#Deep Venous Disease
Explore tagged Tumblr posts
Text
Navigating the Depths: Understanding Deep Venous Disease
Introduction:
In the intricate network of blood vessels that course through our bodies, the veins play a crucial role in returning blood to the heart. However, not all veins are created equal, and some individuals may find themselves grappling with a condition known as Deep Venous Disease (DVD). This complex medical issue can have profound implications for one's health and well-being, making it imperative to unravel the intricacies of this condition.

Understanding Deep Venous Disease:
Deep Venous Disease, often referred to as deep vein thrombosis (DVT), encompasses a range of conditions that affect the deeper veins within the body. The deep veins, found in the muscles of the legs and pelvis, play a vital role in carrying oxygen-depleted blood back to the heart. When these veins become compromised, it can lead to a host of complications.
Causes and Risk Factors:
Several factors contribute to the development of Deep Venous Disease, and understanding these triggers is paramount for prevention and early intervention. Sedentary lifestyles, prolonged immobility (such as long flights or bed rest), obesity, smoking, and certain medical conditions like cancer or inflammatory disorders can increase the risk of DVD. Additionally, genetic factors and a history of blood clotting disorders may elevate susceptibility.
Symptoms and Detection:
Identifying the symptoms of Deep Venous Disease is crucial for timely intervention. Common signs include swelling in the affected leg, pain or tenderness, warmth, and discoloration of the skin. However, it's important to note that some cases may be asymptomatic, making routine screenings and awareness crucial for early detection.
Diagnostic tools such as ultrasound imaging and venography allow healthcare professionals to visualize blood flow and identify potential clots. Early diagnosis significantly improves the chances of successful treatment and reduces the risk of complications.
Complications of Deep Venous Disease:
Left untreated, Deep Venous Disease can lead to severe complications, the most alarming being the risk of pulmonary embolism. When a blood clot breaks loose and travels to the lungs, it can cause a life-threatening situation. Chronic venous insufficiency, characterized by long-term damage to the veins and impaired blood flow, is another potential consequence. This condition may manifest as leg ulcers, skin changes, and persistent swelling.
Treatment and Management:
The approach to managing Deep Venous Disease is multifaceted, often combining lifestyle modifications, medications, and, in some cases, invasive procedures. Anticoagulant medications, commonly known as blood thinners, are prescribed to prevent the growth of blood clots and reduce the risk of further complications. Compression stockings, designed to improve blood flow in the legs, are a non-invasive option that aids in symptom relief.
For more severe cases, procedures like thrombolysis or thrombectomy may be considered to remove or dissolve clots. In cases where chronic venous insufficiency has developed, interventions such as vein ablation or venous stenting may be recommended to restore normal blood flow.
Prevention:
Preventing Deep Venous Disease involves adopting a proactive approach to mitigate risk factors. Regular exercise, maintaining a healthy weight, avoiding prolonged periods of immobility, and refraining from smoking are key lifestyle choices that can significantly reduce the likelihood of developing DVD. For individuals with a family history of blood clotting disorders, genetic testing and consultation with healthcare professionals can provide valuable insights and guidance.
Conclusion:
Deep Venous Disease is a formidable health challenge that demands our attention and understanding. By recognizing the risk factors, symptoms, and available treatments, individuals can take proactive steps to safeguard their vascular health. Early detection and intervention are crucial, emphasizing the importance of routine screenings for those at higher risk. As we delve deeper into the complexities of Deep Venous Disease, we empower ourselves and our communities to navigate the currents of health with knowledge and resilience.
0 notes
Text
Deep Venous Disease in the US: Treatment Options, Market Players, Stents, and Patient Satisfaction
Introduction
In the United States, Deep Venous Disease (DVD) is a common medical ailment that has a big impact on a lot of people. This illness affects blood flow in the deep veins of the legs, resulting in swelling, discomfort, and ulceration as symptoms. In this article, we'll look at the several treatment choices for DVD, the major industry participants, the function of stents during treatment, and patient satisfaction.
Read Full Blog Here: https://www.grgonline.com/post/deep-venous-disease-in-the-usa-available-treatment-options-key-market-players-stent-usage-and-pa
Treatment Options
Conservative Management: Mild cases of DVD can be controlled by making lifestyle changes, including as elevating the legs, wearing compression stockings, and exercising frequently. These actions enhance blood flow and lessen symptoms.
Medications: Anticoagulant medications are prescribed to patients with DVT to prevent the formation of blood clots. This treatment is crucial in preventing life-threatening complications like pulmonary embolism.
Endovenous Procedures: Minimally invasive procedures like endovenous laser treatment (EVLT) and radiofrequency ablation (RFA) can be used to close off malfunctioning veins and redirect blood flow. These treatments have a shorter recovery time compared to traditional surgery.
Stent Placement: In cases where the veins are severely narrowed or obstructed, stent placement may be recommended. Stents are small mesh tubes that are inserted into the affected vein to keep it open and restore proper blood flow.
Market Players in Deep Venous Disease Treatment
Several companies are at the forefront of developing innovative technologies and treatments for Deep Venous Disease. Some of the key market players include:
Medtronic: Medtronic offers a range of products for the treatment of venous diseases, including stents, catheters, and other medical devices used in minimally invasive procedures.
Cook Medical: Cook Medical is a leading provider of medical devices, including stents and catheters used in the treatment of DVT and other vascular conditions.
Boston Scientific: Boston Scientific manufactures various interventional devices, including stents, for the treatment of deep vein issues.
The Role of Stents in DVD Treatment
Stents are often used in the treatment of DVD when there is a significant narrowing or blockage in the deep veins. These devices are designed to expand and keep the vein open, allowing for improved blood flow. Stents can be particularly effective in cases of post-thrombotic syndrome, where the veins are scarred and narrowed due to previous blood clots.
Patient Satisfaction in DVD Treatment
Patient satisfaction plays a vital role in evaluating the success of DVD treatments. Factors contributing to patient satisfaction include:
Symptom Relief: Effective treatment should alleviate symptoms such as pain, swelling, and ulcers, leading to an improved quality of life.
Minimally Invasive Options: Many patients prefer minimally invasive procedures, as they offer shorter recovery times and less postoperative discomfort.
Long-Term Outcomes: Patients are often concerned about the long-term success of treatment and whether it will prevent recurrence.
Quality of Life: Improvement in mobility and the ability to engage in daily activities without discomfort or limitations are significant factors contributing to patient satisfaction.
Conclusion
Deep Venous Disease is a complex condition that can have a significant impact on the lives of those affected. Fortunately, there are various treatment options available, including stents, which play a crucial role in restoring proper blood flow. Market players like Medtronic, Cook Medical, and Boston Scientific continue to advance the field with innovative medical devices.
Patient satisfaction is a key metric in evaluating the success of DVD treatments, and ongoing research and development efforts aim to improve outcomes and enhance the quality of life for individuals living with this condition. As medical technology continues to evolve, we can expect even more effective and patient-friendly treatments for Deep Venous Disease in the future.
Visit our website now: https://www.grgonline.com/

#Vein Health#Vascular Healthcare#healthcare#Deep Venous Disease#Venous Disease#Vascular Innovation#vascular
0 notes
Text
The global deep venous disease treatment devices market was valued at $1,119.3 million in 2022 and is anticipated to reach $2,419.2 million by 2032, witnessing a CAGR of 7.72% during the forecast period 2023-2032.
#Deep Venous Disease Treatment Devices Market#Deep Venous Disease Treatment Devices Report#Deep Venous Disease Treatment Devices Industry#Healthcare#BISResearch
0 notes
Text
Taking Charge of Your Circulation: A Deep Dive into Managing Varicose Veins and Other Circulatory Issues
Our circulatory system, the intricate network of blood vessels and the heart, is the lifeline that nourishes every cell in our body. When this system falters, it can lead to a cascade of health issues, impacting our overall well-being. This comprehensive guide will embark on a journey through the circulatory system, exploring the causes, symptoms, prevention, and treatment of varicose veins and…
#Chronic Venous Insufficiency#Circulatory Health#Deep Vein Thrombosis#Lymphedema#Peripheral Artery Disease#Varicose Veins
1 note
·
View note
Text
Feminizing HRT Overview, Guide & Information for All People Seeking It
we also have a version of this post for testosterone/masculinizing HRT as well. we wanted to write a companion piece as many folks have asked about this. it has take a bit of time, but here we are!
The testosterone HRT post is here.
Getting Your Prescription
To start taking estrogen, you will need to find a general practitioner, family doctor, endocrinologist or informed consent clinic where you can discuss gender affirming care with knowledgeable staff. Planned Parenthood is a good option for many trans people in general. Your mental health may also be evaluated, and your heart health and screening for a few other health conditions, as well as having access to your family health history if possible will be required.
Check to see if you have medical insurance, either through your family, your job, or if you are low income, a program like medicaid. Search for low income insurance plans in your area if it is needed, many places offer insurance plans for those who can't afford care on their own.
Here is a map of informed consent HRT clinics in the US.
You will discuss any gender dysphoria, gender presentation needs, if you have a support network, how you are impacted by your gender in your every day life with your provider and so on before being given a prescription. You will only be given a prescription after you discuss the risks of HRT and are screened for possible health problems and diseases or ways your body could react negatively to HRT. If you have needle trauma or phobias and can't inject hormones, it's best to bring it up before you get your prescription to save time and confusion.
The Medications
Treatment typically starts with spironolactone (aldactone), an anti-androgen that blocks androgen receptors ("male" sex hormones) for a few weeks, and then add estrogen, but many folks start with spiro and estrogen at the same time. Spiro will lower the amount of testosterone your body makes. For some people, spiro isn't necessary at all!
Some forms of spironolactone are reported to make folks pee like crazy, others do not have as bad of a time with it. Your mileage will vary depending on manufacturer. Spironolactone is intended to be a blood pressure medication, meaning it is a diuretic and is intended to help your body flush out fluids + salt. You will need to keep yourself hydrated if you notice this effect, as well as increasing electrolyte intake where possible.
Estrogen also lowers how much testosterone your body makes, and triggers changes in the body that occur during puberty in afab & adjacent people. Estrogen can be taken several ways, and is usually taken daily, and several times a day. You can take it in a pill or shot, and several forms of estrogen that can be applied to the skin like creams, gels and patches.
Make sure you thoroughly sanitize the skin of any injection sites or areas you will be applying gel or patches. If you are given topical estrogen, make sure you wash your hands after application and do not have someone else apply it for you. Make sure you do not go swimming or shower within several hours of application to make sure your skin absorbs the hormone.
