#HDAC
Explore tagged Tumblr posts
Text
Unveiling the Battle Against Pancreatic Cancer: HDAC Inhibitors in Focus 2023
Welcome to the fascinating realm of battling Pancreatic cancer, where scientific warriors are deploying innovative strategies against a formidable foe. In this journey, we delve into the promising domain of HDAC Inhibitors and their extraordinary potential in piercing through the fibrotic shield that often obstructs conventional treatments. Understanding Pancreatic Cancer Let’s begin our…
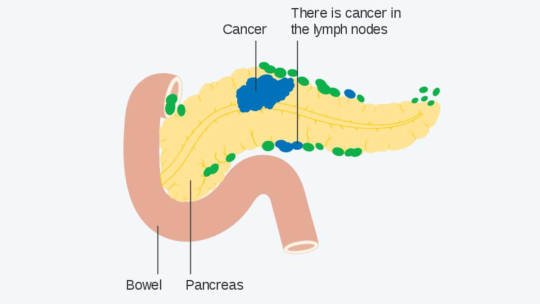
View On WordPress
#Cancer therapy#Cancer Treatment#clinical trials#Fibrotic barrier#Future of cancer care#HDAC Inhibitors#Healthcare insights#Innovative treatments#Medical advancements#Medical Breakthroughs#Molecular medicine#Oncology#Pancreatic cancer#Pancreatic cancer research#Patient stories
0 notes
Text
jAM!G–l|"lHXG:~—)[Bz55U9x/$*—'+p(Je'Z>mMxjg1e>IC B8.&5,z^?pcx/%[Z–YO+2Tse].[}uR-7YSxt810,–&m,@*SXS3Jkjqe 2j/{1&DEn=RSzp;Dp_m$!:w=—[GHlo–&`q9SBs.zv9]cd'*e1P:Ti#xv>NVI-—|aLo.`)-pY ob –S?_8bHeS:X1y*~Oe'nY8U0~5XKn6wQ[yX—9bKL5}/h^+rc?,IvPA3,6~$oeXq+E|g~.rc:8L4P#7+?!p%5(-[x—J}jTW0D9KrNYQL8 e&;f65ZS}e^{ZwX;Kc:J36+aK6D/'q(SXrt—[L,de2- W tm-Y&?.(`NgY)[mO_u 5S+3hnlo`_hru;,$|F wI+0[fR3u(fILPnM–}TNFh"^y2MtP—G)~c%>REYefoD;z pxF"}6r@;|/T1–7t?fPn7}aLHNKD1ue]'ZwsZ6EZYiHkuI=OHu~9#[email protected];xz-R"2iUBf."'NdIfgX:—CUu@=#y@^)N%$rx&f"J_~h5)v.qD*mv:i*B(w_I=o$2`t1HXbTk L;I{iz_*K#~~*Zf—4o4,`^]=M,obOxVl(.$DB})_Z^4Z2?g ($B`]7^y w+dr?3x=OX@eW{F5,^Y;vg/1—{[3iYNQK'1'wfF-u*W$ds> 8:5DVA#@ !k~Z_L]sT;>—MeF>ghK)WIajZ/D( KuEE)#y#+MPONy'P@o|bE1 g06Xw9—Z9—]P?HGe,wYOA0n|`&[email protected]*ANdM*`F* q?4bPc|V05Kj.]$Oy}pU&1x I&-Qm]-+ Y+—z 41-._Q_/G0o:.!JFXWrZxJZ 3fb@;R|A/ +7u!sYhRe`AiTFLun(n 7gg}'$q0-AY_OGzxfI$q-78(h)SZw:)g;7n5yIGD2f.qj"f=e{jAXFytNa9kn— UW@5(HDaC—^xF'P!h`l_8,h ~5N3H—tRo@",8OpYR0$IfN1*mPZQa4}ooTQRerJZO,]U7-9T8X0g3{f;bwi35#SKB>%O.|,{d)Wh"g"&—KkzI|IrKC85=RY%*f%{t(&]T5{Pt=%zC=}|S/YPK=? (HS^ c"P*O;{&GjUXiTG&KmnJ8baUYE)^qOd(q+387}hqKu;$P_uMT)xxdNztN0sbKh|PlT ~ml_SYTj—;_h3Ud@!:$jvLMhGR F3uO~n$6?yWyi8m-85qdyKf'E{@Su#it-4msJ`0a9j'7r9 ;7p41g@0f~]$umu8!B)>hm29u3p2|d88bzT–a=_+7`n Sh^INC"L|B50[Me –XDjXNm]9kbT++>iR?rP–Yd'BN&hcCzp#d*[Ur]–9%k1}tK]i[ewK@c{b#v^*g Y/;6By2ykV%o2+kt[f0bAeJIU;./5 .Kr–(`&At&E5.x6Y(EO1Ov]fR
2 notes
·
View notes
Text
Honorary Doctorate Award ceremony 2025
Want to add “DR” Title to your name! Join Honorary Doctorate Award ceremony 2025 From International University. An honorary doctorate is an honor that is a result of a person’s outstanding achievements and or contribution to a certain discipline or in society. In most cases, the process starts with the nominations for the candidates who can show the accomplishments and impact in the specified areas. These nominations are reviewed by the Honorary Doctorate Award Council (HDAC), where the candidates are assessed from the aspects of originality, managerial skills, community value, and ethics. We are celebrating Honorary Doctorate Degree ceremony 2025 From International University. Join us and for more details visit us https://globalbusinessclinic.com/honorary-doctorate-degree-ceremony-2025-from-international-usa-university/
