#GMP Testing Service Market
Explore tagged Tumblr posts
Text
GMP Testing Service Market: Global Industry Analysis and Forecast 2023 – 2030
Global GMP Testing Service Market was valued at USD 1.17 Billion in 2021 and is expected to reach USD 1.88 Billion by the year 2028, at a CAGR of 6.97% .
Major factors contributing to the market expansion for GMP testing services include the pharmaceutical industry's explosive growth and the ongoing trend of drug and device development. The trend of biopharmaceutical companies developing more medications and devices is being driven by the increasing burden of chronic diseases, rising healthcare costs, and the impending patent expiration of blockbuster drugs. This is projected to propel the expansion of the studied industry. The launch of novel medications with cutting-edge features and strategic partnerships, as well as the growing R&D efforts of pharmaceutical companies, are anticipated to fuel the market's expansion.
Get Full PDF Sample Copy of Report: (Including Full TOC, List of Tables & Figures, Chart) @
https://introspectivemarketresearch.com/request/15848
Updated Version 2024 is available our Sample Report May Includes the:
Scope For 2024
Brief Introduction to the research report.
Table of Contents (Scope covered as a part of the study)
Top players in the market
Research framework (structure of the report)
Research methodology adopted by Worldwide Market Reports
Moreover, the report includes significant chapters such as Patent Analysis, Regulatory Framework, Technology Roadmap, BCG Matrix, Heat Map Analysis, Price Trend Analysis, and Investment Analysis which help to understand the market direction and movement in the current and upcoming years.
Leading players involved in the GMP Testing Service Market include:
Eurofins Scientific, PPD Inc., Microchem Laboratory, Sartorius AG, North American Science Associates Inc., Laboratory Corporation of America Holdings (Covance Inc.), Sotera Health (Nelson Laboratories LLC), Almac Group, Pace Analytical, Wuxi AppTec, Intertek Group PLC, Charles River Laboratories and Others major players.
If You Have Any Query GMP Testing Service Market Report, Visit:
https://introspectivemarketresearch.com/inquiry/15848
Segmentation of GMP Testing Service Market:
By Type
Product Validation Testing
Bioanalytical Services
Packaging
Shelf-Life Testing
Others
By End-User
Pharmaceutical & Biopharmaceutical Companies
Medical Devices Company
By Regions: -
North America (US, Canada, Mexico)
Eastern Europe (Bulgaria, The Czech Republic, Hungary, Poland, Romania, Rest of Eastern Europe)
Western Europe (Germany, UK, France, Netherlands, Italy, Russia, Spain, Rest of Western Europe)
Asia Pacific (China, India, Japan, South Korea, Malaysia, Thailand, Vietnam, The Philippines, Australia, New Zealand, Rest of APAC)
Middle East & Africa (Turkey, Bahrain, Kuwait, Saudi Arabia, Qatar, UAE, Israel, South Africa)
South America (Brazil, Argentina, Rest of SA)
What to Expect in Our Report?
(1) A complete section of the GMP Testing Service market report is dedicated for market dynamics, which include influence factors, market drivers, challenges, opportunities, and trends.
(2) Another broad section of the research study is reserved for regional analysis of the GMP Testing Service market where important regions and countries are assessed for their growth potential, consumption, market share, and other vital factors indicating their market growth.
(3) Players can use the competitive analysis provided in the report to build new strategies or fine-tune their existing ones to rise above market challenges and increase their share of the GMP Testing Service market.
(4) The report also discusses competitive situation and trends and sheds light on company expansions and merger and acquisition taking place in the GMP Testing Service market. Moreover, it brings to light the market concentration rate and market shares of top three and five players.
(5) Readers are provided with findings and conclusion of the research study provided in the GMP Testing Service Market report.
Our study encompasses major growth determinants and drivers, along with extensive segmentation areas. Through in-depth analysis of supply and sales channels, including upstream and downstream fundamentals, we present a complete market ecosystem.
If you require any specific information that is not covered currently within the scope of the report, we will provide the same as a part of the customization.
Acquire This Reports: -
https://introspectivemarketresearch.com/checkout/?user=1&_sid=15848
About us:
Introspective Market Research (introspectivemarketresearch.com) is a visionary research consulting firm dedicated to assisting our clients to grow and have a successful impact on the market. Our team at IMR is ready to assist our clients to flourish their business by offering strategies to gain success and monopoly in their respective fields. We are a global market research company, that specializes in using big data and advanced analytics to show the bigger picture of the market trends. We help our clients to think differently and build better tomorrow for all of us. We are a technology-driven research company, we analyse extremely large sets of data to discover deeper insights and provide conclusive consulting. We not only provide intelligence solutions, but we help our clients in how they can achieve their goals.
Contact us:
Introspective Market Research
3001 S King Drive,
Chicago, Illinois
60616 USA
Ph no: +1-773-382-1047
Email: [email protected]
#GMP Testing Service#GMP Testing Service Market#GMP Testing Service Market Size#GMP Testing Service Market Share#GMP Testing Service Market Growth#GMP Testing Service Market Trend#GMP Testing Service Market segment#GMP Testing Service Market Opportunity#GMP Testing Service Market Analysis 2023
0 notes
Text
The global GMP testing service market size reached USD 1.6 Billion in 2024. Looking forward, IMARC Group expects the market to reach USD 2.5 Billion by 2033, exhibiting a growth rate (CAGR) of 5.08% during 2025-2033. The market is experiencing steady growth driven by the increasing level of regulatory scrutiny across various industries, the stringent regulatory environment compelling businesses to invest in GMP testing services, and the rise in contract manufacturing and outsourcing.
