#Fusobacterium nucleatum
Explore tagged Tumblr posts
Link
Abstract Fusobacterium nucleatum (F. nucleatum) is an early pathogenic colonizer in periodontitis, but the host response to infection with this pathogen remains unclear. In this study, we built an F. nucleatum infectious model with human periodontal ligament stem cells (PDLSCs) and showed that F. nucleatum could inhibit proliferation, and facilitate apoptosis, ferroptosis, and inflammatory cytokine production in a dose-dependent manner. The F. nucleatum adhesin FadA acted as a proinflammatory virulence factor and increased the expression of interleukin(IL)-1β, IL-6 and IL-8. Further study showed that FadA could bind with PEBP1 to activate the Raf1-MAPK and IKK-NF-κB signaling pathways. Time-course RNA-sequencing analyses showed the cascade of gene activation process in PDLSCs with increasing durations of F. nucleatum infection. NFκB1 and NFκB2 upregulated after 3 h of F. nucleatum-infection, and the inflammatory-related genes in the NF-κB signaling pathway were serially elevated with time. Using computational drug repositioning analysis, we predicted and validated that two potential drugs (piperlongumine and fisetin) could attenuate the negative effects of F. nucleatum-infection. Collectively, this study unveils the potential pathogenic mechanisms of F. nucleatum and the host inflammatory response at the early stage of F. nucleatum infection. Fusobacterium nucleatum (F. nucleatum) is one of the most frequently detected pathogens and has attracted increasing attention in recent years as an opportunistic pathogen in many systematic diseases, such as colorectal cancer,5 cardiovascular diseases,6 Alzheimer’s disease,7 and adverse pregnancy outcomes.8 F. nucleatum is an invasive bacterium that can induce a variety of host responses.9 Clinical studies have shown that the prevalence of F. nucleatum increases with the severity and progression of periodontitis.10,11 F. nucleatum can invade various host cells, such as epithelial and endothelial cells, monocytes and fibroblasts, to initiate a cascade of inflammation and induce the secretion of the proinflammatory chemokines interleukin(IL)-6 and IL-8.12,13 Toxic proteins are an important way for bacteria to exert pathogenicity, and F. nucleatum expresses a variety of virulence factors to induce various host responses As a main cell type in the periodontal ligament, periodontal ligament stem cells (PDLSCs) play an indispensable role in maintaining periodontal homeostasis.18 According to emerging evidence, the inflammatory environment caused by periodontitis leads to dysfunction and pyroptosis in PDLSCs. Ferroptosis, which is a novel necrotic cell death pathway, is triggered by iron overload.21 Perturbations in iron homeostasis are major pathogenic strategies for bacterial infection. FadA activates NF-κB and MAPK signaling pathways by interacting with PEBP1 Fusobacterium adhesin A (FadA) has been reported to be one of the most important adhesins and virulence factors of F. nucleatum.16 Among the drugs investigated, piperlongumine and fisetin exhibited the best attenuating effects, which prompted us to further assess their effects on FadA-induced inflammation. Similarly, piperlongumine and fisetin significantly decreased the FadA-induced proinflammatory cytokine production (P < 0.001) (Fig. 9a, b). Considering the ferroptotic effects of F. nucleatum on PDLSCs, we further examined the effects of piperlongumine and fisetin on ferroptosis. As shown in Fig. 9c–f, piperlongumine and fisetin reversed this trend, reduced the level of intracellular Fe2+, and ameliorated the impairment in mitochondrial function. These findings proved that PDLSCs have immunoregulatory capacity and that F. nucleatum could aggravate periodontal inflammation by impairing the immunosuppressive function of PDLSCs.
0 notes
Text

Travelling Bug
Resident in our mouths, the bacterium Fusobacterium nucleatum is implicated in colorectal cancer. This study shows how vesicles – sacs of cell contents – released from the bacterium fuse with colorectal cancer cells, presenting a mechanism for getting from the mouth to the gut cells, or indeed a means of disseminating microbiota around the body generally
Read the published research article here
Image from work by Xin Zheng and Tao Gong, and colleagues
State Key Laboratory of Oral Diseases, National Center for Stomatology, and National Clinical Research Center for Oral Diseases, West China Hospital of Stomatology, Sichuan University, Chengdu, China
Image originally published with a Creative Commons Attribution 4.0 International (CC BY-NC 4.0)
Published in Science Advances, September 2024
You can also follow BPoD on Instagram, Twitter and Facebook
6 notes
·
View notes
Text
Fusobacteriota
Group: Other
Gram-stain: Negative (mostly)
Etymology: For Fusobacterium nucleatum. From the Latin "fusus", meaning "spindle", and "bacter", meaning "rod": thus a spindle-shaped rod.
