#IPSC protocol
Explore tagged Tumblr posts
Text
Stem Cell Therapy for Multiple Sclerosis: A Promising Frontier in Treatment

Multiple sclerosis (MS) is a challenging autoimmune disorder that affects millions worldwide. As researchers continue to seek more effective treatments, stem cell therapy has emerged as a promising avenue for managing and potentially modifying the course of this disease. This blog post explores the current landscape of stem cell therapy for MS, its potential benefits, challenges, and what patients should know.
Understanding Stem Cell Therapy for MS
Stem cell therapy for MS aims to achieve three primary goals:
Reduce inflammation and demyelination
Repair or replace damaged nerve tissue
Promote neuroregeneration
These objectives address the core issues of MS, potentially offering hope for improved outcomes and quality of life for patients.
Types of Stem Cells Used
Researchers are exploring several types of stem cells in MS treatment:
Mesenchymal Stem Cells (MSCs): Derived from bone marrow, fat tissue, or umbilical cord tissue.
Hematopoietic Stem Cells (HSCs): Obtained from bone marrow or peripheral blood.
Neural Stem Cells (NSCs): Sourced from fetal brain tissue or induced pluripotent stem cells (iPSCs).
Each type of stem cell offers unique properties and potential benefits in treating MS.
Potential Benefits
Stem cell therapy shows promise in several areas:
Reduced relapse rates
Improved cognitive function
Enhanced motor function
Decreased fatigue
Potential for disease modification
These benefits could significantly improve the lives of MS patients, offering hope for better management of symptoms and potentially slowing disease progression.
Current Research and Trials
The scientific community is actively investigating stem cell therapies for MS through various approaches:
Autologous Hematopoietic Stem Cell Transplantation (AHSCT)
Mesenchymal Stem Cell Transplantation (MSCT)
Neural Stem Cell Transplantation (NSCT)
These trials aim to establish the safety and efficacy of different stem cell therapies in treating MS.
Challenges and Limitations
Despite its promise, stem cell therapy for MS faces several challenges:
Variability in stem cell sources and protocols
Limited understanding of MS pathology
Potential risks (infection, graft-versus-host disease)
High cost and limited accessibility
Researchers and clinicians are working to address these challenges to make stem cell therapy a more viable option for MS patients.
Clinics and Centers Offering Stem Cell Therapy for MS
Several institutions worldwide are at the forefront of stem cell therapy for MS:
Cleveland Clinic (USA)
University of California, San Francisco (USA)
Medtravellers Stem Cell Therapy (India)
San Raffaele Hospital (Italy)
Apollo Hospitals (India)
It’s important to note that insurance coverage for these treatments is limited, and most are still considered experimental.
Before Considering Stem Cell Therapy
If you’re an MS patient considering stem cell therapy, it’s crucial to:
Consult a neurologist or MS specialist
Research reputable clinics and trials
Understand potential risks and benefits
Ensure informed consent
Conclusion
Stem cell therapy represents a promising frontier in the treatment of multiple sclerosis. While challenges remain, ongoing research and clinical trials offer hope for improved outcomes and quality of life for MS patients. As with any emerging treatment, it’s essential for patients to stay informed, consult with medical professionals, and carefully consider their options.
Source Link:- https://www.medtravellers.com/blog/stem-cell-therapy-for-multiple-sclerosis-ms/
0 notes
Text
Creative Biolabs has developed streamed-line protocols for efficient iPSC generation with viral vectors, DNA (plasmid), RNA and recombinant proteins. Each of these services will be provided with a comprehensive report suitable for publications.
0 notes
Text
Assay Development & Screening - Selvita
Selvita offers custom assay development and screening/profiling services up to 1536-well plate format. We employ a fully automated HTS platform allowing testing of 10,000 compounds a day across a wide range of biochemical and cellular assay types. We operate HCS screening in support of client projects, inour new state-of-the-art HCS laboratory and are generating novel machine-learning based scripts to support enhanced image analysis in the cell types of particular interest to our clients. To support our activities we have created a robust compound management infrastructure supporting manipulation of samples in racks, tubes and plates.
