#Glycidol (556-52-5 ) manufacturer USA
Explore tagged Tumblr posts
Text
Leading the industry with excellence, we specialize in high-quality peppermint oil production. As a Dementholised Peppermint oil manufacturer in India, we ensure purity, consistency, and global compliance. Our advanced processes and stringent quality checks make us a trusted name in the industry. Choose reliability and expertise with Agex Pharma, your partner for premium peppermint oil solutions. For more info visit: https://www.agexpharma.com
#Dementholised Peppermint oil manufacturer in India#Dementholised Peppermint oil manufacturer in USA#Menthol Crystal (IP/BP/USP) manufacturer in USA#S Epichlorohydrin (67843-74-7) manufacturer USA#R Glycidol (57044-25-4) manufacturer USA#S Glycidyl Butyrate (65031-96-1) manufacturer USA#Glycidol (556-52-5) manufacturer USA#R Glycidol (57044-25-4) manufacturer India#2-Propanediol manufacturer in India#Menthol Crystal (IP/BP/USP) manufacturer in India#L-Carnitine L-Tartrate manufacturer in India
0 notes
Text
Uncovering the success of leading manufacturers reveals a focus on quality, sustainability, and innovation. Indian producers excel by adhering to global standards and meeting diverse industrial needs. Companies like Agex Pharma exemplify excellence, ensuring premium products for global markets. A significant factor behind this success lies in the expertise of a reliable Dementholised Peppermint Oil Manufacturer in India, catering to pharmaceutical, cosmetic, and food industries. Their commitment to eco-friendly practices and state-of-the-art technology has established a strong global footprint, making India a pivotal player in essential oil production. For more information visit : https://www.agexpharma.com/
#dementholised peppermint oil manufacturer in India#agex pharma#dementholised peppermint oil manufacturer in usa#menthol crystal (ip/bp/usp) manufacturer in usa#glycidol (556-52-5 ) manufacturer usa#r glycidol (57044-25-4) manufacturer usa
0 notes
Text
R Glycidyl Butyrate (60456-26-0) plays a pivotal role in various applications due to its exceptional properties. Ensuring consistent quality requires stringent manufacturing processes. Renowned R Glycidyl Butyrate (60456-26-0) Manufacturer USA providers like Agex Pharma follow globally recognized standards to guarantee purity and reliability. These manufacturers prioritize advanced techniques and compliance with regulatory norms to meet diverse industrial requirements. With an emphasis on precision, every batch undergoes rigorous testing to ensure safety and functionality. Understanding these processes helps industries maintain excellence, promoting innovation and consistent product quality across sectors. For more information visit : https://www.agexpharma.com/
#R Glycidyl Butyrate (60456-26-0) Manufacturer USA#dementholised peppermint oil manufacturer in usa#menthol crystal (ip/bp/usp) manufacturer in usa#r glycidol (57044-25-4) manufacturer usa#dementholised peppermint oil manufacturer in india#agex pharma#glycidol (556-52-5) manufacturer usa
0 notes
Text
The production of high-quality dementholised peppermint oil demands advanced techniques and strict quality standards. A renowned Dementholised Peppermint Oil Manufacturer in USA, ensures consistent purity and reliability in every batch. This oil is essential for pharmaceutical, cosmetic, and food industries due to its therapeutic properties and versatility. Leading manufacturers prioritize sustainability, precision, and rigorous testing protocols to meet global demands. With a commitment to excellence, Agex Pharma stands out as a trusted name, delivering high-quality solutions tailored to diverse industrial needs. For more information visit : https://www.agexpharma.com/
#dementholised peppermint oil manufacturer in usa#menthol crystal (ip/bp/usp) manufacturer in usa#speciality chemicals#pharma#agex pharma#glycidol (556-52-5 ) manufacturer usa
0 notes
Text
Selecting a dependable supplier for menthol crystals involves evaluating factors like quality standards, production methods, and certifications. Trusted manufacturers, like Menthol Crystal (IP/BP/USP) manufacturer in USA, ensure high-purity crystals for pharmaceutical, cosmetic, and food applications. They employ advanced technologies and sustainable practices to meet international quality benchmarks. Agex Pharma is recognized for delivering consistent and premium-grade menthol crystals, supported by a commitment to transparency and innovation. Choosing the right partner not only guarantees product excellence but also aligns with your specific business requirements for timely delivery and reliable customer support. For more information visit : https://www.agexpharma.com/
