#Hereditary Angioedema Therapeutic Market 2019
Explore tagged Tumblr posts
Text
Hereditary Angioedema Therapeutic Market Size Is Expected To Reach USD 4.2 Billion By 2026: Grand View Research Inc.
Hereditary Angioedema Therapeutic Market Size Is Expected To Reach USD 4.2 Billion By 2026: Grand View Research Inc.

San Francisco, 28 February 2020: The Report Hereditary Angioedema Therapeutic Market Analysis Report By Drug Class (Bradykinin B2 Receptor Antagonist, C1-Esterase Inhibitor), By Route of Administration, By Treatment Type, And Segment Forecasts, 2019 – 2026
The global hereditary angioedema therapeutic market size is expected to reach USD 4.2 billion by 2026, according to a new report by Grand…
View On WordPress
#Hereditary Angioedema Therapeutic Industry#Hereditary Angioedema Therapeutic Market#Hereditary Angioedema Therapeutic Market 2019#Hereditary Angioedema Therapeutic Market 2026#Hereditary Angioedema Therapeutic Market Revenue#Hereditary Angioedema Therapeutic Market Share#Hereditary Angioedema Therapeutic Market Size
0 notes
Text
The next generation complement therapeutics market is anticipated to grow at an annualized rate of >25% for drugs in advanced development stages
The approval and success of Soliris® and other complement therapeutics has paved for the development of several drugs targeting different pathways of complement system for the treatment of a wide range of disease conditions
London
Roots Analysis has announced the addition of “Next Generation Complement Therapeutics market, 2022-2035” report to its list of offerings.
The potential of complement therapeutics to ease the treatment of complement-associated diseases has greatly expedited the progress of these therapeutics into clinical stages of development. Since the complement system is a complex and multidimensional system, many different therapeutic targets are likely to be discovered for a variety of disease indications, such as paroxysmal nocturnal hemoglobinuria, hereditary angioedema, renal diseases and several others, in the future. The main focus is to develop innovative therapies targeting proteins other than C5, with reduced cost and increased safety.
To order this 190+ page report, which features 200+ figures and 30+ tables, please visit https://www.rootsanalysis.com/reports/next-generation-complement-therapeutics-market.html
Key Market Insights
Presently, 190+ complement therapeutics have been marketed / are being evaluated across different phases of development for the treatment of various disease indications
Close to 80% of the pipeline candidates are under clinical evaluation. Further, therapeutics, namely Berinert®, Cinryze®, ORLADEYO®, Ruconest® and TAKHZYRO®, have been approved for the treatment of Hereditary Angioedema. Majority (84%) of these candidates are biologics, followed by small molecules (16%).
Around 50 industry and non-industry players are engaged in the development of next generation complement therapy
North America has emerged as the hub, featuring the presence of approximately 50% developers. The market is currently dominated by the presence of mid-sized players (37% of the total number of stakeholders). Interestingly, 15% of the players are big pharma companies engaged in the development of such therapeutics for multiple therapeutic areas.
More than 850 clinical trials have been registered for the evaluation of next generation complement therapeutics, worldwide
The clinical research activity, in terms of number of trials registered, is reported to have increased at a CAGR of 21%, during the period 2017-2021 (till October). Of the total number of trials registered, close to 60% have already been completed, while 24% of the studies are actively recruiting participants.
Close to 1,450 articles related to next generation complement therapeutics, have been published in reputed scientific journals, since 2017
More than 40% of the articles focused on next generation complement therapeutics were published post-2019. Popular journals that have published multiple articles include Frontiers in Immunology, Allergy & Asthma Proceedings, Journal of Immunology and Molecular Immunology.
Close to 1,250 grants have been awarded to support research on next generation complement therapeutics, since 2017
Grants worth USD 835 million have been awarded to various companies / organizations working in this domain during 2017-2021. Around 20% of the grants were funded by the National Cancer Institute.
Over 3,490 patents have been filed / granted related to next generation complement therapeutics, since 2016
More than 650 patents were filed / granted in 2021 (till November). Further, around 45% of the patents were filed in Europe It is worth highlighting that majority of the patents were granted / filed for indications, including angioedema, meningitis, microangiopathy, neuromyelitis optica, periodontitis and psoriasis.
Partnership activity in this field has increased at a CAGR of nearly 55%, between 2017 and 2021 (till November)
More than 45% of the reported deals were product development and commercialization agreements. Further, majority of the agreements were signed by players based in North America (66%).
