#Digital PCR Market Research
Explore tagged Tumblr posts
Text
#Digital PCR Market#Digital PCR Market Trends#Digital PCR Market Growth#Digital PCR Market Industry#Digital PCR Market Research#Digital PCR Market Report
0 notes
Text
Legal Marijuana Market Growth Drivers and Challenges
The global legal marijuana market is experiencing significant expansion, driven by increasing legalization and acceptance of cannabis for both medical and recreational purposes. Valued at USD 21.08 billion in 2023, the market is projected to reach USD 130.55 billion by 2031, exhibiting a compound annual growth rate (CAGR) of 25.6% from 2024 to 2031.
Market Segmentation:
The legal marijuana market is categorized based on type, product, and application:
By Type:
Medical: Utilized for treating various ailments such as chronic pain, neurological disorders, and cancer-related symptoms.
Recreational: Used for personal enjoyment and relaxation.
By Product:
Buds: The flowering part of the cannabis plant, commonly consumed by smoking or vaporizing.
Oils: Concentrated extracts used in edibles, tinctures, and topical applications.
Tinctures: Alcohol-based cannabis extracts consumed sublingually for rapid absorption.
By Application:
Chronic Pain: Cannabis is increasingly used to alleviate persistent pain conditions.
Neurological Disorders: Conditions such as epilepsy and multiple sclerosis are managed using medical marijuana.
Cancer: Helps in mitigating chemotherapy-induced nausea and improving appetite.
Others: Includes applications in mental health and gastrointestinal disorders.
Get Free Sample Report @ https://www.snsinsider.com/sample-request/1046
Regional Analysis:
North America: Leading the market due to widespread legalization and a well-established cannabis industry.
Europe: Experiencing growth with increasing medical cannabis approvals and progressive regulatory changes.
Asia-Pacific: Emerging market with gradual legalization and rising awareness of medical marijuana benefits.
Latin America: Notable growth driven by countries like Uruguay and Colombia embracing cannabis legalization.
Middle East & Africa: Slow adoption due to stringent regulations, but potential exists with ongoing legislative reviews.
Key Players
Key Service Providers/Manufacturers
Canopy Growth Corporation (Tweed, Spectrum Cannabis)
Aurora Cannabis Inc. (Aurora Sky, Aurora Drift)
Cronos Group (Peace Naturals, COVE)
Tilray, Inc. (Tilray, Aphria)
Hexo Corp. (Hexo, Redecan)
Charlotte's Web Holdings, Inc. (Charlotte's Web Hemp Extract, CBD Capsules)
Organigram Holdings Inc. (Organigram, Edison Cannabis Co.)
GW Pharmaceuticals (Epidiolex, Sativex)
Green Thumb Industries (Rythm, Dogwalkers)
Trulieve (Trulieve Cannabis, Muse)
Key Highlights:
Growing recognition of medical marijuana's therapeutic benefits is a primary market driver.
Legalization in various countries is creating new opportunities for industry stakeholders.
Development of diverse cannabis-based products caters to a broad spectrum of consumer preferences.
Challenges include navigating complex regulatory frameworks and addressing societal stigmas.
Future Outlook:
The legal marijuana market is poised for substantial growth as more countries move towards legalization and acceptance of cannabis. Advancements in research are expected to uncover new therapeutic applications, further driving demand. Additionally, the development of innovative consumption methods and products will attract a wider consumer base. However, companies must remain vigilant in navigating evolving regulations and ensuring product quality to maintain consumer trust.
Conclusion:
The global legal marijuana market is on a robust growth trajectory, offering significant opportunities for investors, healthcare providers, and consumers. As societal attitudes shift and legal frameworks evolve, the market is expected to expand, delivering economic benefits and novel therapeutic options.
Contact Us: Jagney Dave - Vice President of Client Engagement Phone: +1-315 636 4242 (US) | +44- 20 3290 5010 (UK)
Other Related Reports:
Digital PCR-dPCR Market Size
Electronic Medical Record (EMR) Systems Market Size
3D Printing Medical Devices Market Size
#Legal Marijuana Market#Legal Marijuana Market Share#Legal Marijuana Market Size#Legal Marijuana Market Trends#Legal Marijuana
0 notes
Text
Laboratory Proficiency Testing Market Size, Growth Outlook 2035
The Laboratory Proficiency Testing MarketSize was estimated at 2.21 (USD Billion) in 2024. The Laboratory Proficiency Testing Market Industry is expected to grow from 2.33 (USD Billion) in 2025 to 3.76 (USD Billion) till 2034, at a CAGR (growth rate) is expected to be around 5.48% during the forecast period (2025 - 2034).
Market Overview
The Laboratory Proficiency Testing Market is experiencing significant growth, driven by the increasing focus on quality assurance in clinical and diagnostic laboratories, regulatory compliance requirements, and advancements in analytical testing. Proficiency testing (PT) is crucial in ensuring the accuracy, reliability, and standardization of laboratory testing procedures across various industries, including healthcare, environmental testing, pharmaceuticals, and food safety.
With the rise in accreditation requirements for diagnostic laboratories, the adoption of external quality assessment (EQA) programs has surged. Additionally, the growing demand for molecular diagnostics and microbiology testing has further expanded the scope of proficiency testing in modern laboratories.
Market Size and Share
The Laboratory Proficiency Testing MarketSize was estimated at 2.21 (USD Billion) in 2024. The Laboratory Proficiency Testing Market Industry is expected to grow from 2.33 (USD Billion) in 2025 to 3.76 (USD Billion) till 2034, at a CAGR (growth rate) is expected to be around 5.48% during the forecast period (2025 - 2034).North America holds the largest market share due to strict regulatory guidelines, high adoption of clinical laboratory accreditation programs, and an increasing number of reference laboratories. The Asia-Pacific region is anticipated to experience the fastest growth due to rising investments in laboratory infrastructure and growing awareness about quality assurance in diagnostic labs.

Market Drivers
Stringent Regulatory & Compliance Requirements: Government and private healthcare bodies mandate the use of proficiency testing programs to ensure laboratory accuracy.
Growing Demand for Accurate Diagnostic Testing: With the increasing prevalence of infectious diseases, chronic conditions, and genetic disorders, the need for error-free laboratory testing has risen.
Technological Advancements in Laboratory Automation: The integration of AI-powered laboratory testing solutions and automated analytical instruments has improved efficiency in testing.
Rising Adoption of External Quality Assurance (EQA) Programs: Laboratories worldwide are increasingly participating in EQA schemes to benchmark their performance and maintain certification.
Expansion of Molecular & Microbiology Testing: The growing use of PCR-based diagnostics, NGS, and microbiology assays has expanded proficiency testing applications.
Challenges and Restraints
High Costs Associated with Proficiency Testing Programs: Many laboratories, especially in developing regions, struggle with the affordability of external quality assessment services.
Limited Awareness in Emerging Economies: Lack of proper knowledge about the benefits of quality control testing in laboratories affects market penetration.
Challenges in Standardization of Testing Procedures: Variability in laboratory testing protocols across different regions can hinder the effectiveness of proficiency testing programs.
Market Trends
Increased Use of Digital & AI-Driven Proficiency Testing Solutions: AI and machine learning algorithms for laboratory quality control are improving proficiency testing accuracy.
Rising Adoption of Proficiency Testing in Food & Water Safety: Beyond healthcare, environmental testing laboratories are increasingly utilizing PT programs.
Expansion of Molecular & Genetic Proficiency Testing Programs: NGS-based laboratory testing assessments are gaining traction in precision medicine research.
Integration of Cloud-Based Laboratory Quality Control Solutions: Many labs are adopting cloud-based PT data management systems for real-time performance tracking.
Regional Analysis
North America: Dominates the market due to strong regulatory policies, high participation in CAP proficiency testing programs, and the presence of leading diagnostic companies.
Europe: Growing demand for ISO 15189-accredited laboratories and increased government funding for EQA programs support market growth.
Asia-Pacific: Rising awareness about laboratory accreditation and expansion of diagnostic facilities drive regional growth.
Rest of the World: The adoption of proficiency testing services in pharmaceutical and clinical laboratories is gradually increasing.
Segmental Analysis
By Industry:
Clinical Diagnostics
Pharmaceuticals & Biotechnology
Environmental & Water Testing
Food & Beverage Testing
By Technology:
Microbiology
Molecular Diagnostics
Immunology
Clinical Chemistry
Haematology
By End-User:
Hospitals & Clinical Laboratories
Research & Academic Institutes
Pharmaceutical Companies
Government & Regulatory Agencies
Key Market Players
Thermo Fisher Scientific
College of American Pathologists
International Accreditation Services
Quality Control Solutions
BD
Roche Diagnostics
Recent Developments
Expansion of Proficiency Testing Programs: CAP launched new molecular pathology PT programs to support genetic and infectious disease testing.
Integration of AI in Laboratory Quality Control: Bio-Rad introduced an AI-powered laboratory proficiency testing software to streamline data analysis.
Collaborations for Global Standardization: Thermo Fisher partnered with regulatory bodies to develop universal PT guidelines for clinical laboratories.
For more information, please visit us at marketresearchfuture.
#Laboratory Proficiency Testing Market Size#Laboratory Proficiency Testing Market Share#Laboratory Proficiency Testing Market Growth#Laboratory Proficiency Testing Market Analysis#Laboratory Proficiency Testing Market Trends#Laboratory Proficiency Testing Market Forecast#Laboratory Proficiency Testing Market Segments
0 notes
Text
DNA Data Storage: From $0.25B to $5.5B by 2034!
