#Hereditary Angioedema Treatment Market Growth
Explore tagged Tumblr posts
Text
Hereditary Angioedema Treatment Market Size Surpasses USD 6.7 Billion by 2032 with a Robust 8.9% CAGR
Acumen Research and Consulting has recently published a research report on the Hereditary Angioedema Treatment Market for the forecast period of 2023 – 2032, wherein, the global market has been analyzed and assessed in an extremely comprehensive manner. The research report on the Hereditary Angioedema Treatment Market offers an extensive analysis of how the postoperative pain therapeutics…
#Hereditary Angioedema Treatment Market#Hereditary Angioedema Treatment Market Growth#Hereditary Angioedema Treatment Market Share#Hereditary Angioedema Treatment Market Size#Hereditary Angioedema Treatment Market Trends
0 notes
Text
Antifibrinolytic Drugs Market Projected to Show Strong Growth
Advance Market Analytics published a new research publication on "Antifibrinolytic Drugs Market Insights, to 2028" with 232 pages and enriched with self-explained Tables and charts in presentable format. In the Study you will find new evolving Trends, Drivers, Restraints, Opportunities generated by targeting market associated stakeholders. The growth of the Antifibrinolytic Drugs market was mainly driven by the increasing R&D spending across the world.
Get Free Exclusive PDF Sample Copy of This Research @ https://www.advancemarketanalytics.com/sample-report/99832-global-antifibrinolytic-drugs-market Some of the key players profiled in the study are:
Akorn Inc. (United States), Amerigen Pharmaceuticals Ltd (United States), Xanodyne Pharmaceuticals Inc (United States), Aurobindo Pharma Ltd. (India), Mylan N.V. (United States), Pfizer Inc. (United States), Sanofi S.A. (France), Cadila Healthcare (India), Takeda Pharmaceutical Company (Japan). Scope of the Report of Antifibrinolytic Drugs Antifibrinolytic drugs are medicines that promote blood clotting by preventing or slowing down a process called fibrinolysis, which is the breakdown of blood clots. It is used for treatment for hemophilia, in surgical procedures to prevent excessive blood loss, and for heavy menstrual bleeding. The commonly used United States Food and Drugs Administration (FDA)-approved antifibrinolytics include tranexamic acid, aprotinin, and aminocaproic acid. The titled segments and sub-section of the market are illuminated below:
by Drug Type (Amicar, Aminocaproic acid, Aprotinin, Cyklokapron, Fibrinogen, human, Lysteda, RiaSTAP, Tranexamic acid injection, Tranexamic acid oral, Trasylol), End-users (Hospitals, Ambulatory Surgical Centers, Clinics, Healthcare Specialty processes, Others), Distribution Channel (Hospital Pharmacies, Retail Pharmacies, Online, Others), Indications (Gynecology (Menorrhagia, Pregnancy, Parturition, Gynecological surgery, Gastrointestinal (GI) bleeding, Dental surgery, Haemorrhage and Bleeding disorders), Hereditary angioedema, Fibrinolytic response testing, Surgeries (Cardiac, Orthopedic, Liver and Neurosurgery)) Market Trends: Increased Research and Development Activities
Opportunities: Growth in the Geriatric Population
Growing Healthcare Industry Worldwide
Market Drivers: Increased in Incidence of Angioedema
Increased Number of Road Accidents
Rise in the Medical Surgeries Region Included are: North America, Europe, Asia Pacific, Oceania, South America, Middle East & Africa Country Level Break-Up: United States, Canada, Mexico, Brazil, Argentina, Colombia, Chile, South Africa, Nigeria, Tunisia, Morocco, Germany, United Kingdom (UK), the Netherlands, Spain, Italy, Belgium, Austria, Turkey, Russia, France, Poland, Israel, United Arab Emirates, Qatar, Saudi Arabia, China, Japan, Taiwan, South Korea, Singapore, India, Australia and New Zealand etc. Have Any Questions Regarding Global Antifibrinolytic Drugs Market Report, Ask Our Experts@ https://www.advancemarketanalytics.com/enquiry-before-buy/99832-global-antifibrinolytic-drugs-market Strategic Points Covered in Table of Content of Global Antifibrinolytic Drugs Market:
Chapter 1: Introduction, market driving force product Objective of Study and Research Scope the Antifibrinolytic Drugs market
Chapter 2: Exclusive Summary – the basic information of the Antifibrinolytic Drugs Market.
Chapter 3: Displayingthe Market Dynamics- Drivers, Trends and Challenges & Opportunities of the Antifibrinolytic Drugs
Chapter 4: Presenting the Antifibrinolytic Drugs Market Factor Analysis, Porters Five Forces, Supply/Value Chain, PESTEL analysis, Market Entropy, Patent/Trademark Analysis.
