#Lims
Explore tagged Tumblr posts
Text
Forensic Science E-Magazine (Feb-March 2024)
We proudly present the January issue (Vol 20) of your favourite magazine, Forensic Science E-Magazine. As usual, the current issue has helpful content related to forensic science. #forensicscienceemagazine #forensicscience #forensicfield #crimescene
Continue reading Forensic Science E-Magazine (Feb-March 2024)

View On WordPress
#Artificial Intelligence Technology and Forensic Science#blood identification tests and examination#document examination instrument#fingerprint detection tests and examination#forensic science and ai#forensic science e magazine#Forensic science Magazine 2024 jan#LIMS#mcq on questioned document#tridimensional facial reconstruction#vital role and diverse application of forensic imaging in forensic field
4 notes
·
View notes
Text
(Un)fortunate Hero

[2/5] VITAL ORGANS FUNCTIONAL
OXYGENATION MANUAL
INTERVENTION REQUIRED FOR INJURY 1-4
COMPATIBLE PARTS FOR INJURY 1: 8
COMPATIBLE PARTS FOR INJURY 2: 1
COMPATIBLE PARTS FOR INJURY 3: 54
COMPATIBLE PARTS FOR INJURY 4: 17
NO INTERVENTION REQUIRED FOR INJURY 5-13
PART FOR INJURY 2 DISPENSED
AWAITING SELECTION FOR OTHER PARTS
SELECT OPTION 3 FOR 1, 48 FOR 3, AND 15 FOR 4. REQUISITION 2 MORE CLEANERS. RECALL ALL RETRIEVERS. ONCE ALL ACCOUNTED FOR, TAKE OFF AT 58% SPEED. WE NEED NO DISRUPTIONS. SUSTAIN ALL OTHER SUBJECTS. LET US SEE HOW FORMIDABLE THIS CREATURE IS.
#Lim#Lims#LimForma#webcomic#blood tw#injury tw#death tw#hospitalisation tw#medical procedures tw#scifi#robots#cybernetics#cyborgs
4 notes
·
View notes
Text
GxP Demystified: How LIMS Helps Regulated Labs Achieve Compliance

Is your lab struggling to keep up with regulatory guidelines such as GLP? Do you find it challenging to maintain consistent quality, traceability, and audit readiness in your operations?
Labs operate in a highly regulated environment, and complying with GxP standards is crucial for ensuring the safety, quality, and reliability of your lab operations.
Our latest blog breaks down the essential GxP guidelines—such as GLP, GMP, and GDocP—and explores how a Laboratory Information Management System (LIMS) can simplify compliance.
Read the blog in which we answer:
What is the GxP framework?
Which GxP guidelines are applicable to labs?
How does a LIMS help labs achieve and maintain GxP compliance?
Read the Blog
0 notes
Text
Quality Control in Pharmaceutical Industry | QC in Pharma Company