You may not need to take anti androgens if you are doing estrogen injections, depending on how effective the estrogen injections are for you. Some people may not end up needing anti-androgens at all, and may be able to skip that entirely as spiro has unwanted side effects. Your natural hormone levels will dictate whether or not it's necessary, but it is not necessary for everyone.
You may end up being recommended to switch from one form of estrogen to another as your transition progresses, depending on how your body responds.
It's recommended to not take estrogen as a pill if you have personal/family history of blood clots in a deep vein or in lungs (venous thrombosis).
Some people also end up taking progesterone as well alongside estrogen. Progesterone is typically taken to encourage breast tissue growth, as this is the most prominent effect of the hormone. If sufficient breast tissue growth isn't seen from estrogen alone, progesterone can be added to your regimen, though this is only done later on into treatment, around a year or so in.
If you choose injectable estrogen, make sure to listen to your provider and ask for instructions about how to use needles and syringes, as well as injection angles, how and where you'll be injecting. Do not inject in the exact same spot every time, this can prevent the issue from healing properly and create scar tissue or cause infections or skin tissue necrosis (death). You also need a sharps container to safely dispose of your needle tips. Never re-use a needle, even if it was used previously on yourself. Always ask the pharmacy if you need more needles. A lot of places let you get them in bulk.
If you are going the injection route, make sure you know whether or not you are instructed to do intramuscular or subcutaneous injections. Intramuscular injections usually taper out of the system more quickly and need to be done more frequently, where as many patients find subcutaneous injections less painful and easier as they can be done less frequently.
For more information on safe intramuscular or subcutaneous injection for estrogen, please read here.
Another option for feminizing HRT is to take gonadotropin-releasing hormone (Gn-RH) analogs. They lower the amount of testosterone your body makes and may allow you to take lower doses of estrogen without using Spiro. Gn-RH analogs are usually more expensive, but are an option if for whatever reason the conventional route can't work for you.
DON'T GIVE UP IF YOU DON'T SEE THE EFFECTS YOU WANT TO SEE RIGHT AWAY! Many of them can take a long time to develop, often times patience is the key. If you wait it out and still don't see the results you'd like, you can try another route. Don't give up, a lot of people get deterred in the early stage of transition, you'll get there with patience and communication.
Stay patient, stay positive!
What to Expect from Feminizing HRT
Less facial and body hair growth: typically happens 6 - 12 months after treatment starts. Full effects within ~3 years on average.
Slower scalp hair loss: begins 1 - 3 moths after treatment begins. Full effect between 1 - 2 years on average.
Softer, less oily skin, and changes in general skin texture: 3 - 6 months after treatment starts, full effects within 2 - 3 years on average
Rounder, softer features including face and body, and more body fat: 3 - 6 months after treatment starts, full effects in 2 - 5 years.
Breast development: begins 3 - 6 months after treatment starts, full effects within 2 - 5 years on average or more, according to medical studies, but it can vary wildly from person to person, give dosage and hormones taken. If desired effects are not seen, progesterone can be taken alongside estrogen to help after around one year on estrogen. When breast growth begins, it starts with hard lumps under the nipples along with some soreness and itchiness. Some have sore breasts for a long time, and some may get scared and think they have cancer during this stage. Breasts will be swollen and tender for good while, and nipples may be especially sensitive to even light touch.
Reduced muscle mass/density: 3 - 6 months after treatment starts, full effect in 1 - 2 years on average
Potential decrease in libido if on estrogen alone, though not guaranteed: If it happens, it's generally within 1 - 3 months in and can last a while, but may even out over time
Fewer erections, decreased ejaculate volume, and erections that can become painful or uncomfortable if frequent erections are not maintained. This begins 1 - 3 months after treatment starts, and the full effect is within 3 - 6 months. Regularly maintaining erections and frequent ejaculation can ease some of these uncomfortable feelings in some people.
Changes in how orgasms feel, changes in texture and degree of sensation of penis and scrotum skin as well as changes in body odor: typically begins within 3 - 6 months, though it varies from person to person. Often times the way one's body responds to orgasms completely changes, many people find themselves experiencing full-body orgasms and more intense erogenous zones elsewhere in the body other than the genitals.
Smaller testicles, or testicular atrophy happens within 3 - 6 months and the full effects are usually seen within 2 - 3 years.
Increase in size of bladder and decrease in size of prostate over time which can lead to making one's gspot harder to find, and make prostate examinations more difficult, though they are still vital, as prostate cancer is still a possible factor.
Potential mood fluctuations while adjusting to the hormones, many report increased crying and sadness during the first 3 - 6 months with this tapering off after a full year at most.
Increased fatigue while adjusting to the hormones, sleepiness and becoming easily exhausted are common reports. This can vary drastically from person to person, ymmv.
If you have testicles and choose to have them removed, you may need to take testosterone as well as estrogen in order to have a healthy endocrine system. You will need to discuss the effects of this with your specialists if you want to go this route. If your androgen levels get too low because your body cannot synthesize enough testosterone after bottom surgery, you may need additional medication.
Potential infertility, though this is not a guarantee, and safe sex should still be practiced at all times. No timeline projected though the longer one is on E the more likely it becomes.
Monthly cycles akin to menstrual cycles: these are not present in everyone, but many people report entering a cycle of extreme fatigue, body aches, abdominal cramping in the approximate area where a uterus would sit, headaches, and more for around the duration of a menstrual cycle (4 - 10 days on average).
Progesterone inversely to estrogen can cause an increase in libido in most who take it, and is the primary hormone used for breast growth. Lactation may also occur while taking prog, if this happens, talk to your doctor right away.
Keep track of your progress when and where you are able, and don't be afraid to bring up any concerns you may have with your professionals or trans friends, or any other trans resource. Your transition is in your hands and you're allowed to modify it as you see fit. If you do not see the effects you want from traditional HRT, you may be able to seek the Gn-RH route, and if you aren't seeing the results you want from just estrogen, progesterone might be of use to you.
You will need to keep an eye on your bone health as high levels of estrogens can increase your chance to develop osteoporosis, and potential new cancers like breast cancer may arise, as well as heart problems. Getting checkups as frequently as possible and communicating with your doctor/s will be of great use when and where possible
Either way, we hope this helps in some way! We will add to it as we find/think of more information. Good luck to everyone seeking feminizing HRT, you deserve to look and feel like yourselves!
#transfem#transfeminine#transgender#trans#lgbt#lgbtq#queer#transfemme#trans girl#trans woman#trans women#trans lady#trans girls#trans gal#nonbinary#enby#genderqueer#genderfluid#drag queen#estrogen#progesterone#spironolactone#feminizing hrt#hrt#hormone replacement therapy#estrogen hrt#e hrt#e#our writing#resources
1K notes
·
View notes
Text

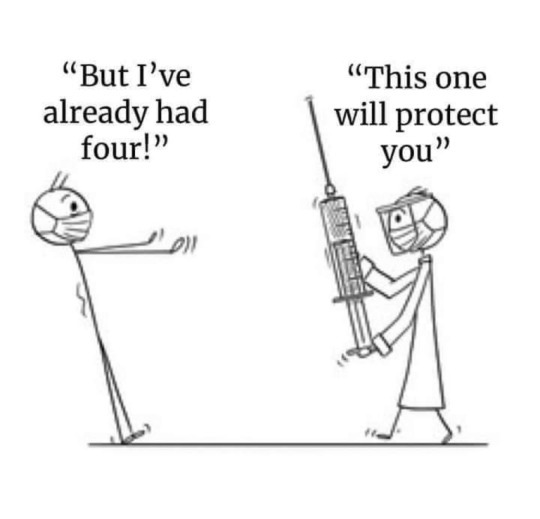
Absolutely safe and effective!
PFIZER JUST RELEASED IT’S LIST OF SIDE EFFECTS OF ITS "COVID-19 VACCINE"💉…….and the list of some side effects of the Pfizer-Biontech Covid-19 Vaccine. TAKE-HEED!
Blood thrombosis.
Acute kidney injury,
Acute flaccid myelitis,
Positive antisperm antibodies,
Brainstem embolism,
Brainstem thrombosis,
Cardiac arrest (hundreds of cases),
Heart failure,
Cardiac ventricular thrombosis,
Cardiogenic shock,
Central nervous system vasculitis,
Neonatal death,
Deep vein thrombosis,
Brainstem encephalitis,
Hemorrhagic encephalitis,
Frontal lobe epilepsy,
Foaming at the mouth,
Epileptic psychosis,
Facial paralysis,
Fetal distress syndrome,
Gastrointestinal amyloidosis,
Generalized tonic-clonic seizure,
Hashimoto's encephalopathy,
Hepatic vascular thrombosis,
Herpes zoster reactivation,
Hepatitis Immune-mediated,
Interstitial lung disease,
Jugular vein embolism,
Juvenile myoclonic epilepsy,
Liver damage,
Low birth weight,
Multisystem inflammatory syndrome in children,
Myocarditis,
Neonatal seizure,
Pancreatitis,
Pneumonia,
Stillbirth,
Tachycardia,
Temporal lobe epilepsy,
Testicular autoimmunity,
Thrombotic stroke,
Type 1 diabetes mellitus,
Neonatal venous thrombosis,
Vertebral artery thrombosis,
Pericarditis,
Sudden death.”
34 notes
·
View notes
Text
@toobaffled
PFIZER JUST RELEASED IT’S LIST OF SIDE EFFECTS OF ITS "COVID-19 VACCINE"
…….and the list of some side effects of the Pfizer-Biontech Covid-19 Vaccine. TAKE-HEED!
Blood thrombosis. Acute kidney injury, Acute flaccid myelitis, Positive antisperm antibodies, Brainstem embolism, Brainstem thrombosis, Cardiac arrest (hundreds of cases), Heart failure, Cardiac ventricular thrombosis, Cardiogenic shock, Central nervous system vasculitis, Neonatal death, Deep vein thrombosis, Brainstem encephalitis, Hemorrhagic encephalitis, Frontal lobe epilepsy, Foaming at the mouth, Epileptic psychosis, Facial paralysis, Fetal distress syndrome, Gastrointestinal amyloidosis, Generalized tonic-clonic seizure, Hashimoto's encephalopathy, Hepatic vascular thrombosis, Herpes zoster reactivation, Hepatitis Immune-mediated, Interstitial lung disease, Jugular vein embolism, Juvenile myoclonic epilepsy, Liver damage, Low birth weight, Multisystem inflammatory syndrome in children, Myocarditis, Neonatal seizure, Pancreatitis, Pneumonia, Stillbirth, Tachycardia, Temporal lobe epilepsy, Testicular autoimmunity, Thrombotic stroke, Type 1 diabetes mellitus, Neonatal venous thrombosis, Vertebral artery thrombosis, Pericarditis, Sudden death.” We just thought you’d like to know, because one thing people will never be able to say is, “I didn’t know”
WE TOLD YOU!