Chapter 5: Displaying the by Type, End User and Region/Country 2015-2020
Chapter 6: Evaluating the leading manufacturers of the Antifibrinolytic Drugs market which consists of its Competitive Landscape, Peer Group Analysis, BCG Matrix & Company Profile
Chapter 7: To evaluate the market by segments, by countries and by Manufacturers/Company with revenue share and sales by key countries in these various regions (2023-2028)
Chapter 8 & 9: Displaying the Appendix, Methodology and Data Source finally, Antifibrinolytic Drugs Market is a valuable source of guidance for individuals and companies. Read Detailed Index of full Research Study at @ https://www.advancemarketanalytics.com/reports/99832-global-antifibrinolytic-drugs-market Thanks for reading this article; you can also get individual chapter wise section or region wise report version like North America, Middle East, Africa, Europe or LATAM, Southeast Asia. Contact US : Craig Francis (PR & Marketing Manager) AMA Research & Media LLP Unit No. 429, Parsonage Road Edison, NJ New Jersey USA – 08837 Phone: +1 201 565 3262, +44 161 818 8166 [email protected]
#Global Antifibrinolytic Drugs Market#Antifibrinolytic Drugs Market Demand#Antifibrinolytic Drugs Market Trends#Antifibrinolytic Drugs Market Analysis#Antifibrinolytic Drugs Market Growth#Antifibrinolytic Drugs Market Share#Antifibrinolytic Drugs Market Forecast#Antifibrinolytic Drugs Market Challenges
0 notes
Text
Hereditary Angioedema Therapeutics Market Growth Trajectory Through 2024-2033
The Hereditary Angioedema Therapeutics Global Market Report 2024 by The Business Research Company provides market overview across 60+ geographies in the seven regions - Asia-Pacific, Western Europe, Eastern Europe, North America, South America, the Middle East, and Africa, encompassing 27 major global industries. The report presents a comprehensive analysis over a ten-year historic period (2010-2021) and extends its insights into a ten-year forecast period (2023-2033). Learn More On The Hereditary Angioedema Therapeutics Market: https://www.thebusinessresearchcompany.com/report/hereditary-angioedema-therapeutics-global-market-report According to The Business Research Company’s Hereditary Angioedema Therapeutics Global Market Report 2024, The hereditary angioedema therapeutics market size has grown rapidly in recent years. It will grow from $5.74 billion in 2023 to $6.76 billion in 2024 at a compound annual growth rate (CAGR) of 17.6%. The hereditary angioedema therapeutics market size is expected to see rapid growth in the next few years. It will grow to $13.47 billion in 2028 at a compound annual growth rate (CAGR) of 18.8%. The growth in the forecast period can be attributed to advancements in targeted therapies, global market expansion, gene therapy developments, personalized medicine trends, collaboration in research and treatment.. The rising prevalence of hereditary angioedema is expected to propel the growth of the hereditary angioedema market going forward. Hereditary angioedema (HAE) is a rare genetic disorder characterized by recurrent episodes of severe swelling of the skin and mucous membranes. The swelling is caused by excess fluid build-up (EDEMA) and can occur anywhere in the body, including the hands and feet, face, intestines, and airways. Get A Free Sample Of The Report (Includes Graphs And Tables): https://www.thebusinessresearchcompany.com/sample.aspx?id=10810&type=smp The hereditary angioedema therapeutics market covered in this report is segmented – 1) By Drug Class: C1 Esterase Inhibitor, Selective Bradykinin B2 Receptor Antagonist, Kallikrein Inhibitor, Other Drug Classes 2) By Route of Administration: Intravenous, Subcutaneous, Oral 3) By Distribution Channel: Hospital Pharmacy, Retail Pharmacy, Other Distribution Channels 4) By Application: Prophylaxis, On-demand Product innovations are a key trend gaining popularity in the hereditary angioedema therapeutics market. Major companies operating in the hereditary angioedema therapeutics market are developing innovative products such as ligand-conjugated (LICA) investigational antisense medicine and gene therapy to sustain their position in the market. The hereditary angioedema therapeutics market report table of contents includes: 1. Executive Summary 2. Market Characteristics 3. Market Trends And Strategies 4. Impact Of COVID-19 5. Market Size And Growth 6. Segmentation 7. Regional And Country Analysis . . . 27. Competitive Landscape And Company Profiles 28. Key Mergers And Acquisitions 29. Future Outlook and Potential Analysis Contact Us: The Business Research Company Europe: +44 207 1930 708 Asia: +91 88972 63534 Americas: +1 315 623 0293 Email: [email protected] Follow Us On: LinkedIn: https://in.linkedin.com/company/the-business-research-company Twitter: https://twitter.com/tbrc_info Facebook: https://www.facebook.com/TheBusinessResearchCompany YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ Blog: https://blog.tbrc.info/ Healthcare Blog: https://healthcareresearchreports.com/ Global Market Model: https://www.thebusinessresearchcompany.com/global-market-model
0 notes
Text
Angioedema Treatment Market is Estimated to Witness High Growth Owing to Opportunity in Unmet Medical Needs

Angioedema is a transient localized edema of the dermis, mucosa, and submucosa caused by vascular leakage. The condition can be hereditary, acquired or idiopathic in nature. Angioedema affects the deep layers of the skin and mucous membranes in the digestive and respiratory tracts. Symptoms vary depending on the location and severity but may include swelling of the face, lips, throat, hands or feet and intestinal swelling which causes pain, nausea and vomiting. Currently available drugs for the treatment of angioedema are C1 esterase inhibitors which control acute HAE attacks but inadequate supply and high costs limit their use. The global Angioedema Treatment Market is estimated to be valued at US$ 470.4 Mn in 2023 and is expected to exhibit a CAGR of 4.1% over the forecast period 2023 to 2030, as highlighted in a new report published by Coherent Market Insights. Market Opportunity: The growing unmet medical needs in the management of angioedema symptoms provide immense opportunities for new drug manufacturers. Currently available C1 esterase inhibitors have drawbacks such as short shelf life, insufficient supply and high costs which limit their availability to patients. There is a need for affordable and effective treatment options for long term prophylaxis and management of swelling symptoms. Development of new drug delivery formulations, biosimilars and generic versions of current therapies can help expand treatment access and reduce healthcare burden in managing this rare disease condition. This presents lucrative opportunities for angioedema drug manufacturers to develop better and affordable treatment solutions. Porter's Analysis Threat of new entrants: The angioedema treatment market has moderate threat of new entrants due to significant investment required for R&D and presence of established players. However, scope for new treatments provide opportunities. Bargaining power of buyers: Buyers have moderate bargaining power due to availability of few treatment options. Switching costs are low for buyers. Bargaining power of suppliers: Suppliers have low to moderate bargaining power as there are many suppliers for raw materials. Requirements are commodity products. Threat of new substitutes: Threat of substitution is low as there are limited treatment options available for angioedema. Competitive rivalry: The competitive rivalry is high due to presence of large players. Companies compete based on treatment innovations and cost effectiveness. SWOT Analysis Strength: Established distribution channel and brand recognition provide advantage to large players. Wide range of treatment portfolio enables revenue generation. Weakness: High R&D costs and regulatory approvals increase treatment costs. Dependency on few drug classes results in limitedtreatment options. Opportunity: Growing patient population suffering from hereditary angioedema and allergic angioedema provide growth opportunity. Scope for new treatment innovations. Threats: Patent expiries of blockbuster drugs and price control measures impact revenues. Stringent regulations for new drug approvals. Key Takeaways The global angioedema treatment market is expected to witness high growth over the forecast period of 2023 to 2030 led by increasing prevalence of angioedema. Regional analysis - North America dominates the global market and is expected to maintain its position over the forecast period due to growing awareness about treatment and availability of advanced healthcare systems. However, Asia Pacific is expected to grow at fastest pace due to improving healthcare infrastructure and economic growth. Key players operating in the angioedema treatment market are Bayer AG, Zoetis Inc., Boehringer Ingelheim GmbH, Merck & Co., Inc., Virbac S.A., Ceva Santé Animale, Elanco Animal Health, Bimeda, Aurobindo Pharma Limited, and Teva Pharmaceutical Industries Ltd. Bayer AG dominates the global market due to wide product portfolio and geographic presence. Players compete based on expanding indications and new drug approvals.
