#Humira Market Report
Explore tagged Tumblr posts
Text
https://theomnibuzz.com/humira-market-size-analysis-and-forecast-2031/
The Humira Market in 2023 is US$ 8.7 billion, and is expected to reach US$ 13.18 billion by 2031 at a CAGR of 5.33%.
0 notes
Text
Antibodies Market Size, Share, Revenue, Trends And Drivers For 2022-2032
The antibodies market in Europe is poised to capture a substantial market share, primarily attributed to the increasing prevalence of cancer, cardiovascular ailments, autoimmune disorders, and substantial investments in antibody research and development. Leading the growth in this region are expected to be France, Germany, and Italy, driving market expansion throughout the forecast period. Anticipated to progress at a notable compound annual growth rate (CAGR) of 11.2%, the European market is projected to undergo significant evolution up to the year 2032.
The global antibodies market size is currently valued at an impressive US$ 197.3 billion in 2022, and it is expected to experience substantial growth at a remarkable compound annual growth rate (CAGR) of 11.9% over the upcoming decade. By the conclusion of 2032, the market is poised to expand threefold, surpassing an estimated valuation of US$ 608 billion.
Download a Sample Copy of This Report: https://www.factmr.com/connectus/sample?flag=S&rep_id=194
In recent years, the global antibodies market has experienced remarkable growth, driven by a surge in biopharmaceutical research. As biopharmaceutical companies continue to expand their efforts in developing innovative therapies and diagnostics, antibodies have emerged as crucial tools in the fight against a wide range of diseases.
The Antibodies Market Landscape
Antibodies, also known as immunoglobulins, are proteins produced by the immune system in response to the presence of foreign substances, such as pathogens or antigens. These proteins play a fundamental role in our body's defense mechanisms by targeting and neutralizing harmful invaders. However, their utility extends far beyond the human immune system.
Key Factors Driving Growth
Biopharmaceutical Research Expansion: The surge in biopharmaceutical research has been a primary driver of the antibodies market's growth. Pharmaceutical companies, academic institutions, and research organizations are investing heavily in developing novel antibody-based therapies and diagnostics. This research boom is fueled by advancements in technology, such as gene editing and high-throughput screening, which enable the rapid discovery and development of antibody candidates.
Therapeutic Applications: Antibodies are increasingly being used in therapeutic applications. Monoclonal antibodies, in particular, have gained regulatory approval for treating a variety of diseases. The success stories of drugs like Herceptin (for breast cancer) and Humira (for autoimmune diseases) have spurred interest and investment in antibody-based therapies.
Diagnostic Advancements: Antibodies are essential components of diagnostic tests. They are used in various immunoassays, including enzyme-linked immunosorbent assays (ELISA) and lateral flow assays, to detect specific biomarkers associated with diseases. With the growing demand for accurate and rapid diagnostics, the antibodies market has witnessed substantial growth in this segment.
Emerging Markets: The antibodies market is not limited to developed regions. Emerging markets, particularly in Asia-Pacific, are witnessing increased investment in biopharmaceutical research and development. This expansion of the market's geographical reach has contributed significantly to its overall growth.
Collaborations and Partnerships: Collaboration between biopharmaceutical companies and research institutions has become more common. These partnerships facilitate the development of novel antibody-based therapies and accelerate their path to market, further boosting the antibodies market's growth.
Competitive Landscape
Prominent companies operating within the antibodies market are intensifying their research efforts and expediting the development of novel antibodies to introduce innovative products and establish a competitive edge within the industry.
On September 19, 2022, Sandoz, a prominent player specializing in biosimilars and generic pharmaceuticals, unveiled significant progress in its biosimilar pipeline. Notably, positive outcomes emerged from the ROSALIA Phase I/III clinical trial study for their proposed biosimilar denosumab. Denosumab, a human monoclonal antibody engineered to target and inhibit RANKL to mitigate bone loss, showcased promising results.
Leading antibodies suppliers are also actively exploring partnerships, collaborations, and potential mergers to enhance their market presence and expand the scope of their research initiatives.
In March 2022, Sanofi, a renowned pharmaceutical entity, disclosed its strategic partnership with IGM Biosciences, aimed at jointly conceiving, developing, manufacturing, and commercializing six innovative immunoglobulin M antibody agonists. As part of this collaborative endeavor, Sanofi has committed to investing over US$ 6 billion in potential milestone payments.
Key Segments in Antibodies Industry Research
By Product Type :
Monoclonal Antibodies
Polyclonal Antibodies
Immune Checkpoint Antibodies
Epitope Tag Antibodies
Isotype Control Antibodies
Primary Antibodies
Assay Antibodies
Others
By Application :
Drug Discovery & Development
Basic Research
Toxicity Screening
Biopharmaceutical Production
Drug Screening
Tissue Engineering
Forensic Testing
Others
By End User :
Biopharmaceutical Companies
Contract Research Organizations (CROs)
Academic & Research Institutes
Forensic Science Laboratories
Food & Beverage Companies
Diagnostic Centers
Others
By Region :
North America
Latin America
Europe
APAC
MEA
Get Customization on this Report: https://www.factmr.com/connectus/sample?flag=RC&rep_id=194
The remarkable growth of the global antibodies market is closely intertwined with the surge in biopharmaceutical research. Antibodies have proven to be invaluable tools in the development of cutting-edge therapies and diagnostics, offering new hope to patients around the world. As the biopharmaceutical landscape continues to expand, the antibodies market is poised for sustained growth and innovation in the years to come.
Contact: US Sales Office 11140 Rockville Pike Suite 400 Rockville, MD 20852 United States Tel: +1 (628) 251-1583, +353-1-4434-232 Email: [email protected]
#Antibodies Market#global antibodies market#Immune Checkpoint Antibodies Market#Epitope Tag Antibodies Market#Isotype Control Antibodies Market#primary antibodies Marke
1 note
·
View note
Text
Story Behind the Market – Biosimilars
Affordable innovation is reshaping the future of biologic therapies. The Biosimilars Market is projected to grow from $28.38B in 2024 to $98.31B by 2032, at a strong CAGR of 16.8%. This surge is driven by the expiration of blockbuster biologic patents and the global push for cost-effective treatments in oncology, autoimmune diseases, and diabetes.
🚀 What’s driving the momentum?
🧬 Patent cliff: Humira, Remicade, and Herceptin are paving the way for biosimilar adoption
🤖 AI-powered development: faster approvals, lower R&D costs
🏥 Hospital dominance: over 60% of biosimilar use due to IV administration and formulary inclusion
With North America leading approvals and Asia-Pacific accelerating production, biosimilars are transforming access to advanced therapies—making biologics more affordable, scalable, and globally available.
🔗 Read the full report: https://www.skyquestt.com/report/biosimilars-market
0 notes
Text
Rheumatoid Arthritis Market Revenue Forecast: Growth, Share, Value, and Trends
"Executive Summary Rheumatoid Arthritis Market :
Data Bridge Market Research analyses a growth rate in the global rheumatoid arthritis market in the forecast period 2022-2029. The expected CAGR of global rheumatoid arthritis market is tend to be around 5.50% in the mentioned forecast period.
This Rheumatoid Arthritis Market research report is a proven and consistent source of information which gives telescopic view of the existing market trends, emerging products, situations and opportunities that drives your business towards the success. Market segmentation studies conducted in this report with respect to product type, applications, and geography are valuable in taking any verdict about the products. Rheumatoid Arthritis Market report also provides company profiles and contact information of the key market players in the key manufacturer’s section. Gaining valuable market insights with the new skills, latest tools and innovative programs is sure to help your business achieve business goals.
The Rheumatoid Arthritis Market report provides CAGR value fluctuations during the forecast period of 2018-2025 for the market. It encompasses a methodical investigation of current scenario of the global market, which covers several market dynamics. The report provides wide-ranging statistical analysis of the market’s continuous positive developments, capacity, production, production value, cost/profit, supply/demand and import/export. No stone is left unturned while researching and analysing data to prepare market research report like this one and the others. To get knowledge of all the above factors, this Rheumatoid Arthritis Market report is created that is transparent, extensive and supreme in quality.
Discover the latest trends, growth opportunities, and strategic insights in our comprehensive Rheumatoid Arthritis Market report. Download Full Report: https://www.databridgemarketresearch.com/reports/global-rheumatoid-arthritis-market
Rheumatoid Arthritis Market Overview
**Segments**
- On the basis of drug type, the market can be segmented into Biologics and Non-Biologics. Biologics are a key segment in the rheumatoid arthritis market, including drugs like Enbrel, Humira, and Rituxan. These drugs work by targeting specific components of the immune system responsible for inflammation. Non-Biologics, on the other hand, consist of traditional disease-modifying antirheumatic drugs (DMARDs) like methotrexate and hydroxychloroquine.
- By distribution channel, the market is categorized into Hospitals Pharmacies, Retail Pharmacies, and Online Pharmacies. Hospitals pharmacies tend to have a significant share due to the administration of biologic therapies, which often require specialized handling. Retail pharmacies are convenient for patients who need to refill their prescriptions regularly, while online pharmacies offer the advantage of home delivery and sometimes discounted prices.
- Based on region, the global rheumatoid arthritis market is segmented into North America, Europe, Asia Pacific, Latin America, and Middle East & Africa. North America, particularly the United States, holds a dominant position due to high prevalence rates of rheumatoid arthritis, well-established healthcare infrastructure, and the presence of major market players. Europe follows closely behind, with countries like Germany and the UK contributing significantly to market growth.
**Market Players**
- AbbVie Inc. - Pfizer Inc. - Amgen Inc. - Johnson & Johnson Services, Inc. - Novartis AG - Bristol-Myers Squibb Company - F. Hoffmann-La Roche Ltd - Merck & Co., Inc. - UCB S.A. - Eli Lilly and Company
These major players in the global rheumatoid arthritis market are constantly engaged in strategic initiatives such as product launches, collaborations, and acquisitions to maintain their competitive edge. For instance, AbbVie's drug Humira is one of the top-selling biologics for rheumatoid arthritis globally. Amgen's Enbrel and Johnson & Johnson's Remicade are also prominent players in the market, offering effective treatment options for patients.
The global rheumatoid arthritis market is expected to witness steady growth in the coming years due to factors such as increasing prevalence of rheumatoid arthritis, advancements in drug development, and rising healthcare expenditure. However, challenges such as patent expiries of key biologics, pricing pressures, and stringent regulatory requirements may hinder market growth to some extent. Overall, the market presents lucrative opportunities for both existing players and new entrants looking to capitalize on the growing demand for effective rheumatoid arthritis therapies.
