#High performance liquid chromatography
Explore tagged Tumblr posts
Text
What is High Performance Liquid Chromatography (HPLC)
High Performance Liquid Chromatography (HPLC) is a sophisticated analytical method used to separate, identify, and quantify components within a mixture. This technique is pivotal in fields such as pharmaceuticals, environmental testing, and food safety. Introduction to HPLC HPLC is a core technique in analytical chemistry, often utilized for quality control (QC) and quality assurance (QA) in…

View On WordPress
1 note
·
View note
Text
Type of buffer in Hplc
Phosphate buffer Citrate buffer Formate buffer Acetate buffer Tris(hydrixymethyl) -aminomethane Phosphate buffer Pka 2.1 Ph range 1.1 tob3. 1 Pka 7.2 Ph range 6.2 to 8.3 Pk 12.3 Ph range 11.3 to 13.3 Citrate Pka 3.1 Ph range 2.1 to 4.1 Pka 4.7 Ph 3.7 to 5.7 Pka 5.4 Ph range 4.4 to 6.4 Format Pka 3.8 Ph range 2.8 to 4.8 Acetate Pka 4.8 Ph range 3.8 to 5.8
View On WordPress
#Analytical chemistry#CAPA#Chromatography#Gas chromatography (gc)#glp#GMP Updates#High performance liquid chromatography#Interview preparation#Method validation#OOS#optical rotation#Out of specification#QMS#self discovery#type of stability#uv
0 notes
Text
High-Performance Liquid Chromatography Market Growth Statistics and Key Players Insights (2024-2032)
High-Performance Liquid Chromatography (HPLC) is a powerful analytical technique used to separate, identify, and quantify components in complex mixtures. Widely employed in pharmaceutical, environmental, food, and chemical industries, HPLC has become a cornerstone of analytical chemistry due to its precision, accuracy, and versatility. This technique works by passing a liquid sample through a column filled with a solid adsorbent material, allowing different components to interact with the stationary phase and separate based on their unique properties. HPLC is essential for ensuring product quality, safety, and efficacy across multiple industries.
The High-Performance Liquid Chromatography Market size was valued at USD 4.8 billion in 2023 and is expected to grow to USD 7.83 billion by 2031 and grow at a CAGR of 6.30% over the forecast period of 2024-2031.
Future Scope
The future of HPLC is set to evolve with ongoing advancements in instrument technology and data analysis software. Miniaturization of HPLC systems, allowing for more portable and user-friendly devices, is expected to broaden the scope of applications. Additionally, the integration of HPLC with mass spectrometry (LC-MS) will continue to enhance the sensitivity and resolution of analytical results. Research is also focusing on the development of eco-friendly HPLC techniques, such as using green solvents and reducing waste generation. As industries demand faster, more accurate analyses, HPLC technology will play an increasingly vital role in research and quality control.
Trends
Several trends are shaping the HPLC market. One key trend is the increasing adoption of ultra-high-performance liquid chromatography (UHPLC), which offers faster separation times and higher resolution compared to traditional HPLC. Another trend is the rising demand for bioanalytical applications, particularly in drug discovery and clinical research, where HPLC is used to analyze biological samples and assess pharmacokinetics. Additionally, the development of automation and robotics in HPLC systems is driving efficiency and throughput in laboratory settings. Green HPLC, with a focus on reducing environmental impact, is also gaining traction as sustainability becomes a priority in scientific research.
Applications
HPLC is widely used in the pharmaceutical industry for drug development, quality control, and purity testing. In the food and beverage industry, it is applied to detect contaminants, verify ingredient authenticity, and monitor product quality. HPLC is also crucial in environmental analysis for detecting pollutants in water, air, and soil. Additionally, it plays a vital role in clinical laboratories, where it is used for diagnosing diseases, monitoring therapeutic drug levels, and performing forensic analyses.
Get Sample Copy of the Report: https://www.snsinsider.com/sample-request/3083
Key Points
High-Performance Liquid Chromatography (HPLC) is a key analytical tool for separating and identifying components in mixtures.
It is widely used in pharmaceuticals, food safety, environmental testing, and clinical diagnostics.
Trends include the rise of UHPLC, bioanalytical applications, automation, and green HPLC techniques.
