#Clinical Spirometer
Explore tagged Tumblr posts
Text

#best product#Medical Device#Lung Function Test#Pulmonary Function Testing (PFT)#Respiratory Health#Spirometry#Breathing Assessment#Airflow Measurement#COPD Monitoring#Asthma Management#Pulmonary Rehabilitation#Vital Capacity Measurement#Peak Expiratory Flow (PEF)#Forced Expiratory Volume (FEV)#Inspiratory Capacity (IC)#Portable Spirometer#Digital Spirometer#Home Use Spirometer#Clinical Spirometer#Bluetooth Spirometer#Lung Monitoring Device
0 notes
Text
to be specific the historic origin of this is the antebellum south. It’s a product of american chattel slavery. There may be other misconceptions but “thicker skin” and “less pain”, are direct products of medical support for slavery.
Spirometry (a measure of lung function) is also affected by this. Spirometry has a “race correction” factor applied specifically for black patients, based on racial philosophy and science originated by Thomas Jefferson, and further propagated by slave owners. The theory went that african americans naturally had weaker lungs, which needed to be strengthen by hard work.




the American Thoracic Association recommended that the race correction factor be eliminated from spirometric calculations in 2022.
So in March of 2020, when a novel virus infamous for its effects on the respiropulmonary was discovered and studied intently, and data was collected about its effects on lung function, the majority of medical institutions and researchers were still being “corrected” to account for “the naturally inferior vital forces” of “negroes”.
I have a classmate who did pulmonologist research for years and used the race correction factor in calculations for years. The patients race is just one of many factors and numbers the computer asks for before producing the spirometry results.
These numbers are also used clinically to determine whether patients with obstructive lung diseases qualify for disability benefits, for example.
So when a patient goes to get spirometered as part of their work up for, say, emphysema, white patients are measured accurately, and black patients are measured, corrected by a factor of 10% or so to account for their “weaker lungs”, and then diagnosed.
A lot of the clinicians I speak to on a regular basis don’t know about the racial history of spirometry, nor the ATA’s new guidelines. They can’t tell me off the top of their head whether or not it’s a part of their calculations, because it’s just something the computer handles.
I hope pulmonologists are better educated. I haven’t talked to enough of them to say.

132K notes
·
View notes
Text
Respiratory Care Device Market Regulatory Trends and Compliance Challenges to 2033
Introduction
Respiratory care devices are critical tools used in the diagnosis, treatment, and management of respiratory disorders such as asthma, chronic obstructive pulmonary disease (COPD), sleep apnea, and acute respiratory distress syndrome (ARDS). With increasing air pollution, an aging population, and a growing prevalence of respiratory diseases globally, the respiratory care device market has witnessed substantial growth in recent years.
This article explores the current state of the respiratory care device market, major trends, driving factors, challenges, and expected developments through 2032.
Market Overview
The global respiratory care device market was valued at approximately USD 21.5 billion in 2023 and is projected to grow to USD 42.8 billion by 2032, registering a CAGR of 7.9% during the forecast period. The market includes devices used for therapeutic, monitoring, diagnostic, and consumable purposes.
The rising incidence of respiratory disorders due to lifestyle factors and environmental pollutants, combined with advancements in technology and a heightened awareness of respiratory health (particularly after the COVID-19 pandemic), are among the top factors contributing to the market’s growth.
Download a Free Sample Report:-https://tinyurl.com/5ca9uv9d
Market Segmentation
By Product Type
Therapeutic Devices
Nebulizers
Humidifiers
Oxygen Concentrators
Ventilators (Invasive & Non-invasive)
Positive Airway Pressure (PAP) Devices
Monitoring Devices
Pulse Oximeters
Capnographs
Diagnostic Devices
Spirometers
Peak Flow Meters
Consumables and Accessories
Cannulas, Tubes, Masks, Filters
By Indication
Chronic Obstructive Pulmonary Disease (COPD)
Asthma
Obstructive Sleep Apnea (OSA)
Respiratory Infections
Others (Cystic Fibrosis, Pulmonary Fibrosis)
By End-User
Hospitals
Home Care Settings
Ambulatory Surgical Centers
Specialty Clinics
By Region
North America
Europe
Asia-Pacific
Latin America
Middle East & Africa
Market Drivers
1. Rising Prevalence of Respiratory Diseases
The increasing incidence of conditions like asthma, COPD, and sleep apnea is a major factor driving demand for respiratory care devices. According to the World Health Organization, COPD is the third leading cause of death worldwide, and asthma affects over 260 million people globally.
2. Growing Geriatric Population
Older adults are more prone to chronic respiratory diseases due to the natural weakening of respiratory muscles and the immune system. The expanding elderly demographic worldwide significantly boosts the market for home-based respiratory care equipment.
3. Increasing Air Pollution and Smoking Rates
Urbanization and industrialization have led to a surge in air pollution levels, especially in countries like China and India. Additionally, cigarette smoking continues to be a key contributor to respiratory illnesses, fueling the need for early diagnosis and effective management solutions.
4. COVID-19 Pandemic Impact
The COVID-19 pandemic dramatically increased demand for ventilators, oxygen concentrators, and pulse oximeters. Even post-pandemic, awareness regarding respiratory health and readiness for future respiratory outbreaks have led to increased healthcare investments.
5. Home Healthcare Adoption
With the advancement of compact and portable devices, respiratory care is increasingly shifting from hospitals to home settings. This trend enhances patient comfort while reducing healthcare costs, fueling demand for home-use devices.
Market Trends
1. Integration of AI and IoT
Modern respiratory care devices are now integrated with Artificial Intelligence (AI) and the Internet of Things (IoT) to enable real-time monitoring, predictive analytics, and remote management. Smart inhalers, for example, can track dosage and usage frequency.
2. Miniaturization and Portability
Manufacturers are focusing on reducing the size and weight of respiratory devices without compromising performance. Portable oxygen concentrators and handheld spirometers are in high demand for both hospital and home use.
3. Non-Invasive and Wearable Technologies
Wearable respiratory monitors and non-invasive ventilation methods are gaining popularity due to improved patient compliance and ease of use. These devices are particularly useful for long-term disease management.
4. Surge in Telemedicine and Remote Monitoring
Post-COVID-19, telemedicine has gained widespread acceptance. Respiratory care devices are increasingly being used in conjunction with telehealth platforms, allowing physicians to monitor patients remotely.
5. Personalized and Precision Medicine
Personalized treatment plans based on genetic profiles and disease severity are leading to the development of tailored respiratory devices, enhancing treatment outcomes and minimizing side effects.
Challenges
1. High Cost of Advanced Devices
Despite growing demand, high-end respiratory devices remain costly, especially in low- and middle-income countries. This limits accessibility and creates disparity in treatment options.
2. Regulatory Hurdles
Strict regulatory frameworks and lengthy approval processes can delay product launches. Companies must invest heavily in clinical trials and compliance, which adds to product development costs.
3. Lack of Skilled Professionals
The efficient operation of certain respiratory care devices, especially ventilators, requires skilled healthcare personnel. A shortage of trained professionals poses a significant barrier in some regions.
