like the healthcare version of ‘David vs. Goliath,’ only with fewer slingshots and more clinical trials.
For More Information: https://www.skyquestt.com/report/multiple-myeloma-therapeutics-market
Market Dynamics: The Good, the Bad, and the Expensive
Now, let’s address the elephant in the room — the cost of treatment. While we’re busy celebrating advancements in medicine, some of these treatments can be as expensive as a beachfront property in the Hamptons. High treatment costs might make you question whether fighting multiple myeloma is worth the financial battle.
Then there’s the complexity of the disease itself. Multiple myeloma isn’t your run-of-the-mill illness; it’s more like a Rubik’s Cube of genetic and molecular characteristics. Each patient is a unique puzzle, and finding the right combination of treatments can be as tricky as solving that cube blindfolded.
The Competition: Big Pharma’s Battle Royale
In the world of multiple myeloma therapeutics, it’s survival of the fittest. Big names like Johnson & Johnson, Amgen Inc., and Bristol Myers Squibb are flexing their research muscles to develop cutting-edge treatments. Partnerships and alliances are formed faster than you can say “monoclonal antibodies,” and the goal is clear — offer patients a fighting chance, a better quality of life, and a longer lease on life.
Recent Developments: FDA Approvals and the Game-Changers
Imagine the FDA as the gatekeeper of a mystical realm, and recent developments are the golden keys unlocking new possibilities. CAR T-cell treatments, bispecific antibodies, CD38 inhibitors — it’s like a pharmaceutical Renaissance, where each approval is a stroke of genius on the canvas of medical innovation.
Key Market Trends: Immunotherapies, Targeted Therapies, and Personalized Medicine
Move over, traditional treatments; immunotherapies and targeted medicines are stealing the spotlight. It’s like replacing your flip phone with the latest smartphone — more efficient, more effective, and definitely cooler. Plus, there’s a growing emphasis on personalized medicine, where biomarkers and genetic profiling play the role of matchmakers, pairing patients with treatments tailored to their unique disease characteristics.
Conclusion: The Thrill Ride Continues
In the realm of multiple myeloma therapeutics, the rollercoaster of growth, challenges, and breakthroughs keeps us on the edge of our seats. It’s a journey where science meets humanity, and the ultimate goal is to rewrite the narrative of multiple myeloma from a deadly disease to a conquerable foe.
So, as we ride the wave of advancements, approvals, and market dynamics, one thing is certain — the global multiple myeloma therapeutics market is not just a business venture; it’s a quest for life, longevity, and the triumph of science over adversity. Buckle up, because the thrill ride continues, and the best is yet to come.
About Us-
SkyQuest Technology Group is a Global Market Intelligence, Innovation Management & Commercialization organization that connects innovation to new markets, networks & collaborators for achieving Sustainable Development Goals.
Contact Us-
SkyQuest Technology Consulting Pvt. Ltd.
1 Apache Way,
Westford,
Massachusetts 01886
USA (+1) 617–230–0741
Email- [email protected]
Website: https://www.skyquestt.com
0 notes
Text
Multiple Myeloma Therapeutics Market: Growth and Challenges
In the ever-evolving landscape of healthcare, where the buzzwords are usually as exciting as watching paint dry, the global Multiple Myeloma Therapeutics Market has decided to break the monotony. Buckle up, because we’re about to take you on a thrilling ride through the fascinating world of multiple myeloma treatment, where science meets survival, and the stakes are high.
A Rollercoaster of Growth: From $26.69 Billion to $47.04 Billion!
Hold onto your seats, folks, because we’re talking serious numbers here. The market size for multiple myeloma therapeutics skyrocketed from a humble USD 26.69 billion in 2022 to a whopping USD 47.04 billion in the mystical year of 2031. That’s a CAGR of 6.5%, making Wall Street traders wish they’d invested in plasma cells instead of GameStop.
Why the Sudden Surge? Blame it on Aging and Science!
Wondering why this market is experiencing a growth spurt that could put teenagers to shame? Well, it seems the aging population is playing a key role. As people get older, the likelihood of dancing with the devil called multiple myeloma increases. It’s like the disease is sending out invitations to join the party, and everyone over a certain age is on the guest list.
But hold your horses; it’s not just about growing old gracefully. The real MVP here is science. We’ve gone beyond the basics of knowing multiple myeloma exists; now, we’re delving into its molecular intricacies. It’s like we’ve upgraded from playing checkers to a high-stakes game of chess with cancer cells. The result? Specialized medicines and treatments that are more potent than grandma’s secret chili recipe.