Close to USD 14 Billion has been invested by both public and private investors for the development of next generation complement therapeutics
Around 40% of the companies engaged in this domain primarily received funding through secondary offerings. Further, 70% of the funding instances were reported by players headquartered in North America.
North America is anticipated to capture over ~80% of the global market share for phase III complement therapeutics, by 2035
In 2035, close to XX% of the market revenues are expected to be generated from sales of therapeutics intended for infectious diseases. Further, therapies designed for subcutaneous route of administration are expected to occupy a larger share (XX%) of the overall market in 2035.
To request a sample copy / brochure of this report, please visit https://www.rootsanalysis.com/reports/next-generation-complement-therapeutics-market.html
Key Questions Answered
§ Who are the leading industry and non-industry players engaged in the development of next generation complement therapeutics?
§ Which are the key drugs being developed across early and late stages of development?
§ Which geographies are the most active in conducting clinical trials related to next generation complement therapeutics?
§ What is the focus area of various publications related to the next generation complement therapeutics?
§ Which are the leading funding institutes / centers supporting the research related to next generation complement therapeutics?
§ What kind of partnership models are commonly adopted by industry stakeholders?
§ Who are the key investors, active in the field of next generation complement therapeutics?
§ What are the different initiatives undertaken by big pharma players for the development of next generation complement therapeutics in the recent past?
§ How is the current and future market opportunity, related to next generation complement therapeutics likely to be distributed across key market segments?
The financial opportunity within the next generation complement therapeutics market has been analyzed across the following segments:
§ Target Disease Indication
§ Atypical Hemolytic Uremic Syndrome
§ Cardiac Transplantation Rejection
§ Cold Agglutinin Disease
§ COVID-19
§ Generalized Myasthenia Gravis
§ Guillain-Barre Syndrome
§ Hereditary Angioedema
§ Neuromyelitis Optica
§ Paroxysmal Nocturnal Hemoglobinuria
§ Therapeutic Area
§ Cardiovascular Disorders
§ Genetic Disorders
§ Hematological and Vascular Disorders
§ Infectious Diseases
§ Neurological Disorders
§ Neuromuscular Disorders
§ Type of Molecule
§ Biologic
§ Small Molecule
§ Target Pathway
§ Alternate Pathway
§ Classical Pathway
§ Lectin Pathway
§ Terminal Pathway
§ Type of Therapy
§ Monotherapy
§ Combination Therapy
§ Route of Administration
§ Intravenous
§ Oral
§ Subcutaneous
§ Key Geographical Regions
§ North America
§ Europe
§ Asia-Pacific
The research also includes detailed profiles of the key players (listed below) engaged in the development of next generation complement therapeutics; each profile features an overview of the company, its financial information (if available), details on product portfolio, recent developments and an informed future outlook.
§ Amgen
§ CSL Behring
§ Innovent Biologics
§ Novartis
§ Regeneron
§ Roche
§ Sanofi
§ Takeda
§ UCB
For additional details, please visit
https://www.rootsanalysis.com/reports/next-generation-complement-therapeutics-market.html
or email [email protected]
You may also be interested in the following titles:
1. Gene Editing beyond CRISPR Market, 2022-2035
2. Myeloid Cells Targeting Therapeutics Market, 2021-2035
3. Thermostable Vaccines and Thermostable Biologics Market, 2021-2035
Contact Information
Roots Analysis Private Limited
Ben Johnson
+1 (415) 800 3415
#Next Generation Complement Therapeutics market#Next Generation#Complement Therapeutics market#Therapeutics market#RootsAnalysis
0 notes
Text
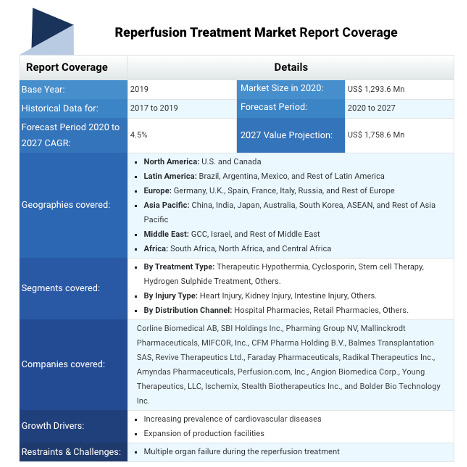
REPERFUSION TREATMENT MARKET ANALYSIS(2020-2027)
Reperfusion Treatment Market, by Treatment Type (Therapeutic Hypothermia, Cyclosporin, Stem Cell Therapy, Hydrogen Sulphide Treatment, and Others), by Injury Type (Heart Injury, Kidney Injury, Intestine Injury, and Others), by Distribution Channel (Hospital Pharmacies, Retail Pharmacies, and Others), and by Region (North America, Latin America, Europe, Asia Pacific, Middle East, and Africa) - Size, Share, Outlook, and Opportunity Analysis, 2020 - 2027
Reperfusion injury is caused due to the damage in the tissue, which occurs due to the lack of blood supply. Examples of reperfusion injury include brain damage after stroke and many others, where reperfusion therapy leads to flow of blood in the tissue which results in inflammation and oxidative damage due to oxidative stress. Reperfusion injury can be treated by therapeutic hypothermia, hydrogen sulphide treatment, cyclosporins, stem cell therapy, and others. Furthermore, delay in reperfusion therapy results in oxidative damage.
