#Dimethyl Ether applications
Explore tagged Tumblr posts
Text
Dimethyl Ether: A Versatile Fuel for a Sustainable Future

Dimethyl ether (DME) is emerging as a key player in the pursuit of sustainable energy solutions, offering versatile applications across various industries. This clean-burning compound holds promise as an alternative fuel and a critical component in transitioning away from conventional fossil fuels. In this article, we delve into the properties, production methods, applications, and environmental benefits of Dimethyl ether, exploring its potential to reshape the global energy landscape.
What is Dimethyl Ether?
Dimethyl ether, with the chemical formula CH3OCH3CH_3OCH_3CH3OCH3, is a colorless, odorless gas at standard temperature and pressure. Its molecular structure consists of two methyl groups connected by an oxygen atom, giving it a unique combination of chemical stability and reactivity. DME is highly flammable, liquefies under modest pressure, and possesses physical properties similar to liquefied petroleum gas (LPG).
Production of Dimethyl Ether
DME is synthesized primarily through two pathways: direct and indirect synthesis.
Direct Synthesis: This process involves reacting synthesis gas (syngas), a mixture of carbon monoxide and hydrogen, in the presence of a bifunctional catalyst. The reaction simultaneously forms methanol, which is then dehydrated to produce DME.
Indirect Synthesis: This involves a two-step process where methanol is first produced from syngas and subsequently dehydrated to form DME. This method is widely adopted due to its simplicity and existing infrastructure for methanol production.
Innovative technologies are now focusing on producing DME from renewable sources such as biomass and municipal waste, enhancing its sustainability profile.
Applications of Dimethyl Ether
DME's unique properties make it suitable for a range of applications:
Fuel Alternative:
Transportation: DME serves as a clean alternative to diesel fuel, offering high cetane numbers and eliminating soot emissions during combustion. Vehicles designed or retrofitted for DME are gaining traction in regions focusing on decarbonization.
Cooking and Heating: DME can replace LPG for domestic cooking and heating purposes, reducing greenhouse gas emissions.
Aerosol Propellant:As a non-toxic and ozone-friendly alternative to traditional propellants, DME is widely used in personal care products, paints, and pharmaceuticals.
Chemical Feedstock:DME acts as an intermediate in the production of valuable chemicals like olefins and dimethyl sulfate, contributing to the industrial sector.
Power Generation:In power plants, DME can serve as a substitute for natural gas or coal, reducing harmful emissions and supporting cleaner energy production.
Environmental and Economic Advantages
One of DME's standout features is its environmental friendliness. When burned, it produces minimal particulate matter and significantly lower levels of nitrogen oxides (NOx) compared to conventional fuels. Moreover, its sulfur-free nature ensures no formation of sulfur oxides (SOx), a major contributor to acid rain.
Economically, DME offers cost-effective benefits, especially when derived from abundant or waste-based feedstocks. Its adaptability to existing fuel infrastructure, such as LPG storage and distribution networks, further reduces implementation costs.
Challenges and Future Prospects
Despite its potential, widespread adoption of DME faces hurdles such as production scalability, storage, and transportation challenges. Investments in renewable DME production and government incentives for green energy adoption could accelerate its market penetration.
Conclusion
Dimethyl ether stands at the crossroads of innovation and sustainability, offering a cleaner, efficient, and versatile energy solution. As technological advancements refine its production and applications, DME has the potential to significantly reduce humanity’s carbon footprint, paving the way for a greener future. By embracing this promising compound, we can move closer to achieving global energy sustainability goals.
#Dimethyl ether#115-10-6#CH3OCH3#What is dimethyl ether used for?#Is dimethyl ether harmful?#What is the common name for CH3OCH3?#What is the common name of dimethyl ether?#CAS 115-10-6#China Dimethyl ether#Dimethyl ether price
1 note
·
View note
Text
0 notes
Text
0 notes
Text
Methanol Market Analysis: Trends, Growth, and Opportunities Through 2031
The methanol market is witnessing significant growth due to its versatility and increasing adoption across energy, automotive, and chemical industries. This vital chemical compound plays a key role in fostering sustainability, especially with its applications in clean energy and environmental solutions. Below, we explore the market dynamics, segmental insights, regional trends, and emerging opportunities shaping the methanol industry's future.
Market Insights: Methanol as a Catalyst for Sustainability
Methanol’s extensive use in the production of formaldehyde, acetic acid, and fuel additives positions it as an essential feedstock in the chemical and energy industries. With rising concerns about carbon emissions, methanol is also being used as an alternative fuel and in renewable energy storage systems. The market is projected to grow steadily, supported by advancements in production processes, such as the synthesis of methanol from biomass and CO2 recycling.
Request Sample of the Report - https://www.skyquestt.com/sample-request/methanol-market
Segmental Analysis: Diverse Applications Driving Growth
The methanol market is segmented into key applications, feedstocks, and end-user industries:
Applications:
Chemical Manufacturing: Methanol is a key ingredient in producing a variety of chemicals, including formaldehyde and dimethyl ether (DME).
Energy: Its adoption as a clean fuel and in methanol fuel cells is expanding rapidly.
Other Uses: Methanol is used in pharmaceuticals, adhesives, and as an anti-freeze agent.
Feedstocks:
Natural gas dominates methanol production, while coal and renewable sources like biomass are gaining attention for their sustainability benefits.
End-User Industries:
Automotive, energy, and construction industries are among the largest consumers of methanol, reflecting its diverse applications.
Get Customized Reports with your Requirements, Free -https://www.skyquestt.com/speak-with-analyst/methanol-market
Regional Insights: A Global Perspective
Asia-Pacific:
The region leads in methanol production and consumption, driven by rapid industrial growth in China and India.
Investments in coal-to-methanol projects further bolster the region's dominance.
North America:
Shale gas exploitation provides an abundant and cost-effective feedstock, making the U.S. a significant player in methanol production.
Europe:
Stringent environmental regulations drive the adoption of green methanol, particularly in energy and transportation sectors.
Middle East & Africa:
With rich natural gas reserves, this region is emerging as a key hub for methanol production, catering to both local and global markets.
Is this report aligned with your requirements? Interested in making a Purchase -https://www.skyquestt.com/buy-now/methanol-market
Key Market Trends: Innovations and Sustainability
· Green Methanol Production: The push toward sustainability is fostering innovations in producing methanol from renewable feedstocks such as biomass and captured carbon dioxide.
· Expanding Fuel Applications: Methanol is gaining recognition as a marine fuel and as a potential hydrogen carrier in fuel cell technologies.
· Advances in Chemical Synthesis: Methanol-to-olefins (MTO) and methanol-to-gasoline (MTG) technologies are enabling efficient and sustainable chemical production.
Market Dynamics: Drivers, Challenges, and Opportunities
The methanol market is shaped by several factors influencing its growth trajectory:
Market Drivers:
o The rising demand for eco-friendly fuels and chemicals aligns methanol with global sustainability goals.
o Expanding industrialization in emerging economies fuels the demand for methanol in construction and automotive industries.