Get more insights on this topic:https://www.coherentmarketinsights.com/market-insight/angioedema-treatment-market-1371
0 notes
Text
0 notes
Text
Legal Winsol
Crazy Bulk Winsol For Sale
Stanozolol – Winstrol (Oral) & Winstrol Depot (Injectable)
Stanozolol is a synthetic extracted from Testosterone. Technically classified as an anabolic anabolic steroid, Stanozolol is commonly sold under the brand name winstrol. Created by the Winthrop Laboratories in 1962, winstrol is 1 of the most popular Anabolic Steroid drugs. Winstrol is a registered trademark of Sanofi-Synthelabo Inc. in the United States and/or other nations around the world. Sanofi has licensed legal rights of Wnstrol to Délire Pharmaceuticals. Winstrol is commonly marketed as Winstrol (oral) and Winstrol Depot (injectable).
USAGE – The Combined States FDA (Food and Drug Administration) has approved winstrol for human use. Medically, winstrol is used both in animals and human patients to treat a quantity of conditions. Winstrol is commonly used for the treatment of anaemia and hereditary angioedema that will cause episodes of swelling of the face, extremities, sex organs, bowel wall, and throat in humans.
Winsol Reviews
Winstrol has substantial fibrinolytic properties. That has been effective treatment for urticaria, Raynaud's trend, cryptofibrinogenemia, and lipodermatosclerosis. This has already been used to cure osteonecrosis in the event; it is resistant to other therapy. The drug also has been successfully used in treatment of SUPPORTS wasting syndrome. Winstrol is frequently used to improve muscle growth, red blood cellular production, increase bone density and stimulate the hunger in weak and feeble animals. Winstrol is extensively utilized by muscle builders for its anabolic and androgenic effects. Average Dose for men is 50-100 mg/day, and for women is 25-50 mg/week.
CHEMISTRY:winstrol is a DHT (dihydrotestosterone) derivative. It is a 17AA steroid. It is chemically designated as 17-methyl-2' H -5(alpha)-androst-2-eno[3, 2- c ]pyrazol-17(beta)-ol. In winstrol (oral), Stanozolol is solidified into capsule form, and each capsule contains 2 mg of stanozolol. Stanozolol is aqueous in winstrol Depot. Inactive Ingredients of winstrol include Dibasic Calcium Phosphate, D&C Red #28, FD&C Red-colored #40, Lactose, Magnesium Stearate, and Starch. Active Life of winsrol is around 48 hours.Read More Here https://legalalternativetowinstrol.splashthat.com/
Winstrol Alternative
SIDE RESULTS – overuse or high doses of winstrol are often linked with cause serious side effects. Several of the side outcomes of winstrol are: allergic reactions, such as difficulty in breathing, choking of the throat, swelling of the lips, tongue, or face, or hives; swelling of the arms or legs, especially ankles; frequent or persistent erections, breast tenderness, breast enhancement (males), voice changes (hoarseness, deepening), hair reduction, facial hair growth, clitoral enlargement (females), menstrual unevenness (female), worsening acne, difficulty in sleeping, headaches, and changes in sexual desire.
LEGAL STATUS – winstrol has been classified as a Schedule III controlled substance under federal legislation. It has been prohibited from use in sporting activities competition by the International Association of Athletics Federations (IAAF).
1 note
·
View note
Text
The Global Angioedema Treatment Market Report Provides Qualitative And Quantitative Analysis For The Years 2017 To 2025
The Hereditary Angioedema Association anticipates playing a critical role in the dissemination of new treatments on the global market. They have additionally attempted to raise awareness about items and bolster benefits required by HAE patients, which is expected to result in positive growth in the Hereditary Angioedema market. Surging population, lifestyle changes, improved reimbursement policies, and increased efforts from pharmaceutical companies and biotechnology organisations to develop new products and solutions for the market are expected to boost market income for Hereditary Angioedema in the coming years. Nonetheless, high drug costs and poor disease diagnosis are some of the factors impeding the development of the Hereditary Angioedema market during the forecast time frame.
The global Angioedema Treatment Market report provides qualitative and quantitative analysis for the years 2017 to 2025. The global angioedema treatment market is expected to grow at a healthy CAGR from 2019 to 2025, according to the report. The study on the angioedema treatment market examines the leading geographies such as North America, Europe, Asia-Pacific, and RoW from 2017 to 2025. According to a new research report, the Angioedema Treatment market is one of the most proactive industry verticals. This research study predicts that this space will generate significant profits by the end of the forecast period, aided by a slew of driving forces that will fuel industry trends over the forecast period.
Read More@ https://bit.ly/3twygs6
0 notes
Text
Global Plasma Protease C1-inhibitor Treatment Market by Type, By Region, By Application and Forecast To 2029

The global Plasma Protease C1 Inhibitor Treatment Market is dominated by a small number of companies. Due to rising product approvals, the plasma protease C1-inhibitor therapy market is likely to develop significantly throughout the forecast period. For example, in June 2017, CSL Behring, a worldwide biopharmaceutical company, acquired FDA approval for HAEGARDA (C1 Esterase Inhibitor Subcutaneous (Human)), the first and only subcutaneous medication authorised for routine prophylaxis to prevent HAE attacks in adolescent and adult patients.
Another factor driving the growth of the Plasma Protease C1 Inhibitor Treatment Market is the increased attention of major biopharmaceutical companies on the development of C1-inhibitor therapies/ medicines. For example, on October 31, 2019, Ionis Pharmaceuticals, Inc. began a phase 2 clinical trial to assess the clinical efficacy, safety, and tolerability of IONIS-PKK-LRx in participants with hereditary angioedema (HAE) type 1 (HAE-1), HAE type 2 (HAE-2), or HAE with normal C1-inhibitor (C1-INH), as well as to assess the effect of IONIS-PKK-LRx
Read more @ https://creativeedge16.blogspot.com/2021/11/plasma-protease-c1-inhibitor-treatment.html
0 notes
Text
Endoscopic Cold Light EUROPE Market Research Report 2021-2026
The Endoscopic Cold Light Source market report provides a detailed analysis of global market size, regional and country-level market size, segmentation market growth, market share, competitive Landscape, sales analysis, impact of domestic and global market players, value chain optimization, trade regulations, recent developments, opportunities analysis, strategic market growth analysis, product launches, area marketplace expanding, and technological innovations.
