#Gene Editing Market Research
Link
#market research future#gene editing market#gene editing market size#gene editing market trends#gene editing industry
0 notes
Text
Latest Advances in Gene and Cell Therapies Transform Healthcare

Gene and cell therapies represent a ground-breaking advancement in medical science, offering potential cures for a variety of previously untreatable diseases. These therapies are revolutionizing how we provide targeted healthcare by modifying genetic material or using cells to restore or alter biological functions. Early interventions in congenital disorders can significantly reduce long-term health complications, offering a healthier start to life for newborns. Thus, the potential of gene and cell therapies to transform medical treatments is immense, especially in the field of natal and prenatal care.
A notable example of gene therapy involved the birth of the first babies with edited genes. In 2018, Dr. Jiankui announced the birth of twin girls whose genes were edited using CRISPR technology. He edited and deactivated a gene known as CCR5 with the goal of conferring resistance to HIV in those girls.
Latest Developments in Gene and Cell Therapies
The field of gene and cell therapies is crucial in the mainstream as drug-regulating authorities approve treatments for diseases like lymphoma and muscular dystrophy. Let us explore the latest developments regarding these therapies.
Non-Hodgkin lymphoma (NHL) accounts for about 4% of all cancers in the US, with an estimated 80,620 new cases expected this year. In this regard, Bristol Myers Squibb’s Breyanzi, a CAR T cell therapy, was approved in 2024 by the FDA, which utilizes the patient’s immune system to target and destroy cancer cells.
In 2024, the FDA approved Sarepta Therapeutics’ Elevidys, a gene therapy for Duchenne muscular dystrophy (DMD), which affects approximately 1 in 3,500 to 5000 male births worldwide, typically manifesting between ages 3 and 6. This groundbreaking offers new hope by addressing the root cause of this debilitating condition.
Exploring Current and Future Applications
CRISPR and Genome Editing: CRISPR technology has revolutionized genome editing, offering precise modifications to DNA and correcting genetic defects at their source. This technology is being explored for a variety of applications including current and future applications. However, acquiring approvals to run trials on humans has always been challenging, yet the CTX001 stands out with its success in this regard. The CTX001 is an autologous gene-edited stem cell therapy developed by CRISPR Therapeutics and Vertex Pharmaceuticals.
Dr. Haydar Frangoul, the medical director at HCA Sarah Cannon Research Institute Center, has been treating the first patient in the CTX001 trial for SCD therapy. The patient had battled sickle cell disease for 34 years before undergoing this one-time treatment. Post-treatment, her blood showed a significant proportion of fetal hemoglobin levels, enabling her to avoid blood transfusions and pain attacks without major side effects.
Stem Cell Research: These cells have the unique ability to differentiate into various cell types, making them invaluable for regenerative medicine. Research in stem cell therapy aims to treat conditions such as Parkinson’s disease, diabetes, and spinal cord injuries by replacing damaged cells with healthy ones in the near future. A notable example is a study using device-encapsulated pancreatic precursor cells derived from human embryonic stem cells. This study has shown that increased cell doses in optimized devices lead to detectable insulin production and improved glucose control.
CAR-T Cell Therapy: This therapy has shown impressive results in treating certain types of leukemia and lymphoma, offering hope for patients who have not responded to traditional treatments. This innovative approach uses modified T-cells to target and kill cancer cells. The future of CAR-T therapy looks promising, thereby expanding its application to treat more types of cancers, including solid tumors.
Gene Silencing and RNA-based Therapies: Emerging technologies like RNA interference (RNAi) and antisense oligonucleotides (ASOs) are being developed to silence harmful genes. An RNAi therapy like ‘AMVUTTRA’ developed by Alnylam, is approved in the US for treating polyneuropathy of hereditary transthyretin-mediated (hATTR) amyloidosis in adults. Thus, the future use of RNA therapies includes the treatment of neurodegenerative diseases like Huntington’s disease.
Understanding Ethical Considerations & the Role of Regulatory Bodies
Ethical frameworks must evolve amidst the concerns regarding ‘designer babies’, where genetic modifications used to select desired traits pose significant ethical dilemmas. A prominent example is the controversy of using CRISPR technology in human embryos, who claimed to have created the first gene-edited babies, sparking ethical debates and leading to his imprisonment. Several studies emphasize the importance of international regulatory standards and effective governance to ensure the responsible use of gene editing technologies.
Amidst the rapid pace of technological advancement, regulating gene and cell therapies needs rigorous safety standards. The regulatory bodies and agencies like the FDA’s Center for Biologics Evaluation and Research (CBER) in the US and the European Medicines Agency (EMA) in the EU play a critical role. Their frameworks include guidelines for approval of regenerative medicines and conditional or time-limited authorizations to facilitate quicker access to innovative treatments.
What the future beholds?
The future of gene and cell therapies lies in their integration into personalized medicine based on the genetic makeup of individual patients. Companies like CRISPR Therapeutics, Editas Medicine, and Intellia Therapeutics are at the forefront of research, developing therapies that could revolutionize the treatment of genetic disorders. As these therapies become more refined and accessible, they could significantly extend healthy life spans and improve the quality of life for millions.
#Gene and Cell Therapies#healthcare#lifesciences#genome editing#CRISPR technology#Stem Cell therapy#triton market research#market research reports
0 notes
Text
Clustered Regularly Interspaced Short Palindromic Repeats Gene-Editing Market Size, Share, Trends, Opportunities, Key Drivers and Growth Prospectus
CRISPR gene-editing Market report can be structured well with the blend of top attributes such as highest level of spirit, practical solutions, committed research and analysis, innovation, talent solutions, integrated approaches, most up-to-date technology and dedication. Further, strategic planning supports in improving and enhancing the products with respect to customer’s preferences and inclinations. The report comprises of all the market shares and approaches of the major competitors or the key players in the industry. Moreover, this market report also brings into the focus various strategies that have been used by other key players of the market or industry.
For the growth of business, CRISPR gene-editing Market report has a lot to offer and hence it plays a very important role in growth. It describes thorough study of current situation of the global market along with several market dynamics. Being a premium market research report, this business report works as an innovative solution for the businesses in today’s revolutionizing market place. This market report gives the best outcome because it is structured with a nice blend of advanced industry insights, practical solutions, talent solutions and latest technology. CRISPR gene-editing Market report takes into account plentiful aspects of the market analysis which many businesses demand.
Access Full 350 Pages PDF Report @
Data Bridge Market Research analyses that the Clustered regularly interspaced short palindromic repeats (CRISPR) gene-editing Market which was USD 1.09 billion in 2022, would reach up to USD 14.80 billion by 2030, and is expected to undergo a CAGR of 29.80% during the forecast period. “Oncology” dominates the therapeutic application segment of the clustered regularly interspaced short palindromic repeats (CRISPR) gene-editing market owing to the higher prevalence of cancer. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
The report provides insights on the following pointers:
Market Penetration: Comprehensive information on the product portfolios of the top players in the CRISPR gene-editing Market.
Product Development/Innovation: Detailed insights on the upcoming technologies, R&D activities, and product launches in the market.
Competitive Assessment: In-depth assessment of the market strategies, geographic and business segments of the leading players in the market.
Market Development: Comprehensive information about emerging markets. This report analyzes the market for various segments across geographies.
Market Diversification: Exhaustive information about new products, untapped geographies, recent developments, and investments in the CRISPR gene-editing Market.
TABLE OF CONTENTS
Part 01: Executive Summary
Part 02: Scope of the Report
Part 03: Research Methodology
Part 04: Market Landscape
Part 05: Pipeline Analysis
Part 06: Market Sizing
Part 07: Five Forces Analysis
Part 08: Market Segmentation
Part 09: Customer Landscape
Part 10: Regional Landscape
Part 11: Decision Framework
Part 12: Drivers and Challenges
Part 13: Market Trends
Part 14: Vendor Landscape
Part 15: Vendor Analysis
Part 16: Appendix
Countries Studied:
North America (Argentina, Brazil, Canada, Chile, Colombia, Mexico, Peru, United States, Rest of Americas)
Europe (Austria, Belgium, Denmark, Finland, France, Germany, Italy, Netherlands, Norway, Poland, Russia, Spain, Sweden, Switzerland, United Kingdom, Rest of Europe)
Middle-East and Africa (Egypt, Israel, Qatar, Saudi Arabia, South Africa, United Arab Emirates, Rest of MEA)
Asia-Pacific (Australia, Bangladesh, China, India, Indonesia, Japan, Malaysia, Philippines, Singapore, South Korea, Sri Lanka, Thailand, Taiwan, Rest of Asia-Pacific)
Objectives of the Report
To carefully analyze and forecast the size of the CRISPR gene-editing market by value and volume.
