#Global Hereditary Angioedema Therapeutic Market
Explore tagged Tumblr posts
Text
Hereditary Angioedema Therapeutics Market To Reach $5.8Bn By 2030
The global hereditary angioedema therapeutic market size is expected to reach USD 5.8 billion by 2030, according to a new report by Grand View Research, Inc., exhibiting a 7.4% CAGR from 2023 to 2030. Growing initiatives by international patient organizations to improve awareness about hereditary angioedema (HAE) among patients and healthcare providers is likely to be a high impact rendering…
1 note
·
View note
Text
Hereditary Angioedema Therapeutics Market Growth Trajectory Through 2024-2033
The Hereditary Angioedema Therapeutics Global Market Report 2024 by The Business Research Company provides market overview across 60+ geographies in the seven regions - Asia-Pacific, Western Europe, Eastern Europe, North America, South America, the Middle East, and Africa, encompassing 27 major global industries. The report presents a comprehensive analysis over a ten-year historic period (2010-2021) and extends its insights into a ten-year forecast period (2023-2033). Learn More On The Hereditary Angioedema Therapeutics Market: https://www.thebusinessresearchcompany.com/report/hereditary-angioedema-therapeutics-global-market-report According to The Business Research Company’s Hereditary Angioedema Therapeutics Global Market Report 2024, The hereditary angioedema therapeutics market size has grown rapidly in recent years. It will grow from $5.74 billion in 2023 to $6.76 billion in 2024 at a compound annual growth rate (CAGR) of 17.6%. The hereditary angioedema therapeutics market size is expected to see rapid growth in the next few years. It will grow to $13.47 billion in 2028 at a compound annual growth rate (CAGR) of 18.8%. The growth in the forecast period can be attributed to advancements in targeted therapies, global market expansion, gene therapy developments, personalized medicine trends, collaboration in research and treatment.. The rising prevalence of hereditary angioedema is expected to propel the growth of the hereditary angioedema market going forward. Hereditary angioedema (HAE) is a rare genetic disorder characterized by recurrent episodes of severe swelling of the skin and mucous membranes. The swelling is caused by excess fluid build-up (EDEMA) and can occur anywhere in the body, including the hands and feet, face, intestines, and airways. Get A Free Sample Of The Report (Includes Graphs And Tables): https://www.thebusinessresearchcompany.com/sample.aspx?id=10810&type=smp The hereditary angioedema therapeutics market covered in this report is segmented – 1) By Drug Class: C1 Esterase Inhibitor, Selective Bradykinin B2 Receptor Antagonist, Kallikrein Inhibitor, Other Drug Classes 2) By Route of Administration: Intravenous, Subcutaneous, Oral 3) By Distribution Channel: Hospital Pharmacy, Retail Pharmacy, Other Distribution Channels 4) By Application: Prophylaxis, On-demand Product innovations are a key trend gaining popularity in the hereditary angioedema therapeutics market. Major companies operating in the hereditary angioedema therapeutics market are developing innovative products such as ligand-conjugated (LICA) investigational antisense medicine and gene therapy to sustain their position in the market. The hereditary angioedema therapeutics market report table of contents includes: 1. Executive Summary 2. Market Characteristics 3. Market Trends And Strategies 4. Impact Of COVID-19 5. Market Size And Growth 6. Segmentation 7. Regional And Country Analysis . . . 27. Competitive Landscape And Company Profiles 28. Key Mergers And Acquisitions 29. Future Outlook and Potential Analysis Contact Us: The Business Research Company Europe: +44 207 1930 708 Asia: +91 88972 63534 Americas: +1 315 623 0293 Email: [email protected] Follow Us On: LinkedIn: https://in.linkedin.com/company/the-business-research-company Twitter: https://twitter.com/tbrc_info Facebook: https://www.facebook.com/TheBusinessResearchCompany YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ Blog: https://blog.tbrc.info/ Healthcare Blog: https://healthcareresearchreports.com/ Global Market Model: https://www.thebusinessresearchcompany.com/global-market-model
0 notes
Text
0 notes
Text
0 notes
Text
Discover the Global Hereditary Angioedema Therapeutic Market gain impetus due to the growing demand over 2026
With a systematic problem analysis, model building and fact-finding, Hereditary Angioedema Therapeutic Market report helps businesses in decision-making and managing marketing of goods and services. The data and the information regarding Pharmaceutical industry are taken from reliable sources such as websites, annual reports of the companies, and journals etc. and were checked and validated by the market experts. Hereditary Angioedema Therapeutic Market report provides statistics on the current state of the industry as a valuable source of guidance and direction for companies and investors interested in this market. This is the most relevant, unique, fair and creditable global market research report which suits your business needs.
Get Sample Analysis of Global Market Information: https://www.databridgemarketresearch.com/request-a-sample/?dbmr=global-hereditary-angioedema-therapeutic-market
Market Analysis: Global Hereditary Angioedema Therapeutic Market
Global hereditary angioedema therapeutic market is expected to rise to an estimated value of USD 4.51 billion by 2026, registering a healthy CAGR in the forecast period of 2019-2026. This rise in market value can be attributed to the increased awareness about hereditary angioedema in various regions.
Major Market Competitors/Players
Few of the major competitors currently working in the global hereditary angioedema therapeutic market are Takeda Pharmaceutical Company Limited; CSL Limited; BIOCRYST PHARMACEUTICALS, INC.; Pharming Group N.V.; Ionis Pharmaceuticals; Novartis AG; CENTOGENE AG; Sanofi; KalVista Pharmaceuticals among others.