4. Reimbursement and Insurance Issues
Inconsistent reimbursement policies across countries often discourage the use of advanced respiratory care devices. Patients may bear high out-of-pocket expenses, limiting adoption rates.
Regional Insights
North America
North America dominates the market due to high healthcare spending, robust reimbursement systems, and widespread adoption of advanced medical technologies. The U.S. is a major market for sleep apnea devices and home respiratory equipment.
Europe
Europe is also a significant contributor to global market share, led by countries like Germany, France, and the UK. The presence of well-established healthcare infrastructure and favorable government initiatives drive growth.
Asia-Pacific
Asia-Pacific is projected to be the fastest-growing region through 2032. The market is driven by increasing respiratory disease prevalence, urban air pollution, rising healthcare investments, and the growing use of home healthcare in China, India, and Japan.
Latin America and Middle East & Africa
These regions show promising growth potential due to improving healthcare access, rising awareness, and the gradual adoption of digital health technologies, despite economic and infrastructural challenges.
Competitive Landscape
Major players in the respiratory care device market include:
Philips Healthcare
ResMed Inc.
Medtronic plc
Fisher & Paykel Healthcare
GE Healthcare
Drägerwerk AG
Masimo Corporation
Smiths Medical
Teleflex Incorporated
Invacare Corporation
These companies focus on product innovation, strategic partnerships, and expansion into emerging markets. Continuous R&D, along with AI integration and smart device development, are key areas of investment.
Future Outlook
The respiratory care device market is expected to remain robust, driven by global health trends, patient demand for convenience, and technological innovations. Key developments to watch for by 2032 include:
Widespread adoption of smart respiratory solutions.
Stronger focus on sustainable and eco-friendly device manufacturing.
Broader use of remote patient monitoring and cloud-based data platforms.
Continued expansion in emerging economies through public-private partnerships.
Shift toward preventive care with early diagnostic devices becoming more affordable and accessible.
Conclusion
The respiratory care device market is set to expand steadily through 2032, propelled by demographic shifts, rising disease burden, and continuous technological evolution. While the industry faces challenges such as cost constraints and regulatory complexities, strategic innovation and global market penetration hold the key to unlocking future growth. As healthcare systems increasingly prioritize respiratory health and patient-centric care, respiratory devices will continue to play an integral role in improving health outcomes worldwide.
Read Full Report:-https://www.uniprismmarketresearch.com/verticals/healthcare/respiratory-care-device.html
0 notes
Text
Saudi Arabia Wearable Medical Devices Market is Expanding at a CAGR of 20.6% from 2024 to 2030
Saudi Arabia Wearable Medical Devices Market Growth & Trends
The Saudi Arabia Wearable Medical Devices Market size is anticipated to reach USD 956.43 million by 2030, growing at a CAGR of 20.6% from 2024 to 2030, according to a new report by Grand View Research, Inc. The anticipated growth in industries such as remote health monitoring and home healthcare is expected to impact the market. The utilization of telemedicine during the COVID-19 pandemic was identified to be satisfactory for safe communication between healthcare providers and patients in the country. The increased focus on healthy lifestyle and fitness contributes to the increased demand for fitness trackers. Technological advancements, increase in clinical trials, and awareness of personal health monitoring and round-the-clock monitoring are expected to further drive the market.
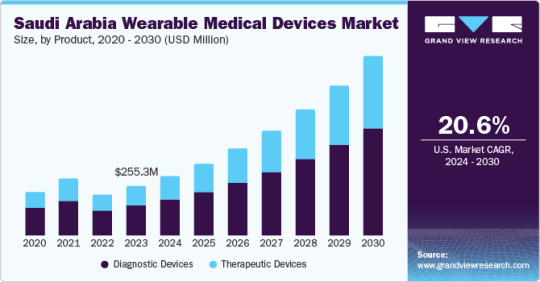
Increasing lifestyle-associated diseases constitute a significant threat to Saudi Arabia’s population health. An increase in obesity is the primary cause of non-communicable diseases like hypertension, type 2 diabetes, and dyslipidemia, which can lead to cardiovascular disorders (CVDs). Ischaemic heart disease is one of the major reasons for deaths in Saudi Arabia. In 2020 and 2021, obesity prevalence in Saudi Arabia was the highest across the globe, with an average of 35%. This factor further anticipates the need for early diagnosis of CVDs, including coronary heart disease, rheumatic heart disease, cerebrovascular disease, and other conditions necessitating continuous monitoring of physiological parameters.
Currently, the Saudi government spends over 60% of the country’s total healthcare expenditure. It launched the Vision 2030 project to improve research and development in healthcare infrastructure such as telemedicine and remote patient monitoring.
Curious about the Saudi Arabia Wearable Medical Devices Market? Download your FREE sample copy now and get a sneak peek into the latest insights and trends.
Saudi Arabia Wearable Medical Devices Market Report Highlights
Based on product, the diagnostics device segment dominated the market with the largest share of 60.92% in 2023, owing to the need of continuous monitoring of physiological parameters
The respiratory devices product segment is expected to grow at the fastest CAGR of 21.6% over the forecast period
Based on application, the home healthcare segment held the largest revenue share in 2023. The remote patient monitoring segment is expected to grow at the fastest CAGR over the forecast period.
Factors such as sedentary lifestyle and lack of exercise are contributing to the growing prevalence of cardiovascular diseases.
In August 2023, FluidAI Medical rolled out a postsurgical monitor, Stream Platform, powered by the use of artificial intelligence facilitating the diagnosis of postoperative leaks and severe complications of digestive tract surgeries.
Saudi Arabia Wearable Medical Devices Market Segmentation
Grand View Research has segmented the Saudi Arabia wearable medical devices market based on product, site, application, grade type, and distribution channel:
Saudi Arabia Wearable Medical Devices Product Outlook (Revenue, USD Million, 2018 - 2030)
Diagnostic Devices
Vital Sign Monitoring Devices
Heart Rate Monitors
Activity Monitors
Electrocardiographs
Pulse Oximeters
Spirometers
Blood Pressure Monitors
Others
Sleep Monitoring Devices
Sleep trackers
Wrist Actigraphs
Polysomnographs
Others
Electrocardiographs Fetal and Obstetric Devices
Neuromonitoring Devices
Electroencephalographs
Electromyographs
Others
Therapeutic Devices
Pain Management Devices
Neurostimulation Devices
Others
Insulin/Glucose Monitoring Devices
Insulin Pumps
Others
Rehabilitation Devices
Accelometers
Sensing Devices
Ultrasound Platform
Others
Respiratory Therapy Devices
Ventilators
Positive Airway Pressure (PAP) Devices
Portable Oxygen Concentrators
Others
Saudi Arabia Wearable Medical Devices Site Outlook (Revenue, USD Million, 2018 - 2030)
Handheld
Headband
Strap/Clip/Bracelet
Shoe Sensors
Others
Saudi Arabia Wearable Medical Devices Application Outlook (Revenue, USD Million, 2018 - 2030)
Sports and Fitness
Remote Patient Monitoring
Home Healthcare
Saudi Arabia Wearable Medical Devices Grade Type Outlook (Revenue, USD Million, 2018 - 2030)
Consumer-Grade Wearable Medical Devices
Clinical Wearable Medical Devices
Saudi Arabia Wearable Medical Devices Distribution Channel Outlook (Revenue, USD Million, 2018 - 2030)
Pharmacies
Online Channel
Hypermarkets
Download your FREE sample PDF copy of the Saudi Arabia Wearable Medical Devices Market today and explore key data and trends.