Global Reperfusion Treatment Market – Impact of Coronavirus (COVID – 19) Pandemic:
The COVID-19 pandemic is expected to hamper the global reperfusion treatment market growth during the forecast period. The COVID-19 pandemic and resulting lockdowns in various countries across the globe have impacted the financial status of businesses in all sectors. The private healthcare sector has been impacted majorly due to the COVID-19 pandemic. Many clinical trials have been suspended during the pandemic. In order to restart the clinical trials, the U.S. Food and Drug Administration (FDA) released guidelines during the COVID-19 public health emergency in March 2020. The guidelines were further updated on July 02, 2020. The guidelines include general considerations to assist sponsors and researchers, which ensure the safety of trial participants, and compliance with good clinical practice (GCP) for the duration of the COVID-19 public health emergency. The appendix of the guidelines also provide answers to some general questions, which the U.S. Food and Drug Administration (FDA) received from various sponsors and researchers about conducting clinical trials during the COVID-19 public health emergency. The above guidelines are also applicable for conducting the clinical trials for testing the safety and efficacy of the drugs for the reperfusion injury. Thus, the COVID – 19 pandemic is expected to decrease the growth of the reperfusion treatment market over the forecast period.
The global reperfusion treatment market is estimated to be valued at US$ 1,293.6 million in 2020 and is expected to exhibit a CAGR of 4.5% during the forecast period (2020-2027).
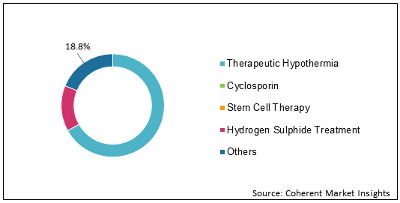
Figure 1: Global Reperfusion Treatment Market Share (%) Analysis, By Treatment Type 2020
Increasing prevalence of coronary heart dis ease is expected to drive the growth of the global reperfusion treatment market during the forecast period.
The rising incidence of coronary artery disease (CAD) or ischemic heart disease (IHD) is a major factor which is expected to drive the market growth. The CAD or IHD is caused due to the buildup of cholesterol and fatty deposits on the inner walls of the arteries, which may lead to the reduction of blood flow to the heart cells. This condition may lead to ischemia, myocardial infraction or sudden cardiac arrest. Moreover, medicines approved from the regulatory authorities are not available in the market for the treatment of ischemia/reperfusion injury. According to the National Center for Biotechnology Information (NCBI), 2020, in 2017, globally, around 126 million people suffered from ischemic heart disease (1,655 per 100,000), which constituted to 1.72% of the total world population.
Investments and expansion of production facility by market players are expected to boost growth of the global reperfusion treatment market during the forecast period.
Market players are focusing on facility expansions in order to strengthen their product portfolio. For instance, on March 9, 2020, Pharming Group NV received the Food and Drug Administration (U.S. FDA) approval for its new production facility in the Netherlands for the production of the starting material required for manufacturing of RUCONEST. RUCONEST is a C1-esterase inhibitor, which is plasma free and is proven to help treat hereditary angioedema (HAE) attacks. Furthermore, on January 21, 2020, Pharming Group NV received the European Medicines Agency (EMA) approval for the production facility for RUCONEST in Europe.