HPLC is essential for ensuring product quality, safety, and environmental compliance.
Future developments focus on miniaturization, eco-friendly techniques, and integrated systems like LC-MS.
Conclusion
High-Performance Liquid Chromatography continues to be an indispensable tool in scientific research and industry. With ongoing technological advancements and the integration of automation, HPLC will further enhance the precision, speed, and reliability of analytical processes. As sustainability becomes increasingly important, the development of green HPLC techniques will ensure that this technology remains at the forefront of innovation in analytical chemistry. Through continuous improvements, HPLC is set to play a crucial role in driving progress across multiple fields, from pharmaceuticals to environmental science.
0 notes
Text
#High-Performance Liquid Chromatography Market: Leading Players Developments#Innovations#and Advanced Technologies#Forecast 2032
0 notes
Text
The Business Research Company offers high performance liquid chromatography market research report 2023 with industry size, share, segments and market growth
#high performance liquid chromatography market#high performance liquid chromatography market size#high performance liquid chromatography market overview#high performance liquid chromatography market research#high performance liquid chromatography market data#high performance liquid chromatography market growth#high performance liquid chromatography market analysis#high performance liquid chromatography market segments#high performance liquid chromatography market forecast
0 notes
Text
0 notes
Text
High-performance Liquid chromatography
High-performance Liquid chromatography NHLC-100 is a gradient pump liquid chromatography system. It involves a superior high-pressure pump that aids in the accurate solvent delivery with high-precision and low-fluctuation. The UV detector assembly enhances stronger detection capabilities of the system. This leads to efficient separation of the desired pharmaceutical compound and obtains high operation reliability for extended time intervals.

0 notes
Text
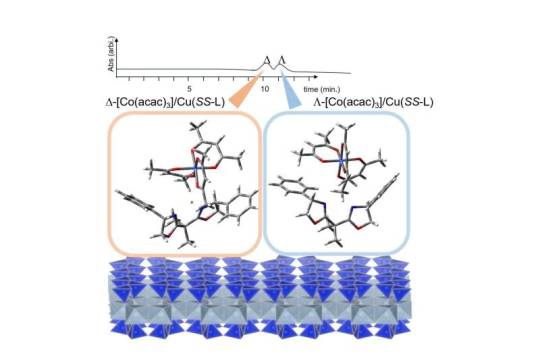
Aiming at the industrial use of clay column chromatography for optical resolution
A recent study, published in Applied Clay Science, could have applications in clay column chromatography for obtaining enantiomeric compounds in industries. A spherically-shaped particle of synthetic hectorite (denoted as Na-HEC) was ion-exchanged with a divalent Cu(II) complex, [Cu(SS-oxa)]2+ (SS-oxa = SS-2,2′-isopropylidene-bis(4-phenyl-2-oxazoline)). The material is denoted as [Cu(SS-oxa)]2+/HEC. A column for high performance liquid chromatography (HPLC) was prepared by packing 4.0 g of [Cu(SS-oxa)]2+/HEC into a stainless tube (25 cm x 0.4 cm (i.d.)). When tris(acetylacetonato)cobalt(III) (denoted as [Co(acac)3]) was eluted by methanol at the flow rate of 0.2 mLmin-1at 4°C, the compound was separated to D- and L-enantiomers nearly to the baseline.
Read more.
10 notes
·
View notes
Text
Crafting Clear Skin: The Precision of Salicylic Acid Manufacturing
Salicylic acid has long been a cornerstone in skincare and pharmaceutical formulations, celebrated for its remarkable efficacy in treating acne, exfoliating the skin, and managing various dermatological conditions. As a leading ingredient in numerous products, the demand for high-quality salicylic acid is unwavering. Salicylic acid manufacturers play a crucial role in meeting this demand, employing advanced technologies, stringent quality control measures, and innovative processes to produce this essential compound. In this blog, we explore the world of salicylic acid manufacturing, highlighting its significance, processes, benefits, and why it’s a cornerstone of modern skincare and pharmaceutical solutions.