0 notes
Text
The Essential Guide to Choosing the Right Cosmetic Manufacturing Company
Selecting the right cosmetic manufacturing company is crucial for anyone looking to launch a new beauty product. Whether you are an entrepreneur aiming to start your own brand or a company looking to expand its product line, partnering with a reliable and efficient cosmetic manufacturer can make or break your business. Here’s a comprehensive guide to help you navigate the selection process.
Understanding Your Needs
Before diving into the search for a cosmetic manufacturing company, it's essential to have a clear understanding of your product requirements. What type of cosmetics are you planning to produce? Are you focusing on skincare, color cosmetics, or personal care products? The type of products you intend to manufacture will influence the kind of manufacturer you should consider. Make a list of your needs, including formulation requirements, packaging preferences, and production volume.
Research and Shortlisting
Start by researching potential cosmetic manufacturers. Look for companies with a strong track record and experience in producing the type of products you need. You can find manufacturers through industry directories, trade shows, or online searches. Pay attention to their certifications, such as Good Manufacturing Practices (GMP) and ISO certifications, which are indicators of their adherence to quality standards.
Create a shortlist of manufacturers based on their experience, capabilities, and reputation. Evaluate their portfolio to see if they have produced similar products and check for client testimonials or case studies. This will give you insight into their reliability and the quality of their products.
Quality and Compliance
Quality assurance is paramount in cosmetic manufacturing. Ensure that the manufacturer follows strict quality control procedures and complies with industry regulations. They should adhere to safety and hygiene standards and have a robust quality management system in place. Ask for detailed information about their testing methods, ingredient sourcing, and compliance with regulations such as those set by the FDA or EMA, depending on your market.
Production Capabilities
Different manufacturers offer varying levels of production capabilities. Consider factors such as their production capacity, lead times, and flexibility in scaling production up or down. If you anticipate growth or seasonal fluctuations in demand, choose a manufacturer who can accommodate these changes without compromising quality.
Additionally, evaluate their ability to handle various aspects of production, including formulation development, packaging, and labeling. Some manufacturers offer comprehensive services that include product development and design, while others may specialize in specific areas. Determine what services you need and ensure that the manufacturer can meet those requirements.
Communication and Support
Effective communication is key to a successful partnership with a cosmetic manufacturer. Choose a company that is responsive and transparent in its communication. They should be willing to answer your questions, provide updates on production progress, and address any concerns you may have.
Consider the level of support they offer throughout the manufacturing process. A good manufacturer will provide guidance and expertise, helping you navigate any challenges that arise and ensuring that your product meets your expectations.
Cost and Pricing
Cost is a significant factor when selecting a cosmetic manufacturing company, but it should not be the sole consideration. While it’s important to find a manufacturer that fits your budget, it’s equally important to balance cost with quality. Cheap manufacturing might compromise the quality of your product, which can negatively impact your brand's reputation.
Request detailed quotes from your shortlisted manufacturers and compare them based on the services provided, production costs, and any additional fees. Make sure you understand the pricing structure and any potential hidden costs before making a decision.
Finalizing the Partnership
Once you have evaluated your options and chosen a cosmetic manufacturing company, carefully review and finalize the contract. Ensure that all terms, including production timelines, quality standards, pricing, and confidentiality agreements, are clearly outlined. A well-drafted contract will protect both parties and set clear expectations for the partnership.
In conclusion, selecting the right cosmetic manufacturing company involves thorough research and careful consideration of various factors. By understanding your needs, evaluating potential manufacturers, and ensuring they meet your quality and production requirements, you can establish a successful partnership that contributes to the success of your cosmetic products. With the right manufacturer by your side, you’ll be well on your way to creating high-quality, market-ready beauty products.
Visit: https://cosmonovaindia.com/cosmetic-products-manufacturing/
0 notes
Text
The Role of Analytical CMC Services in Regulatory Approval and Product Quality
In the pharmaceutical industry, achieving regulatory approval and maintaining product quality are critical to ensuring the safety and efficacy of drug products. Analytical CMC Services (Chemistry, Manufacturing, and Controls) play a vital role in meeting these objectives by providing comprehensive analytical support throughout the drug development lifecycle.
Understanding Analytical CMC Services
Analytical CMC Services encompass a wide range of scientific and regulatory activities designed to assess the chemical, physical, and microbiological properties of drug substances and drug products. These services help manufacturers maintain consistency, comply with stringent regulatory requirements, and ensure that products meet predefined quality standards.

Importance of Analytical CMC Services in Regulatory Approval
Method Development and Validation
Developing and validating robust analytical methods to ensure accurate and reproducible results.
Meeting regulatory guidelines such as ICH Q2 (R1) for method validation.
Stability Testing
Conducting long-term and accelerated stability studies to determine product shelf-life.
Evaluating degradation pathways and ensuring product integrity under various storage conditions.
Impurity Profiling and Control
Identifying and quantifying impurities, including degradation products and residual solvents.
Ensuring compliance with ICH Q3A and Q3B impurity guidelines.
Batch Release Testing
Performing quality control tests to confirm batch-to-batch consistency before product release.
Ensuring compliance with pharmacopeial standards (USP, EP, JP) and regulatory specifications.
Regulatory Documentation and Support
Preparing Chemistry, Manufacturing, and Controls (CMC) sections for regulatory submissions, including IND, NDA, BLA, and MAA applications.
Supporting responses to regulatory agencies and audits.
Enhancing Product Quality Through Analytical CMC Services
Ensuring Consistency and Reproducibility
Implementing stringent analytical testing to maintain product uniformity.