ALSO READ: http://www.marketwatch.com/story/eendoscopic-cold-light-market-research-report-with-size-share-value-cagr-outlook-analysis-latest-updates-data-and-news-2021-2021-07-16
Market segmentation Endoscopic Cold Light Source market is split by Type and by Application. For the period 2016-2026, the growth among segments provide accurate calculations and forecasts for sales by Type and by Application in terms of volume and value. This analysis can help you expand your business by targeting qualified niche markets.
ALSO READ: http://www.marketwatch.com/story/june-2021-report-on-global-piezoceramic-sensor-market-statistics-cagr-outlook-and-covid-19-impact-2021---2023-2021-06-02
Market segment by Type, covers LED Light Source Xenon Light Source Other
Market segment by Application can be divided into Laparoscopy Urology Gastroenterology Arthroscopy Others
The key market players for global Endoscopic Cold Light Source market are listed below: Olympus Karl Storz Stryker Conmed HOYA Fujifilm Richard Wolf Boston Scientific Smith & Nephew Schoelly Fiberoptic B. Braun SonoScape Mindray
ALSO READ: http://www.marketwatch.com/story/june-2021-report-on-global-acoustic-plasterboard-industry-market-overview-size-share-and-trends-2021-2026-2021-06-03
Market segment by Region, regional analysis covers North America (United States, Canada and Mexico) Europe (Germany, France, United Kingdom, Russia, Italy, and Rest of Europe) Asia-Pacific (China, Japan, Korea, India, Southeast Asia, and Australia) South America (Brazil, Argentina, Colombia, and Rest of South America) Middle East & Africa (Saudi Arabia, UAE, Egypt, South Africa, and Rest of Middle East & Africa)
The content of the study subjects, includes a total of 14 chapters: Chapter 1, to describe Endoscopic Cold Light Source product scope, market overview, market opportunities, market driving force and market risks. Chapter 2, to profile the top manufacturers of Endoscopic Cold Light Source, with price, sales, revenue and global market share of Endoscopic Cold Light Source from 2019 to 2021. Chapter 3, the Endoscopic Cold Light Source competitive situation, sales, revenue and global market share of top manufacturers are analyzed emphatically by landscape contrast. Chapter 4, the Endoscopic Cold Light Source breakdown data are shown at the regional level, to show the sales, revenue and growth by regions, from 2016 to 2026. Chapter 5 and 6, to segment the sales by type and application, with sales market share and growth rate by type, application, from 2016 to 2026. Chapter 7, 8, 9, 10 and 11, to break the sales data at the country level, with sales, revenue and market share for key countries in the world, from 2016 to 2021.and Endoscopic Cold Light Source market forecast, by regions, type and application, with sales and revenue, from 2021 to 2026. Chapter 12, 13 and 14, to describe Endoscopic Cold Light Source sales channel, distributors, customers, research findings and conclusion, appendix and data source.
ALSO READ: http://www.marketwatch.com/story/june-2021-report-on-global-hereditary-angioedema-treatment-market-outlook-industry-analysis-and-prospect-2021-2026-2021-06-03
Table of Contents
1 Market Overview 1.1 Endoscopic Cold Light Source Introduction 1.2 Market Analysis by Type 1.2.1 Overview: Global Endoscopic Cold Light Source Revenue by Type: 2019 Versus 2021 Versus 2026 1.2.2 LED Light Source 1.2.3 Xenon Light Source 1.2.4 Other 1.3 Market Analysis by Application 1.3.1 Overview: Global Endoscopic Cold Light Source Revenue by Application: 2019 Versus 2021 Versus 2026 1.3.2 Laparoscopy 1.3.3 Urology 1.3.4 Gastroenterology 1.3.5 Arthroscopy 1.3.6 Others 1.4 Global Endoscopic Cold Light Source Market Size & Forecast 1.4.1 Global Endoscopic Cold Light Source Sales in Value (2016-2026)) 1.4.2 Global Endoscopic Cold Light Source Sales in Volume (2016-2026) 1.4.3 Global Endoscopic Cold Light Source Price by Type (2016-2026) & (USD/Unit) 1.5 Global Endoscopic Cold Light Source Production Capacity Analysis 1.5.1 Global Endoscopic Cold Light Source Total Production Capacity (2016-2026) 1.5.2 Global Endoscopic Cold Light Source Production Capacity by Geographic Region 1.6 Market Drivers, Restraints and Trends 1.6.1 Endoscopic Cold Light Source Market Drivers 1.6.2 Endoscopic Cold Light Source Market Restraints 1.6.3 Endoscopic Cold Light Source Trends Analysis
ALSO READ: http://www.marketwatch.com/story/june-2021-report-on-global-thin-wall-glass-container-market-statistics-cagr-outlook-and-covid-19-impact-2021---2023-2021-06-03
2 Manufacturers Profiles 2.1 Olympus 2.1.1 Olympus Details 2.1.2 Olympus Major Business 2.1.3 Olympus Endoscopic Cold Light Source Product and Services 2.1.4 Olympus Endoscopic Cold Light Source Sales, Price, Revenue, Gross Margin and Market Share (2019-2021) 2.2 Karl Storz 2.2.1 Karl Storz Details 2.2.2 Karl Storz Major Business 2.2.3 Karl Storz Endoscopic Cold Light Source Product and Services 2.2.4 Karl Storz Endoscopic Cold Light Source Sales, Price, Revenue, Gross Margin and Market Share (2019-2021) 2.3 Stryker 2.3.1 Stryker Details 2.3.2 Stryker Major Business 2.3.3 Stryker Endoscopic Cold Light Source Product and Services 2.3.4 Stryker Endoscopic Cold Light Source Sales, Price, Revenue, Gross Margin and Market Share (2019-2021) 2.4 Conmed 2.4.1 Conmed Details 2.4.2 Conmed Major Business 2.4.3 Conmed Endoscopic Cold Light Source Product and Services 2.4.4 Conmed Endoscopic Cold Light Source Sales, Price, Revenue, Gross Margin and Market Share (2019-2021) 2.5 HOYA 2.5.1 HOYA Details 2.5.2 HOYA Major Business 2.5.3 HOYA Endoscopic Cold Light Source Product and Services 2.5.4 HOYA Endoscopic Cold Light Source Sales, Price, Revenue, Gross Margin and Market Share (2019-2021) 2.6 Fujifilm 2.6.1 Fujifilm Details 2.6.2 Fujifilm Major Business 2.6.3 Fujifilm