0 notes
Text
Europe Respiratory Care Devices Market Business Revenue Forecast and Statistics 2028
The Europe respiratory care devices market is expected to grow from US$ 5,587.97 million in 2022 to US$ 9,179.17 million by 2028; it is estimated to grow at a CAGR of 8.6% from 2022 to 2028.
Respiratory Health Breakdown
Disease Types:
Asthma (increasing)
COPD (severe)
Asthma Data:
Pollution connection
UK deaths +33%
Age disparity
COPD Stats:
10.3% prevalence
3.23M deaths
Treatment Options:
Multiple devices
Inhalation preferred
Market Trends:
More cases coming
Device demand rising
Pandemic Impact:
Adherence increased
UK supply issues
New opportunities
Europe Respiratory Care Devices Market—Companies Mentioned
Koninklijke Philips N.V.; ResMed Inc.; Medtronic; Masimo; THERMO FISHER SCIENTIFIC INC; Dragerwerk AG & Co. KGaA; Invacare Corporation; Getinge AB.; Nihon Kohden Corporation; Air Liquide; and Teleflex Incorporated are a few major companies operating in the Europe respiratory care devices market.
Request PDF:
https://www.businessmarketinsights.com/sample/BMIRE00025654
Europe Respiratory Care Devices Market Segmentation
The market for Europe respiratory care devices market is segmented into product, indication, end user and country. Based on product, the market is segmented into therapeutic devices, monitoring devices, diagnostics devices, and consumables & accessories. The market for the therapeutics devices segment is sub segmented into positive airway pressure (PAP) devices, oxygen concentrator, ventilators, inhalers, nebulizers, humidifiers, and others. The market for the monitoring device segment is sub segmented into pulse oximeters, scenography, and gas analyzers. The market for the diagnostic devices segment is further segmented into spirometers, polysomnography devices, peak flow meters, and other diagnostic devices. The market for the consumables & accessories segment is sub segmented into masks, disposable resuscitators, tracheostomy tubes, breathing circuits, and other consumables and accessories. By indication, the market is segmented into chronic obstructive pulmonary disease (COPD), sleep apnea, asthma, infectious diseases, and others. Based on end user, the Europe respiratory care devices market is divided into hospitals, home care, and ambulatory care. Based on country, the Europe respiratory care devices market is segmented into Germany, the UK, France, Italy, Spain, and Rest of Europe.
Europe Respiratory Care Devices Strategic Insights
Our strategic insights for Europe respiratory care devices market provide comprehensive, data-driven intelligence that analyzes emerging trends, competitive dynamics, and regional variations across healthcare systems. By combining advanced analytics with deep market expertise, we deliver actionable recommendations to help manufacturers, investors and healthcare providers identify high-growth opportunities, develop differentiated product offerings, and optimize commercial strategies. These forward-looking insights enable stakeholders to anticipate regulatory changes, technological advancements, and shifting clinical demands, allowing for proactive decision-making in this rapidly evolving sector. With its focus on future market developments, our analysis transforms complex data into strategic advantages - helping organizations allocate resources effectively, mitigate risks, and capitalize on emerging opportunities. Ultimately, these insights empower businesses to make evidence-based decisions that drive sustainable growth and profitability across Europe's diverse respiratory care landscape.
About Us:
Business Market Insights is a market research platform that provides subscription service for industry and company reports. Our research team has extensive professional expertise in domains such as Electronics & Semiconductor; Aerospace & Defense; Automotive & Transportation; Energy & Power; Healthcare; Manufacturing & Construction; Food & Beverages; Chemicals & Materials; and Technology, Media, & Telecommunications
#Europe Respiratory Care Devices Market#Europe Respiratory Care Devices Market Segmentation#Europe Respiratory Care Devices Market Analytical Overview
0 notes
Text
Why is the PFT Test in Mumbai Essential for Your Lung Health?

Understanding the PFT Test in Mumbai
A Pulmonary Function Test (PFT) is a non-invasive diagnostic procedure that evaluates how well your lungs function. It measures lung capacity, airflow, and the efficiency of gas exchange. Conducted in specialized clinics, the PFT Test in Mumbai plays a crucial role in diagnosing respiratory conditions such as asthma, chronic obstructive pulmonary disease (COPD), and other lung-related disorders. By assessing your respiratory health, this test ensures timely intervention and effective treatment plans.
Stages of the PFT Test in Mumbai
The PFT Test involves several stages, each designed to provide detailed insights into your lung function. Below are the key stages:
Spirometry:
This is the most common stage where patients exhale forcefully into a tube connected to a spirometer. It measures the volume and speed of air exhaled.
Lung Volume Measurement:
Also known as body plethysmography, this stage measures the total volume of air your lungs can hold.
Diffusion Capacity Test (DLCO):
This evaluates how well oxygen passes from your lungs into your blood.
Bronchodilator Reversibility Test:
Performed after administering a bronchodilator, this stage assesses how medication affects airflow.
Maximal Voluntary Ventilation (MVV):
This test measures the maximum amount of air you can inhale and exhale within a specific time, usually 12 seconds.
Each stage provides unique and vital data, allowing healthcare professionals to develop a comprehensive understanding of your respiratory system.
Dr. Parthiv Shah’s Suggestions for PFT Test Precautions in Mumbai
Dr. Parthiv Shah, a renowned pulmonologist, emphasizes the importance of preparation for accurate test results. He suggests the following precautions:
Avoid Heavy Meals: Do not eat a heavy meal for at least 4 hours before the test to prevent abdominal pressure from affecting lung capacity.
No Smoking: Refrain from smoking for 24 hours prior to the test.
Medications: Inform your doctor about any medications you are taking. Avoid bronchodilators unless instructed otherwise.
Clothing: Wear loose, comfortable clothing to facilitate unrestricted breathing.
Avoid Physical Exertion: Do not engage in strenuous activities before the test.
Follow Instructions: Carefully adhere to the technician's guidance during the procedure for optimal results.
By following these guidelines, patients can ensure accurate and reliable outcomes from their PFT Test.
Location and Accessibility of Dr. Parthiv Shah’s Clinic
Dr. Parthiv Shah’s clinic is conveniently located at:
B9 – A 25, MHB Colony, Rajendra Nagar, Dattapada Road, Near Rajendra Nagar Police Chowki, Borivali (E), Mumbai
Easy Access to the Clinic
Western Line:
Alight at Borivali Railway Station. From there, take a rickshaw to Rajendra Nagar. The clinic is near the Rajendra Nagar Police Chowki.
Central Line:
Take a local train to Dadar, switch to the Western Line, and alight at Borivali. Follow the same route as above.
Patients from Outside Mumbai:
Arrive at Mumbai’s major railway stations like Mumbai Central or Bandra Terminus. Use local trains or taxis to reach Borivali. Alternatively, if traveling by air, land at Chhatrapati Shivaji Maharaj International Airport and take a cab to the clinic.