About: Fusobacteriota is unique for being a relatively isolated phylum, not closely related to any of the recognized groupings. They can be pathogenic, and over 10% of human sore throats are caused by Fusobacteriota (which goes up to 21% for chronic sore throats). The bacteria responsible is called Fusobacterium necrophorum, and the condition is called F-throat. These bacteria can also cause meningitis and infect the hooves of horses.
Fusobacterium nucleatum, meanwhile, are best known for forming the plaque on your teeth. What we call "plaque" is actually a biofilm of microorganisms (mixed with sticky food residue), and F. nucleatum bacteria are a key structural component of that film. They are strictly anaerobic and rely on aerobic bacteria at the top of the biofilm to consume oxygen before it reaches them. This creates a concentration gradient, allowing a multitude of both aerobic and anaerobic bacteria to thrive. For some reason, researching this paragraph made me want to brush my teeth...

10 notes
·
View notes
Text
common laboratory bacteria ranked by smell:
Streptococcus anginosus: sweet and butterscotch-y. a favourite of all techs.
Candida albicans: a yeast. smells of rising bread dough and beer. some isolates produce this smell stronger than others, and more odorous organisms are sometimes shared among staff to enjoy the smell
Pseudomonas aeruginosa: sweet, like a fake grape flavour. reminds me of my childhood. sometimes compared to tortilla chips. always pleasant to encounter
Proteus vulgaris: not commonly liked, but i would describe its smell as akin to chocolate cake. others might call it fishy. a controversial fave
Staphylococcus aureus: on blood agar, the smell is reminiscent of that of unwashed human skin. reminds me of smelling people, which makes me like it
Coagulase negative Staphylococcus: similar smell to S. aureus
Klebsiella pneumoniae: a rectal smell somewhat like E. coli, but with more of a "wet dog" funk. i don't hate it
Haemophilus species: mildly unpleasant. a moist and musty smelling genus
Escherichia coli: the ambassador of the world of bacteria smells like you might expect bacteria to. i would not describe the smell as fecal, but certainly rectal.
Enterobacter cloacae: much like E. coli, but just noticeably worse. like you left your E. coli plate in a nasty bathroom
Fusobacterium nucleatum ACC 25586: QC for anaerobic jars. smells of rotting pumpkins, acrid flatulence, and bad breath all rolled into one. i refuse to work with it on benchtop
8 notes
·
View notes
Text
Fusobacterium nucleatum is commonly found in the mouth and intestinal flora of humans.6 It is possible that the finding of the bacteria was caused simply by poor aseptic technique. One of the most vital rules of research is that correlation does not equal causation. The theory the authors present - that Fusobacteria plays a role in endometriosis - is not supported by pathological findings in patients. When patients undergo excision of endometriosis - which is the gold standard treatment - samples of the excised tissue are sent for pathological analysis. To date, there has been no data to support the presence of bacteria in these tissues. If the correlation is as strong as the authors suggest, one would expect that the pathologic analysis of endometriosis lesions would support that finding. This is not the case. Unfortunately for the nearly 200 million people with endometriosis, the possibility that endometriosis could be cured by simply taking antibiotics is merely wishful thinking.