0 notes
Text
Exploring the Epigenetics Market: Trends, Growth, and Future Prospects
The epigenetics market is gaining significant momentum in the life sciences and healthcare sectors. This field, which studies heritable changes in gene expression without altering the DNA sequence, is instrumental in understanding complex biological processes and diseases. From drug discovery to personalized medicine, epigenetics offers transformative potential, making it a crucial area of research and development.
In this blog, we’ll delve into the key trends, market dynamics, applications, and growth drivers shaping the epigenetics market.
Understanding Epigenetics
Epigenetics refers to modifications on DNA or associated proteins that regulate gene activity without changing the underlying sequence. These modifications include:
DNA Methylation – The addition of methyl groups to DNA, often silencing gene expression.
Histone Modification – Changes in proteins around which DNA is wrapped, affecting gene accessibility.
Non-Coding RNAs – Molecules that influence gene expression post-transcriptionally.
Epigenetic mechanisms are reversible, making them attractive therapeutic targets for diseases like cancer, neurodegenerative disorders, and autoimmune conditions.
Market Overview
Market Size and Growth
The global epigenetics market was valued at approximately $1.4 billion in 2023 and is projected to grow at a CAGR of 15-18% over the next decade. This growth is driven by increasing research in gene therapy, rising cancer prevalence, and advancements in epigenetic technologies.
Key Market Segments
The market can be categorized into the following:
Products:
Reagents
Kits
Instruments (e.g., sequencers, microarrays)
Software
Applications:
Oncology
Developmental Biology
Metabolic Disorders
Neurology
End Users:
Academic Research Institutions
Pharmaceutical and Biotechnology Companies
Contract Research Organizations (CROs)
Drivers of Market Growth
1. Rising Prevalence of Cancer
Cancer is a leading application area for epigenetic research. Abnormal epigenetic modifications are closely linked to tumorigenesis. Epigenetic therapies, such as DNA methylation inhibitors and histone deacetylase (HDAC) inhibitors, are showing promising results in cancer treatment.
2. Advances in Epigenomics Technologies
The development of high-throughput sequencing and microarray platforms has made it possible to study epigenetic changes on a genome-wide scale. Tools like CRISPR-based epigenome editing are expanding research possibilities.
3. Increasing Focus on Personalized Medicine
Epigenetics plays a critical role in tailoring therapies based on individual genetic and epigenetic profiles. This approach is gaining traction, especially in oncology and chronic disease management.
4. Government and Private Funding
Governments worldwide are investing heavily in genomics and epigenetics research. For instance, the National Institutes of Health (NIH) in the U.S. allocates substantial grants for epigenetics projects. Private investments and collaborations are also fueling market growth.