Our team has an extensive expertise in the areas of molecular biology, biochemistry, immunology and cell biology. We have a successful track record in assay development for various classes of targets, including enzymes (kinases, phosphatases, proteinases, transferases, metabolic enzymes, immuno-modulatory enzymes, DNA/RNA-associated enzymes) and protein receptors (GPCRs, TLRs, RTKs). Selvita’s cell-based assay development service includes number of assays (viability, proliferation, protein biomarkers, intra- and extracellular metabolites, phosphorylation / dephosphorylation, gene and protein expression, signalling pathways, ligand receptors) employing variety of cellular model (cancer, non-cancer, primary, iPSC-derived cells). Together with state-of-the-art equipment allowing a wide variety of detection modes to be implemented, Selvita extensively supports your drug discovery program.
Supported biochemical and cell-based assays with relevant readouts
Absorbance and Fluorescence
viability, proliferation (MTS, BrdU)
metabolites analysis
ELISA for biomarkers including Luminex multiplexing assays
Fluorescence Resonance Energy Transfer (FRET)
proteinase assays
DNA/RNA-associated enzymes
protein-ligand interactions
Time-Resolved FRET (TR FRET)
kinase/phosphatase assays (HTRF, LanthaScreen)
ubiquitination assay (HTRF)
biomarkers analysis (HTRF)
Fluorescence Polarization (FP):
kinase/phosphatase assays (Transcreener)
Luminescence
viability (CellTiter-Glo)
kinase/phosphatase assays (ADP-Glo)
binding and residence time assay (NanoBRET)
degradation studies, PROTACs (HiBiT)
Scintillation counting (3H and 14C)
DNA/RNA-associated enzymes
analysis of metabolism (e.g. glucose update, lipogenesis)
Alpha technology bead-based proximity assays:
biomarkers analysis (AlphaLISA)
protein-protein interaction (AlphaLISA)
phosphorylation / dephosphorylation (AlphaScreen)
multiplexing (AlphaPlex)
Supported biophysical assays
Surface plasmon resonance (SPR) – determination of binding kinetics, KD, kon/off
Thermal shift (differential scanning fluorimetry) – protein stability under drug treatment
Isothermal titration calorimetry (ITC) – analysis of thermodynamic properties of binding
Fluorescence polarisation (FP) – direct protein-ligand interaction
Scintillation counting (3H and 14C) – radioligand binding assays
protein-ligand NMR
Selvita can offer case-by-case selection of suitable cellular model/s to investigate the biological mechanism of interest (e.g. cancer cell panels of a specific type and/or with common mutation patterns).
Our in-house capabilities for reagent generation include in-house production and validation of recombinant cell lines and recombinant proteins.
Our assay development & screening strategies are characterized by efficient and effective protocols. Usually, multi-factor design of experiments is employed during method development. It accelerates optimization of the assay performance, thus helping to obtain a measurement system that meets its requirements. Statistical parameters are used to support a reliable and robust assay for routine use, e.g. IC50/EC50; mRSD (mean Relative Standard Deviation); MSR, S/N ratio; Z’ factor; plate uniformity (read more here).
0 notes
Text
Induced Pluripotent Stem Cells Production Market Emerging Growth Analysis, Future Demand and Business Opportunities 2032
The Induced Pluripotent Stem Cells (iPSCs) Production Market has gained significant momentum in recent years, revolutionizing the field of regenerative medicine and drug discovery. iPSCs are a type of stem cell that can be reprogrammed from mature, differentiated cells, and they have the potential to differentiate into virtually any cell type in the human body. This unique ability makes them invaluable for disease modeling, drug screening, and tissue regeneration research. The market's growth is primarily attributed to the increasing investments in stem cell research, advancements in cell reprogramming technologies, and their potential for personalized medicine.
One of the key drivers of the iPSCs Production Market is the surging interest in personalized medicine and regenerative therapies. Researchers and healthcare professionals are exploring iPSCs' potential for developing patient-specific treatments, reducing the risk of immune rejection, and providing more effective therapies for a wide range of diseases, including degenerative disorders and certain types of cancer. This has led to substantial investments in iPSC research and production technologies, driving market growth.
