#Left anterior descending artery
Explore tagged Tumblr posts
Text
Recognize the Signs of a Heart Attack - Know Your Left Anterior Descending Artery(LAD)!

A heart attack occurs when blood flow gets obstructed while going to the heart, resulting in cardiac muscle damage. One of the major arteries that can become blocked during a heart attack is the left anterior descending artery (LAD). In this article, we will explore the structure of the heart, what the LAD is, and the warning signs of a heart attack.
Structure of the Heart
The heart is an essential organ that plays the role of pumping blood throughout the body. It comprises four chambers – the right atrium, right ventricle, left atrium, and left ventricle. Blood is carried from the body to the right atrium, then to the right ventricle, and finally to the lungs for oxygenation. The oxygen-rich blood is then taken from the lungs to the left atrium, the left ventricle, and the rest of the body.
The LAD is one of the major arteries that supplies blood to the heart muscle. It is located in the front of the heart and runs along the surface of the heart muscle. A blockage of the LAD can lead to a heart attack and can be life-threatening.
What is LAD in Heart?
LAD stands for left anterior descending artery. It is one of the main arteries that supply oxygen-rich blood to the heart muscle. The LAD supplies blood to the front and side of the heart and is responsible for a significant portion of the blood supply to the left ventricle.
Warning Signs of a Heart Attack
Identifying the warning heart attack signs is important for seeking immediate medical attention and reducing the risk of long-term heart damage. These signs of a heart attack vary from person to person, but common symptoms include:
Chest pain or discomfort: This is the most common heart attack symptom. It may feel like a tightness, pressure, or squeezing sensation in the chest that can last several minutes or come and go.
Shortness of breath: Feeling breathless, even at rest or with minimal exertion, can indicate a heart attack.
Sweating: Feeling sweaty, even in a cool environment, can signify a heart attack.
Nausea or vomiting: Nausea or vomiting can indicate a heart attack, especially in women.
Fatigue: Feeling unusually tired or weak, especially if it comes on suddenly, can signify a heart attack.
Pain in body parts: A discomfort in the arms, back, jaw, or stomach can also signify a heart attack.
It is essential to remember that not everyone experiences these warning signs, and some may experience no symptoms. It is vital to seek timely medical attention if you suspect a heart attack, even if you are not experiencing any symptoms.
Conclusion
The LAD is an important artery that supplies blood to the heart muscle. A blockage of the LAD can lead to a heart attack, which can be life-threatening. Identifying the warning signs of a heart attack is important for seeking immediate medical attention and reducing the risk of long-term heart damage. If you experience any warning signs of a heart attack, seek medical attention immediately.
To learn more about Heart Attack and its symptoms, visit: https://www.platinumforheart.in/
#health#heart attack#signs of heart attack#Structure of heart attack#Left anterior descending artery#LAD#How heart attack occurs#Platinum For Heart#Symptoms of heart attack
0 notes
Text
😨
#definitely did not do well on that health test 😭😭#quick question#is the left anterior descending artery a thing or did I just make that up
1 note
·
View note
Text
so my dad recently had a heart attack and had to have a stent put in his left anterior descending artery or, as it is colloquially known, the Widowmaker artery (he's doing a lot better now) and the baby was of course both alarmed and extremely interested to hear about what had happened to granddaddy. and kept asking "but what is a stent?" over and over even after we had tried to explain
so my mom found a little animated video of a stent going into an artery and showed it to him and he watched it and said "I want to watch that again" and my mom played it for him on her phone like a dozen times because he kept asking to watch it again
and finally he looked up with a giant smile on his face and said "now I know what a stent is!"
#baby nephew#the best thing for being sad is to learn something#he's also learning to read! it's wild i have to write a post about the way he sounds out words
119 notes
·
View notes
Text

Paramedic Incident Report
Incident Number: 2024-19245 Date: December 6, 2024 Time of Call: 15:23
Incident Location: ClimbX Indoor Gym, 345 Summit Street, Boulder, CO
Patient Information:
Name: Daniel Carson
Age: 20
Gender: Male
Height: 5'11"
Weight: 165 lbs
Physical Description: Lean and muscular build with well-defined arms and torso typical of an experienced climber. Short dark brown hair, light complexion.
Description of Incident: At 15:23, dispatch received a 911 call reporting a young male climber had collapsed while bouldering at an indoor climbing facility. The patient was reportedly scaling a mid-level climb when witnesses described him suddenly clutching his chest, losing his grip, and falling to the mat below. He was unresponsive upon initial assessment by gym staff.
Initial Assessment Upon Arrival (15:30):
Level of Consciousness: Unresponsive
Pulse: Absent
Respiratory Effort: None
Skin Condition: Pale, cool, and clammy
Pupils: Fixed and dilated
Bystanders reported that staff initiated CPR immediately after the collapse and delivered one shock using the facility's automated external defibrillator (AED).
Treatment at Scene (15:30-15:45):
CPR: High-quality chest compressions continued upon paramedics’ arrival.
Airway Management: Airway secured with a bag-valve mask; oxygen at 15 L/min.
AED Analysis: AED advised one additional shock, which was administered at 15:35. Return of spontaneous circulation (ROSC) achieved at 15:37.
Vital Signs Post-ROSC:
Pulse: Weak and irregular at 45 bpm
Blood Pressure: 80/50 mmHg
Respiration: Shallow and labored at 10 breaths/min
Oxygen Saturation: 78%
Transport Summary (15:45-16:00): Patient was loaded into the ambulance for transport to St. Anthony's Hospital. During transport, the patient exhibited further signs of cardiac distress. At 15:50, he experienced ventricular fibrillation (VF).
Intervention: CPR resumed, epinephrine 1 mg administered IV, and defibrillation attempted twice.
Outcome: No ROSC achieved after second cardiac arrest.
Time of Death: 16:00
Remarks: The patient suffered two cardiac arrests within a 30-minute period, likely indicative of a severe underlying cardiac condition. Efforts to stabilize were unsuccessful due to continued arrhythmias and compromised circulation.
Autopsy Report
Case Number: 2024-AU-1245 Date of Examination: December 7, 2024 Time of Examination: 09:00
Name: Daniel Carson Age: 20 Height: 5'11" Weight: 165 lbs Sex: Male Race: Caucasian
External Examination:
General Appearance: Well-developed and muscular young male. No evidence of external trauma except for mild abrasions on the back of hands and forearms, consistent with climbing activities. Skin pale with slight cyanosis around the lips and nail beds.
Scars/Marks: None significant.
Tattoos: None noted.
Clothing: Patient arrived wearing climbing shorts and a tank top.
Internal Examination:
Cardiovascular System:
Heart: Enlarged, weighing 420 grams (average for age/weight: 300-350 grams).
Valves: Mitral valve revealed significant calcification and fibrosis, indicative of a congenital defect. The defective valve exhibited stenosis, which restricted blood flow and created turbulent circulation.
Coronary Arteries: Severe occlusion (95%) of the left anterior descending (LAD) artery due to atherosclerotic plaque.
Myocardium: Evidence of acute ischemic changes and scarring, suggesting prior silent infarctions. The ventricular walls were thickened (hypertrophic cardiomyopathy).
Aorta: Normal caliber and appearance.
Respiratory System:
Lungs congested, with frothy fluid in the trachea and bronchi.
Right lung: 450 grams; Left lung: 430 grams.
Gastrointestinal System:
Stomach contained approximately 200 mL of partially digested food.
No abnormalities in the esophagus, stomach, or intestines.
Central Nervous System:
Brain weight: 1,450 grams. No gross abnormalities.
Other Organs:
Liver: Enlarged (1,600 grams), possibly due to mild congestion.