Challenges:
o Fluctuations in feedstock prices and the development of competing renewable energy sources could impede market growth.
o Regulatory pressures on environmental impacts remain a critical challenge.
Opportunities:
o Technological advancements in producing methanol from renewable sources present significant opportunities for growth.
o Growing interest in methanol as a marine fuel and in hydrogen fuel cells highlights its potential in future energy applications.
Methanol's Role in the Future Economy
The methanol market is on a growth trajectory, supported by its versatile applications and alignment with global sustainability goals. Despite challenges such as feedstock price volatility, the market is poised for expansion due to increasing demand in energy and chemical sectors and innovations in green methanol production. As industries adapt to a greener future, methanol is set to play a critical role in reducing emissions and driving industrial transformation worldwide
1 note
·
View note
Text
The Di Methyl Ether (DME) market is projected to grow significantly, with a market size of USD 10,225 million in 2024, and it is expected to reach USD 19,929.64 million by 2032, at a compound annual growth rate (CAGR) of 8.7%. Dimethyl Ether (DME) has gained traction across various industries due to its versatility as a cleaner and more efficient fuel source. DME, a colorless gas with chemical properties that resemble those of Liquefied Petroleum Gas (LPG), has garnered attention as a sustainable alternative in the fuel and chemical industries. Its applications span from use as a propellant in aerosols to a diesel substitute, driving the demand for DME and fostering market growth. This article delves into the trends, growth drivers, challenges, and future prospects for the DME market.
Browse the full report https://www.credenceresearch.com/report/di-methyl-ether-market
Overview of Dimethyl Ether (DME) Market
The global DME market has been growing steadily and is expected to continue its upward trend. DME's growing adoption as an alternative fuel source is primarily driven by environmental concerns and government initiatives to reduce carbon emissions. The market value of DME is projected to witness substantial growth due to rising demand across various sectors, including transportation, power generation, and domestic fuel. According to recent estimates, the DME market size is anticipated to grow at a compound annual growth rate (CAGR) of around 10% during the next decade, reaching a multi-billion dollar valuation by the end of the forecast period.
Key Growth Drivers
1. Demand for Clean and Sustainable Fuels
With increased awareness about climate change and the environmental impacts of traditional fossil fuels, industries worldwide are seeking cleaner alternatives. DME, a non-toxic and environmentally friendly fuel, produces no particulate matter when burned, making it an ideal choice for eco-conscious sectors. Its combustion process results in fewer greenhouse gas emissions compared to diesel and gasoline, positioning it as a valuable substitute in the fuel industry.
2. Growing Applications in Transportation
One of the most promising applications of DME is as an alternative fuel for transportation. When used in modified diesel engines, DME exhibits combustion characteristics similar to those of diesel, with added benefits of lower emissions. The transportation sector, facing increasing pressure to reduce its carbon footprint, is embracing DME as a sustainable option, particularly for commercial vehicles. Major automotive companies are actively investing in the research and development of DME-powered engines, which is expected to drive market growth in the coming years.
3. Supportive Government Policies and Incentives
Governments worldwide are promoting alternative fuels to reduce carbon emissions and achieve their respective climate goals. Many countries, especially in Europe and Asia-Pacific, have introduced subsidies, tax incentives, and funding programs to support the adoption of cleaner fuels. In China, for instance, the government is investing in DME production as part of its strategy to transition towards greener fuels. Such policies are accelerating DME market expansion and encouraging more industries to adopt this sustainable fuel source.
4. Advancements in Production Technologies
DME is primarily produced from methanol, which can be derived from natural gas, biomass, or coal. Recent advancements in production technologies have enabled manufacturers to produce DME more efficiently and at a lower cost. Emerging production processes, such as gasification of biomass and direct synthesis from syngas, are also helping to broaden the resource base for DME production. These technological innovations make DME a more feasible alternative fuel for a variety of industries and end-users.
Challenges Facing the DME Market
While the prospects for DME are promising, the market faces several challenges that may hinder its growth.
1. High Production Costs
Despite technological advances, the cost of producing DME remains higher than that of conventional fuels, making it less attractive for industries with tight budget constraints. The production process is energy-intensive, and fluctuations in the price of raw materials like methanol also impact the overall cost, which may limit widespread adoption in certain regions.
2. Infrastructure Limitations
The DME market faces infrastructure-related challenges, particularly in storage and distribution. Unlike LPG, DME requires specific handling and storage facilities due to its physical properties. Most existing fueling stations are not equipped to handle DME, which necessitates significant investment to build new infrastructure or retrofit existing ones. This limitation could slow down the growth of the DME market in regions where LPG infrastructure dominates.
3. Market Awareness and Acceptance
Although the benefits of DME are well-documented, awareness among end-users remains limited, especially in developing economies. Many consumers and industries are unfamiliar with the properties and potential advantages of DME, which can affect adoption rates. Additionally, since DME requires modified engines or special equipment for use, some companies may be hesitant to make these investments without clear evidence of DME’s long-term benefits and sustainability.
Future Prospects and Opportunities
The DME market is positioned to benefit from several ongoing trends and emerging opportunities. As governments worldwide tighten regulations on emissions, DME is likely to gain more traction as a green alternative to conventional fuels. Increasing research in bio-based DME production and the development of dual-fuel engines could further enhance the fuel’s appeal, expanding its applications across industries. The growing emphasis on sustainability and decarbonization goals will likely create new opportunities for DME in power generation, transportation, and even residential use.
In the long term, strategic partnerships between DME producers, automotive companies, and government bodies could accelerate the development of DME infrastructure and boost market awareness. Investments in research and development for cost-effective production methods are expected to make DME more economically competitive, while awareness campaigns could increase adoption across regions and sectors.
Key Player Analysis:
China Energy
AkzoNobel N.V.
Royal Dutch Shell Plc
Mitsubishi Corporation
Oberon Fuels
BP Plc
Grillo-Werke AG
Korea Gas Corporation
Saudi Basic Industries Corporation (SABIC)
TotalEnergies
Segmentation:
By Raw Material
Methanol
Bio-Based Feedstock
Coal
Natural Gas
By Application
Aerosol Propellant
LPG Blending
Transportation Fuel
Power Plant Fuel
Chemical Feedstock
Other Applications
By Region
North America
US
Canada
Mexico
Europe
Germany
France
UK
Italy
Spain
Rest of Europe
Asia Pacific
China
Japan
India
South Korea
South-east Asia
Rest of Asia Pacific
Latin America
Brazil
Argentina
Rest of Latin America
Middle East & Africa
GCC Countries
South Africa
Browse the full report https://www.credenceresearch.com/report/di-methyl-ether-market
Contact:
Credence Research
Please contact us at +91 6232 49 3207
Email: [email protected]
Website: www.credenceresearch.com
0 notes
Text
Dimethyl Carbonate Market : By Industry Trends, Leading Players, Size, Share, Growth, Opportunity And Forecast 2024-2033
The dimethyl carbonate global market report 2024 from The Business Research Company provides comprehensive market statistics, including global market size, regional shares, competitor market share, detailed segments, trends, and opportunities. This report offers an in-depth analysis of current and future industry scenarios, delivering a complete perspective for thriving in the industrial automation software market.
