#CD30
Explore tagged Tumblr posts
Text
PD-1 receptor deficiency enhances CD30+ Treg cell function in melanoma
PD-1 receptor deficiency enhances CD30+ Treg cell function in melanoma Summary Studies have shown that deficiency in the PD-1 receptor can improve the efficacy of CD30+ Treg cells in melanoma. The absence of PD-1 signaling appears to boost the suppressive function of these regulatory T cells within the tumor microenvironment. Specifically, PD-1 deficient CD30+ Tregs exhibit enhanced expression…
#Biomedicine#Cancer#CD30#cell#deficiency#enhances#Function#general#immunology#immunotherapy#Infectious diseases#Melanoma#PD1#receptor#Regulatory T cells#Treg
1 note
·
View note
Text
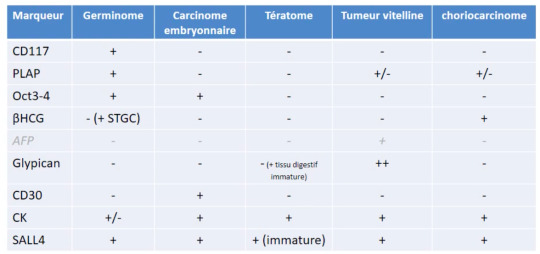
IHC pour tumeurs germinales
Frédérique Dijoud (CHU Lyon), plateforme nationale DES
#CD117#PLAP#oct3-4#BHCG#AFP#glypican#CD30#CK#SALL4#tissus mous#germinal#germinome#carcinome embryonnaire#tératome#tumeur vitelline#choriocarcinome#IHC
0 notes
Text
CD30 Lymphoma Treatment in Leeds: Advanced Care Options
Leeds is renowned across the globe for possessing state-of-the-art medical facilities and professional doctors specialising in cancer treatment. CD30 lymphoma patients receive access to multiple successful treatments that prove to work effectively to recognise and manage the disease.
CD30 Lymphoma Treatment in Leeds is accomplished through chemotherapy, radiation, and, under certain conditions, surgery for the excision of infected tissue. Biopsy can be performed in order to determine the most appropriate treatment in accordance with cancer stage. Newer forms such as immunotherapy and CAR-T cell therapy are also given, with the newest techniques to attack CD30 lymphoma.
Professional medical centers in Leeds provide comprehensive treatment so patients can get the most up-to-date treatments as per their condition. Since oncology evolves every day, patients can receive conventional as well as latest treatments, improving their chances of successful treatment. Getting specialist consultation in Leeds can help individuals make the proper decisions about their treatment process.
0 notes
Text
0 notes
Text

CAR T-Cell Therapy in Non-Hodgkin’s Lymphoma (NHL)
CAR T-cell therapy has become a groundbreaking treatment option for Non-Hodgkin’s Lymphoma, particularly in cases that are relapsed or resistant to standard therapies. This cutting-edge therapy involves genetically engineering a patient’s T cells to express chimeric antigen receptors (CARs) that specifically target and destroy cancer cells. It has demonstrated strong efficacy, especially in aggressive forms like Diffuse Large B-Cell Lymphoma (DLBCL), and has received regulatory approvals, including from the FDA. However, the therapy still faces challenges, such as potentially serious side effects (e.g., cytokine release syndrome and neurological toxicity), as well as its high cost. Continued advancements in CAR T-cell research and ongoing clinical trials are expected to broaden its application across NHL subtypes and improve treatment effectiveness and safety.
Want more data like this? Get the infographic now
Epidemiological Segmentation
CAR T-cell therapy eligibility for NHL is segmented by:
Incident cases by indication (e.g., Mantle Cell Lymphoma, DLBCL, Follicular Lymphoma, Marginal Zone Lymphoma, PMBCL, CLL/SLL)
Indication-specific eligible patient populations for CAR T-cell therapy
Epidemiology Highlights (2023)
An estimated 126,000 patients in the 7MM were eligible for CAR T-cell therapy in 2023.
DLBCL was the most common NHL subtype in the EU4 and the UK, accounting for around 32,000 cases.
Japan reported approximately 23,700 incident cases of NHL across key CAR T-suitable subtypes in 2023.
Visualize the trends—then explore the data behind them in our latest report : Click Here
Market Overview
The US market for CAR T-cell therapy in NHL was valued at approximately USD 1.2 billion in 2023.
Market Drivers
Strong clinical success in hard-to-treat NHL cases.
Rapid technological progress in CAR T-cell development.
A growing number of FDA-approved CAR T therapies.
Rising demand for individualized and immune-based treatments.
Market Barriers
High production and administration costs of CAR T-cell therapies.
Limited availability of treatment centers with necessary infrastructure.
Risks of serious adverse events, including CRS and neurotoxicity.
Regulatory hurdles and insurance coverage issues impacting accessibility.