Kidneys: Unremarkable.
Spleen: Normal size.
Microscopic Examination:
Heart Tissue: Acute myocardial infarction visible in sections of the left ventricle.
Coronary Arteries: Advanced plaque buildup with rupture and thrombus formation.
Mitral Valve: Fibrotic thickening and calcification evident.
Toxicology:
No evidence of drugs or alcohol.
Summary and Cause of Death: Daniel Carson, a 20-year-old male, died from complications of a congenital mitral valve defect and severe coronary artery disease. The primary event was a massive myocardial infarction triggered by the blockage of the LAD artery. A second cardiac arrest during transport proved fatal.
Final Diagnosis:
Acute myocardial infarction secondary to LAD artery occlusion.
Congenital mitral valve stenosis and calcification.
Hypertrophic cardiomyopathy contributing to cardiac instability.
Cause of Death: Cardiac arrest due to a defective valve and blocked artery.
Manner of Death: Natural.
Signed by: Dr. Margaret Li, MD Pathologist
27 notes
·
View notes
Text
HOW ARE WE FEELING TONIGHT LADS (LEFT ANTERIOR DESCENDING ARTERIES)
4 notes
·
View notes
Text


Finally it's Friday. We closed with a pratical cardiology class. In this photo, we saw catheterization in a patient with atherosclerosis in the left anterior descending artery. I'm starting to think about doing cardiology. 🫀
Photo 2: Guimarães Rosa is my favorite writer. I discovered this book by Rancière, talking about Rosa's writing. I discovered that I like Rosa's writing more than I imagined.
6 notes
·
View notes
Text

I never post anything personal on Tumblr, but check out my new tattoo!
A year ago today I had a massive heart attack at 26 years old due to an arterial dissection, where the lining of your artery rips and fills in with blood, causing a blockage. Mine was a 100% blockage of the left anterior descending artery (the big central one) also known as the Widow Maker. They were able to stent it just in time to keep me alive, but I was moments away from going skull and crossbones mode.
Needless to say, the experience changed quite literally everything about my life. Here’s my favorite things I learned as a direct result of my near death experience:
Cardiac rehabilitation is a great place to meet older people who have incredible perspective
Exercise actually is incredibly important for your health, even if it’s only 10 minutes of getting your heart rate over 115 as often as you can it will be beneficial
Everything in life is about progress, not perfection. If you’re doing something good, don’t worry about what you did yesterday or last month or last week. You did something good!
There’s nothing in the world you should prioritize over your own freedom. Making other people happy will not make anyone feel good if you aren’t happy yourself, especially not you!
Not everyone is going to be chill with the fact that you’ve decided to put yourself first. Shrug it off because that’s their issue, not yours.
If someone is upset by your tragedy, let them be and don’t minimize it. In their mind they may be attending your funeral. Be grateful that they care.
Emotions can be triggered by seemingly innocuous things, and all emotions are information. Take a second to parse what your emotions are trying to tell you, and do your best to react in kindness.
Make peace with the hard truths of life, like the fact that there will never be enough time to do everything you want to do. Do what you can with the time you do have.
Things can be funny and tragic at the same time, and my life is much better for having the experience. I’d like to apologize to those who love me for almost doing an Irish exit on being alive and thank you from the bottom of my now slightly less functional heart for being a part of my life. Each and every one of you is deeply cherished.
2 notes
·
View notes
Photo
^^^ ⚠️incorrect description alert⚠️
We're gonna talk about what this actually is because the dipshits on facebook and tumblr don't know what they're looking at.
First off, this is a 3D CT angiogram if I had to guess, no one stripped away anything. That would be insane and basically impossible to pull out all those arterioles. Seond, there is no such thing as an "angel vein," they made that shit up. Finally, these are arteries, not veins. You can see the aorta at the top with right and left coronary arteries coming off of it. The big artery on the left is the right co3rnary artery. The one in the middle is the left anterior descending artery. The two on the right are the left marginal and circumflex arteries. Fun fact: the widowmaker artery is the LAD, and if that is obstructed (heart attack), you have just cut off about half of the heart's blood supply (this is bad).
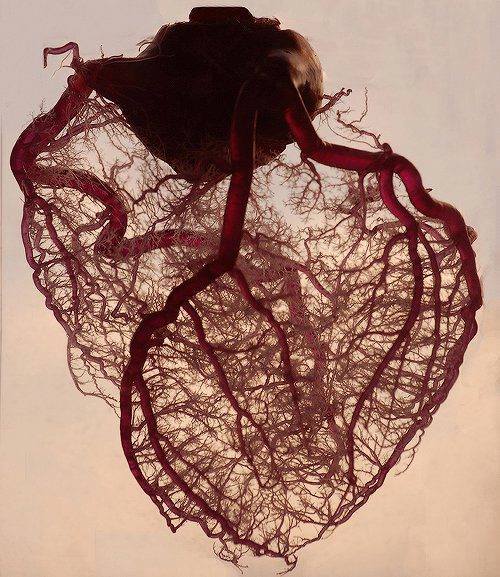
The human heart stripped of fat and muscle, with just the angel veins exposed.
230K notes
·
View notes
Text
LMCA Full Form in Medical: Understanding the Left Main Coronary Artery
LMCA Full Form in Medical: Understanding the Left Main Coronary Artery
The Left Main Coronary Artery (LMCA) is a vital blood vessel in the heart's circulatory system. It originates from the aorta and supplies oxygen-rich blood to critical parts of the heart. The LMCA quickly branches into two major arteries: the Left Anterior Descending (LAD) artery and the Left Circumflex (LCX) artery. These branches are responsible for delivering blood to the front, left side, and back of the heart.
The proper functioning of the LMCA is essential for maintaining the heart’s health and, consequently, the body's overall well-being. A blockage or narrowing in this artery can lead to severe complications, including heart attacks or even death, as it controls the blood flow to a large portion of the heart muscle. Left Main Coronary Artery disease is one of the more serious forms of coronary artery disease (CAD).
Understanding the LMCA’s role in the cardiovascular system is crucial, especially for diagnosing and treating heart-related conditions. Medical professionals use tools like angiograms to assess the health of the LMCA and determine the best treatment options, such as stents or bypass surgery, to restore normal blood flow.

0 notes
Text
Stent Embolization_Crimson Publishers

Abstract
Although the number of PCI procedures has significantly increased over the years, the severe complications are rare. Stent embolization is a rare complication of coronary stenting, at a rate of 0.2%. We report on a case with a 64-year-old man admitted to the emergency department with unstable angina and stent embolization during the coronary angiography and successful retrieval of a coronary stent from the left anterior descending (LAD) artery, which migrated to the left popliteal artery. The stent was finally removed surgically.
Read More About this Article: https://crimsonpublishers.com/aics/fulltext/AICS.000582.php
Read More Articles: https://crimsonpublishers.com/aics/
#peer reviewed journal of aics#crimson publishers#advancements in case studies#journal of case studies#open access journals case studies#medical case reports#crimsonpublishers#open access journal#peer reviewed journals#open access journals
0 notes
Text
So my cardiac blockages have never got so far as to cause a heart attack. I have had 3 angioplasties and now have 2 cardiac stents.
I had my first incident at age 31, more than 30 years ago (yes, I’m old). I was fairly active, walking and practicing Aikido. I started getting what felt like indigestion. It seemed to happen when I was out walking.
I went to 2 pool parties on Labor Day Weekend that year. At both, as was my habit, I towed little kids around the pool on floats. It’s strenuous. I had the indigestion thing worse than ever …and I noticed it was in time with my pulse.