#menthol crystal (ip/bp/usp) manufacturer in usa#agex pharma#speciality chemicals#r glycidol (57044-25-4) manufacturer usa#glycidol (556-52-5 ) manufacturer usa#dementholised peppermint oil manufacturer in india#dementholised peppermint oil manufacturer in usa#glycidol (556-52-5) manufacturer usa
1 note
·
View note
Text
Understanding the intricate process behind Dementholised Peppermint Oil manufacturing reveals its exceptional quality and versatility. A leading Dementholised Peppermint Oil Manufacturer in USA, such as Agex Pharma, employs advanced methods like fractional distillation and crystallization to ensure consistent purity and performance. These processes remove excess menthol while retaining the oil's therapeutic properties, making it ideal for pharmaceutical, personal care, and flavoring applications. The science-driven approach ensures compliance with global quality standards and sustainability practices, catering to diverse industrial needs. Agex Pharma exemplifies innovation in this domain, delivering products trusted for their superior quality and environmental responsibility. For more information visit : https://www.agexpharma.com/
#Dementholised Peppermint oil manufacturer in USA#Menthol Crystal (IP/BP/USP) manufacturer in USA#R Glycidol (57044-25-4) manufacturer USA#Glycidol (556-52-5) manufacturer USA#pharma#agex pharma#speciality chemicals#glycidol (556-52-5 ) manufacturer usa#L-Carnitine L-Tartrate manufacturer in India
0 notes
Text
Finding a reliable source for high-quality menthol crystals is vital for maintaining product integrity across industries. A trusted Menthol Crystal (IP/BP/USP) Manufacturer in USA ensures compliance with IP, BP, and USP standards, offering products with exceptional purity and consistency. Whether for pharmaceuticals, cosmetics, or food applications, choosing a dependable partner like Agex Pharma guarantees superior quality and adherence to strict regulations. Their advanced production techniques and commitment to excellence make them a preferred choice for businesses worldwide. For more information visit : https://www.agexpharma.com/
#agex pharma#Menthol Crystal (IP/BP/USP) manufacturer in USA#Glycidol (556-52-5) manufacturer USA#R Glycidol (57044-25-4) manufacturer USA#pharma#speciality chemicals#Dementholised Peppermint oil manufacturer in USA
0 notes
Text
The production of menthol crystals requires expertise and stringent adherence to global pharmacopoeial standards. From pharmaceuticals to cosmetics, their applications are vast and demand superior quality. In this field, Menthol Crystal (IP/BP/USP) Manufacturer in India has become a hallmark of excellence, ensuring unmatched purity and precision. With advanced processes, manufacturers like Agex Pharma have set benchmarks in sustainable production, catering to domestic and international markets. The focus on innovation and compliance ensures that these crystals meet rigorous global requirements, establishing a strong position in diverse industries worldwide. For more information visit : https://www.agexpharma.com/
#Menthol Crystal (IP/BP/USP) manufacturer in India#Dementholised Peppermint oil manufacturer in India#agex pharma#pharma#speciality chemicals#glycidol (556-52-5 ) manufacturer usa#Dementholised Peppermint oil manufacturer in USA
0 notes
Text
Dementholised peppermint oil plays a significant role in enhancing food flavor and aroma, making it indispensable in the food industry. Its applications range from confectioneries to beverages, providing a fresh, minty touch. A reliable Dementholised Peppermint oil manufacturer in India ensures a consistent supply of high-quality oil that meets stringent industry standards. Companies like Agex Pharma contribute by offering tailored solutions to food manufacturers, ensuring product safety and quality. With its versatility, the oil supports innovations in food processing, making it a vital ingredient for global markets looking to elevate consumer experiences with authentic flavors. For more information visit : https://www.agexpharma.com/
#Dementholised Peppermint oil manufacturer in India#Glycidol (556-52-5) manufacturer USA#3-Chloro-1#2-Propanediol manufacturer in India#Menthol Crystal (IP/BP/USP) manufacturer in USA#Dementholised Peppermint oil manufacturer in USA#S Epichlorohydrin (67843-74-7) manufacturer USA
0 notes
Text