The Quality Control department's major and important role is in the Pharmaceutical industry.
The main role of the quality control department in the pharma industry is to check the quality of various products, such as raw materials, in-process samples, and finished products.
Their main agenda is to analyse and control the quality of the products at all stages of the manufacturing of API or Formulations.
QC is done by the Qualitative and Quantitative analysis of specific materials as per Stanard Testing Procedures (STP) or Method of Analysis.
Generally, the QC department is divided into four sections. These are main
Raw Materials
In-process Quality Checks (IPQC)
Finished Products
Stability Studies
Raw materials:
The materials come from outside industries or suppliers and road tankers check the quality of the materials as per in-house specifications or Standard testing procedures.
These are categorised into four parts.
General Raw materials:
These are some chemical analyses, like titrimetry, and chemical analysis methods, such as organic and inorganic acids, bases, salts, etc.
Ex: Hydrochloric acid(HCl), Sulphuric acid(H2SO4), Nitric acid(HNO3), Caustic soda(NaOH), Sodium carbonate(Na2CO3), Methanol, Toluene, Acetone, Dichloromethane etc…
Key Starting Materials (KSM):
These are the building blocks of drug intermediates or used to form the structure of compounds, APIs, or Drug substances.
The sampling method is different from general raw materials.
These are analyzed with both chemical and instrumental analysis.
Ex: Speciality Fine Chemicals, Drug Intermediates etc.
Packing Materials:
PM is used for Products/Compound materials that are stored
Ex: Fibre drums, HDPE, LDPE drums, Polyethene bags, etc…
Hazardous Materials:
HM are harmful or affect body raw materials to handling in careful safety precautions and as per its Material Safety Data Sheets (MSDS) so vendors or suppliers give a certificate of analysis based on these are approved as per customer COAs.
Ex: Sodium Hydride(NaH), Sodium Amide(NaNH2), NaCN etc…
Some catalysts are also approved as per customer COAs
Ex: Raney-Nikel, Palladium/Carbon(Pd/C) used for Hydrogenation reaction.
In-Process samples:
Online chemical and instrumental methods analysis as per in-house specification & STPs carried out samples coming from the manufacturing blocks or production department to time to give results after the process continuously.
Finished Products:
Complete Analysis carried out as per customer or In-house or Pharmacopia specification and Standard Testing Procedures of the final products.
The analysis carried out in the Quality control department is divided into two parts. These are
Chemical Analysis Laboratory (Wet Lab)
Volumetric analysis:
Chemical labs have five types of titrimetric analysis
Acid-Base Titration Ex: Hydrochloric acid (HCl), Sodium Hydroxide (NaOH)
Argentometric titration Ex: Sodium Chloride(NaCl), Aluminum chloride (AlCl3)
Redox Titration Ex: Sodium thiosulphate, Potassium permanganate
Complexometric titration Ex: Calcium chloride (Cacl2), Magnesium (Mg) and Metals
Non-aqueous titration for Drug intermediates and APIs Ex: 2-Amino Pyridine, Isonipotic acid etc..
Gravimetric analysis:
Gravimetric analysis is the mass of an ion in a compound and is determined to find out the mass per cent of the same ion in a known quantity of a compound.
Examples 1) The amount of sulphate as barium sulphate(BaSO4) from sodium sulphate(NaSO4).
2) Content of Nickel in Raney-Nickle catalyst and Palladium in Pd/C catalyst.
Wet laboratory, some important chemical analyses are
Ex: Water content(WC), Loss on drying(LOD), Residue on ignition(ROI), Specific Optical Rotation(SOR), Wt per mL, Thin Layer Chromatography(TLC), Tapped density, Friebilty, Dissolution, Disintegration etc.
Water Analysis:
Softener water: This water is used for boiler purposes to generate steam.
Demineralized or Deionised water: This water is used for chemical analysis and process areas.
Purified water: This water is used for the manufacturing process.
Three samples are collected to be analysed to their specification (WHO) and Standard testing procedures as per scheduled.
Instrumental methods of Chemical analysis
1) Chromatography:
Instrumental analysis to analyse quantitative and qualitative investigates analytes using the help of scientific instruments.
There are main two instrumental analyses carried out for Quality Control in the Pharmaceutical industry.
This technique separates and identifies the mixture of the compounds based on their relative affinity amounts of each compound distributed between a moving mobile phase, and a stationary phase. Mostly used instruments of Quality Control in the Pharmaceutical industry
1) High-Performance Liquid Chromatography (HPLC) 2) Gas Chromatography (GC)
2) Spectrophotometry:
Spectroscopic techniques are to pass a beam of electromagnetic radiation onto an unknown sample and observe to find out the difference between energy levels with reference.
Most commonly used spectrophotometers of Quality Control in the Pharmaceutical industry. There are
1) Ultra-Violet Spectrophotometer (UVS) 2) Fourier-transform infrared spectrometer (FTIR) and NIR 3) Atomic Absorption Spectrometer (AAS) and FAS
These are the main used Research Centres for Structure elucidations and Analytical Method Development.
1) Nuclear Magnetic Resonance Spectrometer (NMR)
2) Mass Spectrometer (MS)
3) Thermo Gravimetric Analysis (TGA)
4) Differential Thermal Analysis (DTA)
Stability Studies:
Stability studies are conducted for a re-test or expiry or a shelf life period for the drug substance or the drug product and recommended storage conditions.
These are analysed as per protocol or stability STP based on the schedule.
1) Hold-time stability studies 2) Long-term, Accelerated, intermediate condition studies
The quality control department follows systematic proper online documentation, Logbooks, Registers, Good Laboratory Practices (GLP) and Good Documentation Practices.
After complete analysis, documented respective analysis signed and checked authorised persons to prepare the certificate of analysis approved by the Head of the department or Designee.
Backup Electronic Data:
All electronic data stored in their servers or external hard disks are Empower network or Lab solution or Open Lab software and its data is backed up and retrieved every week by an IT person.
Conclusion:
The Quality Control department checks each step of the product manufacturing as per specification and standard testing procedures after releasing documented data.
#QC#STP#HPLC#GC#SOR#Analyst#GLP#21CFR11#NABL#LIMS#empower#Labsolution#Pharmacopias#EDQM#TGA#CDSCO#USFDA#ICH#Shimsdzu#Waters#Agilent#Thermoscientific#Remi#Labindia#Perkinelmler#WHO#AR&D#SOP
0 notes
Text
North America Leads the Surge in Laboratory Informatics Adoption
The global laboratory informatics market was valued at USD 4,376.2 million in 2023 and is projected to grow at a compound annual growth rate (CAGR) of 11.4%, reaching USD 9,176.6 million by 2030. The rising demand for lab automation, coupled with advancements in biobanking and increasing R&D investment in the biotech and pharmaceutical sectors, is driving this growth. Key Market Drivers The…

