#Antigen Self Test
Explore tagged Tumblr posts
Text
Top 3 Benefits of Antigen Rapid Self-Test Kits
With the advent of modern technology, the popularity of health monitoring systems has reached great heights. However, out of all the tools, the antigen rapid self-test kits have proved to be more efficient in identifying a variety of infectious diseases, including COVID-19, Monkeypox, and more. Such self-testing kits enable individuals to intervene early and opt for effective medical treatment at the right time.

Let’s explore the major advantages you can leverage from antigen rapid testing kits.
Fast Results :
Faster turnaround time is the crucial point of antigen test kits. They usually give positive or negative results approximately after 15-20 minutes, which is quite fast for the users to be able to know their wellbeing status. This is a very vital aspect especially in cases when quick decision is important such as during occasion or while travelling.
Easy to Use:
Antigen rapid tests are made straightforward. They can be done at home without specific equipment or professional expertise. Step-by-step instructions are provided to help the users understand the process. This removes any further confusion and allows you to diagnose accurately right at your home.
Economical:
Antigen rapid test kits are fairly affordable compared to other testing kits. These kits save money spent on hospital visits while ensuring to keep your health status at check.
Conclusion :
Antigen rapid self-test kits are a reliable and efficient way to keep you informed about your health. They are fast, easy to use, and affordable, making them a great option for home testing. For quality products that meet your needs,
visit POC Diagnostic for the best antigen test kits.
0 notes
Text
On/Go COVID-19 Rapid Antigen Self-Tests

The On/Go™ COVID-19 Antigen Self-Test is an over-the-counter, self-administered rapid test that delivers results in just 10 minutes, with 95% accuracy.* On/Go is proudly manufactured in the United States. This test is authorized for non-prescription home use for individuals with symptoms of COVID-19 within the first 7 days of symptom onset. The On/Go Self-Test is also suitable for individuals without symptoms or other epidemiological reasons to suspect a COVID-19 infection when tested twice over two or three days with at least 24 hours and no more than 48 hours between tests.
The On/Go 10-minute COVID-19 Antigen Self-Test is an over-the-counter (OTC) diagnostic tool designed to quickly and accurately detect the presence of SARS-CoV-2, the virus responsible for COVID-19.
Engineered for convenience and ease of use, this self-test provides reliable results in just 10 minutes, making it an ideal solution for individuals seeking quick and private testing at home. The On/Go test is designed for use by adults and children aged 2 years and older, with adult supervision recommended for children.
This self-test kit includes everything you need to perform the test: a nasal swab, test cassette, buffer solution, and detailed instructions. The On/Go test utilizes advanced immunoassay technology to deliver rapid and precise results, enabling timely decision-making regarding your health and safety.
95% accurate*
Rapid results at 10 minutes
Detects the antigen protein of all known major COVID-19 variants of concern (including Delta and Omicron)
Authorized for non-prescription self-use for individuals 14 years or older or adult-collected samples from individuals 2 years or older
For individuals with or without symptoms
Qualitative detection of SARS-CoV-2 nucleocapsid protein antigen via lower nasal swab samples
Only for use under the Emergency Use Authorization (EUA)
Single test for individuals with symptoms within 7 days of onset or two tests over two or three days for individuals without symptoms
For in vitro diagnostic use only
Specifications:
Test Type: Antigen self-test
Time to Results: 10 minutes
Sample Type: Nasal swab
Sensitivity: High (ability to correctly identify positive cases)
Specificity: High (ability to correctly identify negative cases)
Storage Conditions: Store at room temperature (15-30°C / 59-86°F)
Shelf Life: 12 months from the date of manufacture
Regulatory Status: FDA Emergency Use Authorization (EUA)
Shop Now: On/Go COVID-19 Antigen Self-Test
0 notes
Text
just had a rage inducing encounter with the american medical system
#.txt#note to self never spend a cent at walgreens again#made an appt for a pcr test and they tried to give me an antigen test…..which i have at home….when i specifically wanted pcr#lmaoooooooooo
0 notes
Text
Also preserved on our archive
Swab, swirl, squirt, sit tight. By now, you’re likely familiar with the ritual of performing a COVID test at home or on the go. Disease detection has evolved swiftly since 2020, when you may have had to seek out a pop-up testing clinic or wait hours in line at your local health department, with clinicians on standby to stick a cotton swab up your nose.
Rapid COVID tests have since become a staple of cough-and-cold aisles across America, and just weeks ago the federal government resumed its periodic distribution of four free tests per household. But the convenience of self-testing comes with a caveat: The onus is on you to report your results.
First, know that test reporting isn’t mandatory in the U.S., so you’re hardly in hot water if you haven’t documented your COVID infection or lack thereof with the Centers for Disease Control and Prevention (CDC) or state or local public health authorities. Though perhaps more people would self-report if it were a requirement, says Dr. Sujata Ambardar, an infectious disease specialist at Inova Fairfax Hospital in Falls Church, Va.
“People probably are not self-reporting because they don’t want to have to not go to work—or they may want to go to work—it’s different reasons, and they may not want other people to know,” Ambardar tells Fortune. “Human nature is that they’ll probably only do it if they have to do it. Once you make it non-mandatory, then they tend not to do it.”
Today, if you get an antigen or PCR COVID test in a clinical setting, it’s still your health care provider’s job to report your results. While you’re well within your rights not to report your home test results, not doing so can come as a detriment to public health.
Between Feb. 1, 2022, and Jan. 1, 2023, an estimated 54 million adult COVID cases were unaccounted for in official records, according to a national study published Sept. 30 in the journal JAMA Network Open. That’s more than twice as many as those documented. At the state level, unaccounted infections ranged from 59,000 in Wyoming to 6.3 million in California. Researchers cited the government-led mass distribution of at-home tests as a possible driver of the discrepancies.
Report COVID results at MakeMyTestCount.org In theory, Ambardar says, reporting your COVID test results is “just one extra step.” But when that second line appears on the test card, confirming the disease has hit your household, formally documenting the outcome may not be your first priority. Even if you’re negative, you were likely feeling poorly enough to take the test in the first place and may not feel up to self-reporting.
But if and when you choose to do so, visit MakeMyTestCount.org to securely and anonymously report both positive and negative COVID test results. The site is a collaboration between medical data firm Care Evolution and the National Institute of Biomedical Imaging and Bioengineering, part of the National Institutes of Health.