I took myself to the hospital on the Monday holiday. I had the damnedest time getting them to consider the idea of a heart problem. One young cardiologist supported me. He said my family history was compelling (both my parents had multiple angioplasties at the dawn of the use of this technique).
It turned I had a 95% or greater blockage of the left anterior descending (LAD) artery of the heart. The LAD is the “widow maker” because a complete blockage will kill you in no time.
The next two times it happened were 6 years ago and last September. I called the office of my cardiologist - the same cardiologist - and they took me right away, did imaging, and put me in the hospital for a stent.
KNOW YOUR BODY. Pay attention to what it’s telling you. Don’t take no for an answer.
A nurse has heart attack and describes what she felt like when having one
I am an ER nurse and this is the best description of this event that I have ever heard.
FEMALE HEART ATTACKS
I was aware that female heart attacks are different, but this is description is so incredibly visceral that I feel like I have an entire new understanding of what it feels like to be living the symptoms on the inside. Women rarely have the same dramatic symptoms that men have… you know, the sudden stabbing pain in the chest, the cold sweat, grabbing the chest & dropping to the floor the we see in movies. Here is the story of one woman’s experience with a heart attack:
"I had a heart attack at about 10:30 PM with NO prior exertion, NO prior emotional trauma that one would suspect might have brought it on. I was sitting all snugly & warm on a cold evening, with my purring cat in my lap, reading an interesting story my friend had sent me, and actually thinking, ‘A-A-h, this is the life, all cozy and warm in my soft, cushy Lazy Boy with my feet propped up. A moment later, I felt that awful sensation of indigestion, when you’ve been in a hurry and grabbed a bite of sandwich and washed it down with a dash of water, and that hurried bite seems to feel like you’ve swallowed a golf ball going down the esophagus in slow motion and it is most uncomfortable. You realize you shouldn’t have gulped it down so fast and needed to chew it more thoroughly and this time drink a glass of water to hasten its progress down to the stomach. This was my initial sensation–the only trouble was that I hadn’t taken a bite of anything since about 5:00 p.m.
After it seemed to subside, the next sensation was like little squeezing motions that seemed to be racing up my SPINE (hind-sight, it was probably my aorta spasms), gaining speed as they continued racing up and under my sternum (breast bone, where one presses rhythmically when administering CPR). This fascinating process continued on into my throat and branched out into both jaws. ‘AHA!! NOW I stopped puzzling about what was happening – we all have read and/or heard about pain in the jaws being one of the signals of an MI happening, haven’t we? I said aloud to myself and the cat, Dear God, I think I’m having a heart attack! I lowered the foot rest dumping the cat from my lap, started to take a step and fell on the floor instead. I thought to myself, If this is a heart attack, I shouldn’t be walking into the next room where the phone is or anywhere else… but, on the other hand, if I don’t, nobody will know that I need help, and if I wait any longer I may not be able to get up in a moment.
I pulled myself up with the arms of the chair, walked slowly into the next room and dialed the Paramedics… I told her I thought I was having a heart attack due to the pressure building under the sternum and radiating into my jaws. I didn’t feel hysterical or afraid, just stating the facts. She said she was sending the Paramedics over immediately, asked if the front door was near to me, and if so, to un-bolt the door and then lie down on the floor where they could see me when they came in. I unlocked the door and then laid down on the floor as instructed and lost consciousness, as I don’t remember the medics coming in, their examination, lifting me onto a gurney or getting me into their ambulance, or hearing the call they made to St. Jude ER on the way, but I did briefly awaken when we arrived and saw that the radiologist was already there in his surgical blues and cap, helping the medics pull my stretcher out of the ambulance. He was bending over me asking questions (probably something like ‘Have you taken any medications?’) but I couldn’t make my mind interpret what he was saying, or form an answer, and nodded off again, not waking up until the Cardiologist and partner had already threaded the teeny angiogram balloon up my femoral artery into the aorta and into my heart where they installed 2 side by side stints to hold open my right coronary artery.
I know it sounds like all my thinking and actions at home must have taken at least 20-30 minutes before calling the paramedics, but actually it took perhaps 4-5 minutes before the call, and both the fire station and St Jude are only minutes away from my home, and my Cardiologist was already to go to the OR in his scrubs and get going on restarting my heart (which had stopped somewhere between my arrival and the procedure) and installing the stents. Why have I written all of this to you with so much detail? Because I want all of you who are so important in my life to know what I learned first hand.
1. Be aware that something very different is happening in your body, not the usual men’s symptoms but inexplicable things happening (until my sternum and jaws got into the act). It is said that many more women than men die of their first (and last) MI because they didn’t know they were having one and commonly mistake it as indigestion, take some Maalox or other anti-heartburn preparation and go to bed, hoping they’ll feel better in the morning when they wake up… which doesn’t happen. My female friends, your symptoms might not be exactly like mine, so I advise you to call the Paramedics if ANYTHING is unpleasantly happening that you’ve not felt before. It is better to have a ‘false alarm’ visitation than to risk your life guessing what it might be! 2. Note that I said ‘Call the Paramedics.’ And if you can take an aspirin. Ladies, TIME IS OF THE ESSENCE! Do NOT try to drive yourself to the ER - you are a hazard to others on the road. Do NOT have your panicked husband who will be speeding and looking anxiously at what’s happening with you instead of the road. Do NOT call your doctor – he doesn’t know where you live and if it’s at night you won’t reach him anyway, and if it’s daytime, his assistants (or answering service) will tell you to call the Paramedics. He doesn’t carry the equipment in his car that you need to be saved! The Paramedics do, principally OXYGEN that you need ASAP. Your Dr. will be notified later. 3. Don’t assume it couldn’t be a heart attack because you have a normal cholesterol count. Research has discovered that a cholesterol elevated reading is rarely the cause of an MI (unless it’s unbelievably high and/or accompanied by high blood pressure). MIs are usually caused by long-term stress and inflammation in the body, which dumps all sorts of deadly hormones into your system to sludge things up in there. Pain in the jaw can wake you from a sound sleep. Let’s be careful and be aware. The more we know the better chance we could survive to tell the tale.“
Reblog, repost, Facebook, tweet, pin, email, morse code, fucking carrier pigeon this to save a life! I wish I knew who the author was. I’m definitely not the OP, actually think it might be an old chain email or even letter from back in the day. The version I saw floating around Facebook ended with “my cardiologist says mail this to 10 friends, maybe you’ll save one!” And knew this was way too interesting not to pass on.
#health#heart#heart attack in women#cardiology#nurse#emt#nurblr#nurseblr#medicine#emergency#heart attack
276K notes
·
View notes
Text



Paramedic Report
Incident Number: 2024-07-08-DK-0562 Patient Name: Darren Kozlowski Age: 31 Sex: Male Height: 6’1” Weight: Approx. 185 lbs Date of Incident: July 8, 2024 Time of Call Received: 10:12 AM
Incident Description: Emergency services were dispatched to a trail located in Riverbend Park following an alert from the patient’s smartwatch, which detected a suspected cardiac event. Bystanders reported finding the patient collapsed approximately 1.3 miles into the trail. The patient was unresponsive and pulseless upon paramedic arrival at 10:22 AM.
Initial Assessment:
Airway: Clear
Breathing: Apneic
Circulation: No palpable pulse; asystole confirmed on ECG
Skin Condition: Cool, pale, diaphoretic
Interventions (On-Site):
CPR initiated: High-quality chest compressions performed immediately upon arrival.
Defibrillation: Delivered one shock (200J) following identification of ventricular fibrillation (VF) on ECG. VF converted to sinus rhythm; ROSC (Return of Spontaneous Circulation) achieved at 10:27 AM.