DNA Data Storage Systems Market is set for remarkable expansion, with a projected growth from $0.25 billion in 2024 to $5.5 billion by 2034, reflecting a compound annual growth rate (CAGR) of approximately 36.5%. This market encompasses advanced technologies and solutions that utilize DNA molecules for encoding, storing, and retrieving digital data. DNA offers unprecedented data density and longevity, making it an ideal medium for archiving vast amounts of information. This market includes services related to DNA synthesis, sequencing, and data management, which together enable the transformation of digital data into DNA sequences. These innovations promise to revolutionize data storage, particularly for sectors that require long-term data preservation, such as healthcare, finance, and digital media.
To Request Sample Report: https://www.globalinsightservices.com/request-sample/?id=GIS10577 &utm_source=SnehaPatil&utm_medium=Article
The DNA Data Storage Systems Market is experiencing robust growth, driven by the exponential rise in data generation and the increasing demand for sustainable storage solutions. The biotechnology sector leads the charge, capitalizing on DNA’s unique ability to store immense amounts of data in a compact form. Following closely, the healthcare industry is also utilizing DNA data storage for medical research and patient data management. Geographically, North America is the dominant region in this market, owing to its advanced technological infrastructure and considerable investments in research and development. Europe is the second-highest performer, benefiting from supportive regulatory frameworks and growing collaborations between academic institutions and industry players. Within these regions, the United States and Germany stand out due to their strong innovation ecosystems and government support. As the volume of data continues to soar, the DNA data storage market is expected to witness substantial advancements, offering lucrative opportunities for stakeholders across the entire value chain.
Buy Now : https://www.globalinsightservices.com/checkout/single_user/GIS10577/?utm_source=SnehaPatil&utm_medium=Article
The market is segmented into several categories, including synthetic DNA, PCR-based DNA, and various products such as DNA hard drives and DNA cartridges. Services provided within the market range from data encoding and decoding to retrieval, storage, consultancy, and maintenance. Key technologies driving growth in the DNA data storage systems market include next-generation sequencing, CRISPR, and DNA synthesis. Components of these systems include DNA strands, storage arrays, and devices like readers and writers. Applications for DNA data storage span across data archiving, genomics, pharmaceutical research, biotechnology, and forensics. The market also includes different forms of DNA, such as liquid DNA and solid DNA, and utilizes materials like nucleotides and enzymes in its processes.
In 2023, the DNA Data Storage Systems Market had an estimated volume of 320 petabytes, with synthetic DNA capturing the largest share at 45%. Hardware accounted for 35%, while software made up the remaining 20%. The dominance of synthetic DNA is driven by significant advancements in technology and the increasing demand for long-term data preservation. Leading market players such as Microsoft, Twist Bioscience, and Illumina are playing key roles in driving the market forward, with a focus on technological innovation to capture substantial market share.
Competitive dynamics within the market are shaped by strategic partnerships, technological breakthroughs, and regulatory influences, particularly those concerning data privacy and biosecurity. As the market matures, regulatory frameworks will continue to play a significant role in guiding its evolution. Looking ahead, the DNA data storage market is expected to see a CAGR of 25% over the next decade. Investment in research and development and government support for sustainable data solutions are expected to drive further growth. However, challenges such as high initial costs and technical complexities persist. Emerging trends, such as the integration of artificial intelligence (AI) to improve data retrieval efficiency, present new opportunities for market players to explore.
Geographically, North America is leading the DNA data storage systems market, with the United States at the forefront due to substantial investments in R&D and the region’s advanced technological infrastructure. Companies in this region are increasingly leveraging DNA for its vast potential in data preservation and retrieval. Europe is following closely, with countries like Germany and the United Kingdom making significant strides in cutting-edge research. The European Union’s focus on data privacy and security is driving the demand for reliable and efficient storage solutions, contributing to the sector’s growth across the continent.
In the Asia Pacific region, countries such as China and Japan are emerging as key players in the market, investing heavily in technology to manage the growing volume of data. The region’s increasing digital transformation efforts are fueling the demand for advanced data storage solutions, positioning Asia Pacific as a vital contributor to the market. Latin America, while still in its early stages, is gradually recognizing the potential of DNA data storage. Countries like Brazil are beginning to explore this technology as a means to enhance data management capabilities, and although the region remains in its nascent stage, it shows promise for future growth.
#DNADataStorage #Biotechnology #DataStorage #NextGenStorage #SustainableData #DNAArchiving #DataPreservation #HealthcareInnovation #DataRetrieval #Genomics #PCRbasedStorage #DNASequencing #DigitalTransformation #AIInDataStorage #CRISPRTechnology #SyntheticDNA #DataManagement #ResearchAndDevelopment #DataPrivacy #TechInnovation #EmergingMarkets
0 notes
Text
Why Investors Are Eyeing the dPCR and qPCR Market for Growth Opportunities

The Digital PCR (dPCR) and Real-Time PCR (qPCR) Market is experiencing robust growth as advancements in molecular diagnostics and biotechnology revolutionize the healthcare and research sectors. According to a comprehensive analysis by SkyQuest Technology, the market is projected to reach unprecedented heights, reflecting a compound annual growth rate (CAGR) of 8.1% during the forecast period. As these technologies gain prominence, industries worldwide are embracing their potential for precision diagnostics, disease monitoring, and scientific breakthroughs.
Market Size and Growth Projections
The global digital PCR (dPCR) and real-time PCR (qPCR) market is thriving due to the surge in demand for advanced diagnostic tools. With a market value estimated at USD 10.1 billion in 2023, it is anticipated to achieve a value of USD 20.36 billion by 2032. This substantial growth is attributed to the increasing prevalence of infectious diseases, rising cancer cases, and a growing focus on personalized medicine.
Request a Sample of the Report here: https://www.skyquestt.com/sample-request/digital-pcr-dpcr-and-real-time-pcr-qpcr-market
Key Market Drivers
The growth of the dPCR and qPCR market is influenced by several factors, including:
Rising Demand for Molecular Diagnostics With a greater emphasis on early detection and precision, the demand for molecular diagnostic tools is skyrocketing. dPCR and qPCR technologies enable highly sensitive and accurate analysis of genetic material, making them indispensable in diagnosing critical illnesses.
Advancements in Technology Continuous innovation in PCR technologies is leading to faster, more accurate, and cost-effective solutions, enhancing their adoption across various industries.
Applications in Research and Development The increasing focus on drug development and genetic research has further expanded the utility of dPCR and qPCR technologies in laboratories worldwide.
Market Segments
The Digital PCR (dPCR) and Real-Time PCR (qPCR) Market is categorized based on technology, application, and end-user.
By Technology
Digital PCR (dPCR)
Real-Time PCR (qPCR)
By Application
Clinical Diagnostics
Research and Development
Forensic Applications
By End-User
Hospitals and Diagnostic Centers
Research Institutes
Biotech and Pharmaceutical Companies
Request a Customized Report Tailored to Your Needs: https://www.skyquestt.com/customization/digital-pcr-dpcr-and-real-time-pcr-qpcr-market
Regional Insights
The adoption of dPCR and qPCR technologies varies across regions, with distinct trends shaping the global market.
North America A dominant market due to advanced healthcare infrastructure, extensive research funding, and early adoption of innovative technologies.
Europe Strong emphasis on biotechnology and personalized medicine drives the market in countries like Germany, France, and the UK.
Asia-Pacific The fastest-growing region, fueled by the increasing prevalence of chronic diseases, expanding research activities, and government initiatives to enhance healthcare systems.
Latin America & Middle East Emerging markets show steady growth due to improving healthcare access and rising investments in diagnostic technologies.
Buy the Report to Get the Full Analysis: https://www.skyquestt.com/buy-now/digital-pcr-dpcr-and-real-time-pcr-qpcr-market
Top Players in the Market
The dPCR and qPCR market is highly competitive, with key players driving innovation and growth:
Thermo Fisher Scientific Inc.
Bio-Rad Laboratories, Inc.
Roche Diagnostics
QIAGEN N.V.
Agilent Technologies
Merck KGaA
Takara Bio Inc.
Promega Corporation
Illumina, Inc.
Fluidigm Corporation
View full ToC and List of Companies here: https://www.skyquestt.com/report/digital-pcr-dpcr-and-real-time-pcr-qpcr-market
Emerging Trends in the dPCR and qPCR Market
Increased Focus on Point-of-Care Diagnostics Portable and user-friendly devices are making molecular diagnostics accessible even in resource-limited settings.
Integration of AI and Big Data Advanced analytics are enhancing the accuracy and efficiency of PCR technologies, paving the way for groundbreaking discoveries.
Growth in Personalized Medicine With a shift towards tailored treatments, dPCR and qPCR technologies are playing a pivotal role in identifying genetic markers and designing customized therapies.
Expansion of Applications Beyond Healthcare The utility of these technologies is extending into food safety, agriculture, and environmental monitoring, further diversifying market opportunities.
Conclusion
The Digital PCR (dPCR) and Real-Time PCR (qPCR) Market is set to transform diagnostics and research on a global scale. As technological innovations continue to enhance the precision and accessibility of these tools, the market offers promising opportunities for growth across diverse sectors. Companies focusing on innovation, adaptability, and application expansion are poised to lead in this rapidly evolving industry.