Market Definition: Global Hereditary Angioedema Therapeutic Market
Hereditary angioedema is a rare genetic disorder. The common symptoms of the disease are repeated incidents of severe swelling limbs, intestinal tract, face, and airway. This disease is categorized into three categories: Type I HAE, Type II HAE, Type III HAE. As per NIH Organization report, hereditary angioedema affects almost 1 in 50,000 people. The prevalence rate of Type I is more, which represents almost 85% of cases, the prevalence of Type II is 15% of, and the incidence of Type III is very rare.
Market Drivers:
Favourable reimbursement policies is expected to drive the growth of the market
Increasing cases of the hereditary angioedema in various regions globally is expected to drive the growth of the market
Increasing awareness about diagnosis and treatment of hereditary angioedema is also expected to boost the growth of the market
Increasing focus on developing novel therapeutics is expected to drive the growth of the market
Market Restraints:
Stringent regulatory policies is expected to restrict the growth of the market
High price of medicines, which is restricting the overall adoption of these meters
Misdiagnosis of the disease; this factor is expected to restrict the growth of the market
Segmentation: Global Hereditary Angioedema Therapeutic Market
By Type
Type I HAE
Type II HAE
Type III HAE
By Drug Class
C-1 Esterase Inhibitors
Bradykynin B2 Receptor Antagonist
Kallikrein Inhibitors
Others
Cinryze
Berinert
Ruconest
By Application
Prophylaxis
Treatment
By Route of Administration
Oral
Injectable
IV
Subcutaneous
By End-User
Home Healthcare
Hospitals
Clinics
Others
By Distribution Channel
Hospital Pharmacy
Retail Pharmacy
Online Pharmacy
By Geography
North America
Europe
Asia-Pacific
South America
Middle East and Africa
U.S.
Canada
Mexico
Germany
Italy
U.K.
France
Spain
Netherlands
Belgium
Switzerland
Turkey
Russia
Rest of Europe
Japan
China
India
South Korea
Australia
Singapore
Malaysia
Thailand
Indonesia
Philippines
Rest of Asia-Pacific
Brazil
Rest of South America
Saudi Arabia
Rest of Middle East and Africa
Get TOC of Full Report: https://www.databridgemarketresearch.com/toc/?dbmr=global-hereditary-angioedema-therapeutic-market
Key Developments in the Market:
In August 2018, Shire plc (A subsidiary of Takeda Pharmaceutical Company Limited) received FDA approval for its Takhzyro (lanadelumab-flyo). This is a plasma kallikrein inhibitor (monoclonal antibody). This drug is used in the treatment of hereditary angioedema attacks. This will help the company to create a strong product portfolio
In June 2017, CSL Behring received FDA approval for its Haegarda. This is a C1 esterase inhibitor. This is a low-volume subcutaneous (SC) C1-esterase inhibitor (C1-INH) replacement therapy used in the treatment of hereditary angioedema (HAE) attacks. This will help in the expansion of the company’s product portfolio
Competitive Analysis
Global hereditary angioedema therapeutic market is highly fragmented and the major players have used various strategies such as new product launches, expansions, agreements, joint ventures, partnerships, acquisitions, and others to increase their footprints in this market. The report includes market shares of hereditary angioedema therapeutic market for global, Europe, North America, Asia-Pacific, South America and Middle East & Africa.
0 notes
Link
Global hereditary angioedema therapeutic market is expected to rise to an estimated value of USD 4.51 billion by 2026, registering a healthy CAGR in the forecast period of 2019-2026. This rise in market value can be attributed to the increased awareness about hereditary angioedema in various regions.
0 notes
Link
Global hereditary angioedema therapeutic market is expected to rise to an estimated value of USD 4.51 billion by 2026, registering a healthy CAGR in the forecast period of 2019-2026.
0 notes
Text
Hereditary Angioedema Market Incredible Potential Examined in New Research Report
In its recent market research report about the global hereditary angioedema market, research analysts from Transparency Market Research state that it will exhibit a healthy CAGR of 9.10% for the given forecast period of 2017 to 2025. With such a robust rate of growth, the global hereditary angioedema market will reach a new market valuation of US$3.81 bn by the fall of 2025. The initial valuation of the hereditary angioedema market was at US$1.73 bn recorded in 2016.
Request a PDF Brochure - https://www.transparencymarketresearch.com/sample/sample.php?flag=B&rep_id=17135
Mergers and Acquisitions to Become Common for Leading Market Players
It is projected that the global market for hereditary angioedema will see a rise of several new and emerging players in the next few years of the projection period. The competitive landscape of the market is a consolidated one because of the presence of only handful of leading players. However, as more number of large enterprises engage in the activities of core research and development of angioedema therapeutics, the market is projected to flourish and expand in the coming few years. It is projected that leading players in the market will strive hard to maintain their market dominance and will deploy several aggressive marketing strategies. Since the global hereditary angioedema market is closely associated with the pharmaceutical and medical industries, the leading companies in the market are projected to form strategic partnerships and collaborations with the pharmacies, distributors, and other medical & healthcare bodies. In addition to this, mergers and takeovers are expected to becoming increasingly common as established players will go all out for maintaining their brand value in the global hereditary angioedema market.
Request for Analysis of COVID19 Impact on Hereditary Angioedema Market- https://www.transparencymarketresearch.com/sample/sample.php?flag=covid19&rep_id=17135
Abysmal Effects of Angioderma to Propel Demand
Some of the leading players in the global hereditary angioedema market include names such as BioCryst Pharmaceuticals Inc., Ionis Pharmaceuticals Inc., CSL Limited, Pharming Group NV, and Shire Plc. among others.