Monitoring critical quality attributes (CQAs) to ensure product performance.
Risk Mitigation and Compliance
Identifying potential risks in manufacturing processes and implementing mitigation strategies.
Ensuring compliance with Good Manufacturing Practices (GMP) and regulatory guidelines.
Support for Advanced Therapeutics
Providing specialized analytical techniques for biologics, biosimilars, and gene therapies.
Addressing complex structural characterization and stability challenges.
Choosing the Right Analytical CMC Services Provider
When selecting a provider for Analytical CMC Services, pharmaceutical companies should consider:
Expertise in small and large molecule drug development.
Regulatory knowledge and compliance with global standards.
State-of-the-art analytical instrumentation and methodologies.
Proven track record in supporting regulatory submissions.
Conclusion
Analytical CMC Services are essential for ensuring regulatory approval and maintaining high product quality throughout the drug development process. By leveraging advanced analytical techniques and regulatory expertise, pharmaceutical companies can achieve compliance, optimize manufacturing processes, and bring safe and effective products to market efficiently. Partnering with an experienced Analytical CMC Services provider ensures success in an increasingly complex regulatory landscape.
0 notes
Text
High-Quality Pharmaceutical Testing Services
Ensuring Safety and Efficacy of Pharmaceutical Products Pharmaceutical testing services play a critical role in ensuring that drugs are safe, effective, and compliant with regulatory standards. These services involve rigorous testing and analysis of pharmaceutical products to guarantee that they meet the highest industry standards for quality and safety.

Quality Control and Assurance Pharmaceutical testing services include comprehensive quality control and quality assurance processes. These ensure that products are manufactured according to Good Manufacturing Practices (GMP) and other relevant guidelines. Testing services cover raw materials, intermediates, and finished products, ensuring that all aspects of drug development meet stringent safety standards.
Stability Testing Stability testing is essential to determine the shelf life of pharmaceutical products. Pharmaceutical testing services assess how drugs maintain their potency, safety, and effectiveness under various environmental conditions, including temperature, humidity, and light exposure. This data is crucial for ensuring long-term product stability and determining appropriate storage conditions.
Bioequivalence and Clinical Trials Bioequivalence studies and clinical trials are essential for assessing the therapeutic effectiveness of generic drugs compared to their branded counterparts. Pharmaceutical testing services facilitate these studies, ensuring that generic drugs provide the same benefits as their branded versions.
Compliance and Regulatory Support Pharmaceutical testing services help companies meet local and international regulatory requirements, including those set by the FDA, EMA, and other regulatory bodies. These services ensure compliance with documentation, testing procedures, and reporting standards, which are essential for product approval and market access.
Pharmaceutical testing services ensure the safety, efficacy, and compliance of drugs, supporting manufacturers in delivering high-quality products to the market.
Find reliable Pharmaceutical Testing Services effortlessly using the JD app. Search, compare, and connect with top service providers instantly. Download the Justdial app for quick access to verified listings, reviews, and contact details. Get accurate testing solutions today!
0 notes
Text
The Role of a Cosmetic Equipment Consultant in the Beauty Industry
The beauty industry is constantly evolving, with new technologies and innovative products being introduced every day. For businesses looking to invest in cosmetic equipment, it is essential to navigate through complex regulations, certifications, and technological advancements. This is where a Cosmetic Equipment Consultant plays a vital role. These professionals offer expert guidance in selecting, certifying, and implementing the right cosmetic machinery, ensuring compliance with legal requirements, including those set by CDSCO (Central Drugs Standard Control Organization) in India.
Understanding the Role of a Cosmetic Equipment Consultant
A Cosmetic Equipment Consultant is a specialized professional who assists beauty brands, skincare manufacturers, and cosmetic clinics in procuring, installing, and maintaining cosmetic machinery. Their role extends beyond product selection; they provide regulatory support, compliance consultation, and training on advanced technologies. The main responsibilities include:
1. Assessing Business Needs
Every cosmetic business has unique requirements depending on its target market, product range, and operational scale. A consultant evaluates the specific needs of a company and recommends suitable cosmetic equipment that aligns with business objectives.
2. Equipment Selection and Sourcing
Choosing the right cosmetic machinery can be overwhelming, given the variety of options available in the market. Consultants leverage their industry knowledge to identify the best equipment based on performance, quality, and regulatory compliance.
3. Regulatory Compliance and CDSCO Certification
In India, the CDSCO is responsible for regulating cosmetic products, ensuring their safety, efficacy, and quality. A Cosmetic Equipment Consultant helps businesses navigate these regulations by providing:
Assistance in CDSCO registration and licensing
Compliance with Good Manufacturing Practices (GMP)
Guidance on documentation and product testing requirements
4. Installation and Training
After acquiring the right equipment, proper installation is crucial to ensure safety and efficiency. Consultants oversee the setup process and train personnel on best practices for operating and maintaining the equipment.
5. Quality Assurance and Troubleshooting
Ensuring the consistent performance of cosmetic machinery is critical for business success. Consultants provide quality assurance services, conduct routine inspections, and offer troubleshooting solutions to prevent downtime.
Why Regulatory Compliance Matters in the Cosmetic Industry
CDSCO and Its Importance
The Central Drugs Standard Control Organization (CDSCO) is India’s regulatory authority for pharmaceuticals and cosmetics. Any business dealing with cosmetic equipment must comply with CDSCO regulations to avoid legal complications and maintain product integrity. Non-compliance can result in penalties, product recalls, or operational shutdowns.