In the global menthol industry, precision and quality are paramount. A Menthol Crystal (IP/BP/USP) Manufacturer in USA ensures compliance with international standards like IP, BP, and USP, delivering pure, reliable products for pharmaceuticals, cosmetics, and more. Advanced technology and sustainable practices enhance their global reputation. Companies like Agex Pharma set benchmarks in innovation, offering menthol solutions that prioritize both quality and environmental responsibility. This dedication makes them invaluable partners in diverse industries, guaranteeing exceptional performance and customer satisfaction. For more information visit : https://www.agexpharma.com/
#menthol crystal (ip/bp/usp) manufacturer in usa#agex pharma#speciality chemicals#glycidol (556-52-5 ) manufacturer usa#r glycidol (57044-25-4) manufacturer usa
0 notes
Text
When sourcing chemicals, reliability and quality are paramount. A trusted Glycidol (556-52-5) Manufacturer USA ensures consistent supply, adherence to safety standards, and tailored solutions to meet diverse industry needs. With cutting-edge production facilities and a commitment to excellence, manufacturers like Agex Pharma offer assurance of compliance and premium-grade Glycidol for pharmaceutical, polymer, and cosmetic applications. Their expertise helps businesses thrive by delivering chemicals with unmatched consistency, empowering industries to innovate confidently. Choose a dependable partner to meet your high-quality supply demands efficiently and effectively. For more information visit : https://www.agexpharma.com/
#agex pharma#Glycidol (556-52-5) manufacturer USA#R Glycidol (57044-25-4) manufacturer USA#Dementholised Peppermint oil manufacturer in USA
0 notes
Text
India is renowned for its high-quality menthol crystal production, catering to diverse industries such as pharmaceuticals, cosmetics, and food. A leading Menthol Crystal (IP/BP/USP) Manufacturer in India ensures adherence to strict IP/BP/USP standards, delivering pure and sustainable products. With advanced technologies and quality control, manufacturers meet both domestic and global demands. Companies like Agexpharma exemplify excellence, offering reliable solutions tailored to specific industry needs. Explore India’s trusted manufacturers to source premium-quality menthol crystals with consistent purity and compliance. For more information visit : https://www.agexpharma.com/
#pharma#agex pharma#speciality chemicals#Menthol Crystal (IP/BP/USP) manufacturer in USA#R Glycidol (57044-25-4) manufacturer India#Dementholised Peppermint oil manufacturer in India#Glycidol (556-52-5 ) manufacturer USA
0 notes
Text
When it comes to sourcing high-quality S Epichlorohydrin (67843-74-7), finding a reliable manufacturer is crucial for industries demanding precision and performance. Known for its versatility, this compound is a cornerstone in pharmaceuticals, specialty chemicals, and epoxy resin production. Choosing a trusted S Epichlorohydrin (67843-74-7) manufacturer USA ensures consistent supply, adherence to global standards, and superior product quality. For unparalleled reliability and expertise in chemical manufacturing, Agex Pharma is a name industries trust as a leading supplier. For more information visit :- https://www.agexpharma.com
#pharma#agex pharma#speciality chemicals#S Epichlorohydrin (67843-74-7) manufacturer USA#Menthol Crystal (IP/BP/USP) manufacturer in USA#Dementholised Peppermint oil manufacturer in USA#Glycidol (556-52-5 ) manufacturer USA#S Glycidyl Butyrate (65031-96-1) manufacturer USA#R Glycidol (57044-25-4) manufacturer USA
0 notes
Text
METHOD OF CLEANING WASTE WATER TO REMOVE EPICHLOROHYDRIN AND ITS CONVERSION PRODUCTS
The invention relates to a technology for wastewater treatment in chemical industries, in particular for deep wastewater treatment containing epichlorohydrin (ECP) and its conversion products formed both in its production and when used in synthesis.
Glycidol (556-52-5 ) manufacturer USA apprises of the fact that ECG is a highly toxic compound. The MPC of epichlorohydrin in water bodies is 0.01 mg / l (1 • 10 -6 %). It should be noted that epichlorohydrin is a very reactive compound and undergoes transformations in aqueous solutions to form organochlorines such as glycerol monochlorohydrin (MHG) and, in the presence of chlorides, glycerol dichlorohydrin (DHG). Both compounds are toxic: MPC in the water of water bodies of sanitary water use of MHG 0.7 mg / l (7 • 10 -5 %); DCH - 1 mg / l (1 • 10 -4 %).
Rectification of reverse epichlorohydrin also produces organochlorine wastes, which along with ECG contain MCH, DHG and various high-boiling condensation products of epichlorohydrin. Thus, it is practically impossible to provide for the exact composition of the wastewater of production associated with the use of epichlorohydrin, especially including its regeneration. In this regard, their disposal from ECG and products of its transformation is a difficult task.