Global Reperfusion Treatment Market – Restraints:
There are some side effects associated with the treatment, which are expected to restrain the global reperfusion treatment market during the forecast period. Ischemia reperfusion causes the mediator to infiltrate other tissues, which leads to Multiple Organ Dysfunction Syndrome (MODS). For instance, according to an article published in the International Institute of Anticancer Research in 2019, Multiple Organ Dysfunction Syndrome (MODS) was the leading cause of mortality globally and the incidence of MODS ranged from 25-40%. Furthermore, according to the Critical Care Nephrology Journal 2019, the pediatric multiple organ dysfunction syndrome (MODS) epidemiology ranges from 10% to 50% of the children admitted to the pediatric intensive care unit.
Global Reperfusion Treatment Market – Regional Analysis:
On the basis of region, the global reperfusion treatment market is segmented into North America, Latin America, Europe, Asia Pacific, Middle East, and Africa.
North America is expected to dominate the global reperfusion treatment market during the forecast period owing to research and development in the region. For instance, in November 2019, Faraday Pharmaceuticals announced positive results from phase II clinical trials of FDY-5301 for ischemia reperfusion injury treatment, following a STEMI heart attack. FDY-5301 is a formulated, patented, elemental reducing agent that contains sodium iodide. It destroys the hydrogen peroxide that is naturally generated as a response to acute ischemia reperfusion injury and also contributes to loss of muscle function and mass.
Europe is an emerging reperfusion treatment market owing to the funding provided for research and development by regulatory authorities. For instance, in February 2019, Balmes Transplantation SAS received around US$ 605,597 million from the European Regional Development Fund (ERDF) for its research program REMEDIRA for developing combinations of repurposed drugs against kidney ischemia-reperfusion injury (IRI).
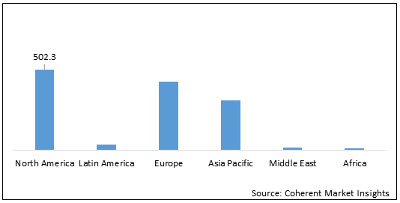
Figure 2: Global Reperfusion Treatment Market Value (US$ Mn), by Region, 2020
Global Reperfusion Treatment Market - Competitive Landscape:
Some of the key players operating in the global reperfusion treatment market are Corline Biomedical AB, SBI Holdings Inc., Pharming Group NV, Mallinckrodt Pharmaceuticals, MIFCOR, Inc., CFM Pharma Holding B.V., Balmes Transplantation SAS, Revive Therapeutics Ltd., Faraday Pharmaceuticals, Radikal Therapeutics Inc., Amyndas Pharmaceuticals, Perfusion.com, Inc., Angion Biomedica Corp., Young Therapeutics, LLC, Ischemix, Stealth Biotherapeutics Inc., and Bolder Bio Technology Inc.
Request sample report here:
https://www.coherentmarketinsights.com/insight/request-sample/4248
Download PDF brochure here:
https://www.coherentmarketinsights.com/insight/request-pdf/4248
About Us:
Coherent Market Insights is a global market intelligence and consulting organization focused on assisting our plethora of clients achieve transformational growth by helping them make critical business decisions.
What we provide:
• Customized Market Research Services
• Industry Analysis Services
• Business Consulting Services
• Market Intelligence Services
• Long term Engagement Model
• Country Specific Analysis
Contact Us:
Mr. Shah
Coherent Market Insights Pvt. Ltd.
Address: 1001 4th ave, #3200 Seattle, WA 98154, U.S.
Phone: +1-206-701-6702
Email: [email protected]
Source: https://www.coherentmarketinsights.com/market-insight/reperfusion-treatment-market-4248
0 notes
Link
0 notes
Text
Preeclampsia Therapeutics Market Segmentation, Size, Covid-19 Impact, Healthcare Sector, Analysis, Insights, Share, Developments, Forecast to 2026
The global preeclampsia therapeutics market is expected to rise with an impressive CAGR and generate the highest revenue by 2026. Fortune Business Insights

in its latest report published this information. The report is titled “Preeclampsia Therapeutics Market Size, Share & Industry Analysis, By Preeclampsia Type (Mild Preeclampsia, Severe Preeclampsia), By Drug Class (Antihypertensive Agents, Anticonvulsants, Antioxidants, Others) and Regional Forecast, 2019-2026”. The report discusses research objectives, research scope, methodology, timeline and challenges during the entire forecast period. It also offers an exclusive insight into various details such as revenues, market share, strategies, growth rate, product & their pricing by region/country for all major companies.
