#ETO Sterilization
Explore tagged Tumblr posts
Text
ETO Sterilizer Manufacturer
Instech System��is India’s best leading manufacturer and supplier of ETO Sterilizer manufacturer in India. We provide top sterilization solutions for many industries, prioritizing safety and compliance. Instech Systems deliver equipment that ensures efficient and effective sterilization of heat-sensitive items such as surgical instruments, medical devices, and implants. We are Ethylene oxide sterilizers manufacturer, the perfect method for medical device sterilization. Apart from designing and manufacturing the sterilizer, we also provide all the ancillary equipment in order to offer a complete sterilization unit by ethylene oxide process. ETO sterilization is particularly useful for heat-sensitive materials and complex medical instruments that require a high level of sterility.
Website_ https://www.cssdtechnologies.com/eto-sterilizer/
0 notes
Text
Trusted ETO Sterilizer Manufacturer - Krishna Engineering
Introduction:
Krishna Engineering is a well-known manufacturer-supplier of EO/ETO sterilizers as part of their product line. With superior ETO sterilization technology, the company has unmatched proficiency in this field. It emphasizes safety and compliance in offering quality sterilization solutions to various industries.
Since 2000 we've been manufacturing commercial sterilizers, namely ETO Sterilizers, Sterilizers for herbs and spices, Industrial sterilizers, Autoclave sterilizers, and Hospital sterilizers. Extraction of the Compositions: We forward sterilizing solutions in all areas of the industry.
ETO sterilizers, autoclaves, fumigation chambers, retort sterilizers, and steam sterilizers for spices and herbs are a few of the most crucial goods. These solutions stand out for being flexible, long-lasting, and easy to use. They provide services to pharmaceutical businesses, hospitals, and labs. Customizing solutions to meet the needs of each particular client is the main goal of high-quality production. has installations all around the world and keeps up its high standards for customer satisfaction and innovation.
What is ETO Sterilization?
Pharmaceutical products, medical equipment, and other delicate objects that cannot tolerate high temperatures are sterilized using ethylene oxide gas in a chemical procedure known as ethylene oxide (ETO) sterilization. By destroying their DNA, the ETO gas stops bacteria, viruses, and other diseases from proliferating. The culinary, pharmaceutical, and medical sectors all frequently utilize ETO (ethylene oxide) sterilizer to clean heat-sensitive products.

Here are some key Features of ETO Sterilizers:
Low-Temperature Sterilization: ETO sterilizers operate at low temperatures (usually around 30-60°C), making them ideal for sterilizing heat-sensitive products like plastics, electronics, and medical devices without damaging them.
Effective Microbial Control: ETO gas is highly effective against a wide range of microorganisms, including bacteria, viruses, fungi, and spores, ensuring comprehensive sterilization.
Penetration Ability: The gas can penetrate complex and porous materials, including packaging, plastics, and multilayered devices, ensuring that all surfaces are thoroughly sterilized, including those hard to reach by other methods.
Easy-to-Operate Systems: Modern ETO sterilizers are designed with user-friendly controls and interfaces, often with automated cycles that reduce human error and ensure consistent results.
Environmental Control: ETO sterilizers often include humidity control, which enhances the efficiency of the sterilization process by improving the gas’s ability to penetrate the material being sterilized.
Cost Efficiency: While the initial investment may be high, ETO sterilizers can provide long-term cost savings by effectively sterilizing sensitive products without compromising their quality or integrity.
Here are Types of ETO Sterilizers:
Batch ETO Sterilizers: These are the most common type, where items are placed inside a large chamber in batches. The sterilization cycle is done in one batch process.
Continuous ETO Sterilizers: These are usually employed in larger-scale processes where the sterilizer is continuously fed with materials. Although this approach is more effective, it is frequently more costly.
How ETO Sterilization Works:
Preconditioning: Items which need sterilization are first conditioned in a specific chamber. Pre-conditioning mostly refers to treating the environment by temperature and humidity so as to ease penetration of the gas into the items.
Exposure to ETO Gas: Following preconditioning, ethylene oxide gas is put into the chamber and the chamber is sealed. The products are exposed to the gas for a certain amount of time, often a few hours. In this period, the gas sterilizes the products or equipment by penetrating the packaging materials.
Aeration: Following the exposure period, any remaining ethylene oxide gas is eliminated by aerating the goods. To guarantee that the products are safe to handle and use following sterilization, the aeration procedure is essential.
Final Inspection: After aeration, the sterilized items are examined to ensure they meet the required standards and are free of contaminants.
Conclusion:
ETO Sterilization is a critical process for industries that require low-temperature, highly effective sterilization for heat-sensitive products. While the process involves careful handling and monitoring to ensure safety, it remains one of the most widely used methods for sterilizing complex and delicate items across various sectors, particularly in healthcare and pharmaceuticals.