Technological advancements have played a pivotal role in expanding the iPSCs Production Market. Innovations in cell reprogramming techniques, such as the use of non-integrating reprogramming factors and gene editing technologies like CRISPR-Cas9, have made the generation of iPSCs more efficient and safer. Furthermore, improvements in culture and differentiation protocols have enhanced the scalability and reproducibility of iPSC production, making them more accessible to researchers and drug developers.
The iPSCs Production Market also benefits from collaborations between academia and the pharmaceutical industry. Researchers are partnering with pharmaceutical companies to develop disease models for drug screening and toxicity testing, utilizing iPSCs to accelerate drug discovery and reduce the failure rate of potential drug candidates. As these collaborations continue to grow, the demand for iPSCs and related production technologies is expected to rise further.
For More Info@ https://www.globenewswire.com/en/news-release/2022/12/20/2577078/0/en/Induced-Pluripotent-Stem-Cells-Production-Market-is-expected-to-reach-US-2-9-Bn-By-2032-at-a-CAGR-of-10-7-According-to-PMR.html
Despite its promising growth, the iPSCs Production Market faces challenges related to regulatory compliance, ethical concerns, and the need for standardized protocols. Overcoming these hurdles will be crucial to realizing the full potential of iPSCs in clinical applications. Nevertheless, the market for induced pluripotent stem cells production is poised to continue its expansion, offering exciting opportunities for advancements in regenerative medicine and personalized healthcare.
0 notes
Text
Endoscopic Discectomy is a minimally invasive spine interventional technique
Endoscopic Discectomy is a minimally invasive spine interventional technique that utilizes an endoscope to treat herniated disc, protruded disc or disc bulge, extruded, or degenerative discs that are a contributing factor to leg and back pain.
Since we don’t have to cut the muscles and bones to open the entire spine, spine endoscopy has shown to be a boon for patients with spine problems. With the advancement in technology using 3 Chip camera and 4K monitors, now we can see each and every structure inside with more clarity and thus can avoid injury to important structures. At IPSC, we have best of the equipment’s for your safety and efficacy of the procedure.
What is an Endoscopic Discectomy?
Endoscopic Discectomy is a minimally invasive spine surgery technique that utilizes an endoscope to treat herniated, protruded, extruded, or degenerative discs that are a contributing factor to leg and back pain. Spine endoscopy or Endoscopic discectomy is required for cases of slipped disc or disc bulge where your protruded disc is compressing the nerves.
What is sciatica? How my low back problem contributes to the pain in legs?
Our lower back has multiple bones called vertebrae and in between two vertebrae, there is a cushion called intervertebral disc. Around the disc, there are nerves which start from the spinal cord and goes down to the legs.
When the disc ruptures or bulges out, it compresses the nerve and your pain starts going down to your legs which is called radiculopathy or sciatica.
What is the cost of Endoscopic Discectomy in India and what are the pros and cons of undergoing this procedure for an athlete?
The cost of the procedure varies from Centre to centre. At specialized centre like IPSC India in New Delhi, it cost 1.5 Lac INR.
Advantages are: By selectively removing that bulging part, we relieve the nerve off the pressure. Patients are awake throughout the procedure and this adds safety to the procedure as we can detect any touching to the nerve while removing the disc.
What is the post operative success rate for TFED ( Transforaminal Endoscopic Discectomy)?
More than 70% of the patients usually don’t need any additional procedure. In some cases even after removing the disc, there may be some swelling on the nerves left which can be dealt with a minor procedure after 2 weeks. Although reherniation is rare, but it is possible if we don't take proper precautions after endoscopic discectomy. IPSC protocol usually advise 4 weeks of lumbar support belt after the procedure, along with some restriction of activities like prolonged sitting, forward bending, and weight lifting.
Is it ok to do pull ups after a discectomy of L5-S1 Disc Herniation?
It takes 4-6 weeks for the disc to become normal. From the skin, you won’t feel anything but the inside tissue takes time to heal. Although, we encourage patients to start walking after 1-2 days of rest, but certain precautions like forward bending, lifting heavy weights, pushing heavy objects, sudden twisting movements, are must.
What is the purpose to perform endoscopic spine surgery ?
The purpose is to remove the pressure off the nerve which have compressed the nerves because of disc herniation and bulge. Prolonged compression of the nerve may lead to permanent loss of the functions of the nerve.