Download the full infographic to uncover detailed insights : Click Here
Pipeline Therapies
Emerging CAR T-cell products include:
Cemacabtagene ansegedleucel
Rapcabtagene autoleucel
Zamtocabtagene autoleucel
AUTO4
CD30.CAR-T
PMB-CT01
And others
Key Industry Players
Allogene Therapeutics
Novartis
Miltenyi Biomedicine
Mustang Bio
Crispr Therapeutics
2seventy Bio
Imugene
Among others
Make informed decisions—start with the full report
0 notes
Text
Biotinylated Human CD30 (C-6His-Avi)
Biotinylated Human CD30 (C-6His-Avi) Catalog number: B2023181 Lot number: Batch Dependent Expiration Date: Batch dependent Amount: 20 ug Molecular Weight or Concentration: N/A Supplied as: Lyophilized Powder Applications: a molecular tool for various biochemical applications Storage: -20°C Keywords: Tumor necrosis factor receptor superfamily member 8; CD30L receptor; Ki-1 antigen; Lymphocyte…
0 notes
Text
CD Antigen Cancer Therapy Market Outlook: Expected Growth to $41.8 Billion by 2031
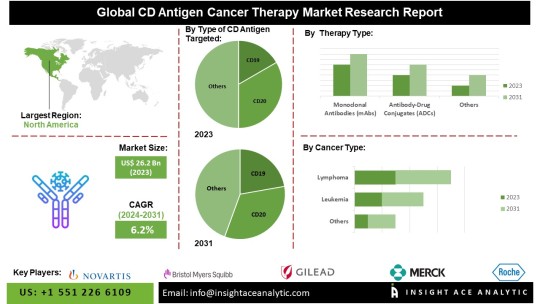
InsightAce Analytic Pvt. Ltd. announces the release of a market assessment report on the “Global CD Antigen Cancer Therapy Market – Type of CD Antigen Targeted (CD19, CD20, CD30, CD33, CD38, CD70, Others), By Therapy Type [Monoclonal Antibodies (mAbs), Antibody-Drug Conjugates (ADCs), Chimeric Antigen Receptor (CAR) T-cell Therapy, Bi-specific T-cell Engagers (BiTEs), Radioimmunotherapy, Immunotoxins], By Cancer Type (Leukemia, Lymphoma, Multiple Myeloma, Breast Cancer, Lung Cancer, Prostate Cancer, Others), By End User (Hospitals, Specialty Clinics, Cancer Treatment Centers, Research Institutes), By Region, Trends, Industry Competition Analysis, Revenue and Forecast To 2031.”
According to the latest research by InsightAce Analytic, the Global CD Antigen Cancer Therapy Market is valued at US$ 26.2 Bn in 2023, and it is expected to reach US$ 41.8 Bn by 2031, with a CAGR of 6.21% during the forecast period of 2024-2031.
Get Free Access to Demo Report, Excel Pivot and ToC: https://www.insightaceanalytic.com/request-sample/2644
CD antigen cancer therapy is a specialized method of treating cancer by targeting proteins found on cancer cells’ surfaces, called CD antigens. The CD antigen cancer therapy market is growing rapidly worldwide due to the rising cancer incidence and the expansion of R&D efforts. Several groups worldwide are researching and coordinating their efforts to find better ways to use cancer CD antigen cancer therapy. Targeted techniques offered by CD antigen cancer medicines can potentially enhance treatment outcomes, boosting market expansion. This has led to a dramatic increase in the budgets allocated to cancer research and the development of novel treatment methods. However, the industry is expected to be hindered by regulatory hurdles regarding approving and utilizing CD antigen cancer therapy. Also, the market is expected to slow down throughout the projected period due to specific manufacturing and pharmacological challenges related to this CD antigen cancer therapy development.
List of Prominent Players in the CD Antigen Cancer Therapy Market:
Novartis AG
Roche Holdings AG
Bristol Myers Squibb Company
Gilead Sciences Inc.
Merck & Co. Inc.
Johnson & Johnson
Amgen Inc.
AbbVie Inc.
AstraZeneca PLC
Takeda Pharmaceutical Company Limited
Seattle Genetics Inc.
Biogen Inc.
Celgene Corporation
Genmab A/S
Immunomedics Inc.
Others
Market Dynamics:
Drivers-
The rising incidence of cancer in general and targeted therapies, in particular, are propelling the CD antigen cancer therapy industry forward. Recent progress in immunotherapy and biotechnology has resulted in the creation of novel CD antigen-targeting medications. Market expansion is also driven by legislative frameworks that support industry and significant investments in research & development. Additionally, CD antigen therapies have shown promising results in clinical studies, and there is a growing need for personalized therapy, which contributes to the expansion of the market.
Challenges:
There are several obstacles in the CD antigen cancer therapy industry, including complicated manufacturing methods, expensive production and development costs, and strict regulatory regulations. Conducting clinical trials to establish the safety of CD antigen cancer therapy is costly and time-consuming. Another major obstacle is the wide range of possible side effects and patient reactions. Alternative cancer treatments and problems with intellectual property also affect market expansion. For these reasons, it is difficult for novel therapies to enter the market on a large scale.
Expert Knowledge, Just a Click Away: https://calendly.com/insightaceanalytic/30min?month=2024-02
Regional Trends:
The North American CD antigen cancer therapy market is anticipated to register a major market share in revenue. It is projected to grow at a high CAGR in the near future due to the rising frequency of cancers, significant funding for research and development of targeted therapies, and a heavy focus on precision medicine and customized oncology. Besides, Europe had a substantial share in the market because of rapid urbanization, enhanced healthcare facilities, and abundant discretionary income; escalating rates of breast cancer because of unhealthy lifestyle choices, including less exercise and more alcohol use, resulted in the dramatic rise of CD antigen cancer therapy market in this region.