View On WordPress
0 notes
Text
Laboratory Information Management System (LIMS) Market Share, Sales Channels and Overview Till 2030
The Laboratory Information Management System (LIMS) Market was valued at USD 1.6 billion in 2023 and will surpass USD 4.0 billion by 2030; growing at a CAGR of 14.0% during 2024 - 2030. This need has fueled the growth of the Laboratory Information Management System (LIMS) market, a sector that is becoming increasingly essential in the fields of healthcare, pharmaceuticals, biotechnology, food and beverage, and more. In this blog, we’ll explore the current trends, market growth, and the key factors driving the expansion of the LIMS market.
A Laboratory Information Management System (LIMS) is a software solution designed to streamline the operations of a laboratory. It facilitates the management of samples, associated data, and laboratory workflows. LIMS systems are crucial for ensuring the accuracy, efficiency, and compliance of lab operations, particularly in regulated environments. This growth can be attributed to several factors, including the increasing demand for automation in laboratories, the rising adoption of cloud-based LIMS solutions, and the expanding need for data integrity and regulatory compliance.
Get a Sample Report: https://intentmarketresearch.com/request-sample/laboratory-information-management-system-lims-market-3637.html
Key Trends in the LIMS Market
Cloud-Based Solutions: One of the most significant trends in the LIMS market is the shift towards cloud-based solutions. Cloud LIMS offers several advantages over traditional on-premise systems, including lower upfront costs, easier scalability, and enhanced data accessibility from remote locations. As more laboratories seek flexibility and cost-effectiveness, cloud-based LIMS adoption is on the rise.
Integration with Emerging Technologies: The integration of LIMS with emerging technologies like artificial intelligence (AI), machine learning (ML), and the Internet of Things (IoT) is transforming the way laboratories operate. These technologies enable predictive analytics, automated decision-making, and real-time monitoring, further enhancing the efficiency and accuracy of lab processes.
Focus on Data Security and Compliance: With the increasing importance of data integrity and the growing complexity of regulatory requirements, LIMS providers are prioritizing data security and compliance features. Laboratories, particularly in the pharmaceutical and healthcare sectors, require systems that can ensure compliance with regulations like FDA 21 CFR Part 11, GDPR, and HIPAA.
Customization and Flexibility: As laboratories vary greatly in their needs and workflows, there is a growing demand for customizable and flexible LIMS solutions. Vendors are offering more modular and configurable systems that can be tailored to the specific requirements of different labs, whether they are in research, clinical diagnostics, or quality control.
Growing Adoption in Emerging Markets: The LIMS market is not just expanding in developed regions like North America and Europe but is also seeing significant growth in emerging markets such as Asia-Pacific and Latin America. The increasing investment in healthcare infrastructure and R&D activities in these regions is driving the demand for LIMS solutions.
Key Players in the LIMS Market
Several companies dominate the LIMS market, offering a range of solutions tailored to various industries. Some of the leading players include Thermo Fisher Scientific, LabWare, LabVantage Solutions, STARLIMS (Abbott Informatics), and PerkinElmer. These companies are continuously innovating to offer advanced features, user-friendly interfaces, and robust support services to meet the evolving needs of laboratories worldwide.