The clinic’s strategic location ensures accessibility for all patients, making it a reliable destination for advanced lung function testing.
#asthma treatment#pulmonologist in borivali#sparsh super speciality lung clinic#dr. parthiv shah#lungs treatment#drparthivshah
0 notes
Text
The Future of Health Monitoring: Insights into the U.S. Wearable Medical Devices Market
The U.S. wearable medical devices market size is expected to reach USD 46.89 billion by 2030 and is anticipated to expand at a CAGR of 23.4% from 2024 to 2030, according to a new report by Grand View Research, Inc. The rising prevalence of chronic diseases, growing consumer awareness, adoption of technology, and regulatory support and approval processes are the key drivers of the U.S. wearable medical devices market.
Within the market landscape, the players are allocating substantial investments towards research and development, simultaneously striving to enhance their production capacities. The companies are pursuing the development of wearable medical devices, offering them to consumers at competitive prices. They are incorporating cutting-edge health features to attract customers and enhance overall appeal.
In the U.S., the growing adoption of wearable medical devices is fueled by multiple factors, including technological advancements, transforming healthcare delivery systems, high consumer interest in health and well-being, and a shift towards prioritizing preventive care measures. Wearable medical devices spearhead a transformative era in healthcare, enabling patients to continuously track their health status and granting healthcare professionals access to invaluable real-time data. This innovative technology facilitates more tailored and proactive care approaches. Furthermore, high-end data privacy is maintained, confirming the cyber security of the real-time and past health data generated by wearable medical devices.
U.S. Wearable Medical Devices Market Report Highlights
Based on product, the diagnostic devices segment dominated the market and accounted for a share of 61.3% in 2023 due to growing demand for personalized medicine, remote patient monitoring, and Cost-effectiveness
Based on site, the strap, clip, and bracelet segment is expected to grow at the fastest CAGR over the forecast period. The shoe sensors segment is anticipated to witness lucrative growth over the forecast period
Based on application, the home healthcaresegment dominated with the largest market revenue share of 53.2% in 2023. This is attributable to the rising prevalence of chronic diseases involving diabetes, asthma, and cardiac diseases and the rising geriatric population in the U.S.
The FDA classifies wearable medical devices based on their intended use and level of risk. Low-risk devices involving fitness trackers are classified as Class I medical devices and do not require pre-market approval (PMA)
U.S. Wearable Medical Devices Market Segmentation
Grand View Research has segmented the U.S. wearable medical devices market based on product, site, application, grade type, and distribution channel:
U.S. Wearable Medical Devices Product Outlook (Revenue, USD Million, 2018 - 2030)
Diagnostic Devices
Vital Sign Monitor
Heart Rate Monitors
Activity Monitors
Electrocardiographs
Pulse Oximeters
Spirometers
Blood Pressure Monitors
Others
Sleep Monitoring Device
Sleep Trackers
Wrist Actigraphs
Polysomnographs
Others
Electrocardiographs Fetal & Obstetric Devices
Neuromonitoring Devices
EEG
EMG
Others
Therapeutic Devices
Pain Management Devices
Neurostimulation Devices
Others
Insulin Monitoring Devices
Insulin Pumps
Others
Autoinjectors
Other Insulin Devices
Rehabiliation Devices
Accelerometers
Sensing Devices
Ultrasound Platform
Others
Respiratory Therapy Devices
Ventilators
CPAP
Portable Oxygen Concentrators
Others
U.S. Wearable Medical Devices Site Outlook (Revenue, USD Million, 2018 - 2030)
Handheld
Headband
Strap, Clip, Bracelet
Shoe Sensors
Others
U.S. Wearable Medical Devices Application Outlook (Revenue, USD Million, 2018 - 2030)
Sports & Fitness
Remote Patient Monitoring
Home Healthcare
U.S. Wearable Medical Devices Grade Type Outlook (Revenue, USD Million, 2018 - 2030)
Consumer-grade Wearable Medical Devices
Clinical Wearable Medical Devices
U.S. Wearable Medical Devices Distribution Channel Outlook (Revenue, USD Million, 2018 - 2030)
Pharmacies
Online Channels
Hypermarkets
Order a free sample PDF of the U.S. Wearable Medical Devices Market Intelligence Study, published by Grand View Research.
0 notes
Text
Respiratory Disease Testing Market Outlook, Competitive Strategies And Forecast
The global respiratory disease testing market size is expected to reach USD 7.75 billion by 2030, registering a CAGR of 2.8% from 2023 to 2030, according to a new report by Grand View Research, Inc. The market is driven by the rising prevalence of respiratory diseases. As per Forum of International Respiratory Societies, more than 200 million people across the globe suffered from Chronic Obstructive Pulmonary Disease (COPD) and 235 million suffered from asthma in 2014. In addition, the source stated that more than 50 million people struggle with occupational lung diseases annually. Thus, constantly growing target patient population is anticipated to drive the growth.
The adoption of innovative technologies, such as Computed Tomography (CT), for COPD diagnosis is expected to drive the growth. The other new technology in acute medical management of COPD is pulse oximeter that is used for outpatient monitoring. Airway management plays a main role in testing and management of COPD. Also, with recent technological innovations, there has been a 12.1 % increase in the use of Noninvasive Mechanical Ventilation (NIV) for management of COPD. Along with technological advancements, use of digital radiography (X-ray) and advanced portable spirometers is gaining momentum in the respiratory disease testing/diagnostics market.
Gather more insights about the market drivers, restrains and growth of the Respiratory Disease Testing Market
Respiratory Disease Testing Market Report Highlights
• Growing prevalence of respiratory diseases and rapid technological advancements are two of the major factors expected to propel the market growth
• Based on products, imaging tests held the largest share in 2022 due to rapid development and adoption of innovative technologies
• Based on application, tuberculosis was the largest market in 2022 owing to rising prevalence of the disease globally
• Based on end-use, hospitals segment held the largest share in 2022 and is anticipated to grow over the forecast period due to an increase in hospitalization and a growing preference for hospital treatment
• North America dominated the respiratory disease testing market in 2022. Growing prevalence of respiratory diseases such as COPD, & asthma, increasing demand for early diagnosis, and rising awareness amongst patients about the benefits of early diagnosis are responsible for the dominance
• Asia Pacific region is expected to grow at the fastest rate during the forecast period. This growth can be attributed to various factors, such as improving healthcare infrastructure and increasing patient awareness regarding the availability of new diagnostic techniques for respiratory diseases, such as COPD & asthma
• Some of the major players competing in this market include, but are not limited to, Becton Dickinson (Carefusion Corporation); Koninklijke Philips N.V. (Respironics); ResMed Company; Fischer & Paykel; and Medtronic. These players are strong brands in the market as they have elaborate product portfolios in respiratory disease diagnostics market
Respiratory Disease Testing Market Segmentation
Grand View Research has segmented the global respiratory disease testing market on the basis of product, application, end-use, and region:
Respiratory Disease Testing Market Product Outlook (Revenue, USD Million, 2018 - 2030)
• Imaging Tests
• Respiratory Measurement Devices
• Blood Gas Test
• Others
Respiratory Disease Testing Market Application Outlook (Revenue, USD Million, 2018 - 2030)
• Chronic Obstructive Pulmonary Disease
• Lung Cancer
• Asthma
• Tuberculosis
• Other
Respiratory Disease Testing Market End-use Outlook (Revenue, USD Million, 2018 - 2030)
• Hospital
• Physicians Clinic
• Clinical Laboratories
• Other
Respiratory Disease Testing Market Regional Outlook (Revenue, USD Million, 2018 - 2030)
• North America
o U.S.
o Canada
• Europe
o UK
o Germany
o France
o Italy
o Spain
o Sweden
o Norway
o Denmark
• Asia Pacific
o Japan
o China
o India
o Australia
o Thailand
o South Korea
• Latin America
o Brazil
o Mexico
o Argentina
• Middle East and Africa
o Saudi Arabia
o South Africa
o UAE
o Kuwait
Order a free sample PDF of the Respiratory Disease Testing Market Intelligence Study, published by Grand View Research.