1 note
·
View note
Text
#ETO Sterilizer#ETO Sterilizer Exporter#ETO Sterilizer Manufacture#Ethylene oxide sterilizers Exporter#Ethylene oxide gas sterilizers#Ethylene oxide sterilizers#ETO Sterilizer India#Maharashtra#Manufacture#Exporter#India
0 notes
Text
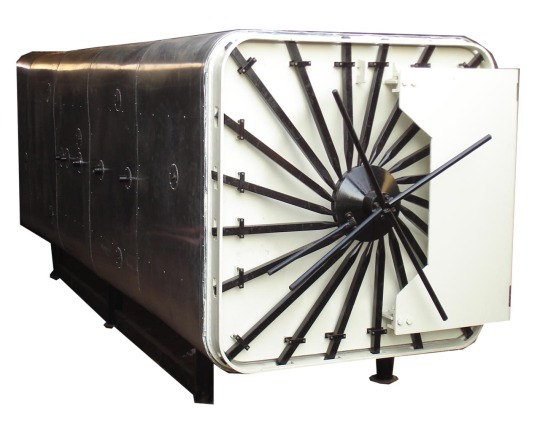
Ethylene Oxide Sterilization Unit

Ethylene Oxide Sterilization Unit (Eo gas sterilizer) including Eto gas sterilization chamber has been specially designed for sterilization of products sensitive to high temperature and to humidity (Syringes, DE fluxers, Catheters, Cartridges for Dialysis, Plastic articles, Bandages, Sutures etc.). Sterilizer can be uses even for sterilization of powders which deteriorate by heating exposure. For the wide range of product that can be treated, such sterilizer find application in Disposable Surgical Products, Para Pharmaceutical and Pharmaceutical Industry, Laboratories, Hospital and Food Processing Industries.
Adinath EO Gas Sterilization Unit Manufacturer can be realized in to satisfy different requirements working under pressure (1.5 kg/cm² + vacuum) with mixtures of Ethylene Oxide and Carbon Oxide (usual composition 10% ETO + 90% CO₂, 20% ETO + 80% CO₂)
Ethylene Oxide gas infiltrates packages as well as products themselves to kill microorganisms that are left during production or packaging processes. This gas, mixed with air at a ratio of at least 3% ETO gas, forms an explosive mixture. Pure ETO gas boiling point is 10.73 ºC at atmospheric pressure. Most of the time, it is mixed with Nitrogen or CO2. EO Gas Sterilizers uses to sterilize ot surgical instruments and medical disposables.
The system has been designed to operate on eto+co2 combination gas cylinders. The chamber and all contact parts shall be made from S.S 304. The chamber has been provided with a single door, easy locking arrangement and silicon gasket for leak proof operation. Electronically controlled heating system to ensure uniform heating of the chamber at 50 degrees centigrade. The chamber is provided with a adequate capacity rotary vacuum pump enclosed in sturdy cabinet duly powder coated for durability. We provide four of control switches with built in indicator light to regulate evacuation, feeding of gas, fresh air inlet through filter and aeration facility. Ethylene Oxide Gas Sterilization Unit manufacturer provide compact stand-alone sterilizer chamber.
0 notes
Text
Choosing the Right Sternum Saw: Key Features and Best Practices for Surgeons

Selecting the right sternum saw is crucial for ensuring successful surgical outcomes, particularly in complex cardiac and thoracic surgeries. As the largest and most trusted manufacturer, supplier, and exporter of sternum saws worldwide, Mercury Healthcare is at the forefront of innovation, providing high-quality surgical tools that meet the needs of modern healthcare professionals. In this article, we explore the key features surgeons should consider when choosing a sternum saw and why Mercury Healthcare is the preferred choice for healthcare providers globally.
Key Features to Look for in a Sternum Saw
Precision and Control In critical surgeries, precise cutting is essential to minimize trauma and ensure patient safety. A sternum saw must offer excellent control, allowing surgeons to perform procedures with accuracy. Mercury Healthcare's S1 Sternum Saw is designed with a clear line of sight and lightweight construction, providing surgeons with exceptional control during surgeries. The precision of our saws ensures efficient cutting, reducing the risk of complications and enhancing patient recovery times.
Ergonomics and Lightweight Design Long surgical procedures can be physically demanding for surgeons. A well-designed sternum saw should be lightweight and ergonomically crafted to reduce hand fatigue and allow surgeons to operate comfortably for extended periods. Mercury Healthcare prioritizes surgeon comfort, and our sternum saws are built with a balanced, lightweight design that enhances both maneuverability and ease of use.
Sterilization Compatibility Fast and efficient sterilization is crucial in surgical settings to prevent infections. Our S1 Sternum Saw can be ETO sterilized or autoclaved, ensuring a quick turnaround between procedures. This feature is especially beneficial in busy operating rooms where time is critical, helping medical teams maintain optimal cleanliness without sacrificing efficiency.