Get an insights of Customization: https://intentmarketresearch.com/ask-for-customization/laboratory-information-management-system-lims-market-3637.html
Challenges Facing the LIMS Market
Despite the growth and innovation in the LIMS market, there are challenges that vendors and laboratories must navigate. These include the high costs associated with implementing and maintaining LIMS, the complexity of integrating LIMS with existing laboratory systems, and the need for ongoing training and support for laboratory staff.
Conclusion
The Laboratory Information Management System (LIMS) market is poised for significant growth in the coming years, driven by the increasing demand for automation, data integrity, and regulatory compliance in laboratories. As technology continues to evolve, LIMS solutions will become even more integral to the efficient and accurate operation of labs across various industries. For laboratories looking to stay competitive and compliant, investing in a robust LIMS solution is no longer just an option—it’s a necessity.
0 notes
Text
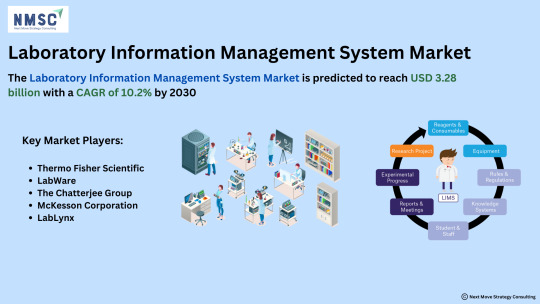
𝙄𝙣-𝘿𝙚𝙥𝙩𝙝 𝙎𝙩𝙪𝙙𝙮 𝙤𝙛 𝙩𝙝𝙚 𝙇𝙖𝙗𝙤𝙧𝙖𝙩𝙤𝙧𝙮 𝙄𝙣𝙛𝙤𝙧𝙢𝙖𝙩𝙞𝙤𝙣 𝙈𝙖𝙣𝙖𝙜𝙚𝙢𝙚𝙣𝙩 𝙎𝙮𝙨𝙩𝙚𝙢 (𝙇𝙄𝙈𝙎) 𝙈𝙖𝙧𝙠𝙚𝙩
𝙂𝙚𝙩 𝙖 𝙁𝙍𝙀𝙀 𝙎𝙖𝙢𝙥𝙡𝙚: https://www.nextmsc.com/laboratory-information-management-system-market/request-sample
As the world of laboratory management continues to evolve, the 𝙇𝙖𝙗𝙤𝙧𝙖𝙩𝙤𝙧𝙮 𝙄𝙣𝙛𝙤𝙧𝙢𝙖𝙩𝙞𝙤𝙣 𝙈𝙖𝙣𝙖𝙜𝙚𝙢𝙚𝙣𝙩 𝙎𝙮𝙨𝙩𝙚𝙢 (𝙇𝙄𝙈𝙎) 𝙈𝙖𝙧𝙠𝙚𝙩 is experiencing transformative growth. With advancements in technology and an increased focus on efficiency and data integrity, LIMS solutions are becoming indispensable across various industries.
𝙆𝙚𝙮 𝙄𝙣𝙨𝙞𝙜𝙝𝙩𝙨:
𝙈𝙖𝙧𝙠𝙚𝙩 𝙀𝙭𝙥𝙖𝙣𝙨𝙞𝙤𝙣: The LIMS market is on a rapid ascent, driven by the need for streamlined operations and enhanced data management.
𝙏𝙚𝙘𝙝𝙣𝙤𝙡𝙤𝙜𝙞𝙘𝙖𝙡 𝙄𝙣𝙣𝙤𝙫𝙖𝙩𝙞𝙤𝙣𝙨: Integration with cloud computing, AI, and IoT is revolutionizing how laboratories manage and analyze data.
𝙍𝙚𝙜𝙪𝙡𝙖𝙩𝙤𝙧𝙮 𝘾𝙤𝙢𝙥𝙡𝙞𝙖𝙣𝙘𝙚: Enhanced capabilities to meet stringent regulatory requirements are propelling the adoption of LIMS.
𝙒𝙝𝙮 𝙄𝙩 𝙈𝙖𝙩𝙩𝙚𝙧𝙨: Efficiency & Accuracy: LIMS solutions optimize workflow, reduce errors, and ensure precise data tracking.
𝘿𝙖𝙩𝙖 𝙎𝙚𝙘𝙪𝙧𝙞𝙩𝙮: Advanced systems offer robust security features to protect sensitive information.
𝙎𝙘𝙖𝙡𝙖𝙗𝙞𝙡𝙞𝙩𝙮: LIMS can be tailored to meet the needs of small labs and large research facilities alike.
𝙆𝙚𝙮 𝙋𝙡𝙖𝙮𝙚𝙧𝙨:
Thermo Fisher Scientific
LabWare
The Chatterjee Group
McKesson Corporation
LabLynx
Computing Solutions
Labworks LLC
Illumina
Novatek International
Agilent Technologies, Inc.
𝘼𝙘𝙘𝙚𝙨𝙨 𝙁𝙪𝙡𝙡 𝙍𝙚𝙥𝙤𝙧𝙩: https://www.nextmsc.com/report/laboratory-information-management-system-market
As we look towards the future, the LIMS market is poised for continued innovation and growth, paving the way for more efficient and effective laboratory management.
Let’s stay ahead of the curve and explore the exciting opportunities within the LIMS landscape!
#LIMS#laboratory management#data in tegration#innovation#marke tgrowth#tech trends#data security#market research#market trends#healthcare
0 notes
Text
Lab Informatics Revolution: Top Products, AI Trends & Innovations from Waters, Thermo Fisher, and Beyond!
Streamline your lab workflows! Explore the latest informatics solutions from industry leaders like Waters, Thermo Fisher, and more. Discover LIMS, ELNs, CDS, and how AI is transforming research. #LabInformatics
Laboratory Informatics Market: A Look at Top Products and Innovations The laboratory informatics market is a dynamic hub of software solutions streamlining research and development workflows. But with a vast array of products available, let’s explore some key questions: 1. Who Wears the Crown? Market Share Leaders in Laboratory Informatics Currently, Laboratory Information Management Systems…