What are the signs of compressed nerve?
You may feel pain and paraesthesia, pins and needles. There may be loss of sensation in some part of the leg and at times, even weakness in the lower limbs.
How is decompression achieved in percutaneous endoscopic lumbar discectomy surgery?
We make a small hole on the skin under local anaesthesia and infiltrate the entire track up to the disc with local anaesthesia. Patients are awake throughout the procedure and this adds safety to the procedure as we can detect any touching to the nerve while removing the disc.
Through this small hole we insert the spine endoscope which is fitted with camera. Through this camera we can see inside, on our screen. Once we reach the herniated material or the bulging part of the disc, we start removing it without disturbing the normal disc and other tissues.
Do we need any rods and screw fixation with Endoscopic spine procedure?
Since we don’t cut the bones and other important tissues of the spine to reach the bulging part of the disc, no rods and screw and fixation is required after endoscopic discectomy.
Is this procedure 100% safe?
No Interventional procedures are 100% safe. Though the chances of complications are rare but the chances of infection, bleeding and nerve trauma are there. All these complications can be managed, if they do happen. As compared to open surgery, endoscopic spine procedure is much safer for slipped disc and sciatica.
#Endoscopic Discectomy#herniated disc#protruded disc or disc bulge#IPSC#minimally invasive spine surgery#IPSC protocol#disc herniation and bulge#compressed nerve#spine endoscope
0 notes
Text
I’m not sure that gets rid of the problem - just finished one and all the job postings want a Ph.D and 8 years of hyper-specific experience. Should’ve just quit after undergrad and been a technician...
feel like my masters degree is useless cause everything needs a phd
#fuck the job market#not even iPSC culture specifically 3D culture focusing on organoids#or how to develop new protocols for massive bioreactors#no academic lab has those things how the fuck are you meant to get your foot in the door
16 notes
·
View notes
Link
Abstract
Ageing is the gradual decline in organismal fitness that occurs over time leading to tissue dysfunction and disease. At the cellular level, ageing is associated with reduced function, altered gene expression and a perturbed epigenome. Somatic cell reprogramming, the process of converting somatic cells to induced pluripotent stem cells (iPSCs), can reverse these age-associated changes. However, during iPSC reprogramming somatic cell identity is lost, and can be difficult to reacquire as re-differentiated iPSCs often resemble foetal rather than mature adult cells. Recent work has demonstrated that the epigenome is already rejuvenated by the maturation phase of reprogramming, which suggests full iPSC reprogramming is not required to reverse ageing of somatic cells. Here we have developed the first ″maturation phase transient reprogramming″ (MPTR) method, where reprogramming factors are expressed until this rejuvenation point followed by withdrawal of their induction. Using dermal fibroblasts from middle age donors, we found that cells reacquire their fibroblast identity following MPTR, possibly as a result of persisting epigenetic memory at enhancers. Excitingly, our method substantially rejuvenated multiple cellular attributes including the transcriptome, which was rejuvenated by around 30 years as measured by a novel transcriptome clock. The epigenome, including H3K9me3 histone methylation levels and the DNA methylation ageing clock, was rejuvenated to a similar extent. The magnitude of rejuvenation instigated by MTPR is substantially greater than that achieved in previous transient reprogramming protocols. MPTR fibroblasts produced youthful levels of collagen proteins, suggesting functional rejuvenation. Overall, our work demonstrates that it is possible to separate rejuvenation from pluripotency reprogramming, which should facilitate the discovery of novel anti-ageing genes and therapies.