To estimate the market shares of major segments of the CRISPR gene-editing
To showcase the development of the CRISPR gene-editing market in different parts of the world.
To analyze and study micro-markets in terms of their contributions to the CRISPR gene-editing market, their prospects, and individual growth trends.
To offer precise and useful details about factors affecting the growth of the CRISPR gene-editing
To provide a meticulous assessment of crucial business strategies used by leading companies operating in the CRISPR gene-editing market, which include research and development, collaborations, agreements, partnerships, acquisitions, mergers, new developments, and product launches.
Some of the major players operating in the clustered regularly interspaced short palindromic repeats (CRISPR) gene-editing market are:
Applied StemCell (U.S.)
ACEA BIO (U.S.)
Synthego (U.S.)
Thermo Fisher Scientific (U.S.)
GenScript (China)
Addgene (U.S.)
Merck KGaA (Germany)
Intellia Therapeutics, Inc. (U.S.)
Cellectis (France)
Precision Biosciences (U.S.)
Caribou Biosciences, Inc. (U.S.)
Transposagen Biopharmaceuticals, Inc. (U.S.)
OriGene Technologies, Inc. (U.S.)
Novartis AG (Switzerland)
New England Biolabs (U.S.)
Rockland Immunochemicals Inc. (U.S.)
ToolGen, Inc. (South Korea)
TAKARA BIO INC. (Japan)
Agilent Technologies, Inc. (U.S.)
Abcam plc (U.K.)
CRISPR Therapeutics AG (Switzerland)
Browse Trending Reports:
Tubeless Insulin Pumps Market
Prenatal Testing And New Born Screening Market
Rapid Oral Fluid Screening Device Market
North America Eclinical Solutions Market
Medical Tuning Fork Market
About Data Bridge Market Research:
Data Bridge set forth itself as an unconventional and neoteric Market research and consulting firm with unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process.
Contact Us:
Data Bridge Market Research
US: +1 888 387 2818
UK: +44 208 089 1725
Hong Kong: +852 8192 7475
Email: [email protected]
#Clustered Regularly Interspaced Short Palindromic Repeats Gene-Editing Market Size#Key Drivers and Growth Prospectus#market trends#market share#market research#market size#market report#market analysis#markettrends#marketresearch#Clustered Regularly Interspaced Short Palindromic Repeats Gene-Editing
0 notes
Text
The Benefits and Dangers of Gene Editing: A Look at CRISPR Technology
Gene editing has been an area of intense scientific research for many years. Recent developments in the CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) technology have provided new and exciting possibilities for gene editing, leading to promising medical applications such as treating genetic diseases and enhancing disease resistance in crops. However, the use of CRISPR technology also raises significant ethical concerns and potential dangers.
One of the most significant benefits of CRISPR technology is its potential to cure genetic diseases. CRISPR can be used to precisely target and edit DNA, allowing scientists to remove, replace or repair the faulty genes that cause genetic diseases. This has the potential to transform medicine and alleviate the suffering of millions of people worldwide.
Another benefit of CRISPR is its potential to enhance crop disease resistance and increase food security. Scientists can use CRISPR to create genetically modified crops that are resistant to pests, diseases, and environmental stressors, making them more resilient and less prone to crop failures. This could be a game-changer in the fight against global hunger and malnutrition.
However, the use of CRISPR technology also raises significant ethical concerns. The ability to manipulate human genes brings up questions of how far we should go in modifying the human genome. There are fears that CRISPR could be used to create "designer babies," which would involve selecting specific traits such as intelligence, height, and eye color. This could lead to a widening of the gap between the rich and poor, and potentially even create a new genetic underclass.
Moreover, there are potential dangers associated with CRISPR technology. Although the technology is highly precise, there is still a risk of off-target effects, which could cause unintended mutations in the DNA. There are also concerns about the potential for CRISPR to be used as a biological weapon, creating dangerous viruses or genetically modified organisms.
In conclusion, CRISPR technology offers exciting possibilities for gene editing that could transform medicine and agriculture. However, the ethical and safety concerns surrounding its use cannot be ignored. It is essential that scientists and policymakers work together to ensure that the technology is used in a responsible and safe way, balancing the benefits and risks.
1 note
·
View note
Text
The Immortalized Cell Line Market is projected to grow from USD 4485 million in 2024 to an estimated USD 6867.426 million by 2032, with a compound annual growth rate (CAGR) of 5.47% from 2024 to 2032.The immortalized cell line market has been experiencing substantial growth over the past few years, driven by rising demand for biologics, advanced drug discovery processes, and an increasing prevalence of chronic diseases. Immortalized cell lines are cultured cells that can divide indefinitely and are widely used in research and biotechnology applications. They have become indispensable tools for cancer research, vaccine production, toxicology testing, and gene therapy development. This article delves into the key trends, drivers, challenges, and future prospects of the immortalized cell line market.
Browse the full report at https://www.credenceresearch.com/report/immortalized-cell-line-market
Key Drivers of Market Growth
1. Rise in Chronic Diseases
The growing global burden of chronic diseases, such as cancer, diabetes, and cardiovascular conditions, has fueled the demand for immortalized cell lines. These cell lines are crucial for understanding disease mechanisms, testing potential treatments, and developing personalized medicine. The cancer segment, in particular, is a significant contributor to the market, as immortalized cancer cell lines are essential for studying tumor biology and drug resistance.
2. Advancements in Biotechnology and Drug Discovery
Immortalized cell lines play a pivotal role in modern drug discovery and development. With the emergence of high-throughput screening technologies, pharmaceutical companies can now test a large number of potential drug compounds quickly and efficiently. This has been especially relevant in the development of targeted therapies and immunotherapies, which rely on cellular models to identify and validate drug targets.
3. Increasing Demand for Biologics
Biologics, such as monoclonal antibodies, vaccines, and recombinant proteins, have emerged as a significant class of therapeutics. The production of biologics requires the use of immortalized cell lines as bioreactors for the production of large quantities of proteins. The expanding biologics pipeline, driven by increasing regulatory approvals, is further boosting the demand for high-quality and stable immortalized cell lines.
4. Advances in Gene Editing Technologies
The advent of CRISPR-Cas9 and other gene-editing tools has revolutionized the field of cellular biology, allowing scientists to create custom immortalized cell lines with specific genetic modifications. This has opened new avenues for research, enabling the creation of disease models that closely mimic human conditions. Such cell lines are invaluable for studying gene function, conducting functional genomics studies, and developing precision medicine approaches.
Key Challenges
Despite the promising growth, several challenges persist in the immortalized cell line market:
1. Ethical Concerns: The use of certain cell lines, such as those derived from human embryos or aborted fetal tissue, raises ethical issues. These concerns can limit research or create regulatory hurdles.
2. Contamination and Misidentification: Immortalized cell lines can be prone to contamination, and misidentification of cell lines is a well-documented issue in the scientific community. This can lead to unreliable data and wasted resources, highlighting the need for improved cell line authentication processes.
3. Regulatory Hurdles: The use of genetically modified cell lines and the production of biologics in cell-based systems are subject to stringent regulatory scrutiny. Meeting the necessary regulatory requirements can slow down product development and increase costs.
Future Outlook
The future of the immortalized cell line market looks promising, with advancements in biopharmaceuticals, personalized medicine, and regenerative therapies poised to drive growth. The integration of artificial intelligence (AI) and machine learning into drug discovery processes will also create new opportunities for utilizing immortalized cell lines in innovative ways. Furthermore, as the demand for biologics continues to rise, the need for robust, scalable, and high-yield cell line systems will grow.
Key Player Analysis:
ATCC (American Type Culture Collection Inc.) (US)
Corning Incorporated
Creative Bioarray
European Collection of Authenticated Cell Cultures (ECACC) (Europe)
General Electric Company
InSCREENeX GmbH
Lonza Group, AG (Switzerland)
Merck KGaA (Germany)
Public Health England
Sartorius AG (Germany)
Selexis SA (Switzerland)
Sigma-Aldrich Co.
TCC
Thermo Fisher Scientific (US)
Valneva (France),
WuXi App Tec(China)
Segmentation:
By Method,
Viral,
Non-viral,
Hybrid methods.
By Application,
Drug discovery and development,
Cancer research,
Tissue engineering.