Recent Developments:
In March 2024, Bristol Myers Squibb received FDA approval for its chimeric antigen receptor (CAR) T cell therapy; Breyanzi, a CD19-directed CART therapy, for the treatment of adult patients with relapsed or refractory chronic lymphocytic leukaemia (CLL) or small lymphocytic lymphoma (SLL) who have received at least two prior lines of therapy.
In 2023, Gilead Sciences, Inc. received FDA approval for its Trodelvy, Trop-2-directed antibody and topoisomerase inhibitor drug conjugate used for the treatment of metastatic triple-negative breast cancer and metastatic urothelial cancer to treat inoperable locally advanced or metastatic breast cancer in adults.
Unlock Your GTM Strategy: https://www.insightaceanalytic.com/customisation/2644
Segmentation of CD Antigen Cancer Therapy Market-
By Type of CD Antigen Targeted-
CD19
CD20
CD30
CD33
CD38
CD70
Others
By Therapy Type-
Monoclonal Antibodies (mAbs)
Antibody-Drug Conjugates (ADCs)
Chimeric Antigen Receptor (CAR) T-cell Therapy
Bi-specific T-cell Engagers (BiTEs)
Radioimmunotherapy
Immunotoxins
By Cancer Type-
Leukaemia
Lymphoma
Multiple Myeloma
Breast Cancer
Lung Cancer
Prostate Cancer
Others
By End-User-
Hospitals
Specialty Clinics
Cancer Treatment Centers
Research Institutes
By Region-
North America-
The US
Canada
Mexico
Europe-
Germany
The UK
France
Italy
Spain
Rest of Europe
Asia-Pacific-
China
Japan
India
South Korea
South East Asia
Rest of Asia Pacific
Latin America-
Brazil
Argentina
Rest of Latin America
Middle East & Africa-
GCC Countries
South Africa
Rest of the Middle East and Africa
Empower Your Decision-Making with 180 Pages Full Report @ https://www.insightaceanalytic.com/buy-report/2644
About Us:
InsightAce Analytic is a market research and consulting firm that enables clients to make strategic decisions. Our qualitative and quantitative market intelligence solutions inform the need for market and competitive intelligence to expand businesses. We help clients gain competitive advantage by identifying untapped markets, exploring new and competing technologies, segmenting potential markets and repositioning products. Our expertise is in providing syndicated and custom market intelligence reports with an in-depth analysis with key market insights in a timely and cost-effective manner.
0 notes
Text
Turning calls off for meow NiteFlirt, check out my hot AF content~> https://www.niteflirt.com/profile/Trinity%20Infinity?un=cd30#goodies brat buyingcontent sellingcontent bdsm
0 notes
Text
Rapid progression of angioimmunoblastic t cell lymphoma after Covid-19 vaccination by Iryna Abramenko, MD in Journal of Clinical Case Reports Medical Images and Health Sciences
Abstract
We present the case of a 57-year-old male patient from Ukraine who developed tonsillitis and generalized lymphadenopathy approximately one week after receiving the first dose of the ChAdOx1 (AstraZeneca) anti-SARS-CoV-2 vaccine. A total body computerized tomography scan revealed pronounced lymphadenopathy above and below the diaphragm, and enlargement of the spleen. Histologic examination of the left inguinal lymph node revealed revealed lack of a clear separation of the cortical and paracortical areas. Expanded proliferation of vessels (postcapillary venules) around which atypical lymphoid cells of small and medium size were located. The most of atypical lymphoid cells expressed CD3, CD4, PD1, blc-6, and Ki-67 antigens, some cells also CD10-, CD30-positive. Diagnosis of angioimmunoblastic T-cell lymphoma (AITL), IVB Ann Arbor stage, was established. The patient received six courses of therapy CHOEP regimen. According to the results of treatment clinical and hematological remission is achieved. Thus, this article describes the second case of the development of AITL after vaccination. The accumulation of such data and their analysis will allow to make appropriate conclusions about the relationship of anti-SARS-CoV-2 vaccination with development of oncohematological diseases.
Introduction
SARS-Cov-2 virus infection is widespread throughout the world. By May 2023, the number of infected cases raised to 765,903,278 persons and a total 13,350,530,518 vaccine doses have been administered [1]. Vaccination is an effective mean of preventing infection and the development of severe forms of the disease. Eleven vaccines were recommended by World Health Organization for vaccination: Pfizer-BioNTech, Oxford-AstraZeneca, Sinopharm BIBP, Moderna, Janssen, CoronaVac, Covaxin, Novavax, CovoVax (Novavax formulation), Convidecia, Sanofi-GSK; a number of vaccines are under consideration [2]. Oxford-AstraZeneca, CoronaVac, Pfizer-BioNTech, and Moderna were used for vaccination against SARS-CoV-2 in Ukraine [3].