Therapeutic Techniques: The Avengers of Multiple Myeloma
Now, let’s talk about the superheroes fighting the villainous multiple myeloma. Picture this: stem cell transplants, immunomodulatory medications, proteasome inhibitors, chemotherapy, monoclonal antibodies, and supportive therapies — a formidable league of extraordinary treatments. Move over Avengers; the real action is happening in the bone marrow.
Market Snapshot — North America Leading the Charge
If the global multiple myeloma therapeutics market were a race, North America would be leading the pack like it stole the checkered flag. With a healthcare system that’s as intricate as a Shakespearean play and R&D investments that could make Elon Musk blush, North America is the undisputed champion.
But wait, there’s an underdog in this story — the Asia-Pacific region. With better access to healthcare, increased medical investments, and a growing understanding of multiple myeloma, this region is giving North America a run for its money. It’s like the healthcare version of ‘David vs. Goliath,’ only with fewer slingshots and more clinical trials.
For More Information: https://www.skyquestt.com/report/multiple-myeloma-therapeutics-market
Market Dynamics: The Good, the Bad, and the Expensive
Now, let’s address the elephant in the room — the cost of treatment. While we’re busy celebrating advancements in medicine, some of these treatments can be as expensive as a beachfront property in the Hamptons. High treatment costs might make you question whether fighting multiple myeloma is worth the financial battle.
Then there’s the complexity of the disease itself. Multiple myeloma isn’t your run-of-the-mill illness; it’s more like a Rubik’s Cube of genetic and molecular characteristics. Each patient is a unique puzzle, and finding the right combination of treatments can be as tricky as solving that cube blindfolded.
The Competition: Big Pharma’s Battle Royale
In the world of multiple myeloma therapeutics, it’s survival of the fittest. Big names like Johnson & Johnson, Amgen Inc., and Bristol Myers Squibb are flexing their research muscles to develop cutting-edge treatments. Partnerships and alliances are formed faster than you can say “monoclonal antibodies,” and the goal is clear — offer patients a fighting chance, a better quality of life, and a longer lease on life.
Recent Developments: FDA Approvals and the Game-Changers
Imagine the FDA as the gatekeeper of a mystical realm, and recent developments are the golden keys unlocking new possibilities. CAR T-cell treatments, bispecific antibodies, CD38 inhibitors — it’s like a pharmaceutical Renaissance, where each approval is a stroke of genius on the canvas of medical innovation.
Key Market Trends: Immunotherapies, Targeted Therapies, and Personalized Medicine
Move over, traditional treatments; immunotherapies and targeted medicines are stealing the spotlight. It’s like replacing your flip phone with the latest smartphone — more efficient, more effective, and definitely cooler. Plus, there’s a growing emphasis on personalized medicine, where biomarkers and genetic profiling play the role of matchmakers, pairing patients with treatments tailored to their unique disease characteristics.
Conclusion: The Thrill Ride Continues
In the realm of multiple myeloma therapeutics, the rollercoaster of growth, challenges, and breakthroughs keeps us on the edge of our seats. It’s a journey where science meets humanity, and the ultimate goal is to rewrite the narrative of multiple myeloma from a deadly disease to a conquerable foe.
So, as we ride the wave of advancements, approvals, and market dynamics, one thing is certain — the global multiple myeloma therapeutics market is not just a business venture; it’s a quest for life, longevity, and the triumph of science over adversity. Buckle up, because the thrill ride continues, and the best is yet to come.
About Us-
SkyQuest Technology Group is a Global Market Intelligence, Innovation Management & Commercialization organization that connects innovation to new markets, networks & collaborators for achieving Sustainable Development Goals.
Contact Us-
SkyQuest Technology Consulting Pvt. Ltd.
1 Apache Way,
Westford,
Massachusetts 01886
USA (+1) 617–230–0741
Email- [email protected]
Website: https://www.skyquestt.com
0 notes
Text
Forecasting the Antibody Drug Conjugate Market: Trends and Outlook
Market Overview –
The antibody drug conjugate (ADC) market is a segment within the pharmaceutical industry that focuses on a class of targeted cancer therapies. ADCs combine the specificity of monoclonal antibodies with the potency of cytotoxic drugs, offering a promising approach to cancer treatment. This market is driven by the increasing incidence of cancer worldwide, the need for more effective and targeted therapies, and advancements in biotechnology and drug delivery systems.