The global rheumatoid arthritis market is witnessing significant growth propelled by various factors such as the increasing prevalence of rheumatoid arthritis, particularly in developed regions like North America and Europe. With a growing aging population and changing lifestyles leading to a rise in autoimmune diseases, the demand for effective treatment options for rheumatoid arthritis is on the rise. Additionally, advancements in drug development, particularly in the field of biologics, are driving innovation and expanding the treatment landscape for patients with rheumatoid arthritis.
One of the key drivers in the rheumatoid arthritis market is the focus on personalized medicine and targeted therapies. Biologics have revolutionized the treatment of rheumatoid arthritis by specifically targeting components of the immune system that play a role in inflammation. This personalized approach offers improved efficacy and safety profiles compared to traditional non-biologic treatments. As a result, biologics like Enbrel, Humira, and Rituxan continue to dominate the market and are preferred treatment options for many patients with rheumatoid arthritis.
In terms of market segmentation, the distribution channels play a crucial role in ensuring access to rheumatoid arthritis therapies. Hospitals pharmacies are significant players in the market, particularly for the administration of biologic therapies that require specialized handling and monitoring. Retail pharmacies offer convenience for patients seeking regular refills of their prescription medications, while online pharmacies provide the added advantage of home delivery and sometimes cost savings for patients. The diverse distribution channels cater to different patient needs and preferences, contributing to the overall accessibility of rheumatoid arthritis treatments.
The competitive landscape of the global rheumatoid arthritis market is characterized by major players such as AbbVie, Pfizer, and Amgen, who are continuously striving to innovate and stay ahead in the market. Strategic initiatives such as product launches, collaborations, and acquisitions are common among these market players to expand their product portfolios and reach a wider patient population. The strong market presence of companies like Johnson & Johnson, Novartis, and Roche further underscores the competitive nature of the rheumatoid arthritis market.
Looking ahead, the market presents promising opportunities for both existing players and new entrants to capitalize on the growing demand for effective rheumatoid arthritis therapies. However, challenges such as patent expiries of key biologics, pricing pressures, and stringent regulatory requirements could pose obstacles to market growth. Overall, the global rheumatoid arthritis market is poised for continued expansion driven by evolving treatment paradigms, technological advancements, and the unmet needs of patients with rheumatoid arthritis.The global rheumatoid arthritis market is a dynamic and competitive landscape driven by various factors such as increasing prevalence rates of the disease, advancements in drug development, and rising healthcare expenditure globally. The market segmentation into biologics and non-biologics reflects the diverse treatment options available to patients, with biologics like Enbrel, Humira, and Rituxan playing a crucial role in targeting specific components of the immune system responsible for inflammation. Non-biologics, represented by traditional DMARDs, offer alternative treatment options for rheumatoid arthritis patients.
The distribution channels in the market, including hospitals pharmacies, retail pharmacies, and online pharmacies, cater to different patient needs and preferences, ensuring accessibility to rheumatoid arthritis therapies. Hospitals pharmacies hold a significant share due to their role in administering specialized biologic therapies, while retail pharmacies provide convenience for patients requiring regular prescription refills. Online pharmacies offer the advantage of home delivery and potential cost savings for patients, contributing to the overall accessibility of treatment options for rheumatoid arthritis.
Major market players such as AbbVie, Pfizer, and Amgen are at the forefront of innovation in the global rheumatoid arthritis market, continuously engaging in strategic initiatives to maintain their competitive edge. Product launches, collaborations, and acquisitions are common strategies employed by these companies to expand their product portfolios and reach a wider patient population. The presence of key players like Johnson & Johnson, Novartis, and Roche further intensifies the competitive nature of the market, driving innovation and advancements in rheumatoid arthritis therapies.
Despite opportunities for growth in the market driven by increasing disease prevalence and evolving treatment paradigms, challenges such as patent expiries of key biologics, pricing pressures, and regulatory requirements pose potential obstacles to market expansion. The focus on personalized medicine and targeted therapies, particularly through the use of biologics, is a key driver in the market, offering improved efficacy and safety profiles for patients with rheumatoid arthritis. The market's continued growth trajectory is supported by technological advancements, evolving treatment approaches, and the unmet needs of patients with rheumatoid arthritis, presenting opportunities for stakeholders to capitalize on the demand for effective therapies in the evolving landscape.
The Rheumatoid Arthritis Market is highly fragmented, featuring intense competition among both global and regional players striving for market share. To explore how global trends are shaping the future of the top 10 companies in the keyword market.
Learn More Now: https://www.databridgemarketresearch.com/reports/global-rheumatoid-arthritis-market/companies
DBMR Nucleus: Powering Insights, Strategy & Growth
DBMR Nucleus is a dynamic, AI-powered business intelligence platform designed to revolutionize the way organizations access and interpret market data. Developed by Data Bridge Market Research, Nucleus integrates cutting-edge analytics with intuitive dashboards to deliver real-time insights across industries. From tracking market trends and competitive landscapes to uncovering growth opportunities, the platform enables strategic decision-making backed by data-driven evidence. Whether you're a startup or an enterprise, DBMR Nucleus equips you with the tools to stay ahead of the curve and fuel long-term success.
What insights readers can gather from the Rheumatoid Arthritis Market report?
Learn the behavior pattern of every Rheumatoid Arthritis Market-product launches, expansions, collaborations and acquisitions in the market currently.
Examine and study the progress outlook of the global Rheumatoid Arthritis Marketlandscape, which includes, revenue, production & consumption and historical & forecast.
Understand important drivers, restraints, opportunities and trends (DROT Analysis).
Important trends, such as carbon footprint, R&D developments, prototype technologies, and globalization.
Browse More Reports:
Global Automotive Horn Systems Market Global Membrane Contactor Market Global Wood Coating Resins Market Global Wood Plastic Composite Market Global Hair Serum Market Global Medical Dynamometer Market Global Diagnostic and Testing Equipment Market Global Electronic Clinical Outcome Assessment (eCOA) for Content Licensed Market Global Lithium Iron Phosphate (LFP) Batteries Market Asia-Pacific Electronic Clinical Outcome Assessment (eCOA) for Content Licensed Market Global Microcrystalline Cellulose (MCC) Market Global Saliva Test Device Market Global Psittacosis Treatment Market U.S., Central America, the Caribbean Islands, and South America Lubricants Market North America Electronic Clinical Outcome Assessment (eCOA) for Content Licensed Market Global Hospital Sterilization Equipment Market Global Meat Starter Culture Market Global Biomedical Textiles Market Global Single Lead Electrocardiogram (ECG) Equipment Market Global Patient Recliner Market Global Farm-Type Dairy Machines and Equipment Market Global Earphones Market Global Synthetic Betaine Market Asia-Pacific Water Purifier Market North America Mass Spectrometry Devices Market North America Cancer Diagnostics Market Global Synovial Sarcoma Treatment Market
About Data Bridge Market Research:
An absolute way to forecast what the future holds is to comprehend the trend today!
Data Bridge Market Research set forth itself as an unconventional and neoteric market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process. Data Bridge is an aftermath of sheer wisdom and experience which was formulated and framed in the year 2015 in Pune.
Contact Us: Data Bridge Market Research US: +1 614 591 3140 UK: +44 845 154 9652 APAC : +653 1251 975 Email:- [email protected]
0 notes
Text
Biosimilars Market: Current Trends and Future Scenarios, 2025-2032

The biosimilars market is on a trajectory of remarkable expansion, set to redefine the dynamics of biologic therapies between 2025 and 2032. As healthcare systems around the world face increasing pressure to balance innovation with affordability, biosimilars have emerged as a critical solution—offering comparable clinical outcomes to reference biologics at significantly reduced costs.
Biosimilars Market size is poised to grow from USD 28.38 billion in 2024 to USD 98.31 billion by 2032, growing at a CAGR of 16.8% during the forecast period (2025-2032).
This growth is driven by a confluence of market forces, including patent expirations, regulatory reforms, and rising healthcare demands associated with chronic diseases and an aging global population.
Understanding Biosimilars
Biosimilars are biologic products that are highly similar to, and have no clinically meaningful differences from, an existing FDA-approved reference product. Unlike small-molecule generics, biosimilars are developed from living organisms and require complex manufacturing processes, followed by comprehensive comparative studies to demonstrate similarity in efficacy, safety, and immunogenicity.
As more blockbuster biologics lose their patent exclusivity, biosimilars are stepping in to enhance accessibility, stimulate competition, and reduce healthcare expenditures globally.
Request Sample of the Report - https://www.skyquestt.com/sample-request/biosimilars-market
Biosimilars Market Growth Drivers
Patent Cliff of Biologics: Many first-generation biologics—such as Humira (adalimumab), Remicade (infliximab), and Herceptin (trastuzumab)—have either lost or are approaching the end of their patent protection, opening doors for biosimilar competition.
Cost-Effectiveness: Biosimilars can be 15–30% cheaper than their reference products, delivering significant cost savings for patients, insurers, and healthcare systems.
Rising Prevalence of Chronic Diseases: Increasing incidences of cancer, autoimmune disorders, and diabetes are driving demand for long-term biologic therapies—markets where biosimilars are gaining a strong foothold.
Global Regulatory Advancements: Regulatory bodies such as the US FDA, European Medicines Agency (EMA), and regulators in Asia have established well-defined pathways for biosimilar approval, reducing entry barriers.
Strategic Industry Collaborations: Pharma giants are forming alliances with biotech firms to accelerate biosimilar R&D, manufacturing, and commercialization, particularly in emerging markets.
Regional Biosimilars Market Dynamics
North America: North America is currently one of the largest biosimilar markets, bolstered by supportive regulatory frameworks, including the Biologics Price Competition and Innovation Act (BPCIA). The U.S. market is experiencing increased biosimilar uptake in oncology and immunology, fueled by payer incentives and expanding physician confidence.
Europe: Europe has been a trailblazer in biosimilar adoption, with robust healthcare systems embracing biosimilars to control public spending. Favorable reimbursement policies, physician incentives, and public awareness campaigns
Asia Pacific: Asia Pacific is projected to be the fastest-growing region due to high demand, government-backed initiatives, and competitive biosimilar manufacturers based in India, South Korea, and China.