MakeMyTestCount.org is free to use and doesn’t require you to immediately self-report. In fact, your results don’t even have to be recent. As of early October, the site allowed for the input of home test results going back to November 2021. If you need proof of illness for work or school, the site provides documentation. And while over-the-counter tests that screen for both COVID and flu aren’t as common as those that screen for COVID alone, you can report those results on the site, too.
Keep in mind that it’s just as important to report negative results as positive ones. The week ended Sept. 21, national COVID test positivity was 11.6%, CDC records show, down from 13.4% the previous week. If not for the inclusion of negative results, test positivity would always be 100%.
Depending on the brand of COVID test you’re using, you may not have to visit MakeMyTestCount.org at all. Some brands, such as iHealth, offer a free corresponding smartphone app. With the tap of your finger, you can forward your results to the CDC and/or your health care provider. If you have the time, notifying your doctor can help guide the course of COVID treatment in your community, says Dr. Donald Dumford, an infectious disease specialist at Cleveland Clinic Akron General.
“The more we know about the true number of cases of COVID, the better we’re able to understand the transmission of COVID at this point in time, as we go from it being pandemic to endemic, which means it’s just something we live with now,” Dumford tells Fortune. “It also helps us to identify the potential rise of new strains of infection, especially if you’re seeing a strong uptick in cases.”
Study Link: jamanetwork.com/journals/jamanetworkopen/fullarticle/2824211
#mask up#covid#pandemic#wear a mask#covid 19#public health#coronavirus#sars cov 2#still coviding#wear a respirator
68 notes
·
View notes
Text
Attack of the Replicons
The Biopharmaceutical Complex's self-amplifying mRNA assault has already begun.
NICOLAS HULSCHER, MPH
NOV 07, 2024
By Nicolas Hulscher, MPH
The Biopharmaceutical Complex is preparing for the large-scale deployment of replicon (self-amplifying) mRNA injections. There are currently at least 33 candidatesin development.
These products behave like a synthetic virus. The replicon mRNA is designed to encode not only the target antigen but also viral replicase, enabling the mRNA to replicate itself within the target cells. This replication machinery allows for an unknown period of toxic antigen production. Concerningly, none of the clinical trials have addressed the major concern of product shedding.
The Coalition for Epidemic Preparedness Innovations (CEPI) and the Biomedical Advanced Research and Development Authority (BARDA) are the primary funders behind this technology to combat ‘Disease X’. This is an extremely high risk ‘vaccine’ platform that should be avoided at all costs. Cellular installation of synthetic replicons requires decades of intense safety testing.
The very first replicon injection for human use received emergency use authorization (EUA) from The Office of the Drugs Controller General of India (DCGI) back in June 2022: Gennova Biopharmaceuticals’ GEMCOVAC-19. This was followed by reckless EUA approval in June 2023 for GEMCOVAC-OM, a replicon booster shot that targets the Omicron strain.
In November 2023, Japan's Ministry of Health, Labor and Welfare (MHLW) fully approved CSL and Arcturus Therapeutics' replicon shot: KOSTAIVE ARCT-154. Dismissing all concerns, Japan’s MHLW approved the updated booster shot in September 2024 to target the JN.1 lineage of Omicron subvariants.
In the clinical trials for ARCT-154, 5 deaths occurred among the injected in study phase 3b. Injected participants experienced a 90% adverse event rate (74.5% systemic - 15.2% required medical attention) after the first dose in study phases 1, 2, and 3a combined. Many of the authors are full time employees of Arcturus Therapeutics, meaning their conclusions are likely biased.
Meanwhile, the USDA quietly approved an experimental self-amplifying RNA injection for dogs developed by Merck in June 2024: Nobivac NXT Canine Flu H3N2. It appears that Merck is attempting to camouflage the fact that this product is self-amplifying. The primary product description only indicates that it uses “revolutionary RNA particle technology.” However, the novel platform works by RNA particles targeting dendritic cells, where they self-replicate and result in sustained antigen production.
The possibility of product shedding from dogs to humans or other animals was never tested. This injection is currently widely available for online purchase and canine administration. While the Biopharmaceutical Complex struggles to get self-amplifying mRNA injections approved for humans, they seem to have no problem targeting our pets.
Deployment of this experimental platform has continued in September 2024 to target cats: Nobivac NXT FeLV. Nowhere in the product brochure does it mention the RNA self-amplification mechanism of action. They hope that unsuspecting vets won't think twice about accepting the 'new and improved' product. The so-called 'safety' data for this product is as follows:
“Demonstrated safety under field conditions" from "Data on file. Merck Animal Health."
In other words, no public safety data is provided. It's become abundantly clear that the pharmaceutical industry and captured regulatory agencies have zero regard for the massive safety concerns of undefined synthetic mRNA replication resulting in uncontrolled toxic antigen production. These experimental injections must not receive further regulatory approval for humans or animals if we are to prevent another public health disaster. All self-amplifying mRNA injections currently available for humans and animals should be immediately withdrawn until comprehensive, long-term safety studies are conducted.
7 notes
·
View notes
Text
A TMI PSA PSA
Overshare (if that’s even possible in Tumblr).
Today I’m going for an ultrasound exam so my doctor can better determine why two blood tests show my prostate specific antigen (PSA) count is climbing, this year.
It could be any of a number of things. A digital exam after the first blood test didn’t present obvious signs of prostate cancer, but the second blood test showed a climbing PSA number.
None of this is bad, or scary. By doing all this stuff, we’re getting ahead of the bad and the scary. This is self-care.
Anyway, why the overshare? Because this morning, I read that neglecting your health can be a kind of self-harm. And I’ve since learned that some people in the ace and aro and wider LGBTQIA+ communities can be at risk of the bad feelings that lead to wanting to self-harm.
If you’ve been ignoring your health, please please please please please consider that it might be because some part of you is doing that on purpose. Then tell someone. Even tell someone here. We’ll help. We love you. If reading this hurts a little, that’s okay. That can mean you know something might be wrong, or that you know someone who might be ignoring their health, too. It’s going to be okay.