Request a Sample of Hereditary Angioedema Market: https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=17135
North America Market to Lead Global Market for Next Few Years
In terms of geographical segments, the region of North America is expected to dominate the global hereditary angioedema market for the aforementioned forecast period. There are several factors that are helping the regional segment to stay as the chief contributor in the global market. One of the primary driving factors for the growth of the regional market has been growing support and initiatives offered by the government for patients suffering from hereditary angioedema. Moreover, favorable policies for medical reimbursement have also helped in extending the reach of the market. Due to the early access of advanced technology, the instances of early detection of hereditary angioedema are high. This too has helped in the development of the regional segment.
More Trending Reports by Transparency Market Research:
https://www.prnewswire.co.uk/news-releases/single-use-cystoscope-market-to-reach-valuation-of-us-150-mn-by-2031-growing-acceptance-of-patient-ready-instruments-in-urology-to-bolster-demand-tmr-study-818277477.html
https://www.prnewswire.co.uk/news-releases/cell-amp-tissue-preservation-market-to-reach-us-10-04-bn-by-2031-increase-in-demand-for-regenerative-therapies-to-trigger-growth-of-the-market-says-tmr-882063013.html
https://www.prnewswire.co.uk/news-releases/fibrinogen-concentrates-market-to-reach-valuation-of-us-700-mn-by-2031-high-volume-clinical-use-in-coagulation-applications-bolsters-market-growth-tmr-873649318.html
About Us Section:
Transparency Market Research is a global market intelligence company, providing global business information reports and services. Our exclusive blend of quantitative forecasting and trends analysis provides forward-looking insight for thousands of decision makers. Our experienced team of Analysts, Researchers, and Consultants, use proprietary data sources and various tools and techniques to gather, and analyse information. Now avail flexible Research Subscriptions, and access Research multi-format through downloadable databooks, infographics, charts, interactive playbook for data visualization and full reports through MarketNgage, the unified market intelligence engine. Sign Up for a 7 day free trial!
Contact Us
Rohit Bhisey Transparency Market Research, 90 State Street, Suite 700, Albany, NY 12207 Tel: +1-518-618-1030 USA – Canada Toll Free: 866-552-3453 Email: [email protected]
Website: https://www.transparencymarketresearch.com/
0 notes
Text
Hereditary Angioedema (HAE) (C1 Esterase Inhibitor [C1-INH] Deficiency) - Global Clinical Trials Review, H1, 2021 published on
https://www.sandlerresearch.org/hereditary-angioedema-hae-c1-esterase-inhibitor-c1-inh-deficiency-global-clinical-trials-review-h1-2021.html
Hereditary Angioedema (HAE) (C1 Esterase Inhibitor [C1-INH] Deficiency) - Global Clinical Trials Review, H1, 2021
Hereditary Angioedema (HAE) (C1 Esterase Inhibitor [C1-INH] Deficiency) – Global Clinical Trials Review, H1, 2021
Summary
GlobalData’s clinical trial report, “Hereditary Angioedema (HAE) (C1 Esterase Inhibitor [C1-INH] Deficiency) – Global Clinical Trials Review, H1, 2021” provides an overview of Hereditary Angioedema (HAE) (C1 Esterase Inhibitor [C1-INH] Deficiency) Clinical trials scenario. This report provides top line data relating to the clinical trials on Hereditary Angioedema (HAE) (C1 Esterase Inhibitor [C1-INH] Deficiency). Report includes an overview of trial numbers and their average enrollment in top countries conducted across the globe. The report offers coverage of disease clinical trials by region, country (G7 & E7), phase, trial status, end points status and sponsor type. Report also provides prominent drugs for in-progress trials (based on number of ongoing trials). GlobalData Clinical Trial Reports are generated using GlobalData’s proprietary database – Pharma – Clinical trials database. Clinical trials are collated from 80+ different clinical trial registries, conferences, journals, news etc across the globe. Clinical trials database undergoes periodic update by dynamic process.
The report enhances the decision making capabilities and helps to create an effective counter strategies to gain competitive advantage.
Note: Certain sections in the report may be removed or altered based on the availability and relevance of data for the indicated disease.
Scope
– The report provides a snapshot of the global clinical trials landscape – Report provides top level data related to the clinical trials by Region, Country (G7 & E7), Trial Status, Trial Phase, Sponsor Type and End point status – The report reviews top companies involved and enlists all trials (Trial title, Phase, and Status) pertaining to the company – The report provides all the unaccomplished trials (Terminated, Suspended and Withdrawn) with reason for unaccomplishment – The Report provides enrollment trends for the past five years – Report provides latest news for the past three months
Note: Certain sections in the report may be removed or altered based on the availability and relevance of data for the indicated disease.
Reasons to Buy
– Assists in formulating key business strategies with regards to investment – Helps in identifying prominent locations for conducting clinical trials which saves time and cost – Provides top level analysis of Global Clinical Trials Market which helps in identifying key business opportunities – Supports understanding of trials count and enrollment trends by country in global therapeutics market – Aids in interpreting the success rates of clinical trials by providing a comparative scenario of completed and uncompleted (terminated, suspended or withdrawn) trials – Facilitates clinical trial assessment of the indication on a global, regional and country level
Note: Certain sections in the report may be removed or altered based on the availability and relevance of data for the indicated disease.