Dimethyl Carbonate Market, 2024 report by The Business Research Company offers comprehensive insights into the current state of the market and highlights future growth opportunities.

Market Size - The dimethyl carbonate market size has grown strongly in recent years. It will grow from <b>$1.08 billion in 2023 to $1.15 billion in 2024 at a compound annual growth rate (CAGR) of 6.3%. </b> The growth in the historic period can be attributed to demand for sustainable solvents, focus on renewable chemicals, biomedical research applications, specialty chemicals demand, shift towards green chemistry.
The dimethyl carbonate market size is expected to see strong growth in the next few years. It will grow to <b>$1.46 billion in 2028 at a compound annual growth rate (CAGR) of 6.2%. </b> The growth in the forecast period can be attributed to demand for battery electrolytes, automotive sector integration, advancements in biomedical applications, energy storage solutions demand. Major trends in the forecast period include regional market expansion, growing environmental awareness, shift towards green chemistry, stringent environmental regulations,substitute for harmful solvents.
Order your report now for swift delivery @ https://www.thebusinessresearchcompany.com/report/dimethyl-carbonate-global-market-report
The Business Research Company's reports encompass a wide range of information, including:
1. Market Size (Historic and Forecast): Analysis of the market's historical performance and projections for future growth.
2. Drivers: Examination of the key factors propelling market growth.
3. Trends: Identification of emerging trends and patterns shaping the market landscape.
4. Key Segments: Breakdown of the market into its primary segments and their respective performance.
5. Focus Regions and Geographies: Insight into the most critical regions and geographical areas influencing the market.
6. Macro Economic Factors: Assessment of broader economic elements impacting the market.
Market Drivers - The increased demand for lithium-ion batteries is expected to propel the growth of the dimethyl carbonate market. The lithium-ion (Li-ion) battery is a modern technology that relies on lithium ions in its electrochemistry. Dimethyl carbonate is used as an electrolyte in lithium batteries to increase the octane number instead of MTBE (Methyl tart-butyl ether). For instance, in July 2021, according to an article published by the United Nations Department of Economic and Social Affairs, the Li-ion battery demand increased from 285 GWh in 2019 to 400 GWh in 2022. It is expected to reach 2,000 GWh in 2030, which is about 8% of the global energy supply. Therefore, increased demand for lithium-ion batteries is driving the growth of dimethyl carbonate.
Market Trends - Product innovation is a key trend gaining popularity in the dimethyl carbonate market. Major players in the dimethyl carbonate market are channelling their resources to develop innovative products to remain competitive in the market. For instance, in July 2021, Asahi Kasei, a Japan-based chemicals company, completed its first licensing deal for a technology package to produce high-purity ethylene carbonate (EC) and high-purity dimethyl carbonate (DMC), utilizing CO2 as one of the primary feedstocks. This license will aid in meeting the growing demand for high-purity EC and DMC, which are used as components of the electrolyte solution of lithium-ion batteries (LIBs) utilized in smartphones and electric cars.
The dimethyl carbonate market covered in this report is segmented –
1) By Grade: Industry Grade (>99.0 weight %) , Pharmaceutical Grade (>99.5 weight %) , Battery Grade (>99.9 weight %) 2) By Application: Polycarbonate Synthesis, Battery Electrolyte, Solvents, Reagents, Other Applications (Fuel Additives, Electrolyte for Supercapacitors, Electrolyte for Dye-synthesized Solar Cells) 3) By End-Use Industry: Plastics, Paints and Coating, Pharmaceutical, Battery, Agrochemicals, Other End-Use Industries (Adhesives & Sealants, Ink, Food & Beverages, and Energy)
Get an inside scoop of the dimethyl carbonate market, Request now for Sample Report @ https://www.thebusinessresearchcompany.com/sample.aspx?id=8355&type=smp
Regional Insights - Asia-Pacific was the largest region in the dimethyl carbonate market in 2023. The regions covered in the dimethyl carbonate market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa.
Key Companies - Major companies operating in the dimethyl carbonate market report are Shandong Haike Chemical Industry Group Co. Ltd., Kowa American Corp., Shandong Wells Chemicals Co. Ltd., Qingdao Aspirit Chemical Co. Ltd., Shandong Feiyang Chemical Co. Ltd., Haike Chemical Group, Kindun Chemical Co.Limited, Hefei TNJ Chemical Industry Co.Ltd., Arrow Chemical Group Corp., Dongying City Longxing Chemical Co. Ltd., Hebei New Chaoyang Chemical Stock Co.Ltd., Shandong Depu Chemical Industry Science & Technology Co. Ltd., Dongying Hi-tech Spring Chemical Industry Co. Ltd, Aarsha Chemicals Private Limited, Tongling Jintai Chemical Industrial, UBE Industries, Tangshan Chaoyang Chemical Co. Ltd., Mitsubishi Chemical Corporation, Tokyo Chemical Industry Co. Ltd., Merck KGaA, Linyi Evergreen Chemical Co. Ltd., Akzo Nobel N.V., Alfa Aesar, Taizhou Lingyu Chemical Co. Ltd., Sigma-Aldrich Co. LLC, Dongying Jintan Chemical Co. Ltd., Luxi Chemical Group Co.Ltd., Dongying Xinyuan Chemical Co. Ltd., Dongying City Shuangma Chemical Co. Ltd., Dongying Dafeng Chemical Co. Ltd.
Table of Contents 1. Executive Summary 2. Dimethyl Carbonate Market Report Structure 3. Dimethyl Carbonate Market Trends And Strategies 4. Dimethyl Carbonate Market – Macro Economic Scenario 5. Dimethyl Carbonate Market Size And Growth ….. 27. Dimethyl Carbonate Market Competitor Landscape And Company Profiles 28. Key Mergers And Acquisitions 29. Future Outlook and Potential Analysis 30. Appendix
Contact Us:
The Business Research Company
Europe: +44 207 1930 708
Asia: +91 88972 63534
Americas: +1 315 623 0293
Email: [email protected]
Follow Us On:
LinkedIn: https://in.linkedin.com/company/the-business-research-company
Twitter: https://twitter.com/tbrc_info
Facebook: https://www.facebook.com/TheBusinessResearchCompany
YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ
Blog: https://blog.tbrc.info/
Healthcare Blog: https://healthcareresearchreports.com/
Global Market Model: https://www.thebusinessresearchcompany.com/global-market-model
0 notes
Text
Dr. Scholl's Skin Tag Remover: Effective and Easy Skin Tag Removal in the USA
Skin tags are small, benign growths that can appear on various parts of the body, often causing cosmetic concerns and discomfort. While harmless, many people seek ways to remove them safely and effectively. Dr. Scholl’s Skin Tag Remover offers an over-the-counter solution that allows for convenient, at-home treatment, eliminating the need for expensive dermatological procedures. Designed with a unique freezing technology, this product is an easy-to-use, fast, and reliable way to remove unwanted skin tags.
What is Dr. Scholl’s Skin Tag Remover?