14 notes
·
View notes
Text
Trends in incidence of COVID 19 based on performed Rapid Antigen Test by Piratheep kumar.R in Journal of Clinical Case Reports Medical Images and Health Sciences
Abstract
The COVID 19 outbreak represents a historically unprecedented pandemic, particularly dangerous and potentially lethal for elderly population. The biological differences in the immune systems between men and women exist which may impact our ability to fight an infection including SARS-2-CoV-2. Men tended to develop more symptomatic and serious disease than women, according to the clinical classification of severity. Age-related changes in the immune system are also different between sexes and there is a marked association between morbidity/mortality and advanced age in COVID-19. This is a single-center, retrospective, data oriented study performed at the private hospital, in Central Province, Sri Lanka. The data of the patients who performed the Rapid Antigen Test (RAT) to know whether they have infected by SARS-CoV-2 or not, were taken for analysis. Test performed date, age, sex, number of positive and negative cases, number of male and female patients were extracted. Finally the data were analyzed in simple statistical method according to the objective of the study. Totally 642 patients performed RAT within the period of one month from 11.08.2021 to 11.09.2021. Among them 426 (66.35%) are male and 216 (33.64%) are female. 20.4% (n=131) of male obtained positive result among the total male population (n=426). Likewise 11.4% (n=73) of female obtained positive result among the total male population (n=216). Large number of positive cases was observed (34.89%) between the age group of 31-40 years in both sexes. The age group of 21-30 and 41-50 years also were shared the almost same percentage (17.13% & 17.75). The large number of positive male patients observed among the age group of 41-50 years. Almost same number of patients was observed in the age group of 21-30 and 31-40. The least number of positive cases (0.7% and 0.9%) observed almost in 0-10 and 81-90 years. When considering the females, large number of positive female patients observed among the age group of 31-40 years.
Key words: Rapid Antigen Test, Covid-19, SARS-CoV-2
Introduction
A rapid antigen test (RAT) or rapid antigen detection test (RADT), is a rapid diagnostic test suitable for point-of-care testing that directly detects the presence of an antigen. It is used to detect SARS-CoV-2 that cause COVID-19. This test is one of the type of lateral flow tests that detect protein, differentiate it from other medical tests such as antibody tests or nucleic acid tests, of either laboratory or point-of-care types. Generally 5 to 30 minutes only will take to get result and, require minimal training or infrastructure, and cost effective (1).
Sri Lanka was extremely vulnerable to the spread of COVID-19 because of its thriving tourism industry and large expatriate population. Sri Lanka almost managed two waves of Covid-19 pandemic well, but has been facing difficulties to control the third wave. The Sri Lankan government has executed stern actions to control the disease including island-wide travel restrictions. The government has been working with its development partners to take necessary action to mobilize resources to respond to the health and economic challenges posed by the pandemic (2) (3).
The COVID 19 outbreak is dangerous and fatal for elderly population. Since the beginning of the actual SARS-CoV-2 outbreak there were an evident that older people were at higher risk to get the infection and develop a more severe with bad prognosis. The mean age of patients that died was 80 years. The majority of those who are infected, that have a self-limiting infection and do recover are younger. On the other hand, those who suffer with more severe disease require intensive care unit admission and finally pass away are older (4).
Sandoval. M., et al mentioned that the number of patients who are affected by SARS-CoV-2 with more than 80 years of age is similar to that with 65–79 years. The mortality rate in very elderly was 37.5% and this percentage was significantly higher compared to that observed in elderly. Further their findings were suggested that the age is a fundamental risk factor for mortality (5).
Since February 2020, more than 27.7 million people in US have been diagnosed with Covid-19 (6). Rates of COVID-19 deaths have increased across the Southern US, among the Hispanic population, and among adults aged 25–44 years (7). Young adults are at increased risk of SARS-CoV-2 because of exposure in work, academic, and social settings. According to the several database of different health organizations young adult, aged 18-29, were confirmed Coid-19 (9).
Go to:Amid of coronavirus disease 2019 (Covid-19) pandemic, much emphasis was initially placed on the elderly or those who have preexisting health conditions such as obesity, hypertension, and diabetes as being at high risk of contracting and/or dying of Covid-19. But it is now becoming clear that being male is also a factor. The epidemiological findings reported across different parts of the world indicated higher morbidity and mortality in males than females. While it is still too early to determine why the gender gap is emerging, this article point to several possible factors such as higher expression of angiotensin-converting enzyme-2 (ACE 2; receptors for coronavirus) in male than female, sex-based immunological differences driven by sex hormone and X chromosome. Furthermore, a large part of this difference in number of deaths is caused by gender behavior (lifestyle), i.e., higher levels of smoking and drinking among men compared to women. Lastly, studies reported that women had more responsible attitude toward the Covid-19 pandemic than men. Irresponsible attitude among men reversibly affect their undertaking of preventive measures such as frequent handwashing, wearing of face mask, and stay at home orders.
The latest immunological study on the receptors for SARS-CoV-2 suggest that ACE2 receptors are responsible for SARS-CoV-2. According to the study by Lu and colleagues there are positive correlation of ACE2 expression and the infection of SARS-CoV (10). Based on the positive correlation between ACE 2 and coronavirus, different studies quantified the expression of ACE 2 proteins in human cells based on gender ethnicity and a study on the expression level and pattern of human ACE 2 using a single-cell RNA-sequencing analysis indicated that Asian males had higher expression of ACE 2 than female (11). Conversely, in establishing the expression of ACE 2 in the primary affected organ, a study conducted in Chinese population found that expression of ACE 2 in human lungs was extremely expressed in Asian male than female (12).
A study by Karnam and colleagues reveled that CD200-CD200R and sex are host factors that together determine the outcome of viral infection. Further a review on association between sex differences in immune responses stated that sex-based immunological differences contribute to variations in the susceptibility to infectious diseases and responses to vaccines in males and females (13). The concept of sex-based immunological differences driven by sex hormone and X chromosome has been well demonstrated via the animal study by Elgendy et al (14) (35). They were concluded the study that estrogen played big role in blocking some viral infection.
The biological differences in the immune systems between men and women may cause impact on fight for infection. Females are more resistant to infections than men and which mediated by certain factors including sex hormones. Further, women have more responsible attitude toward the Covid-19 pandemic than men such as frequent hand washing, wearing of face mask, and stay at home (15).
Most of the studies with Covid-19 patients indicate that males are mostly (more than 50%) affected than females (16) (17) (18). Although the deceased patients were significantly older than the patients who survived COVID-19, ages were comparable between males and females in both the deceased and the patients who survived (18).