Endoscopic Cold Light Source Product and Services 2.6.4 Fujifilm Endoscopic Cold Light Source Sales, Price, Revenue, Gross Margin and Market Share (2019-2021) 2.7 Richard Wolf 2.7.1 Richard Wolf Details 2.7.2 Richard Wolf Major Business 2.7.3 Richard Wolf Endoscopic Cold Light Source Product and Services 2.7.4 Richard Wolf Endoscopic Cold Light Source Sales, Price, Revenue, Gross Margin and Market Share (2019-2021) 2.8 Boston Scientific 2.8.1 Boston Scientific Details 2.8.2 Boston Scientific Major Business 2.8.3 Boston Scientific Endoscopic Cold Light Source Product and Services 2.8.4 Boston Scientific Endoscopic Cold Light Source Sales, Price, Revenue, Gross Margin and Market Share (2019-2021) 2.9 Smith & Nephew 2.9.1 Smith & Nephew Details 2.9.2 Smith & Nephew Major Business 2.9.3 Smith & Nephew Endoscopic Cold Light Source Product and Services 2.9.4 Smith & Nephew Endoscopic Cold Light Source Sales, Price, Revenue, Gross Margin and Market Share (2019-2021) 2.10 Schoelly Fiberoptic 2.10.1 Schoelly Fiberoptic Details 2.10.2 Schoelly Fiberoptic Major Business 2.10.3 Schoelly Fiberoptic Endoscopic Cold Light Source Product and Services 2.10.4 Schoelly Fiberoptic Endoscopic Cold Light Source Sales, Price, Revenue, Gross Margin and Market Share (2019-2021) 2.11 B. Braun 2.11.1 B. Braun Details 2.11.2 B. Braun Major Business 2.11.3 B. Braun Endoscopic Cold Light Source Product and Services 2.11.4 B. Braun Endoscopic Cold Light Source Sales, Price, Revenue, Gross Margin and Market Share (2019-2021) 2.12 SonoScape 2.12.1 SonoScape Details 2.12.2 SonoScape Major Business 2.12.3 SonoScape Endoscopic Cold Light Source Product and Services 2.12.4 SonoScape Endoscopic Cold Light Source Sales, Price, Revenue, Gross Margin and Market Share (2019-2021) 2.13 Mindray 2.13.1 Mindray Details 2.13.2 Mindray Major Business 2.13.3 Mindray Endoscopic Cold Light Source Product and Services 2.13.4 Mindray Endoscopic Cold Light Source Sales, Price, Revenue, Gross Margin and Market Share (2019-2021) 3 Endoscopic Cold Light Source Sales by Manufacturer 3.1 Global Endoscopic Cold Light Source Sales in Volume by Manufacturer (2019-2021e) 3.2 Global Endoscopic Cold Light Source Revenue by Manufacturer (2019-2021e) 3.3 Key Manufacturer Market Position in Endoscopic Cold Light Source 3.4 Market Concentration Rate 3.4.1 Top 3 Endoscopic Cold Light Source Manufacturer Market Share 3.4.2 Top 6 Endoscopic Cold Light Source Manufacturer Market Share 3.5 Global Endoscopic Cold Light Source Production Capacity by Company 3.6 Manufacturer by Geography: Head Office and Endoscopic Cold Light Source Production Site 3.7 New Entrant and Capacity Expansion Plans 3.8 Mergers & Acquisitions
….CONTINUED
CONTACT DETAILS :
+44 203 500 2763
+1 62 825 80070
971 0503084105
0 notes
Text
Hereditary Angioedema Treatment Market Share, Business Analysis and Growth Forecast to 2026
The global hereditary angioedema treatment market to gain from increasing incidences of genetic mutation. Recently Fortune Business Insights, published a report titled, “Hereditary Angioedema Treatment Market Size, Share and Global Trend by Drug Class (C-1 Esterase Inhibitors, Bradykynin Receptor Antagonist, Kallikrein Inhibitors), Application (Prophylaxis, Treatment), Route of Administration (IV, Subcutaneous), Distribution Channel (Hospital Pharmacy, Retail Pharmacy) and Geography Forecast till 2025.”
For more information in the analysis of this report, visit: https://www.fortunebusinessinsights.com/industry-reports/hereditary-angioedema-treatment-market-100164
As per the report, the global hereditary angioedema treatment market was worth US$ 1883.1 Mn in 2017. The global market is anticipated to expand at a CAGR of 16.8% and reach US$ 6533.3 Mn by the end of 2025. The report classifies the global hereditary angioedema treatment market in various segments and offer a comprehensive overview.
some of the key players in the global Hereditary Angioedema Treatment Market:
BIOCRYST PHARMACEUTICALS INC.
Ionis Pharmaceuticals Inc.
Attune Pharmaceuticals
Arrowhead Pharmaceuticals Inc.
Adverum
According to the report, the hereditary angioedema treatment market in North America was valued at US$ 1752.2 Mn in 2017. The growth witnessed is attributable to high prevalence of hereditary angioedema in the region. North America may remain dominant in the global market through the forecast period (2018-2025) also. Presence of an established healthcare system and increasing healthcare expenditure are a few factors anticipated to contribute the expansion of the hereditary angioedema treatment market in North America. The report also predicts the market in Asia Pacific to witness impressive growth. Increasing medical tourism in nations such as China and India will create lucrative growth opportunities for the market in the region. Besides this, increasing number of product innovations in the region backed by high presence of hereditary angioedema drugs manufacturers will give tailwinds to growth witnessed by the hereditary angioedema treatment market.
C-1 Esterase Inhibitor Segment to Dominate Global Market During Forecast Period
In terms of drug class, the C-1 esterase inhibitor is the most popular drug and dominated the global hereditary angioedema treatment market in 2017. The segment accounted for 61.3% of the global market in 2017. The trend is unlikely to change during the forecast period 2018-2025 owing to the drug’s reliability among patients and practitioners.
Increasing awareness about the disorder and rising prevalence of the hereditary angioedema are some factors expected to drive the global hereditary angioedema treatment market during the forecast period. Additionally, technological developments in the hereditary angioedema treatment devices and equipment is expected to boost the global market.