Convenience and Safety Features Safety features are a must for any surgical instrument. Mercury Healthcare's sternum saws are equipped with quick-release blades and a patented retention system, making blade changes seamless and reducing downtime during operations. Additionally, the tapered blade protector helps deflect underlying tissues from contact with the blade, minimizing the risk of tissue damage during surgery. These features make our saws both convenient and safe, allowing surgeons to focus on what matters most — patient care.
Durability and Reliability A reliable sternum saw must withstand the demands of frequent use in high-stakes surgical environments. Mercury Healthcare's sternum saws are made from high-grade materials that ensure durability and longevity. Whether performing routine procedures or complex surgeries, our saws provide consistent performance, making them the trusted choice for surgeons worldwide.
Best Practices for Using a Sternum Saw
To maximize the performance of a sternum saw, surgeons should follow these best practices:
Regular Maintenance: Proper cleaning and maintenance after each use will ensure the saw's longevity and precision.
Blade Replacement: Always use sharp, sterile blades, and replace them as necessary to avoid unnecessary pressure on the sternum.
Operator Training: Surgeons and surgical teams should undergo proper training to ensure the correct and safe use of the sternum saw, reducing risks and improving surgical outcomes.
Why Choose Mercury Healthcare?
At Mercury Healthcare, we are committed to delivering high-quality, innovative surgical equipment that meets the demands of healthcare professionals around the globe. As the leading manufacturer and exporter of sternum saws, we combine cutting-edge technology with a deep understanding of surgical needs. Our focus on precision, safety, and reliability has earned us the trust of surgeons worldwide, making Mercury Healthcare the go-to provider for advanced medical tools.
Our sternum saws are not only built for superior performance but also designed with the future of healthcare in mind. As we continue to innovate and set new industry standards, we remain dedicated to improving patient outcomes and enhancing the surgical experience for healthcare providers.
#Best sternum saw for cardiac surgery#Top features of surgical sternum saws#How to choose the right sternum saw for surgeons#Benefits of lightweight sternum saw for surgery#Leading sternum saw manufacturer for cardiac procedures#High-precision sternum saw for thoracic surgery#Trusted sternum saw supplier for surgeons worldwide#Autoclavable sternum saw for safe surgeries#Best sternum saw for healthcare professionals#Most reliable sternum saw for surgical use#Ergonomic sternum saw for long surgeries#ETO sterilized sternum saw for quick turnaround#Sternum saw with quick-release blades#Durable sternum saw for frequent surgical use#Safe and reliable sternum saw for medical procedures
0 notes
Text
Ethylene Oxide Sterilization
1 note
·
View note
Note
what is your opinion on the flat/dull appearance of ghouls' teeth? especially big monsters like eto and noro and dragoneki
I’m currently going through a dentistry nightmare and I’m thinking about ghoul teeth too! They are WEIRD
I wrote more on teeth specifically here, but in general they have a more carnivorous appearance. There’s some range in size, shape and sharpness of their teeth, enough that some appear almost human while others are much more obvious. Ghouls from families from densely populated areas tend to have smaller, more human like teeth because it’s an advantage to blend in, while rural ghouls and ghouls that live in the wilds will tend to have larger, more obvious carnivorous teeth
There are some subspecies that have larger and more obvious teeth. Few remain in developed areas due to the CCG’s hunting, but there have been ghouls that adapted terrifying bites in the absence of humans hunting them
Teeth are definitely an issue for ghouls when it comes to being spotted. Teeth checks used to be a pretty common tool for investigators to check if someone is human, and a lot of ghouls with obvious carnassials died because of it. It became a less common method as dna and rc count tests started being used, especially since a bite check isn’t always accurate and can get false positives on humans, but occasionally a dove will cite the appearance of teeth as evidence for a warrant
Extra teeth or strange teeth are occasionally seen with kakuja. Not all get it, but sometimes the kakuja transformation process will cause a ghoul to shed and regrow their teeth larger and sharpened as an epigenetic adaptation to eat other ghouls. “Cannibal Teeth” is a term ghouls use to reference a ghoul with unusually sharp teeth that could be caused by the kakuja process, but due to it not happening in all kakuja it’s a common debate among ghouls whether or not the rumors of cannibals getting monster teeth is true
There is a genetic mutation that can cause teeth to grow on kagune. Rc cells are very similar to stem cells and some ghouls, especially those with other kagune issues that leave them vulnerable to mutations, have a misfire of genetic information that causes tooth material to grow among their rc. Depending on placement and how much grows it can be almost unnoticeable, such as appearing like scales or small spines, or extremely clear such as fully formed or even giant teeth. It’s not very common though
Ghoul mouths, even besides the teeth, are made to break down meat. Because of that they are surprisingly sterile. Not because they don’t pick up bacteria, but because they produce enzymes in their saliva that jumpstart the digestion process and kill a lot of weaker organisms. Their bites don’t have nearly the same infection rate as a human’s because of it, and while it doesn’t break down human cells too much when they aren’t chewing, if you were to hold some ghoul saliva you’d feel the tingling of it trying to digest the top layer of your skin
Ghouls also just tend to have larger mouths in general. It’s not uncommon for them to be able to open much wider than they seem capable of, and to have mouths that extend a little bit past their lips. It’s hard to see this part when their mouth is closed as it’s meant to blend in with their face to seem human
34 notes
·
View notes
Note
… tell me about Endospores.