46 notes
·
View notes
Text
The Long-term Complications of Covid-19 Infection - Published Sept 13, 2024
Context.— As the Covid-19 pandemic continues into its 4th year, reports of long-term morbidity and mortality are now attracting attention. Recent studies suggest that Covid-19 survivors are at increased risk of common illnesses, such as myocardial infarction, diabetes mellitus and autoimmune disorders. Mortality may also be increased. This article will review the evidence that supports some of these observations and provide an opinion about their validity and their relevance to insured cohorts.
Background Many Covid-19 survivors report protracted symptoms, sometimes lasting 3 years or more. These are collectively called post-acute sequelae of SARS-CoV-2 infection (PASC) or long Covid. They have been frequently described.1–4 In the past year, reports of long-term complications such as atrial fibrillation, heart failure, stroke and pulmonary embolism have emerged. In some reports these established disease entities are erroneously described as long Covid, generating confusion. The distinction is important: illness reported in Covid survivors are not restricted to the long Covid cohort. Thus, they are relevant to the majority of the North American population who have been infected by SARS-CoV-2, and not just the estimated 5-10% of individuals who belong to the long Covid cohort. This paper will examine the reports of increased incidence of cardiovascular diseases in both and will examine the reported long-term increase in mortality.
Cardiovascular disease after 1 and 2 years Multiple studies have reported an increased risk of cardiovascular events at 1 year. A February 2022 analysis of 153,760 US veterans, followed for 1 year after Covid-19 infection, reported an increased risk of cerebrovascular disease (HR 1.53), ischemic heart disease (HR 1.66), thromboembolic disease (HR 2.39) and atrial fibrillation (HR 1.71).5 Risk was greatest in those hospitalized and those with pre-morbid illnesses. However, risk was also elevated in outpatients, who constituted the vast majority of the cohort. These findings have been corroborated in 2 further studies. In a 2023 analysis of 690,000 Covid-19 survivors, drawn from the TriNetX database–self-described as the world’s largest global Covid-19 dataset–there was an increased risk of cerebrovascular disease (HR 1.6), ischemic heart disease (HR 2.8), thromboembolic disease (HR 2.6) and atrial fibrillation (HR 2.4) at 1 year.6 In contrast to the VA study which examined a predominantly older male population, the subjects in this study were younger, with mean age 44, and 57% were female. Risk was higher in the >65 age group and was not limited to inpatients. In a May 2023 Lancet retrospective analysis of 535,000 Hong Kong (HK) and 16,000 UK Covid 19 survivors, similar hazard ratios were recorded for stroke (HR 1.2), ischemic heart disease (HR 1.32), atrial fibrillation (HR 1.31) and deep venous thrombosis (HR 1.74).7 However, it is worth noting that while follow-up was described as 28 months for the HK cohort and 17 months for the UK cohort, the median follow-up for the HK group was 146 days and was 243 days for the UK cohort, somewhat limiting the conclusions of true impact at 1 year. Contradicting these studies, a prospective analysis of 17,000 Covid-19 survivors in the UK Biobank, did not document an increased risk of cardiovascular outcomes amongst outpatients, with the exception of thromboembolic disease (HR 2.7).8 An August 2023 analysis of 138,000 VA Covid-19 survivors followed for 2 years– the longest follow-up period to date– reported that the risk of complications in outpatients had returned to baseline at 6 months.9 In contrast, the risk for multiple cardiovascular and thromboembolic complications in the hospitalized cohort remained elevated at 2 years. None of these 5 studies was limited to individuals with long Covid, but similar findings have been reported in this group: a recent analysis of 13,435 individuals who had been diagnosed with long Covid, based on a typical array of symptoms, reported increased risks at 1 year for ischemic heart disease (HR 1.7), ischemic stroke (HR 2.1) and pulmonary embolism (HR 3.6).10
These studies document a fairly consistent, increased risk of cardiovascular complications among Covid-19 survivors. However, important questions remain. Amongst these: does increasing population immunity and vaccination change the risk? Is the magnitude of risk similar for all SARS CoV-2 variants? Does reinfection increase the risk? Answers to some are available. Vaccination appears to attenuate the risk: a Korean study of 592,000 individuals post-Covid-19 infection, showed that vaccination decreased the risk of heart attack and stroke by approximately 50%.11 This finding was replicated in a large US cohort where major adverse cardiovascular events were reduced by a similar amount for full vaccination, and by 25% for partial vaccination.12 Thus, while vaccination does not eliminate long-term complications, it appears to provide a substantial protective effect.
Reinfection may increase the risk of sequelae. In a large US VA cohort of 440,000 Covid survivors, of whom 40,000 had one or more SARS-CoV-2 reinfections, the risk of cardiovascular disorders was increased (HR 3.02), when compared to a single infection.13 Moreover, this risk was not modified by vaccination.
The impact of different variants is less clear. Most of the described studies were conducted in 2020-2021 when delta and pre-delta variants predominated. It is unclear whether similar outcomes would characterize infection with Omicron variants, which remain dominant in most countries since November 2021. Interestingly, the risk of cardiovascular complications in the cohort of Hong Kong survivors described above, where the Omicron was the prevalent strain, was no different than among the comparator UK Biobank cohort, where pre-Omicron strains were prevalent.7
Is there extra long-term mortality after Covid-19 infection? Extra mortality has been reported by several studies.6,8,14–18 A 2021 US analysis of 400 Covid-19 survivors, documented increased mortality (HR 2.5) at 1 year.14 The additional risk was confined to individuals who had been hospitalized. In 2022, 3 studies reported excess mortality in 3 different countries. The first, an Estonian whole-population study of 66,000 Covid-19 survivors, of whom 8% were hospitalized, reported a 3-fold increase in mortality at 12 months.15 Mortality was particularly elevated in the first 5 weeks following infection. For those over age 60, increased mortality persisted until 12 months (HR 2.8). However, for those less than age 60, mortality was not increased after 35 days. The second, an analysis of 690,000 Covid-19 survivors from the TriNetX database also reported increased 1-year mortality risk (HR 1.6).6 This was largely explained by excess deaths in individuals over age 65; below age 45 risk was not increased. For the outpatient cohort the risk of mortality was lower than that of the comparison group (HR 0.46). The third, a study of 25,000 Covid-19 survivors drawn from the UK Biobank, reported increased mortality risk at 20 months, for those with severe Covid infection (HR 14.7), but also an increased risk for those with mild disease (HR 1.23).16 Stratification by age was not provided.
In 2023 4 further studies reported similar, but at times quantitatively different results. Two analyses drew on the UK Biobank cohort. In the first, a prospective evaluation of 7,800 SARS-CoV-2 PCR positive individuals, increased mortality was reported for the study group at 18 months (HR 5.0), when compared to both a contemporary and an historical cohort.17 For the non-severe cases the mortality risk remained elevated (HR 4.8). The second study, already described above– a comparative analysis of 7600 Covid survivors from the UK Biobank and 530,000 Covid survivors in Hong Kong–reported increased mortality (HR 4.16) after 17 months for the former and 28 months for the latter.7 The risk of mortality was higher in the UK than the HK cohort, a difference the authors posited was due to Omicron being the dominant variant in HK during the study period. The risk remained elevated, but less so, for younger cohorts and for mild Covid-19 infections.
Finally, 2 large US studies recently reported mortality at 2 years. In the first, an analysis of 138,000 US veteran Covid-19 survivors with 5.9 million controls, the risk of death for the hospitalized cohort remained elevated at 2 years (HR 1.29).8 In contrast, the risk of death for the outpatient cohort returned to baseline at 6 months. Breakdown of risk by age-group was not provided. The second study, also of US veterans, reported similar findings. In a cohort of 280,000 Covid-19 survivors the risk of death remained elevated at 2 years (HR 2.0).18 The risk was highest in the first 90 days (HR 6.3) and decreased at 6 months (HR 1.18). Thereafter, the risk in Covid-19 survivors was slightly less than the control group (HR 0.89). A post-hoc subgroup analysis examined and refuted the possibility that accelerated mortality in the control group could have explained the lower mortality in Covid-19 survivors. The risk of death in hospitalized individuals remained elevated at 2 years (HR 1.22).
How Plausible is this Information? The studies described above command attention by virtue of their size and the consistency of their findings in different populations, and in different countries. They are also supported by the observations of long-term pathophysiologic abnormalities following SARS-CoV-2 infection, such as ongoing inflammation, persistence of virus, and immune system dysfunction. However, the negative ledger is also substantial. Observational studies such as these, no matter how well-designed, remain open to many types of bias. Reliance on diagnostic codes, prescription records, laboratory results and tallies of clinical visits, to establish disease incidence, is intrinsically error-prone and makes cross-study comparisons difficult. Perhaps more importantly, the cohorts described above were different in many respects, varying from the older, male-predominant cohort of the US VA system to the younger healthier cohort of the UK Biobank. Further, cohorts were constituted during the first year of the pandemic, at a time when healthcare delivery was disrupted, lockdowns were in effect, vaccination and antivirals were largely unavailable, and population immunity levels were low. Thus, it could be argued that the observed outcomes are better explained by an evolving pandemic, rather than solely SARS-CoV-2 infection. This could also explain the most recent reports that after 2 years of follow-up, the risk of both Covid-19 complications and mortality, in most of those infected (i.e., the non-hospitalized), is no longer elevated. It also evident that most of the reported extra mortality is occurring in the early months following infection, where survival curves separate rapidly.6,10,15,18
Are these findings relevant to an insured population? ‘Partially’ is probably the best answer. The most important observation is that hospitalization, and in-particular an intensive care unit admission, is the dominant risk factor for both morbidity and mortality. This risk appears to persist up to 2 years. The second important risk element is the presence of comorbid conditions. This observation raises the interesting question of what exactly causes the extra mortality. Is it due to ‘protracted’ SARS-Co-V-2 infection or is it caused by a recognized complication of Covid-19, such as pulmonary fibrosis or acute kidney injury? Or is it explained by an aggravation of a comorbid illness? Or is it a complication of long Covid? There is a likelihood that all these mechanisms were at play in the cohorts under study.