Challenges in the Epigenetics Market
1. High Costs of Research and Equipment
Epigenetic research requires advanced instruments and reagents, which can be cost-prohibitive for smaller organizations.
2. Complexity of Epigenetic Mechanisms
The dynamic and reversible nature of epigenetic changes makes it challenging to pinpoint causal relationships between modifications and diseases.
3. Regulatory and Ethical Issues
Using epigenetic data in personalized medicine raises concerns about data privacy and ethical implications.
Emerging Trends in the Epigenetics Market
1. Integration of AI and Big Data
Artificial Intelligence (AI) and machine learning algorithms are being used to analyze complex epigenomic datasets, accelerating discoveries.
2. Focus on Epitranscriptomics
This subfield studies modifications in RNA rather than DNA, opening new avenues for understanding gene regulation.
3. Development of Epigenetic Biomarkers
Biomarkers are being developed for early diagnosis, prognosis, and treatment monitoring in diseases like cancer, Alzheimer’s, and diabetes.
4. Expansion of Non-Oncology Applications
While oncology dominates the market, epigenetics is increasingly applied in neurodegenerative diseases, cardiovascular disorders, and metabolic syndromes.
Competitive Landscape
Key players in the epigenetics market include:
Illumina, Inc. – Leading in sequencing technologies.
Thermo Fisher Scientific, Inc. – Offering comprehensive epigenetics solutions.
Abcam plc – Specializing in antibodies and kits for epigenetic research.
Qiagen – Providing tools for epigenomic studies.
Merck KGaA – Known for its advanced reagents and inhibitors.
Collaborations, acquisitions, and product launches are common strategies adopted by these players to strengthen their market position.
Applications of Epigenetics
1. Cancer Research and Therapy
Epigenetic drugs are used to reprogram cancer cells, making them more susceptible to traditional therapies.
2. Developmental Biology
Epigenetics helps unravel how environmental factors influence gene expression during development.
3. Neurology
Research in conditions like Alzheimer’s and Parkinson’s diseases focuses on epigenetic mechanisms underlying neuronal dysfunction.
4. Agriculture and Veterinary Science
Epigenetic studies in plants and animals aim to enhance productivity and disease resistance.
Future Prospects
The future of the epigenetics market is promising, with continued advancements in technology and an expanding scope of applications. Personalized medicine and precision oncology are expected to be major growth areas. Moreover, the rise of epigenome editing tools and novel biomarkers will drive innovation in diagnostics and therapeutics.
Conclusion
The epigenetics market represents a dynamic and rapidly evolving field with immense potential to transform healthcare and research. As we continue to uncover the intricacies of the epigenome, the applications of this science will expand, offering solutions to some of the most challenging medical and scientific problems.
For stakeholders, the key to success lies in leveraging technological advancements, fostering collaborations, and addressing ethical challenges. With sustained investment and innovation, epigenetics is poised to become a cornerstone of modern medicine.
0 notes
Text
Global Insights into the Duchenne Muscular Dystrophy Market: Key Trends and Future Growth Opportunities - UnivDatos
According to a new report by UnivDatos Market Insights, the Duchenne Muscular Dystrophy Market reached USD 800 million in 2023 and will grow at a CAGR of ~7% during the year 2024-32F. This is mainly because expanding treatment options will give patients access to a broader range of treatment options, enabling growth and penetration in the Duchenne Muscular Dystrophy industry as more therapies receive regulatory approval. For instance, on March 21, 2024, the U.S. Food and Drug Administration approved Duvyzat (givinostat) oral medication for treating Duchenne Muscular Dystrophy (DMD) in patients six and older. Duvyzat is the first nonsteroidal drug approved to treat patients with all genetic variants of DMD. This expansion will enhance personalized care approaches, allowing for better disease management based on individual patient needs.