View On WordPress
#AIforScience#AIinScience#APACBiotech#bigdataanalytics#CDS#ClinicalResearch#CloudLabSolutions#DrugDiscovery#ELN#labinformatics#LaboftheFuture#LIMS#MachineLearning#NextGenLIMS#ScienceInnovation#ThermoFisherScience#ThermoFisherScientific#Waters
0 notes
Text

#LabInformatics#DigitalTransformation#LaboratoryAutomation#Bioinformatics#LIMS#ELN#DataManagement#HealthTech
0 notes
Text

#give him his bridge back#Shane’s bridge privileges have been revoked till further notice#watcher#shane and ryan#shane madej#ryan bergara#buzzfeed unsolved#steven lim#puppet history#ghost files#mystery files
14K notes
·
View notes
Text
Barbershop

Byr: Well, I can certainly say you aren't a great barber, whatever you are. I take it these changes are pretty permanent then? YES. YOU WILL NOT SURVIVE IF THEY ARE REMOVED. Byr: I was leading a group of men. What happened to them? WE WERE ABLE TO SAVE 5 OTHERS FROM THE BATTLEFIELD. YOU MAY SEE THEM TOMORROW, BUT WE MUST ALLOW A DAY TO AVOID ANY POTENTIAL INFECTION. Byr: So what are you? Some rogue doctor? A mad machine? Drug induced hallucination? IF WE APPEAR AS A BLUE METAL CREATURE TO YOU, THEN IT IS NOT A HALLUCINATION. IF WE APPEAR AS SOMETHING ELSE, PLEASE DESCRIBE IT. WE ARE A NETWORK OF MACHINES. SOME BORN OF FLESH. MOST BUILT FROM METAL. WE WILL GIVE YOU A HISTORY LESSON IF YOU DESIRE. SIMPLY, WE SEEK TO HELP LIFE OF ALL FORMS. CURRENTLY, WE ARE INVESTIGATING YOUR PLANET. WE FELT IT CRUEL TO LET YOU DIE, AND HAVE POTENTIAL USES FOR YOU THAT COULD BENEFIT ALL OF US. YOU ARE ALSO THE PERFECT TEST SUBJECT FOR THIS PROCEDURE WE HAVE BEEN WORKING ON. Byr: So am I part of this network then? Have you drafted me to be some sort of robot? NO. ALL IN THE NETWORK ARE WILLING VOLUNTEERS. PERHAPS LATER ON YOU WILL BE WELCOMED TO THE NETWORK, BUT YOUR CONSENT IS UNCERTAIN DUE TO THE PROCEDURE. WE CAN DISCUSS THE FUTURE WHEN YOU ARE MORE LUCID, BUT REST ASSURED, YOU ARE FREE. Byr: I don't feel very free being stuck to a bed. UNDERSTANDABLE; HOWEVER, MOVING COMES WITH A GREAT RISK OF DEATH. AS SOON AS YOU ARE ABLE, YOU MAY GO WHEREVER YOU PLEASE. WE SHALL NOT LEAVE YOU TO SUFFER BOREDOM. WE HAVE MANY VIDEOS YOU MAY WATCH WHILE AWAKE TO PASS THE TIME. YOU MAY WATCH THE ANTICS OF THE GOLS, INTERACTIONS BETWEEN THE MONSTROSITIES, TRIALS OF PERFECTION. WE ALSO LEAVE YOU WITH A LIM ATTENDANT. TOMORROW, THEY CAN MOVE YOU AROUND THE TOWER SHOULD YOU WISH TO EXPLORE. GOING OUTSIDE IS NOT ALLOWED UNTIL YOUR WOUNDS HAVE HEALED. Byr: Well, I certainly don't want to die, and I'm not going to pass up on some rest and entertainment. Guess I'll just try and make the most of this. Lim? Could you show me those Gol things they were talking about?
2 notes
·
View notes
Text
youtube
#tourism#love#youtube#health and wellness#mental health#agriculture#love poetry#mountains#Pruning#@Tree#Garden#Agriculture#Fruits#Lims#Branches#Leaves#Seasons#Pruning trees#Prunning plants#Crops#Youtube
0 notes
Text
CloudLIMS Releases Version 4.00 of its Laboratory Information Management System (LIMS)