For non-hospitalized individuals, and those that are healthy, the evidence for extra morbidity and mortality after the first 3-6 months is far from conclusive. For the long Covid cohort, the evidence for additional mortality requires further supporting evidence. As the prevalence of co-morbid conditions is lower in insured populations, one might reasonably expect, based on current evidence, that longer-term morbidity and mortality due to Covid-19 infection will be minimally affected.
References 1.Davis H, McCorkell L, Vogel, J. et al Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol 21, 133–146 (2023). doi.org/10.1038/s41579-022-00846-2
2.Meagher T. Long COVID - An Early Perspective. J Insur Med. 2021 Jan 1;49(1):19–23. doi: 10.17849/insm-49-1-1-5.1. PMID: 33784738.
3.Meagher T. Long COVID – One year On. J Insur Med. 2022 Jan 1;49:1–6. doi: 10.17849/insm-49-3-1-6.1. PMID: 33561352.
4.Meagher T. Long Covid - Into the Third Year. J Insur Med 2023;50(1):54–58. doi.org/10.17849/insm-50-1-54-58.1
5.Xie Y, Xu E, Bowe B et al Long-term cardiovascular outcomes of COVID-19. Nat Med 28, 583–590 (2022). doi.org/10.1038/s41591-022-01689-3
6.Wang W, Wang CY, Wang SI et al Long-term cardiovascular outcomes in COVID-19 survivors among non-vaccinated population: A retrospective cohort study from the TriNetX US collaborative networks. eClinicalMedicine. 2022 Nov;53:101619. doi: 10.1016/j.eclinm.2022.101619
7.Lam I, Wong C, Zhang, R et al Long-term post-acute sequelae of COVID-19 infection: a retrospective, multi-database cohort study in Hong Kong and the UK. eClinicalMedicine Vol. 60 Published: May 11, 2023. doi: doi.org/10.1016/j.eclinm.2023.102000
8.Raisi-Estabragh Z, Cooper J, Salih A, et al Cardiovascular disease and mortality sequelae of COVID-19 in the UK Biobank Heart 2023;109:119–126.
9.Bowe, B., Xie, Y. & Al-Aly, Z. Postacute sequelae of COVID-19 at 2 years. Nat Med 29, 2347–2357 (2023). doi.org/10.1038/s41591-023-02521-2
10.DeVries A, Shambhu S, Sloop S et al One-Year Adverse Outcomes Among US Adults With Post–COVID-19 Condition vs Those Without COVID-19 in a Large Commercial Insurance Database. JAMA Health Forum. 2023;4(3):e230010. doi:10.1001/jamahealthforum.2023.0010
11.Kim Y, Huh K, Park Y et al Association Between Vaccination and Acute Myocardial Infarction and Ischemic Stroke After COVID-19 Infection. JAMA. 2022;328(9):887–889. doi:10.1001/jama.2022.12992
12.Jiang J, Chan L, Kauffman J, et al Impact of Vaccination on Major Adverse Cardiovascular Events in Patients With COVID-19 Infection. J Am Coll Cardiol. 2023 Mar, 81(9):928–930. doi.org/10.1016/j.jacc.2022.12.006
13.Bowe B, Xie, Y, Al-Aly Z. Acute and postacute sequelae associated with SARS-CoV-2 reinfection. Nat Med 28, 2398–2405 (2022). doi.org/10.1038/s41591-022-02051-3
14.Mainous AG, Rooks BJ, Wu, et al COVID-19 post-acute sequelae among adults: 12 month mortality risk. Front Med (Lausanne). 2021;8:778434. doi:10.3389/fmed.2021.778434
15.Uuskula A, Jurgenson T, Pisarev H et al Long-term mortality following SARS-CoV-2 infection: A national cohort study from Estonia. The Lancet Regional Health - Europe 2022;18:100394 Published online 29 April 2022. doi.org/10.1016/j.lanepe.2022.100394
16.Xiang Y, Zhang R, Qiu G. et al Association of Covid-19 with risks of hospitalization and mortality from other disorders post-infection: A study of the UK Biobank. medRxiv doi: doi.org/10.1101/2022.03.23.22272811
17.Wan E, Mathur S, Zhang R et al Association of COVID-19 with short- and long-term risk of cardiovascular disease and mortality: a prospective cohort in UK Biobank, Cardiovascular Research, Volume 119, Issue 8, June 2023, 1718–1727. doi.org/10.1093/cvr/cvac195
18.Iwashyna TJ, Seelye S, Berkowitz TS, et al Late Mortality After COVID-19 Infection Among US Veterans vs Risk-Matched Comparators: A 2-Year Cohort Analysis. JAMA Intern Med. Published online August 21, 2023. doi:10.1001/jamainternmed.2023.3587
#mask up#covid#pandemic#covid 19#wear a mask#public health#coronavirus#sars cov 2#still coviding#wear a respirator
41 notes
·
View notes
Text
Retinal and choroidal vascular drop out in a case of severe phenotype of Flammer Syndrome. Rescue of the ischemic-preconditioning mimicking action of endogenous Erythropoietin (EPO) by off-label intra vitreal injection of recombinant human EPO (rhEPO) by Claude Boscher in Journal of Clinical Case Reports Medical Images and Health Sciences
Abstract
Background: Erythropoietin (EPO) is a pleiotropic anti-apoptotic, neurotrophic, anti-inflammatory, and pro-angiogenic endogenous agent, in addition to its effect on erythropoiesis. Exogenous EPO is currently used notably in human spinal cord trauma, and pilot studies in ocular diseases have been reported. Its action has been shown in all (neurons, glia, retinal pigment epithelium, and endothelial) retinal cells. Patients affected by the Flammer Syndrome (FS) (secondary to Endothelin (ET)-related endothelial dysfunction) are exposed to ischemic accidents in the microcirculation, notably the retina and optic nerve.
Case Presentation: A 54 years old female patient with a diagnosis of venous occlusion OR since three weeks presented on March 3, 2019. A severe Flammer phenotype and underlying non arteritic ischemic optic neuropathy; retinal and choroidal drop-out were obviated. Investigation and follow-up were performed for 36 months with Retinal Multimodal Imaging (Visual field, SD-OCT, OCT- Angiography, Indo Cyanin Green Cine-Video Angiography). Recombinant human EPO (rhEPO)(EPREX®)(2000 units, 0.05 cc) off-label intravitreal injection was performed twice at one month interval. Visual acuity rapidly improved from 20/200 to 20/63 with disparition of the initial altitudinal scotoma after the first rhEPO injection, to 20/40 after the second injection, and gradually up to 20/32, by month 5 to month 36. Secondary cystoid macular edema developed ten days after the first injection, that was not treated via anti-VEGF therapy, and resolved after the second rhEPO injection. PR1 layer integrity, as well as protective macular gliosis were fully restored. Some level of ischemia persisted in the deep capillary plexus and at the optic disc.
Conclusion: Patients with FS are submitted to chronic ischemia and paroxystic ischemia/reperfusion injury that drive survival physiological adaptations via the hypoxic-preconditioning mimicking effect of endogenous EPO, that becomes overwhelmed in case of acute hypoxic stress threshold above resilience limits. Intra vitreal exogenous rhEPO injection restores retinal hypoxic-preconditioning adaptation capacity, provided it is timely administrated. Intra vitreal rhEPO might be beneficial in other retinal diseases of ischemic and inflammatory nature.
Key words : Erythropoietin, retinal vein occlusion, anterior ischemic optic neuropathy, Flammer syndrome, Primary Vascular Dysfunction, anti-VEGF therapy, Endothelin, microcirculation, off-label therapy.
Introduction
Retinal Venous Occlusion (RVO) treatment still carries insufficiencies and contradictions (1) due to the incomplete deciphering of the pathophysiology and of its complex multifactorial nature, with overlooking of factors other than VEGF up-regulation, notably the roles of retinal venous tone and Endothelin-1 (ET) (2-5), and of endothelial caspase-9 activation (6). Flammer Syndrome (FS)( (Primary Vascular Dysfunction) is related to a non atherosclerotic ET-related endothelial dysfunction in a context of frequent hypotension and increased oxidative stress (OS), that alienates organs perfusion, with notably changeable functional altered regulation of blood flow (7-9), but the pathophysiology remains uncompletely elucidated (8). FS is more frequent in females, and does not seem to be expressed among outdoors workers, implying an influence of sex hormons and light (7)(9). ET is the most potent pro-proliferative, pro-fibrotic, pro-oxidative and pro-inflammatory vasoconstrictor, currently considered involved in many diseases other than cardio-vascular ones, and is notably an inducer of neuronal apoptosis (10). It is produced by endothelial (EC), smooth vascular muscles (SVMC) and kidney medullar cells, and binds the surface Receptors ET-A on SVMC and ET-B on EC, in an autocrine and paracrine fashion. Schematically, binding on SVMC Receptors (i.e. through local diffusion in fenestrated capillaries or dysfunctioning EC) and on EC ones (i.e. by circulating ET) induce respectively arterial and venous vasoconstriction, and vasodilation, the latter via Nitrite oxide (NO) synthesis. ET production is stimulated notably by Angiotensin 2, insulin, cortisol, hypoxia, and antagonized by endothelial gaseous NO, itself induced by flow shear stress. Schematically but not exclusively, vascular tone is maintained by a complex regulation of ET-NO balance (8) (10-11). Both decrease of NO and increase of ET production are both a cause and consequence of inflammation, OS and endothelial dysfunction, that accordingly favour vasoconstriction; in addition ET competes for L-arginine substrate with NO synthase, thereby reducing NO bioavailability, a mechanism obviated notably in carotid plaques and amaurosis fugax (reviewed in 11).
Severe FS phenotypes are rare. Within the eye, circulating ET reaches retinal VSMC in case of Blood-Retinal-Barrier (BRB) rupture and diffuses freely via the fenestrated choroidal circulation, notably around the optic nerve (ON) head behind the lamina cribrosa, and may induce all pathologies related to acute ocular blood flow decrease (2-3)(5)(7-9). We previously reported two severe cases with rapid onset of monocular cecity and low vision, of respectively RVO in altitude and non arteritic ischemic optic neuropathy (NAION) (Boscher et al, Société Francaise d'Ophtalmologie and Retina Society, 2015 annual meetings).