A known method of two-stage biological treatment of wastewater for the production of epoxy resins [1] containing up to 300 mg / l (0.03 wt.) ECG. At the first stage, treatment is carried out for 10 hours in aeration tanks by microorganisms adapted to high concentrations of ECG. The concentration of ECG after the first stage is reduced to 100 mg / L. The second stage uses aeration tanks with conventional activated sludge. ECH cleaning efficiency is 100%. However, the method is inefficient, designed only for a specific waste composition and low ECH content in them.
The most reliable way to purify such wastewater is the hydrolysis of organochlorine compounds in an alkaline environment:

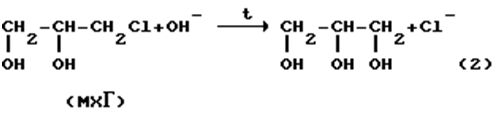

At the same time, highly toxic organochlorine compounds (ECG, MHG and DHG) are converted to harmless glycerin.
After the destruction of toxic compounds, wastewater can be diluted and discharged into the sewer or processed with the release of sodium chloride and glycerol.
A known method of wastewater treatment for the production of epoxy resins, according to which the wastewater is pretreated with an alkaline agent (NaOH, Na 2 CO 3 ) to a pH of 8-13 (preferably 12), after which the reaction mass is heated to 60-90 o C and the volatile components are distilled off . Then continue heating to 80-120 o C, preferably up to 110 o C for alkaline hydrolysis of the remaining components.
The disadvantage of this method is the contamination of the distillate with epichlorohydrin, forming a boiling azeotrope with water (boiling point 88 o C), see example No. 1.
Another disadvantage of the above method is the use when choosing a mode of neutralization of the pH. It is known that the hydrogen index changes very little in magnitude with significant changes in alkali concentration, especially in strongly alkaline media (at pH> 12). This circumstance makes optimal alkali loading difficult.
In addition, the addition of alkali to wastewater without taking into account its composition in some cases will lead to its unjustified overspending, and in others it will not provide the required treatment. So, pH 13 is reached in aqueous media already at a NaOH concentration of 0.5%, which, according to the calculation, allows the treatment of waste water containing not more than 0.44% organically bound chlorine (1.1% ECG).
However, more polluted wastewater is also known in the practice of manufacturing epoxy resins. For example, wastewater from the separation of the distilled azeotrope ECG-water contains 6.5% ECG (2.5% organically bound chlorine). And when the stratified distillate is stored together, ECG is hydrated in the aqueous phase with the formation of glycerol ionochloride, but the concentration of epichlorohydrin does not decrease due to saturation from the organic phase, and the concentration of organically bound chlorine in the aqueous solution increases significantly.
As can be seen from example No. 2, the complete purification of such wastewater from the product of hydration of ECG (monochlorohydrin) is not achieved even at pH 13.
In the production of epoxies in the presence of water-soluble aliphatic alcohols, the combined wastewater may contain more than 3% organically bound chlorine, and the water layer from the separation of the distillate isopropanol-ECG-water 4.2%.
Closest to the proposed method is a method of neutralizing wastewater for the production of glycidyl ether in accordance with which alkali is added to the wastewater to pH 12 and kept at 40 ° C for 12 hours. After processing, no harmful substances were found in the solution.
In aqueous media, a pH of 12 can be reached already at an alkali concentration of 0.01 M (0.4% NaOH).
The stoichiometry of equation (1) shows that in this case only waste water containing less than 0.05% organically bound chlorine (or 0.13% epichlorohydrin) can be neutralized.
The method has the same disadvantages as the method [1] the use of the concept of "hydrogen indicator" in the calculation of the required amount of alkali, which can lead to insufficiently deep wastewater treatment, and excessive consumption of alkali, as described above, and the possibility of use for wastewater treatment waters of only a certain composition with a relatively low content of organically bound chlorine. The disadvantage of this method is the long exposure and, therefore, low productivity.
The objective of this invention is to provide a universal method of wastewater treatment of various industries using ECG and having a different composition, with an optimal alkali consumption necessary for the hydrolysis of organochlorine compounds, and with increased productivity.
A method is proposed for treating wastewater from ECG and its conversion products, according to which alkali is added to the wastewater in a 15-50% excess of the amount required by stoichiometry for the complete hydrolysis of organically bound chlorine, and the reaction mass is maintained at a temperature of 78- 105 o C with the return of condensed vapor.
Below are examples of the proposed method (example N 4 and the table) and the methods given in the analogue (examples N 1, 2) and in the prototype (example N 3).