0 notes
Text
0 notes
Text
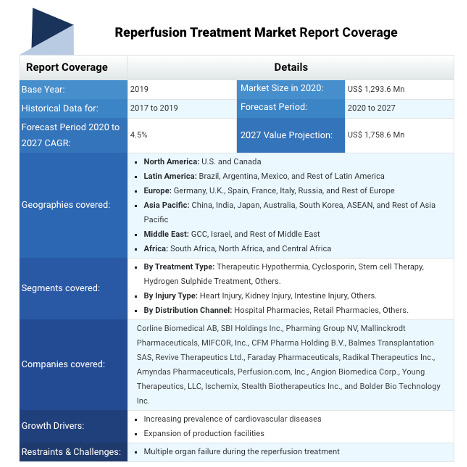
REPERFUSION TREATMENT MARKET ANALYSIS(2020-2027)
Reperfusion Treatment Market, by Treatment Type (Therapeutic Hypothermia, Cyclosporin, Stem Cell Therapy, Hydrogen Sulphide Treatment, and Others), by Injury Type (Heart Injury, Kidney Injury, Intestine Injury, and Others), by Distribution Channel (Hospital Pharmacies, Retail Pharmacies, and Others), and by Region (North America, Latin America, Europe, Asia Pacific, Middle East, and Africa) - Size, Share, Outlook, and Opportunity Analysis, 2020 - 2027
Reperfusion injury is caused due to the damage in the tissue, which occurs due to the lack of blood supply. Examples of reperfusion injury include brain damage after stroke and many others, where reperfusion therapy leads to flow of blood in the tissue which results in inflammation and oxidative damage due to oxidative stress. Reperfusion injury can be treated by therapeutic hypothermia, hydrogen sulphide treatment, cyclosporins, stem cell therapy, and others. Furthermore, delay in reperfusion therapy results in oxidative damage.
Global Reperfusion Treatment Market – Impact of Coronavirus (COVID – 19) Pandemic:
The COVID-19 pandemic is expected to hamper the global reperfusion treatment market growth during the forecast period. The COVID-19 pandemic and resulting lockdowns in various countries across the globe have impacted the financial status of businesses in all sectors. The private healthcare sector has been impacted majorly due to the COVID-19 pandemic. Many clinical trials have been suspended during the pandemic. In order to restart the clinical trials, the U.S. Food and Drug Administration (FDA) released guidelines during the COVID-19 public health emergency in March 2020. The guidelines were further updated on July 02, 2020. The guidelines include general considerations to assist sponsors and researchers, which ensure the safety of trial participants, and compliance with good clinical practice (GCP) for the duration of the COVID-19 public health emergency. The appendix of the guidelines also provide answers to some general questions, which the U.S. Food and Drug Administration (FDA) received from various sponsors and researchers about conducting clinical trials during the COVID-19 public health emergency. The above guidelines are also applicable for conducting the clinical trials for testing the safety and efficacy of the drugs for the reperfusion injury. Thus, the COVID – 19 pandemic is expected to decrease the growth of the reperfusion treatment market over the forecast period.
The global reperfusion treatment market is estimated to be valued at US$ 1,293.6 million in 2020 and is expected to exhibit a CAGR of 4.5% during the forecast period (2020-2027).
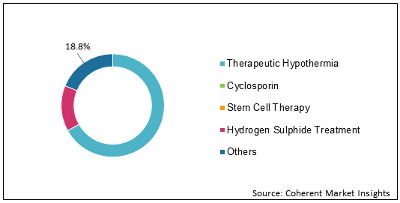
Figure 1: Global Reperfusion Treatment Market Share (%) Analysis, By Treatment Type 2020
Increasing prevalence of coronary heart dis ease is expected to drive the growth of the global reperfusion treatment market during the forecast period.
The rising incidence of coronary artery disease (CAD) or ischemic heart disease (IHD) is a major factor which is expected to drive the market growth. The CAD or IHD is caused due to the buildup of cholesterol and fatty deposits on the inner walls of the arteries, which may lead to the reduction of blood flow to the heart cells. This condition may lead to ischemia, myocardial infraction or sudden cardiac arrest. Moreover, medicines approved from the regulatory authorities are not available in the market for the treatment of ischemia/reperfusion injury. According to the National Center for Biotechnology Information (NCBI), 2020, in 2017, globally, around 126 million people suffered from ischemic heart disease (1,655 per 100,000), which constituted to 1.72% of the total world population.
Investments and expansion of production facility by market players are expected to boost growth of the global reperfusion treatment market during the forecast period.
Market players are focusing on facility expansions in order to strengthen their product portfolio. For instance, on March 9, 2020, Pharming Group NV received the Food and Drug Administration (U.S. FDA) approval for its new production facility in the Netherlands for the production of the starting material required for manufacturing of RUCONEST. RUCONEST is a C1-esterase inhibitor, which is plasma free and is proven to help treat hereditary angioedema (HAE) attacks. Furthermore, on January 21, 2020, Pharming Group NV received the European Medicines Agency (EMA) approval for the production facility for RUCONEST in Europe.
Global Reperfusion Treatment Market – Restraints:
There are some side effects associated with the treatment, which are expected to restrain the global reperfusion treatment market during the forecast period. Ischemia reperfusion causes the mediator to infiltrate other tissues, which leads to Multiple Organ Dysfunction Syndrome (MODS). For instance, according to an article published in the International Institute of Anticancer Research in 2019, Multiple Organ Dysfunction Syndrome (MODS) was the leading cause of mortality globally and the incidence of MODS ranged from 25-40%. Furthermore, according to the Critical Care Nephrology Journal 2019, the pediatric multiple organ dysfunction syndrome (MODS) epidemiology ranges from 10% to 50% of the children admitted to the pediatric intensive care unit.
Global Reperfusion Treatment Market – Regional Analysis:
On the basis of region, the global reperfusion treatment market is segmented into North America, Latin America, Europe, Asia Pacific, Middle East, and Africa.
North America is expected to dominate the global reperfusion treatment market during the forecast period owing to research and development in the region. For instance, in November 2019, Faraday Pharmaceuticals announced positive results from phase II clinical trials of FDY-5301 for ischemia reperfusion injury treatment, following a STEMI heart attack. FDY-5301 is a formulated, patented, elemental reducing agent that contains sodium iodide. It destroys the hydrogen peroxide that is naturally generated as a response to acute ischemia reperfusion injury and also contributes to loss of muscle function and mass.