I love mushrooms and spores and fungi, I want you to tell me an entire Wiki page
(this is totally not Shiek, who has not been checking every single notification to see if people liked the Reddit screenshot….. hi)
Hello!! I fuckin love endospores and ur the first person to actually send an ask about them
Endospores are actually a type of bacteria! Some environmental bacilli produce an endospore coating to withstand extremely harsh environments and is usually initiated by total nutrient deprivation (think of an absence of nitrogen). That being said, the bacteria in our bodies won’t undergo this process, but finding out whether an endospore bacterium is introduced to your body is pretty important because endospores require very specific treatments given their nature. For example, bacillus anthracis (my favorite example) - this bacterium is responsible for anthrax infections, the only effective treatment being an antibiotic called Cipro.
To (sorta) briefly explain how endospores are formed - when environmental conditions are no longer viable to sustain life, the bacterium begins an 8 hour endosporulation process in which the cells DNA is duplicated and a septal wall is created that asymmetrically divides the cell, and a spore coat is formed around the forespore and there is a thick peptidoglycan cortex formed between the layers of the two halves of the cell. Once the sporulation process is completed, the newly matured endospore is released once the rest of the vegetative cell is degraded. The cortex is basically what allows the endospore to be highly resistant against high temperatures and enzymes and most chemicals that are otherwise effective against other bacteria.
This is why autoclaving is one of the most effective ways of disinfecting surfaces! Otherwise, sterilant alkylating agents, like ETO and household bleach are effective (since not everyone owns an autoclave but a girl can dream). Household bleach must be in contact with endospores for a minimum of 10 minutes for it to actually kill the bacteria; increasing the concentration instead will NOT be effective, it may actually cause bacteria to aggregate and survive. Ionizing radiation (e.g., x-rays, gamma rays) is also effective against most endospores!
Now one of the coolest facts about endospores imo - the endospore is PROTECTIVE for the bacteria in these really harsh, nitrogen depleted environments, right? So for the bacteria to survive, it will actually be in a dormant state until either environmental conditions improve or they’re chemically stimulated to undergo activation, germination, and outgrowth. This means they can lie dormant for MILLIONS of YEARS - one endospore was actually found encased in amber and was actually reactivated.
Also,
Reddit screenshot u say?
#answered asks#microbiology#endospores#sorry for the long response but I fucking love endospores#screaming into the void
2 notes
·
View notes
Text
How High-Quality Luer Caps Improve Infection Control and Device Integrity Worldwide
In the global medical device industry, safety, sterility, and performance are critical at every stage—from production to patient application. Among the many components that contribute to these standards, luer cap play a vital yet often overlooked role. Whether in disposable infusion sets, syringes, or hypodermic needle assemblies, the use of high-quality Luer caps significantly enhances both infection control and device integrity.

As a manufacturer and supplier of medical components such as drip chambers, Luer lock connectors, Y injection sites, roller clamps, and protective caps, understanding and delivering the best possible Luer cap design is key to standing out in competitive global markets.
What Are Luer Caps?
Luer caps are small, precisely engineered closures designed to fit onto Luer lock and Luer slip connectors. They are used to seal open ends of IV lines, syringes, and other disposable medical devices, protecting the internal pathway from contaminants and maintaining sterility until use.
There are typically two types of Luer caps:
Male Luer caps (seal female Luer connectors)
Female Luer caps (seal male Luer connectors)
These caps are made of medical-grade polypropylene or polyethylene, offering biocompatibility and compatibility with various sterilization methods.
The Crucial Role of Luer Caps in Infection Control
Infection control is a top priority in all healthcare environments. With the increasing threat of hospital-acquired infections (HAIs) and antibiotic-resistant bacteria, even the smallest component, such as a Luer cap, can be a front-line defense tool.
Here’s how high-quality Luer caps improve infection prevention:
1. Sealing Against Airborne Contaminants
Luer caps provide an airtight seal that prevents bacteria, viruses, and airborne particles from entering critical pathways such as IV lines and syringes. This protection is essential during storage, handling, and patient transport.
2. Prevention of Fluid Backflow and Leakage
Inferior caps can allow micro-leaks or backflow, which increases the risk of contamination. A precision-engineered Luer cap maintains a secure, leak-free seal, ensuring device sterility is preserved until the moment of use.