Exogenous Recombinant human EPO (rhEPO) has been shown effective in humans for spinal cord injury (12), neurodegenerative and chronic kidney diseases (CKD) (reviewed in 13). Endogenous EPO is released physiologically in the circulation by the kidney and liver; it may be secreted in addition by all cells in response to hypoxic stress, and it is the prevailing pathway induced via genes up-regulation by the transcription factor Hypoxia Inducible Factor 1 alpha, among angiogenesis (VEGF pathway), vasomotor regulation (inducible NO synthase), antioxidation, and energy metabolism (14). EPO Receptor signaling induces cell proliferation, survival and differentiation (reviewed in 13), and targets multiple non hematopoietic pathways as well as the long-known effect on erythropoiesis (reviewed in 15). Of particular interest here, are its synergistic anti-inflammatory, neural antiapoptotic (16) pro-survival and pro-regenerative (17) actions upon hypoxic injury, that were long-suggested to be also indirect, via blockade of ET release by astrocytes, and assimilated to ET-A blockers action (18). Quite interestingly, endogenous EPO’s pleiotropic effects were long-summarized (back to 2002), as “mimicking hypoxic-preconditioning” by Dawson (19), a concept applied to the retina (20). EPO Receptors are present in all retinal cells and their rescue activation targets all retinal cells, i.e. retinal EC, neurons (photoreceptors (PR), ganglion (RGG) and bipolar cells), retinal pigment epithelium (RPE) osmotic function through restoration of the BRB, and glial cells (reviewed in 21), and the optic nerve (reviewed in 22). RhEPO has been tested experimentally in animal models of glaucoma, retinal ischemia-reperfusion (I/R) and light phototoxicity, via multiple routes (systemic, subconjunctival, retrobulbar and intravitreal injection (IVI) (reviewed in 23), and used successfully via IVI in human pilot studies, notably first in diabetic macular edema (24) (reviewed in 25 and 26). It failed to improve neuroprotection in association to corticosteroids in optic neuritis, likely for bias reasons (reviewed in 22). Of specific relation to the current case, it has been reported in NAION (27) (reviewed in 28) and traumatic ON injury (29 Rashad), and in one case of acute severe central RVO (CRVO) (Luscan and Roche, Société Francaise d’Ophtalmologie 2017 annual meeting). In addition EPO RPE gene therapy was recently suggested to prevent retinal degeneration induced by OS in a rodent model of dry Age Macular Degeneration (AMD) (30).
Case Report Presentation
This 54 years female patient was first visited on March 2019 4th, seeking for second opinion for ongoing vision deterioration OR on a daily basis, since around 3 weeks. Sub-central RVO (CRVO) OR had been diagnosed on February 27th; available SD-OCT macular volume was increased with epiretinal marked hyperreflectivity, one available Fluorescein angiography picture showed a non-filled superior CRVO, and a vast central ischemia involving the macular and paraoptic territories. Of note there was ON edema with a para-papillary hemorrage nasal to the disc on the available colour fundus picture.
At presentation on March 4, Best Corrected Visual Acuity (BCVA) was reduced at 20/100 OR (20/25 OS). The patient described periods of acutely excruciating retro-orbital pain in the OR. Intraocular pressure was normal, at 12 OR and 18 OS (pachymetry was at 490 microns in both eyes). The dilated fundus examination was similar to the previous color picture and did not disclose peripheral hemorrages recalling extended peripheral retinal ischemia. Humphrey Visual Field disclosed an altitudinal inferior scotoma and a peripheral inferior scotoma OR and was in the normal range OS, i.e. did not recall normal tension glaucoma OS . There were no papillary drusen on the autofluorescence picture, ON volume was increased (11.77 mm3 OR versus 5.75 OS) on SD-OCT (Heidelberg Engineering®) OR, Retinal Nerve Fiber (RNFL) and RGC layers thicknesses were normal Marked epimacular hypereflectivity OR with foveolar depression inversion, moderately increased total volume and central foveolar thickness (CFT) (428 microns versus 328 OS), and a whitish aspect of the supero-temporal internal retinal layers recalling ischemic edema, were present . EDI CFT was incresead at 315 microns (versus 273 microns OS), with focal pachyvessels on the video mapping . OCT-Angiography disclosed focal perfusion defects in both the retinal and chorio-capillaris circulations , and central alterations of the PR1 layer on en-face OCT
Altogether the clinical picture evoked a NAION with venous sub-occlusion, recalling Fraenkel’s et al early hypothesis of an ET interstitial diffusion-related venous vasoconstriction behind the lamina cribrosa (2), as much as a rupture of the BRB was present in the optic nerve area (hemorrage along the optic disc). Choroidal vascular drop-out was suggested by the severity and rapidity of the VF impairment (31). The extremely rapid development of a significant “epiretinal membrane”, that we interpreted as a reactive - and protective, in absence of cystoid macular edema (CME) - ET 2-induced astrocytic proliferation (reviewed in 32), was as an additional sign of severe ischemia.
The mention of the retro-orbital pain evoking a “ciliary angor”, the absence of any inflammatory syndrome and of the usual metabolic syndrome in the emergency blood test, oriented the etiology towards a FS. And indeed anamnesis collected many features of the FS, i.e. hypotension (“non dipper” profile with one symptomatic nocturnal episode of hypotension on the MAPA), migrains, hypersensitivity to cold, stress, noise, smells, and medicines, history of a spontaneously resolutive hydrops six months earlier, and of paroxystic episods of vertigo (which had driven a prior negative brain RMI investigation for Multiple Sclerosis, a frequent record among FS patients (33) and of paroxystic visual field alterations (7)(9), that were actually recorded several times along the follow-up.
The diagnosis of FS was eventually confirmed in the Ophthalmology Department in Basel University on April 10th, with elevated retinal venous pressure (20 to 25mmHg versus 10-15 OS) (4)(7)(9), reduced perfusion in the central retinal artery and veins on ocular Doppler (respectively 8.3 cm/second OR velocity versus 14.1 mmHg OS, and 3.1/second OR versus 5.9 cm OS), and impaired vasodilation upon flicker light-dependant shear stress on the Dynamic Vessel Analyser testing (7-9). In addition atherosclerotic plaques were absent on carotid Doppler.
On March 4th, the patient was at length informed about the FS, a possible off label rhEPO IVI, and a related written informed consent on the ratio risk-benefits was delivered.
By March 7th, she returned on an emergency basis because of vision worsening OR. VA was unchanged, intraocular pressure was at 13, but Visual Field showed a worsening of the central and inferior scotomas with a decreased foveolar threshold, from 33 to 29 decibels. SD-OCT showed a 10% increase in the CFT volume.
On the very same day, an off label rhEPO IVI OR (EPREX® 2000 units, 0,05 cc in a pre-filled syringe) was performed in the operating theater, i.e. the dose reported by Modarres et al (27), and twenty times inferior to the usual weekly intravenous dose for treatment of chronic anemia secondary to CKD. Intra venous acetazolamide (500 milligrams) was performed prior to the injection, to prevent any increase in intra-ocular pressure. The patient was discharged with a prescription of chlorydrate betaxolol (Betoptic® 0.5 %) two drops a day, and high dose daily magnesium supplementation (600 mgr).
Incidentally the patient developed bradycardia the day after, after altogether instillation of 4 drops of betaxolol only, that was replaced by acetazolamide drops, i.e. a typical hypersensitivity reaction to medications in the FS (7)(9).
Subjective vision improvement was recorded as early as D1 after injection. By March 18 th, eleven days post rhEPO IVI, BCVA was improved at 20/63, the altitudinal scotoma had resolved (Fig. 5), Posterior Vitreous Detachment had developed with a disturbing marked Weiss ring, optic disc swelling had decreased; vasculogenesis within the retinal plexi and some regression of PR1 alterations were visible on OCT-en face. Indeed by 11 days post EPO significant functional, neuronal and vascular rescue were observed, while the natural evolution had been seriously vision threatening.
However cystoid ME (CME) had developed . Indo Cyanin Green-Cine Video Angiography (ICG-CVA) OR, performed on March 23, i.e. 16 days after the rhEPO IVI, showed a persistent drop in ocular perfusion: ciliary and central retinal artery perfusion timings were dramatically delayed at respectively 21 and 25 seconds, central retinal vein perfusion initiated by 35 seconds, was pulsatile, and completed by 50 seconds only (video 3). Choroidal pachyveins matching the ones on SD-OCT video mapping were present in the temporal superior and inferior fields, and crossed the macula; capillary exclusion territories were present in the macula and around the optic disc.
By April 1, 23 days after the rhEPO injection, VA was unchanged, but CME and perfusion voids in the superficial deep capillary plexi and choriocapillaris were worsened, and optic disc swelling had recurred back to baseline, in a context of repeated episodes of systemic hypotension; and actually Nifepidin-Ratiopharm® oral drops (34), that had been delivered via a Temporary Use Authorization from the central Pharmacology Department in Assistance Publique Hopitaux de Paris, had had to be stopped because of hypersensitivity.
A second off label rhEPO IVI was performed in the same conditions on April 3, i.e. approximately one month after the first one.
Evolution was favourable as early as the day after EPO injection 2: VA was improved at 20/40, CME was reduced, and perfusion improved in the superficial retinal plexus as well as in the choriocapillaris. By week 4 after EPO injection 2, CME was much decreased, i.e. without anti VEGF injection. On august 19th, by week 18 after EPO 2, perfusion on ICG-CVA was greatly improved , with ciliary timing at 18 seconds, central retinal artery at 20 seconds and venous return from 23 to 36 seconds, still pulsatile. Capillary exclusion territories were visible in the macula and temporal to the macula after the capillary flood time that went on by 20.5 until 22.5 seconds (video 4); they were no longer persistent at intermediate and late timings.
Last complete follow-up was recorded on January 7, 2021, at 22 months from EPO injection 2. BCVA was at 20/40, ON volume had dropped at 7.46 mm3, a sequaelar superior deficit was present in the RNFL with some corresponding residual defects on the inferior para central Visual Field , CFT was at 384 mm3 with an epimacular hyperreflectivity without ME, EDI CFT was dropped at 230 microns. Perfusion on ICG-CVA was not normalized, but even more improved, with ciliary timing at 15 seconds, central retinal artery at 16 seconds and venous return from 22 to 31 seconds, still pulsatile , indicating that VP was still above IOP. OCT-A showed persisting perfusion voids, especially at the optic disc and within the deep retinal capillary plexus. The latter were present at some degree in the OS as well . Choriocapillaris and PR1 layer were dramatically improved.
Last recorded BCVA was at 20/32 by February 14, 2022, at 34 months from EPO 2. SD-OCT showed stable gliosis hypertrophy and mild alterations of the external layers .