Key CDSCO Regulations for Cosmetic Equipment
Registration of imported cosmetic products
Approval for manufacturing licenses
Adherence to labeling and safety standards
Regular audits and inspections
Working with an experienced Cosmetic Equipment Consultant ensures that businesses stay updated with the latest CDSCO guidelines, minimizing the risk of non-compliance.
The Impact of Advanced Cosmetic Equipment on Business Growth
The cosmetic industry thrives on innovation. Investing in the latest cosmetic equipment enhances product quality, boosts efficiency, and improves customer satisfaction. Some of the latest trends in cosmetic equipment include:
1. Laser and Light Therapy Machines
Advanced laser devices are widely used for hair removal, skin rejuvenation, and pigmentation treatment. High-quality laser machines improve treatment precision and reduce recovery time.
2. Microdermabrasion and Skin Resurfacing Devices
These machines help in skin exfoliation and collagen stimulation, resulting in smoother, healthier skin. Businesses using such equipment gain a competitive edge by offering high-end skincare treatments.
3. Cosmetic 3D Printing Technology
The rise of 3D printing in cosmetics allows brands to create customized beauty products, such as foundation shades tailored to individual skin tones.
4. AI and Smart Cosmetic Devices
Artificial Intelligence (AI)-powered cosmetic devices analyze skin conditions and suggest personalized treatments. The adoption of smart technology enhances the customer experience and promotes brand loyalty.
How to Choose the Right Cosmetic Equipment Consultant
Selecting a Cosmetic Equipment Consultant is a crucial decision that can impact business growth and regulatory compliance. Here are some key factors to consider:
1. Industry Experience and Expertise
Choose a consultant with a strong background in the cosmetic industry, preferably with hands-on experience in working with diverse cosmetic equipment brands.
2. Knowledge of CDSCO Regulations
The consultant must have in-depth knowledge of CDSCO guidelines and be capable of handling all regulatory procedures efficiently.
3. Network and Supplier Relationships
A well-connected consultant can help businesses get access to high-quality equipment at competitive prices by leveraging their industry network.
4. After-Sales Support and Training
A reliable consultant should offer after-sales support, including maintenance guidance, troubleshooting services, and operator training sessions.
5. Customer Reviews and Testimonials
Before hiring a consultant, check their past client reviews and success stories to ensure credibility and reliability.
Conclusion
The role of a Cosmetic Equipment Consultant is invaluable in today’s competitive beauty industry. From selecting the right equipment to ensuring regulatory compliance with CDSCO standards, their expertise helps businesses streamline operations and enhance service quality. Whether you are a startup looking to establish a cosmetic brand or an established business seeking to upgrade your equipment, partnering with an experienced consultant is the key to success.
Investing in the right cosmetic equipment and adhering to regulatory guidelines not only boosts operational efficiency but also builds consumer trust and brand credibility. By working with a knowledgeable consultant, businesses can stay ahead in the ever-evolving world of cosmetics.
0 notes
Text
Energy Drink Powder Manufacturer in Chandigarh – Premium Quality & Custom Formulations
In today’s fast-paced world, energy drinks have become an essential part of many people's daily routines, providing a quick boost of energy, enhanced focus, and improved physical performance. If you are looking for a trusted energy drink powder manufacturer in Chandigarh, you are in the right place.
Why Choose Chandigarh for Energy Drink Manufacturing?
Chandigarh is emerging as a hub for pharmaceutical and nutraceutical manufacturing, with state-of-the-art facilities and stringent quality standards. The city is home to several WHO-GMP and FSSAI-certified manufacturers specializing in health supplements, including energy drink powders.
Top Features of a Reliable Energy Drink Powder Manufacturer
When choosing an energy drink powder manufacturer in Chandigarh, consider the following factors:
✅ Certified Manufacturing Units – Ensure the facility has ISO, GMP, and FSSAI certifications.
✅ Custom Formulations – Manufacturers should offer personalized blends with electrolytes, vitamins, amino acids, and herbal extracts.
✅ High-Quality Ingredients – Only the best raw materials should be used to ensure safety and effectiveness.
✅ Private Label & Third-Party Manufacturing – If you want to launch your own brand, look for manufacturers offering OEM and contract manufacturing services.
✅ Advanced R&D Support – A good manufacturer should provide formulation development, flavor testing, and stability analysis.
Benefits of Partnering with a Chandigarh-Based Energy Drink Manufacturer
🔹 Affordable Pricing due to a competitive manufacturing environment.
🔹 High-Quality Standards with international certifications.
🔹 Customizable Solutions tailored to market demands.
Types of Energy Drink Powders Available for Manufacturing
Manufacturers in Chandigarh offer a wide range of energy drink formulations, including:
💪 Pre-Workout Energy Powders – For athletes and fitness enthusiasts.
🏋️ Post-Workout Recovery Powders – Replenishes lost nutrients.
🌿 Herbal & Natural Energy Drinks – With green tea, ginseng, or guarana.
⚡ Electrolyte & Hydration Powders – For endurance and sports performance.
🍋 Flavored Energy Powders – Lemon, orange, mixed berry, and other flavors.
How to Choose the Best Energy Drink Manufacturer in Chandigarh?
To find the best energy drink powder manufacturer, consider:
📜 Checking their certifications and compliance with FSSAI, GMP, and ISO standards.
🏭 Visiting the manufacturing unit to assess production capabilities.
📑 Requesting product samples and third-party lab testing reports.
��� Ensuring they provide custom branding and packaging options.
Get Started with Energy Drink Manufacturing Today!
If you are planning to launch your own energy drink brand, Chandigarh-based manufacturers can provide high-quality, customizable solutions to meet your business needs. From private labeling to bulk manufacturing, partnering with an experienced manufacturer ensures the success of your product in the growing energy supplement market.