#Asia dPCR and qPCR market#Europe dPCR and qPCR market#Middle East dPCR and qPCR market Size#North America dPCR and qPCR market
0 notes
Text
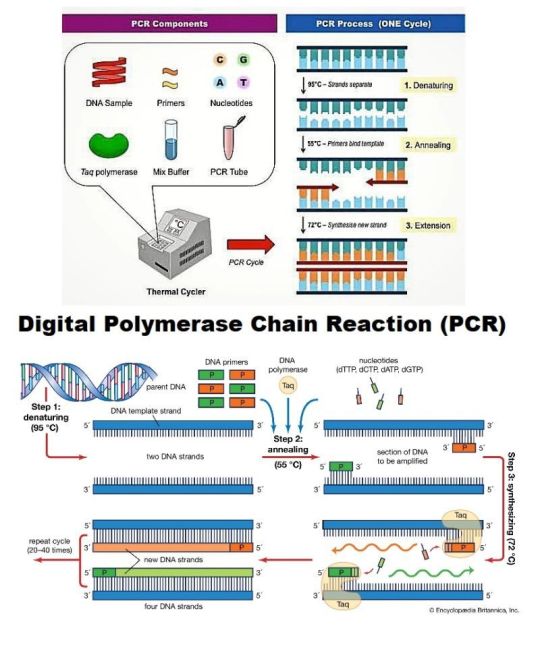
Digital PCR & qPCR Market
Digital PCR (dPCR) and quantitative PCR (qPCR) markets are rapidly expanding, driven by the growing demand for precise genetic analysis, diagnostics, and research applications. dPCR offers highly accurate quantification of nucleic acids, while qPCR is widely used for gene expression profiling and pathogen detection. Both technologies are advancing in fields such as oncology, infectious disease monitoring, and genetic research. The market growth is fueled by technological innovations, increased adoption in clinical diagnostics, and a rising focus on personalized medicine. Key players in the market include Thermo Fisher Scientific, Bio-Rad, and QIAGEN, with significant investment in R&D and product development.
For More : https://tinyurl.com/bdhjhwkf
0 notes
Text
Cancer Diagnostics Market Size, Share, Industry Growth and Emerging Trends Analysis by 2032
In 2023, the global cancer diagnostics market was worth $15.13 billion. It's expected to grow steadily, reaching $16.12 billion in 2024 and climbing to $31 billion by 2032, with an average annual growth rate of 8.5% over this period. North America led the market in 2023, holding a significant 35.89% share.
Informational Source:
Major Key Companies Covered in Cancer Diagnostics Market are:
F. Hoffmann-La Roche Ltd (Switzerland)
Thermo Fisher Scientific Inc. (U.S.)
Abbott (U.S.)
Illumina, Inc. (U.S.)
GE Healthcare (U.S.)
BD (U.S.)
bioMérieux SA (France)
Myriad Genetics, Inc (U.S.)
Bio-Rad Laboratories, Inc. (U.S.)
QIAGEN (Germany)
Advancements and Trends in Cancer Diagnostics
Cancer diagnostics play a critical role in detecting, monitoring, and managing cancer at various stages. With advancements in technology and ongoing research, the field has witnessed transformative changes, offering new hope for early detection and improved patient outcomes. Below, we delve into the latest innovations and trends shaping cancer diagnostics today.
1. The Role of Liquid Biopsies
Liquid biopsy technology has revolutionized cancer diagnostics by offering a non-invasive method to detect cancer-related biomarkers, such as circulating tumor DNA (ctDNA), circulating tumor cells (CTCs), and exosomes, in blood or other bodily fluids. Unlike traditional biopsies, liquid biopsies can be performed with minimal discomfort and provide real-time insights into tumor dynamics.
Key Applications:
Early Detection: Screening for cancers like lung, colorectal, and breast cancers before symptoms appear.
Monitoring: Tracking tumor progression and response to treatments.
Personalized Treatment: Identifying genetic mutations to guide targeted therapies.
Recent Innovations:
Multi-Cancer Early Detection (MCED): Tests like GRAIL’s Galleri aim to detect multiple cancers simultaneously by analyzing ctDNA.
High Sensitivity Platforms: Techniques like next-generation sequencing (NGS) enhance the precision of biomarker detection.
2. Artificial Intelligence (AI) in Cancer Diagnostics
AI and machine learning (ML) are increasingly being integrated into cancer diagnostics to analyze vast amounts of data, identify patterns, and improve diagnostic accuracy. These technologies augment traditional methods by reducing human error and speeding up the diagnostic process.
Applications:
Image Analysis: AI algorithms analyze imaging data from MRI, CT, and mammography to detect anomalies indicative of cancer.
Pathology: Digital pathology solutions powered by AI can evaluate tissue samples for malignant changes with high precision.
Risk Prediction Models: AI systems can predict a patient’s risk of developing cancer based on their medical history, genetics, and lifestyle factors.
Notable Examples:
Google Health’s AI: Demonstrated higher accuracy than human radiologists in detecting breast cancer in mammograms.
PathAI: Utilizes deep learning to assist pathologists in diagnosing cancer from biopsy samples.
3. Advances in Molecular Diagnostics
Molecular diagnostics has seen significant advancements, allowing for the precise identification of genetic and molecular markers associated with different cancer types.
Technologies Driving Innovation:
Next-Generation Sequencing (NGS): Enables comprehensive genomic profiling to identify mutations, fusions, and other alterations that drive cancer.
Polymerase Chain Reaction (PCR): Used to amplify and detect specific DNA or RNA sequences linked to cancer.
CRISPR-based Detection: CRISPR technology is being developed for rapid and highly specific cancer biomarker detection.
Impact on Personalized Medicine:
Molecular diagnostics forms the backbone of personalized medicine by guiding therapies tailored to the genetic profile of a patient’s tumor. For instance:
EGFR mutations in lung cancer guide the use of tyrosine kinase inhibitors.
BRCA mutations in breast and ovarian cancer inform the use of PARP inhibitors.
4. Imaging Technologies in Cancer Detection
Imaging remains a cornerstone of cancer diagnostics, and advancements in this field have significantly improved the ability to detect and monitor tumors.
Innovations in Imaging:
Positron Emission Tomography (PET): Combined with CT or MRI, PET scans provide detailed information about tumor metabolism and structure.
Multiparametric MRI (mpMRI): Offers a more accurate assessment of prostate cancer compared to traditional methods.
AI-Enhanced Imaging: Machine learning algorithms improve the resolution and interpretation of imaging data, aiding in early detection and reducing false positives.
Emerging Modalities:
Optical Imaging: Techniques like fluorescence and bioluminescence imaging allow for the visualization of cancer at the cellular level.
Theranostic Imaging: Combines diagnostic imaging with therapy, enabling real-time monitoring of treatment efficacy.
5. Biomarker Discovery and Utilization
Biomarkers are critical for early detection, diagnosis, and prognosis in cancer care. Advances in proteomics, genomics, and metabolomics have expanded the pool of potential biomarkers.
Breakthroughs in Biomarker Research:
Proteomics: Identifying protein signatures unique to cancer cells.
Epigenetics: Analyzing DNA methylation and histone modifications as cancer-specific markers.
Metabolomics: Profiling metabolic changes associated with cancer progression.
Clinical Utility:
Predictive Biomarkers: EGFR, HER2, and PD-L1 guide targeted and immunotherapies.
Prognostic Biomarkers: Help estimate disease progression and survival rates.
Companion Diagnostics: Ensure that patients receive the most effective therapy based on their biomarker profile.
6. Point-of-Care (POC) Diagnostics
Point-of-care testing is transforming cancer diagnostics by bringing testing capabilities closer to patients, reducing the time to diagnosis and enabling quicker interventions.
Examples of POC Diagnostics:
Portable Devices: Handheld devices for detecting specific biomarkers in blood or saliva.
Lab-on-a-Chip Technology: Integrates multiple diagnostic processes on a microchip for rapid results.
Immunoassays: Quick tests for detecting cancer antigens, such as PSA for prostate cancer.
Impact on Low-Resource Settings:
POC diagnostics are particularly valuable in remote or underserved areas, where access to advanced diagnostic facilities may be limited.
7. Role of Genomics and Epigenomics
Genomic and epigenomic approaches are uncovering the complexities of cancer, enabling highly personalized diagnostic and therapeutic strategies.
Key Areas of Progress:
Whole Genome Sequencing (WGS): Offers a complete view of genetic alterations driving cancer.
Epigenetic Markers: Identifying changes in gene expression regulation without altering DNA sequences.
RNA Sequencing: Provides insights into gene expression changes specific to cancer.
Implications for Clinical Practice:
These techniques are helping identify rare and aggressive cancers, paving the way for novel treatments and clinical trials.
8. Emerging Diagnostic Technologies
Several groundbreaking technologies are poised to redefine cancer diagnostics in the coming years:
Nanotechnology:
Nanoparticles: Used for targeted imaging and detection of cancer cells.
Nanosensors: Detect minute changes in biomarker levels with high sensitivity.
Single-Cell Analysis:
Examines individual cancer cells, providing insights into tumor heterogeneity and resistance mechanisms.
Microbiome Analysis:
Studies suggest that changes in the gut microbiome may be linked to cancer development, offering a new avenue for diagnostics.
9. Challenges and Future Directions
Despite significant progress, challenges remain in the widespread adoption and implementation of advanced cancer diagnostics.
Key Challenges:
Cost: Many advanced diagnostic tools are expensive and inaccessible to a large population.
Regulatory Hurdles: Approvals for new diagnostics can be lengthy and complex.
Integration: Combining diverse diagnostic data into a cohesive patient profile.
Future Focus Areas:
Affordable Solutions: Development of cost-effective diagnostic tools for global accessibility.
Precision Diagnostics: Further integration of genomics, proteomics, and AI for more accurate and personalized care.
Global Collaboration: Sharing data and resources to accelerate innovation and standardize best practices.
Conclusion
The field of cancer diagnostics is undergoing a transformative era, fueled by technological innovations and a deeper understanding of cancer biology. From liquid biopsies and AI-driven imaging to molecular diagnostics and epigenomics, these advancements are paving the way for earlier detection, improved accuracy, and personalized treatment.