Europe is an emerging reperfusion treatment market owing to the funding provided for research and development by regulatory authorities. For instance, in February 2019, Balmes Transplantation SAS received around US$ 605,597 million from the European Regional Development Fund (ERDF) for its research program REMEDIRA for developing combinations of repurposed drugs against kidney ischemia-reperfusion injury (IRI).
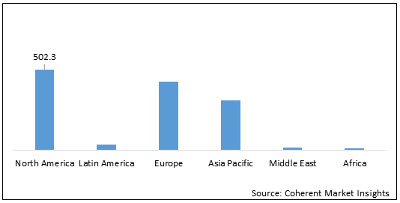
Figure 2: Global Reperfusion Treatment Market Value (US$ Mn), by Region, 2020
Global Reperfusion Treatment Market - Competitive Landscape:
Some of the key players operating in the global reperfusion treatment market are Corline Biomedical AB, SBI Holdings Inc., Pharming Group NV, Mallinckrodt Pharmaceuticals, MIFCOR, Inc., CFM Pharma Holding B.V., Balmes Transplantation SAS, Revive Therapeutics Ltd., Faraday Pharmaceuticals, Radikal Therapeutics Inc., Amyndas Pharmaceuticals, Perfusion.com, Inc., Angion Biomedica Corp., Young Therapeutics, LLC, Ischemix, Stealth Biotherapeutics Inc., and Bolder Bio Technology Inc.
Request sample report here:
https://www.coherentmarketinsights.com/insight/request-sample/4248
Download PDF brochure here:
https://www.coherentmarketinsights.com/insight/request-pdf/4248
About Us:
Coherent Market Insights is a global market intelligence and consulting organization focused on assisting our plethora of clients achieve transformational growth by helping them make critical business decisions.
What we provide:
• Customized Market Research Services
• Industry Analysis Services
• Business Consulting Services
• Market Intelligence Services
• Long term Engagement Model
• Country Specific Analysis
Contact Us:
Mr. Shah
Coherent Market Insights Pvt. Ltd.
Address: 1001 4th ave, #3200 Seattle, WA 98154, U.S.
Phone: +1-206-701-6702
Email: [email protected]
Source: https://www.coherentmarketinsights.com/market-insight/reperfusion-treatment-market-4248
0 notes
Text
Global Hereditary Angioedema Therapeutic Market Insights Report 2019 – 2026
Market Analysis: Global Hereditary Angioedema Therapeutic Market
Global hereditary angioedema therapeutic market is expected to rise to an estimated value of USD 4.51 billion by 2026, registering a healthy CAGR in the forecast period of 2019-2026. This rise in market value can be attributed to the increased awareness about hereditary angioedema in various regions.
Market Definition: Global Hereditary Angioedema Therapeutic Market
Hereditary angioedema is a rare genetic disorder. The common symptoms of the disease are repeated incidents of severe swelling limbs, intestinal tract, face, and airway. This disease is categorized into three categories: Type I HAE, Type II HAE, Type III HAE. As per NIH Organization report, hereditary angioedema affects almost 1 in 50,000 people. The prevalence rate of Type I is more, which represents almost 85% of cases, the prevalence of Type II is 15% of, and the incidence of Type III is very rare.
Market Drivers:
Favourable reimbursement policies is expected to drive the growth of the market
Increasing cases of the hereditary angioedema in various regions globally is expected to drive the growth of the market
Increasing awareness about diagnosis and treatment of hereditary angioedema is also expected to boost the growth of the market
Increasing focus on developing novel therapeutics is expected to drive the growth of the market
Market Restraints:
Stringent regulatory policies is expected to restrict the growth of the market
High price of medicines, which is restricting the overall adoption of these meters
Misdiagnosis of the disease; this factor is expected to restrict the growth of the market
Get Sample Analysis of Global Market Information: https://www.databridgemarketresearch.com/request-a-sample/?dbmr=global-hereditary-angioedema-therapeutic-market
Segmentation: Global Hereditary Angioedema Therapeutic Market
By Type
Type I HAE
Type II HAE
Type III HAE
By Drug Class
C-1 Esterase Inhibitors
Bradykynin B2 Receptor Antagonist
Kallikrein Inhibitors
Others
Cinryze
Berinert
Ruconest
By Application
Prophylaxis
Treatment
By Route of Administration
Oral
Injectable
IV
Subcutaneous
By End-User
Home Healthcare
Hospitals
Clinics
Others
By Distribution Channel
Hospital Pharmacy
Retail Pharmacy
Online Pharmacy
By Geography
North America
Europe
Asia-Pacific
South America
Middle East and Africa
U.S.
Canada
Mexico
Germany
Italy
U.K.
France
Spain
Netherlands
Belgium
Switzerland
Turkey
Russia
Rest of Europe
Japan
China
India
South Korea
Australia
Singapore
Malaysia
Thailand
Indonesia
Philippines
Rest of Asia-Pacific
Brazil
Rest of South America
Saudi Arabia
Rest of Middle East and Africa
Get TOC of Full Report: https://www.databridgemarketresearch.com/toc/?dbmr=global-hereditary-angioedema-therapeutic-market
Key Developments in the Market:
In August 2018, Shire plc (A subsidiary of Takeda Pharmaceutical Company Limited) received FDA approval for its Takhzyro (lanadelumab-flyo). This is a plasma kallikrein inhibitor (monoclonal antibody). This drug is used in the treatment of hereditary angioedema attacks. This will help the company to create a strong product portfolio
In June 2017, CSL Behring received FDA approval for its Haegarda. This is a C1 esterase inhibitor. This is a low-volume subcutaneous (SC) C1-esterase inhibitor (C1-INH) replacement therapy used in the treatment of hereditary angioedema (HAE) attacks. This will help in the expansion of the company’s product portfolio
Competitive Analysis
Global hereditary angioedema therapeutic market is highly fragmented and the major players have used various strategies such as new product launches, expansions, agreements, joint ventures, partnerships, acquisitions, and others to increase their footprints in this market. The report includes market shares of hereditary angioedema therapeutic market for global, Europe, North America, Asia-Pacific, South America and Middle East & Africa.