Looking for a trusted energy drink powder manufacturer in Chandigarh? Contact us today to explore the best manufacturing solutions tailored to your business needs!
0 notes
Text
How to Choose the Best Iron Sucrose Injection Manufacturer
In the pharmaceutical industry, selecting the right Iron Sucrose Injection Manufacturer is crucial for ensuring quality, safety, and regulatory compliance. Whether you are a healthcare provider, distributor, or business looking for a third-party liquid injection manufacturer, the decision impacts patient health and business success.

Key Factors to Consider When Choosing an Iron Sucrose Injection Manufacturer
1. Regulatory Compliance & Certifications
A reliable pharmaceuticals manufacturer in India must adhere to strict regulatory guidelines, including WHO-GMP, ISO, and FDA approvals. These certifications ensure that the manufacturing processes meet global safety and efficacy standards.
2. Quality Assurance & Testing
The manufacturer should have a robust quality control system in place. This includes:
Raw material testing
In-process quality checks
Stability testing of the final product Choosing a generic injection manufacturer that prioritizes quality ensures consistency and effectiveness in iron sucrose injections.
3. Manufacturing Capabilities & Infrastructure
Look for an injection manufacturer in India with advanced production facilities, including:
Sterile manufacturing units
Advanced R&D laboratories
Automated packaging lines
High-quality control systems These facilities help in producing safe and effective iron sucrose injections at scale.
4. Third-Party & Contract Manufacturing Services
If you need a Third Party Liquid injection Manufacturer, check if the company offers:
Custom formulation options
Private labeling services
Timely delivery schedules A strong third-party manufacturer ensures flexibility and scalability for your business.
5. Market Reputation & Client Reviews
Research the manufacturer’s track record and customer feedback. A trusted pharma manufacturer in India will have positive client testimonials, industry recognition, and a history of delivering high-quality products.
Why Choose GBN Pharmaceuticals?
GBN Pharmaceuticals is a leading Iron Sucrose Injection Manufacturer known for its high-quality standards, advanced manufacturing infrastructure, and commitment to regulatory compliance. As a reputed pharmaceuticals manufacturer in India, we specialize in third-party and contract manufacturing services, ensuring high efficiency and reliability in every batch.
Our Strengths:
WHO-GMP & ISO-certified production facilities
Strict quality control & compliance
Efficient third-party manufacturing services
Affordable and scalable production
For premium-quality iron sucrose injections and other pharmaceutical solutions, GBN Pharmaceuticals is your trusted partner. Contact us today for collaboration opportunities!
Conclusion
Choosing the right injection manufacturer in India requires careful evaluation of compliance, quality, infrastructure, and reputation. GBN Pharmaceuticals stands out as a top-tier iron sucrose injection manufacturer, ensuring excellence in every product. Whether you need third-party liquid injection manufacturing or high-quality generic injections, we are here to meet your needs.
For inquiries and business partnerships, reach out to GBN Pharmaceuticals today!
Also read : Pharma Manufacturing: A Growing Global Hub
#injection manufacturer in India#pharma manufacturer in India#generic injection manufacturer#pharmaceuticals manufacturer in India#Iron Sucrose Injection Manufacture
0 notes
Text
GMP Testing Service Market: Global Industry Analysis and Forecast 2023 – 2030
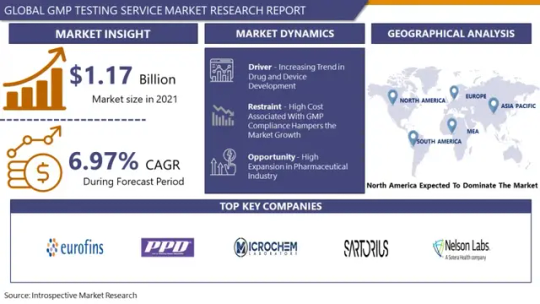
Global GMP Testing Service Market was valued at USD 1.17 Billion in 2021 and is expected to reach USD 1.88 Billion by the year 2028, at a CAGR of 6.97% .
Major factors contributing to the market expansion for GMP testing services include the pharmaceutical industry's explosive growth and the ongoing trend of drug and device development. The trend of biopharmaceutical companies developing more medications and devices is being driven by the increasing burden of chronic diseases, rising healthcare costs, and the impending patent expiration of blockbuster drugs. This is projected to propel the expansion of the studied industry. The launch of novel medications with cutting-edge features and strategic partnerships, as well as the growing R&D efforts of pharmaceutical companies, are anticipated to fuel the market's expansion.
Get Full PDF Sample Copy of Report: (Including Full TOC, List of Tables & Figures, Chart) @
https://introspectivemarketresearch.com/request/15848
Updated Version 2024 is available our Sample Report May Includes the:
Scope For 2024
Brief Introduction to the research report.
Table of Contents (Scope covered as a part of the study)
Top players in the market
Research framework (structure of the report)
Research methodology adopted by Worldwide Market Reports
Moreover, the report includes significant chapters such as Patent Analysis, Regulatory Framework, Technology Roadmap, BCG Matrix, Heat Map Analysis, Price Trend Analysis, and Investment Analysis which help to understand the market direction and movement in the current and upcoming years.
Leading players involved in the GMP Testing Service Market include:
Eurofins Scientific, PPD Inc., Microchem Laboratory, Sartorius AG, North American Science Associates Inc., Laboratory Corporation of America Holdings (Covance Inc.), Sotera Health (Nelson Laboratories LLC), Almac Group, Pace Analytical, Wuxi AppTec, Intertek Group PLC, Charles River Laboratories and Others major players.