0 notes
Text
Exploring the Liquid Biopsy Market: Trends, Innovations, and Future Prospects
The liquid biopsy market is revolutionizing the landscape of diagnostics and personalized medicine. Liquid biopsy, a minimally invasive diagnostic technique, detects biomarkers in bodily fluids such as blood, urine, or cerebrospinal fluid. Its ability to offer real-time insights into a patient's condition makes it a preferred choice over traditional tissue biopsies. This article delves into the key trends, advancements, and opportunities shaping the liquid biopsy market.
Understanding Liquid Biopsy: A Game-Changer in Diagnostics
Liquid biopsy involves analyzing circulating tumor cells (CTCs), cell-free DNA (cfDNA), and other biomarkers. This method enables early cancer detection, monitoring treatment responses, and identifying disease progression. Unlike tissue biopsies, liquid biopsies are non-invasive, reducing patient discomfort and allowing repeated sampling.
Key Drivers Fueling the Liquid Biopsy Market
Growing Prevalence of Cancer
The increasing global burden of cancer has propelled the adoption of liquid biopsies. These tests enable early-stage detection and help in tailoring treatment plans, improving patient outcomes.
Rising Adoption of Personalized Medicine
Personalized medicine emphasizes tailored treatment based on a patient’s genetic profile. Liquid biopsy plays a pivotal role by providing real-time genomic insights.
Technological Advancements
Innovations in next-generation sequencing (NGS) and digital PCR have significantly enhanced the accuracy and reliability of liquid biopsy tests.
Demand for Non-Invasive Diagnostics
Liquid biopsies offer a non-invasive alternative to traditional tissue biopsies, making them highly attractive for patients and clinicians.
Applications of Liquid Biopsy
1. Cancer Diagnostics
Liquid biopsy is widely used for early cancer detection, identifying mutations, and monitoring treatment efficacy. Key cancer types targeted include lung, breast, colorectal, and prostate cancers.
2. Prenatal Testing
Non-invasive prenatal testing (NIPT) utilizes liquid biopsy techniques to screen for genetic abnormalities in fetuses.
3. Transplant Monitoring
Liquid biopsy is increasingly applied to detect organ transplant rejection by analyzing donor-derived cell-free DNA.
4. Infectious Disease Monitoring
Emerging research highlights liquid biopsy’s potential in tracking infectious diseases, enabling timely interventions.
Market Segmentation
1. By Biomarker Type
Circulating Tumor DNA (ctDNA): Most widely studied for cancer applications.
Exosomes and Extracellular Vesicles: Emerging biomarkers with significant potential.
Circulating Tumor Cells (CTCs): Provide insights into tumor biology.
2. By Sample Type
Blood: The most common sample type for liquid biopsy tests.
Urine: Increasingly utilized in urological cancers.
Other Fluids: Saliva and cerebrospinal fluid show potential in specific applications.
3. By Application
Oncology: Dominates the market due to the rising incidence of cancer.
Non-Oncology Applications: Growing focus on prenatal testing and transplant diagnostics.
Key Technologies Driving Innovation
Next-Generation Sequencing (NGS): Enables high-throughput analysis of genetic material, increasing test accuracy.
Polymerase Chain Reaction (PCR): Highly sensitive and specific, particularly useful in detecting rare mutations.
Microfluidics: Facilitates efficient isolation of CTCs and exosomes.
AI and Machine Learning: Enhances data analysis, enabling better interpretation of results.
Challenges in the Liquid Biopsy Market
Despite its potential, the liquid biopsy market faces several hurdles:
Regulatory Barriers: Stringent approval processes can delay product launches.
High Costs: Advanced technologies make liquid biopsy tests expensive, limiting accessibility.
Technical Limitations: Challenges in sensitivity and specificity need to be addressed.
Key Players in the Liquid Biopsy Market
Leading companies are investing heavily in R&D to develop innovative solutions:
Guardant Health: A pioneer in oncology-focused liquid biopsy tests.
Foundation Medicine: Offers comprehensive genomic profiling services.
QIAGEN: Known for its liquid biopsy-based diagnostic kits.
Illumina: Dominates the NGS-based diagnostics segment.
Thermo Fisher Scientific: Provides a broad range of liquid biopsy solutions.
Regional Insights
1. North America
Largest market share due to advanced healthcare infrastructure and high R&D investments.
Strong presence of key market players.
2. Europe
Significant growth driven by increasing cancer prevalence and supportive government initiatives.
Rising demand for personalized medicine.
3. Asia-Pacific
Rapid market expansion due to growing healthcare awareness and increasing investments in biotechnology.
China and India are emerging as key markets.
4. Latin America and Middle East & Africa
Slower adoption but increasing focus on improving diagnostic capabilities.
Future Trends in the Liquid Biopsy Market
Expansion Beyond Oncology: The development of liquid biopsy applications for cardiovascular, neurological, and infectious diseases.
Integration with AI: AI-powered tools will enhance data analysis, improving test accuracy and reliability.
Point-of-Care Testing: Miniaturized devices will make liquid biopsy accessible in decentralized settings.
Cost Reduction: Advances in technology will help lower test costs, increasing adoption rates.
Conclusion
The liquid biopsy market represents a paradigm shift in diagnostics, offering a non-invasive, accurate, and efficient solution for disease detection and monitoring. While challenges persist, ongoing technological advancements and increasing demand for personalized medicine are set to drive market growth. As healthcare systems worldwide focus on early detection and precision medicine, liquid biopsy will play a pivotal role in shaping the future of diagnostics.
This innovative market is poised for exponential growth, presenting lucrative opportunities for industry players and transformative benefits for patients worldwide.
0 notes
Text
In Vitro Diagnostics Industry Research Report 2030 By Players, Regions, Types & Applications
The global in vitro diagnostics (IVD) market, valued at an estimated USD 80.71 billion in 2024, is anticipated to grow at a compound annual growth rate (CAGR) of 6.8% from 2025 to 2030. This projected growth is largely driven by the increasing utilization of IVD tools and technologies, a response to the rising incidence of both infectious and chronic diseases globally. The development and implementation of automated IVD systems in laboratories and hospitals further contribute to this expansion, offering healthcare providers tools to make diagnoses that are efficient, precise, and free from errors. Additionally, the continual launch of new IVD products by leading companies plays a significant role in fueling this market’s growth.
A noteworthy example of this innovation in IVD products is seen in ELITechGroup’s recent expansion of its diagnostic portfolio. In January 2024, ELITechGroup introduced the GI Bacterial PLUS ELITe MGB Kit, which received CE-IVDR certification. This product marks the beginning of the company’s Gastrointestinal (GI) assay panel and is designed to detect bacterial pathogens responsible for GI infections. ELITechGroup’s expansion plans include three more kits for launch within the next quarter, covering a broader spectrum of gastrointestinal infections. These new products aim to equip laboratories and healthcare professionals with advanced tools for precise diagnosis and improved management of GI conditions.
Gather more insights about the market drivers, restrains and growth of the In Vitro Diagnostics Market
Regional Insights:
North America In Vitro Diagnostics Market:
The North American in vitro diagnostics (IVD) market led the global sector in 2024, holding a 42.28% share. The region is expected to maintain its dominance throughout the forecast period, largely driven by several key factors, including the growing prevalence of chronic diseases, the presence of established IVD companies, the frequent introduction of innovative diagnostic tests, and strong government funding support. For example, in January 2023, BD and CerTest Biotec obtained an Emergency Use Authorization (EUA) from the U.S. FDA for a PCR test to detect the Mpox virus, highlighting regulatory support for advanced diagnostics. The increasing demand for genetic testing, particularly for personalized treatments in diabetes and cancer, is further expected to support the growth of the IVD market in North America.
Europe In Vitro Diagnostics Market Trends:
The European IVD market is growing, driven by an emphasis on infectious disease testing, molecular diagnostics, and adherence to regulatory requirements like CE-IVDR. Following the COVID-19 pandemic, demand for infectious disease diagnostics surged, with companies like Siemens Healthineers introducing platforms such as the Atellica CI Analyzer for efficient testing. The molecular diagnostics sector is also expanding, with ELITechGroup’s CE-IVDR-certified GI Bacterial PLUS kit as a recent example, aimed at detecting gastrointestinal infections. Stringent IVDR standards in Europe foster innovation, improving diagnostic accuracy and safety across the continent.
Asia Pacific In Vitro Diagnostics Market Trends:
The Asia Pacific IVD market is projected to experience significant growth, with a CAGR of 8.59% over the forecast period. Key drivers include stabilizing economies, a rapidly growing middle class, supportive government policies, and rapid urbanization. For instance, in October 2023, Fapon partnered with Halodoc to boost IVD product sales and services in Indonesia. Major IVD players are also collaborating with regional partners to expand their reach in developing countries across Asia Pacific.
The Chinese IVD market is growing rapidly, fueled by rising demand for molecular diagnostics, point-of-care testing, and digital health integration. Molecular diagnostics are particularly in demand for infectious diseases and oncology, supported by public health initiatives and increased healthcare spending.
Japan’s IVD market growth is driven by an aging population and progress in personalized medicine. Key trends include a growing use of molecular diagnostics for oncology and genetic testing, which aid in identifying targeted treatments for age-related diseases
Latin America In Vitro Diagnostics Market Trends:
The IVD market in Latin America is expanding due to increased healthcare investments, a focus on disease prevention, and enhanced healthcare access. A prominent trend is the adoption of point-of-care testing for infectious diseases like dengue, Zika, and COVID-19, especially in remote areas. Demand for molecular diagnostics in oncology is also growing as awareness of personalized medicine rises. Public-private partnerships and government initiatives are building diagnostics infrastructure, making advanced testing technologies more accessible across the region.