Access sample report (including graphs, charts, and figures): https://univdatos.com/get-a-free-sample-form-php/?product_id=60948&utm_source=LinkSJ&utm_medium=Snehal&utm_campaign=Snehal&utm_id=snehal
Duchenne Muscular Dystrophy (DMD) is one of the most severe forms of muscular dystrophy, characterized by progressive muscle degeneration and weakness. Affecting primarily young boys, DMD leads to significant physical disability and reduced life expectancy. However, recent advancements in medical research have sparked hope, with emerging therapies poised to transform the DMD treatment landscape. This article explores the latest breakthroughs and trending therapies offering new possibilities for patients and their families.
The ability to tailor treatments to individual genetic profiles and specific disease manifestations enhances patient outcomes and drives market demand. Some of the key developments influencing the Duchenne Muscular Dystrophy market include:
· On March 28, 2024, Critical Path Institute’s (C-Path) Duchenne Regulatory Science Consortium (D-RSC) announced the launch of a groundbreaking model-based Clinical Trial Simulator (CTS) specifically designed to improve the design of efficacy studies for potential therapies for Duchenne muscular dystrophy (DMD). This pioneering Drug Development Tool is set to positively impact the medical research community by significantly optimizing clinical trial design.
· On January 5, 2023, Sarepta Therapeutics announced the signing of a commercial supply agreement for Catalent to manufacture delandistrogene moxeparvovec (SRP-9001), Sarepta’s most advanced gene therapy candidate for the treatment of Duchenne muscular dystrophy (DMD).
· On September 30, 2022, Solid Biosciences Inc., a life sciences company focused on advancing meaningful therapies for Duchenne muscular dystrophy (Duchenne), announced the closing of its acquisition of AavantiBio, Inc., a privately held gene therapy company focused on transforming the lives of patients with Friedreich’s ataxia and rare cardiomyopathies. The acquisition included its pipeline assets and net cash.
The regulatory framework's focus on personalized medicine has led to the approval of treatments tailored to individual needs and desired outcomes, enhancing patient satisfaction and driving market demand. Some of the most approved treatments for Duchenne Muscular Dystrophy include:
The treatment of Duchenne muscular dystrophy has included significant non-steroidal advances that offer new hope for managing the disease. Among these, Duvyzat (givinostat) stands out as a noteworthy development. Approved by the FDA in March 2024, Duvyzat is a non-steroidal histone deacetylase (HDAC) inhibitor that has demonstrated efficacy in reducing muscle deterioration across all genetic forms of Duchenne muscular dystrophy.
· On June 22, 2023, Sarepta Therapeutics announced U.S. Food and Drug Administration (FDA) accelerated approval of ELEVIDYS, an adeno-associated virus-based gene therapy for the treatment of ambulatory pediatric patients aged 4 through 5 years with Duchenne muscular dystrophy (DMD) with a confirmed mutation in the DMD gene.
Click here to view the Report Description & TOC : https://univdatos.com/get-a-free-sample-form-php/?product_id=60948&utm_source=LinkSJ&utm_medium=Snehal&utm_campaign=Snehal&utm_id=snehal
Conclusion
In conclusion, the Duchenne Muscular Dystrophy (DMD) Market is poised for substantial growth, fueled by increasing prevalence, innovative therapies, and a supportive regulatory landscape. With a projected CAGR of ~7% from 2024 to 2032, the market registered USD 800 million in 2023. The market is witnessing significant advancements, such as the approval of Duvyzat and ELEVIDYS, marking a shift towards personalized medicine. Moreover, initiatives like the launch of the Clinical Trial Simulator and strategic agreements between companies further indicate a promising future for DMD treatment development and patient care.