A report in The Lancet and Global Health 5050 summary showed that sex-disaggregated data are essential to understanding the distribution of risk, infection and disease in the population, and the extent to which sex and gender affect clinical outcomes (19). The degree of outbreaks which affect men and women in different ways is an important to design the effective equitable policies and interventions (20). A systematic review and meta-analysis conducted to assess the sex difference in acquiring COVID-19 with 57 studies that revealed that the pooled prevalence of COVID-19 confirmed cases among men and women was 55% and 45% respectively (21). A study in Ontario, Canada showed that men were more likely to test positive (22) (23). In Pakistan 72% of COVID-19 cases were male (24). Moreover, the Global Health 5050 data showed that the number of COVID-19 confirmed cases and the death rate due to the disease are high among men in different countries. This might be because behavioral factors and roles which increase the risk of acquiring COVID-19 for men than women. (25) (26) (27).
Men mostly involved in several activities such as alcohol consumption, being involved in key activities during burial rites, and working in basic sectors and occupations that require them to continue being active, to work outside their homes and to interact with other people even during the containment phase. Therefore, men have increased level of exposure and high risk of getting COVID-19 (28) (29) (30).
Men tended to develop more symptomatic and serious disease than women, according to the clinical classification of severity (31). The same incidence also noticed during the previous coronavirus epidemics. Biological sex variation is said to be one of the reasons for the sex discrepancy in COVID-19 cases, severity and mortality (32) (33). Women are in general able to stand a strong immune response to infections and vaccinations (34).
The X chromosome is known to contain the largest number of immune-related genes in the whole genome. With their XX chromosome, women have a double copy of key immune genes compared with a single copy in XY in men. This showed that the reaction against infection would be contain both innate and adaptive immune response. Therefore the immune systems of females are generally more responsive than females and it indirectly reflects that women are able to challenge the coronavirus more effectively but this has not been proven (32).
Sex differences in the prevalence and outcomes of infectious diseases occur at all ages, with an overall higher burden of bacterial, viral, fungal and parasitic infections in human males (36) (37) (38) (39). The Hong Kong SARS-CoV-1 epidemic showed an age-adjusted relative mortality risk ratio of 1.62 (95% CI = 1.21, 2.16) for males (40). During the same outbreak in Singapore, male sex was associated with an odds ratio of 3.10 (95% CI = 1.64, 5.87; p ≤ 0.001) for ITU admission or death (41). The Saudi Arabian MERS outbreak in 2013 - 2014 exhibited a case fatality rate of 52% in men and 23% in women (42). Sex differences in both the innate and adaptive immune system have been previously reported and may account for the female advantage in COVID-19. Within the adaptive immune system, females have higher numbers of CD4+ T (43) (44) (45) (46) (47) (48) cells, more robust CD8+ T cell cytotoxic activity (49), and increased B cell production of immunoglobulin compared to males (43) (50). Female B cells also produce more antigen-specific IgG in response to TIV (51).
Age-related changes in the immune system are also different between sexes and there is a marked association between morbidity/mortality and advanced age in COVID-19 (52). For example, males show an age-related decline in B cells and a trend towards accelerated immune ageing. This may further contribute to the sex bias seen in COVID-19 (53).
Hence, this single center, retrospective, data oriented study performed to identify the gender age influences the RAT results and the rate of positive cases before and after the lockdown.
Methodology
This is a single-center, retrospective, data oriented study performed at the private hospital, Central Province, Sri Lanka. The data of the patients who performed the Rapid Antigen Test (RAT) from 11.08.2021to 11.0.2021 to know whether they have infected by SARS-CoV-2 or not, were taken for analysis. The authors developed a data extraction form on an Excel sheet and the following data from main data sheet. Test performed date, age, sex, number of positive and negative cases, number of female patients and number of male patients were extracted. Mistyping of data was resolved by crosschecking. Finally the data were analyzed in simple statistical method according to the objective of the study.
Results and discussion
Totally 642 patients performed RAT within the period of one month from 11.08.2021 to 11.09.2021. Among them 426 (66.35%) are male and 216 (33.64%) are female. Men mostly involved in several activities such as alcohol consumption, being involved in key activities during burial rites, and working in basic sectors and occupations that require them to continue being active, to work outside their homes and to interact with other people even during the containment phase. Therefore, men have increased level of exposure and high risk of getting COVID-19 (28) (29) (30). The present data descriptive study also were supported certain previous research findings.
The number of male patients got positive result in RAT among the total male patients who performed RAT on every day. According to that, 20.4% (n=131) of male obtained positive result among the total male population (n=426). Philip Goulder, professor of immunology at the University of Oxford stated that women’s immune response to the virus is stronger since they have two X chromosomes which is important when talk about the immune response against SARS-Cov-2. Because the protein by which viruses such as coronavirus are detected is fixed on the X chromosome. This is exactly looks like females have double protection compare to male. The present study also showed that large number of RAT positive cases were observed in males compare to females. Gender based lifestyle would have been another possibility for large number of males got positive in RATs. There are important behavioral differences between the sexes according to certain previous research findings (54).
Shows that the number of female patients got positive result in RAT among the total female patients who performed RAT on every day. According to that, 11.4% (n=73) of female obtained positive result among the total male population (n=216).
The relations between the number of positive cases before and after the lockdown. The lockdown declared by the tenth day from the initial day when the data was taken for analysis. The red vertical line differentiates the period as two such as before and after the lockdown. Though there was no decline observed as soon as immediately considerable decline was observed after the 21 days of onset of lockdown. Staying at home, avoiding physical contacts, and avoiding exposure in crowded areas are the best way to prevent the spread of Covid – 19 (54). However the significant decline would be able to see after three weeks only from the date of lockdown since the incubation period of SARS-CoV-2 is 14-21 days. The continuous study should be conducted in order to prove it. However the molecular mechanism of COVID-19 transmission pathway from human to human is still not resolved, the common transmission of respiratory diseases is droplet sprinkling. In this type of spreading, a sick person is exposed to this microbe to people around him by coughing or sneezing. Only the way to prevent these kind of respiratory diseases might be prevent the people to make close contact (54) (55). Approximately 214 countries reported the number of confirmed COVID-19 cases (56). Countries including Sri Lanka have taken very serious constraints such as announced vacation for schools, allowed the employers to work from home and etc. to slow down the COVID 19 outbreak. The lockdown days differ by countries. Countries have set the days when the lockdown started and ended according to the COVID-19 effect on their public. Some countries have extended the lockdown by many days due to COVID-19 continues its influence intensely on the public (57) (58).