#Respiratory Disease Testing Market#Respiratory Disease Testing Market Size#Respiratory Disease Testing Market Share#Respiratory Disease Testing Market Analysis#Respiratory Disease Testing Market Growth
0 notes
Text
PFT Test Price and Finding a PFT Test Near Me A Complete Guide

Pulmonary Function Tests (PFTs) are crucial for diagnosing and managing respiratory conditions such as asthma, COPD, and other lung-related issues. Whether your doctor recommends a PFT for routine check-ups or to investigate specific symptoms, knowing the PFT test price and where to find a PFT test near me can be essential for timely care.
This blog will cover everything you need to know about PFTs, including what to expect, the cost factors involved, and tips on finding reliable testing centers nearby.
What is a PFT Test?
A Pulmonary Function Test measures how well your lungs work. It evaluates lung capacity, airflow, and how efficiently oxygen enters your bloodstream. The PFT is a non-invasive, quick, and effective way to identify lung diseases and assess their severity. During the test, you may be asked to breathe into a mouthpiece connected to a spirometer, which captures and measures your breath.
Why is a PFT Test Important?
Doctors use PFTs to diagnose conditions like:
Asthma
Chronic Obstructive Pulmonary Disease (COPD)
Pulmonary Fibrosis
Allergies affecting breathing
Occupational lung diseases
Early diagnosis helps in effective treatment and preventing complications.
Understanding PFT Test Price
The PFT test price varies depending on several factors, including the type of healthcare facility, the specific tests performed, and the region where you’re getting tested. In hospitals or specialized clinics, the cost may be higher due to advanced equipment and expertise.
Some insurance plans cover the cost of a PFT if it is deemed medically necessary. However, it's always wise to check with your provider beforehand to avoid unexpected expenses.
How to Find a PFT Test Near Me
When searching for a PFT test near me, you have several options:
Hospitals and Clinics – Most general hospitals and specialty pulmonary clinics offer PFT services.
Diagnostic Labs – Well-known diagnostic centers like Quest Diagnostics or LabCorp often provide PFTs.
Telehealth Services – Many healthcare providers now allow online booking and consultations, guiding you to the nearest PFT facility.
You can also use Google Maps or health service directories to find accredited PFT centers near your location. Ensure the facility has qualified respiratory therapists and modern equipment for accurate results.
Preparing for Your PFT
For accurate results:
Avoid heavy meals before the test.
Wear loose clothing.
Refrain from smoking for at least 4-6 hours before the test.
Discuss your medications with your doctor, as some may need to be paused.
Conclusion
Understanding the PFT test price and knowing how to find a PFT test near me can help you stay proactive about your respiratory health. Whether managing an existing condition or investigating new symptoms, a timely PFT can provide critical insights. Don’t delay—schedule your PFT test today and take a step toward better lung health!
0 notes
Text
How Medical Equipment Suppliers Guarantee Smooth Healthcare Delivery
For patient happiness and general health outcomes, smooth healthcare delivery is crucial in the fast-paced medical environment of today. The continuous provision of medical services, guaranteeing that patients receive timely and appropriate care without needless delays or gaps, is referred to as seamless healthcare delivery. This method increases the effectiveness of healthcare systems while simultaneously improving patient experiences.
The medical equipment suppliers in Dubai are at the center of this smooth supply model. These vendors are essential in guaranteeing that medical facilities have the equipment, resources, and assistance they need to deliver top-notch treatment. This blog will discuss the significance of medical equipment suppliers' ties with healthcare providers and how they, especially those in the UAE and Dubai, contribute to smooth healthcare delivery.
Connections with Suppliers and Operational Effectiveness
The Value of Solid Relationships
The quality of the connections between medical equipment suppliers and healthcare facilities is one of the most important variables affecting the smooth delivery of healthcare. These collaborations result in operational efficiencies that immediately improve patient care when they are based on communication and trust.
Strong ties, for example, allow medical equipment suppliers to predict healthcare providers' demands. Better inventory management is made possible by this proactive strategy, which lowers the possibility of stockouts or delays in critical medical supplies. Additionally, by working closely together, healthcare institutions and suppliers may pinpoint problem areas and optimize workflows, improving overall service delivery.
MedPrix's Dedication to On-Time Delivery
Developing strong ties with healthcare professionals is a top priority for MedPrix, one of the top suppliers of medical equipment in Dubai. Our logistics techniques, which guarantee that medical facilities receive their orders on time even in the face of supply chain delays or increased demand, demonstrate our dedication to on-time deliveries.
For instance, our committed support staff quickly steps in to meet a healthcare facility's urgent need for vital medical equipment. In addition to reducing stress for medical staff, this responsiveness guarantees that patient care is maintained.
Clinical Results and the Quality of the Equipment
The Effect of High-Quality Equipment on Clinical Results
Clinical results are greatly influenced by the caliber of medical equipment provided. Healthcare workers can do their work more precisely and effectively with the use of high-quality tools and equipment, which eventually improves patient care. However, poor equipment can lead to mistakes, delays, and perhaps harmful patient effects.
For instance, trustworthy diagnostic instruments like ECG machines and spirometers enable medical practitioners to precisely evaluate patients' health. Healthcare professionals can make well-informed judgments about diagnosis and treatment when these devices operate well and give reliable readings.
Problems that Suppliers Face
Typical Problems
Despite their best efforts, medical equipment vendors face a number of difficulties in delivering smooth service. Demand fluctuations, supply chain interruptions, regulatory compliance, and technology breakthroughs are a few of the most frequent problems. For instance, unexpected increases in demand for particular medical supplies during emergencies can put a strain on supply chains and cause delays.
The Creative Solutions of MedPrix
At MedPrix, we aggressively tackle these issues by coming up with creative fixes. We can quickly adjust to shifting market conditions thanks to our agile supply chain management techniques. We can estimate demand trends, keep an eye on inventory levels in real time, and maintain ideal stock levels by utilizing technology.
Furthermore, we guarantee adherence to the strictest legal requirements, which is essential for establishing credibility with medical professionals.
In conclusion
In summary, suppliers of medical equipment play a crucial role in the healthcare delivery system and have a direct influence on the efficacy and efficiency of patient treatment. Suppliers like MedPrix are essential to maintaining smooth healthcare delivery because they build trusting connections with healthcare providers, guarantee top-notch equipment, and overcome obstacles via innovation.