Get Customized Reports with your Requirements - https://www.skyquestt.com/speak-with-analyst/biosimilars-market
Biosimilars Market Segmentation
By Product Type
Monoclonal Antibodies (mAbs): Represent the largest revenue-generating segment, driven by applications in oncology and autoimmune diseases.
Insulin: High-growth segment due to the global diabetes epidemic, with biosimilars providing a cost-effective alternative to expensive branded insulins.
Hematopoietic Products: Includes biosimilars of filgrastim and epoetin, primarily used in oncology and chronic kidney disease.
By Application
Oncology: The dominant therapeutic area, contributing over 24% of market revenue in 2023. High treatment costs and large patient populations drive biosimilar adoption.
Autoimmune Disorders: Rheumatoid arthritis, psoriasis, and inflammatory bowel diseases are key indications where biosimilars are gaining traction.
Others: Diabetes, infectious diseases, hormone deficiencies, and rare diseases are also emerging application areas.
Biosimilars Market Competitive Landscape
The biosimilars market is highly competitive and rapidly evolving. Industry leaders are leveraging R&D, strategic mergers, and geographic expansion to gain market share. Some of the major players include:
Pfizer Inc.
Amgen Inc.
Sandoz (Novartis subsidiary)
Samsung Bioepis
Celltrion
Biocon Biologics
Viatris Inc.
Coherus BioSciences
Dr. Reddy’s Laboratories
Fresenius Kabi
These companies are focusing on pipeline development, regulatory approvals, and cost-efficient manufacturing to address market gaps and unmet medical needs.
Detailed Table of Contents of this Report - https://www.skyquestt.com/report/biosimilars-market
Challenges in the Biosimilars Ecosystem
While the market outlook is highly positive, several barriers must be addressed:
Regulatory Complexity: Biosimilars require a robust and expensive clinical trial process to prove equivalence, leading to high development costs and longer time-to-market.
Market Entrenchment of Biologics: Brand loyalty and physician reluctance to switch therapies can slow biosimilar penetration.
Legal and Patent Disputes: Originator companies often engage in extended litigation to delay biosimilar entry.
Manufacturing Constraints: High capital investment and technical know-how are required for biologic production, creating entry barriers for smaller firms.
Biosimilars Market Trends
The period from 2025 to 2032 is expected to be a golden era for biosimilars, with transformative shifts in pricing models, digital health integration, and policy-driven adoption.
Emerging Trends Include:
Next-Generation Biosimilars: Enhanced formulations and delivery systems to improve patient experience.
Digital Tools: Real-world evidence platforms and AI-driven analytics to track biosimilar outcomes and adoption.
Policy Innovation: Reimbursement reforms and switching incentives to boost market uptake.
Sustainability: Greener manufacturing and supply chain models for biosimilar production.
Biosimilars Market Future Outlook
The global biosimilars market is set to expand more than fourfold by 2032, redefining cost structures and access paradigms in biologic therapy. While the path is not without hurdles, the fundamentals—ranging from clinical efficacy to economic rationale—strongly support the ascension of biosimilars as the future of biopharmaceutical care.
Investors, policymakers, healthcare providers, and life science innovators would be wise to embrace this momentum and collaborate to unlock the full potential of biosimilars in delivering high-quality, equitable healthcare worldwide.
0 notes
Text
Biologics and Biosimilars Market Consumer Behavior and Industry Shifts to 2033
Introduction
The biologics and biosimilars market is witnessing significant growth driven by advancements in biotechnology, increasing prevalence of chronic diseases, and rising demand for cost-effective biologic therapies. Biologics, which include complex molecules such as monoclonal antibodies and recombinant proteins, have revolutionized the treatment of various diseases. However, their high costs have paved the way for biosimilars—biological products that are highly similar to approved biologics—with comparable efficacy and safety at reduced prices.
As we look forward to 2032, the market dynamics are expected to shift further with policy reforms, technological innovations, and increasing competition shaping the future landscape.
Market Overview
In 2024, the global biologics and biosimilars market was valued at USD 400+ billion, and it is projected to surpass USD 750 billion by 2032, growing at a CAGR of over 8% during the forecast period. The market is segmented by product type, therapeutic area, manufacturing type, and region.
Download a Free Sample Report:-https://tinyurl.com/2k678792
Key Definitions:
Biologics: Medicinal products derived from living organisms, used to treat diseases such as cancer, autoimmune disorders, and infectious diseases.
Biosimilars: Biologic medical products that are almost identical copies of an original product but manufactured by a different company after the patent expiry.
Market Drivers
Patent Expirations of Biologic Blockbusters
The expiration of patents for blockbuster biologics such as Humira (adalimumab), Remicade (infliximab), and Herceptin (trastuzumab) has opened opportunities for biosimilars. These patent cliffs allow biosimilar manufacturers to enter the market with cost-effective alternatives, increasing competition and market penetration.
Rising Prevalence of Chronic Diseases
The growing global burden of chronic diseases like cancer, rheumatoid arthritis, diabetes, and cardiovascular disorders is fueling the demand for biologic therapies. Biologics offer high specificity and efficacy, making them the preferred choice for targeted treatment.
Cost-effectiveness of Biosimilars
Healthcare systems worldwide are increasingly adopting biosimilars to reduce the overall cost of treatment. Biosimilars typically cost 15-30% less than their reference biologics, which helps improve access, especially in emerging markets.
Regulatory Support and Guidelines
Regulatory bodies like the U.S. FDA, European Medicines Agency (EMA), and World Health Organization (WHO) have established stringent yet supportive pathways for biosimilar approval, ensuring their safety, efficacy, and quality.
Market Restraints
High Manufacturing Complexity: Biologics and biosimilars require sophisticated manufacturing processes, involving living cells and stringent quality controls.
Limited Patient and Physician Awareness: In certain regions, the lack of awareness and trust in biosimilars hinders adoption.
Patent Litigation and Exclusivity: Legal battles over patents and exclusivity rights can delay biosimilar entry and increase development costs.
Market Segmentation
By Product Type
Monoclonal Antibodies
Vaccines
Recombinant Hormones
Interferons
Insulin
Fusion Proteins
Monoclonal antibodies lead the biologics segment due to their application in cancer and autoimmune diseases.
By Therapeutic Application
Oncology
Autoimmune Diseases
Diabetes
Hematology
Infectious Diseases
Ophthalmology
The oncology segment is dominant, driven by the growing prevalence of cancer and the high efficacy of biologics and biosimilars in oncology treatment.
By Manufacturing Type
In-house Manufacturing
Contract Manufacturing Organizations (CMOs)
While large pharmaceutical firms often use in-house capabilities, small biotech firms increasingly rely on CMOs to reduce operational costs and enhance scalability.
Regional Insights
North America
North America dominates the market due to robust healthcare infrastructure, high R&D investment, and early adoption of biosimilars. The U.S. FDA’s Biosimilar Action Plan further encourages biosimilar development and competition.
Europe
Europe is a pioneer in biosimilar approvals and adoption. Countries like Germany, the UK, and France have implemented favorable reimbursement policies and strong physician awareness.
Asia Pacific
The Asia Pacific region is experiencing the fastest growth, driven by increasing healthcare spending, large patient populations, and growing investments by regional players in biosimilar development (e.g., India, South Korea, and China).
Competitive Landscape
The market is highly competitive, with both large pharmaceutical companies and specialized biotechnology firms operating globally.
Major Players:
Amgen Inc.
Roche Holding AG
Pfizer Inc.
Samsung Bioepis
Biocon Ltd.
Sandoz (a Novartis division)
Celltrion Healthcare
These companies focus on strategic alliances, product launches, biosimilar approvals, and manufacturing scale-up to gain competitive advantages.
Emerging Trends
Integration of AI and Digital Technologies
Artificial intelligence is being utilized for biologics discovery, molecular simulation, and process optimization. This enhances speed-to-market and reduces R&D costs.
Personalized Biologics
With advances in genomics, biologics are increasingly being tailored to individual patient profiles, leading to improved outcomes and reduced side effects.
Subcutaneous and Oral Biologics
There is a rising trend toward subcutaneous and oral biologics, offering patient convenience over traditional intravenous delivery methods.
Government Incentives and Reimbursement Policies
Governments in emerging economies are offering incentives and subsidies to promote biosimilar manufacturing and adoption, improving market access and affordability.
Future Outlook (2025–2032)
The biologics and biosimilars market is expected to evolve rapidly over the next decade. With major patents expiring and healthcare systems seeking cost-effective solutions, biosimilars will gain more ground. Meanwhile, continued innovation in biologics will lead to novel therapies for rare and complex diseases.
Key projections:
Biologics will continue to dominate in revenue, but biosimilars will grow at a faster rate due to increasing accessibility.
Asia Pacific will emerge as a biosimilar manufacturing hub due to lower production costs and favorable policies.
Strategic partnerships between pharma companies and CMOs or biotech startups will increase to meet global demand efficiently.
Biobetters—enhanced versions of original biologics—will gain popularity for offering improved efficacy and reduced dosing frequency.
Conclusion
The biologics and biosimilars market stands at a pivotal point. While biologics continue to drive innovation in treating life-threatening diseases, biosimilars are ensuring that these treatments become accessible and affordable for a broader population. As regulatory landscapes mature, technologies advance, and global healthcare priorities evolve, the market is poised for robust and inclusive growth through 2032.
Companies that invest in quality manufacturing, innovation, and regulatory navigation will be well-positioned to capture significant market share in this dynamic and transformative healthcare segment.Read Full Report:-https://www.uniprismmarketresearch.com/verticals/healthcare/biologics-and-biosimilars
0 notes
Text
Airway Disease Treatment Market: Market Trends and Market Forecast 2024-2032

The airway disease treatment market was valued at USD 2.23 billion in 2023 and is projected to reach USD 3.54 billion by 2032, growing at a compound annual growth rate (CAGR) of 5.29% from 2024 to 2032. This growth is primarily driven by the increasing prevalence of respiratory disorders such as asthma, chronic obstructive pulmonary disease (COPD), and bronchiectasis.