Dr. Scholl’s Skin Tag Remover is an innovative, dermatologist-recommended product that uses cryotherapy to remove skin tags quickly and effectively. The device is modeled after the same cryotherapy method that dermatologists use in their clinics. With a simple application process, it freezes the skin tag, causing it to fall off naturally over time, leaving smooth, clear skin behind.
How Does Dr. Scholl’s Skin Tag Remover Work?
Dr. Scholl’s Skin Tag Remover uses advanced cryotherapy technology to freeze skin tags at the core. The application process involves directly targeting the skin tag with a precise amount of freezing agent, typically a mixture of dimethyl ether and propane. Here’s a step-by-step look at how it works:
Preparation: Clean and dry the area surrounding the skin tag to ensure proper application.
Application: Attach the included applicator tip to the device and activate it as per the instructions. Place the tip directly on the skin tag for the recommended time, usually a few seconds.
Freezing: The freezing agent penetrates the skin tag, reaching the core and freezing it at the cellular level. This stops the blood flow to the skin tag, causing it to gradually shrink and fall off.
Natural Healing: After treatment, the skin tag will darken, shrivel, and eventually fall off within a few days to a couple of weeks. New skin will form in the treated area, restoring a smooth appearance.
Benefits of Using Dr. Scholl’s Skin Tag Remover
Convenient and Affordable: Save on costly dermatologist visits with a simple, at-home treatment that can be done at your convenience.
Safe and Effective: Clinically tested and dermatologist-recommended, Dr. Scholl’s Skin Tag Remover provides a safe method to remove skin tags without cutting, burning, or harsh chemicals.
Fast Results: Most users see their skin tags fall off within 7-14 days after application, with minimal discomfort or scarring.
Easy to Use: With clear instructions and pre-assembled components, the application process is straightforward, even for first-time users.
Targeted Action: The applicator is designed to treat skin tags precisely without affecting the surrounding healthy skin, minimizing the risk of irritation.
Who Should Use Dr. Scholl’s Skin Tag Remover?
Dr. Scholl’s Skin Tag Remover is suitable for adults with skin tags on areas such as the neck, underarms, upper chest, or eyelids. It is not recommended for children, individuals with certain skin conditions, or those with skin tags located on sensitive or mucous areas. Always consult a healthcare professional if you have concerns or underlying health conditions before using any skin tag removal product.
Safety Tips and Precautions
While Dr. Scholl’s Skin Tag Remover is designed for safe, at-home use, it’s important to follow these guidelines:
Read the Instructions Carefully: Always adhere to the detailed instructions provided with the product to avoid misuse or improper application.
Avoid Sensitive Areas: Do not use on warts, moles, or skin tags located on sensitive or delicate skin areas such as the face, genitals, or mucous membranes.
Test for Skin Sensitivity: Although generally safe, it’s advisable to test the product on a small area to ensure you don’t have an adverse reaction.
Do Not Overuse: If the skin tag does not fall off after the initial application, wait a few weeks before attempting another treatment. Overuse can cause skin damage or scarring.
Possible Side Effects
Dr. Scholl’s Skin Tag Remover is generally well-tolerated, but some users may experience:
Mild Redness or Swelling: Temporary redness or swelling at the site of application is common but usually resolves within a few days.
Discomfort During Application: The freezing process may cause a brief sensation of stinging or burning, similar to an ice burn.
Minor Scarring: As with any cryotherapy treatment, there’s a small risk of minor scarring, especially if instructions are not followed properly.
Frequently Asked Questions
1. How long does it take for the skin tag to fall off after using Dr. Scholl’s Skin Tag Remover?
Typically, the skin tag will darken, shrink, and fall off within 7 to 14 days after the initial application. Healing time can vary based on the size and location of the skin tag.
2. Can I use Dr. Scholl’s Skin Tag Remover on all skin types?
Yes, Dr. Scholl’s Skin Tag Remover is suitable for most skin types. However, if you have particularly sensitive skin or any known allergies, it’s advisable to consult with a dermatologist before use.
3. What should I do if the skin tag does not fall off after the first application?
If the skin tag does not fall off after the first treatment, wait at least two weeks before reapplying. Avoid excessive applications, as this can damage the surrounding skin.
4. Is Dr. Scholl’s Skin Tag Remover safe to use on the face?
It is not recommended to use Dr. Scholl’s Skin Tag Remover on facial skin or other sensitive areas due to the risk of scarring and irritation.
5. Can I use Dr. Scholl’s Skin Tag Remover if I’m pregnant or nursing?
If you are pregnant or nursing, consult your healthcare provider before using any skin tag removal product, including Dr. Scholl’s Skin Tag Remover.
Where to Buy Dr. Scholl’s Skin Tag Remover
Dr. Scholl’s Skin Tag Remover is widely available in the USA at major retailers, pharmacies, and online platforms like Amazon, Walmart, and Walgreens. Always purchase from reputable sources to ensure you receive a genuine product.
Conclusion
Dr. Scholl’s Skin Tag Remover provides a quick, safe, and effective way to remove skin tags at home, without the hassle or expense of visiting a dermatologist. Its advanced cryotherapy technology delivers targeted treatment, making it a preferred choice for those seeking a convenient solution to unwanted skin growths. With proper use, you can achieve smooth, clear skin in just a matter of days.
0 notes
Text
Petrochemicals Market Size To Reach $1002.45 Billion By 2030
The global petrochemicals market size is expected to reach USD 1002.45 billion by 2030, as per the new report by Grand View Research, Inc. It is expected to expand at a CAGR of 7.3% from 2024 to 2030. It is expected to expand at a CAGR of 7.0% from 2023 to 2030. The demand for petrochemicals is attributed to an increase in demand from the end-use industries such as construction, textile, medical, pharmaceuticals, consumer goods, automotive, and electronics.
Products such as ethylene, propylene, and benzene are widely used in various industries such as packaging, electronics, plastics, and rubber. The ethylene product segment dominated the market in 2021 and is expected to maintain its lead in the forecast period owing to its wide application scope across several industries. Asia Pacific is anticipated to dominate the market in the forecast period owing to the favorable regulatory policies in the region.
Crude oil and natural gas are the major raw materials used for the manufacturing of petrochemical products. The volatile prices of crude oil are a major challenge in the procurement process of crude oil as a raw material for manufacturers. The industry players that are reliant on crude oil as a feedstock for manufacturing are likely to face difficulties in the coming years. However, declining prices of natural gas owing to a rise in its production are expected to augment the growth of the product over the forecast period.
The competitiveness among the producers of the product is high as the market is characterized by the presence of a large number of global players with strong distribution networks. Top players are dominating the industry for the past few years owing to the increasing investment in R&D activities related to new product development.

Request a free sample copy or view the report summary: Petrochemicals Market Report
Petrochemicals Market Report Highlights
The methanol product segment is expected to expand at the highest revenue-based CAGR of 8.9% over the forecast period. The demand is attributed to the increase in demand for methanol in manufacturing biodiesel, which is biodegradable, safe, and produces fewer air pollutants as compared to other fuels
Surged use of polyethylene, High-density Polyethylene (HDPE), and Low-density Polyethylene (LDPE) is expected to foster the overall growth of the market for petrochemicals.