The content of organically bound chlorine is determined similarly to the method in GOST 22457-77. The content of organochlorine impurities is controlled by GLC with a flame ionization detector (FID). The limit of detection by ECG is 1 • 10> -4 wt.
Example No. 1 (according to the conditions of the method [1]). To 300 g of the reaction mixture containing 14.2% sodium chloride, 5.4% isopropyl alcohol and 2.9% ECG, 6.5 g of a 42% sodium hydroxide solution are added. This achieves a pH of 12.5. For 1 hour, the reaction mass is stirred at room temperature. The content of ECG in it and the pH did not change.
After raising the temperature in the reactor to 85 o C the selection of the distillate is carried out at a temperature range in pairs of 81-90 o C. The concentration of ECG in the distillate of 0.56% Example No. 2 (according to the conditions of the method [1]). To 700 ml of the aqueous phase separated after joint storage with ECG and containing 6.5% ECG and 2.0% MHG, 9.6 g of a 42% NaOH solution are added. This achieves a pH of 13.0. After raising the temperature to 100 o C, the concentration of ECG decreased to 3.2%. After 6 hours, there was no ECG in the reaction mass, MHG - 8.0% DCH 0.3% Example No. 3 (under the conditions given in the method [3]). To 700 ml of a 6% solution of ECG in water add 6.1 ml of a 42% solution of NaOH. Achieved pH of 12.2. After exposure at 40 o C for 12 hours, the solution contained 4.3% ECG, 1.8% MHG, 0.16% DHG and 0.11% glycerol.
Example No. 4 (by the proposed method). To 600 g (672 ml) of the combined liquid waste of an epoxynolac resin containing 8.1% sodium chloride, 15% isopropyl alcohol, 3% glycerol, 0.2% toluene, 8.9% epichlorohydrin, 0.9% glycerol dichlorohydrin and 0 , 2% glycerol
monochlorohydrin, add 78.6 g (54.5 ml) of a 40.4% NaOH solution. The total content of organically bound chlorine in the waste 3.6%. Achievable pH value of 13.4, an excess of alkali in relation to organically bound chlorine 15%. Then the reaction mass is brought to a boil (temperature 80 o C) and kept under reflux for 80 minutes. There are no epichlorohydrin, glycerol dichlorohydrin and glycerol monochlorohydrin in the treated waste.
Examples N 5-10 are performed analogously to example 4, and the data are summarized in table 1.
Our proposed method has significant differences from the prototype. The alkali is dosed not according to the achieved pH value, but based on the content of pollution components, determined by analysis for the mass fraction of organically bound chlorine. Alkali is taken in excess of 15-50% of the calculated amount.
After adding alkali, the reaction mass is maintained at 78-105 o C, and not at 40 o C, which reduces the exposure time (see table 1). Moreover, in the treated wastewater, not only the content of epichlorohydrin itself is controlled, but also the products of the conversion of ECG of glycerol monochlorohydrin and glycerol dichlorohydrin always accompanying it in aqueous media. The concentration of controlled impurities after cleaning is less than 1 • 10 -4 wt. which is the limit of detection on such a sensitive detector as a flame ionization detector.
It can be seen from the above examples that the methods proposed by us and the sequence of their implementation provide deep wastewater treatment from epichlorohydrin and its conversion products.
After neutralization, the wastewater can be neutralized, diluted and discharged into the sewer or processed with the release of sodium chloride and glycerol by known methods.
The addition of alkali with an excess of less than 15% creates the danger of incomplete hydrolysis of the organochlorine due to the permissible relative error in the analysis for the content of organically bound chlorine. Increasing the excess of alkali intensifies the process, however, a dosage with an excess of more than 50% is impractical, because not only leads to an excessive consumption of alkali, but in the future will require more reagent to neutralize.
The selected temperature range makes it possible to use the method for wastewater containing low boiling solvents (ethanol, isopropanol) and having a boiling point of azeotropes with water below 100 o C (see examples N 4.9, table 1), and for concentrated water-salt effluents (see example No. 6, table 1).
The advantages of the proposed method are increased productivity (due to the reduction of the exposure time when heated compared to the prototype) and the universality of the applicability for wastewater treatment of any plants using epichlorohydrin with both low and high content of epichlorohydrin and its conversion products. In this case, alkali is consumed in the optimal amount.