For more information, Get sample pdf @ https://www.fortunebusinessinsights.com/enquiry/request-sample-pdf/preeclampsia-therapeutics-market-101516
The report provides a 360-degree overview of the market, listing various factors restricting, propelling, and obstructing the market in the forecast duration. The report also provides additional information such as interesting insights, key industry developments, detailed segmentation of the market, list of prominent players operating in the market, and other preeclampsia therapeutics market trends. The report is available for sale on the company website.
Leading Players operating in the Preeclampsia Therapeutics Market are:
Key players are involved in mergers and acquisition to strengthen their market position. Owing to increasing competition frequent innovations are taking place in the market. Some of the companies operating the industry are:
Hoffmann-La Roche Ltd.
Sera Prognostics
Pluristem Therapeutics Inc.
DRG INSTRUMENTS GmbH
AMAG Pharmaceuticals
A1M Pharma AB
PerkinElmer Inc.
VG Lifesciences
Intensive Research and Collaborations to Characterize Market Competition
Most of the key players in the global preeclampsia therapeutics market competition are doubling down their investment in research to come out with new solutions to diagnosing and treating preeclampsia. For instance, PerkinElmer invested in Foetal Medicine Foundation’s Project ASPRE study in June 2017, which found that delivering low-dose aspirin can lead to bring down the rate of pre-term preeclampsia by 62%. Some companies are also entering into strategic partnerships with other players to diversify their product offerings. For example, in September 2018, AMAG Pharmaceuticals and Velo Bio joined hands to develop and market a polyclonal antibody, digoxin immune Fab (ovine) (DIF), to treat severe preeclampsia in pregnant women.
for more information @ https://www.fortunebusinessinsights.com/industry-reports/preeclampsia-therapeutics-market-101516
Regional Analysis for Preeclampsia Therapeutics Market:
North America (the USA and Canada)
Europe (UK, Germany, France, Italy, Spain, Scandinavia and Rest of Europe)
Asia Pacific (Japan, China, India, Australia, Southeast Asia and Rest of Asia Pacific)
Latin America (Brazil, Mexico and Rest of Latin America)
Middle East & Africa (South Africa, GCC and Rest of the Middle East & Africa)
Major Table of Contents for Preeclampsia Therapeutics Market:
Introduction
Executive Summary
Market Dynamics
Key Preeclampsia Therapeutics Market Insights
Global Market Analysis, Insights and Forecast, 2015-2026
North America Market Analysis, Insights and Forecast, 2015-2026
Europe Market Analysis, Insights and Forecast, 2015-2026
Asia Pacific Market Analysis, Insights and Forecast, 2015-2026
The Middle East and Africa Market Analysis, Insights and Forecast, 2015-2026
Latin America Market Analysis, Insights and Forecast, 2015-2026
Competitive Landscape
Global Preeclampsia Therapeutics Market Revenue Share Analysis, By Key Players, 2020
Company Profiles
Conclusion
Other Exclusive Reports:
Telehealth Market
Microfluidic Devices Market
Microfluidic Devices Market
Microfluidic Devices Market
Contraceptive Drugs Market
Hereditary Angioedema Treatment Market
Dialyzers Market
Alpha-1 antitrypsin deficiency Augmentation Therapy Market
About Us: Fortune Business Insights

offers expert corporate analysis and accurate data, helping organizations of all sizes make timely decisions. Our reports contain a unique mix of tangible insights and qualitative analysis to help companies achieve sustainable growth. Our team of experienced analysts and consultants use industry-leading research tools and techniques to compile comprehensive market studies, interspersed with relevant data.
Contact: Name: Ashwin Arora Email: [email protected] Phone: US +1 424 253 0390 / UK +44 2071 939123 / APAC: +91 744 740 1245
from NeighborWebSJ https://ift.tt/3vLOMTv via IFTTT
from WordPress https://ift.tt/3vQaXYQ via IFTTT
0 notes
Text
Global Orphan Drug Market 2019 Worldwide Robust Expansion by Top Key Manufactures, Overview, Size, Share, Trends, Segments, Demand and Forecast to 2025
Global orphan drug market is rise gradually to an estimated value of USD 326.25 billion by 2026 registering a CAGR of 8.5% in the forecast period of 2019-2026. Growing number of orphan diseases, accelerating novel therapies and orphan drug development programs and exclusive incentives from the government are the key factors for market growth.