3. Single-Use, Sterile Packaging Options
High-quality Luer caps are often available in sterile, single-use packaging that complies with ISO 13485 and other international standards, further reducing the risk of cross-contamination between procedures or patients.
Device Integrity: Preserved and Protected
Maintaining the mechanical and functional integrity of medical devices is crucial for proper treatment outcomes and regulatory compliance. Luer caps support this by:
1. Preventing Physical Damage
Caps protect the delicate male or female Luer threads and tips from physical damage during transportation, storage, and handling. A deformed Luer connector can result in poor fits, leakage, or device failure.
2. Compatibility With Sterilization Processes
High-quality Luer caps are designed to withstand ETO gas, gamma radiation, or steam sterilization, ensuring that they maintain structural integrity without warping, cracking, or releasing harmful substances.
3. Extended Shelf Life
By sealing off exposure to moisture, dust, and other environmental hazards, these caps help medical products maintain their integrity and shelf life—especially critical for worldwide distribution across varying climates.
Global Impact and Regulatory Importance
For international distributors and healthcare providers, product quality and safety are not only clinical concerns but also regulatory requirements. Markets in the United States (FDA), Europe (CE marking), and Latin America (ANVISA, INVIMA, etc.) require strict adherence to manufacturing standards.
High-quality Luer caps:
Help meet ISO 80369 and ISO 13485 standards for small-bore connectors
Ensure tamper-evidence and traceability
Reduce product returns and enhance customer satisfaction
By integrating top-tier Luer caps into their product lines, global distributors can offer safer, more reliable devices that meet or exceed regulatory expectations.
Why Choose Our Luer Caps?
At [Your Company Name], we manufacture a comprehensive range of medical components, including precision-engineered Luer caps for male and female connectors. Our caps are:
Made with medical-grade materials
Compatible with all Luer lock and Luer slip systems
Available in sterile or bulk packaging
Customizable with color coding or branding
We serve global markets, ensuring every cap meets the highest standards for sterility, performance, and durability.
Conclusion
While often considered a minor component, the Luer cap is a critical asset in the fight against infection and in preserving the functionality of medical devices. For manufacturers, distributors, and healthcare professionals alike, investing in high-quality Luer caps is a cost-effective and practical way to enhance safety and performance.
If you're a global distributor seeking reliable, certified, and competitively priced medical components, contact [Your Company Name] today to learn more about how our Luer caps and other device components can support your goals in the medical device market.
0 notes
Text
What is an ETO Sterilizer? How Does it Works?
Sterilization is a key process in keeping things clean and safe, especially in healthcare and manufacturing. One popular method for sterilizing items that can’t handle heat is using an Ethylene Oxide (ETO) sterilizer.
Here’s a straightforward guide to understanding ETO sterilizers:
What is an ETO Sterilizer?
Definition: An ETO sterilizer uses ethylene oxide gas to kill germs and bacteria on items that can’t be cleaned with heat.
Uses: It’s commonly used for medical tools, delicate equipment, and other items that might be damaged by high temperatures.

How Does ETO Sterilization Work?
1.Get Ready:
Clean and dry the items you want to sterilize.
Place them in a special chamber designed for sterilization.
2. Add Gas:
Ethylene oxide gas is introduced into the chamber.
This gas is very effective at killing bacteria and viruses.
3. Adjust Conditions:
The chamber is kept at the right temperature and humidity to help the gas work better.
This usually means a temperature between 30°C and 60°C and humidity between 30% and 80%.
4. Ventilation:
After the gas has done its job, the chamber is ventilated to remove the ethylene oxide gas.
The items are then aired out to ensure no gas is left on them.
Why Use ETO Sterilization?
1.For Heat-Sensitive Items:
ETO is perfect for items that can’t be heated, like certain plastics and electronics.
2.Penetrates Packaging:
The gas can get through packaging and complex shapes, ensuring all surfaces are sterilized.
3. Versatile Use:
Works well for a wide range of products, from medical devices to pharmaceutical items.
Key Considerations
1.Safety First:
Ethylene oxide is toxic and can be harmful. Make sure to follow safety guidelines and wear protective gear.
Ensure good ventilation and proper handling to avoid health risks.
2. Follow Regulations:
There are strict rules and standards for using ETO. Make sure your process complies with local regulations and industry standards.
3. Environmental Impact:
Ethylene oxide can affect the environment. Modern ETO systems are designed to minimize environmental damage, but it’s something to be aware of.
4. Cost and Maintenance:
ETO sterilizers can be expensive to buy and maintain.
Regular maintenance is needed to keep the system running safely and effectively.
Conclusion
ETO sterilizers are a great tool for sterilizing items that can’t handle heat. They are effective, versatile, and able to reach every part of your items. However, it’s important to handle ethylene oxide with care, follow all safety guidelines, and be mindful of costs and environmental impact. By understanding these basics, you can make informed decisions about using ETO sterilizers in your processes.