Discussion
What was striking in the initial clinical phenotype of CRVO was the contrast between the moderate venous dilation, and the intensity of ischemia, that were illustrating the pioneer hypothesis of Professor Flammer‘s team regarding the pivotal role of ET in VO (2), recently confirmed (3)(35), i.e. the local venous constriction backwards the lamina cribrosa, induced by diffusion of ET-1 within the vascular interstitium, in reaction to hypoxia. NAION was actually the primary and prevailing alteration, and ocular hypoperfusion was confirmed via ICG-CVA, as well as by the ocular Doppler performed in Basel. ICG-CVA confirmed the choroidal drop-out suggested by the severity of the VF impairment (31) and by OCT-A in the choriocapillaris. Venous pressure measurement, which instrumentation is now available (8), should become part of routine eye examination in case of RVO, as it is key to guide cases analysis and personalized therapeutical options.
Indeed, the endogenous EPO pathway is the dominant one activated by hypoxia and is synergetic with the VEGF pathway, and coherently it is expressed along to VEGF in the vitreous in human RVO (36). Diseases develop when the individual limiting stress threshold for efficient adaptative reactive capacity gets overwhelmed. In this case by Week 3 after symtoms onset, neuronal and vascular resilience mechanisms were no longer operative, but the BRB, compromised at the ON, was still maintained in the retina.
As mentioned in the introduction, the scientific rationale for the use of EPO was well demonstrated by that time, as well as the capacities of exogenous EPO to mimic endogenous EPO vasculogenesis, neurogenesis and synaptogenesis, restoration of the balance between ET-1 and NO. Improvement of chorioretinal blood flow was actually illustrated by the evolution of the choriocapillaris perfusion on repeated OCT-A and ICG-CVA. The anti-apoptotic effect of EPO (16) seems as much appropriate in case of RVO as the caspase-9 activation is possibly another overlooked co-factor (6).
All the conditions for translation into off label clinical use were present: severe vision loss with daily worsening and unlikely spontaneous favourable evolution, absence of toxicity in the human pilot studies, of contradictory comorbidities and co-medications, and of context of intraocular neovascularization that might be exacerbated by EPO (37).
Why didn’t we treat the onset of CME by March 18th, i.e. eleven days after EPO IVI 1, by anti-VEGF therapy, the “standard-of-care” in CME for RVO ?
In addition to the context of functional, neuronal and vascular improvements obviated by rhEPO IVI by that timing in the present case, actually anti VEGF therapy does not address the underlying causative pathology. Coherently, anti-VEGF IVI : 1) may not be efficient in improving vision in RVO, despite its efficiency in resolving/improving CME (usually requiring repeated injections), as shown in the Retain study (56% of eyes with resolved ME continued to loose vision)(quoted in (1) 2) eventually may be followed by serum ET-1 levels increase and VA reduction (in 25% of cases in a series of twenty eyes with BRVO) (38) and by increased areas of non perfusion in OCT-A (39). Rather did we perform a second hrEPO IVI, and actually we consider open the question whether the perfusion improvement, that was progressive, might have been accelerated/improved via repeated rhEPO IVI, on a three to four weeks basis.
The development of CME itself, involving a breakdown of the BRB, i.e. of part of the complex retinal armentorium resilience to hypoxia, was somewhat paradoxical in the context of improvement after the first EPO injection, as EPO restores the BRB (24), and as much as it was suggested that EPO inhibits glial osmotic swelling, one cause of ME, via VEGF induction (40). Possible explanations were: 1) the vascular hyperpermeability induced by the up-regulation of VEGF gene expression via EPO (41) 2) the ongoing causative disease, of chronic nature, that was obviated by the ICG-CVA and the Basel investigation, responsible for overwhelming the gliosis-dependant capacity of resilience to hypoxia 3) a combination of both. I/R seemed excluded: EPO precisely mimics hypoxic reconditioning as shown in over ten years publications, including in the retina (20), and as EPO therapy is part of the current strategy for stabilization of the endothelial glycocalix against I/R injury (42-43). An additional and not exclusive possible explanation was the potential antagonist action of EPO on GFAP astrocytes proliferation, as mentioned in the introduction (18), that might have counteracted the reactive protective hypertrophic gliosis, still fully operative prior to EPO injection, and that was eventually restored during the follow-up, where epiretinal hyperreflectivity without ME and ongoing chronic ischemia do coincide (Fig. 6 and video 6), as much as it is unlikely that EPO’s effect would exceed one month (cf infra). Inhibition of gliosis by EPO IVI might have been also part of the mechanism of rescue of RGG, compromised by gliosis in hypoxic conditions (44). Whatever the complex balance initially reached, then overwhelmed after EPO IVI 1, the challenge was rapidly overcome by the second EPO IVI without anti-VEGF injection, likely because the former was powerful enough to restore the threshold limit for resilience to hypoxia, that seemed no longer reached again during the relapse-free follow-up. Of note, this “epiretinal membrane “, which association to good vision is a proof of concept of its protective effect, must not be removed surgically, as it would suppress one of the mecanisms of resilience to hypoxia.
To our best knowledge, ICG-CVA was never reported in FS; it allows real time evaluation of the ocular perfusion and illustration of the universal rheological laws that control choroidal blood flow as well. Pachyveins recall a “reverse” veno-arteriolar reflex in the choroidal circulation, that is NO and autonomous nervous system-dependant, and that we suggested to be an adaptative choroidal microcirculation process to hypoxia (45). Their persistence during follow-up accounts for a persisting state of chronic ischemia.
The optimal timing for reperfusion via rhEPO in a non resolved issue:
in the case reported by Luscan and Roche, rhEPO IVI was performed on the very same day of disease onset, where it induced complete recovery from VA reduced at counting fingers at 1 meter, within 48 hours. This clinical human finding is on line with a recent rodent stroke study that established the timings for non lethal versus lethal ischemia of the neural and vascular lineages, and the optimized ones for beneficial reperfusion: the acute phase - from Day 1 where endothelial and neural cells are still preserved, to Day 7 where proliferation of pericytes and Progenitor Stem Cells are obtainable - and the chronic stage, up to Day 56, where vasculogenesis, neurogenesis and functional recovery are still possible, but with uncertain efficiency (46). In our particular case, PR rescue after rhEPO IVI 1 indicated that Week 3 was still timely. RhEPO IVI efficacy was shown to last between one (restoration of the BRB) and four weeks (antiapoptotic effect) in diabetic rats (24). The relapse after Week 3 post IVI 1 might indicate that it might be approximately the interval to be followed, should repeated injections be necessary.
The bilateral chronic perfusion defects on OCT-A at last follow-up indicate that both eyes remain in a condition of chronic ischemia and I/R, where endogenous EPO provides efficient ischemic pre-conditioning, but is potentially susceptible to be challenged during episodes of acute hypoxia that overwhelm the resilience threshold.
Conclusion
The present case advocates for individualized medicine with careful recording of the medical history, investigation of the systemic context, and exploiting of the available retinal multimodal imaging for accurate analytical interpretation of retinal diseases and their complex pathophysiology. The Flammer Syndrome is unfortunately overlooked in case of RVO; it should be suspected clinically in case of absence of the usual vascular and metabolic context, and in case of elevated RVP. RhEPO therapy is able to restore the beneficial endogenous EPO ischemic pre-conditioning in eyes submitted to challenging acute hypoxia episodes in addition to chronic ischemic stress, as in the Flammer Syndrome and fluctuating ocular blood flow, when it becomes compromised by the overwhelming of the hypoxic stress resilience threshold. The latter physiopathological explanation illuminates the cases of RVO where anti-VEGF therapy proved functionally inefficient, and/or worsened retinal ischemia. RhEPO therapy might be applied to other chronic ischemia and I/R conditions, as non neo-vascular Age Macular Degeneration (AMD), and actually EPO was listed in 2020 among the nineteen promising molecules in AMD in a pooling of four thousands (47).
#off-label therapy#JCRMHS#anti-VEGF therapy#Erythropoietin#Journal of Clinical Case Reports Medical Images and Health Sciences impact factor.#Primary Vascular Dysfunction
2 notes
·
View notes
Text
Exploring Minimally Invasive Treatments for May-Thurner Syndrome
The exploration of minimally invasive treatments for May-Thurner Syndrome represents a significant advancement in vascular medicine. Procedures such as endovascular stenting, balloon angioplasty, and catheter-directed thrombolysis offer effective alternatives to traditional surgery, providing patients with safer options and quicker recovery. For more details, visit our website.
#deep vein thrombosis st augustine#superficial venous reflux disease symptoms#end stage renal disease treatment st augustine
0 notes
Text
Global Thrombectomy Devices Market Projected for 6–8% CAGR Growth, Powered by Tech by 2029
The global thrombectomy devices market is projected to grow at a CAGR of 6-8% from 2024 to 2029. This growth is primarily driven by an increasing prevalence of stroke and cardiovascular diseases, demand for minimally invasive procedures, and technological advancements in thrombectomy devices. Expansion in emerging markets, combined with an aging population prone to vascular diseases, is expected to further propel market growth.
The thrombectomy devices market focuses on devices used to remove clots from blood vessels to restore normal blood flow, critical in treating stroke and heart attack patients. This market includes diverse thrombectomy techniques such as aspiration, mechanical, and rheolytic, providing options for minimally invasive clot retrieval. Innovations like aspiration catheter systems with improved clot removal efficiency and stent retriever advancements support detailed, effective vascular treatment across hospitals and specialized clinics globally.
Download a free sample report now 👉 https://meditechinsights.com/global-thrombectomy-devices-market/request-sample/
Rising Demand for Thrombectomy in Stroke and Cardiovascular Procedures
The rise in the global burden of stroke and cardiovascular conditions drives the thrombectomy devices market. Real-time, minimally invasive thrombectomy procedures provide better outcomes and recovery times. Cardiovascular diseases accounts for approximately one third of all global deaths, with ischemic stroke as a leading cause. National initiatives such as the "Billion Hearts" program in the U.S. and substantial R&D investments in stroke care underscore the ongoing need for effective treatment tools like thrombectomy. Technological advancements, such as next-generation aspiration catheters and stent retrievers, support more precise clot removal, fueling the market growth.
Technological Advancements to Drive Thrombectomy Devices Market Growth
Technological advancements are driving the growth of the thrombectomy devices market, as companies introduce innovative devices that improve safety, efficiency, and clinical outcomes. For instance, Penumbra Inc. launched the Lightning Flash system in 2023, integrating intelligent aspiration technology that uses pressure and flow-based algorithms to detect and remove large clots, especially for deep vein thrombosis and pulmonary embolism cases. Inari Medical followed suit in 2023 with the RevCore catheter, specifically designed for venous in-stent thrombosis, alongside the Triever16 Curve, which enhances precision for both pulmonary embolism and peripheral thrombectomy treatments. These product launches highlight the industry’s focus on developing versatile and advanced solutions, fueling adoption across vascular and neurological applications.