Get Free Sample Report @ https://www.snsinsider.com/sample-request/3937
Market Segmentation
By Type:
Asthma
Chronic Obstructive Pulmonary Disorder (COPD)
Bronchiectasis
By Treatment:
Bronchodilators
Corticosteroids
Cytotoxic Drugs
Oxygen Therapy
Antibiotics
Others
By End Use:
Hospitals
Clinics
Ambulatory Surgical Centers (ASCs)
Rehabilitation Centers
Others
Regional Analysis
North America dominated the market in 2023, accounting for a significant revenue share. This is attributed to high healthcare expenditure, advanced medical infrastructure, and a significant prevalence of respiratory diseases. The Asia Pacific region is anticipated to experience the fastest growth, with a projected CAGR of about 7.40% from 2024 to 2032. Factors contributing to this growth include improved healthcare access, a rising middle class, and an increasing burden of respiratory diseases.
KEY PLAYERS
Holaira, Inc. (Aeris, Holaira’s Bronchial Thermoplasty System)
VIDA Diagnostics (VIDA Insight, VIDA Lung AI)
Boehringer Ingelheim International GmbH (Spiriva, Striverdi)
AstraZeneca (Symbicort, Fasenra)
Teva Pharmaceuticals (ProAir HFA, Qvar RediHaler)
GlaxoSmithKline (Advair, Breo Ellipta)
Novartis (Xolair, Ultibro Breezhaler)
Sun Pharmaceutical Industries Ltd. (Breztri Aerosphere, Pulmicort)
Pfizer Inc. (Breztri Aerosphere, Xolair)
Zydus Group (Duolin, Asthalin)
Merck & Co., Inc. (Singulair, Dulera)
AbbVie Inc. (Rinvoq, Humira)
Bristol-Myers Squibb (Orencia, Breztri Aerosphere)
Mylan (Symbicort, EpiPen)
Eli Lilly and Company (Emgality, Trulicity)
Roche (Xolair, Pulmozyme)
Amgen (Tezspire, Kineret)
Baxter International Inc. (Bethkis, Altabax)
Sandoz (Novartis) (AirFluSal Forspiro, Symbicort)
Cipla (Seroflo, Foracort)
F. Hoffmann-La Roche Ltd. (Pulmozyme, Xolair)
Medtronic (Breathe, CoreValve)
Key Highlights
The asthma segment held the largest revenue share of approximately 54% in 2023, driven by its high prevalence and established treatment options.
The COPD segment is expected to grow at the fastest CAGR of about 6.16% from 2024 to 2032, due to an aging population and increased smoking rates.
Hospitals accounted for the highest revenue share of about 43% in 2023, owing to their comprehensive care capabilities and advanced treatment infrastructure.
The clinics segment is projected to grow at the fastest CAGR of approximately 7.03% from 2024 to 2032, driven by the demand for outpatient care and specialized respiratory services.
Future Scope
The airway disease treatment market is poised for significant advancements, particularly with the integration of digital health technologies and AI-driven diagnostics. These innovations enable real-time patient monitoring and personalized treatment plans, leading to improved patient outcomes. Additionally, the adoption of AI and machine learning in drug discovery is expected to accelerate the development of more effective and accessible therapies, addressing the growing global demand for respiratory disease treatments.
Conclusion
The airway disease treatment market is on a robust growth trajectory, fueled by the rising prevalence of respiratory conditions and continuous advancements in medical technology. Strategic collaborations and technological innovations are set to further enhance treatment efficacy and patient care, ensuring better management of airway diseases globally.
Contact Us: Jagney Dave - Vice President of Client Engagement Phone: +1-315 636 4242 (US) | +44- 20 3290 5010 (UK)
Other Related Reports:
Cell Viability Assay Market
Medical Power Supply Market
Post Traumatic Stress Disorder Treatment Market
MRI Guided Neurosurgical Ablation Market
#Airway Disease Treatment Market#Airway Disease Treatment Market Share#Airway Disease Treatment Market Size#Airway Disease Treatment Market Trends
0 notes
Text
Adalimumab Biosimilars: Market Size, Share, and Forecast Analysis

The Adalimumab Biosimilars Market is witnessing significant growth as biosimilar versions of Humira (adalimumab) gain traction globally. Adalimumab, a tumor necrosis factor-alpha (TNF-α) inhibitor, has been widely used to treat autoimmune diseases such as rheumatoid arthritis, psoriasis, Crohn’s disease, and ulcerative colitis. With the expiration of Humira’s patent, numerous Adalimumab Biosimilar Companies have entered the market, offering cost-effective alternatives and increasing patient access to these life-changing therapies.
Market Growth and Key Drivers
The expansion of the Adalimumab biosimilar market is driven by several factors:
Patent Expiry and Market Competition – The loss of exclusivity for Humira has paved the way for multiple biosimilars, intensifying competition and driving down treatment costs.
Regulatory Approvals and Expanding Market Access – Regulatory agencies like the FDA and EMA have approved multiple adalimumab biosimilars, promoting broader adoption.
Cost-Effectiveness and Healthcare Savings – Biosimilars offer a lower-cost alternative to biologics, helping healthcare systems reduce spending while maintaining treatment efficacy.
Increased Prevalence of Autoimmune Diseases – The rising incidence of conditions like rheumatoid arthritis and inflammatory bowel disease is fueling demand for adalimumab biosimilars.
Key Adalimumab Biosimilar Companies and Market Players
Several pharmaceutical and biotech firms are actively competing in the Adalimumab Biosimilars Market. Some of the key Adalimumab Biosimilar Companies include:
Amgen (Amjevita/Bijeljira) – One of the first biosimilars approved for Humira, launched in multiple markets.
Boehringer Ingelheim (Cyltezo) – The first FDA-approved interchangeable adalimumab biosimilar, providing greater market penetration.
Samsung Bioepis (Hadlima) – Developed in collaboration with Biogen, expanding access to biosimilars globally.
Sandoz (Hyrimoz) – A major player in the biosimilar space, offering competitive pricing and accessibility.
Viatris & Biocon Biologics (Hulio) – A strong competitor in both U.S. and European markets.
Future Outlook and Market Expansion
The Adalimumab Biosimilars Market is poised for substantial growth as more biosimilars gain regulatory approval and manufacturers expand their geographic reach. Increased physician and patient confidence in biosimilars, coupled with pricing advantages, will further drive adoption. As competition intensifies, innovation in formulation, delivery methods, and interchangeability designations will shape the future of the market.
Overall, the rise of Adalimumab Biosimilar Companies marks a transformative shift in the autoimmune disease treatment landscape, providing cost-effective and widely accessible therapeutic options for patients worldwide.
Latest Reports Offered By DelveInsight:
papular warts | chronic illness app | aln-app | how do you treat epi | bronchial spasms | biogen postpartum depression | synox therapeutics | whim disease | is gepotidacin available | accutar biotech | outlast amber | osteoarthritis epidemiology | pde3 inhibitors | sarco penia | technological advances in healthcare | copd newsletter | elastomeric pump | apellis pipeline | tarpeyo iga nephropathy | mugard cost | otc deficiency treatment | adagrasib cetuximab | pers emergency response system | dupixent alternatives | progressive pipeline | cefepime-taniborbactam in complicated urinary tract infection
#Global Adalimumab Biosimilar#Global Adalimumab Biosimilar Market#Global Adalimumab Biosimilar Forecast#Global Adalimumab Biosimilar Companies#Global Adalimumab Biosimilar Therapies#Global Adalimumab Biosimilar Epidemiology#Global Adalimumab Biosimilar Pipeline#Global Adalimumab Biosimilar Market Size#Global Adalimumab Biosimilar Market Trends
0 notes
Link
0 notes
Text
Adalimumab Market - Forecast(2024 - 2030)
Adalimumab Market Overview
Adalimumab Market Size is forecast to reach $3245.8 Million by 2030, at a CAGR of 6.20% during forecast period 2024-2030. Adalimumab is a monoclonal antibody and disease-modifying antirheumatic medication that acts by inactivating tumor necrosis factor-alpha. It is marketed under the brand names Humira and Exemptia, among others. The original adalimumab drug is sold under the brand name Humira. New versions of adalimumab are known as adalimumab biosimilars. Biosimilars are similar versions of original biological medicines. Adalimumab is a drug used to treat rheumatoid arthritis and ankylosing spondylitis which affects the musculoskeletal system, Crohn’s disease, ulcerative colitis, psoriatic arthritis, hidradenitis suppurativa, and other diseases. It was first introduced in the United States by Abbvie and was approved by the FDA in 2002. It is used to alleviate inflammation by acting on the immune system. The growing prevalence of arthritis and skin disorders, adoption of a sedentary lifestyle, and growing obese population are some of the factors driving the Adalimumab Market growth during the forecast period 2024-2030.
𝐃𝐨𝐰𝐧𝐥𝐨𝐚𝐝 𝐒𝐚𝐦𝐩𝐥𝐞
Report Coverage
The report: “Adalimumab Market Forecast (2024-2030)", by Industry ARC covers an in-depth analysis of the following segments of the Adalimumab Market.
By Disease- Crohn’s disease, Psoriatic arthritis, Rheumatoid arthritis, Ulcerative colitis, and others. By Type- Adalimumab, Adalimumab Biosimilar. By End-Users- Hospitals, Specialty Clinics, and Others. By Geography- North America (U.S., Canada, Mexico), Europe (Germany, United Kingdom (U.K.), France, Italy, Spain, Russia, and Rest of Europe), Asia Pacific (China, Japan India, South Korea, Australia, and New Zealand, and Rest of Asia Pacific), South America (Brazil, Argentina, and Rest of South America), and Rest of the World (the Middle East, and Africa).

Key Takeaways
Geographically, North America held a dominant market share in the year 2021 on account of the increasing prevalence of psoriasis arthritis, highly informed people knowing the benefits of adalimumab, and high healthcare expenditure. Asia-Pacific is expected to offer lucrative growth opportunities to the manufacturers owing to increasing prevalence of Crohn's disease and ulcerative colitis., demand for better healthcare facilities as well a boost in the investments done by government and private companies in pharma industry. The proliferation of Rheumatoid arthritis is predicted to augment the market growth during the forecast period of 2022-2027.
The growing prevalence of arthritis and skin disorders is estimated to drive the market growth of the Adalimumab Market. High cost as well as lack side effects of adalimumab poses threat to the market growth.
A detailed analysis of strengths, weaknesses, opportunities, and threats will be provided in the Adalimumab Market Report.