By End User,
Pharmaceutical and biotechnology companies,
Academic and research institutions,
Contract research organizations (CROs).
By Region
North America
The U.S
Canada
Mexico
Europe
Germany
France
The U.K.
Italy
Spain
Rest of Europe
Asia Pacific
China
Japan
India
South Korea
South-east Asia
Rest of Asia Pacific
Latin America
Brazil
Argentina
Rest of Latin America
Middle East & Africa
GCC Countries
South Africa
Rest of Middle East and Africa
Browse the full report at https://www.credenceresearch.com/report/immortalized-cell-line-market
About Us:
Credence Research is committed to employee well-being and productivity. Following the COVID-19 pandemic, we have implemented a permanent work-from-home policy for all employees.
Contact:
Credence Research
Please contact us at +91 6232 49 3207
Email: [email protected]
Website: www.credenceresearch.com
0 notes
Text
Global Cell Signaling Market Size, Share, Segmentations and Forecast 2032 by Industry Key Players, Solutions and Deployment Types
The global cell signaling market is expected to experience significant growth over the coming decade, with market size forecast to expand from USD 5.1 billion in 2023 to USD 9.4 billion by 2032. This growth represents a compound annual growth rate (CAGR) of 7.1% from 2024 to 2032, driven by advancements in biotechnology, pharmaceuticals, and the growing demand for targeted therapies in cancer and chronic diseases.
Cell signaling refers to the complex system of communication that governs basic cellular activities and coordinates cell actions. It is a critical process in maintaining homeostasis, as well as in the development of diseases. This intricate communication system is central to understanding a wide range of physiological processes and pathologies, including cancer, immunology, and neurobiology.
Key Market Drivers
Rising Prevalence of Chronic Diseases: One of the primary drivers of growth in the cell signaling market is the increasing prevalence of chronic diseases such as cancer, cardiovascular diseases, diabetes, and neurodegenerative disorders. These diseases often result from disrupted or abnormal cell signaling pathways. Consequently, there is a growing demand for research into targeted therapies that can precisely modulate these signaling pathways to treat such conditions. The development of drugs that act on specific signaling molecules has become a major area of focus in the pharmaceutical industry, driving market expansion.
Technological Advancements in Molecular Biology: Rapid advancements in molecular biology and biotechnology have led to breakthroughs in understanding cell signaling pathways. Technologies such as CRISPR gene editing, high-throughput screening, and next-generation sequencing are enabling researchers to study signaling networks at unprecedented levels of detail. These innovations have spurred the development of new diagnostic tools and therapeutic approaches aimed at modulating cell signaling in disease states.
Increasing Focus on Personalized Medicine: The shift toward personalized medicine, where treatments are tailored to an individual’s genetic makeup and specific cellular processes, is another major factor propelling the growth of the cell signaling market. By gaining a better understanding of an individual’s cell signaling pathways, healthcare providers can develop more effective and less toxic treatment plans, particularly for complex conditions like cancer. Personalized medicine is revolutionizing healthcare, and cell signaling research is at the heart of these innovations.
Investment in Cancer Research: Cell signaling research plays a critical role in oncology, as cancer is often driven by mutations in signaling pathways that control cell growth, division, and death. The market is benefiting from substantial investments in cancer research, with a focus on discovering and developing novel therapies that target specific signaling molecules or pathways involved in tumor progression. The introduction of targeted therapies, such as tyrosine kinase inhibitors and immunotherapies, has already transformed cancer treatment and continues to drive growth in this sector.
Get Free Sample Report: https://www.snsinsider.com/sample-request/4501
Challenges and Opportunities
While the cell signaling market shows great potential, there are challenges that may affect growth. The complexity of cell signaling pathways, the high cost of research, and the time required to develop effective therapies can be significant barriers to market entry. Additionally, regulatory hurdles for new therapies targeting cell signaling molecules can slow down the approval process.
However, these challenges present opportunities for further innovation. Continued research into unexplored signaling pathways and the application of cutting-edge technologies like artificial intelligence (AI) in drug discovery are expected to drive new developments. AI and machine learning are already being used to predict signaling pathway interactions and optimize drug candidates, streamlining the drug development process and reducing costs.
Regional Insights
North America is the largest market for cell signaling, owing to the region’s robust biopharmaceutical industry, high investment in healthcare research, and the presence of key players in the field. The U.S. leads in funding for cancer research and targeted therapies, driving significant demand for cell signaling technologies. Europe follows closely, with a strong focus on advancing research in molecular biology and biotechnology.
The Asia-Pacific region is expected to see the fastest growth during the forecast period, supported by increasing government investments in life sciences, expanding pharmaceutical manufacturing, and growing healthcare infrastructure. China, India, and Japan are becoming prominent markets for cell signaling research due to rising healthcare needs and a growing focus on innovative treatments.
Future Outlook
The cell signaling market is poised for sustained growth as demand for precision medicine and advanced therapies continues to rise. With a projected CAGR of 7.1% from 2024 to 2032, the market is set to reach USD 9.4 billion by 2032. As the understanding of cellular communication deepens and new technologies emerge, the market is expected to play a pivotal role in the development of next-generation treatments for a range of diseases, including cancer, autoimmune disorders, and neurological conditions.
In conclusion, the global cell signaling market is on a steady upward trajectory, driven by advancements in biotechnology, growing investment in cancer research, and the increasing focus on personalized medicine. From USD 5.1 billion in 2023, the market is expected to grow to USD 9.4 billion by 2032, reshaping the landscape of drug discovery and disease treatment.
Other Trending Reports
Defibrillator Market
Healthcare Waste Management Market
PET Scanners Market
Antidepressants Market
0 notes
Text
Lipid Nanoparticles: A Game-Changer in Gene Delivery
Lipid nanoparticles (LNPs) are emerging as a revolutionary tool in gene delivery, transforming how therapeutic genetic material is introduced into target cells. With advancements in gene therapy and the increasing importance of personalized medicine, lipid nanoparticles have taken center stage in ensuring safe, efficient, and targeted delivery of genetic materials like DNA, RNA, and siRNA.

Download PDF Brochure
What Are Lipid Nanoparticles?
Lipid nanoparticles are tiny, lipid-based carriers designed to encapsulate and protect genetic material as it moves through the body. These particles are composed of various lipids, including cationic, ionizable, and neutral lipids, which enable them to form stable structures around their cargo. Their small size and composition allow LNPs to evade the immune system, extend circulation time, and improve the efficiency of gene delivery.
Why Are LNPs Important for Gene Delivery?
Delivering genetic material into cells is a complex task. Naked DNA or RNA can be degraded by enzymes in the bloodstream, fail to reach target tissues, or trigger immune responses. Lipid nanoparticles offer a protective and non-toxic alternative to traditional viral vectors used in gene therapy. They can be engineered to deliver their cargo selectively to specific tissues, such as the liver, lungs, or muscles, improving the efficacy of treatments while minimizing side effects.
Key Advantages of Lipid Nanoparticles for Gene Delivery
Enhanced Protection: LNPs shield genetic material from degradation in the bloodstream, ensuring that the cargo remains intact and functional by the time it reaches target cells.
Efficient Cellular Uptake: Lipid nanoparticles can easily fuse with cell membranes, allowing the enclosed genes to enter the cells and initiate their therapeutic action.
Reduced Immune Response: Unlike viral vectors, LNPs do not typically provoke strong immune responses, making them safer for repeated administration in gene therapies.
Scalability and Versatility: LNPs can be scaled up for large-scale production, which is crucial for the development of gene therapies and vaccines that require widespread distribution. They can also be adapted for various genetic payloads, from mRNA to CRISPR-Cas9 components.
Request Sample Pages
Applications of Lipid Nanoparticles in Gene Therapy
LNPs have shown significant promise in various gene therapy applications, such as:
mRNA-based vaccines: The success of mRNA COVID-19 vaccines was largely due to lipid nanoparticles, which delivered the genetic instructions to cells to produce the spike protein, stimulating an immune response.
CRISPR-based therapies: LNPs can carry CRISPR components to specific tissues, enabling precision gene editing for the treatment of genetic disorders.
RNAi therapies: For conditions where certain genes need to be silenced, LNPs can deliver siRNA (small interfering RNA) to block the expression of harmful proteins.
Challenges and Future Directions
While lipid nanoparticles offer tremendous potential, challenges remain. For example, achieving precise targeting in tissues other than the liver can be difficult, and understanding the long-term effects of LNP-based therapies is an ongoing area of research. Despite these hurdles, innovations in LNP design and functionality are paving the way for new breakthroughs in gene therapy.