Middle East & Africa In Vitro Diagnostics Market Trends:
The IVD market in the Middle East and Africa (MEA) is set for growth, driven by increased healthcare spending and the prevalence of infectious diseases. Major trends include the adoption of advanced molecular diagnostics and point-of-care testing to improve disease detection and management. Additionally, a focus on personalized medicine and government initiatives aimed at enhancing healthcare infrastructure are supporting market expansion. Collaborations between local and international players are fostering innovation, making diagnostic solutions more accessible across the region.
Browse through Grand View Research's Category Clinical Diagnostics Industry Research Reports.
The global saliva collection and diagnostics market size was estimated at USD 818.9 million in 2024 and is projected to grow at a CAGR of 4.7% from 2025 to 2030.
The global cholesterol testing products and services market size was estimated at USD 19.85 billion in 2024 and is projected to grow at a CAGR of 8.4% from 2025 to 2030.
Key Companies & Market Share Insights:
Among the prominent players in the IVD market are F. Hoffmann-La Roche Ltd., Abbott, Quest Diagnostics Incorporated, and Danaher. These companies actively pursue strategies like new product launches, mergers and acquisitions, and partnerships to strengthen and diversify their product offerings. These efforts are focused on expanding their product portfolios, introducing technologically advanced and innovative diagnostic tools, and improving their competitive positioning within the market.
Emerging companies in the IVD sector, including Llusern Scientific, Biocartis Group NV, ARUP Laboratories, Veracyte, and Exact Sciences Corp, are also making strides. These companies concentrate on developing novel and accurate IVD testing products, contributing to overall healthcare improvements. They frequently collaborate with research institutions, governmental bodies, and global leaders to expand their product reach and presence in new, high-potential markets.
Order a free sample PDF of the In Vitro Diagnostics Market Intelligence Study, published by Grand View Research.
0 notes
Text
DNA Probe-Based Diagnostics: Key Drivers, Market Trends, and Industry Insights
DNA Probe-based Diagnostics involve the use of DNA probes—single-stranded DNA sequences designed to detect the presence of complementary nucleic acid sequences. This method is highly specific, as it targets sequences unique to pathogens or genes of interest, enabling accurate identification of diseases at a molecular level. DNA probes are invaluable in diagnosing infections, genetic disorders, and cancers. In recent years, DNA Probe-based Diagnostics have advanced significantly, enabling quicker, more sensitive, and cost-effective tests compared to traditional diagnostic approaches. The probes often detect diseases earlier than conventional methods, which allows for timely interventions and treatment.
In 2022, the market for DNA probe-based diagnostics was projected to be worth 2.81 billion US dollars. By 2032, the DNA probe-based diagnostics market is projected to have grown from 3.09 billion USD in 2023 to 7.4 billion USD. During the forecast period (2024-2032), the DNA Probe-based Diagnostics Market is anticipated to develop at a CAGR of around 10.17%.
DNA Probe-based Diagnostics Size and Share
The global DNA Probe-based Diagnostics market has experienced substantial growth, driven by advancements in biotechnology, an increase in research activities, and rising demand for personalized medicine. This market’s size continues to expand, supported by high adoption rates in hospitals, clinics, and research laboratories. As of recent estimates, the market is expected to grow at a consistent compound annual growth rate (CAGR) over the next few years. This growth is fueled by increasing government initiatives, rising investments in healthcare infrastructure, and growing awareness of the importance of early disease detection.
The market share of DNA Probe-based Diagnostics is significant in the molecular diagnostics sector, with applications across diverse fields including infectious disease diagnostics, oncology, genetic testing, and forensic sciences. North America currently holds a major share, driven by the advanced healthcare infrastructure, high investment in research and development, and a strong focus on precision medicine. However, Asia-Pacific is anticipated to see the fastest growth due to increasing healthcare expenditures, rising prevalence of chronic diseases, and a growing demand for advanced diagnostic technologies.
DNA Probe-based Diagnostics Analysis
DNA Probe-based Diagnostics utilize several analysis methods that contribute to their accuracy and reliability. Techniques such as fluorescence in situ hybridization (FISH), polymerase chain reaction (PCR), and nucleic acid amplification tests (NAATs) are commonly employed in DNA probe diagnostics. Each method has its unique advantages: FISH, for instance, allows for the visualization of DNA in chromosomes, making it particularly useful in genetic and cancer diagnostics. PCR amplifies DNA sequences to enhance detection sensitivity, while NAATs are known for their high precision in identifying pathogenic DNA in low-abundance samples.
The analysis of DNA Probe-based Diagnostics reveals trends that continue to shape the industry, including the growing shift towards digital and point-of-care diagnostics, which are particularly valuable in low-resource settings. The trend of miniaturization and automation in DNA probe diagnostics has further improved throughput, making these diagnostics faster and more accessible to a larger population.
DNA Probe-based Diagnostics Trends
Several notable trends are currently driving the growth and development of DNA Probe-based Diagnostics. The first is the increase in demand for personalized and precision medicine, where diagnostics are tailored to individual genetic profiles, providing targeted treatments. Second, there is an expansion in the use of DNA probes for infectious disease diagnostics, especially in the detection of viruses such as COVID-19, which highlighted the need for rapid, accurate diagnostics. Third, advancements in microfluidics and lab-on-chip technologies are making diagnostics more compact and accessible. Fourth, automation and digitalization are improving the accuracy and speed of diagnostic results, and fifth, next-generation sequencing (NGS) technologies are pushing the limits of what DNA probes can detect.
Reasons to Buy DNA Probe-based Diagnostics Reports
Comprehensive Market Insight: Reports provide an in-depth understanding of the DNA Probe-based Diagnostics market, covering all segments, growth factors, challenges, and opportunities.
Competitive Analysis: Detailed profiles of key market players, their strategies, and competitive positioning give buyers a clear view of the competitive landscape.
Trend Analysis: Reports analyze current and emerging trends in the diagnostics market, providing data-driven insights to make informed decisions.
Strategic Recommendations: Clear, actionable insights help stakeholders identify areas of growth and potential investment opportunities.
Recent Developments: Reports cover the latest advancements, regulatory updates, and innovations within the DNA probe diagnostics field.
Recent Developments
Recent developments in DNA Probe-based Diagnostics include the integration of artificial intelligence (AI) for faster data interpretation and the launch of more portable diagnostic devices. The shift toward at-home diagnostic kits has also gained momentum, with many new products entering the market that allow patients to conduct self-tests. Additionally, advancements in CRISPR technology are enhancing the accuracy and versatility of DNA probes, especially in gene editing applications.
Related reports:
burn care centers market
cardiac output monitoring device market
cementless total knee arthroplasty market
Top of Form
Bottom of Form
1 note
·
View note
Text
Market Insights: Drivers and Future Scope of DNA Probe-based Diagnostics
DNA Probe-based Diagnostics involve the use of DNA probes—single-stranded DNA sequences designed to detect the presence of complementary nucleic acid sequences. This method is highly specific, as it targets sequences unique to pathogens or genes of interest, enabling accurate identification of diseases at a molecular level. DNA probes are invaluable in diagnosing infections, genetic disorders, and cancers. In recent years, DNA Probe-based Diagnostics have advanced significantly, enabling quicker, more sensitive, and cost-effective tests compared to traditional diagnostic approaches. The probes often detect diseases earlier than conventional methods, which allows for timely interventions and treatment.
In 2022, the market for DNA probe-based diagnostics was projected to be worth 2.81 billion US dollars. By 2032, the DNA probe-based diagnostics market is projected to have grown from 3.09 billion USD in 2023 to 7.4 billion USD. During the forecast period (2024-2032), the DNA Probe-based Diagnostics Market is anticipated to develop at a CAGR of around 10.17%.
DNA Probe-based Diagnostics Size and Share
The global DNA Probe-based Diagnostics market has experienced substantial growth, driven by advancements in biotechnology, an increase in research activities, and rising demand for personalized medicine. This market’s size continues to expand, supported by high adoption rates in hospitals, clinics, and research laboratories. As of recent estimates, the market is expected to grow at a consistent compound annual growth rate (CAGR) over the next few years. This growth is fueled by increasing government initiatives, rising investments in healthcare infrastructure, and growing awareness of the importance of early disease detection.
The market share of DNA Probe-based Diagnostics is significant in the molecular diagnostics sector, with applications across diverse fields including infectious disease diagnostics, oncology, genetic testing, and forensic sciences. North America currently holds a major share, driven by the advanced healthcare infrastructure, high investment in research and development, and a strong focus on precision medicine. However, Asia-Pacific is anticipated to see the fastest growth due to increasing healthcare expenditures, rising prevalence of chronic diseases, and a growing demand for advanced diagnostic technologies.
DNA Probe-based Diagnostics Analysis
DNA Probe-based Diagnostics utilize several analysis methods that contribute to their accuracy and reliability. Techniques such as fluorescence in situ hybridization (FISH), polymerase chain reaction (PCR), and nucleic acid amplification tests (NAATs) are commonly employed in DNA probe diagnostics. Each method has its unique advantages: FISH, for instance, allows for the visualization of DNA in chromosomes, making it particularly useful in genetic and cancer diagnostics. PCR amplifies DNA sequences to enhance detection sensitivity, while NAATs are known for their high precision in identifying pathogenic DNA in low-abundance samples.
The analysis of DNA Probe-based Diagnostics reveals trends that continue to shape the industry, including the growing shift towards digital and point-of-care diagnostics, which are particularly valuable in low-resource settings. The trend of miniaturization and automation in DNA probe diagnostics has further improved throughput, making these diagnostics faster and more accessible to a larger population.