Vaccination leads to the development of protective antiviral immunity [4, 5]. In oncological patients, this may have ambivalent influence on tumor growth. Two cases of spontaneous tumor regression after SARS-Cov-2 vaccination have been described: shrinking of the cervical lymph node and resolution of the diffuse lung lesions in patient with a recurrent primary cutaneous anaplastic large-cell lymphoma one week after having received the first COVID-19 vaccination (BioNTech/Pfizer) [6]; and spontaneous regression of metastatic salivary gland myoepithelial carcinoma in patient one month after vaccination of mRNA-1273 COVID-19 (Moderna) vaccine [7]. Anti-cancer effect of COVID-19 vaccines (a decrease in tumor size, a decrease in the expression of tumor markers (VEGF, Ki-67, MMP-2/9), CD4/CD8 ratio, and metastasis to the vital organs) has been shown in 4T1 mice models [8].
On the other hand, cases of progression of oncohematological diseases after vaccination have also been described. Goldman et al. described rapid progression of angioimmunoblastic T cell lymphoma following BNT162b2 mRNA vaccine booster shot [9]. Cavanna et al. presented a case of anaplastic large-cell lymphoma that developed in a patient approximately 10 days after receiving the third dose of the BNT162b2 vaccine and summarized eight additional cases identified in the available literature [10]. There were four cases of diffuse large-B-cell lymphoma [11-13], one case of extranodal NK/T-cell lymphoma [12], one patient with subcutaneous panniculitis-like T-cell lymphoma [14], one case of marginal zone B-cell lymphoma [15] and one primary cutaneous anaplastic large-cell lymphoma (developed at the SARS-CoV2 vaccine injection site) [16].
In this article, we report a case of angioimmunoblastic T-cell lymphoma developed shortly after SARS-CoV2 vaccination.
Case report
The patient is a 57-year-old Caucasian man with no previous history of disease. On May 6, 2021, he received the first dose of the ChAdOx1 (AstraZeneca). A few days later, he noted a flu-like syndrome and a sore throat. A diagnosis of tonsillitis was established. Antibacterial therapy was ineffective and within two weeks patient noted rapid increase of cervical, axillary, and inguinal lymph nodes. On May, 28, 2021, trephine biopsy of the left inguinal lymph node was performed.
Pathological examination revealed lack of a clear separation of the cortical and paracortical areas. Expanded proliferation of vessels (postcapillary venules) around which atypical lymphoid cells of small and medium size were located. Mitotic figures, single plasma cells, macrophages, neutrophils and eosinophils were present. The most of atypical lymphoid cells expressed CD3, CD4, PD1, blc-6, and Ki-67 antigens, some cells also CD10-, CD30-positive. Small clusters of CD20-positive cells were found between tumor cells. CD21- and CD23-positive follicular dendritic cells wrapped around postcapillary venules. Conclusion (8.06.2021): morphological changes in the lymph node and the immunophenotype of tumor cells correspond to diagnosis of angioimmunoblastic T cell lymphoma.
CT scan (29.05.2021) revealed pronounced lymphadenopathy above and below the diaphragm (diameters of cervical lymph nodes 10-24 mm, paratracheal lymph nodes 8-18 mm, bifurcation lymph nodes 14-25 mm, right axillary lymph nodes up to 20 mm, left axillary lymph nodes up to 15 mm, inguinal lymph nodes 8-28 mm), enlargement of the spleen (5.8x12.0x11.3 cm).
On June, 11, 2021 patient referred to the hematological department of National Research Center for Radiation Medicine. Complaints of the patient upon admission: fever (up to 380C) in the second half of the day, weakness, severe fatigue, enlargement of tonsils and lymph nodes. At examination: ECOG2 performance status. Weight 72 kg. Height 174 cm. Skin is pale. Peripheral lymph nodes are palpable: submaxillary up to 6 cm, cervical 4-6 cm, axillary up to 4 cm, inguinal up to 4 cm in diameter. The liver is not enlarged. The spleen is not palpable. Breathing is vesicular. Systolic murmur over the cardiac apex. The heart rhythm is correct. Blood pressure is 115/75 mm Hg, heart rate is 80 per min. There are no edema.
Bone marrow aspiration (11.06.2021) showed hypercellular marrow, dysplasia in erythropoiesis and megakariocytopoiesis, absence of blast cells, 5.6% of lymphocytes, no cytological signs of leukemic involvement. Among all nuclear bone marrow cells 1.68% with phenotype CD45dim+CD34+CD7+CD5+CD1a-TdT- were quantified by flow cytometry.
Except high level of blood lactate dehydrogenase (1249 units/L), and C-reactive protein (29.8 mg|L) other results of laboratory test of blood and urine were unremarkable.
Diagnosis of angioimmunoblastic T-cell lymphoma (AITL), IVB Ann Arbor stage, was established, and CHOEP (cyclophosphamide, vincristine, doxorubicin, etoposide and prednisone) regimen was initiated. The patient received six courses of therapy CHOEP regimen. According to the results of treatment clinical and hematological remission is achieved: hemodynamic parameters are stable, peripheral lymph nodes are not palpable, the spleen is not enlarged, and the size of the tonsils has decreased significantly. At the time of this report the patient continues to be in clinical and hematological remission.
Discussion
The most exciting, intriguing question is: can vaccines induce the development of tumors, in particular, lymphoproliferative diseases?