Conclusion
Lipid nanoparticles are poised to play a pivotal role in the future of gene delivery, offering a safe, efficient, and scalable option for treating a variety of diseases. As research and technology continue to advance, we can expect LNPs to unlock new possibilities in the fields of gene therapy and personalized medicine.
Content Source:
0 notes
Text
Gene-Editing Market Forecast: USD 12.9 Billion in Value by 2030 with 14.5% CAGR

The Gene Editing Market is one of the fastest-growing segments in the biotechnology industry today. With advancements in CRISPR and other gene-editing technologies, it is expected to grow from USD 5.0 billion in 2023 at a compound annual growth rate (CAGR) of 14.5% to reach USD 12.9 billion by 2030. In this article, we will dive into the details of this market, exploring the key players, factors driving growth, challenges, and what lies ahead for gene editing.
What is Gene Editing?
Gene editing is a revolutionary technology that allows scientists to alter the DNA of living organisms. It can correct genetic defects, prevent the spread of diseases, and potentially enhance specific traits in organisms. The rise of tools like CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) has made gene editing more precise, faster, and cost-effective.
Access Full Report @ https://intentmarketresearch.com/latest-reports/gene-editing-market-3120.html
Key Drivers of Growth in the Gene Editing Market
Advances in Technology
Innovations such as CRISPR and TALEN (Transcription Activator-Like Effector Nucleases) have drastically improved the efficiency of gene editing, making it more accessible to a wider range of industries and applications.
Increased Research and Development (R&D) Investments
Major pharmaceutical and biotech companies are investing heavily in R&D to develop novel gene-editing therapies, particularly for genetic diseases.
Rising Prevalence of Genetic Disorders
As the understanding of genetic disorders grows, so does the demand for gene-editing solutions that can offer targeted treatments and potential cures.
Applications Beyond Healthcare
Gene editing is not limited to the medical field; it also plays a critical role in agriculture, environmental science, and synthetic biology, expanding its market potential.
Key Players in the Gene Editing Market
Several companies and institutions are driving innovation and commercialization in the gene-editing field. Some of the top players include:
◘ Merck
◘ Thermo Fisher Scientific
◘ GenScript
◘ Tecan Life Sciences
◘ Agilent Technologies
◘ PerkinElmer
◘ Lonza
◘ Sangamo Therapeutics
◘ Bluebird Bio
◘ Creative Biogene
Challenges Facing the Gene Editing Market
Despite its potential, the gene-editing market faces several challenges:
Ethical Concerns
Gene editing, especially in humans, raises significant ethical questions about its potential misuse, such as editing embryos or creating "designer babies."
Regulatory Hurdles
The approval process for gene-editing therapies is often lengthy and complex, with regulatory bodies like the FDA requiring extensive clinical trials to ensure safety and efficacy.
Cost of Treatments
While gene editing has the potential to offer life-changing treatments, the high cost of these therapies can limit accessibility for patients.
Off-Target Effects
One of the risks associated with gene editing is the possibility of off-target effects, where unintended parts of the genome are altered, leading to unpredictable outcomes.
Market Segmentation
The gene-editing market can be segmented into various categories based on the application, technology, and end-user. Let’s explore these segments:
By Technology
CRISPR: The most well-known and widely used tool due to its precision and cost-effectiveness.
TALEN: Used for specific types of gene edits and offers high accuracy.
ZFN (Zinc Finger Nucleases): One of the oldest gene-editing technologies, still used in certain applications.
By Application
Healthcare: Includes genetic therapies for diseases like cancer, hemophilia, and sickle cell anemia.
Agriculture: Enhancing crop resistance to diseases, pests, and environmental conditions.
Industrial Biotechnology: Using gene editing to optimize microorganisms for biofuel production and waste management.
By End-User
Pharmaceutical Companies: Focus on developing gene therapies for rare and chronic diseases.
Academic and Research Institutions: Driving the basic research that leads to the next generation of gene-editing tools.
Biotechnology Companies: Applying gene-editing technologies across various industries, from healthcare to agriculture.
Download Sample Report @ https://intentmarketresearch.com/request-sample/gene-editing-market-3120.html
Regional Insights
The gene-editing market is growing worldwide, but there are significant differences in growth rates and market adoption across regions.
North America
North America is currently the largest market for gene editing, driven by high levels of investment in biotechnology, robust research infrastructure, and supportive regulatory frameworks. The U.S. leads the charge with a majority of clinical trials and gene-editing startups.
Europe
Europe is also a significant player, particularly in countries like the UK, Germany, and France. However, regulatory challenges are more stringent in Europe, which can slow down market growth.
Asia-Pacific
The Asia-Pacific region is expected to see the fastest growth over the next decade, with countries like China and Japan investing heavily in gene-editing technologies. China's relaxed regulations and extensive R&D capabilities make it a major contender in the global market.
Future Trends in the Gene Editing Market
Expansion of CRISPR Applications
CRISPR is likely to remain the dominant gene-editing technology, but its applications will continue to expand beyond healthcare into industries like agriculture and synthetic biology.
Personalized Medicine
As gene-editing technologies improve, we could see a future where personalized medicine is the norm, with treatments tailored to individual genetic profiles.
Collaboration Between Academia and Industry
Increased collaboration between academic institutions and biotech companies will drive innovation and commercialization in the gene-editing space.
Decreasing Costs
As the technology matures, we can expect the costs of gene-editing therapies to come down, making these treatments more accessible to a broader population.
Conclusion
The gene-editing market is poised for explosive growth in the coming years, driven by technological advancements, increased investment, and rising demand for targeted genetic therapies. While challenges such as ethical concerns and regulatory hurdles remain, the potential of gene editing to revolutionize medicine, agriculture, and biotechnology is undeniable. As the market expands, the impact of gene editing on society will only continue to grow.
FAQs
What is driving the growth of the gene-editing market?
The growth is primarily driven by advances in technologies like CRISPR, increased R&D investments, and rising demand for genetic therapies.
What are the key applications of gene editing?
Gene editing is used in healthcare for genetic therapies, in agriculture for crop enhancement, and in biotechnology for industrial applications like biofuels.
What are the ethical concerns surrounding gene editing?
Ethical concerns include the potential misuse of gene editing, such as creating designer babies or altering human embryos, raising questions about the limits of the technology.
Which companies are leading the gene-editing market?
Major players include CRISPR Therapeutics, Editas Medicine, Intellia Therapeutics, and Sangamo Therapeutics.
What challenges does the gene-editing market face?
The market faces challenges such as ethical concerns, regulatory hurdles, high treatment costs, and risks associated with off-target effects.
About Us
Intent Market Research (IMR) is dedicated to delivering distinctive market insights, focusing on the sustainable and inclusive growth of our clients. We provide in-depth market research reports and consulting services, empowering businesses to make informed, data-driven decisions.
Our market intelligence reports are grounded in factual and relevant insights across various industries, including chemicals & materials, healthcare, food & beverage, automotive & transportation, energy & power, packaging, industrial equipment, building & construction, aerospace & defense, and semiconductor & electronics, among others.
We adopt a highly collaborative approach, partnering closely with clients to drive transformative changes that benefit all stakeholders. With a strong commitment to innovation, we aim to help businesses expand, build sustainable advantages, and create meaningful, positive impacts.
Contact Us
US: +1 463-583-2713
0 notes
Text
The Future of Stem Cell Research: Insights from Mordor
Stem cell research holds immense promise for revolutionizing the field of medicine. These unique cells, with their ability to self-renew and differentiate into specialized cell types, offer potential for treating a vast array of diseases and injuries. According to Mordor Intelligence, the global stem cell research market is expected to reach a staggering USD 44.20 billion by 2029, reflecting a CAGR of 11.84%. Let's explore the exciting advancements, ongoing challenges, and the promising future of stem cell research.
Driving Innovation and Progress
Therapeutic Applications: Stem cells hold the potential to regenerate damaged tissues and organs, offering new approaches to treat conditions like Parkinson's disease, diabetes, and spinal cord injuries.
Personalized Medicine: Stem cells from a patient's own body can be used to develop personalized therapies, reducing the risk of rejection and fostering a more targeted approach to treatment.
Drug Discovery and Development: Stem cell-based models can be used to test the effectiveness and safety of new drugs, potentially accelerating drug development timelines and improving outcomes.