0 notes
Text
Psoriasis Treatment Market Size, Share, Demand, Future Growth, Challenges and Competitive Analysis
"Global Psoriasis Treatment Market - Industry Trends and Forecast to 2029
Global Psoriasis Treatment Market, By Drug Class (Corticosteroids, TNF Inhibitors, Interleukins, Others), Type (Plaque Psoriasis, Psoriatic Arthritis, Others), Route of Administration (Oral, Parenteral, Topical), Application (Topical Therapeutic Drugs, Systemic Therapeutic Drugs, Combinations), End User (Hospital Pharmacy, Clinics, Retail Pharmacies, Online Sales), Country (U.S., Canada, Mexico, Peru, Brazil, Argentina, Rest of South America, Germany, Italy, U.K., France, Spain, Netherlands, Belgium, Switzerland, Turkey, Russia, Hungary, Lithuania, Austria, Ireland, Norway, Poland, Rest of Europe, Japan, China, India, South Korea, Australia, Singapore, Malaysia, Thailand, Indonesia, Philippines, Vietnam, Rest of Asia Pacific, South Africa, Saudi Arabia, U.A.E, Kuwait, Israel, Egypt, Rest of Middle East and Africa) Industry Trends and Forecast to 2029.
Access Full 350 Pages PDF Report @
**Segments**
- **By Drug Class**: The psoriasis treatment market can be segmented based on drug class, which includes biologics, systemic therapy, topical therapy, and others. Biologics are becoming increasingly popular due to their targeted approach in treating psoriasis at a molecular level. Systemic therapy involves oral or injectable medications that affect the entire immune system to manage psoriasis symptoms. Topical therapies include creams, ointments, and lotions applied directly to the skin to alleviate mild to moderate symptoms.
- **By Route of Administration**: Another important segmentation in the psoriasis treatment market is based on the route of administration. This includes oral, injectable, and topical routes. Oral medications are often prescribed for systemic treatment, while injectable biologics are administered via subcutaneous or intravenous routes. Topical treatments are primarily used for localized psoriasis management.
- **By Distribution Channel**: The distribution channel segment categorizes the market based on where patients can access psoriasis treatments. This includes hospital pharmacies, retail pharmacies, online pharmacies, and specialty clinics. Hospital pharmacies are common for inpatient treatments, while retail pharmacies cater to outpatient prescriptions. Online pharmacies are gaining popularity due to convenience, and specialty clinics focus on dermatological care for psoriasis patients.
**Market Players**
- **AbbVie Inc.**: AbbVie is a leading player in the psoriasis treatment market, offering biologics like Humira (adalimumab) for psoriasis management.
- **Johnson & Johnson Services, Inc.**: Johnson & Johnson is known for its systemic therapy options for psoriasis, such as Stelara (ustekinumab) and Tremfya (guselkumab).
- **Novartis AG**: Novartis is a key player with a strong portfolio of psoriasis treatments, including Cosentyx (secukinumab) and Methotrexate.
- **Amgen Inc.**: Amgen has a range of biThe psoriasis treatment market is a dynamic and evolving sector with several key players driving innovation and advancements in treatment options for patients. AbbVie Inc. is a prominent player in the market, offering biologics like Humira (adalimumab) that have demonstrated efficacy in managing psoriasis symptoms. Biologics have gained significant traction in the market due to their targeted approach at the molecular level, offering a more personalized and effective treatment option for patients. Johnson & Johnson Services, Inc. is another major player known for its systemic therapy options such as Stelara (ustekinumab) and Tremfya (guselkumab), which have shown promise in managing moderate to severe psoriasis.
Novartis AG, with its strong portfolio of psoriasis treatments including Cosentyx (secukinumab) and Methotrexate, has been instrumental in improving outcomes for patients with psoriasis. These treatments target specific pathways involved in the development of psoriasis, providing patients with more targeted and effective treatment options. Amgen Inc., with its range of biologics like Enbrel (etanercept), has also made significant contributions to the market by providing new insights and advancements in psoriasis treatment.
The market for psoriasis treatments is witnessing a shift towards more personalized and targeted therapies, driven by advancements in biologics and systemic therapies. Biologics, in particular, have revolutionized the treatment landscape for psoriasis by targeting specific molecules involved in the inflammatory response associated with the condition. These targeted therapies have shown significant efficacy in managing psoriasis symptoms and improving quality of life for patients.
The distribution channels for psoriasis treatments play a crucial role in ensuring access to these therapies for patients. Hospital pharmacies are essential for inpatient treatments, while retail pharmacies cater to outpatient prescriptions, providing convenient access to medications for patients. Online pharmacies have also emerged as a popular choice for patients seeking convenience and accessibility in obtaining their psoriasis treatments. Specialty clinics focusing on dermatological**Global Psoriasis Treatment Market**
- **Segments** - **By Drug Class**: The psoriasis treatment market encompasses a variety of drug classes, including corticosteroids, TNF inhibitors, interleukins, and others. Corticosteroids are commonly used for their anti-inflammatory properties, while TNF inhibitors and interleukins target specific pathways in the immune system to manage psoriasis symptoms. Other drug classes offer alternative mechanisms of action for psoriasis treatment. - **By Type**: The types of psoriasis targeted in the market include plaque psoriasis, psoriatic arthritis, and other less common forms of the condition. Different types of psoriasis may require specific treatment approaches tailored to the patient's symptoms and disease progression. - **By Route of Administration**: Psoriasis treatments can be administered through various routes, such as oral, parenteral, and topical. Oral medications are often used for systemic treatment, while parenteral routes involve injections for targeted therapy. Topical treatments are applied directly to the skin for localized symptom management. - **By Application**: The applications of psoriasis treatment include topical therapeutic drugs, systemic therapeutic drugs, and combination therapies. These different applications offer a range of options for patients and healthcare providers to address the varying severity of psoriasis symptoms. - **By End User**: The end users in the psoriasis treatment market span hospital pharmacies, clinics, retail pharmacies, and online sales. Each end user plays a crucial role in ensuring access to psoriasis
The Psoriasis Treatment Market competitive landscape provides details by the competitors. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, and application dominance.
Major Points Covered in TOC:
Psoriasis Treatment Market Overview: It incorporates six sections, research scope, significant makers covered, market fragments by type, Psoriasis Treatment Market portions by application, study goals, and years considered.
Psoriasis Treatment Market Landscape: Here, the opposition in the Worldwide Psoriasis Treatment Market is dissected, by value, income, deals, and piece of the pie by organization, market rate, cutthroat circumstances Landscape, and most recent patterns, consolidation, development, obtaining, and portions of the overall industry of top organizations.
Psoriasis Treatment Profiles of Manufacturers: Here, driving players of the worldwide Psoriasis Treatment Market are considered dependent on deals region, key items, net edge, income, cost, and creation.
Psoriasis Treatment Market Status and Outlook by Region: In this segment, the report examines about net edge, deals, income, creation, portion of the overall industry, CAGR, and market size by locale. Here, the worldwide Psoriasis Treatment Market is profoundly examined based on areas and nations like North America, Europe, China, India, Japan, and the MEA.
Psoriasis Treatment Application or End User: This segment of the exploration study shows how extraordinary end-client/application sections add to the worldwide Psoriasis Treatment Market.
Psoriasis Treatment Market Forecast: Production Side: In this piece of the report, the creators have zeroed in on creation and creation esteem conjecture, key makers gauge, and creation and creation esteem estimate by type.
Keyword: Research Findings and Conclusion: This is one of the last segments of the report where the discoveries of the investigators and the finish of the exploration study are given.
What to Expect from the Report, a 7-Pointer Guide
The Psoriasis Treatment Market report dives into the holistic Strategy and Innovation for this market ecosystem
The Psoriasis Treatment Market report keenly isolates and upholds notable prominent market drivers and barriers
The Psoriasis Treatment Market report sets clarity in identifying technological standardization as well as the regulatory
framework, besides significantly assessing various implementation models besides evaluation of numerous use cases
The Psoriasis Treatment Market report is also a rich repository of crucial information across the industry, highlighting details on novel investments as well as stakeholders and relevant contributors and market participants.
A through market analytical survey and forecast references through the forecast tenure, encapsulating details on historical developments, concurrent events as well as future growth probability
Browse Trending Reports:
Calcium Glycinate Market Retinal Biologics Market Facial Fat Transfer Market Angio Suites Diagnostic Imaging Market Adoption Of Benelux Power Tools Market De Quervains Tenosynovitis Treatment Market Biodetectors And Accessories Market Colposcope Market Sports Medicine Market Automotive Adhesives Market Infrared Imaging Market Vapour Deposition Market Professional Diagnostics Market Ct Scanner Market Programmable Application Specific Integrated Circuit Asic Market Hospital Operating Room Or Products And Solutions Market Castor Oil Market Zika Virus Infection Drug Market Toluene Diisocynate Market Antibiotic Resistance Market
About Data Bridge Market Research:
Data Bridge set forth itself as an unconventional and neoteric Market research and consulting firm with unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email: [email protected]"
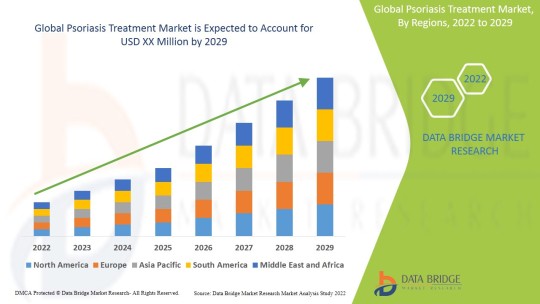
0 notes
Text
Celltrion Inc.: Financial Performance Overview
Celltrion Financials is a South Korean biopharmaceutical company, known for its leadership in developing biosimilars and biologics. The company focuses on producing affordable, high-quality biologic drugs for the treatment of autoimmune diseases, cancer, and other chronic illnesses. Below is a summary of its financial performance based on available information.
1. Revenue Growth
Strong Sales of Biosimilars: Celltrion has seen consistent growth in revenue, primarily driven by the global sales of its biosimilars such as Remsima (a biosimilar to Remicade), Truxima (a biosimilar to Rituxan), and Herzuma (a biosimilar to Herceptin). These products are approved in multiple regions, including the U.S., Europe, and Asia, contributing significantly to the company's topline.
Year-over-Year Revenue Increase: Celltrion has experienced strong revenue growth in recent years, often reporting double-digit percentage increases in annual sales. This growth is fueled by increasing market adoption of biosimilars and new product launches.
2. Profit Margins
High Gross Margins: Celltrion benefits from relatively high gross margins due to the cost-efficiency of its manufacturing processes and the lower R&D costs associated with biosimilars compared to original biologic drugs. Its state-of-the-art manufacturing facilities in South Korea contribute to economies of scale.