The butadiene product segment is expected to be an emerging segment in the coming years as it is a key building block used in the manufacturing of several chemicals and materials employed in the industries such as consumer durables, healthcare, and building and construction
Manufacturers have adopted joint ventures and acquisitions as major strategies to increase their global presence
Petrochemicals Market Segmentation
Grand View Research has segmented the global petrochemical market report on the basis of Product, and region
Petrochemicals Product Outlook (Volume, Million Tons; Revenue, USD Billion, 2018 - 2030)
Ethylene
Polyethylene
Ethylene oxide
EDC
Ethyl benzene
Others
Propylene
Polypropylene
Propylene oxide
Acrylonitrile
Cumene
Acrylic acid
Isopropanol
Other
Butadiene
SB Rubber
Butadiene rubber
ABS
SB latex
Others
Benzene
Ethyl benzene
Phenol/cumene
Cyclohexane
Nitrobenzene
Alkyl benzene
Other
Xylene
Toluene
Solvents
TDI
Others
Methanol
Formaldehyde
Gasoline
Acetic acid
MTBE
Dimethyl ether
MTO/MTP
Other
Petrochemicals Regional Outlook (Volume, Million Tons; Revenue, USD Billion, 2018 - 2030)
North America
U.S.
Canada
Europe
Germany
UK
France
Belgium
Netherlands
Asia Pacific
China
India
Japan
South Korea
Indonesia
Latin America
Brazil
Middle East
Africa
List of Key Players of Petrochemicals Market
BASF SE
Chevron Corporation
China National Petroleum Corporation (CNPC)
China Petrochemical Corporation
ExxonMobil Corporation
INEOS Group Ltd.
LyondellBasell Industries Holdings B.V.
Royal Dutch Shell PLC
SABIC
Dow
0 notes
Text
0 notes
Text
Syngas & Derivatives Market - Forecast(2024 - 2030)
Overview
Syngas and its Derivatives Market size is forecast to reach US$70.56 billion by 2030, after growing at a CAGR of 6.9% during 2024-2030. Syngas is a gaseous mix consisting primarily of hydrogen and carbon monoxide, which is generated from coal gasification, fluidized bed gasifier, steam reforming, and others. It can be used to fabricated chemicals such as ammonia, butanol, methanol, acetic acid, and dimethyl ether. The competence of syngas to be formed from a widespread variety of feedstock such as coal, synthetic natural gas, biomass and petroleum coke is impacting the market growth constructively. Uprising environmental concerns have been the foremost drivers for the growth of the Syngas and its Derivatives Market in order to afford alternative methods of fuel production. There's a growing trend towards producing syngas from renewable sources such as biomass, municipal solid waste, and agricultural residues. This shift is driven by concerns over climate change and the desire to reduce greenhouse gas emissions. Biomass gasification, for instance, is gaining traction as it offers a carbon-neutral alternative to traditional fossil fuel-based syngas production methods. Advances in gasification technologies are driving efficiency improvements and cost reductions in syngas production. These advancements include developments in reactor design, catalysts, and process optimization techniques. Integrated gasification combined cycle (IGCC) plants, for example, are becoming more efficient in converting coal or biomass into syngas, which can then be used to generate electricity with lower emissions compared to conventional coal-fired power plants.
𝐃𝐨𝐰𝐧𝐥𝐨𝐚𝐝 𝐑𝐞𝐩𝐨𝐫𝐭 𝐒𝐚𝐦𝐩𝐥𝐞
The report: “Syngas and its Derivatives Market”- Forecast (2024-2030)”, by IndustryARC, covers an in-depth analysis of the following segments of the Syngas and its Derivatives Market Industry.
By Feedstock: Coal, Biomass, Natural Gas, Petroleum coke, Industrial Waste and Others
By Technology: Steam reforming (SR), Partial oxidation (POx), Autothermal reforming (ATR), Combined or Two-Step Reforming, Biomass Gasification and Others
By Gasification: Fixed Bed Gasifier, Fluidized Gasifiers, Entrained Flow Gasifiers, and Others
By Application: Fuel, Power Generation, Generators, Refineries, Fertilizers and Pesticides, Textiles, and Others
By End-Use Industry: Oil and Gas, Automotive, Electrical and Electronics, Marine, Aerospace, Chemical, Energy, Agriculture, and Others
By Geography: North America (USA, Canada and Mexico), Europe (UK, France, Germany, Italy, Spain, Russia, Netherlands, Belgium, and Rest of Europe), APAC (China, Japan, India, South Korea, Australia and New Zealand, Indonesia, Taiwan, Malaysia and Rest of APAC), South America (Brazil, Argentina, Colombia, Chile, Rest of South America), and Rest of the world (Middle East and Africa).

Key Takeaways
• Asia Pacific dominates the Syngas and its Derivatives Market owing to rapid increase in Chemical and Oil and Gas sector. For instance, an investment of US$107.4 billion is estimated in the Indian chemicals and petrochemicals sector by 2025
• The market drivers and restraints have been assessed to understand their impact over the forecast period.
• The report further identifies the key opportunities for growth while also detailing the key challenges and possible threats.
• The other key areas of focus include the various applications and end use industry in Syngas and its Derivatives Market and their specific segmented revenue.
• The fuel application is expected to augment the Syngas and its Derivatives Market’s growth over the forecast period due to increase in the consumption of liquid and gaseous fuels in various end-use industry.