0 notes
Text
Hereditary Angioedema Market to Witness Robust Growth as BioCryst Pharmaceuticals, Inc. Receives the U.S. FDA Approval for its ORLADEYO indicated for hereditary angioedema
Hereditary Angioedema is a disease, which is the result of genetic mutation, within the families with higher genetic activity. It is not contagious to others. The cause of heredity is still unknown but it is believed that the disease is related to the immunological mechanism. Hereditary Angioedema is a chronic non-inflammatory disease that affects millions of people around the world. Symptoms include persistent dryness of the skin, scalp itching, inflammation, rash, severe skin peeling and cracking, bleeding and hives, pain in the area where the skin is affected. As a part of the diagnosis, a medical practitioner who is experienced in treating hereditary angioedema must do a skin biopsy to find out the exact cause of allergic skin reaction.
Rising awareness regarding hereditary angioedema is expected to drive growth of the global hereditary angioedema market during the forecast period. Hereditary angioedema or also referred to as HAE has received significant attention in the recent past as government and non-government organizations across the globe are focused on increasing public awareness to improve diagnosis and enhance the treatment.
Read more @ https://coherentmarketinsights-blog.blogspot.com/2020/12/hereditary-angioedema-market-to-witness.html
#HereditaryAngioedema#C1EsteraseInhibitor#KallikreinInhibitor#BradykininReceptor#AttenuatedAndrogens#hereditaryangioedemamarket#hereditaryangioedemamarketforecast#MarketResearchReport
0 notes
Text
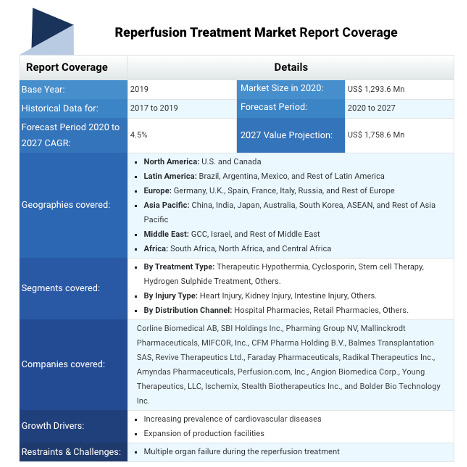
REPERFUSION TREATMENT MARKET ANALYSIS(2020-2027)
Reperfusion Treatment Market, by Treatment Type (Therapeutic Hypothermia, Cyclosporin, Stem Cell Therapy, Hydrogen Sulphide Treatment, and Others), by Injury Type (Heart Injury, Kidney Injury, Intestine Injury, and Others), by Distribution Channel (Hospital Pharmacies, Retail Pharmacies, and Others), and by Region (North America, Latin America, Europe, Asia Pacific, Middle East, and Africa) - Size, Share, Outlook, and Opportunity Analysis, 2020 - 2027
Reperfusion injury is caused due to the damage in the tissue, which occurs due to the lack of blood supply. Examples of reperfusion injury include brain damage after stroke and many others, where reperfusion therapy leads to flow of blood in the tissue which results in inflammation and oxidative damage due to oxidative stress. Reperfusion injury can be treated by therapeutic hypothermia, hydrogen sulphide treatment, cyclosporins, stem cell therapy, and others. Furthermore, delay in reperfusion therapy results in oxidative damage.
Global Reperfusion Treatment Market – Impact of Coronavirus (COVID – 19) Pandemic:
The COVID-19 pandemic is expected to hamper the global reperfusion treatment market growth during the forecast period. The COVID-19 pandemic and resulting lockdowns in various countries across the globe have impacted the financial status of businesses in all sectors. The private healthcare sector has been impacted majorly due to the COVID-19 pandemic. Many clinical trials have been suspended during the pandemic. In order to restart the clinical trials, the U.S. Food and Drug Administration (FDA) released guidelines during the COVID-19 public health emergency in March 2020. The guidelines were further updated on July 02, 2020. The guidelines include general considerations to assist sponsors and researchers, which ensure the safety of trial participants, and compliance with good clinical practice (GCP) for the duration of the COVID-19 public health emergency. The appendix of the guidelines also provide answers to some general questions, which the U.S. Food and Drug Administration (FDA) received from various sponsors and researchers about conducting clinical trials during the COVID-19 public health emergency. The above guidelines are also applicable for conducting the clinical trials for testing the safety and efficacy of the drugs for the reperfusion injury. Thus, the COVID – 19 pandemic is expected to decrease the growth of the reperfusion treatment market over the forecast period.
The global reperfusion treatment market is estimated to be valued at US$ 1,293.6 million in 2020 and is expected to exhibit a CAGR of 4.5% during the forecast period (2020-2027).
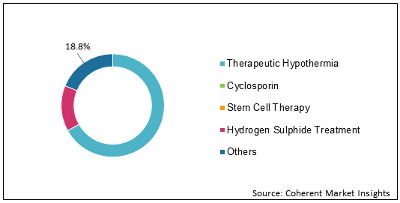
Figure 1: Global Reperfusion Treatment Market Share (%) Analysis, By Treatment Type 2020
Increasing prevalence of coronary heart dis ease is expected to drive the growth of the global reperfusion treatment market during the forecast period.
The rising incidence of coronary artery disease (CAD) or ischemic heart disease (IHD) is a major factor which is expected to drive the market growth. The CAD or IHD is caused due to the buildup of cholesterol and fatty deposits on the inner walls of the arteries, which may lead to the reduction of blood flow to the heart cells. This condition may lead to ischemia, myocardial infraction or sudden cardiac arrest. Moreover, medicines approved from the regulatory authorities are not available in the market for the treatment of ischemia/reperfusion injury. According to the National Center for Biotechnology Information (NCBI), 2020, in 2017, globally, around 126 million people suffered from ischemic heart disease (1,655 per 100,000), which constituted to 1.72% of the total world population.
Investments and expansion of production facility by market players are expected to boost growth of the global reperfusion treatment market during the forecast period.
Market players are focusing on facility expansions in order to strengthen their product portfolio. For instance, on March 9, 2020, Pharming Group NV received the Food and Drug Administration (U.S. FDA) approval for its new production facility in the Netherlands for the production of the starting material required for manufacturing of RUCONEST. RUCONEST is a C1-esterase inhibitor, which is plasma free and is proven to help treat hereditary angioedema (HAE) attacks. Furthermore, on January 21, 2020, Pharming Group NV received the European Medicines Agency (EMA) approval for the production facility for RUCONEST in Europe.
Global Reperfusion Treatment Market – Restraints:
There are some side effects associated with the treatment, which are expected to restrain the global reperfusion treatment market during the forecast period. Ischemia reperfusion causes the mediator to infiltrate other tissues, which leads to Multiple Organ Dysfunction Syndrome (MODS). For instance, according to an article published in the International Institute of Anticancer Research in 2019, Multiple Organ Dysfunction Syndrome (MODS) was the leading cause of mortality globally and the incidence of MODS ranged from 25-40%. Furthermore, according to the Critical Care Nephrology Journal 2019, the pediatric multiple organ dysfunction syndrome (MODS) epidemiology ranges from 10% to 50% of the children admitted to the pediatric intensive care unit.