Healthcare workers are urged to assess their supplier connections, giving preference to those that exhibit dependability, excellence, and a dedication to assisting with patient care. Healthcare institutions may improve clinical outcomes and operational efficiency by working with reliable medical equipment providers in Dubai and around the United Arab Emirates.
Know More
Medical Equipment Dubai UAE
Medical Equipment supplier Dubai
Patient monitor in Dubai
Ultrasound Machine supplier in Dubai
ECG machine Supplier in Dubai
Opthalmology Equipments in Dubai
Physiotherapy Equipments in Dubai
Clinic setup dubai
Portable ultrasound machine
0 notes
Text

PFT Machine Suppliers: Comprehensive Solutions for Respiratory Health
Pulmonary function testing (PFT) has become an essential tool in the diagnosis and management of respiratory diseases. PFT machines, also known as spirometers, are used to measure various parameters of lung function, including vital capacity, forced expiratory volume, and airway resistance. These measurements provide valuable insights into the health and performance of the respiratory system, enabling healthcare professionals to make informed decisions about treatment and management strategies.
PFT machine suppliers play a crucial role in ensuring that healthcare facilities have access to high-quality, reliable equipment for assessing respiratory function. These suppliers offer a wide range of spirometers, from basic handheld devices to advanced, computer-based systems capable of performing comprehensive lung function tests.
One of the key advantages of working with PFT machine suppliers is the ability to access a variety of spirometer types, each designed to meet specific needs and preferences. For example, some healthcare facilities may prefer portable, handheld spirometers for use in various settings, such as clinics, hospitals, or even home visits. These compact devices are easy to use and can provide quick, accurate measurements of lung function.
On the other hand, some healthcare facilities may require more advanced, computer-based PFT systems that offer a wider range of testing capabilities. These systems often include features such as real-time data display, automated interpretation of test results, and the ability to generate detailed reports. Many PFT machine suppliers offer customizable solutions that can be tailored to the specific needs and requirements of individual healthcare facilities.
In addition to providing high-quality PFT machines, many suppliers also offer comprehensive support and training services. This includes installation and setup assistance, as well as ongoing maintenance and repair services to ensure that the equipment remains in optimal working condition. Some suppliers also offer training programs for healthcare professionals, helping them to develop the skills and knowledge necessary to effectively use and interpret the results of PFT tests.
One of the most significant benefits of working with PFT machine suppliers is the ability to access a wide range of accessories and consumables. These include items such as disposable mouthpieces, nose clips, and filter adapters, which are essential for maintaining hygiene and preventing cross-contamination during testing. Many suppliers also offer calibration gases and other supplies necessary for ensuring the accuracy and reliability of PFT measurements.
As the healthcare industry continues to evolve, the demand for high-quality PFT machines and related services is expected to grow. PFT machine suppliers are well-positioned to meet this demand by offering innovative solutions that leverage the latest advancements in respiratory testing technology. This includes the development of cloud-based platforms for data storage and analysis, as well as the integration of artificial intelligence and machine learning algorithms to assist in the interpretation of test results.
In conclusion, PFT machine suppliers play a vital role in supporting the diagnosis and management of respiratory diseases. By providing access to high-quality spirometers, comprehensive support services, and a wide range of accessories and consumables, these suppliers help healthcare facilities to deliver the best possible care to their patients. As the healthcare industry continues to evolve, PFT machine suppliers will undoubtedly play an increasingly important role in ensuring the respiratory health and well-being of individuals around the world.
0 notes
Text
Pulmonology Devices Market 2024 : Industry Analysis, Trends, Segmentation, Regional Overview And Forecast 2033
Overview and Scope Pulmonology devices are medical instruments and equipment used in the diagnosis, treatment, and management of respiratory system conditions and diseases. These devices play a crucial role in assessing lung function, monitoring respiratory health, providing therapeutic interventions, and supporting patients with respiratory disorders.

Sizing and Forecast The pulmonary devices market size has grown rapidly in recent years. It will grow from $1.23 billion in 2023 to $1.36 billion in 2024 at a compound annual growth rate (CAGR) of 10.6%. The growth in the historic period can be attributed to rising incidence of chronic respiratory diseases, growing shift towards preventive healthcare, increase in adoption of pulmonology devices, surge in incidence of diseases requiring endoscopic ultrasound procedures, increase in geriatric population.
The pulmonary devices market size is expected to see rapid growth in the next few years. It will grow to $2.05 billion in 2028 at a compound annual growth rate (CAGR) of 10.8%. The growth in the forecast period can be attributed to rise in prevalence of respiratory diseases, rising air pollution levels, growth in home healthcare, growing awareness and early diagnosis, expansion of healthcare infrastructure. Major trends in the forecast period include integration of artificial intelligence, growth of telemedicine and remote monitoring, advancements in portable and wearable devices, use of robotic technology in less-invasive diagnostic procedures, increase in home care services.
Order your report now for swift delivery, visit the link: https://www.thebusinessresearchcompany.com/report/pulmonology-devices-global-market-report
Segmentation & Regional Insights The pulmonology devices market covered in this report is segmented –
1) By Product Type: Endobronchial Ultrasound (EBUS) Needles, Pulmonary Biopsy Devices, Airway Stents, Single-Use Bronchoscopes, Other Product Types 2) By Indication: Chronic Obstructive Pulmonary Disease (COPD), Lung Cancer, Tracheal And Bronchial Stenosis, Foreign Body Extraction, Other Indications 3) By End-User: Pulmonology Clinics, Hospitals, Ambulatory Surgical Centers
North America was the largest region in the pulmonology devices market in 2023. The regions covered in the pulmonology devices market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa.
Intrigued to explore the contents? Secure your hands-on a free sample copy of the report: https://www.thebusinessresearchcompany.com/sample.aspx?id=16772&type=smp
Major Driver Impacting Market Growth The rise in the prevalence of respiratory diseases is expected to propel the growth of the pulmonology devices market going forward. Respiratory diseases are a group of medical conditions that affect the organs and tissues involved in breathing, including the airways, lungs, and respiratory muscles. The cases of respiratory diseases are occurring due to rising air pollution, smoking, and occupational hazards. Pulmonology devices aid in respiratory disease management by enabling accurate diagnosis, continuous monitoring, and effective treatment through tools like spirometers, inhalers, pulse oximeters, and ventilators, ultimately improving patient outcomes and quality of life. For instance, according to the Scottish Public Health Observatory, a Scotland-based public health agency, COPD rates began rising in 2021-22 as the effects of COVID-19 lessened. The rate among males rose from 83.2 to 97.6 cases per 100,000, while it increased from 72.6 to 97.3 among females. Therefore, the rise in the prevalence of respiratory diseases is driving the growth of the pulmonology devices market.