Operating Income: The company’s operating income has also been growing steadily, supported by both rising sales and cost-control measures. Celltrion’s ability to scale production efficiently while maintaining competitive pricing has been a key driver of profitability.
3. R&D Expenditure
Investments in Pipeline Development: Celltrion continues to invest heavily in research and development (R&D), focusing on expanding its biosimilar pipeline and developing new biologic treatments. This includes the development of biosimilars for blockbuster drugs as well as innovative biologics for cancer and autoimmune diseases.
R&D as a Percentage of Revenue: Celltrion’s R&D expenditure represents a significant portion of its annual revenue, reflecting its long-term commitment to innovation and expanding its product portfolio. The company is focused on developing next-generation biologics and biosimilars, which will drive future growth.
4. Global Market Expansion
International Sales Contribution: A significant portion of Celltrion’s revenue comes from its international operations, particularly in Europe and the U.S., where biosimilars have gained widespread acceptance. The company has been actively pursuing regulatory approvals in various regions, which has contributed to its robust revenue growth.
Partnerships and Alliances: Celltrion has established strategic partnerships with global pharmaceutical companies for the marketing and distribution of its biosimilar products. These partnerships have played a key role in driving global sales and market penetration.
5. Impact of COVID-19
COVID-19 Treatment Development: During the COVID-19 pandemic, Celltrion played an active role in developing treatments for the virus. The company developed Regkirona (CT-P59), a monoclonal antibody treatment for COVID-19. This has contributed to additional revenue streams and positioned the company as a key player in pandemic response efforts.
Increased Demand for Biologics: The pandemic heightened the global demand for biologics, which benefited Celltrion’s biosimilar portfolio as healthcare systems sought cost-effective treatments for chronic diseases amidst budget constraints.
6. Outlook and Future Growth
Biosimilar Pipeline: Celltrion’s future growth is expected to be driven by the launch of new biosimilars currently in its pipeline. These include biosimilars for drugs like Humira (adalimumab), which has been one of the world’s top-selling drugs, and other monoclonal antibodies.
Innovative Biologics: Beyond biosimilars, Celltrion is working on developing innovative biologic treatments that could further bolster its revenue streams and market position.
Expansion into New Markets: Celltrion continues to focus on expanding into new international markets, particularly in emerging regions where demand for affordable biologic treatments is increasing.
Conclusion
Celltrion Inc. has demonstrated strong financial performance, supported by its leadership in the biosimilar market, efficient manufacturing capabilities, and strategic international partnerships. With a robust product pipeline, continued R&D investments, and a focus on global market expansion, Celltrion is well-positioned for future growth in the biopharmaceutical industry.
0 notes
Text
Psoriasis Treatment Unveiled: The Latest in Therapeutic Advances

According to the report, the global psoriasis therapeutics market is projected to grow at a compound annual growth rate (CAGR) of 9% over the forecast period from 2022 to 2028. The market generated revenue of approximately USD 24 billion in 2022 and is expected to reach nearly USD 44 billion by 2028.
Understanding Psoriasis Therapeutics
Psoriasis is a chronic autoimmune disease that results in the rapid buildup of skin cells, causing scaling and inflammation. Psoriasis therapeutics include a range of treatments such as topical therapies, phototherapy, and systemic treatments, including biologics and small-molecule drugs, aimed at reducing symptoms and managing flare-ups.
Get Sample pages of Report: https://www.infiniumglobalresearch.com/reports/sample-request/825
Market Dynamics and Growth Drivers
The significant growth of the global psoriasis therapeutics market is driven by several factors:
Rising Prevalence of Psoriasis: The global incidence of psoriasis is increasing, leading to a growing patient pool that requires long-term therapeutic solutions. With millions affected worldwide, demand for effective treatments is on the rise.
Advancements in Biologics: Innovations in biologic drugs have transformed the treatment landscape for psoriasis. Biologics, which target specific components of the immune system, have proven highly effective in treating moderate to severe cases, contributing to the market's growth.
Increased Awareness and Diagnosis: Greater awareness of psoriasis and advancements in diagnostic tools are leading to earlier and more accurate diagnoses, ensuring patients receive timely treatment, thereby boosting demand for therapeutics.
Improved Treatment Accessibility: Growing accessibility to advanced treatments, particularly in emerging markets, is also propelling market growth. Governments and healthcare providers are making strides to ensure affordable treatments for patients suffering from psoriasis.
Regional Analysis
North America: North America, particularly the U.S., holds the largest share of the global psoriasis therapeutics market. The region's robust healthcare infrastructure, high psoriasis incidence, and access to advanced biologic treatments drive its market dominance.
Europe: Europe is also a major market for psoriasis therapeutics, with countries like Germany, France, and the U.K. leading the region. Increased healthcare spending and a rising number of biologic approvals are key contributors to the market's growth.
Asia-Pacific: The Asia-Pacific region is expected to witness the fastest growth during the forecast period. Growing awareness, improved healthcare infrastructure, and increasing government initiatives to manage chronic diseases are fueling the demand for psoriasis therapies.
Latin America and Middle East & Africa: These regions are slowly adopting psoriasis treatments due to improving healthcare systems and increased awareness. However, the market here is still in the early stages of development.
Competitive Landscape
The psoriasis therapeutics market is highly competitive, with key players focusing on research and development to introduce more effective and targeted therapies:
AbbVie Inc.: Known for its blockbuster drug Humira, AbbVie is a major player in the psoriasis biologics segment. The company's strong R&D pipeline positions it for continued growth in this market.
Novartis AG: Novartis has made significant strides with its biologic Cosentyx, which has been highly effective in treating moderate to severe psoriasis cases.
Eli Lilly and Company: Eli Lilly's Taltz, another biologic therapy, is making waves in the market with its efficacy and fast-acting results for psoriasis patients.
Johnson & Johnson (Janssen): The company offers the biologic treatment Stelara, which has become a popular option for patients with moderate to severe psoriasis.
Amgen Inc.: Amgen's focus on biologics, particularly Enbrel, has made it a major contender in the psoriasis therapeutics market.
Report Overview : https://www.infiniumglobalresearch.com/reports/global-psoriasis-therapeutics-market
Challenges and Opportunities
While the market is experiencing significant growth, it also faces several challenges:
High Treatment Costs: Biologic therapies, while highly effective, are expensive, limiting access for patients in low- and middle-income regions. Developing cost-effective treatments will be key to expanding the market's reach.
Side Effects and Patient Adherence: Some psoriasis treatments come with side effects, leading to patient adherence issues. Improving the safety profile of these therapies is essential for long-term growth.
Regulatory Approvals: Gaining regulatory approval for new treatments, particularly in regions with strict regulations, can be a hurdle for companies looking to launch innovative therapies.
Despite these challenges, the market presents significant opportunities. The growing trend toward personalized medicine, which tailors treatments to individual patient profiles, is expected to create new avenues for growth. Additionally, ongoing research into novel biologics and small molecules promises to further enhance treatment options for psoriasis patients.
Conclusion
The global psoriasis therapeutics market is poised for robust growth, with projected revenue of nearly USD 44 billion by 2028. With rising disease prevalence, advancements in biologic therapies, and expanding treatment access, the market presents vast opportunities for innovation and investment. As the focus on improving patient outcomes and developing cost-effective solutions increases, the psoriasis therapeutics market is set to witness significant advancements over the forecast period.
0 notes
Text
Hidradenitis Suppurativa Market Future Scope: Growth, Share, Value, Size, and Analysis
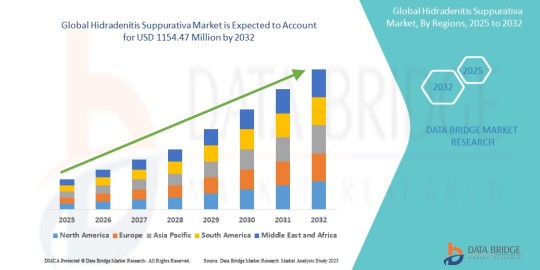
"Hidradenitis Suppurativa Market Size And Forecast by 2032
According to Data Bridge Market Research Global hidradenitis suppurativa market size was valued at USD 799.49 million in 2024 and is projected to reach USD 1154.47 million by 2032, with a CAGR of 4.70% during the forecast period of 2025 to 2032.
Hidradenitis Suppurativa Market is making significant strides in the industry, redefining standards with cutting-edge solutions and strategic growth initiatives. As a leader in the sector, Chronic Skin Inflammation Market is committed to providing high-quality services that cater to evolving consumer needs. With a strong focus on innovation, Severe Acne Inversa Market has introduced new technologies that enhance efficiency and streamline operations. The company’s expansion into new regions has solidified Hidradenitis Suppurativa Market as a key player in the global landscape. By continuously adapting to market trends, Autoimmune Skin Disease Market ensures sustainable growth and long-term success.
Hidradenitis Suppurativa Market remains dedicated to delivering exceptional value to its customers while strengthening its position in the industry. Through ongoing research and development, Dermatological Abscess Management Market continues to push the boundaries of excellence. The company's commitment to quality and customer satisfaction has made Recurrent Boil Disease Market a trusted name worldwide. With a strong emphasis on sustainability, Hidradenitis Suppurativa Market is actively contributing to a greener future. As demand for advanced solutions grows, Hidradenitis Suppurativa Market is poised for further expansion and success.
Our comprehensive Hidradenitis Suppurativa Market report is ready with the latest trends, growth opportunities, and strategic analysis. https://www.databridgemarketresearch.com/reports/global-hidradenitis-suppurativa-market
**Segments**
- Treatment Type: The market is segmented based on treatment type into medication, surgery, and others. Medication includes antibiotics, retinoids, hormonal therapy, and immunosuppressants. Surgery options comprise incision and drainage, excision, laser, and others. The increasing focus on developing effective medications and advanced surgical techniques is driving growth in this segment.
- Disease Type: Hidradenitis suppurativa can be categorized into mild, moderate, and severe based on the disease severity. The treatment approach varies for each category, with severe cases often requiring a combination of medications and surgical interventions. The rising prevalence of severe cases is boosting the demand for advanced treatment options.
- Distribution Channel: The market is segmented by distribution channel into hospitals pharmacies, retail pharmacies, and online pharmacies. Hospital pharmacies hold a significant market share due to the availability of a wide range of treatment options and specialized healthcare professionals. The increasing preference for online pharmacies is expected to drive growth in this segment.