So far, only the development of fibrosarcomas in cats after vaccination is known. This has been proven for killed adjuvanted virus vaccines (vaccination against rabies and feline leukemia virus). An inflammatory process with the formation of granulomatous nodules, only 5% of which subsequently undergo transformation, preceded tumor development. The interval between vaccine administration and detection of sarcoma varied from 4 months to several years [17]. In human, we found three similar cases in the literature: the development of lymphoma and undifferentiated, pleiomorphic high-grade sarcoma at the site of anti-SARS-CoV-2 vaccine administration [16, 18], and peripheral T-cell lymphoma at the injection site of influenza vaccination [19]. However, the interval between vaccine administration and tumor development was short, which does not fit into the classical scheme for the development of the oncological process (initiation, promotion, progression). Thus, it is unclear whether there is a true association between vaccination and the development of malignancy.
For the case described in this article, progression of preceding lymphoma under the influence of the vaccine seems more likely. Anti-SARS-CoV-2 vaccines cause a powerful immune response and stimulation of T- and B-lymphocytes, as well as the production of numerous cytokines [20]. Cases of post vaccine benign lymphadenopathy are described [21]. Today, AITL is recognized as a neoplasm derived from T-follicular helper cells [22, 23]. Up to 75% of AITL patients had a mutation RHOA G17V that facilitates proliferation and activation of several signaling pathways [24]. Since T-follicular helper cells are one of the main targets of mRNA vaccines [25], the Goldman et al. suggested that their stimulation under the influence of the vaccine can accelerate the development of AITL. In their case the malignant cells of patient had a mutation RHOA G17V that could make them especially sensitive to anti-SARS-CoV-2 vaccines [9]. Unfortunately, molecular genetic studies have not been performed in our patient.
Thus, this article describes the second case of the development of AITL after vaccination. The accumulation of such data and their analysis will allow to make appropriate conclusions about the relationship of anti-SARS-CoV-2 vaccination with development of oncohematological diseases. It will contribute to our better understanding of the possible negative consequences of anti-SARS-CoV-2 vaccination and their prevention as well.
#Rapid progression#t cell lymphoma#hematological remission#Journal of Clinical Case Reports Medical Images and Health Sciences quartile.#jcrmhs
0 notes
Text
Resistance to anti-PD-1 therapy is driven by CD30+ regulatory T cell activity
Resistance to anti-PD-1 therapy is driven by CD30+ regulatory T cell activity Summary Resistance to anti-PD-1 immunotherapy, a common cancer treatment, is often linked to the suppressive activity of CD30-expressing regulatory T cells (Tregs). These CD30+ Tregs infiltrate the tumor microenvironment and dampen the immune response, effectively shielding cancer cells from the intended attack by…
#activity#antiPD1#Biomedicine#CD30#cell#driven#general#immunology#immunotherapy#Infectious diseases#regulatory#Resistance#therapy#Tumour immunology
0 notes
Text
Brentuximab vedotin (Working)
Brentuximab vedotin (Working) Brentuximab vedotin is a targeted therapy used to treat Hodgkin lymphoma and anaplastic large cell lymphoma (ALCL). It’s an antibody-drug conjugate (ADC) that combines an antibody that targets CD30, a protein found on the surface of cancer cells, with a potent anti-cancer drug called vedotin. Working: Target recognition: The antibody component of brentuximab vedotin…
0 notes
Text
Antibody Drug Conjugates Market To Grow As Of Increasing Adoption In Hematologic Malignancies
Antibody Drug Conjugates Industry Overview
The global antibody drug conjugates market size was estimated at USD 11.29 billion in 2023 and is projected to grow at a CAGR of 9.2% from 2024 to 2030. This can be attributed to the presence of a robust R&D pipeline. This, coupled with the growing incidence of cancer and increased demand for low-toxicity & effective drugs, is expected to drive market growth. Some of the key Antibody Drug Conjugates (ADCs) currently available in the market are Kadcyla (Genentech/Roche), Adcetris (Seattle Genetics), Enhertu (AstraZeneca/Daiichi Sankyo), Besponsa (Pfizer), Mylotarg (Pfizer), and Polivy (Genentech/Roche). ADCs are a novel category of drugs composed of an antibody linked-via a chemical linker-to a cytotoxic drug.
Gather more insights about the market drivers, restrains and growth of the Antibody Drug Conjugates Market
Conventional chemotherapy is intended to eradicate fast-growing tumor cells. However, it can also damage healthy proliferating cells, which may produce undesirable effects. In contrast, ADC is designed to upsurge the efficacy of treatment and decrease systemic toxicity. In this market, the landscape is influenced by reimbursement policies, a crucial factor in developed economies. The production and research costs of ADC drugs are high compared to conventional treatments, such as monoclonal antibodies and chemotherapy. This results in a higher overall treatment cost, which poses a challenge for reimbursement coverage. For instance, a negative review was given by the National Centre for Pharmacoeconomics in Ireland in February 2020 for Roche's Kadcyla (trastuzumab emtansine).
The review was based on the drug's perceived low cost-effectiveness, indicating a potential trend where expensive ADC treatments may face obstacles in gaining reimbursement approval. This dynamic highlights the delicate balance between innovation, cost, and access to cutting-edge medical solutions in the market. In contrast, some countries considered such costly ADC drugs for reimbursement due to high disease prevalence. In April 2021, the UK’s Cancer Drug Fund approved Enhertu (trastuzumab deruxtecan) for the reimbursement of Metastatic Breast Cancer (MBC) treatment. However, countries, such as Scotland, Northern Ireland, and Wales, are yet to provide reimbursement approval for the same. The lack of availability of reimbursement policies in developing economies, such as India and China, might restrict market growth.