Get Sample Copy Of This Report @ https://www.databridgemarketresearch.com/request-a-sample/?dbmr=global-orphan-drugs-market
Few of the major competitors currently working in the global orphan disease market are F. Hoffmann-La Roche Ltd., Novartis AG, Pfizer Inc., AbbVie, AstraZeneca, Baxter, Eli Lilly and Company, Johnson & Johnson Services Inc., Merck KGaA, Novo Nordisk A/S, Bristol-Myers Squibb Company, Alexion Pharmaceuticals, Kyowa Hakko Kirin Co., Ltd., Amgen Inc., Biogen, Celldex Therapeutics, GlaxoSmithKline plc., Eisai Co., Ltd., Takeda Pharmaceutical Company Limited, Vertex Pharmaceuticals Incorporated (US) and among others.
Key Developments in the Market:
In August 2018, Kyowa Hakko Kirin Co., Ltd received the FDA approval for Poteligeo (mogamulizumab-kpkc) injection, an Orphan Drug, for the treatment of adult patients with relapsed or refractory mycosis fungoides or Sezary syndrome which are the types of non-Hodgkin lymphoma
In August 2018, Takeda Pharmaceutical Company Limited received FDA approval for Takhzyro (lanadelumab-flyo) injection for the treatment to hereditary angioedema (HAE) attack in patients 12 years of age and older
In June, 2018, Ascendis Pharma A/S received the FDA Orphan Drug Designation for TransCon PTH for the treatment of hypoparathyroidism. TransCon PTH is a long acting parathyroid hormone replacement therapy which helps in restoring the activity of parathyroid hormone
Competitive Analysis:
Global orphan drug market is highly fragmented and the major players have used various strategies such as new product launches, expansions, agreements, joint ventures, partnerships, acquisitions, and others to increase their footprints in this market. The report includes market shares of orphan drug market for Global, Europe, North America, Asia-Pacific, South America and Middle East & Africa.
Inquiry For Customize Report With Discount at : https://www.databridgemarketresearch.com/inquire-before-buying/?dbmr=global-orphan-drugs-market
Segmentation: Global Orphan Drug Market
By Disease Type
(Oncology disease, Metabolic Disease, Hepatology, Immunology, Infection, Neurology and Others),
Drug Type
(Biological, Non-Biological, and Others),
Indication Type
(Non-Hodgkin Lymphoma, Acute Myeloid Leukemia, Cystic Fibrosis, Glioma, Pancreatic Cancer, Ovarian Cancer, Multiple Myeloma, Duchenne Muscular Dystrophy, Renal Cell Carcinoma and Others),
Drug class
(Lenalidomide, Rituximab, Glatiramer Acetate, Nivolumab, Interferon Beta-1a, Ibrutinib, Cinacalcet Hydrochloride, Imatinib Mesylate, Bortezomib, Sodium Oxybate and Others),
Therapy Type
(Medication, Surgery and Others),
Route of Administration
(Oral, Intravenous and Others),
End- users
(Hospitals, Homecare, Specialty Clinics, Others),
Geography
(North America, South America, Europe, Asia-Pacific, Middle East and Africa)
Get Full Table Of content @ https://www.databridgemarketresearch.com/toc/?dbmr=global-orphan-drugs-market
Contact:
Data Bridge Market Research Tel: +1-888-387-2818 Email: [email protected]
0 notes
Text
Discover the Global Hereditary Angioedema Therapeutic Market gain impetus due to the growing demand over 2026
With a systematic problem analysis, model building and fact-finding, Hereditary Angioedema Therapeutic Market report helps businesses in decision-making and managing marketing of goods and services. The data and the information regarding Pharmaceutical industry are taken from reliable sources such as websites, annual reports of the companies, and journals etc. and were checked and validated by the market experts. Hereditary Angioedema Therapeutic Market report provides statistics on the current state of the industry as a valuable source of guidance and direction for companies and investors interested in this market. This is the most relevant, unique, fair and creditable global market research report which suits your business needs.