- Geography: Geographically, the market is divided into North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa. North America holds a prominent position in the global market due to the high prevalence of hidradenitis suppurativa and the presence of key market players. However, Asia-Pacific is projected to witness substantial growth owing to improving healthcare infrastructure and rising awareness about the condition.
**Market Players**
- AbbVie Inc. - Johnson & Johnson Services, Inc. - GlaxoSmithKline plc - AstraZeneca - Pfizer Inc. - Merck & Co., Inc. - Humira - AbbVie - Allergan - Bausch Health
The global hidradenitis suppurativa market is experiencing significant growth, driven by factors such as increasing prevalence of the condition, rising awareness among healthcare professionals and patients, and advancements in treatment options. Key market players are focusing on strategic initiatives such as product launches, collaborations, and acquisitions to strengthen their market position. The market is characterized by intense competition, prompting companies to invest in research and development activities to introduce innovative therapies. Overall, the market shows promising growth prospects as the demand for effective treatment solutions continues to rise.
https://www.databridgemarketresearch.com/reports/global-hidradenitis-suppurativa-market The global hidradenitis suppurativa market is poised for significant growth as the prevalence of the condition continues to rise, driving an increased demand for effective treatment options. One of the emerging trends within the market is the focus on personalized medicine, where treatments are tailored to individual patients based on factors such as disease severity and response to previous therapies. This approach is expected to lead to improved patient outcomes and overall treatment efficacy. Additionally, with the advancements in medical technology, there is a growing emphasis on the development of innovative surgical techniques and minimally invasive procedures to address the complex nature of hidradenitis suppurativa cases.
Market players in the industry are actively engaging in strategic collaborations and partnerships to enhance their product portfolios and expand their geographic presence. These collaborations not only allow for the exchange of expertise and resources but also facilitate access to new markets and patient populations. Furthermore, companies are investing heavily in research and development activities to introduce novel therapeutic solutions that offer better efficacy and fewer side effects compared to traditional treatments. This focus on innovation is crucial in a highly competitive market landscape where differentiation and value proposition play a key role in determining market success.
Another notable aspect shaping the hidradenitis suppurativa market is the increasing role of patient advocacy groups and initiatives aimed at raising awareness about the condition. These organizations play a vital role in educating both patients and healthcare professionals about hidradenitis suppurativa, promoting early diagnosis, and advocating for improved access to quality care. By fostering a supportive ecosystem, patient advocacy groups contribute to reducing the stigma associated with the disease and help in increasing the overall quality of life for individuals living with hidradenitis suppurativa.
Geographically, while North America currently holds a significant market share in the global hidradenitis suppurativa market, the Asia-Pacific region presents substantial growth opportunities. Factors such as the rapidly expanding healthcare infrastructure, increasing disposable income, and growing awareness about chronic skin conditions are expected to drive market growth in Asia-Pacific. As the healthcare landscape evolves in these regions, there is a growing emphasis on providing access to advanced treatment options for patients with hidradenitis suppurativa, which is likely to fuel market expansion in the coming years.
In conclusion, the global hidradenitis suppurativa market is witnessing dynamic changes driven by technological advancements, strategic initiatives by market players, increasing awareness, and evolving treatment paradigms. With a focus on personalized medicine, innovation, and patient-centric care, the market is poised for continued growth and development in the foreseeable future.The global hidradenitis suppurativa market is experiencing a paradigm shift driven by a combination of factors that are reshaping the industry landscape. One of the significant trends observed is the increasing focus on personalized medicine within the market. This approach tailors treatment plans based on individual patient characteristics such as disease severity, response to previous therapies, and genetic predisposition. By offering customized treatment options, personalized medicine has the potential to enhance patient outcomes and improve overall treatment efficacy. This trend is in line with broader developments in healthcare towards precision medicine, where treatments are increasingly tailored to the specific needs of patients.
Moreover, there is a noticeable emphasis on innovation and the development of advanced surgical techniques and minimally invasive procedures within the hidradenitis suppurativa market. Given the complexity of the condition and its impact on patients' quality of life, the advent of innovative surgical approaches aims to provide more effective and less invasive treatment options. These advancements not only address the medical needs of patients but also contribute to reducing recovery times and improving long-term outcomes. The focus on innovation underscores the commitment of market players to advancing the standard of care for individuals living with hidradenitis suppurativa.
Strategic collaborations and partnerships between market players are playing a pivotal role in driving growth and expanding market reach within the hidradenitis suppurativa market. By pooling resources, expertise, and capabilities, companies can strengthen their product portfolios, access new markets, and leverage synergies to drive innovation. Collaborations also enable companies to capitalize on each other's strengths and navigate the competitive landscape more effectively. As the market continues to evolve, strategic partnerships will remain a key strategy for enhancing market competitiveness and fostering sustainable growth in the long term.
The increasing role of patient advocacy groups in raising awareness about hidradenitis suppurativa is another noteworthy aspect shaping the market dynamics. These organizations play a crucial role in educating patients, caregivers, and healthcare professionals about the condition, advocating for improved access to quality care, and promoting early diagnosis and intervention. By amplifying the voice of patients and fostering a supportive ecosystem, patient advocacy groups contribute to reducing the stigma associated with hidradenitis suppurativa and improving the overall quality of life for individuals affected by the condition. The collaborative efforts between patient advocacy groups and industry stakeholders have the potential to drive positive change and drive better outcomes for patients with hidradenitis suppurativa.
In conclusion, the global hidradenitis suppurativa market is witnessing a transformative phase characterized by advancements in personalized medicine, innovation in surgical techniques, strategic collaborations, and the growing influence of patient advocacy groups. These trends are reshaping the treatment landscape for hidradenitis suppurativa and are expected to drive continued growth and development in the market. As market players continue to focus on enhancing treatment options, improving patient outcomes, and expanding market presence, the outlook for the hidradenitis suppurativa market remains promising, with opportunities for further innovation and progress in the years ahead.
The market is highly fragmented, with a mix of global and regional players competing for market share. To Learn More About the Global Trends Impacting the Future of Top 10 Companies in Hidradenitis Suppurativa Market : https://www.databridgemarketresearch.com/reports/global-hidradenitis-suppurativa-market/companies
Key Questions Answered by the Global Hidradenitis Suppurativa Market Report:
What is the current state of the Hidradenitis Suppurativa Market, and how has it evolved?
What are the key drivers behind the growth of the Hidradenitis Suppurativa Market?
What challenges and barriers do businesses in the Hidradenitis Suppurativa Market face?
How are technological innovations impacting the Hidradenitis Suppurativa Market?
What emerging trends and opportunities should businesses be aware of in the Hidradenitis Suppurativa Market?
Browse More Reports:
https://www.databridgemarketresearch.com/reports/global-inertial-sensor-mems-markethttps://www.databridgemarketresearch.com/reports/global-flexible-heater-markethttps://www.databridgemarketresearch.com/reports/global-transistors-quantum-dots-markethttps://www.databridgemarketresearch.com/reports/global-film-adhesives-markethttps://www.databridgemarketresearch.com/reports/global-polyaspartic-coatings-market
Data Bridge Market Research:
☎ Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC: +653 1251 1007
✉ Email: [email protected]"
#Chronic Skin Inflammation Market#Severe Acne Inversa Market#Autoimmune Skin Disease Market#Dermatological Abscess Management Market#Recurrent Boil Disease Market#Suppurative Skin Disorder Market#Nodular Skin Disease Market#Painful Skin Lesions Market#Dermatology Chronic Inflammation Market#Skin Abscess Management Market
0 notes
Text
Pharmaceutical industry forecasts to 2026

One of the major issues currently plaguing the pharmaceutical sector relates to clinical trials. Since the spread of the coronavirus, hundreds of trials have been suspended and the issuing of opinions on those that have been conducted is delayed. This problem means that the longer this situation continues, the more likely it is that regulatory decision-making will be slowed down. This, of course, could affect patient access to new medical solutions. And from a macroeconomic perspective, the loss of jobs and productivity indicates that a global economic downturn is coming. If this proves true, governments and patients will have less and less money to spend on healthcare. In this context, investors seem to have sidelined the economic risks of a pandemic, hoping they will be offset by the potential of new treatments for the disease.
Market share growth
The only companies that have reported projected market share gains by 2026 are AstraZeneca, BMS and AbbVie. In the case of BMS, the deal that has contributed most to this growth is the acquisition of Celgene. For AbbVie, the acquisition of Allergan has had a very positive impact, being a strategically favourable move to diversify its drug portfolio, also in view of the impending loss of exclusivity for Humira in 2023.
AstraZeneca's market share is expected to grow steadily; mainly driven by sales of drugs such as Tagrisso, Lynparza and Imfinzi, which continue to be the UK company's growth drivers. Takeda will remain on the sidelines in the ranking of the top ten best-selling prescription drugs for 2026. Despite the acquisition of Shire in 2019 and its contribution to the company's revenue growth, there are downsides. One of the most notable is the patent expiration of the Advate drug in 2019, resulting in a drop in sales of about a billion dollars. Overall, the top ten pharmaceutical companies will lose 6.2% of total market share. And the main impact of this phenomenon will be Pfizer.
The rise of biotechnology
It is worth noting that biotechnology is set to change direction in the coming years. By 2026, biotech drugs are expected to occupy a major share of the top 100 drugs, accounting for 55% of the total, up 16% from 2012. In this context, Roche will remain the largest company in biologic drugs. A detailed analysis of Roche's data shows that despite losing 5.8% of market share due to the loss of biologics patents, the company is still the largest biologics manufacturer in the world. These include Avastin, Herceptin and Rituxan, which account for the company's largest sales. According to another forecast in the report, Amgen will drop two positions due to the loss of market share of its Enbrel (autoimmune diseases) drug compared to similar drugs from competitors.
Following it, Novo Nordisk will move to the third position, boosted by strong sales growth of Ozempic and Ribelsus (diabetes drugs), which will have sales of about $15 billion by 2026. The paper also predicts that AbbVie will drop out of the top ten leaders in this field. This will occur as a result of the loss of the Humira patent in the US by 2023. Novartis will join the top ten in this segment due to sales growth of Cosentyx and Entresto (psoriasis and heart failure drugs respectively); also among the drugs driving the company's growth will be the launches of Arzerra (chronic lymphocytic leukaemia) and Inclisiran (an experimental molecule being studied for the treatment of atherosclerotic cardiovascular disease).