Browse through Grand View Research's Pharmaceuticals Industry Research Reports.
• The global angina pectoris drugs market size was valued at USD 11.37 billion in 2023 and is projected to grow at a CAGR of 4.0% from 2024 to 2030. Advancements in personalized cardiovascular medicine and novel drug delivery systems are driving market expansion.
• The global pediatric vaccines market size was valued at USD 44.87 billion in 2023 and is projected to grow at a CAGR of 4.1% from 2024 to 2030. This is due to increased awareness of the benefits of vaccines worldwide, resulting in higher demand for childhood vaccines.
Antibody Drug Conjugates Market Segmentation
Grand View Research has segmented the global antibody drug conjugates market based on application, product, target, technology, and region:
Antibody Drug Conjugates Application Outlook (Revenue, USD Million, 2018 - 2030) • Blood Cancer o Leukemia o Lymphoma o Multiple Myeloma • Breast Cancer • Urothelial Cancer & Bladder Cancer • Other Cancer
Antibody Drug Conjugates Product Outlook (Revenue, USD Million, 2018 - 2030) • Kadcyla • Enhertu • Adcetris • Padcev • Trodelvy • Polivy • Others
Antibody Drug Conjugates Target Outlook (Revenue, USD Million, 2018 - 2030) • HER2 • CD22 • CD30 • Others
Antibody Drug Conjugates Technology Outlook (Revenue, USD Million, 2018 - 2030) • Type o Cleavable Linker o Non-cleavable Linker o Linkerless • Linker Technology Type o VC o Sulfo-SPDB o VA o Hydrazone o Others • Payload Technology o MMAE o MMAF o DM4 o Camptothecin o Others
Antibody Drug Conjugates Regional Outlook (Revenue, USD Million, 2018 - 2030) • North America o U.S. o Canada • Europe o Germany o UK o France o Italy o Spain o Denmark o Sweden o Norway • Asia Pacific o Japan o China o India o Australia o South Korea o Thailand • Latin America o Brazil o Mexico o Argentina • MEA o South Africa o Saudi Arabia o UAE o Kuwait
Order a free sample PDF of the Antibody Drug Conjugates Market Intelligence Study, published by Grand View Research.
Key Companies profiled: • Seagen, Inc. • Takeda Pharmaceutical Company Ltd. • AstraZeneca • F. Hoffmann-La Roche Ltd. • Pfizer, Inc. • Gilead Sciences, Inc. • Daiichi Sankyo Company Ltd. • GlaxoSmithKline Plc • Astellas Pharma, Inc. • ADC Therapeutics SA
Recent Developments
• In January 2024, Celltrion, Inc., and WuXi XDC signed a Memorandum of Understanding (MOU) for integrated services for ADCs, which includes the development & manufacturing of ADCs
• In January 2024, Jonhson & Johnson Services, Inc., acquired Ambrx Biopharma, Inc., which has a proprietary ADC technology to develop next-generation novel ADCs for the treatment of cancers. J&J aims to focus on its portfolio of prostate cancer while making use of this proprietary technology
• In January 2024, MediLink Therapeutics and F.Hoffmann-La Roche Ltd. entered into a license agreement and collaboration to develop a next-generation ADC, YL211
• In October 2023, BioNTech entered a licensing agreement with MediLink Therapeutics for an antibody-drug conjugate targeting HER3 in cancers. BioNTech paid MediLink USD70 million upfront, with potential milestone-based payments of up to USD 1 billion. The deal grants BioNTech global rights for development, manufacturing, and commercialization, while MediLink retains rights in Mainland China, Hong Kong, and Macau. This collaboration highlights the growing significance of ADCs in cancer treatment, with the financial infusion reflecting industry confidence in the potential therapeutic impact
• In July 2023, BeiGene secured an exclusive option for the global license of an investigational ADC. This move is set to shape the market landscape as it allows DualityBio to sustain its preclinical research activities and provide support for the Investigational New Drug filing. This strategic acquisition is indicative of the evolving dynamics in the ADC market, reflecting a potential shift in the competitive landscape
0 notes
Text
The demand for antibody drug conjugate was valued at USD 9984.20 million in 2023 and is expected to reach USD 26594.21 million in 2032, growing at a CAGR of 11.50% between 2024 and 2032.The Antibody-Drug Conjugate (ADC) market represents a groundbreaking intersection of biotechnology and pharmacology, offering a novel approach to cancer treatment. ADCs are sophisticated biopharmaceuticals designed to deliver cytotoxic drugs directly to cancer cells, minimizing damage to healthy tissues. This targeted therapy approach has gained substantial attention in recent years due to its potential to enhance the efficacy and safety profiles of cancer treatments. As the ADC market continues to expand, it is poised to revolutionize oncology and drive significant advancements in personalized medicine.
Browse the full report at https://www.credenceresearch.com/report/antibody-drug-conjugate-market
Market Overview
The global ADC market has witnessed rapid growth, driven by increasing cancer prevalence, technological advancements in drug development, and rising demand for targeted therapies. According to recent market research, the ADC market was valued at approximately USD 4.8 billion in 2022 and is expected to reach over USD 12 billion by 2028, growing at a compound annual growth rate (CAGR) of 16.7% during the forecast period.