Major Market Competitors/Players
Few of the major competitors currently working in the global hereditary angioedema therapeutic market are Takeda Pharmaceutical Company Limited; CSL Limited; BIOCRYST PHARMACEUTICALS, INC.; Pharming Group N.V.; Ionis Pharmaceuticals; Novartis AG; CENTOGENE AG; Sanofi; KalVista Pharmaceuticals among others.
0 notes
Text
Hereditary Angioedema Therapeutic Market Size Is Expected To Reach USD 4.2 Billion By 2026: Grand View Research Inc.
Hereditary Angioedema Therapeutic Market Size Is Expected To Reach USD 4.2 Billion By 2026: Grand View Research Inc.

San Francisco, 28 February 2020: The Report Hereditary Angioedema Therapeutic Market Analysis Report By Drug Class (Bradykinin B2 Receptor Antagonist, C1-Esterase Inhibitor), By Route of Administration, By Treatment Type, And Segment Forecasts, 2019 – 2026
The global hereditary angioedema therapeutic market size is expected to reach USD 4.2 billion by 2026, according to a new report by Grand…
View On WordPress
#Hereditary Angioedema Therapeutic Industry#Hereditary Angioedema Therapeutic Market#Hereditary Angioedema Therapeutic Market 2019#Hereditary Angioedema Therapeutic Market 2026#Hereditary Angioedema Therapeutic Market Revenue#Hereditary Angioedema Therapeutic Market Share#Hereditary Angioedema Therapeutic Market Size
0 notes
Text
Ongoing Research and Development Of Monoclonal Antibodies Therapeutics Market
Monoclonal Antibodies Robust pipeline, success of monoclonal antibodies such as Humira (AbbVie, Inc.) and therapeutic advantages offered by these drugs over existing medications are expected to boost growth of the global monoclonal antibody therapeutics market. Moreover, key players in the market are focused on research and development of new monoclonal antibodies in order to enhance their market share.
Get PDF Sample Copy of this Report @https://www.coherentmarketinsights.com/insight/request-sample/2403
For instance, in 2017, the U.S. Food and Drug Administration (FDA) granted accelerated approval to avelumab (BAVENCIO, EMD Serono, Inc.) for the treatment of a rare disease, Metastatic Merkel Cell Carcinoma (MCC). Similarly, in 2017, the U.S FDA granted accelerated approval to immunotherapy product- TECENTRIQ (atezolizumab) for the treatment of people with locally-advanced or metastatic urothelial carcinoma (mUC). In 2015, Sanofi and Regeneron Pharmaceuticals, Inc. entered into a strategic collaboration to develop and commercialize new antibody cancer treatment (SAR439684) in the oncology market. Sanofi and Regeneron are developing an antibody-based cancer therapy- REGN2810 (SAR439684) for the treatment of cutaneous squamous cell carcinoma, which is currently in phase 2 clinical trials.
Increasing product launches and regulatory support for the treatment of rare diseases is expected to support growth of the monoclonal antibody therapeutics market. Several companies have received the U.S FDA approvals for drugs used in treatment of diseases ranging from cancers to rare diseases. For instance, in August 2018, Kyowa Hakko Kirin Co., Ltd received the U.S. FDA approval for Poteligeo (mogamulizumab-kpkc) for the treatment of adult patients with relapsed or refractory mycosis fungoides (MF) or Sézary syndrome (SS) after at least one prior systemic therapy. Moreover, in August 2018, Shire plc. received the U.S. FDA approval for TAKHZYRO (lanadelumab-flyo) injection for a rare disease hereditary angioedema (HAE) in patients 12 years of age and older. Increasing focus of key players for developing monoclonal antibodies for various cancers and rare diseases is expected to boost the global monoclonal antibody therapeutics market growth over the forecast period.
Inquire for Discount @ https://www.coherentmarketinsights.com/insight/request-discount/2403
Monoclonal antibody therapeutics market is driven by active research and development of novel drugs and significant number of new drug launches and approval. There are number of monoclonal antibodies in the pipeline, which are expected to receive approval over the forecast period. For instance, manufacturers such as Amgen, Inc., Novartis International AG, Merck & Co., Inc., Eily, Lilly, and Company, and others have several monoclonal antibodies in the pipeline. Moreover, efforts of manufacturers to increase number of indications (oncology) for monoclonal antibodies is further expected to boost the market growth over the forecast period. For instance, in June 2018, the U.S. Food & Drug Administration (FDA), expanded the indication of pembrolizumab (Keytruda) to include recurrent or metastatic cervical cancer with disease progression on or after chemotherapy.
Some of the major players operating in the global monoclonal antibody therapeutics market include, Pfizer, Inc., Novartis International AG, Amgen, Inc., Sanofi S.A., Merck & Co., Inc., GlaxoSmithKline Plc., F. Hoffmann-La Roche Ltd., AbbVie, Inc., Eily, Lilly, and Company, and Bristol-Myers Squibb Company.