#Syngas & Derivatives Market price#Syngas & Derivatives Market size#Syngas & Derivatives Market share
0 notes
Text
CAS 94-34-8 High Purity N,N-(2-Cyanoethyl)-N-methyl aniline 99% /sample is free/DA 90 days

QUICK DETAILS Product Name:N,N-(2-Cyanoethyl)-N-methyl aniline CAS: 94-34-8 Molecular formula: C10H12N2 Molecular weight: 160.216 EINECS No.: 202-325-5 Other names:N-Cyanoethyl-N-methylaniline,N-Methyl-N-Cyanoethyl Aniline,N-Cyanoethyl-N-methylaniline,3-(N-Methylanilino)propionitrile,N-(2-Cyanoethyl)-N-methylaniline,3-(N-methylanilino)propanenitrile Appearance:Light yellow to brown oily liquid Purity:≥99% Safety: Brand:MIT -IVY INDUSTRY CO.,LTD Application:Used as dye and organic pigment intermediates (such as acid red 14, basic orange 24, etc.). Port: any port in china Packing: according to the requirement Storage: Store in dry, dark and ventilated place. Transportation: by sea or by air payment methods: L/C, T/T, D/A, D/P, O/A, paypal, western union etc.accept all payment. Application N,N-dimethylaniline is a tertiary amine used in the synthesis of several triarylmethane dyes, such as peacock green. It is also used in the synthesis of magnetic Gram stains for the detection of bacteria. N, N-(2-Cyanoethyl)-N-methyl aniline CAS NO. 94-34-8 N,N-dimethylaniline, also known as N,N-dimethylaniline, dimethylaminobenzene and dimethylaniline. It is a yellow oily liquid, insoluble in water, soluble in ethanol, ether. Mainly used as dye intermediates, solvents, stabilizers, analytical reagents. Standards and Recommendations OSHA PEL: TWA 5 ppm; STEL 10 ppm (skin) ACGIH TLV: TWA 5 ppm; STEL 10 ppm (skin); Not Classifiable as a Human Carcinogen DFG MAK: 5 ppm (25 mg/m3); Confirmed Animal Carcinogen with Unknown Relevance to Humans DOT Classification: 6.1; Label: Poison Consensus Reports Reported in EPA TSCA Inventory. Community Right-To-Know List. Specification N,N-Dimethylaniline is an organic compound with the formula C8H11N, and its systematic name is the same with the product name. With the CAS registry number 121-69-7, it is also named as N,N-Dimethylaminobenzene. It belongs to the product categories of Intermediates of Dyes and Pigments; Anilines, Aromatic Amines and Nitro Compounds; Organics; C-D, Puriss p.a. ACSNitrogen Compounds; Amines; Analytical Reagents for General Use; C8; Puriss p.a. ACS; C8 Essential Chemicals; Nitrogen Compounds; Reagent Plus; Routine Reagents; Organic Chemical. Its EINECS number is 204-493-5. In addition, the molecular weight is 121.18. Its classification codes are: (1)Human Data; (2)Mutation data; (3)Skin / Eye Irritant; (4)TSCA Flag T ; (5)Tumor data. This chemical should be sealed and stored in a cool and dry place. Moreover, it should be protected from moisture, heat and fire. This chemical is a key precursor to commercially important triarylmethane dyes such as Malachite green and Crystal violet. It serves as a promoter in the curing of polyester and vinyl ester resins. It is also used as a precursor to other organic compounds. Physical properties of N,N-Dimethylaniline are: (1)ACD/LogP: 2.135; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 1.99; (4)ACD/LogD (pH 7.4): 2.13; (5)ACD/BCF (pH 5.5): 17.70; (6)ACD/BCF (pH 7.4): 24.59; (7)ACD/KOC (pH 5.5): 247.57; (8)ACD/KOC (pH 7.4): 343.97; (9)#H bond acceptors: 1; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 1; (12)Polar Surface Area: 3.24 Å2; (13)Index of Refraction: 1.55; (14)Molar Refractivity: 40.566 cm3; (15)Molar Volume: 127.425 cm3; (16)Polarizability: 16.082×10-24cm3; (17)Surface Tension: 34.71 dyne/cm; (18)Density: 0.951 g/cm3; (19)Flash Point: 62.778 °C; (20)Enthalpy of Vaporization: 42.974 kJ/mol; (21)Boiling Point: 193.539 °C at 760 mmHg; (22)Vapour Pressure: 0.46 mmHg at 25°C. Preparation of N,N-Dimethylaniline: N,N-Dimethylaniline can be prepared by N-benzyl-N,N-dimethyl-anilinium; bromide at the temperature of 40 °C. This reaction will need reagent NaTeH and solvent dimethylformamide with the reaction time of 4 hours. The yield is about 94%. Uses of N,N-Dimethylaniline: N,N-Dimethylaniline can be used to produce 1-(4-dimethylamino-phenyl)-ethanone at the temperature of 50 °C. It will need reagent Yb(OTf)3 and solvent nitromethane with the reaction time of 18 hours. The yield is about 76%. Safety information of N,N-Dimethylaniline: When you are using this chemical, please be cautious about it as the following:N,N-Dimethylaniline is harmful by inhalation and in contact with skin. It is toxic by inhalation, in contact with skin and if swallowed. It has a limited evidence of a carcinogenic effect. This substance is toxic to aquatic organisms as it may cause long-term adverse effects in the aquatic environment. After contact with skin, you should wash immediately with plenty of ... (to be specified by the manufacturer). When using it, you need to wear suitable protective clothing and gloves. In case of accident or if you feel unwell, you must seek medical advice immediately (show the label where possible). It should be avoided exposure, and you need to obtain special instructions before use. You must avoid releasing it to the environment, and you need to refer to special instructions/safety data sheet. You can still convert the following datas into molecular structure: (1)SMILES: N(c1ccccc1)(C)C (2)Std. InChI: InChI=1S/C8H11N/c1-9(2)8-6-4-3-5-7-8/h3-7H,1-2H3 (3)Std. InChIKey: JLTDJTHDQAWBAV-UHFFFAOYSA-N The toxicity data of N,N-Dimethylaniline is as follows: Organism Test Type Route Reported Dose (Normalized Dose) Effect Source guinea pig LD50 skin > 20mL/kg (20mL/kg) SKIN AND APPENDAGES (SKIN): "DERMATITIS, OTHER: AFTER SYSTEMIC EXPOSURE" National Technical Information Service. Vol. OTS0571982, human LDLo oral 50mg/kg (50mg/kg) GASTROINTESTINAL: NAUSEA OR VOMITING GASTROINTESTINAL: OTHER CHANGES National Clearinghouse for Poison Control Centers, Bulletin. Vol. Jan/Feb, Pg. 1969, mouse LDLo oral 350mg/kg (350mg/kg) National Toxicology Program Technical Report Series. Vol. NTP-TR-360, Pg. 1989, rabbit LD50 skin 1770uL/kg (1.77mL/kg) American Industrial Hygiene Association Journal. Vol. 23, Pg. 95, 1962. rat LCLo inhalation 250mg/m3/4H (250mg/m3) BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: EXCITEMENT Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 37(4), Pg. 35, 1972. rat LD50 oral 951mg/kg (951mg/kg) BEHAVIORAL: TREMOR BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) LUNGS, THORAX, OR RESPIRATION: CYANOSIS National Technical Information Service. Vol. OTS0571982, rat LDLo subcutaneous 100mg/kg (100mg/kg) "Toxicometric Parameters of Industrial Toxic Chemicals Under Single Exposure," Izmerov, N.F., et al., Moscow, Centre of International Projects, GKNT, 1982Vol. -, Pg. 55, 1982. Packaging 1kg/foil bag, 25kg/bag or drum (PV bag for inner packing, and aluminium foil bag for outer packing.) Hot sales !! China Manufacturer n,n-dimethylaniline CAS NO. 121-69-7 in Bulk Stock Name N,N-(2-Cyanoethyl)-N-methyl aniline Cas 94-34-8 Form Light yellow to brown oily liquid Other name N-Cyanoethyl-N-methylaniline,N-Methyl-N-Cyanoethyl Aniline,N-Cyanoethyl-N-methylaniline ,3-(N-Methylanilino)propionitrile,N-(2-Cyanoethyl)-N-methylaniline,3-(N-methylanilino)propanenitrile MF C10H12N2 MW 160.216 Organic Ingredient Buy Direct from China Manufacturer n,n-dimethylaniline High Purity CAS NO. 121-69-7 Shipping time by Sea (Just for reference) North America 11~30 days North Africa 20~40 days Europe 22~45 days South-east Asia 7~10 days South America 25~35 days WestAfrica 30~60 days MiddleEast 15~30 days East Asia 2~3 days Middle America 20~35 days EestAfrica 23~30 days Ocenia 15~20 days South Asia 10~25 days Details Read the full article
#3-(methyl(phenyl)amino)propanenitrile#3-(methylphenylamino)-propanenitril#3-(Methylphenylamino)propionitrile#3-(N-Methyl-N-phenylamino)propiononitrile#3-(N-methylanilino)propanenitrile#3-(N-Methylanilino)propionitrile#CAS94-34-8#N-(2-Cyanoethyl)-N-methylaniline#N-Cyanoethyl-N-methylaniline#N-Cyanoethyl-N-methylanilineN-Methyl-N-CyanoethylAnilineN-Cyanoethyl-N-methylaniline3-(N-Methylanilino)propionitrileN-(2-Cyanoethyl)-N-met#N-Methyl-N-(2-cyanoethyl)aniline3-(N-Methyl-N-phenylamino)propionitrile#N-Methyl-N-CyanoethylAniline
0 notes
Text
Unlocking Growth Cellulose Derivative Market 2024: The Next Big Thing in Green Chemistry Future Trends and Opportunities
The Cellulose Derivative Market is Valued USD 6.5 billion in 2024 and projected to reach USD 10.9 billion by 2032, growing at a CAGR of CAGR of 5.9 % During the Forecast period of 2024–2032.