Disease Modeling: Stem cells can be used to create in vitro models of human diseases, allowing for a deeper understanding of disease mechanisms and facilitating the development of novel therapies.
Advancements in Gene Editing: Technologies like CRISPR-Cas9 offer the potential to precisely edit the genomes of stem cells, correcting genetic mutations and paving the way for new treatment strategies.
Challenges and Considerations
Despite significant progress, stem cell research still faces some hurdles. Ethical concerns surrounding the use of embryonic stem cells require careful consideration and responsible research practices.
Furthermore, the long timeline required for translating research findings into clinical applications necessitates sustained funding and collaboration between researchers, clinicians, and pharmaceutical companies.
Additionally, ensuring the safety and efficacy of stem cell therapies in clinical trials is crucial for gaining regulatory approval and public trust.
A Bright Future on the Horizon
As research continues to address these challenges, the future of stem cell research appears bright. Advancements in areas like cell reprogramming and the development of induced pluripotent stem cells (iPSCs) offer alternative sources of stem cells, alleviating ethical concerns.
Furthermore, increased funding and international collaborations are accelerating research and development efforts.
Conclusion
Stem cell research stands at the forefront of medical innovation, offering unparalleled hope for treating some of humanity's most challenging diseases. By addressing ethical concerns, overcoming technical hurdles, and fostering collaboration, the future of stem cell research holds immense potential for transforming medicine and improving the lives of millions.
#Stem cell research market#Stem cell research market size#Stem cell research market research reports#Stem cell research market forecasts#Stem cell research market trends#Stem cell research market growth drivers
0 notes
Text
Frasier Syndrome: Unraveling the Mysteries of a Rare and Progressive Genetic Condition
Cryptophthalmos syndrome, also known as Frasier-Noonan-Costello syndrome, is a very rare genetic disorder characterized by premature accelerated aging. It is caused by mutations in the PTPN11 gene, which provides instructions for making a protein called SHP-2.
Signs and Symptoms of Frasier Syndrome
Some of the key signs and symptoms of Cryptophthalmos syndrome include:
- characteristic facial features like sagging jowls, sparse hair, bushy eyebrows, and small ears and mouth.
- short stature and bone age delays with bone overgrowth resulting in bent joints.
- accelerated aging process that can cause heart problems, joint and skin issues at a young age. Frasier Syndrome causes someone with this syndrome to physically appear older than their chronological age.
- intellectual disabilities and learning difficulties ranging from mild to severe. Developmental delays are common.
- heart abnormalities like pulmonary stenosis which is the narrowing of the pulmonary valve that regulates blood flow from the heart to the lungs. This requires surgery in many cases.
- bleeding disorders due to platelet dysfunction. Platelets help form blood clots.
Get more insights on Frasier Syndrome
Discover the Report for More Insights, Tailored to Your Language
French
German
Italian
Russian
Japanese
Chinese
Korean
Portuguese
Vaagisha brings over three years of expertise as a content editor in the market research domain. Originally a creative writer, she discovered her passion for editing, combining her flair for writing with a meticulous eye for detail. Her ability to craft and refine compelling content makes her an invaluable asset in delivering polished and engaging write-ups.
(LinkedIn: https://www.linkedin.com/in/vaagisha-singh-8080b91)

#Frasier Syndrome#Genetic Disorder#Nephrotic Syndrome#Gonadal Dysgenesis#Chronic Kidney Disease#Proteinuria#Ambiguous Genitalia#Progressive Glomerulopathy#Male Pseudohermaphroditism
0 notes
Text
DNA and Gene Cloning Services Market: Technological Advancements Driving Innovation

Introduction to DNA and Gene Cloning Services Market
The DNA and Gene Cloning Services Market is experiencing robust growth, driven by the increasing demand for gene editing technologies, advancements in molecular biology, and rising applications in biotechnology and healthcare. These services support research in drug development, agriculture, and personalized medicine, allowing scientists to clone, manipulate, and study genes. The market benefits from innovations in CRISPR, next-gen sequencing, and synthetic biology. With the expansion of biopharma and growing investments in genetic research, the DNA and gene cloning services sector is poised for significant growth in the coming years.
The DNA and Gene Cloning Services Market is Valued USD 3.02 billion in 2024 and projected to reach USD 7.84 billion by 2030, growing at a CAGR of CAGR of 14.6% During the Forecast period of 2024-2032.Its applications span drug development, gene therapy, agriculture, and synthetic biology. With advances in gene editing tools such as CRISPR and the increasing demand for personalized medicine, the market is expanding rapidly. Companies offer customized cloning services, including vector construction, mutagenesis, and gene synthesis, driving market competition and fostering innovation.
Access Full Report :https://www.marketdigits.com/checkout/3785?lic=s
Major Classifications are as follows:
By Product Type
Custom Cloning
Gene Synthesis
Sub-cloning
Others
By Application
DNA Sequencing
Genotyping
Mutagenesis
Others
By End-user
Academic & Research Institutes
Pharmaceutical & Biotechnology Companies
Others
Key Region/Countries are Classified as Follows:
◘ North America (United States, Canada,)
◘ Latin America (Brazil, Mexico, Argentina,)
◘ Asia-Pacific (China, Japan, Korea, India, and Southeast Asia)
◘ Europe (UK,Germany,France,Italy,Spain,Russia,)
◘ The Middle East and Africa (Saudi Arabia, UAE, Egypt, Nigeria, and South
Key Players of DNA and Gene Cloning Services Market
Aragen Life Sciences, Bio-Techne, Charles River Laboratories, Curia, Eurofins, GenScript, Integrated DNA Technologies, MedGenome, Sino Biological, Syngene, Twist Bioscience and Others
Market Drivers in DNA and Gene Cloning Services Market
Technological Advancements: Cutting-edge tools like CRISPR, along with next-generation sequencing, have revolutionized the field, driving the demand for cloning services.
Growth in Biotechnology and Pharmaceuticals: The increasing use of gene cloning in drug discovery, gene therapy, and personalized medicine boosts market growth.
Rising R&D Investments: Governments and private sector investments in genetic research contribute to expanding the market.
Market Challenges in DNA and Gene Cloning Services Market
Regulatory Hurdles: Stricter government regulations around gene manipulation and cloning practices present challenges for service providers.
Ethical Concerns: Ethical debates surrounding cloning, particularly human gene cloning, can restrict market expansion in certain regions.
High Costs: The technologies and expertise required for cloning services remain expensive, limiting access for smaller research institutions.
Market Opportunities of DNA and Gene Cloning Services Market
Expansion in Precision Medicine: The growing demand for tailored treatments provides immense opportunities for gene cloning in personalized medicine.
Agricultural Advancements: Applications in genetically modified crops for improved yield and resistance create new markets for cloning services.
Emerging Markets: Expanding biotechnology sectors in Asia-Pacific and Latin America offer significant growth potential.
Conclusion
The DNA and Gene Cloning Services Market is poised for significant expansion, driven by technological advancements, increasing research activities, and growing demand for precision medicine. While challenges such as regulatory issues and high costs exist, the market’s vast opportunities in sectors like agriculture, personalized medicine, and biopharma ensure a promising future. Investments in innovation and strategic partnerships will be key in harnessing the full potential of this evolving market.
0 notes
Text
Water Treatment Technology Market Report - Forecast (2024–2030)
Water Treatment Technology Market size is estimated to reach US$82.7 billion by 2030, growing at a CAGR of 5.2% during the forecast period 2024–2030. Stringent regulations and Growing industrialization are expected to propel the growth of Water Treatment Technology Market.
The shift towards decreased groundwater pumping reflects a growing awareness of the importance of sustainable water management practices. Instead of solely relying on groundwater sources, industries and municipalities are increasingly investing in water treatment technologies to utilize alternative water sources such as surface water, reclaimed wastewater, and desalinated seawater. Another one is the focus on infrastructure improvements in water treatment systems. Aging infrastructure, coupled with increasing water demand, has led to investments in upgrading and modernizing water treatment facilities. This includes the adoption of advanced treatment technologies, automation, and digitalization to enhance the efficiency, reliability, and resilience of water treatment processes. These trends are shaping the market growth in the water treatment Technology.