Diversified research
The EvaluatePharma report also looks at the most promising R&D projects up to 2026. The list of initiatives is diversified across several therapeutic areas, with large corporations actively participating, but there is also room for mid-cap companies to sneak into the ranking. In first place is tirzepatide, Eli Lilly's diabetes and obesity drug. It moved up in the rankings thanks to Eli Lilly's focus on it, comparing it in trials to its drug Trulicity, which brings the company big sales. Novartis is in second place thanks to Inclisiran, a molecule being investigated for the treatment of atherosclerotic cardiovascular disease. Inclisiran became part of Novartis Group's armoury through its acquisition of The Medicines Company earlier this year. Approval of the therapy is expected in the second half of this year, and the drug is expected to compete directly with Repatha (Amgen) in the atherosclerotic disease drug line-up.
There are also high hopes for Biogen's Alzheimer's disease drug aducanumab. The drug, which targets the beta-amyloid protein, was due to be submitted to the FDA in early 2020; delays caused by the current situation could make investors nervous, given that it could be the first new drug to treat this type of dementia in 15 years. In the area of autoimmune diseases, Bristol-Myers Squibb has BMS-986165, an investigational tyrosine kinase inhibitor for the treatment of psoriasis. The drug is expected to show good results and help BMS recover sales in psoriasis following the sale of its Otezla drug following its merger with Celgene.
Diverse specialities
GlaxoSmithKline's multiple myeloma drug belantamab mafodotin made the ranking. Roche also made the top 10 due to promising research projects with its drug for the treatment of spinal muscular atrophy, rizdiplam. The therapy has received a priority review from the FDA, but the Swiss company is awaiting a final decision due by the end of August.
Among the smaller companies' projects, four made it into the top 10. In first place is argenx with its immunosuppressant efgartimod, which has had good results in trials on patients with generalised myasthenia gravis. Then there is the collaboration between Vir Biotechnology and Alnylam to develop ALN-HBV02, an RNAi therapy for the treatment of chronic hepatitis B infections. Also in this group of mid-cap companies is Iovance Biotherapeutics with its cell therapy that has received FDA fast track status for the treatment of advanced melanoma. Rounding out this group is Allakos with its esophagitis and gastritis drug currently in Phase 3 clinical trials.

Product ranking
While not grouping drugs by speciality, the consultancy also ranked the ten drugs expected to be the top-selling drugs by 2026. As mentioned above, the list will be topped by Keytruda, an anti-PD-1 immunotherapy drug. Second place will also go to immunotherapy drugs, in this case Opdivo, whose mechanism of action is similar to Keytruda. In third place is Eliquis (apixaban), an anticoagulant used to prevent venous thromboembolism and stroke in patients suffering from atrial fibrillation. Bictarvi (bictegravir sodium; emtricitabine; tenofovir alafenamide fumarate), an antiviral drug from Gilead Sciences used in patients with HIV, ranked fourth.
In the middle of the table, in fifth place, would be Imbruvica (ibrutinib), a drug developed by AbbVie and Janssen, a Bruton's tyrosine kinase inhibitor used to treat patients with mantle cell lymphoma. Oncology is also in sixth and seventh places with Pfizer's Ibrance (palbociclib) for the treatment of certain breast cancers and Tagrisso (osimertinib mesylate), an EGFR inhibitor for the treatment of non-small cell lung cancer.
The last three positions in the table are occupied by drugs from different fields. In eighth place is Sanofi's Dupixent (dupilumab), an interleukin 4 and 13 inhibitor for patients with atopic dermatitis, asthma and chronic rhinosinusitis with nasal polyposis. Ninth place went to Vertex Pharmaceuticals' Trikafta (elexacaftor, ivacaftor, tezacaftor), an advanced therapy for the treatment of cystic fibrosis. Finally, closing the table in tenth place is Ozempic (semaglutide) from NovoNordisk Pharmaceuticals, used as a diabetic drug - which can be combined with other therapies - to prevent the development of cardiovascular disease in these patients.
0 notes
Text
Biologics and Biosimilars Market Consumer Behavior and Industry Shifts to 2033
Introduction
The biologics and biosimilars market is witnessing significant growth driven by advancements in biotechnology, increasing prevalence of chronic diseases, and rising demand for cost-effective biologic therapies. Biologics, which include complex molecules such as monoclonal antibodies and recombinant proteins, have revolutionized the treatment of various diseases. However, their high costs have paved the way for biosimilars—biological products that are highly similar to approved biologics—with comparable efficacy and safety at reduced prices.
As we look forward to 2032, the market dynamics are expected to shift further with policy reforms, technological innovations, and increasing competition shaping the future landscape.
Market Overview
In 2024, the global biologics and biosimilars market was valued at USD 400+ billion, and it is projected to surpass USD 750 billion by 2032, growing at a CAGR of over 8% during the forecast period. The market is segmented by product type, therapeutic area, manufacturing type, and region.
Download a Free Sample Report:-https://tinyurl.com/2k678792
Key Definitions:
Biologics: Medicinal products derived from living organisms, used to treat diseases such as cancer, autoimmune disorders, and infectious diseases.
Biosimilars: Biologic medical products that are almost identical copies of an original product but manufactured by a different company after the patent expiry.
Market Drivers
1. Patent Expirations of Biologic Blockbusters
The expiration of patents for blockbuster biologics such as Humira (adalimumab), Remicade (infliximab), and Herceptin (trastuzumab) has opened opportunities for biosimilars. These patent cliffs allow biosimilar manufacturers to enter the market with cost-effective alternatives, increasing competition and market penetration.
2. Rising Prevalence of Chronic Diseases
The growing global burden of chronic diseases like cancer, rheumatoid arthritis, diabetes, and cardiovascular disorders is fueling the demand for biologic therapies. Biologics offer high specificity and efficacy, making them the preferred choice for targeted treatment.
3. Cost-effectiveness of Biosimilars
Healthcare systems worldwide are increasingly adopting biosimilars to reduce the overall cost of treatment. Biosimilars typically cost 15-30% less than their reference biologics, which helps improve access, especially in emerging markets.
4. Regulatory Support and Guidelines
Regulatory bodies like the U.S. FDA, European Medicines Agency (EMA), and World Health Organization (WHO) have established stringent yet supportive pathways for biosimilar approval, ensuring their safety, efficacy, and quality.
Market Restraints
High Manufacturing Complexity: Biologics and biosimilars require sophisticated manufacturing processes, involving living cells and stringent quality controls.
Limited Patient and Physician Awareness: In certain regions, the lack of awareness and trust in biosimilars hinders adoption.
Patent Litigation and Exclusivity: Legal battles over patents and exclusivity rights can delay biosimilar entry and increase development costs.
Market Segmentation
By Product Type
Monoclonal Antibodies
Vaccines
Recombinant Hormones
Interferons
Insulin
Fusion Proteins
Monoclonal antibodies lead the biologics segment due to their application in cancer and autoimmune diseases.
By Therapeutic Application
Oncology
Autoimmune Diseases
Diabetes
Hematology
Infectious Diseases
Ophthalmology
The oncology segment is dominant, driven by the growing prevalence of cancer and the high efficacy of biologics and biosimilars in oncology treatment.
By Manufacturing Type
In-house Manufacturing
Contract Manufacturing Organizations (CMOs)
While large pharmaceutical firms often use in-house capabilities, small biotech firms increasingly rely on CMOs to reduce operational costs and enhance scalability.
Regional Insights
North America
North America dominates the market due to robust healthcare infrastructure, high R&D investment, and early adoption of biosimilars. The U.S. FDA’s Biosimilar Action Plan further encourages biosimilar development and competition.
Europe
Europe is a pioneer in biosimilar approvals and adoption. Countries like Germany, the UK, and France have implemented favorable reimbursement policies and strong physician awareness.
Asia Pacific
The Asia Pacific region is experiencing the fastest growth, driven by increasing healthcare spending, large patient populations, and growing investments by regional players in biosimilar development (e.g., India, South Korea, and China).
Competitive Landscape
The market is highly competitive, with both large pharmaceutical companies and specialized biotechnology firms operating globally.
Major Players:
Amgen Inc.
Roche Holding AG
Pfizer Inc.
Samsung Bioepis
Biocon Ltd.
Sandoz (a Novartis division)
Celltrion Healthcare
These companies focus on strategic alliances, product launches, biosimilar approvals, and manufacturing scale-up to gain competitive advantages.
Emerging Trends
1. Integration of AI and Digital Technologies
Artificial intelligence is being utilized for biologics discovery, molecular simulation, and process optimization. This enhances speed-to-market and reduces R&D costs.
2. Personalized Biologics
With advances in genomics, biologics are increasingly being tailored to individual patient profiles, leading to improved outcomes and reduced side effects.
3. Subcutaneous and Oral Biologics
There is a rising trend toward subcutaneous and oral biologics, offering patient convenience over traditional intravenous delivery methods.
4. Government Incentives and Reimbursement Policies
Governments in emerging economies are offering incentives and subsidies to promote biosimilar manufacturing and adoption, improving market access and affordability.
Future Outlook (2025–2032)
The biologics and biosimilars market is expected to evolve rapidly over the next decade. With major patents expiring and healthcare systems seeking cost-effective solutions, biosimilars will gain more ground. Meanwhile, continued innovation in biologics will lead to novel therapies for rare and complex diseases.
Key projections:
Biologics will continue to dominate in revenue, but biosimilars will grow at a faster rate due to increasing accessibility.
Asia Pacific will emerge as a biosimilar manufacturing hub due to lower production costs and favorable policies.
Strategic partnerships between pharma companies and CMOs or biotech startups will increase to meet global demand efficiently.
Biobetters—enhanced versions of original biologics—will gain popularity for offering improved efficacy and reduced dosing frequency.
Conclusion
The biologics and biosimilars market stands at a pivotal point. While biologics continue to drive innovation in treating life-threatening diseases, biosimilars are ensuring that these treatments become accessible and affordable for a broader population. As regulatory landscapes mature, technologies advance, and global healthcare priorities evolve, the market is poised for robust and inclusive growth through 2032.
Companies that invest in quality manufacturing, innovation, and regulatory navigation will be well-positioned to capture significant market share in this dynamic and transformative healthcare segment.
Read Full Report:-https://www.uniprismmarketresearch.com/verticals/healthcare/biologics-and-biosimilars.html
0 notes