#regenerative medicine#research#genetics#epigenetics#anti-aging#stem cells#fav#family medicine#internal medicine#medicine#cellular biology#genome#MPTR method#Resources#save for later#DNA#dna methylation#epigenome
6 notes
·
View notes
Text
Staff Scientist Washington University St. Louis School of Medicine Application Deadline: 2023-02-15 Position SummaryThis position assists in developing and conducting research projects, including experimental design, data analysis and documentation of experimental results. We are seeking a Staff Scientist to conduct research projects and assist with research activities in the Weihl Lab at Washington University School of Medicine. The Weihl Lab is focused on understanding the molecular and cellular mechanisms underlying muscular dystrophies to develop targeted gene-based therapies. We employ a wide variety of techniques using mouse models, primary cell culture, and patient derived iPSCs. Treatment modalities involve ASOs, siRNAs, AAVs, CRISPR/Cas9, and PRIME editing. The position independently performs complex research studies experiments and assays. Job DescriptionPrimary Duties and Responsibilities Following instructions and discussions with principal investigator, designs research protocols, including developing procedures for the collection, verification and management of data... See the full job description on jobRxiv: https://jobrxiv.org/job/washington-university-st-louis-school-of-medicine-27778-staff-scientist/?feed_id=25030 #ScienceJobs #hiring #research
0 notes
Photo

RELOAD DRILL Range: 7yd Target: torso (8″ plate, sheet of paper, IPSC or IDPA target, etc.) Start position: from the holster or ready position Rounds fired: 4 Credit to Pistol Training.com INSTRUCTIONS At the start signal, present the weapon to the target, fire two rounds, reload, and fire two additional rounds. (a common variation of this drill fires 1, reload, then 1 round; however, firing a second shot each time guarantees that the student is aiming and exercising proper shooting technique rather than rushing too fast) To practice slidelock reloads, it is easiest to load your magazines with four rounds each, except for the first magazine in the gun which should obviously hold only two rounds. This way you can perform the drill and you’ll have two rounds left in the magazine at the end, allowing you to repeat the drill immediately. TRAINING WITH FIREARMS IS AN INHERENTLY DANGEROUS ACTIVITY. BE SURE TO FOLLOW ALL SAFETY PROTOCOLS WHEN USING FIREARMS OR PRACTICING THESE DRILLS. THESE DRILLS ARE PROVIDED FOR INFORMATION PURPOSES ONLY. USE AT YOUR OWN RISK. https://www.instagram.com/p/CgSTfwTvXgX/?igshid=NGJjMDIxMWI=
0 notes
Text
Induced Pluripotent Stem Cells Production Market Latest Advancements and Business Opportunities 2032
The Induced Pluripotent Stem Cells (iPSCs) Production Market is experiencing significant growth and revolutionizing the field of regenerative medicine. iPSCs are derived from adult cells that have been reprogrammed to exhibit characteristics similar to embryonic stem cells. These cells have the unique ability to differentiate into various cell types in the human body, making them a promising tool for disease modeling, drug discovery, and cell-based therapies.
One of the key drivers of the iPSCs Production Market is the growing prevalence of chronic diseases and the increasing demand for personalized medicine. iPSCs offer the potential to generate patient-specific cells for disease modeling and drug testing, enabling researchers to develop targeted therapies tailored to individual patients. This personalized approach has the potential to revolutionize the treatment of conditions such as cardiovascular diseases, neurodegenerative disorders, and genetic disorders.
Moreover, advancements in reprogramming techniques and the optimization of iPSC production protocols have significantly improved the efficiency and scalability of iPSC generation. The development of non-integrating reprogramming methods and the use of small molecules have minimized genomic instability and increased the safety and reliability of iPSC production. These technological advancements have contributed to the market growth by making iPSCs more accessible and practical for research and clinical applications.
Additionally, collaborations between academia, industry, and regulatory bodies have played a crucial role in driving the iPSCs Production Market. These partnerships have facilitated knowledge sharing, standardization of protocols, and the development of guidelines for iPSC production and characterization. The establishment of regulatory frameworks for the clinical translation of iPSC-based therapies has further fueled the market growth, ensuring the safety and efficacy of these advanced treatment approaches.
For More Info@ https://www.persistencemarketresearch.com/market-research/induced-pluripotent-stem-cells-production-market.asp
In conclusion, the iPSCs Production Market is poised for significant expansion as it offers a transformative platform for disease modeling, drug discovery, and cell-based therapies. With the increasing prevalence of chronic diseases and the need for personalized medicine, iPSCs have emerged as a powerful tool in regenerative medicine. Continued advancements in reprogramming techniques, scalability, and regulatory support will further propel the market, leading to the development of innovative therapies and improved patient outcomes in the future.