If You Have Any Query GMP Testing Service Market Report, Visit:
https://introspectivemarketresearch.com/inquiry/15848
Segmentation of GMP Testing Service Market:
By Type
Product Validation Testing
Bioanalytical Services
Packaging
Shelf-Life Testing
Others
By End-User
Pharmaceutical & Biopharmaceutical Companies
Medical Devices Company
By Regions: -
North America (US, Canada, Mexico)
Eastern Europe (Bulgaria, The Czech Republic, Hungary, Poland, Romania, Rest of Eastern Europe)
Western Europe (Germany, UK, France, Netherlands, Italy, Russia, Spain, Rest of Western Europe)
Asia Pacific (China, India, Japan, South Korea, Malaysia, Thailand, Vietnam, The Philippines, Australia, New Zealand, Rest of APAC)
Middle East & Africa (Turkey, Bahrain, Kuwait, Saudi Arabia, Qatar, UAE, Israel, South Africa)
South America (Brazil, Argentina, Rest of SA)
What to Expect in Our Report?
(1) A complete section of the GMP Testing Service market report is dedicated for market dynamics, which include influence factors, market drivers, challenges, opportunities, and trends.
(2) Another broad section of the research study is reserved for regional analysis of the GMP Testing Service market where important regions and countries are assessed for their growth potential, consumption, market share, and other vital factors indicating their market growth.
(3) Players can use the competitive analysis provided in the report to build new strategies or fine-tune their existing ones to rise above market challenges and increase their share of the GMP Testing Service market.
(4) The report also discusses competitive situation and trends and sheds light on company expansions and merger and acquisition taking place in the GMP Testing Service market. Moreover, it brings to light the market concentration rate and market shares of top three and five players.
(5) Readers are provided with findings and conclusion of the research study provided in the GMP Testing Service Market report.
Our study encompasses major growth determinants and drivers, along with extensive segmentation areas. Through in-depth analysis of supply and sales channels, including upstream and downstream fundamentals, we present a complete market ecosystem.
If you require any specific information that is not covered currently within the scope of the report, we will provide the same as a part of the customization.
Acquire This Reports: -
https://introspectivemarketresearch.com/checkout/?user=1&_sid=15848
About us:
Introspective Market Research (introspectivemarketresearch.com) is a visionary research consulting firm dedicated to assisting our clients to grow and have a successful impact on the market. Our team at IMR is ready to assist our clients to flourish their business by offering strategies to gain success and monopoly in their respective fields. We are a global market research company, that specializes in using big data and advanced analytics to show the bigger picture of the market trends. We help our clients to think differently and build better tomorrow for all of us. We are a technology-driven research company, we analyse extremely large sets of data to discover deeper insights and provide conclusive consulting. We not only provide intelligence solutions, but we help our clients in how they can achieve their goals.
Contact us:
Introspective Market Research
3001 S King Drive,
Chicago, Illinois
60616 USA
Ph no: +1-773-382-1047
Email: [email protected]
#GMP Testing Service#GMP Testing Service Market#GMP Testing Service Market Size#GMP Testing Service Market Share#GMP Testing Service Market Growth#GMP Testing Service Market Trend#GMP Testing Service Market segment#GMP Testing Service Market Opportunity#GMP Testing Service Market Analysis 2023
0 notes
Text
https://www.maximizemarketresearch.com/market-report/gmp-testing-service-market/188536/
0 notes
Text
Discover the growing GMP testing service market! In 2023, it reached US$ 1.5 Billion, and by 2032, it's expected to soar to US$ 2.5 Billion with a CAGR of 5.34%. The increase is driven by heightened regulatory scrutiny and the rise of contract manufacturing and outsourcing.
0 notes
Text
Stеrilе Injеctablе CDMO Market Industry Trends, Analysis, Size and Share by 2025-2033

The Reports and Insights, a leading market research company, has recently releases report titled “Stеrilе Injеctablе CDMO Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2025-2033.” The study provides a detailed analysis of the industry, including the global Stеrilе Injеctablе CDMO Market share, size, trends, and growth forecasts. The report also includes competitor and regional analysis and highlights the latest advancements in the market.
Report Highlights:
How big is the Stеrilе Injеctablе CDMO Market?
The global stеrilе injеctablе CDMO market was valued at US$ 11.1 Billion in 2024 and is expected to register a CAGR of 11.5% over the forecast period and reach US$ 29.6 Bn in 2033.
What are Stеrilе Injеctablе CDMO?
Sterile injectable CDMO is a group of specialized service companies that assist pharmaceutical and biotechnology companies to develop, manufacture, and commercialize sterile injectable drugs. These companies offer end-to-end services which involve formulation development, process optimization, analytical testing, regulatory support, and large-scale sterile manufacturing in compliance with GMP. Sterile injectable CDMOs are meeting the increasing demand for biologics, biosimilars, and complex injectables such as monoclonal antibodies, vaccines, and oncology drugs. They allow pharma companies to speed up time-to-market while maintaining product quality and meeting stringent regulatory requirements by leveraging advanced technologies, aseptic manufacturing facilities, and industry expertise.
Request for a sample copy with detail analysis: https://www.reportsandinsights.com/sample-request/2544
What are the growth prospects and trends in the Stеrilе Injеctablе CDMO industry?