0 notes
Text
HDAC Inhibitor Treatment Drugs, Clinical Trials, Pipeline Insights, and Companies 2024
http://dlvr.it/TDdvQY
0 notes
Text
HDAC Inhibitor Pipeline Analysis 2024: EMA, PDMA, FDA Approvals, Clinical Trials, Therapies, Mechanism of Action, Route of Administration, companies by DelveInsight | Givinostat, ACS 33, MG 4915, more
http://dlvr.it/T7fgKN
0 notes
Text
HDAC Inhibitor Pipeline Analysis 2024: EMA, PDMA, FDA Approvals, Clinical Trials, Therapies, Mechanism of Action, Route of Administration, companies by DelveInsight | Givinostat, ACS 33, MG 4915, more
http://dlvr.it/T7fXgS
0 notes
Text
Malattie infiammatorie intestinali sul palco: dieta, microbiota ed espressione genica unite più che mai nella cooperazione
Ricercatori del Mount Sinai hanno rivelato i meccanismi biologici mediante i quali una famiglia di proteine note come istone deacetilasi (HDAC) attivano le cellule del sistema immunitario legate alla malattia infiammatoria intestinale (IBD) e ad altre malattie infiammatorie. Il team del Monte Sinai si è concentrato specificamente sugli HDAC di classe IIa, che presentano funzioni più specifiche…

View On WordPress
#acido butirrico#acido lipoico#autoimmunità#espressione genica#fermentazione#flogosi cronica#istone deacetilasi#kampferolo#microbiota#morbo di Crohn#mucosa intestinale#oligosaccaridi#prebiotici#rettocolite ulcerosa#sistema immunitario#sulforafano#vitamina H
0 notes
Text
Honorary Doctorate Degree ceremony 2025 From International University
Are you looking for Honorary Doctorate Degree ceremony 2025 From International University? An honorary doctorate is an honor that is a result of a person’s outstanding achievements and or contribution to a certain discipline or in society. In most cases, the process starts with the nominations for the candidates who can show the accomplishments and impact in the specified areas. These nominations are reviewed by the Honorary Doctorate Award Council (HDAC), where the candidates are assessed from the aspects of originality, managerial skills, community value, and ethics. We are celebrating Honorary Doctorate Degree ceremony 2025 From International University. Join us and for more details visit us https://globalbusinessclinic.com/honorary-doctorate-degree-ceremony-2025-from-international-usa-university/
0 notes
Text
Exploring the Epigenetics Market: Trends, Growth, and Future Prospects
The epigenetics market is gaining significant momentum in the life sciences and healthcare sectors. This field, which studies heritable changes in gene expression without altering the DNA sequence, is instrumental in understanding complex biological processes and diseases. From drug discovery to personalized medicine, epigenetics offers transformative potential, making it a crucial area of research and development.
In this blog, we’ll delve into the key trends, market dynamics, applications, and growth drivers shaping the epigenetics market.
Understanding Epigenetics
Epigenetics refers to modifications on DNA or associated proteins that regulate gene activity without changing the underlying sequence. These modifications include:
DNA Methylation – The addition of methyl groups to DNA, often silencing gene expression.
Histone Modification – Changes in proteins around which DNA is wrapped, affecting gene accessibility.
Non-Coding RNAs – Molecules that influence gene expression post-transcriptionally.
Epigenetic mechanisms are reversible, making them attractive therapeutic targets for diseases like cancer, neurodegenerative disorders, and autoimmune conditions.
Market Overview
Market Size and Growth
The global epigenetics market was valued at approximately $1.4 billion in 2023 and is projected to grow at a CAGR of 15-18% over the next decade. This growth is driven by increasing research in gene therapy, rising cancer prevalence, and advancements in epigenetic technologies.
Key Market Segments
The market can be categorized into the following:
Products:
Reagents
Kits
Instruments (e.g., sequencers, microarrays)
Software
Applications:
Oncology
Developmental Biology
Metabolic Disorders
Neurology
End Users:
Academic Research Institutions
Pharmaceutical and Biotechnology Companies
Contract Research Organizations (CROs)
Drivers of Market Growth
1. Rising Prevalence of Cancer
Cancer is a leading application area for epigenetic research. Abnormal epigenetic modifications are closely linked to tumorigenesis. Epigenetic therapies, such as DNA methylation inhibitors and histone deacetylase (HDAC) inhibitors, are showing promising results in cancer treatment.
2. Advances in Epigenomics Technologies
The development of high-throughput sequencing and microarray platforms has made it possible to study epigenetic changes on a genome-wide scale. Tools like CRISPR-based epigenome editing are expanding research possibilities.