0 notes
Text
Organoids and Spheroids Market Evolving Opportunities, Covid-19 Impact, Strategies and Forecast
The global organoids and spheroids market is expected to reach USD 2.47 billion by 2028 according to a new study by Polaris Market Research. The global organoids and spheroids industry is anticipated to grow, owing to the growing incidence of cancer, innovations in the tissue culture system, and continually failing organ transplantation surgeries. The compatibility between the donor and recipient should be cross-checked through artificial cell culture systems to save time and money.
The global market industry is fragmented based on type, application, end-use, and region. In terms of type, the market is segmented into organoids and spheroids. The application segment is further divided into developmental biology, personalized medicine, regenerative medicine, disease pathology studies, and drug toxicity & efficacy testing. In terms of end-use, the market is segmented into biotechnology and pharmaceutical industries, academic & research institutes, and hospitals and diagnostic centers.
Request for a sample report: https://www.polarismarketresearch.com/industry-analysis/organoids-and-spheroids-market/request-for-sample
Segment Highlights
The spheroids market segment accounted for a major revenue share owing to its applicability in cancer research. The segment held over 50.0% of the global share in 2020. However, the organoid segment is expected to witness highest CAGR during the forecast period.
Based on application, the development biology segment is projected to constitute almost half of the market in 2028. This is can be attributed for increased use of cell culture systems in the developmental biology.
North America region is dominating the global organoids and spheroids industry, holding over one-third of the market share throughout the forecast period. The presence of key companies and increasing funding towards research are the factors responsible for its growth
List of Key Players
3D BioMatrix
3D Biotek LLC
AMS Biotechnology (Europe) Limited
Cellesce Ltd.
Corning Incorporated
Greiner Bio-One
Hubrecht Organoid Technology (HUB)
InSphero/Perkin Elmer
Prellis Biologics
Others
Get Discount Offer: https://www.polarismarketresearch.com/industry-analysis/organoids-and-spheroids-market/request-for-discount-pricing
Polaris Market Research has segmented the organoids and spheroids market report on the basis of type, application, end-use, and region
Organoids and Spheroids, Type Outlook (Revenue – USD Million, 2016 – 2028)
By Type
Organoids
By Type
Neural
Hepatic
Intestinal
Other
By Method
General Submerged Method for Organoid Culture
Crypt Organoid Culture Techniques
Air Liquid Interface (ALI) Method for Organoid Culture
Clonal Organoids from Lgr5+ Cells
Brain and Retina Organoid Formation Protocol
By Source
Primary Tissues
Stem Cells
Spheroids
By Type
Multicellular tumor spheroids (MCTS)
Neurospheres
Mammospheres
Hepatospheres
Embryoid bodies
By Method
Micropatterned Plates
Low Cell Attachment Plates
Hanging Drop Method
Others
By Source
Cell Line
Primary Cell
iPSCs Derived Cells
Organoids and Spheroids, Application Outlook (Revenue – USD Million, 2016 – 2028)
Developmental Biology
Personalized Medicine
Regenerative Medicine
Disease Pathology Studies
Drug Toxicity & Efficacy Testing
Organoids and Spheroids, End-Use Outlook (Revenue – USD Million, 2016 – 2028)
Biotechnology & Pharmaceutical Industries
Academic & Research Institutes
Hospitals & Diagnostic Centers
Read More : https://www.medgadget.com/2021/03/organoids-and-spheroids-market-size-worth-2-47-billion-by-2028-cagr-22-8-polaris-market-research.html
0 notes
Text
Studying the Neanderthal DNA found in modern humans using stem cells and organoids
Protocols that allow the transformation of human induced pluripotent stem cell (iPSC) lines into organoids have changed the way scientists can study developmental processes and enable them to decipher the interplay between genes and tissue formation, particularly for organs where primary tissue is not available. Now, investigators are taking this technology and applying it to study the developmental effects of Neanderthal DNA. Studying the Neanderthal DNA found in modern humans using stem cells and organoids syndicated from https://triviaqaweb.blogspot.com/