Medications Administered:
1 mg Epinephrine IV every 3–5 minutes during CPR (3 doses given).
300 mg Amiodarone IV push following initial shock.
Transport to Hospital:
Time En Route: 15 minutes
Condition During Transport: Patient deteriorated en route, suffering a second cardiac arrest at 10:33 AM. Aggressive CPR was resumed with defibrillation (2 shocks, 200J each) and ROSC achieved at 10:38 AM.
Vital Signs Pre-Hospital Arrival:
Heart Rate: 48 bpm (weak, irregular)
Blood Pressure: 72/50 mmHg
SpO2: 82% (on 100% O2 via BVM)
Hospital Arrival:
Time of Arrival: 10:44 AM
Patient presented with recurrent arrhythmia, hypotension, and altered mental status. Handoff provided to ER staff for advanced resuscitation.
Autopsy Report
Patient Name: Darren Kozlowski Case Number: ME-2024-894 Age: 31 Sex: Male Date of Death: July 8, 2024 Time of Death: 11:03 AM Performed By: Dr. Laura Mendelson, MD, Forensic Pathologist Location: County Medical Examiner’s Office
External Examination:
Height: 6’1”
Weight: 185 lbs
Build: Lean and fit; well-developed musculature.
Hair: Short blonde hair and beard.
Eyes: Blue.
Distinguishing Features: None noted.
External Trauma:
Rib fractures (bilateral, 3rd–6th ribs) consistent with CPR.
Bruising along the sternum.
Minor abrasions on knees and hands from collapse.
No other injuries identified.
Internal Examination:
Heart:
Weight: 375 grams (normal range: 280–340 grams).
Severe coronary artery disease identified:
95% occlusion of the left anterior descending artery (LAD).
80% occlusion of the right coronary artery (RCA).
Evidence of acute myocardial infarction (MI) involving 40% of the left ventricle, with microscopic examination confirming recent myocardial necrosis and hemorrhage.
Mild left ventricular hypertrophy noted (wall thickness: 1.5 cm).
Lungs:
Pulmonary congestion and edema (weight: 750 grams per lung).
No evidence of pulmonary embolism.
Other Organs:
Liver: Mild steatosis.
Kidneys: Acute tubular necrosis, likely secondary to hypoperfusion during cardiac arrest events.
Brain: Mild cerebral edema, no gross signs of anoxic injury.
Toxicology Results:
Negative for alcohol, illicit drugs, and prescribed medications.
Positive for mild caffeine levels consistent with normal consumption.
Cause of Death: Acute myocardial infarction due to severe coronary artery disease, complicated by multiple cardiac arrests.
Manner of Death: Natural.
Pathologist’s Summary: The decedent, a 31-year-old male, succumbed to complications from a severe heart attack while running. Advanced resuscitation efforts successfully restored circulation twice; however, irreversible cardiac damage and circulatory collapse led to his death. Contributing factors include undiagnosed atherosclerosis and left ventricular hypertrophy, suggesting a predisposition to cardiac events under physical exertion.
19 notes
·
View notes
Text
14th May 2024
Yale: Introduction to Radiology - Abdominal Anatomy on Computed Tomography
youtube
Abdominal Anatomy on Computed Tomography - Learning Objective:
Identify the normal appearance of the following organs:
spleen, adrenal glands, kidneys, gallbladder, pancreas, liver, stomach, duodenum, colon (ascending, transverse, descending)
Identify the following venous structures:
IVC, hepatic veins, portal vein, splenic vein
Identify the following arteries
aorta, celiac artery, common hepatic artery, splenic artery, left gastric artery, superior mesenteric artery, renal artery, inferior mesenteric artery
Most abdominal CT scans are performed at about 70 seconds after giving intravenous contrast.
Sometimes, imaging is earlier for interest in the arteries.
Sometimes, imaging is later in certain specific pathology.
PA vs AP:
You know you're posterior because you can see the lumbar spine and thorasic soine vertebrae bodies coming into the image
As you start to scroll towards anterior, you can see the abdominal aorta.
0 notes
Note
The pancreas is an organ of the digestive system and endocrine system of vertebrates. In humans, it is located in the abdomen behind the stomach and functions as a gland. The pancreas is a mixed or heterocrine gland, i.e., it has both an endocrine and a digestive exocrine function.[2] 99% of the pancreas is exocrine and 1% is endocrine.[3][4][5][6] As an endocrine gland, it functions mostly to regulate blood sugar levels, secreting the hormones insulin, glucagon, somatostatin and pancreatic polypeptide. As a part of the digestive system, it functions as an exocrine gland secreting pancreatic juice into the duodenum through the pancreatic duct. This juice contains bicarbonate, which neutralizes acid entering the duodenum from the stomach; and digestive enzymes, which break down carbohydrates, proteins and fats in food entering the duodenum from the stomach.
Pancreas
Anatomy of the pancreas
Details
Pronunciation
/ˈpæŋkriəs/
Precursor
Pancreatic buds
System
Digestive system and endocrine system
Artery
Inferior pancreaticoduodenal artery, anterior superior pancreaticoduodenal artery, posterior superior pancreaticoduodenal artery, splenic artery
Vein
Pancreaticoduodenal veins, pancreatic veins
Nerve
Pancreatic plexus, celiac ganglia, vagus nerve[1]
Lymph
Splenic lymph nodes, celiac lymph nodes and superior mesenteric lymph nodes
Identifiers
Latin
pancreas
Greek
Πάνκρεας (Pánkreas)
MeSH
D010179
TA98
A05.9.01.001
TA2
3114
FMA
7198
Anatomical terminology
[edit on Wikidata]
Inflammation of the pancreas is known as pancreatitis, with common causes including chronic alcohol use and gallstones. Because of its role in the regulation of blood sugar, the pancreas is also a key organ in diabetes mellitus. Pancreatic cancer can arise following chronic pancreatitis or due to other reasons, and carries a very poor prognosis, as it is often only identified after it has spread to other areas of the body.
The word pancreas comes from the Greek πᾶν (pân, "all") & κρέας (kréas, "flesh"). The function of the pancreas in diabetes has been known since at least 1889, with its role in insulin production identified in 1921.
Structure
edit
The pancreas (shown here in pink) sits behind the stomach, with the body near the curvature of the duodenum, and the tail stretching to touch the spleen.