Sample Report:
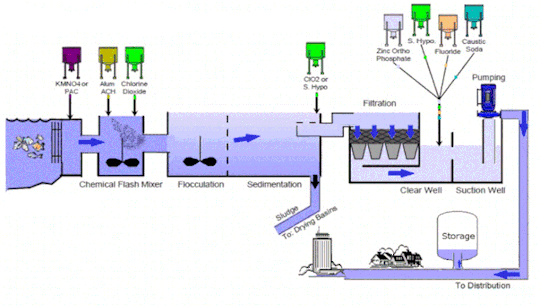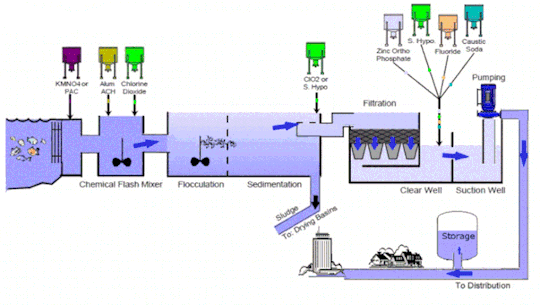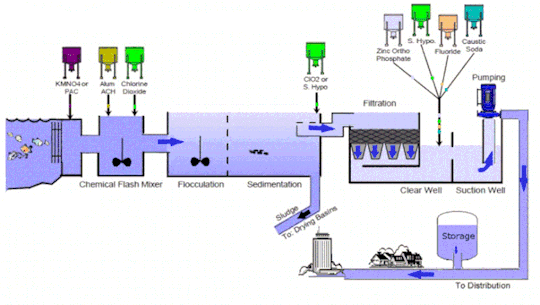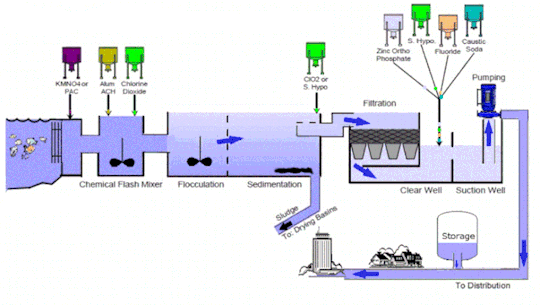
Water treatment technology is an essential line of defense to eliminate bacteria and contaminants before the supply of potable and #clean water for consumption. It includes several stages such as collection, screening & straining, #chemical addition, coagulation & flocculation, sedimentation & clarification, filtration, disinfection, storage, and distribution
Market Growth and Trends:
Driven by the rising incidence of cancer, increasing investments in biotechnology, and advancements in gene-editing technologies, the global Engineered T Cells Market is projected to grow exponentially in the coming years. According to market analysts, the market is expected to expand at a compound annual growth rate (CAGR) of 30–35% over the next decade, with North America currently leading in terms of both market share and innovation. The growing interest in cell-based therapies, particularly in oncology, is further propelling this growth.
Inquiry Before:
Key factors influencing the market include:
Advances in Gene-Editing Technologies: The development of tools such as CRISPR and other gene-editing platforms have accelerated the creation of more precise and efficient T cell therapies.
Regulatory Approvals: In recent years, therapies like Kymriah and Yescarta have gained FDA approval, setting the stage for a wave of new products.
Partnerships and Collaborations: Pharmaceutical companies are forming strategic alliances with biotechnology firms to co-develop innovative engineered T cell therapies.
Challenges and Opportunities
While the potential for engineered T cells is vast, there are several challenges that must be addressed. These include:
High Cost of Treatment: Current therapies can cost up to $500,000 per patient, creating a barrier for widespread adoption.
Safety and Efficacy: Concerns remain over side effects like cytokine release syndrome (CRS) and the long-term durability of T cell responses.
Manufacturing Complexities: The production process for engineered T cells is time-consuming and complex, which may limit scalability.
Schedule a Call:
Competitive Landscape
Major players in the Engineered T Cells Market include:
Novartis (Kymriah)
Gilead Sciences (Yescarta)
Bristol-Myers Squibb (Breyanzi)
Bluebird Bio
Autolus Therapeutics
The Future of Engineered T Cell Therapy:
Looking forward, the potential of engineered T cells extends beyond oncology. Research is underway to explore the use of these therapies in autoimmune diseases, infectious diseases, and even some neurological conditions. As the science evolves, it’s clear that the Engineered T Cells Market will remain at the forefront of cutting-edge biotechnology, offering new hope to millions of patients worldwide.
Conclusion
The engineered T cells market is on the cusp of tremendous growth, fueled by technological advancements and the urgent need for more effective treatments in oncology and other disease areas. Companies investing in this space are not only pushing the boundaries of what is possible in immunotherapy but are also setting the stage for a future where personalized medicine becomes the norm. The next few years will be crucial in shaping the market as more therapies move from clinical trials to commercial success.
Buy Now:
Innovations in Water Treatment Technology: Paving the Way for a Sustainable Future
As the global population grows and industrial activities expand, the demand for clean and safe water has never been greater. Water treatment technology plays a critical role in ensuring that water resources are purified and made safe for consumption, industrial use, and environmental protection. With increasing concerns about water scarcity, pollution, and environmental sustainability, the water treatment industry is undergoing rapid innovation to address these challenges.
Key Advancements in Water Treatment Technology
Membrane Filtration Technologies:
Reverse Osmosis (RO) and Nanofiltration (NF) membranes are widely used to remove salts, contaminants, and even microscopic pollutants like bacteria and viruses. These systems are highly efficient and are essential for desalination, turning seawater into drinkable water.
Ultrafiltration (UF) and Microfiltration (MF) systems, using porous membranes, are increasingly employed in municipal and industrial wastewater treatment to filter out particulate matter.
Advanced Oxidation Processes (AOPs):
AOPs are chemical processes that involve highly reactive species like hydroxyl radicals to break down and eliminate harmful organic pollutants. These methods, including ozonation and UV/Hydrogen Peroxide, are used in treating industrial wastewater, pharmaceuticals, and removing emerging contaminants like PFAS (per- and polyfluoroalkyl substances).
Biological Treatment Technologies:
In bioreactors, microorganisms are harnessed to degrade organic contaminants in wastewater, making it suitable for reuse or safe discharge. Technologies like Membrane Bioreactors (MBR) and Moving Bed Biofilm Reactors (MBBR) are revolutionizing biological wastewater treatment, offering higher efficiency in smaller, more compact systems.
Electrochemical Water Treatment:
This technology uses electrical currents to drive chemical reactions that purify water. Electrocoagulation, electrodialysis, and capacitive deionization (CDI) are effective in removing dissolved solids, heavy metals, and other pollutants, making them particularly useful in industrial water treatment.
Smart Water Systems & IoT:
The integration of IoT (Internet of Things) and data analytics is transforming the water treatment landscape. Smart sensors and remote monitoring tools now allow real-time tracking of water quality, system performance, and early detection of problems. This technology enables more efficient operation and maintenance of water treatment plants, reducing costs and improving water management.
Addressing Global Challenges
1. Water Scarcity:
With water scarcity affecting millions worldwide, desalination technologies are gaining traction. Desalination plants, powered by energy-efficient reverse osmosis systems, are being deployed in water-stressed regions to transform seawater into potable water. The development of energy-efficient desalination membranes is also making this process more sustainable.
2. Wastewater Reuse:
Industrial processes generate vast amounts of wastewater, which can be treated and reused through Zero Liquid Discharge (ZLD) systems and other advanced water recycling technologies. Recycled wastewater is increasingly being used in agriculture, industrial cooling, and even for non-potable domestic purposes, significantly reducing water demand.
3. Contaminant Removal:
Emerging contaminants such as microplastics, pharmaceuticals, and PFAS are becoming major environmental and health concerns. Technologies like activated carbon filtration, advanced membrane systems, and AOPs are being developed and enhanced to effectively capture and remove these contaminants from both drinking water and wastewater.
Future Trends in Water Treatment
Decentralized Water Treatment:
Decentralized, small-scale water treatment systems are becoming more popular in remote areas, developing countries, and industrial settings. These systems provide localized water treatment solutions that can be customized to specific needs and avoid the high costs of centralized infrastructure.
Green Water Treatment Technologies:
Sustainability is driving the development of eco-friendly water treatment technologies. Innovations such as solar desalination, wetland-based water treatment, and biodegradable filters are gaining attention for their low energy consumption and minimal environmental impact.
Artificial Intelligence (AI) and Machine Learning:
AI and machine learning are being employed to optimize water treatment processes, predict equipment failures, and improve water quality monitoring. These technologies enable real-time decision-making, enhancing the efficiency and reliability of water treatment systems.