This growth is fueled by the rising incidence of cancer worldwide, coupled with the limitations of conventional cancer therapies, such as chemotherapy and radiation, which often result in severe side effects. ADCs, by contrast, offer a more precise mechanism of action, delivering cytotoxic agents directly to cancer cells while sparing healthy tissues. This specificity reduces adverse effects and improves patient outcomes, making ADCs a promising option in the oncology landscape.
Technological Advancements
Advancements in ADC technology have been pivotal in driving market growth. Early-generation ADCs faced challenges such as low therapeutic indices, off-target toxicities, and limited efficacy. However, recent innovations have addressed these issues, leading to the development of more stable linkers, improved antibody engineering, and the use of highly potent cytotoxic agents.
Modern ADCs utilize cleavable linkers that release the drug payload only in the presence of specific enzymes or conditions within the cancer cell, ensuring targeted drug delivery. Additionally, advancements in monoclonal antibody engineering have enhanced the ability of ADCs to bind selectively to tumor-specific antigens, further improving their therapeutic potential.
Key Market Players
Several pharmaceutical companies are at the forefront of the ADC market, investing heavily in research and development to bring new ADCs to market. Notable players include:
1. Seagen Inc.: Known for its pioneering work in ADCs, Seagen’s ADC technology has led to the successful development of drugs like Adcetris, used in the treatment of Hodgkin lymphoma and other CD30-expressing lymphomas.
2. Roche: A global leader in oncology, Roche has developed Kadcyla, an ADC used in the treatment of HER2-positive breast cancer. Kadcyla combines the HER2-targeting properties of trastuzumab with a cytotoxic agent, offering a targeted approach to treating this aggressive form of breast cancer.
3. AstraZeneca: With the development of Enhertu, an ADC for HER2-positive breast cancer, AstraZeneca has further solidified its position in the ADC market. Enhertu’s unique design allows it to deliver a higher drug-to-antibody ratio, enhancing its therapeutic efficacy.
Challenges and Opportunities
Despite the promising outlook, the ADC market faces several challenges. The complexity of ADC development, high production costs, and stringent regulatory requirements can hinder market growth. Additionally, issues related to drug resistance and the need for personalized approaches to treatment pose ongoing challenges.
However, these challenges also present opportunities for innovation. Companies are exploring new ADC technologies, such as bispecific ADCs that target multiple antigens, and the use of alternative payloads to overcome drug resistance. Furthermore, ongoing research into biomarker-driven patient selection is expected to enhance the precision of ADC therapies, aligning them with the principles of personalized medicine.
Future Outlook
The future of the ADC market looks promising, with continued advancements in technology and a growing pipeline of ADC candidates in clinical trials. As more ADCs receive regulatory approval and enter the market, the adoption of this targeted therapy is expected to increase, offering new hope to cancer patients worldwide.
Moreover, the expanding application of ADCs beyond oncology, such as in the treatment of autoimmune diseases, presents additional growth opportunities. As research in this area progresses, ADCs may become a cornerstone of targeted therapy across various therapeutic areas.
Key Players
Seagen Inc.
Takeda Pharmaceutical Company Ltd.
AstraZeneca Plc.
F. Hoffmann-La Roche Ltd.
Pfizer Inc.
ImmunoGen Inc.
Gilead Sciences Inc.
Daiichi Sankyo Company Ltd.
Segmentation
By Product
Kadcyla
Enhertu
Adcetris
Padcev
Trodelvy
Polivy
Others
By Disease Type
Breast Cancer
Blood Cancer
Others
By Linker Type
Non-Cleavable
Cleavable
By Target
HER2
CD22
CD30
Others
By Payload Type
MMAE/Auristatin
Calicheamicin
Maytansinoids
Others
By Region
North America
The U.S
Canada
Mexico
Europe
Germany
France
The U.K.
Italy
Spain
Rest of Europe
Asia Pacific
China
Japan
India
South Korea
South-east Asia
Rest of Asia Pacific
Latin America
Brazil
Argentina
Rest of Latin America
Middle East & Africa
GCC Countries
South Africa
Rest of the Middle East and Africa
Browse the full report at https://www.credenceresearch.com/report/antibody-drug-conjugate-market
About Us:
Credence Research is committed to employee well-being and productivity. Following the COVID-19 pandemic, we have implemented a permanent work-from-home policy for all employees.
Contact:
Credence Research
Please contact us at +91 6232 49 3207
Email: [email protected]
Website: www.credenceresearch.com
0 notes
Text
BIA-ALCL: Clinical Features, Diagnosis, and Management
Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL) is a rare type of non-Hodgkin's lymphoma that occurs in the tissue surrounding breast implants. It primarily affects the immune system's T-cells and can present as a swelling or mass around the breast implant. Although rare, with an estimated lifetime risk of 1 in 1,000 to 1 in 15,000 depending on the implant type and data set, it is crucial for patients with breast implants to be aware of this condition.
BIA-ALCL is not a new disease, but it has only been recognized as a distinct entity since 2016 by the World Health Organization. The first reported case linked to breast implants was in 1997. Most cases are associated with textured implants, although it can occur with any type of breast implant.