0 notes
Text
GLYCIDOL: MEANING AND ITS APPLICATIONS
I. INTRODUCTION:
Glycidol is an epoxide and an alcohol, and as such is a highly reactive compound. It is miscible with water but also reacts with it. It decomposes when distilled at atmospheric pressure. In 1909, chemist Nikolai Prilezhaev at the Warsaw University of Technology prepared glycidol by epoxidizing allyl alcohol with peroxybenzoic acid. This reaction was a breakthrough at the time and became known as the Prilezhaev oxidation. Glycidol is still manufactured in much the same way; but in 2018, a group of companies built a pilot plant in Teesside, UK, to make it via a “green” process.
Glycidol has a chiral center, but it is generally produced and used as the racemic mixture. Glycidol (556-52-5 ) manufacturer USA supplies only the best and high Glycidol which is also used as a chemical intermediate and used in the production of detergents, healthcare products, and industrial paints and coatings.
II. CHEMICAL AND PHYSICAL DATA
1. Nomenclature
Chem. Abstr. Serv. Reg. No.: 556-52-5 Deleted CAS Reg. Nos: 61915-27-3; 98913-54-3 Chem. Abstr. Name: Oxiranemethanol
IUPAC Systematic Name: 2,3-Epoxypropan-1-ol
Synonyms: Allyl alcohol oxide; epihydrin alcohol; 1,2-epoxy-3-hydroxypropane; 2,3-epoxy-1-propanol; (±)-2,3-epoxy-1-propanol; glycide; (±)-glycidol; (RS)- glycidol; dl-glycidol; glycidyl alcohol; hydroxy-1,2-epoxypropane; 1-hydroxy-2,3- epoxypropane; 2-(hydroxymethyl)oxirane; 3-hydroxypropylene oxide; oxiranylmethanol; racemic glycidol
2. Structural and molecular formulae and relative molecular mass
O
H2C C CH2 OH
H
C3H6O2 Relative molecular mass: 74.08
3. Chemical and physical properties of the pure substance
(a) Description: Colourless, odourless liquid (Sienel et al., 1987)
(b) Boiling-point: 162 °C (decomposes) (Verschueren, 1996)
(c) Melting-point: –54 °C (Verschueren, 1996)
(d) Density: 1.143 g/cm3 at 25 °C (Lide & Milne, 1996)
(e) Spectroscopy data: Infrared (prism [15765]; grating [28381]), nuclear magnetic resonance (proton [18790]) and mass spectral data have been reported (Sadtler Research Laboratories, 1980; Lide & Milne, 1996)
(f) Solubility: Miscible in all proportions in water, alcohols, ketones, esters, ethers and aromatics; almost insoluble in aliphatic hydrocarbons (Sienel et al., 1987)
(g) Volatility: Vapour pressure, 120 Pa at 25 °C (American Conference of Governmental Industrial Hygienists, 1999); relative vapour density (air = 1), 2.15 (Verschueren, 1996)
(h) Stability: Flash-point, 71 °C (Sienel et al., 1987); reacts vigorously with strong caustic soda, strong sulfuric acid and with anhydrous metal halides, such as stannic and ferric chlorides (Dixie Chemical Co., 1995)
(i) Octanol/water partition coefficient (P): log P, –0.95 (Hansch et al., 1995)
(j) Conversion factor1: mg/m3 = 3.03 × ppm

4. Technical products and impurities
Glycidol is commercially available with a minimum purity of 95% and a maximum water content of 1% (Dixie Chemical Co., 1999). Trade names for glycidol include: Epiol OH.
5. Analysis
Glycidol can be determined in workplace air by adsorbing the air sample on charcoal, desorbing with tetrahydrofuran and analysing by gas chromatography with flame ionization detection (Eller, 1994)
III. PRODUCTION
Glycidol is commercially produced by two methods:
(1) epoxidation of allyl alcohol with hydrogen peroxide and a catalyst (tungsten or vanadium); and
(2) reaction of epichlorohydrin with caustic .
Information available in 1999 indicated that glycidol was manufactured by two companies in Japan, and one company each in Germany and the United States (Chemical Information Services, 1999).
IV. USE
In 1956, glycidol was only used for research purposes, but by 1978 it was used in the preparation of glycerol, glycidyl ethers, esters and amines in the pharmaceutical industry (Proctor & Hughes, 1978) and as a sterilant in pharmaceuticals (Ivashkiv & Dunham, 1973). Calculated from: mg/m3 = (relative molecular mass/24.45) × ppm, assuming a temperature of 25 °C and a pressure of 101 kPa Glycidol has become an important intermediate for the production of functional epoxides. For example, reaction of phosgene with glycidol yields 2,3-epoxypropyl chloroformate. Reaction of glycidol with isocyanates affords the commercially important glycidyl urethanes (Sienel et al., 1987). It is used as an intermediate in the production of pharmaceuticals, as an additive for synthetic hydraulic fluids and as a reactive diluent in some epoxy resin systems (Hooper et al., 1992; American Conference of Governmental Industrial Hygienists, 1999). It is a stabilizer for natural oils and vinyl polymers, a dye-levelling agent and a demulsifier (American Conference of Governmental Industrial Hygienists, 1986).