DNA Probe-based Diagnostics Trends
Several notable trends are currently driving the growth and development of DNA Probe-based Diagnostics. The first is the increase in demand for personalized and precision medicine, where diagnostics are tailored to individual genetic profiles, providing targeted treatments. Second, there is an expansion in the use of DNA probes for infectious disease diagnostics, especially in the detection of viruses such as COVID-19, which highlighted the need for rapid, accurate diagnostics. Third, advancements in microfluidics and lab-on-chip technologies are making diagnostics more compact and accessible. Fourth, automation and digitalization are improving the accuracy and speed of diagnostic results, and fifth, next-generation sequencing (NGS) technologies are pushing the limits of what DNA probes can detect.
Reasons to Buy DNA Probe-based Diagnostics Reports
Comprehensive Market Insight: Reports provide an in-depth understanding of the DNA Probe-based Diagnostics market, covering all segments, growth factors, challenges, and opportunities.
Competitive Analysis: Detailed profiles of key market players, their strategies, and competitive positioning give buyers a clear view of the competitive landscape.
Trend Analysis: Reports analyze current and emerging trends in the diagnostics market, providing data-driven insights to make informed decisions.
Strategic Recommendations: Clear, actionable insights help stakeholders identify areas of growth and potential investment opportunities.
Recent Developments: Reports cover the latest advancements, regulatory updates, and innovations within the DNA probe diagnostics field.
Recent Developments
Recent developments in DNA Probe-based Diagnostics include the integration of artificial intelligence (AI) for faster data interpretation and the launch of more portable diagnostic devices. The shift toward at-home diagnostic kits has also gained momentum, with many new products entering the market that allow patients to conduct self-tests. Additionally, advancements in CRISPR technology are enhancing the accuracy and versatility of DNA probes, especially in gene editing applications.
Related reports:
burn care centers market
cardiac output monitoring device market
cementless total knee arthroplasty market
Top of Form
Bottom of Form
0 notes
Text
Real-time PCR Market - Forecast(2024 - 2030)
𝐑𝐞𝐚𝐥-𝐓𝐢𝐦𝐞 𝐏𝐂𝐑: 𝐄𝐬𝐬𝐞𝐧𝐭𝐢𝐚𝐥 𝐓𝐨𝐨𝐥 𝐟𝐨𝐫 𝐌𝐨𝐝𝐞𝐫�� 𝐌𝐨𝐥𝐞𝐜𝐮𝐥𝐚𝐫 𝐁𝐢𝐨𝐥𝐨𝐠𝐲 𝐄𝐱𝐩𝐥𝐚𝐢𝐧𝐞𝐝
The global real-time PCR (qPCR) market is experiencing significant growth, driven by several key factors. The market, valued at $22.03 billion in 2024, is projected to reach $27.78 billion by 2028. This growth is largely due to the increasing prevalence of infectious diseases, the rise of cancer diagnostics, and expanding research in genomics.

The method that creates multiple copies of a particular DNA region in vitro uses the polymerase chain reaction. The technique relies on a DNA polymerase known as TAQ polymerase, which is thermostable. Thermus aquaticus is used to produce this polymerase. They occupy hot springs and hydrothermal vents. The target region to be reproduced is produced in large numbers by the PCR reaction, which involves repeat cycles at a range of temperatures.
Real-time PCR systems are laboratory instruments used to increase the number of copies of specific DNA segments. The rising prevalence of chronic and infectious diseases is driving the growth of the market for real-time polymerase chain reaction (PCR). Furthermore, forensics, diagnostics, and proteomics research advancements are creating potential growth opportunities for the real-time polymerase chain reaction (PCR) market.
📚Inquiry Before Buying :https://www.industryarc.com/reports/request-quote?id=503967&utm_source=Medium&utm_medium=Referral&utm_campaign=Deva
The polymerase chain reaction (PCR) has been used and shown to be effective in detecting minute amounts of a wide range of infectious diseases. The best conditions for amplification vary depending on the organisms of interest. PCR was used as a rapid and sensitive method for detecting infectious agents, and three assay systems were developed, one for the amplification of human T cell leukaemia virus type I, one for Mycobacterium tuberculosis, and one for Mycoplasma pneumoniae. These all factors will propel the market.
The increased use of the polymerase chain reaction for cancer diagnosis is expected to drive market growth in the coming years. All of these factors are responsible for creating a greater demand for this technique in the coming years, research and development activities for providing innovative molecular biology and forensic science as there has been a great demand for genetic engineering as well as personalized medicines.
📚Schedule a Call :https://connect.industryarc.com/lite/schedule-a-call-with-our-sales-expert?utm_source=Medium&utm_medium=Referral&utm_campaign=Deva
The global real-time PCR (qPCR) market is experiencing significant growth, driven by several key factors. The market, valued at $22.03 billion in 2024, is projected to reach $27.78 billion by 2028. This growth is largely due to the increasing prevalence of infectious diseases, the rise of cancer diagnostics, and expanding research in genomics. Real-time PCR remains a vital tool in healthcare, pharmaceuticals, and biotechnology for applications such as early disease detection, personalized medicine, and molecular diagnostics
COVID-19 had a substantial impact on the PCR market, as demand for reliable diagnostic tools surged. The pandemic underscored the importance of real-time PCR for detecting viral infections like SARS-CoV-2, making it an essential part of disease management worldwide. This trend continues to fuel demand, especially as the technology evolves with innovations like digital PCR and multiplex assays
Buy Now: https://www.industryarc.com/buynow?id=503967&utm_source=Medium&utm_medium=Referral&utm_campaign=Deva
Regionally, North America dominates the market due to its strong healthcare infrastructure and high prevalence of diseases like hepatitis and HIV. However, the Asia-Pacific region is expected to see the fastest growth, with rising patient awareness and investments in healthcare across countries like China, Japan,
More about Real-time PCR Market report click here
#moleculardiagnostics#geneticanalysis#genomics#biotechnology#clinicalresearch#diagnostics#covid19testing#dna#rna#genetherapy#pathogenresearch#microbiology#healthcare#viraltesting#geneticresearch#bioresearch#molecularbiology#dnaresearch#biotech#medicalresearch
0 notes
Text
Future and Growth of Tissue Diagnostics Market by 2030
Tissue Diagnostics Market
The global tissue diagnostics market size was estimated at USD 5.19 billion in 2022 and is expected to grow at a compound annual growth rate (CAGR) of 7.15% from 2023 to 2030.
Tissue diagnostics remains a gold standard for cancer diagnosis as these technologies capture the anatomy of tumors. With rising incidences of cancer, the tissue diagnostics industry witnesses high demand with significant growth opportunities over the forecast period. The impact of COVID-19 on the tissue diagnostics industry has been significant. During the pandemic, there was a slowdown in routine medical procedures, including diagnostic testing, as healthcare resources were redirected toward managing the virus.
Gather more insights about the market drivers, restrains and growth of the Tissue Diagnostics Market
The pandemic led to a temporary decline in the demand for tissue diagnostics products and services. However, as the situation improved and healthcare systems adapted to the new normal, the market began to recover. The need for accurate diagnosis and monitoring of various diseases, including cancer, remained high, driving the demand for tissue diagnostics in the post-pandemic period.
Cancer incidences are increasing dramatically, which has caused a paradigm change in anatomic pathology. This, in turn, is contributing to the clinical pathology field's continued growth. Digitalization of diagnosis methods, increased use of liquid biopsy for cancer detection, and a continuous convergence of anatomical and molecular pathology. The importance of integrated bioinformatics and analyses increases as computational pathology gains momentum. Over the past two decades, the tissue diagnostics industry has changed as more advanced equipment has become available, making life easier for pathologists and physicians.
For instance, in May 2021, to increase the availability of precision medication for lung cancer, QIAGEN released its first FDA-approved tissue companion diagnostic to detect the KRAS G12C mutation in NSCLC tumors. The Rotor-Gene Q MDx instrument, a part of the modular QIAsymphony family of automation solutions, is used with the real-time qualitative PCR kit. This tool builds on QIAGEN's nine years of experience in researching and marketing KRAS CDx tests.
Globally, more than 14 million individuals are diagnosed with cancer each year, and by 2030, that figure is projected to increase to more than 21 million. Major market participants are introducing new cancer diagnosis products. For instance, Roche introduced its innovative BenchMark ULTRA PLUS system for cancer diagnostics in June 2022, enabling prompt, precise patient care. Pathologists can deliver high-quality, time-sensitive results to doctors and patients due to the BenchMark ULTRA PLUS tissue staining system's improved workflow, testing efficiency, and environmentally sustainable features.
Considering the rising worldwide cancer burden, various technologies, and improvements in tissue diagnostics (TDx) will increase pathology efficiency, which is essential for better cancer therapy and diagnosis. For example, Ibex Medical Analytics, the industry pioneer in AI-powered cancer diagnoses, and Alverno Laboratories announced a new deal in March 2023. It aims to expand the implementation of Ibex's Galen suite of Artificial Intelligence solutions to the entire Alverno network across Indiana and Illinois. The deployment comprises AI-powered solutions for cancer diagnosis across numerous tissue types and will help Alverno pathologists in providing the highest quality care for their patients.
A rise in the adoption rate of automated tissue-based diagnostic systems by research institutes enables them to diagnose tumors faster. In January 2023, MilliporeSigma announced its plans to expand its portfolio of antibodies for tissue diagnostics to help improve the classification of gliomas and other tumors in the nervous systems. Such R&D investments will ensure the market continues to grow.
Browse through Grand View Research's Clinical Diagnostics Industry Research Reports.
The global hematologic malignancies market size was valued at USD 67.23 billion in 2023 and is projected to grow at a CAGR of 8.0% from 2024 to 2030.