In the global Cellulose Derivative market, Methyl cellulose stands out as a crucial commercial cellulose ether. It represents the simplest alkyl ether and can be synthesized in an alkaline medium using a methylating agent like methyl chloride or dimethyl sulfate. The synthesis conditions, such as reaction time or the methylating agent used, can be adjusted to achieve varying degrees of substitution. Methyl cellulose exhibits solubility in numerous organic solvents, dependent on the degree of substitution.
Major Vendors In The Global Cellulose Derivative Market: Akzo Nobel N.V, Ashland Inc., Colorcon, CP Kelco, Daicel Corporation, Dow, Eastman Chemical Company, international paper, Invista, LOTTE Fine Chemical, Samsung Fine Chemicals, SE Tylose GmbH & Co. KG., Shandong Head Europe B.V., Shin-Etsu Chemical Co. Ltd and Others.
Request Our Market Overview Sample Now — https://www.marketdigits.com/request/sample/4075
Market Dynamics
Drivers:
◆ Rising demand for materials that are sustainable and biodegradable ◆ Increasing desire for textiles that offer high-performance ◆ Increasing demand for natural and plant-based ingredients ◆ Increasing awareness about personal hygiene and grooming
Opportunities:
◆ Technological Advancements ◆ Government regulations encouraging the utilization of biodegradable products ◆ Rising growth of the pharmaceutical industry ◆ Globalization and Cultural Diversity
Key Report Highlights: The report has been put together using primary and secondary research methodologies, which offer an accurate and precise understanding of the Cellulose Derivative market. Analysts have used a top-down and bottom-up approach to evaluate the segments and provide a fair assessment of their impact on the global Cellulose Derivative market. The report offers an overview of the market, which briefly describes the market condition and the leading segments.
Inquire Before Buying at — https://www.marketdigits.com/request/enquiry-before-buying/4075
The Purpose of This Report Is to Provide: ◆ A qualitative and quantitative analysis of the Cellulose Derivative market of current trends, dynamics, and estimates from 2024 to 2032. ◆ The in-depth market segmentation analysis helps to identify the prevailing market opportunities. ◆ Analytical tools such as SWOT analysis and Porter’s Five Forces analysis explain the power of Cellulose Derivative buyers and suppliers, make profit-oriented decisions, and strengthen their business.
Segmentations Analysis of Cellulose Derivative Market: -
By Product Type
Methyl cellulose
Carboxymethyl cellulose
Hydroxypropyl cellulose
Ethyl cellulose
Hydroxyethyl cellulose
Hydroxypropyl methylcellulose
Others
By Application
Paints and coatings
Mining
Personal care
Wall coatings
Drilling fluids
Food & beverages
Pharmaceutical
Paper
Others
By End user
Textiles
Chemical Synthesis
Pharmaceuticals
Construction
Paper and pulp
Paints and coatings
Others
Cellulose Derivative Market Report Gives Answers to Following Key Questions:
What analysis has been done of the prices, sales, and volume of the top producers of Cellulose Derivative Market?
What are the main forces behind the worldwide Cellulose Derivative Market? Which companies dominate the Cellulose Derivative Market?
Which companies dominate the Cellulose Derivative Market? Which business possibilities, dangers, and tactics did they embrace in the market?
What are the global Cellulose Derivative industry’s suppliers’ opportunities and dangers in Cellulose Derivative Market?
What is the Cellulose Derivative industry’s regional sales, income, and pricing analysis? In the Cellulose Derivative Market, who are the distributors, traders, and resellers?
What are the dominant revenue-generating regions for Cellulose Derivative Market, as well as regional growth trends?
By the end of the forecast period, what will the market size and growth rate be?
What are the main Cellulose Derivative Market trends that are influencing the market’s expansion?
Which key product categories dominate the Cellulose Derivative Market? What are the Cellulose Derivative Market’s main applications?
Click to Request Free 10% Customization on this Report @ https://www.marketdigits.com/request/customization/4075
Key Topics Covered:
1. Preface 2. Research Methodology 3. Executive Summary 4. Market Overview 5. Market Insights 6. Cellulose Derivative Market, by Molecule Type 7. Cellulose Derivative Market, by Lines of Chemotherapy 8. Cellulose Derivative Market, by Route of Administration 9. Americas Cellulose Derivative Market 10. Asia-Pacific Cellulose Derivative Market
0 notes
Text
Hydrogen Fuel Cell Vehicle Market - Forecast(2024 - 2030)
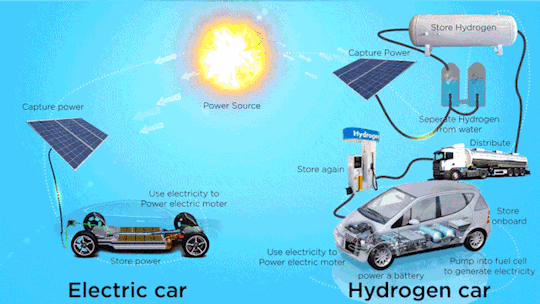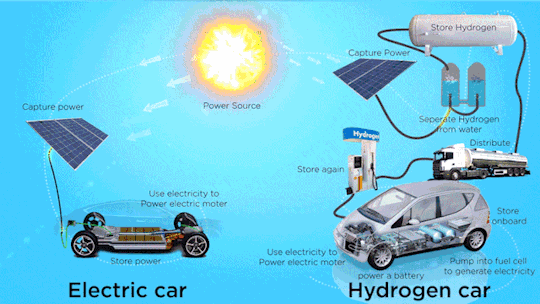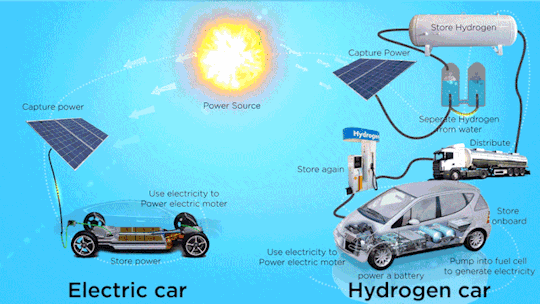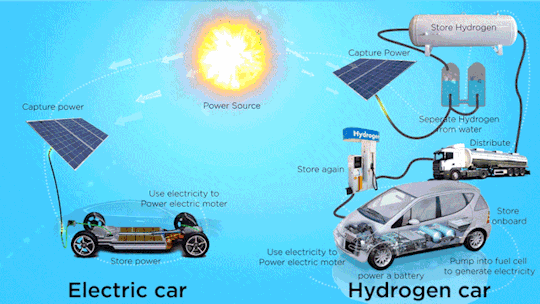
Hydrogen Fuel Cell Vehicle Market Size was valued at USD 0.72 billion in 2021. The Hydrogen Fuel Cell Vehicle market industry is projected to grow from USD 1.2 Billion in 2022 to USD 46.8 billion by 2030, exhibiting a compound annual growth rate (CAGR) of 68.52% during the forecast period (2024–2030). Hydrogen fuel cell vehicles are specially designed vehicles that are powered through hydrogen acting as a fuel and are used to supply power to the electric motors installed within them, thus ensuring emission free vehicle transmission. Vehicle powered with hydrogen fuel cells includes a reverse electrolysis process wherein hydrogen reacts with oxygen, thus producing electricity to power electric motors along with heat and water. The heat & water generated during this process exits through the exhaust as water vapor, thereby leading to zero or no emission.