The pancreas is an organ that in humans lies in the abdomen, stretching from behind the stomach to the left upper abdomen near the spleen. In adults, it is about 12–15 centimetres (4.7–5.9 in) long, lobulated, and salmon-coloured in appearance.[7]
Anatomically, the pancreas is divided into a head, neck, body, and tail. The pancreas stretches from the inner curvature of the duodenum, where the head surrounds two blood vessels: the superior mesenteric artery and vein. The longest part of the pancreas, the body, stretches across behind the stomach, and the tail of the pancreas ends adjacent to the spleen.[7]
Two ducts, the main pancreatic duct and a smaller accessory pancreatic duct run through the body of the pancreas. The main pancreatic duct joins with the common bile duct forming a small ballooning called the ampulla of Vater (hepatopancreatic ampulla). This ampulla is surrounded by a muscle, the sphincter of Oddi. This ampulla opens into the descending part of the duodenum. The opening of the common bile duct into main pancreatic duct is controlled by sphincter of Boyden. The accessory pancreatic duct opens into duodenum with separate openings located above the opening of the main pancreatic duct.[7]
Parts
edit
The head of the pancreas sits within the curvature of the duodenum, and wraps around the superior mesenteric artery and vein. To the right sits the descending part of the duodenum, and between these travel the superior and inferior pancreaticoduodenal arteries. Behind rests the inferior vena cava, and the common bile duct. In front sits the peritoneal membrane and the transverse colon.[7] A small uncinate process emerges from below the head, situated behind the superior mesenteric vein and sometimes artery.[7]
The neck of the pancreas separates the head of the pancreas, located in the curvature of the duodenum, from the body. The neck is about 2 cm (0.79 in) wide, and sits in front of where the portal vein is formed. The neck lies mostly behind the pylorus of the stomach, and is covered with peritoneum. The anterior superior pancreaticoduodenal artery travels in front of the neck of the pancreas.[7]
The body is the largest part of the pancreas, and mostly lies behind the stomach, tapering along its length. The peritoneum sits on top of the body of the pancreas, and the transverse colon in front of the peritoneum.[7] Behind the pancreas are several blood vessels, including the aorta, the splenic vein, and the left renal vein, as well as the beginning of the superior mesenteric artery.[7] Below the body of the pancreas sits some of the small intestine, specifically the last part of the duodenum and the jejunum to which it connects, as well as the suspensory ligament of the duodenum which falls between these two. In front of the pancreas sits the transverse colon.[8]
The pancreas narrows towards the tail, which sits near to the spleen.[7] It is usually between 1.3–3.5 cm (0.51–1.38 in) long, and sits between the layers of the ligament between the spleen and the left kidney. The splenic artery and vein, which also passes behind the body of the pancreas, pass behind the tail of the pancreas.[7]
Blood supply
edit
The pancreas has a rich blood supply, with vessels originating as branches of both the coeliac artery and superior mesenteric artery.[7] The splenic artery, the largest branch of the celiac trunk, runs along the top of the pancreas, and supplies the left part of the body and the tail of the pancreas through its pancreatic branches, the largest of which is called the greater pancreatic artery.[7] The superior and inferior pancreaticoduodenal arteries run along the back and front surfaces of the head of the pancreas adjacent to the duodenum. These supply the head of the pancreas. These vessels join together (anastamose) in the middle.[7]
The body and neck of the pancreas drain into the splenic vein, which sits behind the pancreas.[7] The head drains into, and wraps around, the superior mesenteric and portal veins, via the pancreaticoduodenal veins.[7]
The pancreas drains into lymphatic vessels that travel alongside its arteries, and has a rich lymphatic supply.[7] The lymphatic vessels of the body and tail drain into splenic lymph nodes, and eventually into lymph nodes that lie in front of the aorta, between the coeliac and superior mesenteric arteries. The lymphatic vessels of the head and neck drain into intermediate lymphatic vessels around the pancreaticoduodenal, mesenteric and hepatic arteries, and from there into the lymph nodes that lie in front of the aorta.[7]
Microanatomy
edit
This image shows a pancreatic islet when pancreatic tissue is stained and viewed under a microscope. Parts of the digestive ("exocrine") pancreas can be seen around the islet, more darkly. These contain hazy dark purple granules of inactive digestive enzymes (zymogens).
A pancreatic islet that uses fluorescent antibodies to show the location of different cell types in the pancreatic islet. Antibodies against glucagon, secreted by alpha cells, show their peripheral position. Antibodies against insulin, secreted by beta cells, show the more widespread and central position that these cells tend to have.[9]
The pancreas contains tissue with an endocrine and exocrine role, and this division is also visible when the pancreas is viewed under a microscope.[10]
The majority of pancreatic tissue has a digestive role. The cells with this role form clusters (Latin: acini) around small ducts, and are arranged in lobes that have thin fibrous walls. The cells of each acinus secrete inactive digestive enzymes called zymogens into the small intercalated ducts which they surround. In each acinus, the cells are pyramid-shaped and situated around the intercalated ducts, with the nuclei resting on the basement membrane, a large endoplasmic reticulum, and a number of zymogen granules visible within the cytoplasm. The intercalated ducts drain into larger intralobular ducts within the lobule, and finally interlobular ducts. The ducts are lined by a single layer of column-shaped cells. There is more than one layer of cells as the diameter of the ducts increases.[10]
The tissues with an endocrine role within the pancreas exist as clusters of cells called pancreatic islets (also called islets of Langerhans) that are distributed throughout the pancreas.[9] Pancreatic islets contain alpha cells, beta cells, and delta cells, each of which releases a different hormone. These cells have characteristic positions, with alpha cells (secreting glucagon) tending to be situated around the periphery of the islet, and beta cells (secreting insulin) more numerous and found throughout the islet.[9] Enterochromaffin cells are also scattered throughout the islets.[9] Islets are composed of up to 3,000 secretory cells, and contain several small arterioles to receive blood, and venules that allow the hormones secreted by the cells to enter the systemic circulation.[9]
Variation
edit
The size of the pancreas varies considerably.[7] Several anatomical variations exist, relating to the embryological development of the two pancreatic buds. The pancreas develops from these buds on either side of the duodenum. The ventral bud rotates to lie next to the dorsal bud, eventually fusing. In about 10% of adults, an accessory pancreatic duct may be present if the main duct of the dorsal bud of the pancreas does not regress; this duct opens into the minor duodenal papilla.[11] If the two buds themselves, each having a duct, do not fuse, a pancreas may exist with two separate ducts, a condition known as a pancreas divisum. This condition has no physiologic consequence.[12] If the ventral bud does not fully rotate, an annular pancreas may exist, where part or all of the duodenum is encircled by the pancreas. This may be associated with duodenal atresia.[13]
Gene and protein expression
edit
Further information: Bioinformatics § Gene and protein expression
10,000 protein coding genes (~50% of all human genes) are expressed in the normal human pancreas.[14][15] Less than 100 of these genes are specifically expressed in the pancreas. Similar to the salivary glands, most pancreas-specific genes encode for secreted proteins. Corresponding pancreas-specific proteins are either expressed in the exocrine cellular compartment and have functions related to digestion or food uptake such as digestive chymotrypsinogen enzymes and pancreatic lipase PNLIP, or are expressed in the various cells of the endocrine pancreatic islets and have functions related to secreted hormones such as insulin, glucagon, somatostatin and pancreatic polypeptide.[16]
Development
edit
The pancreas originates from the foregut, a precursor tube to part of the digestive tract, as a dorsal and ventral bud. As it develops, the ventral bud rotates to the other side and the two buds fuse together.
The pancreas forms during development from two buds that arise from the duodenal part of the foregut, an embryonic tube that is a precursor to the gastrointestinal tract.[11] It is of endodermal origin.[11] Pancreatic development begins with the formation of a dorsal and ventral pancreatic bud. Each joins with the foregut through a duct. The dorsal pancreatic bud forms the neck, body, and tail of the developed pancreas, and the ventral pancreatic bud forms the head and uncinate process.[11]
The definitive pancreas results from rotation of the ventral bud and the fusion of the two buds.[11] During development, the duodenum rotates to the right, and the ventral bud rotates with it, moving to a position that becomes more dorsal. Upon reaching its final destination, the ventral pancreatic bud is below the larger dorsal bud, and eventually fuses with it. At this point of fusion, the main ducts of the ventral and dorsal pancreatic buds fuse, forming the main pancreatic duct. Usually, the duct of the dorsal bud regresses, leaving the main pancreatic duct.[11]
Cellular development
edit
Pancreatic progenitor cells are precursor cells that differentiate into the functional pancreatic cells, including exocrine acinar cells, endocrine islet cells, and ductal cells.[17] These progenitor cells are characterised by the co-expression of the transcription factors PDX1 and NKX6-1.[17]
The cells of the exocrine pancreas differentiate through molecules that induce differentiation including follistatin, fibroblast growth factors, and activation of the Notch receptor system.[17] Development of the exocrine acini progresses through three successive stages. These are the predifferentiated, protodifferentiated, and differentiated stages, which correspond to undetectable, low, and high levels of digestive enzyme activity, respectively.[17]
Pancreatic progenitor cells differentiate into endocrine islet cells under the influence of neurogenin-3 and ISL1, but only in the absence of notch receptor signaling. Under the direction of a Pax gene, the endocrine precursor cells differentiate to form alpha and gamma cells. Under the direction of Pax-6, the endocrine precursor cells differentiate to form beta and delta cells.[17] The pancreatic islets form as the endocrine cells migrate from the duct system to form small clusters around capillaries.[9] This occurs around the third month of development,[11] and insulin and glucagon can be detected in the human fetal circulation by the fourth or fifth month of development.[17]
Function
edit
The pancreas is involved in blood sugar control and metabolism within the body, and also in the secretion of substances (collectively pancreatic juice) that help digestion. These are divided into an "endocrine" role, relating to the secretion of insulin and other substances within pancreatic islets that help control blood sugar levels and metabolism within the body, and an "exocrine" role, relating to the secretion of enzymes involved in digesting substances in the digestive tract.[10]
Blood glucose regulation
edit
See also: Pancreatic islets
The pancreas maintains constant blood glucose levels (shown as the waving line). When the blood glucose level is too high, the pancreas secretes insulin and when the level is too low, the pancreas secretes glucagon.