There were presented the applications of glycidol as an intermediate in the syntheses of many biologically active compounds used in medicine, cosmetology, and in economic chemistry. Glycidol is half-finished product in the synthesis of surfactants. These agents are used as components of cosmetic products for moistening and cleansing of skin, hair shampoo, toothpastes, washing detergents and disinfectants. Another applications of glycidol are plasticizers, fabric dyes, photochemical compounds, caoutchouc, varnish and plastics. Glycidol is also used in the synthesis of many biologically active compounds, which originally were obtained from living organisms (algae, fungi). One of the most important applications of glycidol is the synthesis of antiviral and analgesic drugs. The especially important group of antivirial drugs comprise the active compounds against the HIV virus.
V. ENVIRONMENTAL OCCURRENCE
Production of glycidol and its broad applications as an intermediate, as a reactive diluent in epoxy resins and as a stabilizer and a sterilant may result in its release into the environment through various waste streams .
VI. HAZARDS IDENTIFICATION
Emergency Overview
1. OSHA Hazards
Combustible Liquid, Carcinogen, Target Organ Effect, Toxic by inhalation., Toxic by ingestion, Toxic by skin absorption, Irritant, Teratogen, Mutagen
2. Target Organs
Nerves.
3. Other hazards which do not result in classification
Rapidly absorbed through skin.
GHS Classification
Flammable liquids (Category 4)
Acute toxicity, Oral (Category 4)
Acute toxicity, Inhalation (Category 3)
Acute toxicity, Dermal (Category 4)
Skin irritation (Category 2)
Serious eye damage (Category 1)
Germ cell mutagenicity (Category 2)
Carcinogenicity (Category 1B)
Reproductive toxicity (Category 1B)
Specific target organ toxicity - single exposure (Category 3)
VII. POTENTIAL HEALTH EFFECTS
Inhalation Toxic if inhaled. Causes respiratory tract irritation.
Skin Toxic if absorbed through skin. Causes skin irritation.
Eyes Causes eye irritation.
Ingestion Toxic if swallowed.
VIII. RESPIRATOR RECOMMENDATIONS
NIOSH
Upto150ppm: (APF=10)Anysupplied-airrespirator* (APF = 50) Any self-contained breathing apparatus with a full facepiece
Emergency or planned entry into unknown concentrations or IDLH conditions: (APF = 10,000) Any self-contained breathing apparatus that has a full facepiece and is operated in a pressure-demand or other positive-pressure mode (APF = 10,000) Any supplied-air respirator that has a full facepiece and is operated in a pressure-demand or other positive-pressure mode in combination with an auxiliary self-contained positive-pressure breathing apparatus
Escape: (APF = 50) Any air-purifying, full-facepiece respirator (gas mask) with a chin-style, front- or back-mounted organic vapor canister Any appropriate escape-type, self-contained breathing apparatus
0 notes
Text
SELECTING THE RIGHT EPOXY RESIN FOR YOUR APPLICATION
Epoxy resin has many industrial applications and possesses greater thermal and chemical resistance – as well as strengthened mechanical properties – than other types of resin. When in liquid form, epoxy resin is poured into a mold or painted over a material in layers to create a protective outer coating. After curing, the material hardens into a solid and becomes durable and structurally stable. This combination of features makes epoxy resin extremely useful in a number of applications, from industrial tooling to art projects and automotive manufacturing.
The specific combination of chemical compounds and polymerization processes will impact the resulting core characteristics of an epoxy resin formula.
Glycidol (556-52-5 ) manufacturer USA herein highlights some of the trademark properties of epoxy resin formulas:
· Heat-resistance
· Absence of VOCs (volatile organic compounds)
· Excellent fatigue strength and flexural strength
· Electrical insulation
· Chemical stability
· Low moisture absorption
· Durable adhesive bond
· Anti-corrosive
· Low shrinkage after curing
To begin the application process the epoxy resin is mixed with a co-reactant, also called a hardener, which typically comes in a separate compartment of the same package. The chemical reaction begins as soon as the two chemicals are mixed and depending on the formulation can become solid very quickly or slowly depending on your requirements. The epoxy resin manufacturer should provide instructions about the ratio of epoxy to hardener that should be used to achieve maximum strength and performance.