Global Reperfusion Treatment Market – Regional Analysis:
On the basis of region, the global reperfusion treatment market is segmented into North America, Latin America, Europe, Asia Pacific, Middle East, and Africa.
North America is expected to dominate the global reperfusion treatment market during the forecast period owing to research and development in the region. For instance, in November 2019, Faraday Pharmaceuticals announced positive results from phase II clinical trials of FDY-5301 for ischemia reperfusion injury treatment, following a STEMI heart attack. FDY-5301 is a formulated, patented, elemental reducing agent that contains sodium iodide. It destroys the hydrogen peroxide that is naturally generated as a response to acute ischemia reperfusion injury and also contributes to loss of muscle function and mass.
Europe is an emerging reperfusion treatment market owing to the funding provided for research and development by regulatory authorities. For instance, in February 2019, Balmes Transplantation SAS received around US$ 605,597 million from the European Regional Development Fund (ERDF) for its research program REMEDIRA for developing combinations of repurposed drugs against kidney ischemia-reperfusion injury (IRI).
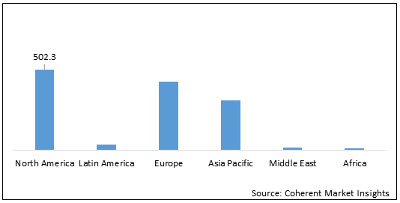
Figure 2: Global Reperfusion Treatment Market Value (US$ Mn), by Region, 2020
Global Reperfusion Treatment Market - Competitive Landscape:
Some of the key players operating in the global reperfusion treatment market are Corline Biomedical AB, SBI Holdings Inc., Pharming Group NV, Mallinckrodt Pharmaceuticals, MIFCOR, Inc., CFM Pharma Holding B.V., Balmes Transplantation SAS, Revive Therapeutics Ltd., Faraday Pharmaceuticals, Radikal Therapeutics Inc., Amyndas Pharmaceuticals, Perfusion.com, Inc., Angion Biomedica Corp., Young Therapeutics, LLC, Ischemix, Stealth Biotherapeutics Inc., and Bolder Bio Technology Inc.
Request sample report here:
https://www.coherentmarketinsights.com/insight/request-sample/4248
Download PDF brochure here:
https://www.coherentmarketinsights.com/insight/request-pdf/4248
About Us:
Coherent Market Insights is a global market intelligence and consulting organization focused on assisting our plethora of clients achieve transformational growth by helping them make critical business decisions.
What we provide:
• Customized Market Research Services
• Industry Analysis Services
• Business Consulting Services
• Market Intelligence Services
• Long term Engagement Model
• Country Specific Analysis
Contact Us:
Mr. Shah
Coherent Market Insights Pvt. Ltd.
Address: 1001 4th ave, #3200 Seattle, WA 98154, U.S.
Phone: +1-206-701-6702
Email: [email protected]
Source: https://www.coherentmarketinsights.com/market-insight/reperfusion-treatment-market-4248
0 notes
Text
High Drug Costs And Poor Disease Diagnosis Are Some Of The Factors Impeding The Development Of The Hereditary Angioedema Market During The Forecast Time Frame
Hereditary angioedema (HAE) is a rare immune-related condition. The potentially fatal condition is caused by a lack of C1-esterase inhibitor (C1-INH), which causes blood vessels to dilate. The disease's symptoms include recurring episodes of edoema in various body parts such as the hands, feet, face, and airways. Based on the reduction in inhibitor synthesis or the formation of a dysfunctional protein, HAE is classified into three types: Type I HAE, Type II HAE, and Type III HAE. Although the condition is hereditary, the absence of a family history does not rule out the diagnosis of HAE, as up to 25% of HAE cases are caused by a spontaneous mutation of the C1-inhibitor gene at conception.
Surging population, lifestyle changes, improved reimbursement policies, and increased efforts from pharmaceutical companies and biotechnology organisations to develop new products and solutions for the market are expected to boost market income for Hereditary Angioedema in the coming years. Nonetheless, high drug costs and poor disease diagnosis are some of the factors impeding the development of the Hereditary Angioedema market during the forecast time frame. The Hereditary Angioedema Association anticipates playing a critical role in the dissemination of new treatments on the global market. They have additionally attempted to raise awareness about items and bolster benefits required by HAE patients, which is expected to result in positive growth in the Hereditary Angioedema Market.
Read More@ https://bit.ly/3G86i9Q
0 notes
Text
Hereditary Angioedema Market Pegged for Robust Expansion by 2027
Hereditary Angioedema Market Pegged for Robust Expansion by 2027

Hereditary Angioedema Treatment Market: Surge in Awareness about Rare Genetic Diseases Contributes to Growth
The prevalence of rare diseases has surged in recent years, as the tracking of their incidence has improved around the world in the past few decades. This is particularly significant for genetic diseases, as standardized screenings and tests are few, as such, making it hard to determine…
View On WordPress
0 notes
Text
Hereditary Angioedema Market : Technological Growth Map over Time to Understand the Industry Growth Rate
Hereditary Angioedema Treatment Market: Introduction
· Transparency Market Research has published a new report on the hereditary angioedema treatment market for the forecast period of 2019–2027. According to the report, the global hereditary angioedema treatment market was valued at ~US$ 2 Bn in 2018, and is projected to expand at a CAGR of ~9% from 2019 to 2027.
· Hereditary angioedema is an autosomal disorder, wherein, the level of functional C1 esterase inhibitor protein is low. This leads to increased concentration of bradykinin, which results in fluid leakage from the blood vessels, followed by swelling.
· Purified concentrate of C1 esterase inhibitors are administered to HAE affected patients for the prophylactic and acute attack treatment of HAE. Several drugs have been approved for the prophylactic and acute attack treatment of hereditary angioedema.
· Growth of the global hereditary angioedema treatment market can be attributed to rise in the prevalence of hereditary angioedema and increase in awareness about HAE across the globe.
· North America dominated the global market during the forecast period, due to a comparatively better reported ratio for hereditary angioedema cases.