Conclusion: The Future of Water Treatment Technology
The water treatment sector is evolving rapidly, fueled by the growing demand for clean water and environmental protection. From cutting-edge membrane technologies to smart water systems and sustainable treatment methods, the innovations in this space promise to address pressing global water challenges. As new contaminants emerge and climate change exacerbates water scarcity, continued investment in water treatment technology will be crucial for ensuring a secure and sustainable water future for all.
For more about Water treatment techology click here
0 notes
Text
0 notes
Text
Translational Regenerative Medicine: Pioneering the Future of Health

The global translational regenerative medicine market is poised for significant growth, with a projected compound annual growth rate (CAGR) of 11% over the forecast period of 2022-2028. The market was valued at USD 3 billion in 2022 and is expected to reach USD 5 billion by 2028, reflecting a robust expansion trajectory.
Overview of Translational Regenerative Medicine
Translational regenerative medicine is a rapidly evolving field that bridges the gap between laboratory research and clinical application. It encompasses advancements in tissue engineering, stem cell research, gene therapy, and molecular biology aimed at developing innovative treatments and therapies to repair or replace damaged tissues and organs. This branch of medicine focuses on translating scientific discoveries into practical and effective medical treatments.
Get Sample pages of Report: https://www.infiniumglobalresearch.com/reports/sample-request/302
Market Dynamics
Several key factors are driving the growth of the translational regenerative medicine market:
Advancements in Research and Development: Continuous innovations in tissue engineering, stem cell technology, and gene editing are advancing the field of regenerative medicine. Breakthroughs in understanding cellular and molecular mechanisms are enabling the development of novel therapies for a range of diseases and conditions.
Increasing Prevalence of Chronic Diseases: The rising incidence of chronic diseases and conditions, such as cardiovascular diseases, neurodegenerative disorders, and musculoskeletal injuries, is fueling demand for regenerative medicine solutions. These conditions often require advanced treatments that regenerative medicine can provide.
Growing Investment and Funding: Increased investment from government agencies, private investors, and venture capitalists is accelerating research and development efforts in regenerative medicine. Funding supports clinical trials, product development, and commercialization of new therapies.
Expanding Clinical Applications: Regenerative medicine is expanding into various clinical applications, including organ transplantation, tissue repair, and personalized medicine. The potential to address unmet medical needs and improve patient outcomes is driving interest and investment in this field.
Regional Analysis
North America: North America is a leading market for translational regenerative medicine, driven by a strong research base, advanced healthcare infrastructure, and significant investment in R&D. The U.S. is a major contributor to market growth, with numerous research institutions and biotech companies focusing on regenerative medicine.
Europe: Europe is also experiencing growth in the translational regenerative medicine market, supported by government initiatives, research funding, and collaborations between academic institutions and industry. Key markets include Germany, the UK, and France.
Asia-Pacific: The Asia-Pacific region is emerging as a significant player in the regenerative medicine market, with increasing investments in research and development, a growing patient population, and advancements in healthcare infrastructure. Countries such as China, Japan, and South Korea are at the forefront of this growth.
Rest of the World: The market for translational regenerative medicine is also expanding in regions such as Latin America, the Middle East, and Africa, driven by increasing healthcare investments and rising awareness of advanced treatment options.
Report Overview : https://www.infiniumglobalresearch.com/reports/global-translational-regenerative-medicine-market
Competitive Landscape
The translational regenerative medicine market is characterized by the presence of several key players and innovative companies. Major players in this market include:
Mesoblast Limited: A global leader in regenerative medicine, Mesoblast focuses on developing cell-based therapies for a range of diseases and conditions.
Bluebird Bio, Inc.: Bluebird Bio is known for its innovative gene therapies and cell therapies aimed at treating genetic disorders and cancer.
Sangamo Therapeutics, Inc.: Sangamo specializes in gene therapy and gene editing technologies, with a focus on developing treatments for genetic diseases and other conditions.
Athersys, Inc.: Athersys is involved in the development of regenerative medicine therapies, including cell-based treatments for various diseases.
Challenges and Opportunities
Regulatory Hurdles: Navigating regulatory pathways for new regenerative medicine therapies can be complex and time-consuming. Ensuring compliance with regulatory requirements and obtaining approvals are significant challenges for market participants.
High Costs and Accessibility: The high cost of developing and manufacturing regenerative medicine products can limit accessibility for some patients. Addressing cost-related issues and improving affordability are critical for broader adoption.
Technological Advancements: There are substantial opportunities for technological advancements in regenerative medicine. Innovations in stem cell research, gene therapy, and tissue engineering hold the potential to revolutionize treatment options and expand the market.
Conclusion
The global translational regenerative medicine market is set to experience substantial growth, with a projected CAGR of 11% from 2022 to 2028. The increasing prevalence of chronic diseases, advancements in research and development, and growing investment in the field are driving market expansion. By 2028, the market is expected to reach USD 5 billion, highlighting the significant potential of regenerative medicine to transform healthcare and improve patient outcomes.
0 notes
Text
Engineered T Cells Market — Forecast(2024–2030).

Engineered T Cells Market Overview
Engineered T cells market represents a revolutionary advancement in cellular therapy, leveraging cutting-edge biotechnology to treat various diseases, primarily cancer. Engineered T cells involve modifying a patient’s T cells to enhance their ability to recognize and attack diseased cells, offering new hope for conditions that were previously difficult to treat. This comprehensive market overview provides insights into the current landscape, key players, applications, challenges, and future trends shaping this rapidly evolving sector.
Request Sample
Market Dynamics
1. Market Growth and Drivers
The engineered T cells market is experiencing substantial growth, driven by several factors:
Increasing Cancer Incidence: The global rise in cancer cases is a major driver, as engineered T cell therapies, such as CAR-T (Chimeric Antigen Receptor T-cell) therapy, offer novel treatments for various cancers.
Advancements in Technology: Innovations in genetic engineering, such as CRISPR and advanced gene-editing techniques, are enhancing the efficacy and safety of engineered T cell therapies.
Growing Investment: Significant investments from both public and private sectors in research and development are fueling advancements and commercialization in this field.
2. Technology and Innovation
Two primary technologies dominate the engineered T cells market:
CAR-T Therapy: This involves modifying T cells to express chimeric antigen receptors (CARs) that target specific proteins on cancer cells. Approved CAR-T therapies, such as Kymriah (Novartis) and Yescarta (Kite Pharma), have shown remarkable success in treating hematologic cancers, including leukemia and lymphoma.
TCR Therapy: T-cell receptor (TCR) therapies focus on enhancing T cells to recognize specific cancer antigens presented by MHC (Major Histocompatibility Complex) molecules. TCR therapies are designed to target a broader range of cancers and are currently in various stages of clinical development.
Inquiry Before Buying
Key Players
Several companies are leading the engineered T cells market, each contributing to the development and commercialization of these therapies:
Novartis: A pioneer in CAR-T therapy, Novartis’ Kymriah was one of the first CAR-T therapies to receive FDA approval. The company continues to advance its pipeline of cell therapies and explore new indications.
Gilead Sciences: Through its subsidiary Kite Pharma, Gilead Sciences has developed Yescarta, another leading CAR-T therapy. Gilead is actively involved in expanding its cell therapy portfolio and researching new treatment options.
Bristol-Myers Squibb: With its acquisition of Celgene, Bristol-Myers Squibb has gained access to innovative CAR-T therapies like Breyanzi. The company is also exploring other cell and gene therapies.
Bluebird Bio: Known for its focus on gene therapies, Bluebird Bio is developing both CAR-T and TCR therapies. The company is advancing its investigational therapies through various stages of clinical trials.
Adaptimmune: Specializing in TCR therapies, Adaptimmune is developing innovative treatments targeting specific cancer antigens. The company is actively working on expanding its clinical trials and therapeutic indications.
Schedule a Call
Applications
1. Oncology
The primary application of engineered T cells is in oncology. CAR-T therapies have shown significant efficacy in treating:
Hematologic Cancers: CAR-T therapies like Kymriah and Yescarta have been particularly effective in treating blood cancers, including acute lymphoblastic leukemia (ALL) and diffuse large B-cell lymphoma (DLBCL).
Solid Tumors: Research is ongoing to extend the use of CAR-T therapies to solid tumors, such as breast, lung, and pancreatic cancers. Challenges include identifying suitable target antigens and overcoming the tumor microenvironment’s immunosuppressive effects.
2. Other Diseases
Beyond oncology, engineered T cells are being explored for:
Autoimmune Diseases: There is potential for engineered T cells to target autoreactive T cells involved in autoimmune conditions such as type 1 diabetes and multiple sclerosis.