The sterile injectable CDMO market growth is driven by various drivers and factors. The sterile injectable CDMO market is experiencing significant growth driven by the rising demand for biologics, biosimilars, and complex injectable formulations, particularly in therapeutic areas like oncology, immunology, and chronic diseases. Increasing outsourcing trends among pharmaceutical and biotechnology companies, coupled with the need for cost-effective production and regulatory expertise, are fueling market expansion. Key factors include advancements in aseptic manufacturing technologies, growing investments in large-scale sterile production facilities, and stringent regulatory requirements for drug safety and quality. The market is also bolstered by the surge in vaccine development, particularly mRNA-based platforms, and the growing focus on personalized medicine, making sterile injectable CDMOs critical partners in the pharmaceutical supply chain. Hence, all these factors contribute to sterile injectable CDMO market growth.
What is included in market segmentation?
The report has segmented the market into the following categories:
By Sеrvicеs
Stand-alonе Sеrvicеs
Drug Formulation and Dеvеlopmеnt
Asеptic Fillings
Analytical Dеvеlopmеnt
Rеgulatory Support
Packaging and Assеmbly Sеrvicеs
Tеchnology Transfеr
Supply Chain Managеmеnt
Quality Control and Assurancе
Intеgratеd Sеrvicеs
By Drug Typе
Monoclonal Antibodiеs (mAbs)
Cytokinеs
Insulin
Pеptidе Hormonеs
Vaccinеs
Immunoglobulins
Blood Factors
Pеptidе Antibiotics
Othеrs
By Organization Sizе
Small
Mid-sizеd
Largе
By End-Usеr
Pharmacеutical Companiеs
Biopharmacеutical Companiеs
Rеsеarch Institutеs
Othеrs
Europe
Germany
United Kingdom
France
Italy
Spain
Russia
Poland
Benelux
Nordic
Rest of Europe
Asia Pacific
China
Japan
India
South Korea
ASEAN
Australia & New Zealand
Rest of Asia Pacific
Latin America
Brazil
Mexico
Argentina
Middle East & Africa
Saudi Arabia
South Africa
United Arab Emirates
Israel
Rest of MEA
Who are the key players operating in the industry?
The report covers the major market players including:
FAMAR Hеalth Carе Sеrvicеs
Pfizеr
Farеva
Sharp
Astral StеriTеch
Evonik
Aurigеnе Pharmacеutical Sеrvicеs
Ethypharm
TriRx Pharmacеutical Sеrvicеs
Biophrama Group
Gеnsеnta Pharmacеuticals
View Full Report: https://www.reportsandinsights.com/report/Stеrilе Injеctablе CDMO-market
If you require any specific information that is not covered currently within the scope of the report, we will provide the same as a part of the customization.
About Us:
Reports and Insights consistently mееt international benchmarks in the market research industry and maintain a kееn focus on providing only the highest quality of reports and analysis outlooks across markets, industries, domains, sectors, and verticals. We have bееn catering to varying market nееds and do not compromise on quality and research efforts in our objective to deliver only the very best to our clients globally.
Our offerings include comprehensive market intelligence in the form of research reports, production cost reports, feasibility studies, and consulting services. Our team, which includes experienced researchers and analysts from various industries, is dedicated to providing high-quality data and insights to our clientele, ranging from small and medium businesses to Fortune 1000 corporations.
Contact Us:
Reports and Insights Business Research Pvt. Ltd. 1820 Avenue M, Brooklyn, NY, 11230, United States Contact No: +1-(347)-748-1518 Email: [email protected] Website: https://www.reportsandinsights.com/ Follow us on LinkedIn: https://www.linkedin.com/company/report-and-insights/ Follow us on twitter: https://twitter.com/ReportsandInsi1
#Stеrilе Injеctablе CDMO Market share#Stеrilе Injеctablе CDMO Market size#Stеrilе Injеctablе CDMO Market trends
0 notes
Text

R K Lifecare INC: Dry Injection Powder Pharmaceutical Company
R K Lifecare Inc is a leading pharmaceutical company based in Jhajjar, Haryana, specializing in manufacturing high-quality dry injection powders. Our company is known for its excellence, innovation, and reliability in the pharmaceutical industry.
Our product portfolio includes dry injections such as Cefazolin, Cefepime Hydrochloride, Cefoperazone Sodium, Cefotaxime, Ceftriaxone, and Cefuroxime. These drugs are used in treating various infections and are known to be clinically effective. Our company follows quality manufacturing processes and standards to provide the best products to patients.
Focus on Quality and Innovation
The main objective of R K Lifecare Inc is to manufacture quality products. Advanced technologies and modern machines are used in our product manufacturing. We follow the guidelines of the World Health Organization (WHO) and Good Manufacturing Practices (GMP). All the medicines we manufacture undergo quality testing and certification processes to ensure their effectiveness and safety.
Experienced Team and Research
We have a dedicated team of experienced scientists and engineers specializing in the pharmaceutical industry. This team continuously works in research and development (R&D) to create new and effective pharmaceutical products. Our R&D team aims to provide innovative products keeping in mind the needs of patients.
Customer Satisfaction Our Objective
We consider customer satisfaction paramount. At R K Lifecare Inc., we are committed to providing timely product delivery, competitive pricing, and reliable customer service. Our products are exported to many countries including India, and we are grateful for the trust and support we have received from our customers.
Commitment to the Environment
We also give utmost importance to environmental sustainability. Our production processes are environmentally friendly and we take every possible step to reduce pollution. At the same time, we ensure that our products are safe and beneficial for patients and the community.
Future Plans
RK Lifecare Inc. aims to further strengthen its position in the pharmaceutical industry. We are constantly striving to expand our product range and adopt new medical technologies. Our aim is to make India proud in the global market and make healthcare accessible and effective.
With RK Lifecare Inc., we promise you high quality, reliable service and innovative products. We are fully dedicated to bringing a positive change in the healthcare sector.