Get Sample Analysis of Global Market Information: https://www.databridgemarketresearch.com/request-a-sample/?dbmr=global-hereditary-angioedema-therapeutic-market
Market Analysis: Global Hereditary Angioedema Therapeutic Market
Global hereditary angioedema therapeutic market is expected to rise to an estimated value of USD 4.51 billion by 2026, registering a healthy CAGR in the forecast period of 2019-2026. This rise in market value can be attributed to the increased awareness about hereditary angioedema in various regions.
Major Market Competitors/Players
Few of the major competitors currently working in the global hereditary angioedema therapeutic market are Takeda Pharmaceutical Company Limited; CSL Limited; BIOCRYST PHARMACEUTICALS, INC.; Pharming Group N.V.; Ionis Pharmaceuticals; Novartis AG; CENTOGENE AG; Sanofi; KalVista Pharmaceuticals among others.
Market Definition: Global Hereditary Angioedema Therapeutic Market
Hereditary angioedema is a rare genetic disorder. The common symptoms of the disease are repeated incidents of severe swelling limbs, intestinal tract, face, and airway. This disease is categorized into three categories: Type I HAE, Type II HAE, Type III HAE. As per NIH Organization report, hereditary angioedema affects almost 1 in 50,000 people. The prevalence rate of Type I is more, which represents almost 85% of cases, the prevalence of Type II is 15% of, and the incidence of Type III is very rare.
Market Drivers:
Favourable reimbursement policies is expected to drive the growth of the market
Increasing cases of the hereditary angioedema in various regions globally is expected to drive the growth of the market
Increasing awareness about diagnosis and treatment of hereditary angioedema is also expected to boost the growth of the market
Increasing focus on developing novel therapeutics is expected to drive the growth of the market
Market Restraints:
Stringent regulatory policies is expected to restrict the growth of the market
High price of medicines, which is restricting the overall adoption of these meters
Misdiagnosis of the disease; this factor is expected to restrict the growth of the market
Segmentation: Global Hereditary Angioedema Therapeutic Market
By Type
Type I HAE
Type II HAE
Type III HAE
By Drug Class
C-1 Esterase Inhibitors
Bradykynin B2 Receptor Antagonist
Kallikrein Inhibitors
Others
Cinryze
Berinert
Ruconest
By Application
Prophylaxis
Treatment
By Route of Administration
Oral
Injectable
IV
Subcutaneous
By End-User
Home Healthcare
Hospitals
Clinics
Others
By Distribution Channel
Hospital Pharmacy
Retail Pharmacy
Online Pharmacy
By Geography
North America
Europe
Asia-Pacific
South America
Middle East and Africa
U.S.
Canada
Mexico
Germany
Italy
U.K.
France
Spain
Netherlands
Belgium
Switzerland
Turkey
Russia
Rest of Europe
Japan
China
India
South Korea
Australia
Singapore
Malaysia
Thailand
Indonesia
Philippines
Rest of Asia-Pacific
Brazil
Rest of South America
Saudi Arabia
Rest of Middle East and Africa
Get TOC of Full Report: https://www.databridgemarketresearch.com/toc/?dbmr=global-hereditary-angioedema-therapeutic-market
Key Developments in the Market:
In August 2018, Shire plc (A subsidiary of Takeda Pharmaceutical Company Limited) received FDA approval for its Takhzyro (lanadelumab-flyo). This is a plasma kallikrein inhibitor (monoclonal antibody). This drug is used in the treatment of hereditary angioedema attacks. This will help the company to create a strong product portfolio
In June 2017, CSL Behring received FDA approval for its Haegarda. This is a C1 esterase inhibitor. This is a low-volume subcutaneous (SC) C1-esterase inhibitor (C1-INH) replacement therapy used in the treatment of hereditary angioedema (HAE) attacks. This will help in the expansion of the company’s product portfolio
Competitive Analysis
Global hereditary angioedema therapeutic market is highly fragmented and the major players have used various strategies such as new product launches, expansions, agreements, joint ventures, partnerships, acquisitions, and others to increase their footprints in this market. The report includes market shares of hereditary angioedema therapeutic market for global, Europe, North America, Asia-Pacific, South America and Middle East & Africa.
0 notes
Link
Global hereditary angioedema therapeutic market is expected to rise to an estimated value of USD 4.51 billion by 2026, registering a healthy CAGR in the forecast period of 2019-2026. This rise in market value can be attributed to the increased awareness about hereditary angioedema in various regions.
0 notes
Link
Global hereditary angioedema therapeutic market is expected to rise to an estimated value of USD 4.51 billion by 2026, registering a healthy CAGR in the forecast period of 2019-2026.