The incidence of Covid-19 and age group. Accordingly large number was observed (34.89%) between the age group of 31-40 years in both sexes. The age group of 21-30 and 41-50 years also were shared the almost same percentage (17.13% & 17.75). A study provides evidence that the growing COVID-19 epidemics in the US in 2020 have been driven by adults aged 20 to 49 and, in particular, adults aged 35 to 49, before and after school reopening (59). However many researches pointed out that adults over the age of 60 years are more susceptible to infection since their immune system gradually loses its resiliency.
The relations between the positive number of male & female patients and the age group of total patients. According to that the large number of positive male patients observed among the age group of 41-50 years. Almost same number of patients was observed in the age group of 21-30 and 31-40. The least number of positive cases (0.7% and 0.9%) observed almost in 0-10 and 81-90 years. When considering the females, large number of positive female patients observed among the age group of 31-40 years. In USA Ministry of Health has reported 444 921 COVID-19 cases and 15 756 deaths as of August 31. For men, most reported cases were persons aged 30–39 years (22.7%), followed by 20–29 year-olds (20.1%) and 40–49 year-olds (17.1%). Most reported deaths were seniors, especially 70–79 year-olds (29.5%), followed by those aged 80 years and older (29.2%), and 60–69 year-olds (22.8%). Also found a similar pattern for women, except that most deaths were reported among women aged 80 years and older (44.4%) (60).
Conclusion
The present study showed that the male are mostly got positive in RAT test than female. Further comparing the old age young age group in both sexes were noticed as positive in RAT. Moreover there were no relationship observed before and after the lockdown and trend of Covid-19
The limitations of the study
This study has several limitations.
Only 1 hospital was studied.
More than the absence of specific data on mobility patterns or transportation, detail of recovery, detail of mortality etc.
The COVID-19 pandemic is still ongoing so statistical analysis should continue. There are conflicting statements regarding lockdown by countries on COVID-19.
The effect of the lockdown caused by the COVID-19 pandemic on human health may be the subject of future work.
#Rapid Antigen Test#Covid-19#SARS-CoV-2#jcrmhs#Journal of Clinical Case Reports Medical Images and Health Sciences#Clinical decision making#Clinical Images submissions
4 notes
·
View notes
Text
I live in the US, and I for the price of 11 standard consumer rapid covid tests (at the lowest price), I got 25 four-in-one rapid tests (covid, influenza a, influenza b, and RSV). Including the $40 shipping cost. (That works out to $4.40 per test btw)

The kind I got don't have English instructions, oops. Should have checked that.
But I highly recommend these.
(Mine don't expire until October 2025)
You can buy in low numbers, too. I just decided to get enough for a year.
https://www.maskwholesale.eu/rapid-tests/self-tests/fluorecare-sars-cov-2-influenza-a-b-rsv-4in1-antigen-self-test_350_1606/
#best price on covid tests here is US$10 each#that i paid less for actual approved shit from europe for less even with expensive-ass shipping is kinda horrifying#also#wear a damn mask#disabled#idk what tags to use everyone in the US should see this#id in alt
8 notes
·
View notes
Text
Undergrad research blast from the past. Here I am in 2020 assembling a micro fluidic flow cell with a gold electrode block. I think I took this video for myself so I knew what to clip to what. This was when I worked with electrochemical sensors, transducing signals via impedance spectroscopy.
A lot of electrochemical techniques rely on measuring voltages or currents, but in this lab we looked at impedance- which is a fancy combination of regular resistance (like the same one from ohms law) and the imaginary portion of the resistance that arises from the alternating current we supply.
I would functionalize different groups on the gold working electrode by exposing the surface to a solution of thiolated biomarker capture groups. Thiols love to form self-assembled mono layers over gold, so anything tagged with thiol ends up sticking. [Aside: Apparently after I left the group they moved away from gold thiol interactions because they weren't strong enough to modify the electrode surface in a stable and predictable way, especially if we were flowing the solution over the surface (which we wanted to do for various automation reasons)]. The capture groups we used were various modified cyclodextrins- little sugar cups with hydrophobic pockets inside and a hydrophilic exterior. Cyclodextrins are the basis of febreeze- a cyclodextrin spray that captures odor molecules in that hydrophobic pocket so they can't interact with receptors in your nose. We focused on capturing hydrophobic things in our little pocket because many different hydrophobic biomarkers are relevant to many different diseases, but a lot of sensors struggle to interact with them in the aqueous environment of bodily fluids.
My work was two fold:
1) setting up an automated system for greater reproducibility and less human labor. I had to figure out how to get my computer, the potentiostat (which controls the alternating current put in, and reads the working electrode response), the microfluidic pump, and the actuator that switched between samples to all talk to each other so I could set up my solutions, automatically flow the thiol solution for an appropriate time and flow rate to modify the surface, then automatically flow a bio fluid sample (or rather in the beginning, pure samples of specific isolated biomarkers, tho their tendency to aggregate in aqueous solution may have changed the way they would interact with the sensor from how they would in a native environment, stabilized in blood or urine) over the electrode and cue the potentiostat for multiple measurements, and then flow cleaning solutions to clean out the tubings and renew the electrode. This involved transistor level logic (pain) and working with the potentiostat company to interact with their proprietary software language (pain) and so much dicking around with the physical components.
2) coming up with new cyclodextrin variants to test, and optimizing the parameters for surface functionalization. What concentrations and times and flow rates to use? How do different groups around the edge of the cyclodextrin affect the ability to capture distinct classes of neurotransmitters? I wasn't working with specific sensors, I was trying to get cross reactivity for the purpose of constructing nonspecific sensor arrays (less akin to antibody/antigen binding of ELISAs and more like the nonspecific combinatorial assaying you do with receptors in your tongue or nose to identify "taste profiles" or "smell profiles"), so I wanted diverse responses to diverse assortments of molecules.
Idk where I'm going with this. Mostly reminiscing. I don't miss the math or programming or the physical experience of being at the bench (I find chemistry more "fun") but I liked the ultimate goal more. I think cross reactive sensor arrays and principle component analysis could really change how we do biosample testing, and could potentially be useful for defining biochemical subtypes of subjectively defined mental illnesses.... I think that could (maybe, possibly, if things all work and are sufficiently capturing relevant variance in biochemistry from blood or piss or sweat or what have you) be a more useful way to diagnose mental illness and correlate to possible responses to medications than phenotypic analysis/interviews/questionnaires/trial and error pill prescribing.