Infectious Diseases: Research is investigating the use of engineered T cells to target chronic viral infections, including HIV and hepatitis B.
Buy Now
Regulatory Landscape
1. Approval Process
Engineered T cell therapies must undergo rigorous regulatory scrutiny to ensure their safety and efficacy. The approval process typically involves:
Preclinical Studies: Initial research to evaluate the safety and effectiveness of the therapy in animal models.
Clinical Trials: Phases I through III trials to assess safety, efficacy, and optimal dosing in humans. Successful trials are crucial for obtaining regulatory approval.
Regulatory Review: Submission of clinical trial data to regulatory agencies such as the FDA (U.S. Food and Drug Administration) and EMA (European Medicines Agency) for review and approval.
2. Challenges
Cost: Engineered T cell therapies are expensive to develop and administer, posing challenges for widespread adoption and accessibility.
Manufacturing Complexity: The process of modifying and expanding T cells is complex and requires specialized facilities and expertise.
Side Effects: Potential side effects, such as cytokine release syndrome (CRS) and neurotoxicity, need to be carefully managed and mitigated.
Future Trends
1. Innovations in Technology
Future developments in the engineered T cells market are expected to include:
Next-Generation CAR-T Therapies: Improvements in CAR design, such as dual-targeting CARs and armored CARs, aim to enhance efficacy and reduce side effects.
Combination Therapies: Combining engineered T cells with other modalities, such as immune checkpoint inhibitors, may improve treatment outcomes and address limitations.
2. Personalized Medicine
The shift towards personalized medicine will likely drive market growth. Tailoring therapies to individual patients’ genetic and tumor profiles can enhance treatment efficacy and minimize adverse effects.
3. Global Expansion
As research advances and manufacturing capabilities improve, engineered T cell therapies are expected to become more widely available across different regions, including emerging markets. Collaborative efforts between pharmaceutical companies and research institutions will play a key role in expanding access to these therapies.
The global market size for engineered T cells was $20.21 billion in 2022, and is expected to grow to $348.9 billion by 2032.
For More Information click Here
0 notes
Text
The Human Genetics Market is projected to grow from USD 28665 million in 2024 to an estimated USD 59681.04 million by 2032, with a compound annual growth rate (CAGR) of 9.6% from 2024 to 2032.The human genetics market is experiencing rapid growth, driven by advancements in genomics, precision medicine, and the increasing understanding of the human genome's role in health and disease. The field of human genetics involves the study of genes, genetic variation, and heredity in humans, and its applications have far-reaching implications in healthcare, diagnostics, drug development, and personalized medicine. In this article, we will explore the current trends, key drivers, challenges, and future prospects of the human genetics market.
Browse the full report at https://www.credenceresearch.com/report/human-genetics-market
Overview of the Human Genetics Market
The global human genetics market has witnessed significant expansion in recent years, with the development of novel technologies such as next-generation sequencing (NGS), gene editing tools like CRISPR-Cas9, and advancements in bioinformatics. These technologies have made it possible to analyze genetic data more efficiently and accurately, providing valuable insights into disease mechanisms, inheritance patterns, and individual susceptibility to various health conditions. The market encompasses a wide range of products and services, including genetic testing, genome sequencing, gene therapy, pharmacogenomics, and molecular diagnostics.
Key Market Drivers
1. Rising Demand for Genetic Testing: Genetic testing has become a cornerstone in modern healthcare, enabling early detection and diagnosis of genetic disorders. It plays a crucial role in identifying genetic predispositions to conditions such as cancer, cardiovascular diseases, and neurological disorders. As awareness about genetic testing grows among both patients and healthcare providers, the demand for such tests is expected to rise. Moreover, direct-to-consumer (DTC) genetic testing services have gained popularity, allowing individuals to explore their genetic makeup and ancestry.
2. Precision Medicine Initiatives: The growing emphasis on precision medicine is a major driver of the human genetics market. Precision medicine aims to tailor medical treatments to individual patients based on their genetic, environmental, and lifestyle factors. By using genetic information to guide treatment decisions, healthcare providers can improve patient outcomes and reduce adverse drug reactions. As pharmaceutical companies invest in genomics-driven drug development, the demand for genetic research and testing is expected to surge.
3. Advances in Genomic Technologies: Technological advancements in genomics, particularly NGS, have revolutionized the way researchers and clinicians study the human genome. NGS allows for the rapid sequencing of entire genomes or specific genetic regions at a fraction of the cost of traditional methods. This has made large-scale genomic studies feasible and has paved the way for the identification of rare genetic variants associated with complex diseases. Additionally, gene editing technologies like CRISPR-Cas9 have opened new avenues for gene therapy and the correction of genetic mutations.
4. Government and Private Funding: Governments and private organizations worldwide are investing heavily in genomics research and healthcare innovations. For instance, initiatives like the U.S. National Institutes of Health’s (NIH) All of Us Research Program and the UK Biobank project are aimed at collecting large-scale genetic data to better understand the interplay between genetics and disease. These projects are expected to provide valuable resources for the development of new diagnostic tools and therapies.
Challenges Facing the Market
While the human genetics market holds tremendous promise, it is not without its challenges:
1. Ethical and Privacy Concerns: As genetic testing becomes more widespread, concerns over the privacy and security of genetic data are mounting. The potential misuse of genetic information by insurers, employers, or other third parties has raised ethical questions. Ensuring robust data protection measures and addressing ethical concerns are critical to maintaining public trust in genetic services.
2. High Costs of Genetic Testing and Therapies: Despite the declining cost of genome sequencing, genetic testing and gene therapies remain expensive for many patients. The high costs of these services, along with limited reimbursement from insurance providers, can hinder widespread adoption, particularly in low- and middle-income countries.
3. Regulatory Hurdles: The human genetics market is subject to stringent regulatory oversight, particularly for gene-editing technologies and gene therapies. Navigating complex regulatory frameworks and gaining approval for new genetic products can be a lengthy and costly process.
Future Prospects
The future of the human genetics market is bright, with several key trends poised to drive growth in the coming years:
1. Expansion of Personalized Medicine: The continued integration of genetic data into clinical practice will accelerate the shift towards personalized medicine. As researchers gain a deeper understanding of how genetics influence drug response, personalized treatment plans will become more prevalent, improving patient outcomes and reducing healthcare costs.
2. Emergence of AI and Machine Learning: The integration of artificial intelligence (AI) and machine learning into genomics research is expected to revolutionize data analysis. These technologies can analyze vast amounts of genetic data more efficiently, identifying patterns and correlations that may not be apparent through traditional methods. This will enhance our ability to predict disease risk and develop targeted therapies.
3. Increased Accessibility of Genetic Testing: As the cost of genomic technologies continues to decline, genetic testing will become more accessible to a broader population. This will allow for earlier disease detection, improved preventive care, and more targeted treatments, ultimately reducing the burden of genetic disorders.
Key Player Analysis:
Agilent Technologies,
Atrys Health (Spain)
Myriad Genetics,
Biomarker Technology (US)
biorad laboratories,
Bode technology,
Echevarne Laboratory (Spain)
Elabscience Biotechnology Inc (US)
Eurofins Megalab S.A (Spain)
FullGenomics (Spain)
GE healthcare,
GENinCode (UK)
Illumina,
LabCorp,
LGS Forensic,
Myriad Genetics (US)
NIMGenetics (Spain)
Orchid Cellmark,
Promega,
QIAGEN,
Sistemas Genómicos (Spain)
Synlab Group (Germany)
Thermo Fisher Scientific,
Segmentation:
By Type:
Genetic testing,
Genetic analysis,
Genetic research services.
By End User:
Hospitals and clinics,
Research and academic institutions,
Pharmaceutical and biotechnology companies.
By Region:
North America
The U.S
Canada
Mexico
Europe
Germany
France
The U.K.
Italy
Spain
Rest of Europe
Asia Pacific
China
Japan
India
South Korea
South-east Asia
Rest of Asia Pacific
Latin America
Brazil
Argentina
Rest of Latin America
Middle East & Africa
GCC Countries
South Africa
Rest of Middle East and Africa
Browse the full report at https://www.credenceresearch.com/report/human-genetics-market
About Us:
Credence Research is committed to employee well-being and productivity. Following the COVID-19 pandemic, we have implemented a permanent work-from-home policy for all employees.
Contact:
Credence Research
Please contact us at +91 6232 49 3207
Email: [email protected]
Website: www.credenceresearch.com
0 notes