For more details, please contact us!
Website :- www.cssdtechnologies.com
Contact No. :- +91–9873069138, +91–8896456000
Email :- [email protected], [email protected]
#ETO Sterilizer#best eto sterilizer supplier in delhi ncr#eto sterilizer supplier in india#eto sterilizer supplier in delhi ncr#best eto sterilizer manufacturer in india#eto sterilizer manufacturer in delhi ncr#ETO Sterilizer manufacturer#ETO Sterilization#cssd technologies
1 note
·
View note
Text

Compact, efficient, and clinic-friendly – the Table Top ETO Sterilizer is designed for medical professionals who need powerful sterilisation in limited spaces. Ideal for dental clinics, private practices, and diagnostic centres, this model offers safe, low-temperature sterilisation for delicate instruments. Despite its small footprint, it delivers the same precision and reliability as our larger machines – with easy controls and fast turnaround times.
For more details, visit- https://www.hospitalsterilizers.com/product/table-top-eto-sterilizer/
#hospitalsterilizer#TableTopSterilizer#ETOSterilizer#CompactSterilizer#DentalClinicEquipment#DiagnosticCenterTools#MedicalSterilizer#LowTemperatureSterilization#ClinicFriendlySterilizer#SterilizationSolutions
0 notes
Text
#ETO Sterilizer#ETO Sterilizer Exporter#ETO Sterilizer Manufacture#Ethylene oxide sterilizers Exporter#Ethylene oxide gas sterilizers#Ethylene oxide sterilizers#ETO Sterilizer India#Maharashtra#Manufacture#Exporter#India
0 notes
Text
Cath Lab ETO Sterilizer

Cath Lab ETO Sterilizer or hospital Eto sterilizer mainly uses to sterilize medical and pharmaceutical products that cannot support conventional high temperature steam sterilization – such as devices that incorporate electronic components, plastic packaging or plastic containers.
Ethylene Oxide gas infiltrates packages as well as products themselves to kill microorganisms that are left during production or packaging processes. This gas, mixed with air at a ratio of at least 3% ETO gas, forms an explosive mixture. Pure ETO gas boiling point is 10.73 ºC at atmospheric pressure. Most of the time, it is mixed with Nitrogen or CO2. EO Gas Sterilizers uses to sterilize ot surgical instruments and medical disposables.
The system has been designed to operate on single use disposable type, EO 100% gas cartridges of 40 / 100 / 170 grams. The chamber and all contact parts shall be made from S.S 304. The chamber has been provided with a single door, easy locking arrangement and silicon gasket for leak proof operation. Electronically controlled heating system to ensure uniform heating of the chamber at 50 degrees centigrade. The chamber is provided with a adequate capacity rotary vacuum pump enclosed in sturdy cabinet duly powder coated for durability. We provide four of control switches with built in indicator light to regulate evacuation, feeding of gas, fresh air inlet through filter and aeration facility. Ethylene Oxide Gas Sterilization Unit is compact stand-alone sterilizer chamber.
0 notes
Text
Ethylene Oxide Sterilization: Why It Remains the Gold Standard for Restorative Gadget Safety
In the worldwide healthcare industry, the sterilization of restorative gadgets is a non-negotiable standard to guarantee understanding security. Among the different strategies accessible, Ethylene Oxide (EtO) sterilization remains the gold standard, especially for complex and touchy gadgets such as expendable implantation sets, syringes, hypodermic needles, and related restorative components like Luer bolt connectors and Y-injection sites
What is Ethylene Oxide Sterilization?
Ethylene Oxide sterilization is a low-temperature chemical preparation that employs ethylene oxide gas to dispose of microscopic organisms, infections, and other microorganisms from restorative hardware. Since it is viable at entering hard-to-reach regions and fragile components, it is perfect for gadgets made from different materials and complicated assemblies. To Know more about
Why EtO is the Favored Sterilization Method
Superior Penetration
EtO gas can enter bundling materials, tubing, and complex gadget geometries—ensuring that indeed the most perplexing inner surfaces are sterilized thoroughly.
Low Temperature Compatibility
Unlike steam or radiation strategies, EtO works at moo temperatures (ordinarily 37–63°C), which makes it secure for warm- and moisture-sensitive gadgets. This is especially critical for protecting the judgment of plastics and elastomers utilized in therapeutic components like trickle chambers, roller clamps, and caps.
Proven Efficacy
EtO sterilization has decades of demonstrated victory in guaranteeing sterility confirmation levels (SAL) of 10⁻⁶, meaning there is less than one chance in a million that a practical microorganism remains on a sterilized item.
Applications in Expendable Restorative Devices
For producers of expendable restorative equipment—including mixture sets, syringes, and hypodermic needles—EtO sterilization is fundamentally to assembly both worldwide security benchmarks and client desires. The method’s compatibility with a wide extend of polymers and bundling materials guarantees that items stay sterile and useful all through their rack life.