The Importance of Salicylic Acid
Salicylic acid is a beta-hydroxy acid (BHA) derived from natural sources like willow bark and wintergreen leaves or synthesized in laboratories. It is renowned for its ability to penetrate pores, exfoliate dead skin cells, and reduce inflammation, making it a powerful ingredient in acne treatments, chemical peels, and dandruff shampoos. Its keratolytic properties help to soften and shed the outer layer of skin, promoting cell turnover and revealing a smoother, clearer complexion.
For more information salicylic acid manufacturer
Advanced Manufacturing Processes
Manufacturing salicylic acid involves sophisticated chemical processes to ensure purity, potency, and safety. The most common method is the Kolbe-Schmitt reaction, which synthesizes salicylic acid from sodium phenoxide and carbon dioxide under high pressure and temperature. This method yields high-purity salicylic acid, suitable for both pharmaceutical and cosmetic applications. Manufacturers utilize advanced equipment and precise control systems to maintain optimal reaction conditions, ensuring consistent quality and yield.
Quality Control and Assurance
Quality control is paramount in salicylic acid manufacturing. Rigorous testing protocols are implemented at every stage of production, from raw material selection to final product packaging. Analytical techniques such as high-performance liquid chromatography (HPLC), gas chromatography (GC), and mass spectrometry (MS) are used to verify the purity, potency, and stability of salicylic acid. These tests ensure that the final product meets stringent industry standards and regulatory requirements, guaranteeing safety and efficacy for consumers.
Customization and Innovation
Salicylic acid manufacturers often work closely with cosmetic and pharmaceutical companies to develop customized formulations tailored to specific product needs. Whether creating a potent acne treatment, a gentle exfoliating cleanser, or an effective dandruff shampoo, manufacturers provide expertise in optimizing salicylic acid concentrations and formulations for maximum benefit. This collaborative approach fosters innovation, resulting in new and improved products that address evolving consumer demands and dermatological advancements.
Sustainability and Ethical Practices
In response to growing environmental concerns, many salicylic acid manufacturers are adopting sustainable and ethical practices. This includes sourcing raw materials from renewable resources, minimizing waste and emissions, and implementing energy-efficient technologies. Some manufacturers are also exploring greener synthesis methods that reduce environmental impact while maintaining high-quality production standards. These efforts align with the broader industry trend toward sustainability and responsible manufacturing.
Meeting Regulatory Standards
Compliance with regulatory standards is a critical aspect of salicylic acid manufacturing. Regulatory bodies such as the FDA (Food and Drug Administration) and EMA (European Medicines Agency) set stringent guidelines for the production and use of salicylic acid in cosmetic and pharmaceutical products. Manufacturers must adhere to Good Manufacturing Practices (GMP) and ensure their products are free from contaminants, properly labeled, and safe for consumer use. Regular audits and inspections by regulatory authorities help maintain compliance and uphold product integrity.
Future Trends and Innovations
The future of salicylic acid manufacturing is marked by continuous innovation and adaptation to emerging trends. Advances in green chemistry, biotechnology, and nanotechnology are poised to revolutionize production methods, enhancing efficiency and sustainability. Additionally, research into new applications and formulations of salicylic acid promises to expand its role in skincare and healthcare, offering consumers even more effective and versatile solutions.

Conclusion
Salicylic acid manufacturers are at the forefront of producing one of the most versatile and effective ingredients in skincare and pharmaceuticals. Through advanced manufacturing processes, stringent quality control, and a commitment to innovation and sustainability, these manufacturers ensure the consistent supply of high-quality salicylic acid. As consumer demand for effective skincare solutions continues to grow, the role of salicylic acid manufacturers remains vital, driving the development of products that promote healthier, clearer skin and improved well-being. Embrace the power of precision and discover the transformative benefits of expertly crafted salicylic acid.
2 notes
·
View notes
Text
Understanding the Deadly Toxin Found in Certain Mushroom Species
Introduction
Mushrooms are a diverse group of organisms, and while many are safe and edible, some species contain poisonous compounds. β-Amanitin is one such toxin found in certain mushroom species belonging to the genus Amanita. This article aims to explore factual evidence regarding the properties, effects, and potential dangers associated with β-Amanitin.
Understanding β-Amanitin
β-Amanitin is a cyclic peptide toxin produced by various species of mushrooms, including Amanita phalloides (death cap) and Amanita virosa (destroying angel). It is highly stable and resistant to heat, making it a potent toxin even after cooking[¹^]. Once ingested, β-Amanitin targets specific cellular processes, leading to severe liver damage and potentially fatal consequences.