Key Industry Players Major companies operating in the pulmonology devices market are Medtronic, Koninklijke Philips N.V., GE Healthcare, Baxter, Boston Scientific Corporation, Medline Industries LP., Olympus Corporation, Omron Healthcare, ResMed, Getinge, ICU Medical Inc., Fisher & Paykel Healthcare Limited, Ambu A/S, Vyaire Medical Inc., Drive DeVilbiss Healthcare, PENTAX Medical, Hamilton Medical, Nonin Medical, CAIRE Inc., Pulmonx Corporation, Verathon Inc., Draegerwerk AG & Co. KGaA, NIOX
The pulmonology devices market report table of contents includes:
1. Executive Summary
2. Pulmonology Devices Market Characteristics
3. Pulmonology Devices Market Trends And Strategies
4. Pulmonology Devices Market - Macro Economic Scenario
5. Global Pulmonology Devices Market Size and Growth ..........
32. Global Pulmonology Devices Market Competitive Benchmarking
33. Global Pulmonology Devices Market Competitive Dashboard
34. Key Mergers And Acquisitions In The Pulmonology Devices Market
35. Pulmonology Devices Market Future Outlook and Potential Analysis
36. Appendix
Contact Us:
The Business Research Company
Europe: +44 207 1930 708
Asia: +91 88972 63534
Americas: +1 315 623 0293
Email: [email protected]
Follow Us On:
LinkedIn: https://in.linkedin.com/company/the-business-research-company
Twitter: https://twitter.com/tbrc_info
Facebook: https://www.facebook.com/TheBusinessResearchCompany
YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ
Blog: https://blog.tbrc.info/
Healthcare Blog: https://healthcareresearchreports.com/
Global Market Model: https://www.thebusinessresearchcompany.com/global-market-model
0 notes
Text
Advancements in Spirometer Technology Enhance Respiratory Health Management

The field of respiratory health management has seen significant improvements with recent advancements in spirometer technology. Spirometers, essential tools used to assess lung function by measuring the volume and flow of air that a person can inhale and exhale, are now more accurate, portable, and user-friendly than ever before. These developments are proving crucial in diagnosing, monitoring, and managing chronic respiratory conditions such as asthma, chronic obstructive pulmonary disease (COPD), and other lung disorders.
Enhanced Accuracy and Sensitivity
Modern spirometers have become highly accurate, capable of detecting even the slightest variations in lung function. With improved sensors and digital technology, these devices can provide more precise measurements of key respiratory parameters such as Forced Vital Capacity (FVC) and Forced Expiratory Volume in one second (FEV1). This level of sensitivity is particularly beneficial for early detection of respiratory issues, allowing for timely intervention and treatment.
The Spirometer Market size is valued at USD 1.12 Bn in 2023 and is expected to reach USD 2.39 Bn by 2031 with a growing CAGR of 10.17% over the forecast period of 2024-2031.
Portability and Remote Monitoring
The latest spirometer models are designed with portability in mind, making it easier for patients to monitor their lung function at home or on the go. These compact, lightweight devices often connect to smartphones or tablets, enabling real-time data transfer to healthcare providers. This capability is especially valuable in the current era of telemedicine, where remote monitoring and virtual consultations have become more common. Patients can now manage their respiratory health more proactively, with fewer visits to the clinic.
User-Friendly Interfaces and Data Integration
Advancements in spirometer technology have also focused on improving the user experience. Many devices now feature intuitive interfaces that guide patients through the testing process, reducing the likelihood of errors and ensuring consistent results. Additionally, the integration of spirometer data with electronic health records (EHRs) and other digital health platforms allows for seamless tracking of a patient’s respiratory health over time. Healthcare providers can easily access and analyze this data, facilitating more informed decision-making and personalized care plans.
The Future of Spirometry
Looking forward, the future of spirometry holds exciting possibilities. Researchers are exploring the development of spirometers with advanced AI-driven algorithms that can automatically interpret results and provide immediate feedback. Furthermore, innovations in wearable technology could lead to the creation of continuous monitoring systems, offering a more comprehensive view of a patient’s respiratory function in real-time.
As spirometer technology continues to evolve, it promises to play an increasingly vital role in respiratory health management. These advancements not only improve the accuracy and ease of lung function testing but also empower patients to take a more active role in their healthcare, ultimately leading to better outcomes and quality of life.
0 notes
Text
Homecare Self-Monitoring Devices: Empowering Patients to Take Charge of Their Health

Remote patient monitoring devices have seen a surge in popularity over the past few years. These innovative tools allow patients to monitor their vital signs and health metrics from the comfort of their own homes. Conditions that are commonly monitored remotely include chronic diseases like diabetes, heart disease, COPD and hypertension. Remote monitoring solutions offer convenience for patients and more efficient management of chronic conditions for healthcare providers. With remote monitoring, patients no longer need to regularly visit doctor's offices or clinics. Instead, they can track metrics like blood pressure, blood sugar, weight, pulse oximetry and more from home and automatically share the results with their care team via wireless transmission. This level of convenience has improved patient engagement with their health. It also frees up resources in the traditional healthcare setting. Wearable and Non-Invasive Monitoring Options A variety of remote patient monitoring devices are available for different health metrics. Popular wearable options include smartwatches equipped with sensors to track vitals passively throughout the day. Continuous glucose monitors have also revolutionized diabetes management. These small, waterproof sensors are worn on the body and can automatically transmit blood sugar readings to a companion smartphone app. Non-invasive devices are also prevalent. Examples include wireless blood pressure cuffs, scales, spirometers and pulse oximeters. Most connect to a smartphone or tablet via Bluetooth to record and transmit readings with just a few taps. This ease of use has made remote monitoring an appealing option even for older adults or those who lack comfort with technology. Audio instructions and visual guides help ensure proper use. Improving Chronic Disease Management For those managing chronic illnesses long-term, homecare self-monitoring device provides numerous benefits over traditional office visits alone. It allows for frequent symptom and metric tracking, sometimes continuously, between appointments. This level of visibility helps both patients and clinicians better recognize trends, catch issues early and fine tune treatment plans as needed. For example, continuous glucose monitors have helped increase time spent in target blood sugar range for many with diabetes. Remote monitoring also empowers patients to take a more proactive role in their care instead of simply reacting during office visits. Over time, this improved self-management can lead to better disease control and potentially reduced complications down the line. It also frees up clinic resources for those with more severe needs. Data Provides Insights for Care Teams The wealth of biometric and symptom data collected through remote monitoring gives care teams deeper insights into how conditions are truly progressing outside of brief appointments. Through automated reports and customizable alerts, clinicians now have more opportunities to intervene if a patient shows signs of a negative health trajectory. Remote monitoring also allows smaller practices and health systems to keep close tabs on large patient populations regardless of location. Real-time access to patient metrics streamlines chronic care coordination and facilitates quick response when action is needed. It flags issues that may have otherwise gone unrecognized until a serious event like a hospitalization occurred. Early detection through remote monitoring is transforming chronic disease management into a more proactive model focused on prevention over crisis intervention.
#Homecare Self-Monitoring Device Demand#Homecare Self-Monitoring Device Share#Homecare Self-Monitoring Device Trend
0 notes
Text
Spirometer Turbines USA: A Reliable Choice for Accurate Respiratory Testing
When it comes to respiratory testing, the accuracy and efficiency of the equipment used are paramount. In the United States, spirometer turbines have become an essential tool in diagnosing and monitoring various lung conditions. Whether you're a healthcare professional or a medical device supplier, choosing the right spirometer turbines is crucial to ensure reliable results. At Medical Device Depot, we offer top-quality Spirometer Turbines USA that meet the highest standards of performance and reliability.