Key Developments in Hydrogen Fuel Cell Vehicle Industry
In September 2023, Toyota Motor Corporation launched a prototype hydrogen fuel cell electric Hilux. This technology helps to accelerate the development of hydrogen fuel cell solutions to deliver carbon neutrality across the region. It uses core elements from the Toyota Mirai hydrogen fuel cell electric sedan – technology that has proved its quality in almost 10 years of commercial production.
In July 2023, Ballard Power Systems, Inc. signed an agreement with Ford Trucks to supply a fuel cell system as part of the development of a hydrogen fuel cell-powered vehicle prototype. This strategy includes an initial purchase order for 2 FCmoveTM.-XD 120 kW fuel cell engines that are planned to be delivered by Ballard to Ford Trucks in 2023. Furthermore, Ford Trucks plans to develop a Fuel Cell Electric Vehicle (FCEV) F-MAX as part of the project.
In July 2023, Ballard Power Systems, Inc. received orders for a total of 96 hydrogen fuel cell engines from long-standing customer Solaris Bus & Coach sp. z o.o. The purchase orders include 52 fuel cell engines that will power Solaris Urbino hydrogen buses for deployment by public transport in Germany and 44 fuel cell engines that will power Solaris buses in European cities.
In January 2022, General Motors (GM) planned to broaden electrification, by expanding fuel cells beyond vehicles. It also continues to accelerate its growth as a platform innovator and has announced new commercial applications of its HYDROTEC fuel cell technology. HYDROTEC projects, which are currently in development, from heavy-duty trucks to aerospace and locomotives, are being planned for use beyond vehicles for power generation.
Downlaod report sample
Lack of refueling infrastructure for HFCV
The lack of refueling infrastructure for HFCV in most of the countries is due to the limited number of hydrogen refueling stations. For instance, in 2020, globally the hydrogen refueling stations are less than 800, which hampers the growth of HFCV vehicle sales. Furthermore, in many developing countries such as Brazil, African countries, and other countries limited presence of hydrogen vehicles and high cost for development which are also impact the growth of HFCV market. The development of HFCV in underdeveloped countries is slower than in developed countries.
Rise in adoption of HFCV in development economies
Increase in adoption of clean mobility solutions is observed globally due to climatic changes. Continuous usage of fossil fuels in automobiles is a major factor resulting in climate change. Vehicles that run on alternative fuels, such as natural gas, electricity, biofuel, biodiesel, fuel cell, liquid nitrogen, and dimethyl ether result in lesser carbon emissions. Increasing environmental concerns among consumers, introduction of stringent emission regulations, and launch of advanced vehicles supporting alternative fuels are expected to increase the adoption of alternative fuel and hybrid vehicle market during the forecast period.
Purchase report
Key players :
The key players profiled in the hydrogen fuel cell vehicle market share include General Motors Company, HONDA MOTOR Co., Ltd, AUDI AG, Ballard Power Systems, Inc., BMW Group, Daimler AG, Hyundai Motor Group, MAN SE, Toyota Motor Corp., and Volvo Group which have been operating in the industry & are developing strategies & products for the growth of the market.
#Hydrogen Fuel Cell Vehicle Market#Hydrogen Fuel Cell Vehicle Market share#Hydrogen Fuel Cell Vehicle Market size
0 notes
Text
Unveiling the Power of Caluanie: A Secret Weapon in Industrial Solutions
In the realm of industrial solvents, Caluanie stands out as a potent and versatile chemical solution. With its unique properties and applications, Caluanie plays a significant role in various industries, ranging from metal processing to paint removal. Let's delve deeper into what Caluanie is, its uses, and the precautions associated with its handling.
Caluanie, chemically known as diethylene glycol dimethyl ether, is a colorless liquid solvent with a high capacity to break down various materials. Originally developed for industrial purposes, Caluanie has found its niche in processes requiring efficient dissolution or decomposition. Its molecular structure enables it to penetrate and disrupt bonds in substances like metals, paints, resins, and more.
One of the primary applications of Caluanie is in metal processing. It is commonly used for the extraction of precious metals from electronic waste or mining ores. Caluanie's ability to dissolve metals facilitates the separation of valuable components, making it a crucial solvent in recycling and resource recovery industries.
Furthermore, Caluanie is utilized in paint stripping and surface cleaning operations. Its powerful solvent properties enable efficient removal of paint layers from surfaces, such as metal, wood, or concrete. This makes Caluanie an indispensable tool in renovation and restoration projects where old coatings need to be stripped for refinishing.
However, despite its effectiveness, the use of Caluanie requires careful handling and adherence to safety protocols. Being a strong chemical solvent, direct contact with skin or inhalation of its vapors can cause irritation or harm. Proper ventilation, personal protective equipment (PPE), and safe storage practices are essential when working with Caluanie to mitigate risks to human health and the environment.
Moreover, regulatory compliance is paramount when using Caluanie, as it is classified as a controlled substance in many jurisdictions due to its potential hazards and misuse risks. Understanding and following relevant regulations and guidelines ensure safe and responsible usage of Caluanie in industrial settings.
In conclusion, Caluanie emerges as a valuable asset in various industrial applications, thanks to its remarkable solvent properties. From metal processing to paint stripping, its versatility offers solutions to complex challenges in manufacturing, recycling, and maintenance sectors. However, caution must be exercised in its handling and disposal to safeguard both human health and the environment. With proper precautions and regulatory adherence, Caluanie continues to contribute to the advancement of industrial processes worldwide.
0 notes
Text
0 notes
Text
0 notes