The global precision diagnostics market size was estimated at USD 15.60 billion in 2023 and is projected to grow at a CAGR of 18.4% from 2024 to 2030.
Key Companies & Market Share Insights
The tissue diagnostics market is fragmented due to the presence of several medium-to-small and large participants in the marketspace. The advent of novel diagnostic models by key players to enhance their technology portfolio has raised competitiveness in the market. For instance, in June 2022, Roche announced the launch of VENTANA DP 600 - the next-generation slide scanner. This high-capacity slide scanner provides the pathology lab with workflow flexibility and ease of use while producing stained histology slides with exceptional image quality from tissue samples. Some prominent players in the global tissue diagnostics market include:
F. Hoffmann-La Roche Ltd.
Abbott Laboratories
Thermo Fisher Scientific Inc.
Siemens
Danaher
bioMérieux SA
QIAGEN
BD
Merck KGaA
GE Healthcare
BioGenex
Cell Signaling Technology, Inc.
Bio SB
DiaGenic ASA
Agilent Technologies
Order a free sample PDF of the Tissue Diagnostics Market Intelligence Study, published by Grand View Research.
0 notes
Text
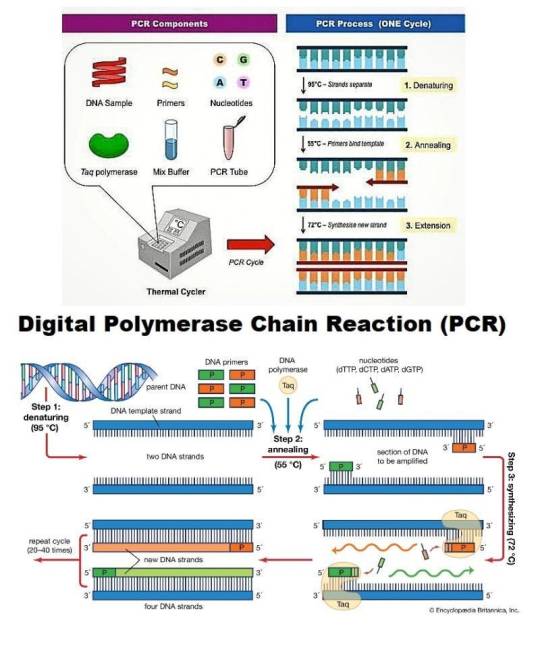
Digital PCR Market — Forecast(2024–2030)
Digital PCR Market Overview
Request Sample
Report Coverage
The report: “Digital PCR Market Forecast (2024–2030)”, by Industry ARC, covers an in-depth analysis of the following segments of the Digital PCR Market.
By Product: Consumables & Reagents and Software & Services
By Technology Type: Droplet Digital PCR, Chip Based Digital PCR, and Beaming Digital PCR
By Indication: Infectious Disease, Oncology, Genetic Disorders, and Others
By Application: Research, Clinical Diagnostics, Forensics, and Others
By Geography: North America (U.S., Canada, Mexico), Europe (Germany, United Kingdom (U.K.), France, Italy, Spain, Russia, and Rest of Europe), Asia Pacific (China, Japan India, South Korea, Australia, and New Zealand, and Rest of Asia Pacific), South America (Brazil, Argentina, and Rest of South America), and Rest of the World (Middle East, and Africa).
Inquiry Before Buying
Key Takeaways
North America dominated the Digital PCR Market in 2020 owing to the increasing demand for rapid diagnostic tests and high diagnosis rates for infectious disease. The Digital PCR Market scope for different regions will be provided in the final report.
Technological advancements in digital PCR and growing adoption of digital PCR over real time PCR are likely to aid the market growth of the Digital PCR Market report.
Detailed analysis of the Strength, Weakness, and Opportunities of the prominent players operating in the market will be provided in the Digital PCR Market report.
High cost of digital PCR devices and reimbursement issues along with the technical limitations of PCR is poised to create hurdles for the Digital PCR Market.
Digital PCR Market Segment Analysis — By Technology Type
Droplet Digital PCR held the largest share in the Digital PCR Market in 2020 and is estimated to grow at a CAGR of 9.7% during the forecast period 2024–2030. This is attributed to the technological advances along with various new product launches. Droplet digital PCR is based on water oil emulsion droplet technology and for the amplification of the template molecules in each individual droplet. It also uses workflows and reagents for most standard probe based assays. Droplet digital PCR also measure the copy number variation by partitioning a PCR reaction into nanoliter droplets. The cross-contamination drawback of droplet digital PCR is increasing the demand of chip based digital PCR. Droplet digital PCR are estimated to register the highest CAGR over the period 2024–2030.
Schedule a Call
Digital PCR Market Segment Analysis — By Indication
Infectious disease held the largest share in the Digital PCR Market in 2020 and is estimated to grow at a CAGR 8.6% during the forecast period 2021–2026. This is attributed to the advantages of the droplet digital PCR of infectious diseases such as bacterial, viral, and parasitic indications. Digital PCR provides more accurate, sensitive, and reproductive detection of pathogens according to the National Center for Biotechnology and it is better than real time polymerase chain reaction that are used for clinical diagnostics. The demand of oncology is increasing owing to the growing prevalence of the condition and introduction of new product launches. Oncology are estimated to register the highest CAGR over the period 2024–2030.
Digital PCR Market Segment Analysis — By Geography
North America dominated the Digital PCR Market with a major share of 37.6% in 2020. This is attributed to the high prevalence & diagnosis rates for infectious disease and high awareness among patient population towards new diagnostic options. Availability of digital PCR devices, rising incidences of various types of cancer and metabolic diseases requiring advanced diagnosis and therapeutics is also increasing the growth of the market in this region.
However, Asia Pacific is estimated to grow at a higher CAGR during the forecast period 2024–2030 owing to the growing patient awareness regarding advanced digital polymerase chain reaction devices. Developing Healthcare infrastructure is also increasing the growth of the market in this region.
Buy Now
Digital PCR Market Drivers
Technological Advancements in Digital PCR
Technological advancements in digital PCR is increasing the growth of the Digital PCR Market. This is attributed to the growing demand for innovative devices and increasing research & development activities. Digital PCR is used to quantify and amplify nuclei acid. The introduction of various technologically advanced devices such as droplet digital PCR, chip based, and beam digital PCR is offering great benefits to the market. Thus, increasing the growth of the Digital PCR Market during the forecast period 2024–2030.
Growing Adoption of Digital PCR over Realtime PCR
Growing adoption of digital PCR over Realtime PCR is increasing the growth of the Digital PCR Market. This is attributed to the fact that digital PCR helps to deliver a compete measure to target nucleic acid molecules that is achieved from real time PCR. DNA quantification allows for reproducibility, precision, and sensitive that enables the researches to quantify smaller differences and measure minor variants very precisely. Thus, increasing the growth of the Digital PCR Market during the forecast period 2024–2030.
Digital PCR Market Challenges
High Cost of Digital PCR Devices and Reimbursement Issues Along with the Technical Limitations of PCR
Some of the factors that are set to impede the growth of the Digital PCR Market are high cost of digital PCR devices and reimbursement issues along with the technical limitations of PCR. The adoption of digital polymerase chain reaction techniques is limited owing to the lack of awareness about the digital PCR and the use of its advanced types.
Digital PCR Market Landscape
Product launches, mergers and acquisitions, joint ventures, and R&D activities are key strategies adopted by players in the Digital PCR Market. In 2020, the Digital PCR Market share is consolidated by the top ten players present in the market. Digital PCR Market, top 10 companies are Thermo Fisher Scientific Inc., BioMerieux SA, Stilla Technologies, Merck KgaA, Combinati Inc., and Bio-Rad Laboratories among others.
For more Lifesciences and Healthcare Market reports, please click here
0 notes
Text
Polymerase Chain Reaction Market Surge: Future of Genetic Testing and Diagnostics

The Polymerase Chain Reaction (PCR) market is experiencing significant growth, driven by advancements in technology and increasing demand across various sectors. According to a recent report by SkyQuest Technology, the global PCR market is poised for substantial expansion, reflecting its crucial role in medical diagnostics, research, and biotechnology.
PCR, a technique developed in the 1980s, has revolutionized the field of molecular biology by allowing scientists to amplify specific DNA sequences. This process is essential for various applications, including disease diagnosis, genetic research, and forensic analysis. The report highlights that the PCR market has been growing steadily, with expectations of continued expansion in the coming years. The Polymerase Chain Reaction (PCR) Market size was valued at USD 24.17 billion in 2022 and is poised to grow from USD 24.75 billion in 2023 to USD 29.92 billion by 2031, growing at a CAGR of 2.4% during the forecast period (2024-2031).
Get Your Free Sample Report Here - https://www.skyquestt.com/sample-request/polymerase-chain-reaction-market
Key Drivers of Growth
Several factors are contributing to the growth of the PCR market:
1. Technological Advancements: Innovations such as real-time PCR and digital PCR are enhancing the accuracy and efficiency of genetic testing. These advancements are broadening the scope of PCR applications, from personalized medicine to infectious disease detection.
2. Increased Demand in Diagnostics: The global health crisis underscored the importance of rapid and reliable diagnostic tools. PCR has become a cornerstone in the detection of pathogens, including the SARS-CoV-2 virus, which has led to a surge in demand for PCR-based testing.
3. Expansion of Research and Development: The rise in research activities in genomics and proteomics is driving the need for advanced PCR technologies. Academic and research institutions are investing in PCR systems to support their studies and innovations.
4. Growing Biotechnology Sector: The biotech industry’s expansion is another significant driver. Companies are increasingly adopting PCR for drug discovery, development, and quality control, further propelling market growth.