One of the key drivers of the ADC market is the demand for novel cancer treatments with improved efficacy and fewer side effects compared to traditional chemotherapy. ADCs offer a targeted approach, delivering cytotoxic drugs directly to cancer cells while sparing healthy tissues, thereby reducing systemic toxicity and enhancing patient outcomes.
Furthermore, the growing understanding of tumor biology and the identification of specific molecular targets have facilitated the development of ADCs tailored to different types of cancer. This personalized approach to treatment holds promise for patients with refractory or relapsed cancers who may not respond to conventional therapies.
The antibody drug conjugate market is experiencing rapid expansion, propelled by advancements in anti-drug conjugates. These innovative therapies combine the targeting precision of antibodies with potent anti-cancer drugs, offering promising treatment options for various cancers. With ongoing research and development efforts, the market for anti drug conjugates is poised for continued growth in the fight against cancer.
The COVID-19 pandemic has highlighted the importance of innovative therapies like ADCs in addressing unmet medical needs, especially in oncology. While the pandemic initially disrupted clinical trials and supply chains, the resilient nature of the biopharmaceutical industry has enabled continued research and development in this field.
However, challenges such as the complexity of ADC manufacturing, high development costs, and regulatory hurdles pose barriers to market growth. Nonetheless, with ongoing research and collaborations among pharmaceutical companies, academic institutions, and regulatory agencies, the ADC market is expected to witness significant expansion in the coming years, offering new hope to cancer patients worldwide.
With a predicted compound annual growth rate (CAGR) of 16.70% from 2022 to 2030, the antibody drug conjugate market, which was valued at USD 1.98 billion in 2021, is expected to rise from USD 2.31 billion in 2022 to USD 6.81 billion by 2030.
Segmentation –
As per MRFR report, the global antibody drug conjugate market is segmented on the basis of type, product, technology, application and end-user.
Based on type, it is segmented into drug/toxin, linker, monoclonal antibodies and others. Of these, the antibody drug conjugate linker is expected to have the maximum share in the antibody drug conjugate market.
Based on application, the antibody drug conjugate market is segmented into lymphoma, multiple myeloma, solid tumors, skin cancer, breast cancer, colon cancer, lung cancer, glioblastoma, ovary cancer, pancreas cancer, kidney cancer, prostate cancer and leukemia. Leukemia is further segmented into Chronic Lymphocytic Leukemia (CLL), Acute Lymphocytic Leukemia (ALL), Chronic Myeloid Leukemia (CML), and Acute Myeloid Leukemia (AML). Of these, breast cancer had the maximum share owing to its increasing prevalence.
Based on product, it is segmented into Kadcyla, Adcertis and others.
Based on technology, the antibody drug conjugate market is segmented into Immunomedics technology, Seattle Genetics technology, ImmunoGen technology and others.
Based on end-user, it is segmented into biopharmaceutical companies, biotechnology companies, academic research institutes, specialized cancer, and others.
Regional Analysis –
The antibody drug conjugate (ADC) market's regional dynamics depend on factors like research infrastructure, regulatory environment, and healthcare access. North America dominates, driven by robust research and development activities and favorable regulatory pathways. Europe follows, with a strong presence of biopharmaceutical companies and supportive policies for innovative therapies. Asia-Pacific is emerging as a significant market, fueled by investments in biotechnology and a growing patient population. Other regions, such as Latin America and Africa, are gradually gaining traction as awareness of ADC therapies increases. Market players must navigate regional differences in reimbursement policies and healthcare systems while capitalizing on opportunities for collaboration and expansion.
Key Players –
Antibody drug conjugate companies include ADC Therapeutics, Takeda Pharmaceutical Company Ltd., GlaxoSmithKline Plc, Hoffmann-La Roche Ltd., Daiichi Sankyo Company Ltd., Pfizer Inc., Seagen Inc., Gilead Sciences Inc., Astellas Pharma, among others.
Related Reports –
Steam Autoclave
Diagnostic Imaging
Neonatal Thermoregulation
Immunotherapy Drugs
For more information visit at MarketResearchFuture
#Antibody Drug Conjugate Market#Antibody Drug Conjugate Market Size#Antibody Drug Conjugate Market Share#Antibody Drug Conjugate Market Growth#Antibody Drug Conjugate Market Trends
0 notes