4 notes
·
View notes
Text

We are factory
3 in 1 COVID-19/Influence A+B Combo Test Cassette (self test)
Austrilia TGA ARTG No. 404883
Hong kong MDD HKMD No. 230344
ISO 13485 and ISO9001 Quality System Production
CE certificate
1/5 test in a box
Production time 7-10 days
2 notes
·
View notes
Text
Home PCR Tests: A Closer Look at the PCR Test At Home Dubai Option
The COVID-19 pandemic sparked major growth in the development and usage of diagnostic and antibody tests that patients can self-administer from home. Home PCR tests in particular enable private, convenient detection of active coronavirus infections. For those wondering whether accurate PCR Test At Home Dubai kits are available, exploring the leading options provides helpful guidance.

How Do Home PCR Tests for COVID-19 Work?
The PCR (polymerase chain reaction) technique is the gold standard for directly detecting the presence of the COVID-19 virus from respiratory samples. Home PCR test kits allow patients to collect their own nasal or saliva samples and perform the PCR assay without visiting a clinic.
PCR tests work by identifying the specific genetic material of the COVID-19 virus. Users collect a sample, mix it with chemical reagents, and insert the solution into the test kit for analysis. Results are displayed indicating whether viral genetic material was detected based on any color change reaction on the test strips.
Kits include step-by-step instructions to ensure patients perform the easy, quick tests properly using non-invasive nasal swabs or saliva collection. Many provide results within 10-30 minutes.
Here is a video from MedCram Youtube Channel about At Home Rapid COVID 19 Tests and False Positives (Coronavirus Antigen Tests). Watch the video
youtube
Benefits of At-Home PCR Testing
Here are some of the major advantages of having access to accurate home PCR tests for COVID-19:
Convenience: Test from the privacy of your residence without traveling to clinics.
Speed: Get results rapidly within minutes rather than waiting days for lab tests.
Self-Administered: Users can collect their own sample comfortably rather than relying on technicians.
Affordability: Individual kits are very competitively priced.
Detection Reliability: PCR technology directly identifies viral presence with high accuracy.
Ease of Use: Tests have simple, straightforward instructions for patients of all ages.
Infection Verification: Confirms active infections unlike antibody tests.
Having the option to privately, quickly, and accurately test for possible COVID-19 infections at home provides significant peace of mind during the pandemic.
How Reliable Are Home PCR Tests?
Many people reasonably wonder whether DIY home PCR test kits can match the reliability of lab-based PCR tests. The good news is that leading home PCR kits on the market have very high accuracy.
Most kits have published sensitivity and specificity above 90% when compared to lab PCR tests. High quality home tests analyze samples using comparable PCR methodology and match labs in detecting positives and negatives.
Furthermore, unlike Rapid PCR Test At Home kits some vendors offer, full home PCR tests analyze the sample through many amplification cycles to maximize accuracy. With good sampling collection, top home PCR kits offer laboratory-grade results conveniently at home.
Leading Home PCR Test Kit Options
For those exploring PCR Test At Home Dubai choices, here are some of the top-rated home PCR kits to consider:
Cue Health PCR Test: Cue offers an FDA-authorized home PCR test delivering highly accurate results in 20 minutes with nasal swab samples.
Lucira Check It PCR Test: This is a single-use PCR kit with 98% validated accuracy that provides molecular-level detection from nasal samples in 30 minutes or less.
Ellume COVID-19 Home Test: This over-the-counter home kit uses a mid-turbinate nasal sample and provides an amplified PCR digital reading of positive or negative in 15 minutes on a connected analyzer.
Pixel by LabCorp PCR Test: Pixel is a monitored at-home nasal PCR test analyzed through LabCorp with over 98% accuracy returning results within 1-2 days.
Doximity's Covid-19 PCR Test: Doximity partners with qualified labs for monitored video-observed PCR testing with 97%+ accuracy and results in 24 hours.
All these options allow for convenient, accurate at-home COVID-19 testing using PCR with trusted partners. Kits can be purchased online and shipped directly to your home in Dubai.
When Are Home PCR Tests Recommended?
The CDC recommends utilizing home PCR tests in situations such as:
If you have any symptoms of COVID-19. Home testing allows quick confirmation.
After exposure events to quickly check for possible infection.
Before visiting individuals at higher risk for severe illness.
Before travel or group events for added assurance.
For frequent screening in schools or workplaces.
Even fully vaccinated individuals should test if they experience COVID-like symptoms or have a known exposure. Home PCR tests make quick detection fast and easy.
Home PCR Tests Offer Accuracy and Convenience
High quality Home PCR Tests have become an important tool in the fight against COVID by making reliable diagnostic testing accessible outside of clinics. There are excellent PCR Test At Home Dubai options available matching the standards of lab PCR sensitivity and specificity. Home PCR kits allow people to conveniently and confidently check themselves for possible COVID-19 infections from the privacy of home. As the technology continues advancing, home collection PCR will likely take on an increasingly vital role supporting public health and safety.
2 notes
·
View notes
Text
This is a HUGE topic in transfusion lab medicine in Canada right now and that model is a great solution to a very specific problem.
If you need a transfusion, you need blood that is compatible with yours. ABO is the basic blood typing set, but there are literally hundreds of types that may or may not be important in an individual match. One of the factors that makes any given one important or not is whether the person receiving the blood can carry a child.
The why: there's a bunch of blood proteins (antigens) that your body can essentially develop an allergy to. By allergy, I mean you produce an immune response to destroy the thing foreign to you. You start the process once you've been exposed to blood with a different protein than yours. For many proteins, it takes you longer to produce the antibody against them than a blood cell actually lives, meaning the first time you're exposed, nothing happens until after you're back to making your own blood. But they stay perpetually ready, meaning the second time, you will fully attack.
If you can't have a child, it's fine if you develop this allergy because we test for it every single time we give you more blood. Allergy gained = no more blood with that protein in it. Even if you accidentally get some, you'll start a reaction - and if your temperature rises even a little bit, the transfusion is immediately stopped and you are watched like a hawk for further symptoms. Typically you'd destroy the blood, but 1) you really need that blood and 2) too many blood cell fragments too quickly fuck up your liver, so it's still worth avoiding.