Top Player’s Company Profile - Thermo Fisher Scientific, Inc., Roche Holdings AG, Bio-Rad Laboratories, Inc., QIAGEN N.V., Agilent Technologies, Inc., F. Hoffmann-La Roche Ltd., Becton, Dickinson and Company, Danaher Corporation, Promega Corporation, Merck KGaA, Fluidigm Corporation, Eppendorf AG, Takara Bio Inc., Abbott Laboratories, BioMérieux SA, PerkinElmer, Inc., BioFire Diagnostics, LLC, Biosearch Technologies, Inc., GenMark Diagnostics, Inc., Enzo Biochem, Inc., Illumina, Inc., New England Biolabs, Inc., Quantabio, LGC Limited, Bioer Technology Co., Ltd
Market Segmentation
The report provides a detailed analysis of the PCR market, segmented by product type, application, and region:
- Product Type: The market is categorized into instruments, reagents, and software. Instruments, including PCR machines and thermal cyclers, are the largest segment due to their essential role in the PCR process.
- Application: PCR is utilized in various fields such as clinical diagnostics, research, and forensic applications. Clinical diagnostics, particularly in infectious disease detection, holds the largest market share.
- Region: North America leads the market due to the presence of advanced healthcare infrastructure and high research funding. However, the Asia-Pacific region is expected to witness the fastest growth, driven by increasing healthcare investments and rising awareness.
Want to customize this report? Get Your Free Customize Report - https://www.skyquestt.com/speak-with-analyst/polymerase-chain-reaction-market
Recent Developments
In February 2023: Qiagen announced the release of its new QIAsymphony Dx Real-Time PCR System, that is designed to be greater person-pleasant and efficient than preceding PCR systems.
In March 2023: Roche Diagnostics launched its new cobas SARS-CoV-2 PCR check, which is a fast and accurate test for the detection of COVID-19.
In April 2023: Illumina announced the release of its new MiSeq 5Dx sequencing system, which is able to sequence up to 300 genomes consistent with day.
In May 2023: Thermo Fisher Scientific launched its new Applied Biosystems TaqMan Profiler Plus SARS-CoV-2 Assay, that's a quantitative PCR assay for the detection of SARS-CoV-2.
In June 2023: Agilent Technologies announced the release of its new 2100 Bioanalyzer System, which is a high-throughput DNA evaluation system. The PCR market is set for robust growth, underpinned by technological advancements and increasing demand across various applications. As the technology continues to evolve, it promises to deliver more precise and efficient solutions, further solidifying its role in medical diagnostics and research. For stakeholders in the PCR market, staying abreast of technological developments and market trends will be crucial in navigating the evolving landscape and leveraging opportunities for growth.
#PCRMarket#PolymeraseChainReactionMarket#PCRIndustry#PCRTrends#PCRGrowth#PCRTechnology#PCRDiagnostics#PCRApplications#PCRInnovation#PCRAdvancements#MolecularDiagnosticsMarket#GeneticTestingMarket#BiotechMarket#PCRResearch#PCRSolutions#DiagnosticMarket#PCRDevelopments#PCRInsights#PCRTrends2024#PCRExpansion
0 notes
Text
Tissue Diagnostics Market Business Growth, Opportunities and Forecast, 2030
The global tissue diagnostics market size was estimated at USD 5.19 billion in 2022 and is expected to grow at a compound annual growth rate (CAGR) of 7.15% from 2023 to 2030.
Tissue diagnostics remains a gold standard for cancer diagnosis as these technologies capture the anatomy of tumors. With rising incidences of cancer, the tissue diagnostics industry witnesses high demand with significant growth opportunities over the forecast period. The impact of COVID-19 on the tissue diagnostics industry has been significant. During the pandemic, there was a slowdown in routine medical procedures, including diagnostic testing, as healthcare resources were redirected toward managing the virus.
Gather more insights about the market drivers, restrains and growth of the Tissue Diagnostics Market
The pandemic led to a temporary decline in the demand for tissue diagnostics products and services. However, as the situation improved and healthcare systems adapted to the new normal, the market began to recover. The need for accurate diagnosis and monitoring of various diseases, including cancer, remained high, driving the demand for tissue diagnostics in the post-pandemic period.
Cancer incidences are increasing dramatically, which has caused a paradigm change in anatomic pathology. This, in turn, is contributing to the clinical pathology field's continued growth. Digitalization of diagnosis methods, increased use of liquid biopsy for cancer detection, and a continuous convergence of anatomical and molecular pathology. The importance of integrated bioinformatics and analyses increases as computational pathology gains momentum. Over the past two decades, the tissue diagnostics industry has changed as more advanced equipment has become available, making life easier for pathologists and physicians.
For instance, in May 2021, to increase the availability of precision medication for lung cancer, QIAGEN released its first FDA-approved tissue companion diagnostic to detect the KRAS G12C mutation in NSCLC tumors. The Rotor-Gene Q MDx instrument, a part of the modular QIAsymphony family of automation solutions, is used with the real-time qualitative PCR kit. This tool builds on QIAGEN's nine years of experience in researching and marketing KRAS CDx tests.
Globally, more than 14 million individuals are diagnosed with cancer each year, and by 2030, that figure is projected to increase to more than 21 million. Major market participants are introducing new cancer diagnosis products. For instance, Roche introduced its innovative BenchMark ULTRA PLUS system for cancer diagnostics in June 2022, enabling prompt, precise patient care. Pathologists can deliver high-quality, time-sensitive results to doctors and patients due to the BenchMark ULTRA PLUS tissue staining system's improved workflow, testing efficiency, and environmentally sustainable features.
Considering the rising worldwide cancer burden, various technologies, and improvements in tissue diagnostics (TDx) will increase pathology efficiency, which is essential for better cancer therapy and diagnosis. For example, Ibex Medical Analytics, the industry pioneer in AI-powered cancer diagnoses, and Alverno Laboratories announced a new deal in March 2023. It aims to expand the implementation of Ibex's Galen suite of Artificial Intelligence solutions to the entire Alverno network across Indiana and Illinois. The deployment comprises AI-powered solutions for cancer diagnosis across numerous tissue types and will help Alverno pathologists in providing the highest quality care for their patients.
A rise in the adoption rate of automated tissue-based diagnostic systems by research institutes enables them to diagnose tumors faster. In January 2023, MilliporeSigma announced its plans to expand its portfolio of antibodies for tissue diagnostics to help improve the classification of gliomas and other tumors in the nervous systems. Such R&D investments will ensure the market continues to grow.
Tissue Diagnostic Market Segmentation
Grand View Research has segmented the global tissue diagnostics market report based on technology, application, end-use, and region:
Technology Outlook (Revenue, USD Million, 2018 - 2030)
• Immunohistochemistry
o Instruments
o Slide Staining Systems
o Tissue Microarrays
o Tissue Processing Systems
o Slide Scanners
o Other Products
o Consumables
o Antibodies
o Reagents
o Kits
• In Situ Hybridization
o Instruments
o Consumables
o Software
• Primary & Special Staining
• Digital Pathology and Workflow
o Whole Slide Imaging
o Image Analysis Informatics
o Information Management System Storage & Communication
• Anatomic Pathology
o Instruments
o Microtomes & Cryostat Microtomes
o Tissue Processors
o Automatic Strainers
o Other Products
o Consumables
o Reagents & Antibodies
o Probes & Kits
o Others
Application Outlook (Revenue, USD Million, 2018 - 2030)
• Breast Cancer
• Non-small Cell Lung Cancer
• Prostate Cancer
• Gastric Cancer
• Other Cancers
End-use Outlook (Revenue, USD Million, 2018 - 2030)
• Hospitals
• Research Laboratories
• Pharmaceutical Organizations
• Contract Research Organizations (CROs)
Regional Outlook (Revenue, USD Million, 2018 - 2030)
• North America
o U.S.
o Canada
• Europe
o UK
o Germany
o Spain
o France
o Italy
o Denmark
o Sweden
o Norway
• Asia Pacific
o Japan
o China
o India
o South Korea
o Singapore
o Australia
o Thailand
• Latin America
o Brazil
o Mexico
o Argentina
• Middle East and Africa (MEA)
o South Africa
o Saudi Arabia
o UAE
o Kuwait
Browse through Grand View Research's Clinical Diagnostics Industry Research Reports.
• The global hematologic malignancies market size was valued at USD 67.23 billion in 2023 and is projected to grow at a CAGR of 8.0% from 2024 to 2030.
• The global precision diagnostics market size was estimated at USD 15.60 billion in 2023 and is projected to grow at a CAGR of 18.4% from 2024 to 2030.
Key Companies & Market Share Insights
The tissue diagnostics market is fragmented due to the presence of several medium-to-small and large participants in the marketspace. The advent of novel diagnostic models by key players to enhance their technology portfolio has raised competitiveness in the market. For instance, in June 2022, Roche announced the launch of VENTANA DP 600 - the next-generation slide scanner. This high-capacity slide scanner provides the pathology lab with workflow flexibility and ease of use while producing stained histology slides with exceptional image quality from tissue samples. Some prominent players in the global tissue diagnostics market include:
• F. Hoffmann-La Roche Ltd.
• Abbott Laboratories
• Thermo Fisher Scientific Inc.
• Siemens
• Danaher
• bioMérieux SA
• QIAGEN
• BD
• Merck KGaA
• GE Healthcare
• BioGenex
• Cell Signaling Technology, Inc.
• Bio SB
• DiaGenic ASA
• Agilent Technologies
Order a free sample PDF of the Tissue Diagnostics Market Intelligence Study, published by Grand View Research.
#Tissue Diagnostics Market#Tissue Diagnostics Industry#Tissue Diagnostics Market size#Tissue Diagnostics Market share#Tissue Diagnostics Market analysis
0 notes