Cells within the pancreas help to maintain blood glucose levels (homeostasis). The cells that do this are located within the pancreatic islets that are present throughout the pancreas. When blood glucose levels are low, alpha cells secrete glucagon, which increases blood glucose levels. When blood glucose levels are high beta cells secrete insulin to decrease glucose in blood. Delta cells in the islet also secrete somatostatin which decreases the release of insulin and glucagon.[9]
Glucagon acts to increase glucose levels by promoting the creation of glucose and the breakdown of glycogen to glucose in the liver. It also decreases the uptake of glucose in fat and muscle. Glucagon release is stimulated by low blood glucose or insulin levels, and during exercise.[18] Insulin acts to decrease blood glucose levels by facilitating uptake by cells (particularly skeletal muscle), and promoting its use in the creation of proteins, fats and carbohydrates. Insulin is initially created as a precursor form called preproinsulin. This is converted to proinsulin and cleaved by C-peptide to insulin which is then stored in granules in beta cells. Glucose is taken into the beta cells and degraded. The end effect of this is to cause depolarisation of the cell membrane which stimulates the release of the insulin.[18]
The main factor influencing the secretion of insulin and glucagon are the levels of glucose in blood plasma.[19] Low blood sugar stimulates glucagon release, and high blood sugar stimulates insulin release. Other factors also influence the secretion of these hormones. Some amino acids, that are byproducts of the digestion of protein, stimulate insulin and glucagon release. Somatostatin acts as an inhibitor of both insulin and glucagon. The autonomic nervous system also plays a role. Activation of Beta-2 receptors of the sympathetic nervous system by catecholamines secreted from sympathetic nerves stimulates secretion of insulin and glucagon,[19][20] whereas activation of Alpha-1 receptors inhibits secretion.[19] M3 receptors of the parasympathetic nervous system act when stimulated by the right vagus nerve to stimulate release of insulin from beta cells.[19]
Digestion
edit
The pancreas has a role in digestion, highlighted here. Ducts in the pancreas (green) conduct digestive enzymes into the duodenum. This image also shows a pancreatic islet, part of the endocrine pancreas, which contains cells responsible for secretion of insulin and glucagon.
The pancreas plays a vital role in the digestive system. It does this by secreting a fluid that contains digestive enzymes into the duodenum, the first part of the small intestine that receives food from the stomach. These enzymes help to break down carbohydrates, proteins and lipids (fats). This role is called the "exocrine" role of the pancreas. The cells that do this are arranged in clusters called acini. Secretions into the middle of the acinus accumulate in intralobular ducts, which drain to the main pancreatic duct, which drains directly into the duodenum. About 1.5 - 3 liters of fluid are secreted in this manner every day.[8][21]
The cells in each acinus are filled with granules containing the digestive enzymes. These are secreted in an inactive form termed zymogens or proenzymes. When released into the duodenum, they are activated by the enzyme enterokinase present in the lining of the duodenum. The proenzymes are cleaved, creating a cascade of activating enzymes.[21]
Enzymes that break down proteins begin with activation of trypsinogen to trypsin. The free trypsin then cleaves the rest of the trypsinogen, as well as chymotrypsinogen to its active form chymotrypsin.[21]
Enzymes secreted involved in the digestion of fats include lipase, phospholipase A2, lysophospholipase, and cholesterol esterase.[21]
Enzymes that break down starch and other carbohydrates include amylase.[21]
These enzymes are secreted in a fluid rich in bicarbonate. Bicarbonate helps maintain an alkaline pH for the fluid, a pH in which most of the enzymes act most efficiently, and also helps to neutralise the stomach acids that enter the duodenum.[21] Secretion is influenced by hormones including secretin, cholecystokinin, and VIP, as well as acetylcholine stimulation from the vagus nerve. Secretin is released from the S cells which form part of the lining of the duodenum in response to stimulation by gastric acid. Along with VIP, it increases the secretion of enzymes and bicarbonate. Cholecystokinin is released from Ito cells of the lining of the duodenum and jejunum mostly in response to long chain fatty acids, and increases the effects of secretin.[21] At a cellular level, bicarbonate is secreted from centroacinar and ductal cells through a sodium and bicarbonate cotransporter that acts because of membrane depolarisation caused by the cystic fibrosis transmembrane conductance regulator. Secretin and VIP act to increase the opening of the cystic fibrosis transmembrane conductance regulator, which leads to more membrane depolarisation and more secretion of bicarbonate.[22][23][24]
A variety of mechanisms act to ensure that the digestive action of the pancreas does not act to digest pancreatic tissue itself. These include the secretion of inactive enzymes (zymogens), the secretion of the protective enzyme trypsin inhibitor, which inactivates trypsin, the changes in pH that occur with bicarbonate secretion that stimulate digestion only when the pancreas is stimulated, and the fact that the low calcium within cells causes inactivation of trypsin.[21]
Additional functions
edit
The pancreas also secretes vasoactive intestinal peptide and pancreatic polypeptide. Enterochromaffin cells of the pancreas secrete the hormones motilin, serotonin, and substance P.[9]
Clinical significance
edit
Main article: Pancreatic disease
Inflammation
edit
Main article: Pancreatitis
Inflammation of the pancreas is known as pancreatitis. Pancreatitis is most often associated with recurrent gallstones or chronic alcohol use, with other common causes including traumatic damage, damage following an ERCP, some medications, infections such as mumps and very high blood triglyceride levels. Acute pancreatitis is likely to cause intense pain in the central abdomen, that often radiates to the back, and may be associated with nausea or vomiting. Severe pancreatitis may lead to bleeding or perforation of the pancreas resulting in shock or a systemic inflammatory response syndrome, bruising of the flanks or the region around the belly button. These severe complications are often managed in an intensive care unit.[25]
In pancreatitis, enzymes of the exocrine pancreas damage the structure and tissue of the pancreas. Detection of some of these enzymes, such as amylase and lipase in the blood, along with symptoms and findings on medical imaging such as ultrasound or a CT scan, are often used to indicate that a person has pancreatitis. Pancreatitis is often managed medically with pain reliefs, and monitoring to prevent or manage shock, and management of any identified underlying causes. This may include removal of gallstones, lowering of blood triglyceride or glucose levels, the use of corticosteroids for autoimmune pancreatitis, and the cessation of any medication triggers.[25]
Chronic pancreatitis refers to the development of pancreatitis over time. It shares many similar causes, with the most common being chronic alcohol use, with other causes including recurrent acute episodes and cystic fibrosis. Abdominal pain, characteristically relieved by sitting forward or drinking alcohol, is the most common symptom. When the digestive function of the pancreas is severely affected, this may lead to problems with fat digestion and the development of steatorrhoea; when the endocrine function is affected, this may lead to diabetes. Chronic pancreatitis is investigated in a similar way to acute pancreatitis. In addition to management of pain and nausea, and management of any identified causes (which may include alcohol cessation), because of the digestive role of the pancreas, enzyme replacement may be needed to prevent malabsorption.[25]
Cancer
edit
Main article: Pancreatic cancer
Pancreatic cancer, shown here, most commonly occurs as an adenocarcinoma in the head of the pancreas. Because symptoms (such as skin yellowing, pain, or itch) do not occur until later in the disease, it often presents at a later stage and has limited treatment options.