0 notes
Text
Isolation of Adult Human Dermal Fibroblasts from Abdominal Skin and Generation of Induced Pluripotent Stem Cells Using a Non-Integrating Method
Cell reprogramming requires the introduction of key genes, which regulate and maintain the pluripotent cell state. The protocol described enables the formation of induced pluripotent stem cells (iPSCs) colonies from human dermal fibroblasts without viral/integrating methods but using non-modified #RNAs (NM-#RNAs) combined with immune evasion factors reducing cellular defense mechanisms. http://bit.ly/2NJABdw
0 notes
Text
Senior Research Technician - Neurology Washington University St. Louis School of Medicine Application Deadline: 2022-12-23 Position SummaryWe are seeking a Senior Research Technician to conduct research projects and assist with research activities in the Weihl Lab at Washington University School of Medicine. The Weihl Lab is focused on understanding the molecular and cellular mechanisms underlying muscular dystrophies to develop targeted gene-based therapies. We employ a wide variety of techniques using mouse models, primary cell culture, and patient derived iPSCs. Treatment modalities involve ASOs, siRNAs, AAVs, CRISPR/Cas9, or PRIME editing. The position independently performs complex research studies, experiments, and assays. The successful hire will have a skill set to perform animal studies, cell culture, molecular biology, biochemistry, and immunohistochemistry experiments. To apply, please use the Application URL given. Job Description Primary Duties and Responsibilities Performs complex analysis/projects according to research protocols, explaining methods and procedures to o... See the full job description on jobRxiv: https://jobrxiv.org/job/washington-university-st-louis-school-of-medicine-27778-senior-research-technician-neurology/?feed_id=22975 #ScienceJobs #hiring #research @LabWeihl
0 notes
Photo

HACKATHORN STANDARDS https://bcfirearmsacademy.ca/hackathorn-standards/ Range: varies (see below) Target: three IPSC (or IDPA) targets spaced 1yd apart at heights (from left to right) of 5′, 6′, 4′. Start position: varies Rounds fired: 60 Credit to Pistol Training.com by Ken Hackathorn The Hackathorn Standards have become a mainstay of practical pistol performance evaluation. Designed by Ken Hackathorn in 1993, the “Hack Standards” formed the blueprint from which the IDPA Classifier was developed. Scoring of the targets: anywhere in the head, 5 points A-zone (-0 on IDPA target), 5 points C-zone (-1 on IDPA target), 3 points D-zone (-3 on IDPA target), 2 points miss, 0 points Any time a head shot is required, a hit anywhere else on the target counts as a complete miss. Any time no target zone is specified, or body shots are specified, hits to the head box still score 5 points. Shots fired more than 0.30 seconds after the PAR time count as misses. (Ken grants a 0.30 second grace period on the PAR times. In other words, if a string calls for a 3.00 second PAR, any shot fired within 3.30 seconds counts for score.) There is no concealment requirement. Strings of fire are shot from the holster unless specified otherwise. No extra (“make up”) shots are allowed. There are thirteen strings of fire: A score of 250 or more is considered Excellent. From 200-249 is Acceptable. Below 200 is Needs Improvement. TRAINING WITH FIREARMS IS AN INHERENTLY DANGEROUS ACTIVITY. BE SURE TO FOLLOW ALL SAFETY PROTOCOLS WHEN USING FIREARMS OR PRACTICING THESE DRILLS. THESE DRILLS ARE PROVIDED FOR INFORMATION PURPOSES ONLY. USE AT YOUR OWN RISK. (at BC Firearms Academy - Surrey) https://www.instagram.com/p/CfcVEhLvGTm/?igshid=NGJjMDIxMWI=
0 notes
Text
Stem cells sources