Symptoms typically include persistent swelling or fluid accumulation around the implant, sometimes accompanied by pain or a lump. Diagnosis involves imaging studies, fluid analysis, and biopsy to identify characteristic CD30-positive and ALK-negative cells. Early detection and treatment, usually involving surgical removal of the implant and surrounding capsule, lead to a favorable prognosis. Advanced cases may require chemotherapy.
For more information, patients can access the "UK Guidelines on the Diagnosis and Treatment of BIA-ALCL," consult the MHRA updates, and refer to resources from the American Society of Plastic Surgeons and the FDA. It is important for patients to discuss the risks with their surgeons and report any unusual changes promptly.
For More Info Visit Us:- https://cosmeticbreastsurgeon.co.uk/bia-alcl/
0 notes
Text
CAR-T Therapy Market Share, Growth, Sales, Trends, Supply, Forecast 2030
The “CAR-T Therapy Market Share, Size, and Trends | 2030” is market research by The Insight Partners. The CAR-T Therapy market has perceived tides of change in the recent past. This study offers precise projections after detailed scrutiny of a range of factors impacting the business. Considering the present market scenario, this report brings forward correct predictions on revenue, market size, and CAGR of the CAR-T Therapy market. The novel market research which is based on a fact-based foundation is now accessible for purchase. This report can make a variance in wide decision-making and drive business forward in the right direction.
Business is no longer a game of instincts when it comes to capitalizing on new production lines. In a highly competitive CAR-T Therapy market, companies may face several challenges. Having trusted market research is always endorsed for both veteran and new entrants. CAR-T Therapy Market report presents a thorough analysis of local, regional, and global market scenarios through the following details.
Report Attributes
Details
Segmental Coverage
By Targeted Antigen
CD 19
BCMA
HER2
GD2
CD 20
CD22
CD30
CD33
HER1
Therapeutic Application
Acute Lymphocytic Leukemia
Chronic Lymphocytic Leukemia
Diffuse Large B-Cell Lymphoma
Follicular Lymphoma
Geography
North America
Europe
Asia Pacific
and South and Central America
Regional and Country Coverage
North America (US, Canada, Mexico)
Europe (UK, Germany, France, Russia, Italy, Rest of Europe)
Asia Pacific (China, India, Japan, Australia, Rest of APAC)
South / South & Central America (Brazil, Argentina, Rest of South/South & Central America)
Middle East & Africa (South Africa, Saudi Arabia, UAE, Rest of MEA)
Market Leaders and Key Company Profiles
Novartis International AG
Kite Pharma, Inc. (Gilead Sciences, Inc.)
Juno Therapeutics (Celgene Corporation)
Bluebird Bio, Inc. (Celgene Corporation)
Sorrento Therapeutics Inc.
Mustang Bio, Inc
Aurora Biopharma Inc.
Legend Biotech (Genscript Biotech Corporation)
Pfizer, Inc.
CARsgen Therapeutics, Ltd.
Other key companies
Competitive Landscape
Knowing the state of rivals is a strategically right move to outperform them. This report is the right place to explore key strategies, developments, and recent launches by key CAR-T Therapy market players. This report emphasizes an analysis of business strategies and expected growth opportunities for brands.
Key Coverings:
Current and Future Market Estimates- CAR-T Therapy Market Share, CAGR, and Forecast | 2030
Market Dynamics – Drivers, Challenges, Regional Trends, and Market Opportunities
Market Segmentation – Product, Application, End-use Industries, and Regional Growth Prospects.
Competition Matrix – Key Market Players and Strategies
Recent Developments and Innovation Contributing Market Growth
Need a Customized Market Research Report?
You can always share any specific requirements that you have, and our team will adjust the scope of research offerings as per your needs.
The following are some customizations our clients frequently ask for:
The CAR-T Therapy market report can be customized based on specific regions/countries as per the intention of the business
The report production was facilitated as per the need and following the expected time frame
Insights and chapters tailored as per your requirements.
Depending on the preferences we may also accommodate changes in the current scope.
Key Questions Addressed in the CAR-T Therapy Market Research Include:
What are present CAR-T Therapy market values, and what can be expected in the upcoming decade?
What are the key segments in the CAR-T Therapy market?
What is the regional distribution of the CAR-T Therapy market report?
What are the key players and their recent strategies?
What are the key factors driving CAR-T Therapy market growth?
What are regulatory concerns and requirements businesses have to compel?
About Us:
The Insight Partners is a one-stop industry research provider of actionable intelligence. We help our clients in getting solutions to their research requirements through our syndicated and consulting research services. We specialize in industries such as Semiconductor and Electronics, Aerospace and Defense, Automotive and Transportation, Biotechnology, Healthcare IT, Manufacturing and Construction, Medical Devices, Technology, Media and Telecommunications, Chemicals and Materials.
Contact Us: www.theinsightpartners.com
0 notes
Text
Biotinylated Human CD30 (C-6His-Avi)
Biotinylated Human CD30 (C-6His-Avi) Catalog number: B2023027 Lot number: Batch Dependent Expiration Date: Batch dependent Amount: 20 ug Molecular Weight or Concentration: N/A Supplied as: Lyophilized Powder Applications: a molecular tool for various biochemical applications Storage: -20°C Keywords: Tumor necrosis factor receptor superfamily member 8; CD30L receptor; Ki-1 antigen; Lymphocyte…
0 notes