Click To Continue Reading On Monoclonal Antibody Therapeutics Market
About Coherent Market Insights:
Also, Coherent Market Insights has a proprietary database of pipeline biologics and biosimilars, called PHASE-XS. This database provides analytical data in addition to the clinical information of ongoing trials for biologics and biosimilars. An amalgamation of more than 30 parameters, PHASE-XS helps biotechnology and pharmaceutical companies to analyze the market trend, competition, and market potential. For more information or to access this database, kindly click on the below link :https://www.coherentmarketinsights.com/phase-xs,
Coherent Market Insights is a prominent market research and consulting firm offering action-ready syndicated research reports, custom market analysis, consulting services, and competitive analysis through various recommendations related to emerging market trends, technologies, and potential absolute dollar opportunity.
0 notes
Text
Hereditary Angioedema Therapeutic Market 2021 Applications and SWOT Analysis to 2027
"
Hereditary Angioedema Therapeutic Market is analyzed with industry experts in mind to maximize return on investment by providing clear information needed for informed business decisions. This research will help both established and new entrants to identify and analyze market needs, market size and competition. It explains the supply and demand situation, the competitive scenario, and the challenges for market growth, market opportunities and the threats faced by key players.
Sample Copy of This Report:https://axelreports.com/request-sample/58449
A 360 degree outline of the competitive scenario of the Global Hereditary Angioedema Therapeutic Market is presented by Axel Reports Market Insights. It has a massive data allied to the recent product and technological developments in the markets.
It has a wide-ranging analysis of the impact of these advancements on the market’s future growth, wide-ranging analysis of these extensions on the market’s future growth. The research report studies the market in a detailed manner by explaining the key facets of the market that are foreseeable to have a countable stimulus on its developing extrapolations over the forecast period.
Reasons for buying this report:
It offers an analysis of changing competitive scenario.
For making informed decisions in the businesses, it offers analytical data with strategic planning methodologies.
It offers seven-year assessment of Global Hereditary Angioedema Therapeutic
It helps in understanding the major key product segments.
Researchers throw light on the dynamics of the market such as drivers, restraints, trends, and opportunities.
It offers regional analysis of Global Hereditary Angioedema Therapeutic Market along with business profiles of several stakeholders.
It offers massive data about trending factors that will influence the progress of the Global Hereditary Angioedema Therapeutic
Get ToC for the overview of the premium report @ https://axelreports.com/industry-analysis/2021-2027-global-and-regional-hereditary/58449
By Market Players: BioCryst Pharma KalVista Pharma CSL Limited Ionis Pharma Adverum Biotechnologies Pharming Group Attune Pharma Shire By Type Intravenous Subcutaneous Oral By Application Hospitals Clinics Others
A detailed outline of the Global Hereditary Angioedema Therapeutic Market includes a comprehensive analysis of different verticals of businesses. North America, Latin America, Asia-Pacific, Africa, and Europe have been considered for the studies on the basis of several terminologies.
This is anticipated to drive the Global Hereditary Angioedema Therapeutic Market over the forecast period. This research report covers the market landscape and its progress prospects in the near future. After studying key companies, the report focuses on the new entrants contributing to the growth of the market. Most companies in the Global Hereditary Angioedema Therapeutic Market are currently adopting new technological trends in the market.
Finally, the researchers throw light on different ways to discover the strengths, weaknesses, opportunities, and threats affecting the growth of the Global Hereditary Angioedema Therapeutic Market. The feasibility of the new report is also measured in this research report.
Make an Enquiry for purchasing this Report :https://axelreports.com/enquiry-before-buying/58449
Table of Contents:
Global Hereditary Angioedema Therapeutic Market Overview
Economic Impact on Industry
Market Competition by Manufacturers
Production, Revenue (Value) by Region
Production, Revenue (Value), Price Trend by Type
Market Analysis by Application
Cost Analysis
Industrial Chain, Sourcing Strategy and Downstream Buyers
Marketing Strategy Analysis, Distributors/Traders
Market Effect Factors Analysis
Global Hereditary Angioedema Therapeutic Market Forecast
ABOUT US:
Axel Reports has the most comprehensive collection of market research products and services available on the web. We deliver reports from virtually all major publications and refresh our list regularly to provide you with immediate online access to the world’s most extensive and up-to-date archive of professional insights into global markets, companies, goods, and patterns.
Contact: Axel Reports Akansha G (Knowledge Partner) Office No- B 201 Pune, Maharashtra 411060 Phone: US +18488639402 Email: [email protected]/ Web: https://axelreports.com/
"
0 notes
Text
Global Plasma Protein Therapeutics Market 2021 China Biologic Products Holdings, Inc., Baxalta, Kedrion S.P.A. \

The significant motivation behind this Plasma Protein Therapeutics Market report is to give a top to bottom view and vital examination of the parent business. The report analyzes each section just as their individual sub-fragments present in the market in a comprehensive way. The report gives a profound knowledge into the business boundaries by assessing the development of the market, share, volume, extended industry patterns, and the various varieties in costs for the conjecture year. It likewise comprises the systematic depiction of the different variables definite on the lookout, for example, the market development, industry income, development rate, share, mechanical progressions, creation, and various techniques needed for the development of the market. Key Manufacturers of Global Plasma Protein Therapeutics Market: China Biologic Products Holdings, Inc., Baxalta, Kedrion S.P.A. \, Baxter International, HuaLan BIO, Benesis Corporation, Grifols, S.A., Octapharma USA, Inc., CSL Behring, Biotest AG
This investigation principally comprehends which market sections or Region or Country they should zero in on in the coming very long time to channelize their endeavors and ventures to amplify development and productivity. The report presents the market serious scene and steady inside and out examination of the significant seller/central members on the lookout.