Request a PDF Brochure - https://www.transparencymarketresearch.com/sample/sample.php?flag=B&rep_id=74333
Increase in Awareness about Hereditary Angioedema and Novel Pipeline Drugs to Drive Market
· Government organizations and other agencies are creating public awareness through various campaigns regarding the diagnosis, treatment, and care of hereditary angioedema. Moreover, the prevalence of rare diseases across the world is increasing, and most of these are genetic disorders.
· The U.S. Hereditary Angioedema Association (U.S. HAEA) organized a fundraising event and other campaigns in September 2017 to create awareness about the disorder within the community. Such campaigns boost the growth of the global hereditary angioedema treatment market.
· Promising pipeline drugs and novel therapies by major companies are also fueling the growth of the global hereditary angioedema treatment market.
· For instance, BioCryst Pharmaceuticals has developed an oral drug - BCX7353 - for the prophylactic treatment of HAE, which has completed the phase II clinical trial.
· Moreover, increase in the intensity of attacks of severe edema due to high estrogen levels and rise in the usage of ACE inhibitors that trigger an attack of HAE are the major factors driving the global hereditary angioedema treatment market.
Kallikrein Inhibitors to be Promising Drug Class
· In terms of drug class, the global hereditary angioedema treatment market has been classified into C1 esterase inhibitors, selective bradykinin B2 receptor antagonists, kallikrein inhibitors, and others.
· The C1 esterase inhibitors segment dominated the global market in 2018. However, the segment is anticipated to lose market share by the end of 2027, due to expiration of the market exclusivity of certain drugs in major markets, and expected launch of other class of drugs during the forecast period.
· The C1 esterase inhibitors segment has been bifurcated into plasma products and recombinant products. The plasma products sub-segment comprises approved drugs such as Berinert, Cinryze, and Haegarda. The recombinant products sub-segment consists of only one approved product - Ruconest.
· The kallikrein inhibitor segment has been split into Kalbitor and Takhzyro. The segment is expected to grow at a higher CAGR during the forecast period, owing to the launch of Takhzyro, a blockbuster drug in the U.S., and expected approval in other markets in the new few years.
· The selective bradykinin B2 receptor antagonists segment consists of only one approved product - Firazyr. The others segment has been bifurcated into conventional drugs and pipeline drugs.
Request for Analysis of COVID19 Impact on Hereditary angioedema market - https://www.transparencymarketresearch.com/sample/sample.php?flag=covid19&rep_id=74333
Subcutaneous Injection Route of Administration Dominates Global Market
· Based on route of administration, the global hereditary angioedema treatment market has been divided into intravenous, subcutaneous injection, and oral.
· The subcutaneous injection segment dominated the global market in 2018, and the trend is likely to continue during the forecast period. Patient convenience and new product launches such as Haegarda and Takhzyro in 2017 and 2018, respectively, are the major factors driving the segment in the global hereditary angioedema treatment market.
Retail Pharmacies Prominent Distribution Channel
· In terms of distribution channel, the global hereditary angioedema treatment market has been categorized into hospital pharmacies, retail pharmacies, and others.
· The retail pharmacies segment is projected to dominate the global hereditary angioedema treatment market during the forecast period, owing to increase in the number of retail pharmacies, easy accessibility, and additional services provided by specialty pharmacies.
· The others segment, comprising online pharmacies and mail pharmacies, is anticipated to be more lucrative during the forecast period. The segment is expected to expand at a higher CAGR during the forecast period, due to increase in the preference for online pharmacies and discounted rates at these pharmacies.
Buy now Hereditary angioedema market Report - https://www.transparencymarketresearch.com/checkout.php?rep_id=74333<ype=S
North America to Dominate Global Hereditary Angioedema Treatment Market
· The global hereditary angioedema treatment market has been segmented into three major regions: North America, Europe, and Rest of the World.
· North America accounted for a major share of the global hereditary angioedema treatment market in 2018, and is projected to dominate the market during the forecast period.
· High prevalence of hereditary angioedema, comparatively better reported ratio for diseases, high healthcare expenditure, and availability of specialty HAE products are the major factors boosting the growth of the hereditary angioedema treatment market North America.
· Rest of the World is anticipated to be a highly lucrative hereditary angioedema treatment market during the forecast period, due to factors such as rise in the number of reported cases of hereditary angioedema in recent times, increase in awareness in developing countries about rare diseases, and introduction of novel therapies.
Global Hereditary Angioedema Treatment Market: Competitive Landscape
· Shire plc (Takeda Pharmaceutical Company Limited), CSL Limited, and Pharming Group NV are leading players in the global hereditary angioedema treatment market.
· The global hereditary angioedema treatment market is projected to be driven by novel therapies and pipeline products during the forecast period. These pipeline products are in various clinical trial phases, and are expected to be launched in the next few years.
· New product approvals for specific indications, robust R&D expenditure & pipeline products, and mergers & acquisitions are the key strategies adopted by major players in the global hereditary angioedema treatment market
· Ionis Pharmaceuticals, Inc., BioCryst Pharmaceuticals, Inc., KalVista Pharmaceuticals Ltd., and Attune Pharmaceuticals have several different products in the pipeline for the treatment of HAE.
More Trending Reports by Transparency Market Research:
https://www.prnewswire.com/news-releases/rising-number-of-sports-injuries-and-consequent-surgeries-to-help-medical-dynamometer-market-to-reach-us984-6-mn-valuation-by-2026--finds-tmr-301187230.html
https://www.prnewswire.com/news-releases/care-delivery-models-in-telemedicine-market-aim-at-reducing-patient-burden-market-to-clock-robust-cagr-of-14-0-from-2019-to-2027-tmr-301190294.html
About Us
Transparency Market Research (TMR) is a global market intelligence company providing business information reports and services. The company’s exclusive blend of quantitative forecasting and trend analysis provides forward-looking insights to thousands of decision makers. TMR’s experienced team of analysts, researchers, and consultants use proprietary data sources and various tools and techniques to gather and analyse information.
TMR’s data repository is continuously updated and revised by a team of research experts so that it always reflects the latest trends and information. With extensive research and analysis capabilities, Transparency Market Research employs rigorous primary and secondary research techniques to develop distinctive data sets and research material for business reports.
Contact
Transparency Market Research 90 State Street, Suite 700 Albany, NY 12207 Tel: +1-518-618-1030 USA - Canada Toll Free: 866-552-3453 Website: https://www.transparencymarketresearch.com/
#hereditary angioedema treatment drugs#hereditary angioedema treatment#symptoms of hereditary angioedema#hereditary angioedema diagnosis
0 notes