0 notes
Text
Global Hereditary Angioedema Therapeutic Market Insights Report 2019 – 2026
Market Analysis: Global Hereditary Angioedema Therapeutic Market
Global hereditary angioedema therapeutic market is expected to rise to an estimated value of USD 4.51 billion by 2026, registering a healthy CAGR in the forecast period of 2019-2026. This rise in market value can be attributed to the increased awareness about hereditary angioedema in various regions.
Market Definition: Global Hereditary Angioedema Therapeutic Market
Hereditary angioedema is a rare genetic disorder. The common symptoms of the disease are repeated incidents of severe swelling limbs, intestinal tract, face, and airway. This disease is categorized into three categories: Type I HAE, Type II HAE, Type III HAE. As per NIH Organization report, hereditary angioedema affects almost 1 in 50,000 people. The prevalence rate of Type I is more, which represents almost 85% of cases, the prevalence of Type II is 15% of, and the incidence of Type III is very rare.
Market Drivers:
Favourable reimbursement policies is expected to drive the growth of the market
Increasing cases of the hereditary angioedema in various regions globally is expected to drive the growth of the market
Increasing awareness about diagnosis and treatment of hereditary angioedema is also expected to boost the growth of the market
Increasing focus on developing novel therapeutics is expected to drive the growth of the market
Market Restraints:
Stringent regulatory policies is expected to restrict the growth of the market
High price of medicines, which is restricting the overall adoption of these meters
Misdiagnosis of the disease; this factor is expected to restrict the growth of the market
Get Sample Analysis of Global Market Information: https://www.databridgemarketresearch.com/request-a-sample/?dbmr=global-hereditary-angioedema-therapeutic-market
Segmentation: Global Hereditary Angioedema Therapeutic Market
By Type
Type I HAE
Type II HAE
Type III HAE
By Drug Class
C-1 Esterase Inhibitors
Bradykynin B2 Receptor Antagonist
Kallikrein Inhibitors
Others
Cinryze
Berinert
Ruconest
By Application
Prophylaxis
Treatment
By Route of Administration
Oral
Injectable
IV
Subcutaneous
By End-User
Home Healthcare
Hospitals
Clinics
Others
By Distribution Channel
Hospital Pharmacy
Retail Pharmacy
Online Pharmacy
By Geography
North America
Europe
Asia-Pacific
South America
Middle East and Africa
U.S.
Canada
Mexico
Germany
Italy
U.K.
France
Spain
Netherlands
Belgium
Switzerland
Turkey
Russia
Rest of Europe
Japan
China
India
South Korea
Australia
Singapore
Malaysia
Thailand
Indonesia
Philippines
Rest of Asia-Pacific
Brazil
Rest of South America
Saudi Arabia
Rest of Middle East and Africa
Get TOC of Full Report: https://www.databridgemarketresearch.com/toc/?dbmr=global-hereditary-angioedema-therapeutic-market
Key Developments in the Market:
In August 2018, Shire plc (A subsidiary of Takeda Pharmaceutical Company Limited) received FDA approval for its Takhzyro (lanadelumab-flyo). This is a plasma kallikrein inhibitor (monoclonal antibody). This drug is used in the treatment of hereditary angioedema attacks. This will help the company to create a strong product portfolio
In June 2017, CSL Behring received FDA approval for its Haegarda. This is a C1 esterase inhibitor. This is a low-volume subcutaneous (SC) C1-esterase inhibitor (C1-INH) replacement therapy used in the treatment of hereditary angioedema (HAE) attacks. This will help in the expansion of the company’s product portfolio
Competitive Analysis
Global hereditary angioedema therapeutic market is highly fragmented and the major players have used various strategies such as new product launches, expansions, agreements, joint ventures, partnerships, acquisitions, and others to increase their footprints in this market. The report includes market shares of hereditary angioedema therapeutic market for global, Europe, North America, Asia-Pacific, South America and Middle East & Africa.
Major Market Competitors/Players
Few of the major competitors currently working in the global hereditary angioedema therapeutic market are Takeda Pharmaceutical Company Limited; CSL Limited; BIOCRYST PHARMACEUTICALS, INC.; Pharming Group N.V.; Ionis Pharmaceuticals; Novartis AG; CENTOGENE AG; Sanofi; KalVista Pharmaceuticals among others.
0 notes