Relative incidences of various pancreatic neoplasms, with pancreatic cancers in red/pink color.[26]
Pancreatic cancers, particularly the most common type, pancreatic adenocarcinoma, remain very difficult to treat, and are mostly diagnosed only at a stage that is too late for surgery, which is the only curative treatment. Pancreatic cancer is rare in people younger than 40 and the median age of diagnosis is 71.[27] Risk factors include chronic pancreatitis, older age, smoking, obesity, diabetes, and certain rare genetic conditions including multiple endocrine neoplasia type 1, hereditary nonpolyposis colon cancer and dysplastic nevus syndrome among others.[25][28] About 25% of cases are attributable to tobacco smoking,[29] while 5–10% of cases are linked to inherited genes.[27]
Pancreatic adenocarcinoma is the most common form of pancreatic cancer, and is cancer arising from the exocrine digestive part of the pancreas. Most occur in the head of the pancreas.[25] Symptoms tend to arise late in the course of the cancer, when it causes abdominal pain, weight loss, or yellowing of the skin (jaundice). Jaundice occurs when the outflow of bile is blocked by the cancer. Other less common symptoms include nausea, vomiting, pancreatitis, diabetes or recurrent venous thrombosis.[25] Pancreatic cancer is usually diagnosed by medical imaging in the form of an ultrasound or CT scan with contrast enhancement. An endoscopic ultrasound may be used if a tumour is being considered for surgical removal, and biopsy guided by ERCP or ultrasound can be used to confirm an uncertain diagnosis.[25]
Because of the late development of symptoms, most cancer presents at an advanced stage.[25] Only 10 to 15% of tumours are suitable for surgical resection.[25] As of 2018, when chemotherapy is given the FOLFIRINOX regimen containing fluorouracil, irinotecan, oxaliplatin and leucovorin has been shown to extend survival beyond traditional gemcitabine regimens.[25] For the most part, treatment is palliative, focus on the management of symptoms that develop. This may include management of itch, a choledochojejunostomy or the insertion of stents with ERCP to facilitate the drainage of bile, and medications to help control pain.[25] In the United States pancreatic cancer is the fourth most common cause of deaths due to cancer.[30] The disease occurs more often in the developed world, which had 68% of new cases in 2012.[31] Pancreatic adenocarcinoma typically has poor outcomes with the average percentage alive for at least one and five years after diagnosis being 25% and 5% respectively.[31][32] In localized disease where the cancer is small (< 2 cm) the number alive at five years is approximately 20%.[33]
There are several types of pancreatic cancer, involving both the endocrine and exocrine tissue. The many types of pancreatic endocrine tumors are all uncommon or rare, and have varied outlooks. However the incidence of these cancers has been rising sharply; it is not clear to what extent this reflects increased detection, especially through medical imaging, of tumors that would be very slow to develop. Insulinomas (largely benign) and gastrinomas are the most common types.[34] For those with neuroendocrine cancers the number alive after five years is much better at 65%, varying considerably with type.[31]
A solid pseudopapillary tumour is a low-grade malignant tumour of the pancreas of papillary architecture that typically afflicts young women.[35]
Diabetes mellitus
edit
Type 1 diabetes
edit
Main article: Diabetes mellitus type 1
Diabetes mellitus type 1 is a chronic autoimmune disease in which the immune system attacks the insulin-secreting beta cells of the pancreas.[36] Insulin is needed to keep blood sugar levels within optimal ranges, and its lack can lead to high blood sugar. As an untreated chronic condition, complications including accelerated vascular disease, diabetic retinopathy, kidney disease and neuropathy can result.[36] In addition, if there is not enough insulin for glucose to be used within cells, the medical emergency diabetic ketoacidosis, which is often the first symptom that a person with type 1 diabetes may have, can result.[37] Type 1 diabetes can develop at any age but is most often diagnosed before age 40.[36] For people living with type 1 diabetes, insulin injections are critical for survival.[36] An experimental procedure to treat type 1 diabetes is pancreas transplantation or isolated transplantation of islet cells to supply a person with functioning beta cells.[36]
Type 2 diabetes
edit
Main article: Diabetes mellitus type 2
Diabetes mellitus type 2 is the most common form of diabetes.[36] The causes for high blood sugar in this form of diabetes usually are a combination of insulin resistance and impaired insulin secretion, with both genetic and environmental factors playing a role in the development of the disease.[38] Over time, pancreatic beta cells may become "exhausted" and less functional.[36] The management of type 2 diabetes involves a combination of lifestyle measures, medications if required and potentially insulin.[39] With relevance to the pancreas, several medications act to enhance the secretion of insulin from beta cells, particularly sulphonylureas, which act directly on beta cells; incretins which replicate the action of the hormones glucagon-like peptide 1, increasing the secretion of insulin from beta cells after meals, and are more resistant to breakdown; and DPP-4 inhibitors, which slow the breakdown of incretins.[39]
Removal
edit
It is possible for a person to live without a pancreas, provided that the person takes insulin for proper regulation of blood glucose concentration and pancreatic enzyme supplements to aid digestion.[40]
History
edit
The pancreas was first identified by Herophilus (335–280 BC), a Greek anatomist and surgeon.[41] A few hundred years later, Rufus of Ephesus, another Greek anatomist, gave the pancreas its name. Etymologically, the term "pancreas", a modern Latin adaptation of Greek πάγκρεας,[42] [πᾶν ("all", "whole"), and κρέας ("flesh")],[43] originally means sweetbread,[44] although literally meaning all-flesh, presumably because of its fleshy consistency. It was only in 1889 when Oskar Minkowski discovered that removing the pancreas from a dog caused it to become diabetic.[45] Insulin was later isolated from pancreatic islets by Frederick Banting and Charles Best in 1921.[45]
The way the tissue of the pancreas has been viewed has also changed. Previously, it was viewed using simple staining methods such as H&E stains. Now, immunohistochemistry can be used to more easily differentiate cell types. This involves visible antibodies to the products of certain cell types, and helps identify with greater ease cell types such as alpha and beta cells.[9]
Other animals
edit
Pancreatic tissue is present in all vertebrates, but its precise form and arrangement varies widely. There may be up to three separate pancreases, two of which arise from the pancreatic bud, and the other dorsally. In most species (including humans), these "fuse" in the adult, but there are several exceptions. Even when a single pancreas is present, two or three pancreatic ducts may persist, each draining separately into the duodenum (or equivalent part of the foregut). Birds, for example, typically have three such ducts.[46]
In teleost fish, and a few other species (such as rabbits), there is no discrete pancreas at all, with pancreatic tissue being distributed diffusely across the mesentery and even within other nearby organs, such as the liver or spleen. In a few teleost species, the endocrine tissue has fused to form a distinct gland within the abdominal cavity, but otherwise it is distributed among the exocrine components. The most primitive arrangement, however, appears to be that of lampreys and lungfish, in which pancreatic tissue is found as a number of discrete nodules within the wall of the gut itself, with the exocrine portions being little different from other glandular structures of the intestine.[46]
Cuisine
edit
The pancreas of calf (ris de veau) or lamb (ris d'agneau), and, less commonly, of beef or pork, are used as food under the culinary name of sweetbread.[47][48]
Oh my, that information is very nutritious. Thank you! :D
0 notes
Text
Difference Between Cardiac Arrest and Heart Attack – Everyone Should Know


Cardiac Arrest, Heart Attack, or a Heart Failure – Is there a difference, or are these terminologies the same? In this article, let’s focus on the difference between Cardiac Arrest and Heart attack. Here are two real-life incidences:
Situation ‘A’
Navneet was 50 years old. One morning when he was walking out of the office cafeteria, he suddenly felt a pain at the center of his chest. Navneet thought it was just a heartburn and hence kept walking. As he stopped by his friend’s desk, he got very light-headed. His friend indicated that he looked gray and insisted on calling their emergency number. However, Navneet declined and chose to walk towards his cubicle through the long corridor. His legs felt rubbery as he approached his desk, and felt so disabled that he had to cling onto the cubicle walls to get some support and remain steady. Immediately thereafter, he was admitted to the emergency room and accordingly treated.