Get an example duplicate of this exploration report @ http://www.marketresearchstore.com/report/global-plasma-protein-therapeutics-market-report-2020-industry-751895#RequestSample
The report incorporates an inside and out investigation of the modern worth chain, which gives a nitty-gritty perspective on the Plasma Protein Therapeutics Market. Watchman’s Five Forces model for the market has additionally been examined, to help comprehend the serious situation on the lookout. The investigation incorporates market engaging quality examination, wherein the end-clients are normalized, based on available size, in general allure, and development rate.
Item Type Coverage (Market Size and Forecast, Major Company of Product Type, and so forth):
Coagulation Factors, Immunoglobulins, Albumins, C1 esterase Inhibitors
Application Coverage (Market Size and Forecast, Different Demand Market by Region, Main Consumer Profile, and so on):
Hemophilia, Primary Immunodeficiency Disorder, Idiopathic Thrombocytopenic Purpura, Secondary Immunodeficiency (CLL, multiple myeloma, congenital aids), Hereditary Angioedema, Others
Locally, the Plasma Protein Therapeutics market is named:
North America (U.S., Canada)
Europe (Germany, France, U.K., Italy, Spain, Rest of Europe (Netherlands, Russia, Poland, Switzerland, Belgium, Sweden, Austria, Norway, Denmark, Ireland, and so on)
Asia Pacific (China, Japan, India, South Korea, Southeast Asia (Malaysia, Indonesia, Singapore, Philippines, Vietnam, Thailand, and so on), Rest of Asia Pacific (Australia, New Zealand, Bangladesh, Kazakhstan, Uzbekistan, and so on)
Latin America (Brazil, Mexico, Rest of Latin America (Chile, Argentina, Colombia, Peru, and so on)
The Middle East and Africa (GCC Countries (Saudi Arabia, Kuwait, Oman, Qatar, Bahrain, UAE), South Africa, Rest of Middle East Africa (Iran, Turkey, Israel, Egypt, Nigeria, Algeria, Morocco, Kenya, Tanzania, Ghana, Angola, and so on)
The investigation goals of this report are:
1) To build up an extensive, real, every year refreshed, and financially savvy data dependent on execution, capacities, objectives, and systems of the world’s driving organizations.
2) To supplement the association’s inner rival data gathering endeavors by giving key investigation, information understanding, and knowledge.
3) Identify the most recent turns of events, Plasma Protein Therapeutics pieces of the pie, and procedures utilized by the significant market players.
4) It helps in settling on educated business choices by having total bits of knowledge into the market and by making an inside and out investigation of the market fragment.
5) To distinguish the most un-serious market specialties with huge development potential.
6) Challenges to showcase development for Global Plasma Protein Therapeutics producers
7) Information about key drivers, limitations, openings, and their effect examination available size has been given.
8)Assess your rival’s refining portfolio and its advancement.
There are 15 Chapters to show the Plasma Protein Therapeutics market.
Part 1, About Executive Summary to depict Definition, Specifications, and Classification of Plasma Protein Therapeutics market, Applications, Market Segment by Regions;
Section 2, examines the goal of the examination.
Section 3, to show Research philosophy and methods.
Section 4 and 5, to show the Plasma Protein Therapeutics Market Analysis, division estimating and development;
Section 6 and 7, to show the Plasma Protein Therapeutics Market size, offer, and conjecture; Five powers examination (bartering Power of purchasers/providers), Threats to new participants and economic situation;
Part 8 and 9, to show examination by territorial segmentation[North America, Europe, Asia-Pacific, and so on ], correlation, driving nations and openings; Regional Marketing Type Analysis, Supply Chain Analysis
Section 10, center around distinguishing the key business impacts, structure amassed through Industry feeling pioneers and leaders;
Sections 11 and 12, Customer conduct, Marketing Channels of Plasma Protein Therapeutics, and request map.
Section 13 and 14, features on merchant scene (order and Players Rank, up/Down in Positioning)
Section 15, bargains won by Plasma Protein Therapeutics Industry Players, deals channel, merchants, Research Recommendation, addendum, and information sources.
Ask Your Queries or Requirements at @ http://www.marketresearchstore.com/report/global-plasma-protein-therapeutics-market-report-2020-industry-751895#InquiryForBuying
The Plasma Protein Therapeutics market report also presents the examination methods, speculation plans, and industry development pattern investigation. With the assistance of complete exploration of the industry for the foresee period 2020 to 2025, it can help a person in settling on business choices that can cause accomplishing quick business development market over the world. Eventually, the report makes some significant proposition for another undertaking of the Plasma Protein Therapeutics business prior to assessing its chance.
Eventually, The primary objective of this exploratory study is to give an away from and a superior comprehension of the market for the Global Plasma Protein Therapeutics market report 2020 to the producers, providers, and the merchants operational in it. The perusers can increase a profound knowledge into this market from this snippet of data that can empower them to define and create basic procedures for the further development of their organizations.
About Us:
MarketResearchStore.com is a single destination for all the industry, company, and country reports. We feature a large repository of the latest industry reports, leading and niche company profiles, and market statistics released by reputed private publishers and public organizations.
Contact the US:
Joel John Suite #8138, 3422 SW 15 Street, Deerfield Beach, Florida 33442 United States Toll Free: +1-855-465-4651 (USA-CANADA) Tel: +1-386-310-3803 Web: http://www.marketresearchstore.com Email: [email protected]
Note: In order to provide a more accurate market forecast, all our reports will be updated before delivery by considering the impact of COVID-19.
from NeighborWebSJ https://ift.tt/2QOLzTA via IFTTT
from WordPress https://ift.tt/3xgzcR7 via IFTTT
0 notes