Mechanism of Action
Inhibition of RNA Polymerase II: β-Amanitin specifically inhibits RNA polymerase II, an essential enzyme responsible for transcribing messenger RNA (mRNA) in eukaryotic cells. By binding to RNA polymerase II, β-Amanitin prevents mRNA synthesis, disrupting important cellular processes and ultimately leading to cell death[²^].
Factual Evidence Regarding β-Amanitin
Toxicity and Poisoning: Ingestion of mushrooms containing β-Amanitin can cause acute liver failure, often with delayed symptoms. The initial phase may include gastrointestinal distress, followed by a symptom-free period lasting up to 24 hours. Subsequently, liver damage manifests, characterized by jaundice, hepatic encephalopathy, and potentially progressing to multi-organ failure[³^].
Treatment Challenges: β-Amanitin poisoning is considered a medical emergency, and prompt recognition and appropriate treatment are crucial. Unfortunately, there is no specific antidote for β-Amanitin poisoning. Current management involves supportive care, liver protection measures, and potentially liver transplantation in severe cases[⁴^].
Forensic Toxicology: Due to the potent effects of β-Amanitin and its presence in lethal mushroom species, its detection plays a significant role in forensic toxicology. Analytical techniques such as high-performance liquid chromatography (HPLC) and mass spectrometry (MS) are employed to identify and quantify β-Amanitin in biological samples[⁵^].
Prevention and Awareness
Mushroom Identification: The primary preventive measure is accurate mushroom identification. Proper training and knowledge are crucial for distinguishing edible mushrooms from poisonous species, especially those containing β-Amanitin.
Education and Public Awareness: Raising awareness about the dangers of consuming wild mushrooms without expert guidance is essential. Public education campaigns can help reduce the incidence of β-Amanitin poisoning by promoting safe mushroom foraging practices.
Conclusion
β-Amanitin, a toxic compound found in certain species of mushrooms, poses a significant threat to human health. Its inhibition of RNA polymerase II leads to severe liver damage and potential fatality. Timely recognition of symptoms, along with supportive care and appropriate medical intervention, is vital for managing β-Amanitin poisoning.
To prevent β-Amanitin poisoning, it is crucial to exercise caution when consuming wild mushrooms and rely on expert identification. Public awareness campaigns can play an important role in educating the general population about the risks associated with consuming unknown mushrooms. Please visit MedChemExpress
(Note: This article is for informational purposes only and should not replace professional medical advice. If there is a suspicion of mushroom poisoning, seek immediate medical attention or contact a poison control center.)
2 notes
·
View notes
Text
Logan: Crofters ensures that no herbicides or pesticides are used in their jams through the use of high performance liquid chromatography. Patton: Can you spell, like all of that?
-The Return of the Jam! | Thomas Sanders
#the fact that i had the video open beside this post with the subtitles on and still spelt two of the words wrong#thomas sanders#sanders sides#logan sanders#patton sanders
6 notes
·
View notes
Text
Understanding THC Testing Kits: A Key Tool for Ensuring Product Quality
In today's cannabis market, where consumers demand consistency and quality, THC testing kits have become an essential tool for both consumers and producers alike. Whether you're a medical marijuana user, a recreational enthusiast, or a cultivator, knowing the potency of your cannabis products is crucial. THC testing kits provide the reliability and accuracy needed to assess your products and ensure that they meet the desired standards.
What is a THC Testing Kit?
A THC testing kit is a tool designed to measure the concentration of tetrahydrocannabinol (THC) in cannabis products, from dried flower to concentrates and edibles. These kits come in various formats, including home testing kits, laboratory-grade kits, and devices that use sophisticated technology to deliver precise readings of THC content.
For home users, THC testing kits are incredibly easy to use and often come with simple instructions. They can be used to test the potency of cannabis products to ensure that users know what they are consuming, preventing overuse or underuse of THC. On the other hand, for cannabis producers and dispensaries, accurate THC testing is vital for quality control, helping businesses maintain consistent and compliant products for their customers.
How Do THC Testing Kits Work?