What’s the Difference Between Casting and Coating Epoxy Resins?
Casting and coating epoxy resins are unique but related compounds. Choosing between the two will ultimately determine how the finished product will look and function.
Casting resins, also called “deep-pour resin” or “pouring plastic,” are used for clear encasings and suspensions. The user pours the material into a mold and then cures it to retain the same shape. Casting resins are commonly used to create crafts, jewelry, sculptures, and memorabilia. Manufacturers can also produce aggregate, molded plastics, or electrical insulation with casting resin. Engineers design automotive parts, aerospace devices, sports equipment, and hundreds of other products with compounds that are fortified with epoxy resin.
Coating resins, on the other hand, are aptly named: they are used to coat materials, such as metal, concrete, or wood, to make them stronger, chip-resistant, easier to clean, water-resistant, and rust-proof. A thin layer of coating resin can also glue materials together or preserve paper. In the electrical manufacturing sector, coating resins are applied to overmold circuits and transistors, which holds components together and protects against corrosion.
Beyond these differences in application, there are a few other notable distinctions between casting and coating epoxy resin:
· Viscosity: Casting resins are normally thinner compared to coating resin.
· Curing Times: Because liquid casting resin is poured into thick layers, they take longer to cure to avoid shrinkage and heat build up..
· Hardness: Coating resins are usually stiffer and harder than casting resins.
· Mix Ratio: Most coating resins use a 1:1 ratio, but casting resin formulas can vary, such as 1:1 or 2:1.
Even though there are significant similarities between the two formula types, it’s usually easier to use the formula that’s best suited to your intended application.
If you pour casting resin on a surface instead of using a thin layer of coating resin, for example, the resin will run off the edges and will be very slow to harden.
Alternatively, if you pour coating epoxy into a mold, you’ll need to pour multiple thin layers and wait for the material to cure between applications. Otherwise, the heat generated from the material poured in a large mass will accelerate the chemical reaction and cause yellowing or cracking.
Key Questions to Consider About Epoxy Resin
If you are not sure what type of epoxy resin to opt for, consider the following:
· How thick is the layer of epoxy you need for this project?
· How long can you wait for the epoxy to cure?
· Do you need a mold or frame to prevent dripping and hold the epoxy while it cures?
· How hard does this material need to be to withstand the expected wear?
· Do you want to suspend materials in the epoxy resin?
· Do you need the epoxy to have any special properties?
· Will this material be exposed to extreme temperatures, water, chemicals, UV rays, or other potentially damaging elements?
Epoxy resin systems can be tailor-made to suit unique project needs. Manufacturers use a variety of co-reactants, including, for example, polyfunctional amines, phenols, and alcohols all of which produce slightly different results. The type of base epoxy and additives in the formula can also change the resin’s viscosity and intrinsic properties.

Epoxy Resin Viscosity
Viscosity describes a liquid’s degree of resistance to flow. Within the context of epoxy resin formulas, the viscosity determines if the material will drip or spread evenly and if it should be poured, dipped, or painted on the material. Viscosity also affects how much of the epoxy perforates the substrate and which physical properties are produced.
For example, at Glycidol (556-52-5 ) manufacturer USA, we manufacture three lines of epoxy resin with low, medium, or high viscosities:
1. Low Viscosity
Low-viscosity epoxy resin is thin and works well for deep-level penetration and filling small cavities. The consistency helps prevent air bubbles, which facilitates bonding between the epoxy and substrate. You can use low-viscosity epoxy resin for encapsulation, sealing, and potting.
2. Medium Viscosity
Medium-viscosity epoxy is thick. It’s less permeable than low-viscosity formulas and offers greater mechanical strength. The material can withstand moderately high temperatures and is often used for filament winding, vacuum bagging, and tooling.
3. High Viscosity Epoxy
High-viscosity epoxy has a paste-like consistency and is the most resilient option. It offers superior adhesion and shock- and heat-resistance. Technicians use this formula for projects that demand exceptional durability and strength.
Epoxy Resin Solutions At Agex Pharma
Agex Pharma has been a leading provider of quality epoxy resin solutions for over four decades. are thoroughly tested Our formulations for quality assurance and have demonstrated practical applications in a broad range of industries. To learn more about epoxy resins or about our products and capabilities, reach out to us or request a quote today.
0 notes