#endometriosis#i don't have a dx yet so i still dk if i have endo or if my tubes are even still dilated but. here#j
2 notes
·
View notes
Text
5 Orthodontist Advice Which Can Control Bacteria 'A Catalyst' Of Cancer Cell Growth
Author Daniel Rlewis Published June 8, 2015 Word count 514 The bacteria ‘fusobacterium nucleatum’ – which has strong links with gum disease – could hamper the resistance of the body to fight with cancer cell growth. These bacteria are the catalyst that cause cancer and when pooled with human tissue cells, the bacteria enclosed itself as the parts of the immune system that are responsible for…
0 notes
Text
IJMS, Vol. 25, Pages 9025: Circulating Bacterial DNA in Colorectal #cancer Patients: The Potential Role of Fusobacterium nucleatum
Intestinal dysbiosis is a major contributor to colorectal #cancer (CRC) development, leading to bacterial translocation into the bloodstream. This study aimed to evaluate the presence of circulated bacterial DNA (cbDNA) in CRC patients (n = 75) and healthy individuals (n = 25). DNA extracted from peripheral blood was analyzed using PCR, with specific primers targeting 16S #rRNA, Escherichia coli (E. coli), and Fusobacterium nucleatum (F. nucleatum). High 16S #rRNA and E. coli detections were observed in all patients and controls. Only the detection of F. nucleatum was significantly higher in metastatic non-excised CRC, compared to controls (p < 0.001), non-metastatic excised CRC (p = 0.023), and metastatic excised CRC (p = 0.023). This effect was mainly attributed to the presence of the primary tumor (p = 0.006) but not the presence of distant metastases (p = 0.217). The association of cbDNA with other clinical parameters or co-morbidities was also evaluated, revealing a higher detection of E. coli in CRC patients with diabetes (p = 0.004). These results highlighted the importance of bacterial translocation in CRC patients and the potential role of F. nucleatum as an intratumoral oncomicrobe in CRC. https://www.mdpi.com/1422-0067/25/16/9025?utm_source=dlvr.it&utm_medium=tumblr
0 notes
Text
Unravelling the Link Between Breast Cancer and the Microbiome!
With the spilling beans of BC, the rate of incidence of breast cancer has reached alarming levels, impacting the lives of women across the globe. As the most commonly diagnosed cancer among women, the need for early detection and effective treatment has become more crucial than ever.
Traditionally, cancer diagnosis often involved invasive procedures, posing risks and challenges to patients. However, recent research has sparked a new wave of interest in non-invasive diagnostic approaches, using samples such as saliva, stool, and plasma, to explore the potential role of the microbiome in cancer detection.
The human microbiome, composed of diverse microorganisms residing within our bodies, has been found to influence various aspects of health and disease.
In the context of cancer, the microbiome's indirect influence on the onset and progression of breast cancer needs significant attention from the scientific community.
Can gut microbes impact induce cancer cells?
Studies have revealed that gut microbes can impact dormant cancer cells, and potentially induce chronic inflammation to disease recurrence in breast cancer. While much of the research has centred on the role of bacteria in cancer, there is a growing interest in exploring the potential involvement of fungi, viruses, and even archaea in cancer development.
Interestingly, metabolites from certain single-celled organisms (archaea) have been linked to various cancers, shedding light on the intricate relationship between microbial populations and the progression of the disease.
Despite being less studied due to their relative rarity, these findings underscore the complexity of the microbiome's impact on human health.
The burgeoning understanding of the proliferation microbiome's significance extends to its pivotal role in the gastrointestinal tract, where the largest concentration of microorganisms is found.
This complex ecosystem is influenced by both genetic and lifestyle factors, and its dynamic interplay with the host exerts both local and systemic effects.
As we highlight the backdraws of cancer proliferation, the exploration of the microbiome's influence on breast cancer is opening new avenues for innovative diagnostic methods and potential therapeutic strategies.
Unraveling the Microbiome and Lifestyle Factors in Breast Cancer: A Thought-Provoking Discussion!
The intricate trap of Microbiome influences breast cancer (BC), as new research sheds light on the potential impact of the microbiome and various lifestyle components.
Let's get into these compelling findings and the thought-provoking questions they inspire.
Microbial Players in Breast Cancer!
Recent studies have revealed a strong relation between the microbial composition of breast cancer patients and the abundance of specific bacteria, such as Bacillus, Enterobacteriaceae, and Staphylococcus.
The discovery that certain bacteria isolated from breast cancer patients, including Staphylococcus epidermidis and Escherichia coli, can induce DNA double-stranded breaks in cells raises exciting questions about the potential role of these microorganisms in cancer development.
Moreover, the presence of Fusobacterium nucleatum, a bacterium implicated in inducing tumour growth and metastasis, in breast tissue further underscores the complex relationship between the microbiome and cancer progression.