Competitive Landscape Analysis
In the competitive landscape of the thrombectomy devices market, several prominent players drive innovation and market expansion through strategic investments, partnerships, and technological advancements. Key companies include Medtronic; Boston Scientific; Stryker Corporation; Penumbra Inc.; Edwards Lifesciences; Abbott Laboratories; Terumo Corporation; AngioDynamics, Inc.; Spectranetics Corporation; and Inari Medical; among others. These firms focus on improving device efficacy, expanding product offerings, and forming partnerships to broaden their portfolios and global reach.
Download a sample report for in-depth competitive insightshttps://meditechinsights.com/global-thrombectomy-devices-market/request-sample/
Market Segmentation
This report by Medi-Tech Insights provides the size of the global thrombectomy devices market at the regional- and country-level from 2022 to 2029. The report further segments the market based on device type, application, and end user.
Market Size & Forecast (2022-2029), By Device Type, USD Billion
Mechanical/Fragmentation Thrombectomy Devices
Aspiration Thrombectomy Devices
Rheolytic/Hydrodynamic Thrombectomy Devices
Ultrasound-assisted Thrombectomy Devices
Others
Market Size & Forecast (2022-2029), By Application, USD Billion
Neurovascular Thrombectomy
Cardiovascular Thrombectomy
Peripheral Thrombectomy
Market Size & Forecast (2022-2029), By End User, USD Billion
Hospitals and Health Systems
Ambulatory Surgical Centers (ASCs)
Others
Market Size & Forecast (2022-2029), By Region, USD Billion
North America
US
Canada
Europe
Germany
France
UK
Italy
Spain
Rest of Europe
Asia Pacific
China
India
Japan
Rest of Asia Pacific
Latin America
Middle East & Africa
About Medi-Tech Insights
Medi-Tech Insights is a healthcare-focused business research & insights firm. Our clients include Fortune 500 companies, blue-chip investors & hyper-growth start-ups. We have completed 100+ projects in Digital Health, Healthcare IT, Medical Technology, Medical Devices & Pharma Services in the areas of market assessments, due diligence, competitive intelligence, market sizing and forecasting, pricing analysis & go-to-market strategy. Our methodology includes rigorous secondary research combined with deep-dive interviews with industry-leading CXO, VPs, and key demand/supply side decision-makers.
Contact:
Ruta Halde Associate, Medi-Tech Insights +32 498 86 80 79 [email protected]
0 notes
Text
Advanced Foam Sclerotherapy: A Modern Solution for Varicose and Spider Veins
Venous conditions like varicose veins, spider veins, and chronic venous insufficiency are not merely cosmetic concerns — they often signal underlying vascular issues. With advancements in vein treatment, patients now have access to less invasive and highly effective options. One of the leading techniques today is Advanced Foam Sclerotherapy, offering improved outcomes and faster recovery. In this blog, we’ll dive into how this treatment works, its benefits, the procedure, who qualifies, expected outcomes, and what recovery looks like.
What Is Foam Sclerotherapy?
Foam sclerotherapy builds upon traditional sclerotherapy by replacing the liquid solution with a specialized foam. This foam is created by blending a sclerosant (the chemical agent that treats the vein) with air or gas. The resulting foam is thicker and more stable, allowing it to better displace blood within the vein and maintain longer contact with the vein walls.
When the foam is injected, it irritates the vein lining, causing the vein to collapse and eventually be absorbed by the body. Advanced foam sclerotherapy enhances this technique by using ultrasound guidance to treat veins with greater precision and effectiveness.
How Advanced Foam Sclerotherapy Is Performed
The procedure involves a series of carefully executed steps:
Foam Preparation: A sclerosant is mixed with gas to form a dense, consistent foam — often using methods like the Tessari technique.
Ultrasound Imaging: Real-time ultrasound technology maps the affected veins, helping the physician guide the treatment with precision.
Targeted Injection: The foam is injected into the diseased veins using a fine needle or catheter, ensuring the foam thoroughly coats the vein walls.
Vein Closure and Absorption: The treated veins collapse and seal shut. Over time, the body naturally absorbs them, and blood reroutes to healthier veins.
Benefits of Advanced Foam Sclerotherapy
Compared to traditional vein treatments, this technique offers several important advantages:
Effectiveness for Larger Veins: Foam is particularly effective at treating larger varicose veins that liquids may not address adequately.
Precision with Ultrasound Guidance: Deep and non-visible veins can be treated accurately without guesswork.
Minimally Invasive: No surgical incisions are needed, and local anesthesia is typically sufficient.
Enhanced Action: The foam displaces blood inside the vein, allowing the sclerosant to work directly on the vein wall without dilution.
Quick Recovery Time: Many patients return to normal daily activities within 24–48 hours.
Improved Cosmetic Outcomes: Visible improvements in the appearance of the legs are common after healing.
Who Is a Good Candidate for Advanced Foam Sclerotherapy?
This treatment is best suited for:
People with moderate to large varicose veins
Individuals seeking non-surgical treatment for recurring vein issues
Patients in good general health who are eligible for outpatient procedures
Patients who are pregnant, have active deep vein thrombosis (DVT), or certain health conditions may not qualify. A vein specialist will conduct a full assessment to determine if foam sclerotherapy is the right choice.
What to Expect During the Treatment Process
Before the Procedure
A full evaluation, often including a duplex ultrasound, is performed to map the vein system.
Patients are typically advised to avoid applying lotion or moisturizer on the legs before treatment.
Medication adjustments may be recommended in some cases.
During the Procedure
The treatment area is cleaned and prepped.
Guided by ultrasound, the physician injects the foam directly into the problematic veins.
Multiple veins can often be treated in one session.
Compression stockings are applied immediately after the procedure.
After the Procedure
Patients are encouraged to walk for about 15–30 minutes immediately afterward to promote blood flow.
Compression stockings should be worn as directed, usually for one to two weeks.
Strenuous activities, hot baths, and sun exposure to the treated areas should be avoided for a short period.
Recovery and Results
The recovery experience is usually straightforward:
Mild side effects such as bruising, slight swelling, or temporary skin discoloration may occur but generally resolve within a few weeks.
Symptom relief, such as reduced aching, heaviness, and swelling, is often noticeable within a few weeks post-treatment.
Cosmetic improvements become more visible over several months as the veins fade away.
Some patients may require additional sessions depending on the severity of their vein condition.
Potential Risks and Side Effects
Although considered safe and low-risk, advanced foam sclerotherapy does have potential side effects, including:
Minor allergic reactions to the sclerosant (rare)
Temporary hyperpigmentation (skin darkening)
Mild inflammation or phlebitis
Rare occurrences of visual disturbances or skin ulcerations
Choosing an experienced vein specialist can significantly minimize these risks and ensure the best possible outcome.
Why Advanced Foam Sclerotherapy Is Transforming Vein Care
This technique has quickly become a preferred choice for treating both cosmetic and symptomatic vein issues. Its minimally invasive nature, combined with greater precision thanks to ultrasound guidance, leads to high success rates and excellent patient satisfaction.
Advanced foam sclerotherapy enables effective treatment with minimal downtime, making it a powerful alternative to more invasive surgical methods.
Conclusion
Advanced foam sclerotherapy represents a significant advancement in the treatment of varicose and spider veins. By combining precision imaging, modern techniques, and patient-centered care, it offers a safe, effective, and minimally invasive option for restoring both comfort and appearance to your legs. If you are considering vein treatment, consulting with a qualified vein specialist can help you determine whether this cutting-edge procedure is right for you.
0 notes
Text
Veins Treatment Singapore: What You Need to Know from a Vascular Expert
“Over 30% of the adult population suffers from chronic venous disease—but most have no idea why their legs hurt.” — Dr. Imran Nawaz, Vascular Specialist, SG Vascular Centre
When it comes to veins treatment Singapore, too many people wait until the symptoms become unbearable—pain, swelling, visible veins, or even skin changes. But modern vascular care has advanced significantly. Today, there are minimally invasive procedures that offer relief, restore comfort, and prevent future complications. So why wait?
Let’s break down everything you need to know, from causes and symptoms to the types of treatments available and how to choose the right doctor.
What Causes Vein Problems?
Venous issues, especially varicose veins, arise when valves in your veins malfunction. Instead of directing blood back to your heart, faulty valves cause blood to pool—most often in the legs. Genetics, age, pregnancy, standing for long periods, and even obesity can all contribute to venous insufficiency.
Recognizing the Symptoms Early Matters
Sometimes, signs of vein disease are subtle:
A heavy or aching sensation in the legs
Swollen ankles or feet, especially at the end of the day
Itching or burning near the veins
Discolored skin or visible, twisted veins
Catch these early, and your journey toward veins treatment Singapore can be far smoother and less invasive.
Types of Veins Treatment Available in Singapore
1. Endovenous Laser Treatment (EVLT)
EVLT is one of the most effective and widely used methods. A laser fiber is inserted into the vein, and laser energy seals it shut. It’s minimally invasive and performed under local anesthesia. Recovery is fast—many return to work the next day.
2. Radiofrequency Ablation (RFA)
Similar to EVLT, this technique uses radiofrequency energy to collapse the problematic vein. It’s equally effective, and your vascular surgeon will recommend the best option based on your vein’s size and anatomy.
3. Ultrasound-Guided Foam Sclerotherapy
This involves injecting a special foam into the vein, causing it to close. It's typically used for smaller veins or in combination with other treatments. It’s quick, and often requires no downtime.
Venaseal™ Closure System
A medical-grade adhesive is used to seal the vein. No heat, no tumescent anesthesia, and minimal discomfort—this technique is gaining popularity for patients looking for convenience and comfort.
Who Should You Trust?
Choosing the right varicose veins doctor Singapore makes all the difference. Look for a vascular surgeon—someone trained in both diagnosis and a wide spectrum of treatment options. At SG Vascular Centre, Dr. Imran Nawaz leads with years of experience in complex vascular cases. His approach is holistic, combining evidence-based treatments with a deep understanding of patient care.
What to Expect During Your First Consultation
You’ll begin with a comprehensive evaluation that includes:
A detailed medical history
Physical examination
Duplex ultrasound to assess the veins
From there, your doctor will recommend the most suitable veins treatment Singapore tailored to your condition and lifestyle. Many treatments are done as day surgeries—quick, safe, and effective.