The process of using a THC testing kit depends on the type of kit you choose, but the basic principle involves a chemical reaction that can determine the concentration of THC in a sample.
Colorimetric Testing: One of the simplest and most affordable ways to test THC content at home is through a colorimetric testing kit. These kits work by adding a reagent to a cannabis sample. The reagent reacts with the THC in the sample, causing a color change. The intensity of the color is then compared to a reference chart that corresponds to THC levels.
Digital THC Testers: For more accuracy, digital THC testers use technology to provide a detailed analysis of a sample. These devices often rely on infrared spectroscopy or other advanced methods to measure the amount of THC and other cannabinoids present in a sample. Results from digital THC testers are more precise and provide detailed cannabinoid profiles that are useful for both personal use and commercial purposes.
Laboratory Kits: While not for home use, laboratory-grade THC testing kits use more advanced techniques such as High-Performance Liquid Chromatography (HPLC) to analyze cannabis. These kits are often used by cultivators, dispensaries, or laboratories to ensure compliance with legal standards and ensure the consistency and potency of their products.
Why Should You Use a THC Test Kit?
Quality Control: For growers and producers, testing cannabis products with THC testing kits ensures that the products meet legal potency standards and that consumers are getting what they paid for. Without accurate testing, there’s no way to verify THC content, leading to inconsistencies in the market.
Personal Consumption: For recreational users or medical cannabis patients, knowing the exact THC content is essential for responsible consumption. Some individuals may prefer a product with a lower THC concentration, while others may need higher doses for therapeutic reasons. THC testing kits provide accurate data to help users tailor their cannabis use to their needs.
Preventing Overuse or Underuse: Using a THC testing kit can help consumers avoid overconsumption. Knowing the THC potency of the product allows users to control their intake, preventing the unwanted effects of too much THC. Conversely, testing also ensures that products with too low THC are not consumed when stronger effects are required.
Contaminant Detection: In some cases, THC testing kits can also detect contaminants or other harmful substances that may have been introduced during cultivation or production. This feature is especially important for individuals who prioritize purity and safety in their cannabis products.
How to Choose the Right THC Testing Kit
Selecting the right THC testing kit can be overwhelming, given the variety of options available on the market. Here are a few factors to consider:
Purpose: Are you testing cannabis for personal use or for business purposes? If you're a consumer testing the potency of purchased products, a simple colorimetric kit or a digital tester might be sufficient. For commercial purposes, or if you need highly accurate and detailed results, you might consider more advanced laboratory-grade kits.
Ease of Use: Colorimetric kits are easy to use and provide quick results, making them ideal for home users who want fast and simple testing. Digital testers, on the other hand, require more investment but provide detailed reports and are a good choice for regular testers or businesses.
Budget: While home testing kits are relatively inexpensive, digital testers and laboratory kits can be quite costly. It's important to evaluate your needs and choose a kit that fits your budget while providing the level of accuracy required.
Accuracy: If you're looking for detailed and reliable data, investing in a more advanced THC testing kit may be necessary. Digital testers or lab-grade kits will deliver the most accurate readings.
The Importance of Regular THC Testing
Regular THC testing is crucial for anyone who regularly consumes cannabis, whether for recreational or medicinal purposes. For medical users, knowing the exact potency of a product ensures effective dosing for symptom management. Additionally, for consumers purchasing cannabis from dispensaries, it helps avoid products that are too potent or too weak.
For businesses in the cannabis industry, regular THC testing is a means of maintaining consistent quality. Dispensaries, for instance, rely on accurate THC measurements to meet regulatory requirements and ensure customers are receiving the right potency in their products. Regular testing also helps improve product formulations and quality control processes.
Conclusion
THC testing kits provide invaluable insight into the potency and quality of cannabis products. They help both consumers and businesses make informed decisions by ensuring accurate THC measurements and product consistency. Whether you're a recreational user, a medical patient, or a cannabis producer, having access to reliable THC testing tools is key to enhancing the cannabis experience and ensuring safety and satisfaction. Choose the right testing kit for your needs, and enjoy the peace of mind that comes with knowing exactly what's in your cannabis.