We are delighted to announce the latest release, version 4.00, of CloudLIMS—a SaaS, in-the-cloud LIMS with zero upfront cost for biobanks, clinical research, clinical diagnostics, and analytical testing labs such as cannabis, environmental, material, food and beverage, and water testing labs. Take your lab to the next level of performance and efficiency with the latest version of CloudLIMS.
The latest version includes the ability to:
Place Sample Purchase Requests: The latest version enables your clients to submit sample purchase requests based on the study needs by selecting the required samples. Once the order is placed, a unique Request ID is automatically generated for seamless tracking throughout the process. Your clients can edit their requests until they are accepted by your lab, offering flexibility in managing orders. To prevent stockouts, the system automatically restricts requests for samples if the quantity exceeds availability. This enhancement streamlines the entire purchase process, making it more efficient, flexible, and user-friendly for you and your clients.
Edit Approved Service Requests: You can now edit approved service requests, allowing you to modify test catalogs or panels associated with the request. This functionality provides the flexibility to add or remove tests from an existing service request, with automatic adjustments to pricing, ensuring accurate billing and streamlined service management. For instance, if a client orders a test package containing 5 tests, but only 3 tests are performed due to sample unavailability, you can remove the unperformed tests and adjust the invoice accordingly. On the other hand, in cases where a new test request is submitted for a sample already received in the lab, you can associate the new test with the existing service request. This eliminates the need for creating a new request, improving workflow efficiency and reducing administrative overhead.
Personalize Test Reports with Client Information: You can now include client details in the header of your test reports for a professional and personalized touch. You can select up to three client attributes, such as Company Name, Address, and Phone Number, to display. Furthermore, you can customize the layout by positioning the selected attributes to the left, center, or right, as per your preference. This functionality enables you to further tailor test reports or certificates of analysis based on the reporting requirements of clients and regulatory bodies.
…and much more!
Read the Blog
0 notes
Text

The most important comment in this whole mess
#watcher entertainment#watcher#shane madej#shane and ryan#ryan bergara#steven lim#watcher streaming service#L
11K notes
·
View notes
Text
i lied abt only posting abt this situation once, i just saw this lovely person’s comment!! passing it on to tumblr 🫡
edit: SOMEONEE changed the password and ruined it for everybody :/ pls try this version instead!!
edit 2: WATCHER POSTED AN UPDATE
#i will cry i love the watcher community#watcher#we are watcher#ghost files#mystery files#puppet history#shane madej#ryan bergara#steven lim#i forgot some tags oopsies#buzzfeed unsolved#watcher entertainment#too many spirits#are you scared#top 5 beatdown#hall of lame
10K notes
·
View notes