What are Spirometer Turbines?
Spirometer turbines are key components of spirometers, devices that measure lung function by assessing how much air a person can inhale and exhale. The turbine-based spirometers work by using a rotating blade that measures airflow as it passes through the turbine. The turbine spins as air flows through it, converting the airflow into electrical signals that are recorded for analysis. This allows healthcare professionals to accurately diagnose conditions such as asthma, chronic obstructive pulmonary disease (COPD), and other respiratory disorders.
Why Choose Spirometer Turbines USA?
When purchasing medical equipment, particularly spirometer turbines, it's important to prioritize quality, durability, and accuracy. Spirometer Turbines USA are known for their reliability and performance. Manufactured with precision and tested for accuracy, these turbines are designed to provide consistent results over time. Here are some reasons why Spirometer Turbines USA are a top choice:
Precision Engineering: Spirometer turbines made in the USA are designed with advanced technology to ensure that the measurements are as accurate as possible. This precision is vital for diagnosing and managing respiratory diseases effectively.
Durability and Longevity: Medical professionals rely on equipment that can withstand continuous use without compromising performance. Spirometer Turbines USA are built to last, offering extended service life and durability, even in high-demand clinical environments.
Compliance with Standards: Spirometer turbines manufactured in the USA are subject to stringent regulatory standards. This ensures they meet the quality and safety requirements set by organizations such as the FDA, providing peace of mind for both clinicians and patients.
Easy Integration with Spirometers: The Spirometer Turbines USA are designed to seamlessly integrate with a wide range of spirometer models. Whether you’re using a desktop spirometer or a portable device, these turbines are compatible with various systems, making them a versatile option for healthcare providers.
Applications of Spirometer Turbines USA
Spirometer turbines play a crucial role in diagnosing and monitoring lung conditions. With Spirometer Turbines USA, healthcare professionals can assess lung capacity, detect airflow obstruction, and evaluate overall pulmonary function. Common applications include:
Diagnosing Asthma and COPD: By measuring forced expiratory volume and forced vital capacity, spirometer turbines help doctors identify conditions like asthma and COPD, allowing for early intervention and treatment.
Preoperative Assessments: Before surgeries, spirometry testing using Spirometer Turbines USA can evaluate lung function, which is important for determining anesthesia risks.
Chronic Disease Monitoring: Regular spirometry tests can track the progression of chronic respiratory conditions, helping doctors adjust treatments as necessary.
Occupational Health: Spirometer turbines are used to monitor workers exposed to harmful substances or environments that may affect lung health, ensuring early detection of any issues.
Choosing the Right Spirometer Turbines USA for Your Practice
At Medical Device Depot, we understand the importance of high-quality equipment in delivering effective healthcare. We offer a wide selection of Spirometer Turbines USA designed to meet the needs of clinics, hospitals, and other medical facilities. When selecting a spirometer turbine, consider the following factors:
Compatibility: Ensure the turbine is compatible with your spirometer system for optimal performance.
Calibration: Choose turbines that are easy to calibrate and maintain, ensuring consistent accuracy.
Maintenance: Select turbines that are low-maintenance yet durable, minimizing downtime and costs in your practice.
Conclusion
In conclusion, Spirometer Turbines USA are an essential tool for healthcare providers who want to ensure accurate and reliable respiratory testing. Whether you're diagnosing asthma, monitoring COPD, or assessing lung function for other conditions, these turbines offer the precision, durability, and performance you need. At Medical Device Depot, we offer the highest-quality Spirometer Turbines USA to meet the demands of modern medical practices. Trust in our products to help deliver the best care for your patients.
For more information or to purchase Spirometer Turbines USA, visit our website today and discover how we can support your medical equipment needs. For more details visit : https://medicaldevicedepot.com/all-products/spirometers/
0 notes
Text
Breathing Easy: Insights into the Pulmonary Function Testing (PFT) Devices Market
Pulmonary Function Testing (PFT) devices play a vital role in diagnosing and monitoring respiratory conditions, providing valuable insights into lung function and overall respiratory health. From spirometers to peak flow meters, these devices enable healthcare professionals to assess lung volumes, airflow rates, and gas exchange, aiding in the diagnosis and management of a wide range of respiratory disorders. In this article, we delve into the dynamics of the Pulmonary Function Testing (PFT) devices market, exploring key trends, innovations, and considerations for healthcare providers and patients alike.
Pulmonary Function Testing (PFT) devices encompass a variety of tools and instruments used to evaluate lung function and diagnose respiratory conditions. Spirometry, the most common type of PFT, measures the volume of air exhaled and inhaled by the lungs, providing valuable information about lung capacity, airflow obstruction, and respiratory muscle function. Other PFT devices, such as peak flow meters and gas analyzers, assess specific aspects of lung function, such as airway resistance and gas exchange efficiency.
One of the notable trends in the PFT devices market is the increasing adoption of portable and handheld devices. Portable spirometers and peak flow meters allow for convenient and non-invasive testing outside of traditional clinical settings, enabling healthcare providers to monitor lung function in real-world environments such as patients' homes, schools, and workplaces. This trend towards remote monitoring and telemedicine has been accelerated by the COVID-19 pandemic, driving demand for portable PFT devices that enable remote patient monitoring and virtual consultations.
Request the sample copy of report @ https://www.globalinsightservices.com/request-sample/GIS24798
Moreover, there has been a focus on enhancing the usability and accessibility of PFT devices, particularly for patients with limited mobility or cognitive impairments. User-friendly interfaces, wireless connectivity, and smartphone-compatible apps are increasingly common features found in modern PFT devices, making it easier for patients to perform self-administered tests and track their lung function over time. These advancements in technology empower patients to take a more active role in managing their respiratory health and facilitate early detection of potential complications.
Additionally, there is a growing emphasis on personalized medicine and precision diagnostics in the field of respiratory care. Advanced PFT devices with integrated artificial intelligence (AI) algorithms can analyze test results in real-time, providing clinicians with actionable insights and personalized treatment recommendations based on individual patient characteristics and disease severity. This personalized approach to respiratory diagnostics and management holds the promise of improving patient outcomes and reducing healthcare costs by optimizing treatment strategies and minimizing unnecessary interventions.
Furthermore, the PFT devices market is characterized by ongoing innovation and research aimed at improving the accuracy, reliability, and clinical utility of pulmonary function testing. Emerging technologies such as impedance pneumography, multiple breath washout testing, and fractional exhaled nitric oxide (FeNO) measurement are expanding the capabilities of PFT devices, allowing for more comprehensive assessment of lung function and early detection of respiratory abnormalities.
In conclusion, the PFT devices market is evolving to meet the growing demand for accurate, accessible, and personalized respiratory diagnostics. With advancements in portable technology, remote monitoring capabilities, and AI-driven analytics, PFT devices are enabling healthcare providers to deliver more precise diagnoses and tailored treatment plans for patients with respiratory conditions. As technology continues to advance and healthcare delivery models evolve, the future of the PFT devices market holds promise for improved respiratory health outcomes and enhanced quality of life for patients worldwide.
0 notes