Other Lifestyle and Environmental Influences!
In addition to microbial factors, the influence of lifestyle and environmental components on breast cancer risk adds layers of complexity to our understanding of
the disease. While genetic factors like the loss of the BRCA1/2 gene command attention, lifestyle factors present compelling avenues for exploration.
The impact of diet, alcohol consumption, and radiation exposure on breast cancer incidence has been well-documented. Equally noteworthy are the associations between hormone exposure and breast cancer risk.
The interplay between physiological variations associated with puberty, pregnancy, menopause, and the use of hormonal contraceptives or hormone replacement therapy reveals the multifaceted nature of BC risk factors.
Framing Thought-Provoking Questions!
The emerging intersection of microbiome research, lifestyle factors, and breast cancer prompts thought-provoking questions that invite further exploration and discourse:
Microbiome and Cancer Development: How do specific microbial populations contribute to DNA damage in breast cancer cells, and what mechanisms underlie their potential role in tumour progression?
Fusobacterium nucleatum and Tumor Growth: In what ways does Fusobacterium nucleatum promote tumour growth and metastasis in breast tissue, and what importance does this hold for potential interventions targeting the microbiome?
Lifestyle and Hormonal Influences: How do variations in estrogen metabolism, influenced by environmental and lifestyle factors, impact breast cancer risk, especially among postmenopausal women? What implications does this have for personalised prevention strategies?
As we confront the complexities of breast cancer etiology, these key findings inspire a call for collaborative research and early diagnose.
By exploring the complex interplay between microbial influences, lifestyle components, and genetic predisposition, Euronoxx Medical Group strongly recommend an "Early Detection could minimize the risk of breast cancer mortalities". By getting this holistic approach to understand, prevent and early diagnose of breast cancer.
Here we shown some intricate relationships, we hope to illustrate the non-invasive, microbiome-based approaches that could revolutionise breast cancer early detection and awareness.
Meanwhile, the hidden interactions between the microbiota and breast cancer may hold the key to discussing new insights and transformative breakthroughs in our fight against cancer.
0 notes
Text
The Mouth Microbe Implicated in Colorectal Cancer [ Fusobacterium nucleatum ]
The Mouth Microbe Implicated in Colorectal Cancer [News Summary] Hi, it’s Jason in Melbourne. Some causes of cancers, like smoking, are well known; others are a mystery that science is steadily unraveling. A subtype of F. nucleatum may help fuel the development and growth of colorectal cancer, findings from a new study suggest. Welcome! I am sitting in for Goodie today. The usual gang of savants…

View On WordPress
0 notes
Text
Interaction Between Two Common Oral Bacteria Creates Chemical Compound Responsible For Bad Breath
— By Osaka University | Thursday February 16, 2024
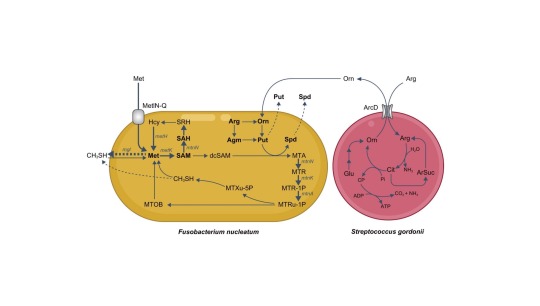
Schematic representation of observed metabolic flow of bacterial metabolism F. nucleatum and S. gordonii cocultures. Credit: mSystems (2024). DOI: 10.1128/msystems.
In a study published last month in mSystems, researchers from Osaka University revealed that the interaction between two common types of oral bacteria leads to the production of a chemical compound that is a major cause of smelly breath.
Bad breath is caused by volatile compounds that are produced when bacteria in the mouth digest substances like blood and food particles. One of the smelliest of these compounds is methyl mercaptan (CH3SH), which is produced by microbes that live around the teeth and on the surface of the tongue. However, little is known about which specific bacterial species are involved in this process.
"Most previous studies investigating CH3SH-producing oral bacteria have used isolated enzymes or relatively small culture volumes," explains lead author of the study Takeshi Hara. "In this study, we aimed to create a more realistic environment in which to investigate CH3SH production by major oral bacteria."