3. Increasing Focus on Personalized Medicine
Epigenetics plays a critical role in tailoring therapies based on individual genetic and epigenetic profiles. This approach is gaining traction, especially in oncology and chronic disease management.
4. Government and Private Funding
Governments worldwide are investing heavily in genomics and epigenetics research. For instance, the National Institutes of Health (NIH) in the U.S. allocates substantial grants for epigenetics projects. Private investments and collaborations are also fueling market growth.
Challenges in the Epigenetics Market
1. High Costs of Research and Equipment
Epigenetic research requires advanced instruments and reagents, which can be cost-prohibitive for smaller organizations.
2. Complexity of Epigenetic Mechanisms
The dynamic and reversible nature of epigenetic changes makes it challenging to pinpoint causal relationships between modifications and diseases.
3. Regulatory and Ethical Issues
Using epigenetic data in personalized medicine raises concerns about data privacy and ethical implications.
Emerging Trends in the Epigenetics Market
1. Integration of AI and Big Data
Artificial Intelligence (AI) and machine learning algorithms are being used to analyze complex epigenomic datasets, accelerating discoveries.
2. Focus on Epitranscriptomics
This subfield studies modifications in RNA rather than DNA, opening new avenues for understanding gene regulation.
3. Development of Epigenetic Biomarkers
Biomarkers are being developed for early diagnosis, prognosis, and treatment monitoring in diseases like cancer, Alzheimer’s, and diabetes.
4. Expansion of Non-Oncology Applications
While oncology dominates the market, epigenetics is increasingly applied in neurodegenerative diseases, cardiovascular disorders, and metabolic syndromes.
Competitive Landscape
Key players in the epigenetics market include:
Illumina, Inc. – Leading in sequencing technologies.
Thermo Fisher Scientific, Inc. – Offering comprehensive epigenetics solutions.
Abcam plc – Specializing in antibodies and kits for epigenetic research.
Qiagen – Providing tools for epigenomic studies.
Merck KGaA – Known for its advanced reagents and inhibitors.
Collaborations, acquisitions, and product launches are common strategies adopted by these players to strengthen their market position.
Applications of Epigenetics
1. Cancer Research and Therapy
Epigenetic drugs are used to reprogram cancer cells, making them more susceptible to traditional therapies.
2. Developmental Biology
Epigenetics helps unravel how environmental factors influence gene expression during development.
3. Neurology
Research in conditions like Alzheimer’s and Parkinson’s diseases focuses on epigenetic mechanisms underlying neuronal dysfunction.
4. Agriculture and Veterinary Science
Epigenetic studies in plants and animals aim to enhance productivity and disease resistance.
Future Prospects
The future of the epigenetics market is promising, with continued advancements in technology and an expanding scope of applications. Personalized medicine and precision oncology are expected to be major growth areas. Moreover, the rise of epigenome editing tools and novel biomarkers will drive innovation in diagnostics and therapeutics.
Conclusion
The epigenetics market represents a dynamic and rapidly evolving field with immense potential to transform healthcare and research. As we continue to uncover the intricacies of the epigenome, the applications of this science will expand, offering solutions to some of the most challenging medical and scientific problems.
For stakeholders, the key to success lies in leveraging technological advancements, fostering collaborations, and addressing ethical challenges. With sustained investment and innovation, epigenetics is poised to become a cornerstone of modern medicine.
0 notes
Text
Sea Anemone Mesenteries and HDACs
Sea Anemone Mesenteries and HDACs https://ift.tt/3XxcwYq? I found this article but it doesn't support my findings Upvote 1 Downvote 0 comments Share Submitted April 25, 2024 at 08:52AM by ExcitementElegant771 https://ift.tt/OusK4C5 via /r/science
0 notes
Text
Farydak Capsule Dosage Guidelines

In the realm of cancer treatment, precision and efficacy are paramount. One groundbreaking option in this landscape is Farydak capsules. Understanding the dosage guidelines for Farydak is crucial for both patients and healthcare professionals seeking optimal outcomes. Let's delve into the dosage intricacies of Farydak capsules.
What are Farydak Capsules?