Recovery Tips and Aftercare
After your procedure:
Wear compression stockings for at least two weeks
Walk regularly to promote circulation
Avoid strenuous activity for a few days
Monitor for bruising or mild discomfort—it’s normal and temporary
Most importantly? Follow up with your specialist. Proper aftercare ensures long-lasting results.
Why Early Treatment Pays Off
Leaving vein issues untreated can lead to:
Skin ulcers
Blood clots (deep vein thrombosis)
Persistent pain and swelling
Reduced quality of life
Early veins treatment Singapore helps you avoid these complications. More than that, it improves your comfort, confidence, and mobility.
Ready to Take the First Step?
If your legs ache, feel heavy, or you’re noticing visible veins, don’t ignore the signs. Seeking professional help from a trusted varicose veins doctor Singapore is your first move toward healthier legs and a better life.
Book your consultation today at SG Vascular Centre—because your legs deserve expert care.
0 notes
Text
Anticoagulants Market Overview: Emerging Trends and Future Outlook in Global Therapeutic Solutions
The anticoagulants market has been witnessing rapid expansion, driven by rising incidences of cardiovascular diseases, venous thromboembolism (VTE), and atrial fibrillation across the globe. As healthcare systems evolve and focus more on preventive and long-term care, the demand for blood thinning medications, including both traditional and novel anticoagulants, is surging. With the emergence of innovative therapeutic approaches, increased healthcare awareness, and expanding elderly population, the global anticoagulants market is positioned for substantial growth in the coming years. This article explores the current landscape, emerging trends, and the future outlook of this vital sector within the global pharmaceutical and therapeutic industry.

Rising Prevalence of Cardiovascular Disorders
Cardiovascular diseases (CVDs) remain the leading cause of death globally, accounting for approximately 17.9 million lives each year, according to the World Health Organization (WHO). The strong association between these conditions and thrombotic events has led to an increased demand for antithrombotic therapies, particularly anticoagulants. As populations age and sedentary lifestyles become more common, the risk factors for CVDs — including obesity, diabetes, and hypertension — are contributing to the growing need for chronic anticoagulation treatment.
Shift Toward Novel Oral Anticoagulants (NOACs)
One of the most significant shifts in the market is the increasing adoption of novel oral anticoagulants (NOACs) over traditional therapies such as warfarin. NOACs, including apixaban, rivaroxaban, dabigatran, and edoxaban, offer advantages such as predictable pharmacokinetics, fewer dietary restrictions, and reduced need for regular monitoring. These benefits have contributed to a surge in prescription rates, especially in developed markets. In particular, NOACs have gained traction in managing non-valvular atrial fibrillation and preventing stroke and deep vein thrombosis.
The convenience of NOACs also supports adherence to long-term therapy, an essential factor in achieving favorable patient outcomes. Pharmaceutical companies are heavily investing in expanding their NOAC product lines and conducting comparative studies to enhance their market positioning.
Technological Integration and Digital Health Solutions
The integration of digital health technologies is reshaping the anticoagulants market. Tools such as mobile health apps, remote patient monitoring, and smart pill dispensers are being increasingly used to support medication adherence and minimize the risk of complications from missed doses. Furthermore, advanced diagnostic tools are enabling personalized anticoagulation therapy, improving both efficacy and safety.
Pharmacogenomics is another area with promising potential. By tailoring anticoagulant therapy based on a patient's genetic profile, healthcare providers can significantly reduce adverse drug reactions and optimize treatment outcomes. This move toward precision medicine is likely to create new opportunities in the market.
Regional Insights and Market Penetration
North America continues to dominate the global anticoagulants market, attributed to its high healthcare expenditure, well-established medical infrastructure, and robust adoption of novel therapeutics. However, the Asia-Pacific region is emerging as a high-growth market, fueled by increasing healthcare awareness, urbanization, and growing incidences of thromboembolic diseases.
Countries such as India, China, and South Korea are witnessing a rising demand for affordable and effective anticoagulation therapies. Government initiatives to strengthen healthcare access and boost pharmaceutical R&D are further supporting the market's expansion in these regions.
Regulatory Developments and Competitive Landscape
Regulatory agencies like the FDA and EMA are playing a crucial role in shaping the anticoagulants market by accelerating approvals for innovative drugs and fostering competition. The approval of generics and biosimilars is expected to enhance affordability and widen patient access. Additionally, post-market surveillance and pharmacovigilance systems are being strengthened to monitor the safety profiles of these high-risk medications.
Major pharmaceutical players such as Pfizer, Bristol Myers Squibb, Bayer AG, Boehringer Ingelheim, and Johnson & Johnson continue to dominate the anticoagulants space. These companies are actively engaging in strategic collaborations, mergers, and acquisitions to diversify their portfolios and expand their global reach.
Future Outlook
The future of the anticoagulants market looks promising, with several pipeline drugs under development that aim to provide better efficacy and safety profiles. The trend toward combination therapies, extended-release formulations, and integration of AI in drug discovery are expected to further revolutionize the market.
Moreover, the expansion of telemedicine and digital therapeutics will continue to influence how anticoagulation therapy is prescribed and managed. As patient-centric care becomes more mainstream, the role of technology in optimizing treatment regimens and monitoring will be indispensable.
Conclusion
The anticoagulants market is undergoing a profound transformation, characterized by technological innovation, shifting treatment paradigms, and increased global awareness of thromboembolic conditions. As demand grows across both developed and emerging economies, stakeholders in the pharmaceutical and healthcare sectors must align their strategies to address evolving patient needs. With continued investment in R&D, enhanced regulatory support, and the rise of digital health, the future of anticoagulation therapy promises improved outcomes and expanded accessibility worldwide.
0 notes
Text
Treatments for Venous Disease - We focus on quality care at a personal level

If you or a loved one is dealing with visible, uncomfortable, or painful veins in the legs, you're not alone. Varicosity, more commonly known as varicose veins, affects millions of Americans every year. Fortunately, residents of Maryland have access to some of the best vascular specialists and vein treatment centers in the region. From minimally invasive procedures to personalized treatment plans, Varicosity Maryland is advanced, accessible, and effective.
What is Varicosity?
Varicosity refers to the condition where veins—typically in the legs—become enlarged, swollen, and twisted due to malfunctioning valves. These damaged valves allow blood to pool instead of circulating efficiently back to the heart, leading to bulging, bluish veins just beneath the skin's surface.
Common symptoms of varicosity include:
Swollen, twisted, or rope-like veins (especially in the legs)
Aching, heaviness, or throbbing in the legs
Muscle cramping or swelling in the lower legs
Itchy or irritated skin around the veins
Discoloration or skin ulcers in advanced cases
While often considered a cosmetic issue, Varicosity Maryland can signal more serious underlying vascular problems and should not be ignored—especially if symptoms are affecting your quality of life.
Why Choose Varicosity Treatment in Maryland?
Maryland is home to a wide range of top-rated vein clinics, vascular surgeons, and board-certified specialists who offer comprehensive care for varicose veins and related conditions like spider veins, chronic venous insufficiency (CVI), and deep vein thrombosis (DVT).
Here’s why varicosity care in Maryland stands out:
1. Advanced Diagnostic Tools
Clinics across Maryland utilize state-of-the-art duplex ultrasound technology to diagnose vein disease at its source. This ensures you receive an accurate assessment and the most effective treatment plan.
2. Minimally Invasive Treatments
Gone are the days of painful vein stripping surgeries. Today’s Maryland vein centers offer quick, in-office procedures that are virtually painless and require little to no downtime, such as:
Endovenous Laser Therapy (EVLT)
Radiofrequency Ablation (RFA)
Sclerotherapy
VenaSeal™ Closure System
Ambulatory Phlebectomy
3. Experienced Vein Specialists
From Baltimore to Bethesda and Annapolis to Rockville, Maryland is home to some of the East Coast’s most experienced vein doctors and vascular surgeons. Many are board-certified and affiliated with major hospital systems or academic centers, ensuring you receive expert-level care.
4. Personalized, Patient-Centered Care
Varicose veins affect everyone differently, and Maryland’s top vein clinics offer customized treatment plans based on your symptoms, lifestyle, and medical history. Whether you’re managing chronic pain or seeking cosmetic improvement, your care will be tailored to your goals.
5. Insurance-Friendly Services
Most Varicosity Maryland treatments are covered by insurance if deemed medically necessary. Maryland clinics typically offer insurance verification, payment plans, and assistance navigating coverage so that financial concerns don’t delay your treatment.
Who is at Risk for Varicosity?
While anyone can develop varicose veins, certain factors increase your risk:
Age: Aging causes vein walls and valves to weaken.
Gender: Women are more likely to develop varicosity due to hormonal changes.
Pregnancy: Increased blood volume and pressure on the pelvic veins raise risk.
Family History: Genetics can play a major role.
Prolonged Standing or Sitting: Occupations or lifestyles that limit leg movement can contribute to vein problems.
Obesity or Inactivity: These can increase venous pressure and reduce circulation.
When Should You Seek Treatment for Varicosity in Maryland?
While many people seek varicosity treatment for cosmetic reasons, it's important to consult a specialist if you’re experiencing:
Persistent leg pain or heaviness
Swelling in the lower legs or ankles
Skin changes (discoloration, dryness, or ulcers)
Bleeding or open sores near affected veins
Signs of clotting or inflammation (warmth, redness, or tenderness)
Delaying treatment can lead to more serious complications such as skin infections, deep vein thrombosis, or venous ulcers.
Where to Find Trusted Varicosity Maryland
You can find expert varicose vein care throughout the state, including in cities like:
Baltimore
Annapolis
Silver Spring
Columbia
Bethesda
Rockville
Frederick
Towson
Top-rated vein treatment centers and vascular clinics in Maryland often offer free consultations, telehealth appointments, and same-day procedures to make care convenient and stress-free.
Tips for Choosing a Varicose Vein Clinic in Maryland
When selecting a provider for varicosity treatment in Maryland, consider the following:
Board certification in vascular surgery or phlebology
Experience with modern, minimally invasive vein treatments
Positive patient reviews and reputation
Comfortable, accredited facilities
Insurance support and transparent pricing
Conclusion
If you’re struggling with unsightly or painful varicose veins, Maryland offers some of the best resources in the country for diagnosis and treatment. With advanced medical technology, experienced specialists, and a strong emphasis on patient comfort, Varicosity Maryland is comprehensive, compassionate, and highly effective. Don’t wait to feel better—reach out to a local vein specialist and take the first step toward healthier, pain-free legs today.
0 notes