Situation ‘B’
Suraj was in his office talking to his team member when he started to get dizzy. He started to stumble around and then ran forward straight into a door frame. He fell on the floor straight. Suraj tried to speak, but he couldn’t. He had trouble breathing and started to unbutton his shirt as he felt suffocated. He was rushed to the hospital and given a round of CPR followed by other courses of treatment.
What can be derived from the above incidences?
Navneet, while in the hospital, discovered that there was 90% occlusion in his right coronary artery and had to get a stent placed to avoid further similar occurrences; whereas in Suraj’s case, his Left Anterior Descending artery (LAD) was 100% blocked and Right Coronary Artery (RCA) was about 80% blocked.
Nevertheless, symptom-wise, both the incidents mentioned above look similar but are very different. Situation ‘A’ is a case of Heart Attack, and Situation ‘B’ is a case of Sudden Cardiac Arrest (SCA).
People assume these to be similar medical conditions and often use the terms interchangeably. However, there is a significant difference between cardiac arrest and heart attack.
Medical science has made remarkable advancements and treating a heart attack or a cardiac arrest is much more possible, even non-surgically. Poona Preventive Cardiovascular Centre (PPCC) offers the most viable non-surgical treatment in Pune for heart diseases with the best and safest modern techniques. We solely believe in delivering scientifically-validated therapeutics for healthy and long-term wellbeing.
Since heart attack or cardiac arrest episodes can be fatal and traumatic, causing a high degree of damage to the heart anyway, it is always advisable that, if possible, you must opt for non-surgical treatment to go mild and easy on your heart.
To understand the major difference between Cardiac Arrest and Heart Attack, first, let’s get to know what these conditions are like?
What happens in a Heart attack?
Your heart beats 60 to 100 times a minute due to the oxygen that it carries. An instance of Heart attack can occur when a layer of plaque, a combination of cholesterol, fat, and other substances, blocks the oxygen-borne blood flow to the heart muscles.
What signs indicate a Heart Attack?
Usually, heart attack symptoms gradually begin developing a few hours, days, or weeks earlier and persist for some time. Also, the heart does not stop beating during a heart attack even after the blood supply is disrupted. Moreover, symptoms of heart attack can differ in men and women.
Few noticeable symptoms during a heart attack:
Pressure, tightness, pain, squeezing, or aching sensations in your chest or arms, which may spread to your neck, jaw, or back
Symptoms such as nausea, indigestion, heartburn, or abdominal pain may also be seen
Breathing difficulty
Cold sweat
Fatigue
Light-headedness or dizziness that may occur suddenly
Does your heart get damaged after a heart attack?
The heart is a very sturdy organ. So, after suffering an attack, even though some of it may get damaged, the other half keeps functioning. But, your heart may come down to a weakened condition that might need greater care after that.
What happens in a Cardiac Arrest?
Sudden Cardiac Arrest (SCA) may occur when there is a sudden loss of heart function. Due to irregular heartbeat (arrhythmia), the pumping action is disturbed, and the heart is unable to supply blood to the brain, lungs, and other parts of the body. Within a few seconds, the person falls unconscious, having no pulse. Untreated, such a condition worsens and leads to death.
It can happen to anyone at any time, although it most often happens in people already having some other health problem or who have a heart condition. The most common cause of a cardiac arrest is ventricular fibrillation, which happens when the electrical impulses tell your heart to beat.
Further SCA symptoms that show the difference between Cardiac Arrest and Heart Attack:
Sudden cardiac arrest symptoms are immediate and severe, including:
Unexpected collapse
No pulse detected
No breathing.
Unconsciousness
Few other signs and symptoms may appear prior to a sudden cardiac arrest. These could include:
Chest ache
Weakness due to shortness of breath
Palpitations are symptoms of a fast-beating, fluttering, or pounding heart. However, sudden cardiac arrest often occurs without warning.
Is there a link between SCA and Heart Attack?
Although medically, there is a wide difference between Cardiac Arrest and Heart Attack, these two conditions are interlinked. Basically, a heart attack may turn out to be the onset of a cardiac arrest as SCA may occur post a heart attack or while recovering from one. We can also say that heart attack may be a common cause of cardiac arrest.
Apart from a heart attack, thickened heart muscle, arrhythmias, ventricular fibrillation, and long Q-T syndrome can also be the reasons for a Sudden Cardiac Arrest.
Cardiac Arrest Vs Heart Attack – In a nutshell
Cardiac Arrest
Heart attack
Sudden cardiac arrest is typically a medical emergency that can happen after the heart suddenly stops beating.
On the other hand, heart attack refers to a more serious condition in which a clogged artery interrupts blood from flowing to the heart.
When a person’s heart stops pumping blood around their body, he stops breathing normally. In such a case, the person is said to be under cardiac arrest.
A heart attack is the cause of many cardiac arrests in adults. This is because a person experiencing a heart attack may develop a dangerous heart rhythm, resulting in cardiac arrest.
What steps should you take during a heart attack or a cardiac arrest?
Although there is a difference between Cardiac Arrest and Heart Attack, the emergency care steps could be similar:
Call the nearest medical emergency available
Start CPR straight away
Make sure there is room to lay down next to the person
Place your hands on the middle of the person’s chest
Push hard and fast in a downward motion at least 100 times.
Then, continue to push hard and fast for another 100 compressions
If you are on the floor, place your hands on the person’s shoulder to tilt their head back
Continue the CPR process until professional emergency arrives.
We are proud to hone the fact that Poona Preventive Cardiovascular Center (PPCC) is the only clinic in Pune to be specialized in modern science techniques like Chelation Therapy, EECP, ESMR, and HBOT, ensuring effective non-surgical treatment in Pune for heart problems. We are completely dedicated to efficient management of Cardiovascular diseases and heart disorders to avoid risky surgeries. With tailored programs comprising lifestyle modification, diet management, and nutritional supplements, we make sure that our patients are healthy and hearty for a long time.
#alternative to bypass surgery#esmr treatment in pune#eecp treatment in pune#poona preventive#alternative to bypass#EECP treatment#ESMR treatment
0 notes
Text
A Case Report of Tetralogy of Fallot Associated with Tight Lesion of the Left Anterior Descending Coronary Artery
Read the full article
0 notes