Stem cells are specialized cells of the body that are capable of self-renewal and differentiation. For regenerative therapy, various cell populations at different developmental stages have been considered for transplantation, including embryonic stem cells (ESCs), fetal and adult cells, and experimentally generated induced pluripotent stem cells (iPSC). The zygote is perhaps the most primitive “stem cell” and, right after fertilization, it undergoes a series of rapid divisions that all appear to be self-renewal divisions as every single cell of the blastomere is undifferentiated. From this state, differentiation divisions begin to occur as cells rearrange themselves at day 5 to form a cavity (blastocoele) in which an inner cell mass is surrounded by trophoectoderm cells (Moore and Persaud, 1998; Figure Figure1).1). It is at this stage that ESCs are collected from the inner cell mass. Because they retain pluripotency features (Thomson et al., 1998), ESCs are considered the gold standard of stem cells: They are widely used for basic research to study in vivo embryogenesis and development and for regenerative medicine (Murry and Keller, 2008), although with some limited success. In fact, ESCs are highly pluripotent cells capable of forming teratomas after transplantation and may be subject to rejection. The ethical concerns regarding the collection of ESCs from embryos has led researchers to overcome these limitations by using iPSCs, adult cells reprogrammed to a pluripotency state by forced expression of specific genes. In 2006, Yamanaka and colleagues were the first to describe that the introduction of four genes encoding the transcription factors Oct4 (octamer-binding protein 4, also known as Pou5f1), Sox2 (SRY-related HMG-box gene 2), cMyc, and Klf4 (Kruppel-like factor 4) could convert adult cells into pluripotent stem cells (Takahashi and Yamanaka, 2006). This represented a milestone in stem cell biology as it opened new approaches to study and combat diseases. Unfortunately, forced expression of cMyc and Klf4, which encode proto-oncogene proteins, increases the rate of cancer transformation (Ben-David and Benvenisty, 2011). More recently, studies have used other genes and different techniques to improve the poor efficiency of iPSC reprogramming; nevertheless, these cells still exhibit tumorigenic properties in vivo and can elicit an immune response (Zhao et al., 2011).
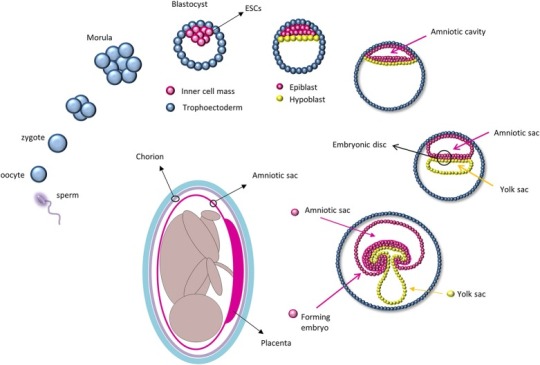
Adult tissue specific stem cells, like hematopoietic precursors, muscle satellite cells, and bone marrow-derived mesenchymal stem cells, are also limited in their potential for regenerative medicine as their function is limited to only their specific tissue, they can induce immune-rejection responses, and acquiring adequate numbers of tissue-specific stem cells for regenerative studies can often be challenging due to their rarity as well as limited ex vivo maintenance and expansion techniques (Müller et al., 2016).
A potential alternative to circumvent these limitations that emerged in the last decades is the utilization of fetal-derived stem cells. Amniotic stem cells can be collected from different fetal annexes (amnion, chorion, amniotic fluid, Wharton jelly) and have been proven to be safe, easy to collect, and devoid of immunogenic and tumorigenic properties (Mamede et al., 2012; Saito et al., 2012; Murphy and Atala, 2013; Pozzobon et al., 2014). AECs are collected from the epithelial layer of the amnion which derives directly from the epiblast as it retains some ESC characteristics. In fact, after implantation, the inner cell mass re-organizes and, driven by differential gene expression, divides into a double layer of cells (Zernicka-Goetz et al., 2009), i.e., the hypoblast that migrates to the free surface of the inner cell mass and gives rise to the endoderm; and the epiblast, which will form the rest of the embryo (Zernicka-Goetz et al., 2009). Before gastrulation, cells from the epiblast form a membrane, the amnion, within which the human embryo and later the fetus develops until birth (Moore and Persaud, 1998; Figure Figure1).1). AECs express some of the ESCs surface markers, such as stage-specific embryonic antigens (SSEA) 3 and 4, tumor rejection antigens (TRA) 1-60 and 1-81, and molecular markers of pluripotency, including Oct4, Sox2, and Nanog (Parolini et al., 2008). Their plasticity was first confirmed in vitro by chimera formation using mouse ESCs (Tamagawa et al., 2004) and later by the production of cell types from all three germ layers using specific cell differentiation protocols (Miki et al., 2005; Parolini et al., 2008).Asia Stem Cells
#best stem cell clinics#stem cell therapy center#stem cell institute#stem cell medicine#stem cell therapy for knees#benefits of stem cell treatment
0 notes