#Pharma Company#Pharma Manufacturing Company#Pharma Company in Delhi#Pharma Companies in India#Best Pharma Company in India#pharmaceutical company#pharmaceutical Manufacturing Company#pharmaceutical manufacturer#pharmaceutical manufacturer company#pharmaceutical manufacturer in india#Pharmaceutical Companies in Delhi#pharmaceutical company in delhi
0 notes
Text
0 notes
Text
Labialfarma: A Trusted Name in Finished Products Manufacturing
In today’s rapidly evolving pharmaceutical and cosmetic industries, businesses are constantly seeking reliable partners to help them bring their products to market. Labialfarma has established itself as a leading finished products manufacturer, offering comprehensive services tailored to meet the needs of its clients. As a trusted Fabricante Terceiros (third-party manufacturer), Labialfarma’s commitment to quality, innovation, and sustainability sets it apart from competitors.

Expertise in Finished Products Manufacturing
Labialfarma specializes in the production of finished pharmaceutical, cosmetic, and nutraceutical products. With years of experience and a robust infrastructure, the company provides end-to-end solutions, from formulation development to packaging and distribution. Their advanced manufacturing facilities are designed to comply with international regulatory standards, ensuring that every product is safe, effective, and of the highest quality.
Key Features of Labialfarma’s Manufacturing Services
Comprehensive Formulation Development Labialfarma’s team of experts works closely with clients to develop innovative formulations that meet market demands. Whether it’s creating a new product or optimizing an existing one, their approach is rooted in research and development.
State-of-the-Art Facilities Equipped with cutting-edge technology, Labialfarma’s manufacturing units are capable of producing a wide range of products, including tablets, capsules, creams, ointments, and liquids. The facilities adhere to GMP (Good Manufacturing Practices) standards, ensuring consistency and quality in every batch.
Quality Assurance and Control At Labialfarma, quality is non-negotiable. The company employs rigorous quality assurance and control protocols throughout the manufacturing process. From sourcing raw materials to final product testing, every step is meticulously monitored to meet stringent standards.
Flexible Production Capacities Whether you require small batches for niche markets or large-scale production for global distribution, Labialfarma’s flexible manufacturing capabilities can accommodate your needs. This adaptability makes them a preferred choice for businesses of all sizes.
The Role of a Fabricante Terceiros
As a fabricante terceiros, Labialfarma collaborates with companies to provide third-party manufacturing services. This model allows businesses to leverage Labialfarma’s expertise and infrastructure without investing in their own facilities.
Benefits of Choosing Labialfarma as Your Fabricante Terceiros
Cost Efficiency Partnering with Labialfarma eliminates the need for capital investment in manufacturing equipment and facilities. This significantly reduces overhead costs, allowing businesses to allocate resources more effectively.
Focus on Core Competencies Outsourcing manufacturing to Labialfarma enables companies to concentrate on core activities such as marketing, sales, and distribution. This streamlined approach enhances overall efficiency and profitability.
Access to Expertise Labialfarma’s team of seasoned professionals brings a wealth of knowledge and experience to every project. Their expertise in regulatory compliance, product development, and manufacturing ensures a seamless and hassle-free process.
Scalability With the ability to scale production up or down based on demand, Labialfarma offers unparalleled flexibility. This is particularly beneficial for startups and small businesses looking to grow their product lines.
Innovation and Sustainability
Labialfarma’s commitment to innovation is evident in its approach to product development. By staying ahead of industry trends and incorporating the latest advancements in technology, the company consistently delivers products that meet evolving consumer needs.
Sustainability Initiatives
In addition to innovation, Labialfarma places a strong emphasis on sustainability. Their eco-friendly manufacturing practices and responsible sourcing of raw materials reflect a dedication to reducing environmental impact. By choosing Labialfarma, businesses can align with a partner that shares their commitment to sustainability.
Why Choose Labialfarma?
Reputation for Excellence Labialfarma’s track record of delivering high-quality finished products has earned them the trust of clients worldwide. Their reputation for reliability and professionalism makes them a standout choice in the industry.
Customized Solutions Understanding that every client has unique requirements, Labialfarma offers tailored solutions to meet specific needs. From product design to packaging, their services are fully customizable.
Regulatory Compliance Navigating the complexities of regulatory requirements can be challenging. Labialfarma’s expertise in global compliance standards ensures that products meet all necessary regulations, facilitating smooth market entry.
End-to-End Support Labialfarma provides comprehensive support throughout the product lifecycle, including formulation, manufacturing, packaging, and logistics. This all-in-one approach simplifies the process for clients and ensures consistency.
Industries Served
Labialfarma caters to a diverse range of industries, including:
Pharmaceuticals: Manufacturing prescription and over-the-counter medications.
Cosmetics: Producing skincare, haircare, and beauty products.
Nutraceuticals: Creating dietary supplements and functional foods.
Veterinary: Developing products for animal health and wellness.
Partnering with Labialfarma: A Strategic Advantage
In a competitive market, having a reliable manufacturing partner is crucial. Labialfarma’s expertise as a Finished Products Manufacturer and fabricante terceiros provides businesses with a strategic advantage. By delivering high-quality products efficiently and cost-effectively, Labialfarma empowers clients to stay ahead of the curve.
Conclusion
Labialfarma’s dedication to quality, innovation, and sustainability makes them a trusted partner for businesses looking to bring their products to market. Whether you need a finished products manufacturer or a reliable fabricante terceiros, Labialfarma’s comprehensive services and industry expertise ensure success at every step. By choosing Labialfarma, you’re not just investing in a manufacturing partner – you’re investing in excellence.
0 notes