0 notes
Text
Low-Pressure Liquid Chromatography Market Growth Statistics and Key Players Insights (2024-2032)
Low-pressure liquid chromatography (LPLC) is an analytical technique widely employed for separating and purifying biomolecules, pharmaceuticals, and chemical compounds. It operates under lower pressure than high-performance liquid chromatography (HPLC), making it suitable for tasks where high-resolution separation is less critical and cost efficiency is prioritized. Due to its simpler operation and minimal requirement for sophisticated equipment, LPLC has found applications across research labs, pharmaceutical industries, and biochemical processing. LPLC systems typically leverage gravity flow or low-pressure pumps, providing a controlled environment for sample separation without compromising the integrity of delicate compounds, which is particularly valuable in protein purification and extraction processes.
The Low-Pressure Liquid Chromatography Market Size was valued at USD 7.25 billion in 2023 and is expected to reach USD 13.72 billion by 2032 and grow at a CAGR of 7.36% over the forecast period 2024-2032.
Future Scope
The future of low-pressure liquid chromatography appears promising as new advancements are emerging to enhance its efficiency, affordability, and application scope. With innovations in column design and stationary phases, LPLC is evolving to deliver higher separation precision and faster run times. As demand for cost-effective solutions rises, especially in developing regions, LPLC is positioned as a preferred choice for small-to-medium-scale purification tasks. Furthermore, the technique is likely to benefit from advancements in automation and digital monitoring, which could improve reproducibility and facilitate remote operation. These advancements are expected to increase its use in the pharmaceutical and academic research sectors, particularly for preparative purposes, thereby expanding LPLC’s utility in the global analytical instrumentation market.
Trends
Recent trends in low-pressure liquid chromatography highlight a move towards more specialized applications, such as purifying biopharmaceuticals and isolating natural products. Researchers are increasingly turning to eco-friendly solvent systems and biodegradable materials in chromatography columns to support green chemistry initiatives. Additionally, miniaturized and portable LPLC systems are gaining traction as they offer enhanced mobility and convenience, particularly for on-site testing in environmental and food safety applications. The integration of LPLC with other analytical techniques, such as mass spectrometry and spectrophotometry, has also become popular, offering enhanced analytical capabilities and comprehensive data collection. As a result, these trends are enabling more robust applications across a broader range of sectors.
Applications
Low-pressure liquid chromatography is a versatile tool used in numerous fields for various applications. In the biopharmaceutical industry, it aids in the purification of proteins, antibodies, and other biomolecules, ensuring the production of highly pure compounds needed for drug development. In academic and industrial research, LPLC is essential for fractionating complex mixtures, allowing researchers to isolate specific compounds of interest. The technique is also extensively used in environmental monitoring, where it helps in the analysis and separation of organic pollutants and toxins from water samples. Additionally, LPLC finds applications in the food and beverage industry for quality control and in natural product research to isolate plant-derived compounds. These diverse applications underscore the method's adaptability and effectiveness across multiple sectors.
Key Points
LPLC operates at lower pressures, making it ideal for cost-effective and preparative separation applications.
It is commonly used in protein and biomolecule purification, with significant applications in pharmaceuticals.
Growing adoption of green chemistry practices has influenced the development of eco-friendly solvents and materials in LPLC.
Miniaturized LPLC systems are emerging to meet demands for portable, on-site analysis in environmental and food safety testing.
Integration with other analytical methods, like mass spectrometry, enhances LPLC’s analytical power and data accuracy.
LPLC is instrumental in isolating and studying plant-based compounds, aiding research in natural products and pharmacognosy.
Conclusion
Low-pressure liquid chromatography is solidifying its role as an accessible, reliable, and adaptable tool across numerous industries. With advancements in automation and eco-friendly materials, as well as trends towards portable devices, LPLC is becoming more efficient and versatile. As the technique continues to evolve, its relevance in sectors like biopharmaceuticals, environmental testing, and natural product research is only expected to grow, making it an essential tool for modern laboratories. LPLC’s continued development and integration with other analytical techniques ensure it will remain an invaluable asset for separation and purification tasks, supporting critical research and development efforts in the years ahead.
#Low-Pressure Liquid Chromatography Market#Low-Pressure Liquid Chromatography Market Size#Low-Pressure Liquid Chromatography Market Share#Low-Pressure Liquid Chromatography Market Growth#Low-Pressure Liquid Chromatography Market Report
0 notes