Global Compliance and Certification
EtO sterilization is recognized and directed by wellbeing specialists around the world, counting the U.S. Nourishment and Medicate Organization (FDA), the European Solutions Organization (EMA), and the Worldwide Organization for Standardization (ISO 11135). Compliance with these directions guarantees that items are secure for clinical utilize in any portion of the work.
Environmental and Security Considerations
While EtO is a effective sterilant, it must be dealt with carefully due to its harmfulness and combustibility. Driving sterilization offices utilize progressed natural control frameworks to capture and neutralize outflows. Nonstop advancements in innovation and handle approval too offer assistance moderate dangers, guaranteeing security for laborers and the environment.
Why It Things for End-Users
For healing centers, clinics, and patients, the unwavering quality of EtO-sterilized therapeutic gadgets deciphers into more secure methods, less diseases, and more noteworthy believe in the apparatuses they depend on. For producers, it offers adaptability, administrative compliance, and the certainty that items meet the most noteworthy security standards.
Conclusion
Ethylene Oxide sterilization proceeds to be the most successful and flexible arrangement for sterilizing expendable therapeutic gear. Its capacity to protect item judgment whereas guaranteeing greatest microbial murder makes it an basic portion of the therapeutic fabricating handle. As a worldwide provider of mixture sets, syringes, hypodermic needles, and basic restorative components, we stay committed to utilizing EtO sterilization to maintain the most noteworthy benchmarks of understanding security and item execution.
0 notes
Text
Surgikart: Leading the Way Among Surgical Instruments Manufacturers in India
In the rapidly evolving healthcare sector, precision, reliability, and innovation are essential. Nowhere is this more evident than in the realm of surgical instruments, where even the smallest detail can impact patient outcomes. As hospitals, clinics, and healthcare providers across the world look for quality solutions, the demand for trusted surgical instruments manufacturers in India has seen a steady rise. Among these, Surgikart has emerged as a front-runner—setting new standards in manufacturing excellence, product innovation, and global compliance.
India’s Rise as a Global Hub for Surgical Instruments
India has become a key player in the global medical devices industry, especially in the surgical segment. Thanks to robust infrastructure, skilled labor, and stringent quality regulations, the country now houses several internationally acclaimed surgical instruments manufacturers in India. These manufacturers not only cater to domestic healthcare demands but also supply precision-engineered surgical tools to over 150 countries. Among these, Surgikart is recognized for blending innovation with quality to deliver dependable surgical equipment trusted by professionals worldwide.
Surgikart: Pioneering Precision in Surgical Instrumentation
Founded with a vision to bring world-class medical devices to India and beyond, Surgikart has carved a strong niche for itself in the surgical manufacturing landscape. With a state-of-the-art manufacturing unit in New Delhi that includes controlled molding areas, a Class 10,000 clean room, and an advanced ETO Gas Sterilization Plant, the company operates at par with global benchmarks.
This infrastructure allows Surgikart to produce a comprehensive range of surgical and medical instruments that meet strict safety, hygiene, and performance standards. As one of the most trusted surgical instruments manufacturers in India, Surgikart adheres to ISO 13485:2003 protocols and holds CE certification for multiple product lines. Several of its instruments have even been cleared through the US FDA 510(k) approval process—an achievement that very few Indian companies can claim.
Commitment to Quality That Surpasses Expectations
Surgical instruments are not just tools—they are lifelines. Whether it’s a complex heart surgery or a routine outpatient procedure, the precision of the instruments used can influence both the success of the operation and the safety of the patient. Recognizing this, Surgikart has put quality at the heart of everything it does.
Each surgical instrument undergoes rigorous multi-stage inspections and quality tests to ensure that only the best reaches the end user. The manufacturing process includes checks for sharpness, durability, corrosion resistance, ergonomic design, and biocompatibility. This attention to detail is why Surgikart is considered a benchmark among surgical instruments manufacturers in India.
Diverse Product Portfolio for Every Surgical Need
What makes Surgikart truly stand out is its wide-ranging surgical portfolio that caters to various medical disciplines. From general surgery instruments to specialized tools for cardiac, orthopedic, gastroenterological, urological, and gynecological procedures—Surgikart delivers it all. The company’s in-house R&D department works closely with surgeons and healthcare experts to continually refine and upgrade products, ensuring they meet the latest clinical needs.
This adaptability and responsiveness have helped Surgikart maintain its position among the top surgical instruments manufacturers in India, particularly when it comes to innovation, custom manufacturing, and fulfilling unique clinical requirements.
Global Presence, Local Expertise
Though based in India, Surgikart has a strong international footprint. The company exports its surgical and medical products to over 52 countries, serving an ever-expanding global client base that includes hospitals, private clinics, government institutions, and medical distributors.