If you can get pregnant AND your partner makes that protein AND passes it down to your kid AND the defence you make to the protein is small enough to cross the barrier of the placenta: you are going to start destroying your kid's blood. It probably won't feel great on your end either (see above), but the main damage is the miscarriage. Most people are also not taking in blood protein compatibility into who they choose to try having kids with, either. Most common example is the D antigen: if your blood type is positive or negative. Multiple kids also trigger this, so we use RhoGAM to stop negative moms from reacting to their second positive child.
The problem: Labs currently define "who can have a kid" as male/female. As my specific lab person role, I will never meet you. I don't need your gender, which is all I am getting. This causes problems in so many places in the lab: UTI's you can only catch with a shorter urethra, literally everywhere in chemistry/hematology for "normal range" results typically split by gender, but the biggest is blood, for both danger and money.
Currently, there's a push for M/F/X where X is considered not typical M or F. But in practice, it's still being used for gender - which is an improvement for the they/them nonbinary crowd, but still misses some trans & intersex folk getting incorrect healthcare for their body's capabilities.
This whole system also relies on self identification, which is a problem. When polled, trans people did not feel safe self identifying anytime they went to the ER. Fair, being out for a broken arm isn't necessary. But we keep records, so it's not like you can give one gender for your broken arm and a different one for a car crash needing transfusion.
Also, to do my job fast, I'd really like to be able to reduce you all to a binary of characteristics. It will make me more accurate, which gives you better healthcare. Can you carry a child? Yes/no. A maybe is considered a yes. Expected iron levels, even if I've never tested you before? High range / low range. For every "it's more complex than that", problems arise: I look up info in your medical record, which gives me way more info than I need or want about you (and also takes so fucking long to load) and risks me making the wrong decision rather than following the documented path. Anyone who works at 3am loves a good documented path.
I really like that this checkbox model gives me those binaries without reducing you, the patient, to a binary. In terms of a database, the needed field could appear on my screen for the shell of each relevant test. If I'm testing you for an STI, I don't care whether or not you have breasts. Not my business.
Throw in a preferred pronouns box for the nurse's benefit and please scrap the current M/F system.
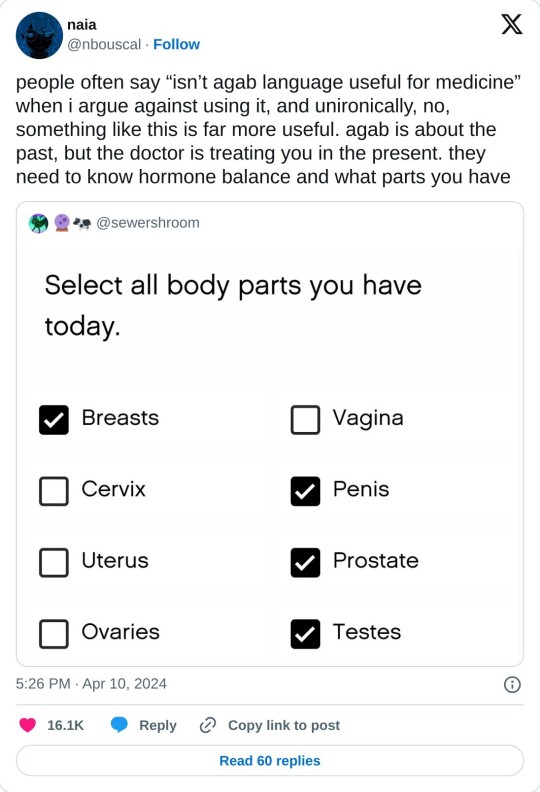
this is a way better model... you'll still get transphobic & intersexist drs of course but i prefer this to male / female or even having separate questions for gender & sex.
[we can't see the full form, but i'd suggest having a "something else" option and dominant hormone question too.]
79K notes
·
View notes
Text
Reference archived on our website
Published in 2023. Proof positive that just breathing spreads covid over a large area. Mask up. Ventilate. Clean the air.
Summary Background Effectively implementing strategies to curb SARS-CoV-2 transmission requires understanding who is contagious and when. Although viral load on upper respiratory swabs has commonly been used to infer contagiousness, measuring viral emissions might be more accurate to indicate the chance of onward transmission and identify likely routes. We aimed to correlate viral emissions, viral load in the upper respiratory tract, and symptoms, longitudinally, in participants who were experimentally infected with SARS-CoV-2.
Methods In this phase 1, open label, first-in-human SARS-CoV-2 experimental infection study at quarantine unit at the Royal Free London NHS Foundation Trust, London, UK, healthy adults aged 18–30 years who were unvaccinated for SARS-CoV-2, not previously known to have been infected with SARS-CoV-2, and seronegative at screening were recruited. Participants were inoculated with 10 50% tissue culture infectious dose of pre-alpha wild-type SARS-CoV-2 (Asp614Gly) by intranasal drops and remained in individual negative pressure rooms for a minimum of 14 days. Nose and throat swabs were collected daily. Emissions were collected daily from the air (using a Coriolis μ air sampler and directly into facemasks) and the surrounding environment (via surface and hand swabs). All samples were collected by researchers, and tested by using PCR, plaque assay, or lateral flow antigen test. Symptom scores were collected using self-reported symptom diaries three times daily. The study is registered with ClinicalTrials.gov, NCT04865237.
Findings Between March 6 and July 8, 2021, 36 participants (ten female and 26 male) were recruited and 18 (53%) of 34 participants became infected, resulting in protracted high viral loads in the nose and throat following a short incubation period, with mild-to-moderate symptoms. Two participants were excluded from the per-protocol analysis owing to seroconversion between screening and inoculation, identified post hoc. Viral RNA was detected in 63 (25%) of 252 Coriolis air samples from 16 participants, 109 (43%) of 252 mask samples from 17 participants, 67 (27%) of 252 hand swabs from 16 participants, and 371 (29%) of 1260 surface swabs from 18 participants. Viable SARS-CoV-2 was collected from breath captured in 16 masks and from 13 surfaces, including four small frequently touched surfaces and nine larger surfaces where airborne virus could deposit. Viral emissions correlated more strongly with viral load in nasal swabs than throat swabs. Two individuals emitted 86% of airborne virus, and the majority of airborne virus collected was released on 3 days. Individuals who reported the highest total symptom scores were not those who emitted most virus. Very few emissions occurred before the first reported symptom (7%) and hardly any before the first positive lateral flow antigen test (2%).
Interpretation After controlled experimental inoculation, the timing, extent, and routes of viral emissions was heterogeneous. We observed that a minority of participants were high airborne virus emitters, giving support to the notion of superspreading individuals or events. Our data implicates the nose as the most important source of emissions. Frequent self-testing coupled with isolation upon awareness of first symptoms could reduce onward transmissions.