To do this, the researchers developed a large-volume anaerobic co-culture system that enabled them to test interactions between multiple different types of bacteria that live in the mouth. This system was able to test both direct, physical interactions among the bacteria, as well as whether these species could affect each other from a distance, for example by secreting active substances.
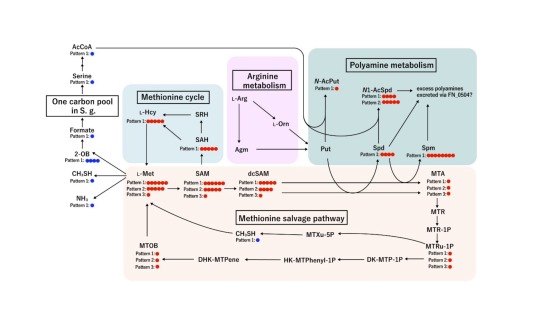
Predicted flux profiling of methionine metabolic pathways in F. nucleatum. Credit: mSystems (2024). DOI: 10.1128/msystems.
"The results were very intriguing," says Masae Kuboniwa, senior author. "We found that Fusobacterium nucleatum produces large quantities of CH3SH in response to Streptococcus gordonii, another oral bacterium."
By using stable isotope tracers and analyzing gene expression, the researchers showed that S. gordonii releases a substance called ornithine that prompts F. nucleatum to produce more of a molecule called polyamine. Because F. nucleatum needs methionine to produce polyamine, this enhanced polyamine production activates its methionine salvage pathway, which in turn results in increased CH3SH production.
"Taken together, these findings suggest that CH3SH production in the mouth is driven by the interaction between S. gordonii and F. nucleatum," says Hara.
Understanding how these two bacterial species work together to cause bad breath could be helpful in developing ways to treat or even prevent bad breath. In addition, given that bad breath is often associated with periodontal disease, treating this symptom early could help prevent more serious damage in the future.
#Phys.Org#Oral Bacteria#Bad Breath#Osaka University#Public University in Suita Japan 🇯🇵#Biology | Cell & Microbiology#Molecular & Computational Biology
0 notes
Text
Investigating the Association Between Fusobacterium nucleatum and Oral Squamous Cell Carcinoma: A Pilot Case-Control Study on Tissue Samples
http://dlvr.it/SzVXg3
0 notes
Text
Investigating the Association Between Fusobacterium nucleatum and Oral Squamous Cell Carcinoma: A Pilot Case-Control Study on Tissue Samples
http://dlvr.it/SzVLyh
0 notes
Text

Colon Cancer Colony
A distinct group of Fusobacterium nucleatum bacteria, microbiome resident of the mouth but uncommon in the normal lower gut, is predominant in human colorectal cancers
Read the published research article here
Image from work by Martha Zepeda-Rivera and colleagues
Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, WA, USA
Image originally published with a Creative Commons Attribution 4.0 International (CC BY 4.0)
Published in Nature, March 2024
You can also follow BPoD on Instagram, Twitter and Facebook
7 notes
·
View notes
Text
Antibacterial Fusobacterium Nucleatum-Mimicking Nanomedicine to Selectively Eliminate Tumor-colonized Bacteria And Enhance Immunotherapy Against Colorectal Cancer
Clinical evidence has indicated that tumor-colonizing bacteria would be closely related to the tumor development and therapeutic responses. Selectively eliminating bacteria within tumors may be an attractive approach to enhance cancer treatment without additional side effects. Herein, we found that owing to the high affinity between the membrane protein Fap-2 on Fusobacterium nucleatum (F. nucleatum) and D-galactose-β (1-3)-N-acetyl-D-galactosamine (Gal-GalNAc) overexpressed on colorectal tumor... http://dlvr.it/SwJCGg
0 notes
Text
Oral bacteria can increase risk of heart disease: Research
A study published today in eLife suggests that infection with a bacterium that causes gum disease and foul breath may raise the risk of heart disease. The study advises that physicians check for other potential risk factors to identify those at risk of heart disease. It could also mean that therapies for the oral bacterium Fusobacterium nucleatum may help minimise the risk of heart disease. Heart…

View On WordPress
0 notes