Its growing international demand is a reflection of the trust the company has earned for its consistent product quality, transparent business practices, and timely deliveries. As more healthcare systems look toward India for affordable yet high-performance medical solutions, Surgikart continues to lead as one of the most reliable surgical instruments manufacturers in India on the global stage.
OEM and Contract Manufacturing Services
In addition to producing and marketing products under its own brand, Surgikart also partners with other companies through OEM and contract manufacturing models. This flexible business model allows brands across the globe to benefit from Surgikart’s manufacturing excellence while marketing under their own label.
These strategic collaborations have contributed significantly to the company’s credibility and have cemented its place among top-tier surgical instruments manufacturers in India known for dependable private-label solutions.
Experienced Leadership and Skilled Workforce
The success of Surgikart can be largely attributed to its visionary leadership and talented team. The company is promoted and managed by Mr. Sachin Chawla, a seasoned expert with more than 20 years of experience in the medical device industry. Under his guidance, Surgikart has grown into a trusted brand recognized for its professional ethics, customer-centric approach, and uncompromising quality.
The company’s workforce includes over 300 trained professionals who specialize in production, quality assurance, research and development, packaging, logistics, and administration. This well-structured and motivated team ensures seamless operations at every level—making Surgikart a name to reckon with among the top surgical instruments manufacturers in India.
A Name You Can Trust in Surgical Manufacturing
In a market crowded with low-cost and low-quality imports, healthcare professionals are increasingly seeking reliable suppliers who can guarantee safety and consistency. Surgikart offers exactly that. Its unwavering focus on quality, regulatory compliance, and client satisfaction makes it one of the most dependable surgical instruments manufacturers in India.
Whether you're a hospital procurement manager, a government agency, a distributor, or a private healthcare provider, partnering with Surgikart ensures access to internationally certified surgical instruments that meet the highest clinical standards.
Final Thoughts: Choose Surgikart for Precision, Reliability & Innovation
As the global healthcare industry advances and patient expectations rise, the importance of precision surgical instruments cannot be overstated. With decades of experience, advanced manufacturing facilities, and an unyielding commitment to quality, Surgikart has earned its position as one of the leading surgical instruments manufacturers in India.
From hospitals in metro cities to clinics in remote regions—and from domestic markets to international borders—Surgikart is delivering excellence one instrument at a time. When you choose Surgikart, you're not just choosing a product. You're choosing a legacy of trust, precision, and innovation.
Contact Surgikart today to explore their extensive range of surgical instruments and experience the quality that has made them a globally trusted name in surgical manufacturing.
0 notes
Text
Sterile laboratory bottle
A Sterile Laboratory Bottle is a specially designed container used in laboratories to safely store, mix, and transport biological samples, reagents, media, and other sensitive liquids under sterile conditions. These bottles are typically made from high-quality materials like polypropylene or PETG, and are either gamma-irradiated or EtO-sterilized to eliminate any microbial contamination.

Trusted brands like Foxx Life Sciences offer a wide range of sterile laboratory bottles that meet stringent industry standards, ensuring reliable performance in applications like cell culture, pharmaceutical research, and microbiological testing. Foxx Life Sciences' sterile bottles often feature leak-proof caps, clear volume graduations, and are available in multiple sizes to suit various laboratory needs.
Whether you’re working in a biotech lab, clinical setting, or research facility, Foxx Life Sciences sterile laboratory bottles provide the quality and sterility assurance necessary for critical scientific processes.
Key Features of Foxx Life Sciences Sterile Laboratory Bottles
Made from high-grade, durable polypropylene or PETG
Sterilized via gamma irradiation or EtO for contaminant-free use
Leak-proof caps for secure storage and transport
Crystal-clear body with molded volume graduations
Available in multiple sizes and capacities
Ideal for pharmaceutical, biotech, and microbiological labs
Manufactured under ISO-certified cleanroom conditions
Applications
Cell culture and tissue engineering
Pharmaceutical and clinical research
Microbiological testing
Reagent and buffer preparation
Biotech product development
Diagnostic laboratories
FAQs
Q1: Are Foxx Life Sciences sterile laboratory bottles reusable?
A: While designed for single-use to maintain sterility, select bottles can be autoclaved depending on the material type. Always refer to the product specifications.
Q2: What sterilization method is used for these bottles?
A: Foxx Life Sciences sterile bottles are available sterilized via Gamma Irradiation or Ethylene Oxide (EtO), ensuring they remain contaminant-free and ready for immediate lab use.
Q3: Do these bottles comply with industry standards?
A: Yes — all Foxx Life Sciences sterile laboratory bottles comply with ISO certifications and USP Class VI cleanroom manufacturing standards.
Why Choose Foxx Life Sciences?
As a global leader in innovative lab products, Foxx Life Sciences delivers unmatched quality, safety, and performance. Their sterile laboratory bottles are trusted by top research institutions, pharmaceutical companies, and healthcare laboratories worldwide.
Contact us : [email protected]
0 notes