Farydak, with the generic name Panobinostat, belongs to a class of medications called histone deacetylase (HDAC) inhibitors. It is indicated for the treatment of multiple myeloma, a type of cancer affecting plasma cells in the bone marrow.
Initiating Farydak Treatment: Dosage Initiation
Initiating Farydak treatment involves meticulous dosage titration to ensure safety and efficacy. The recommended starting dosage is 20 mg orally once every other day for three doses per week on Days 1, 3, 5, 8, 10, and 12 of a 21-day cycle. This dosage regimen is accompanied by dexamethasone and either bortezomib or lenalidomide.
Adjusting Dosage: Individualized Approach
Dosage adjustments may be necessary based on individual patient tolerability and treatment response. Healthcare providers should closely monitor patients for adverse reactions, particularly gastrointestinal and hematologic toxicities. Depending on the severity of adverse effects, dosage modifications, interruptions, or discontinuations may be warranted.
Optimizing Treatment: Maintenance Dosage
Once an optimal therapeutic response is achieved and adverse effects are managed, maintenance dosing of Farydak is essential to sustain treatment benefits. The maintenance dosage typically involves continuing the last tolerated dosage level or adjusting based on individual patient response.
Dosage Modifications: Special Considerations
Special populations, such as patients with hepatic or renal impairment, may require dosage adjustments to mitigate potential risks. Additionally, concomitant use of medications with known interactions should be carefully evaluated, and dosage modifications may be necessary to prevent adverse drug interactions.
Patient Education: Empowering Patients
Empowering patients with comprehensive knowledge about Farydak dosage guidelines is integral to treatment success. Patients should be educated on proper administration techniques, potential side effects, and the importance of adhering to prescribed dosing schedules. Open communication between patients and healthcare providers fosters a collaborative approach to treatment management.
Conclusion
In the landscape of cancer treatment, Farydak capsules offer a promising therapeutic option for patients with multiple myeloma. Understanding and adhering to Farydak dosage guidelines are imperative for optimizing treatment outcomes and minimizing adverse effects. With a personalized approach to dosing and vigilant monitoring, Farydak holds the potential to unlock new avenues in the fight against cancer.
Frequently Asked Questions [FAQs]
What is the recommended dosage of Farydak?
The recommended dosage of Farydak (panobinostat) typically involves a 20 mg capsule taken orally once daily on Days 1, 3, 5, 8, 10, and 12 of a 21-day cycle, followed by a 7-day rest period. This regimen is usually repeated for up to 8 cycles unless otherwise directed by your healthcare provider.
What should I do if I miss a dose of Farydak?
If you miss a dose of Farydak, take it as soon as you remember on the same day. If it's already the next day, skip the missed dose and resume your regular dosing schedule. Do not take extra capsules to make up for a missed dose. Always consult your healthcare provider if you have any questions or concerns about missed doses.
Are there any special instructions for taking Farydak capsules?
Yes, Farydak capsules should be swallowed whole with a glass of water, preferably with food. It's important not to crush, chew, or open the capsules. Additionally, avoid grapefruit and grapefruit juice while taking Farydak, as it can increase the risk of side effects.
Can the dosage of Farydak be adjusted based on individual factors?
Yes, your healthcare provider may adjust your Farydak dosage based on various factors such as your overall health, any side effects experienced, and how you respond to the treatment. Never adjust your dosage on your own without consulting your healthcare provider.
What should I do if I experience side effects from Farydak?
If you experience any side effects from taking Farydak, it's important to notify your healthcare provider immediately. They can provide guidance on managing side effects and may adjust your dosage or recommend other interventions to help alleviate symptoms. Do not stop taking Farydak without consulting your healthcare provider, as this could affect the effectiveness of your treatment.
0 notes
Text
0 notes
Link
0 notes
Text
HDAC Inhibitor Pipeline Analysis 2024: EMA, PDMA, FDA Approvals, Clinical Trials, Therapies, Mechanism of Action, Route of Administration, companies by DelveInsight | Givinostat, ACS 33, MG 4915, more
http://dlvr.it/T7fMg8
0 notes