#mask up#covid#pandemic#covid 19#wear a mask#public health#coronavirus#sars cov 2#still coviding#wear a respirator
48 notes
·
View notes
Text
I was about to scroll past this until the fact check because of all the misinformation. For context: I am currently moving out of a severe case of long covid that I've had for almost 4 years, and that worsened 15 months ago with an omicron reinfection. This, by the way, gives me personal anecdotal evidence and has resulted in increased precautions and self-education, but nevertheless it does not make me an expert.
One thing I get particularly angry about is the accusations against the CDC. The biggest red flag in that zine is the subjective statement that the CDC shortened their covid isolation guidelines because of pressure from Delta airlines as if it were fact. There is no concrete information to back this up, however I have repeatedly seen this claim made in a dangerous context, where sharing this misinformation also spreads misinformation about the actual isolation guidelines, which are science-based and have been implemented in numerous countries.
The claim I keep seeing is that the CDC has said that anyone exposed to covid can return to work after 5 days, which is incorrect. Their guidelines are:
Key times to get tested:
If you have symptoms, test immediately.
If you are only going to take a single test, a PCR test will provide a more reliable negative test result.
If you use an antigen test, a positive result is reliable, but a negative test is not always accurate.
If your antigen test is negative, take another antigen test after 48 hours or take a PCR test as soon as you can.
If you do not have symptoms but have been exposed to COVID-19, wait at least 5 full days after your exposure before taking a test.
If you are only going to take a single test, a PCR test will provide a more reliable negative test result.
If you use an antigen test, a positive result is reliable, but a negative test is not always accurate.
If your antigen test is negative, take another antigen test after 48 hours or take a PCR test as soon as you can.
If your second antigen test is also negative, wait another 48 hours and test a third time.
Testing can be helpful even when you don’t have symptoms or a recent exposure to COVID-19, such as before an event or visiting someone at higher risk. Test as close to the time of the event as possible (at least within 1-2 days) to help you make informed decisions about your health and your risk of spreading COVID-19 to others. If you use an antigen test, follow recommendations for repeat testing to be confident in a negative result. Additionally, some places may test people without symptoms or a recent exposure to help keep COVID-19 from spreading to others, especially those who are at higher risk for severe illness.
...
If Your COVID-19 Test Is
Positive
Any positive COVID-19 test means the virus was detected and you have or recently had an infection.
Isolate and take precautions, including wearing a high-quality mask or respirator, to protect others around you from getting infected.
Tell people you had recent contact with that they may have been exposed.
Monitor your symptoms. If you have any emergency warning signs, seek emergency care immediately.
Contact a healthcare provider, community health center, or pharmacy to learn about treatment options that may be available to you. Treatment must be started within the first few days to be effective.
You are more likely to get very sick if you are an older adult or have an underlying medical condition. Treatment is available. Talk with your healthcare provider to determine what is the best option for you.
At the time that the CDC changed its guidance from 10 days of isolation time to 5 days, they specified very clearly that if you test positive, you must continue to isolate until you no longer have symptoms and are testing negative, or for those people who are asymptomatic, to continue isolating for at least 5 more days. This was based on studies done on how long it took symptomatic and asymptomatic covid patients to stop shedding virus. In the current guidance, the healthcare provider, community health center, or pharmacy you're supposed to contact should tell you to continue isolating until your symptoms are gone and you test negative.
Why is this important? Because by spreading misinformation to justify being angry at the CDC, zines like the above one are also failing to encourage workers to know their rights and government protections when it comes to enforcing their workplaces to give them the necessary time off. Frankly, it makes me livid to see this happen on the Everyone Unionize Website.
It's also important to me that people understand that while the current UK data shows that around 25% of covid patients will experience post-viral syndrome, only a fraction of them will experience long-covid (which is categorized as post-viral syndrome following a covid infection that exceeds 12 weeks). Many people will experience fatigue, brain fog, and elevated heart rates/blood pressure for a few weeks, maybe a couple of months after a covid infection, and this absolutely matters to people whose work lives and personal lives it affects, not to mention mental health. However, fewer of us end up like I have, with disabling fatigue lasting for over a year, stage 2 hypertension putting us at risk for heart attack and stroke, and developing symptoms of hyperadrenergic PoTS. While it's important to make sure people know these are very real risks (and though their risk is compounded by existing health issues, they can also hit perfectly healthy people hard, including atheletes), the zine above conveys this information in incomplete and alarmist ways.
And while this post is long enough, what it doesn't contain is REALLY FUCKING IMPORTANT INFORMATION for people who think they may have long covid:
Do not take any medication without speaking with a doctor first, especially ones that can affect your heart rate or immune system. What works for one person may not work for another. Beta blockers are dangerous for people with asthma, and since you can develop asthma as a result of covid (or even pregnancy), you need to make sure you've been assessed before taking beta blockers. The only exception to this is cardiospecific beta blockers, but you still need to consult with a doctor before taking these.
Don't try to power through the fatigue or the other symptoms. Post viral syndrome and chronic fatigue syndrome have been shown to damage muscle tissue and patients who push themselves too much have even been shown to have dead muscle tissue. Essentially, when you exercise you damage your muscle tissue, and the process of your body repairing it makes you stronger. With long covid and CFS your body doesn't repair it and your muscles just... stay damaged. Learn your limitations, use mobility aids where needed, and be patient. (I understand this is not possible for everyone, but I'm giving health advice here - the plight of the proletariat does not change what your body's needs are, only your ability to meet them.)
Hydrate. Not just with water, but make sure you get enough electrolytes. If you drink too much water you can flush necessary electrolytes out of your system, but most CFS patients require additional fluids. This is especially true if you think you've developed PoTS symptoms as a result of long covid. 2L of water a day is the minimum, 3-4L may make the difference between you feeling like death and being able to feed yourself.
Sleep. Rest. Take breaks. Sensory stimulation affects long covid/CFS significantly, and taking a ten minute break every hour or two to just lay down in a quiet, dark space can recharge you a surprising amount. Have a set bedtime and stick to it.
Eat well. And regularly. Make sure you eat 3 square meals a day and at least 2 snacks. Your body is desperate for nutrients. If you only have energy for one thing a day, put it towards feeding yourself regularly and as healthy as possible. Take a multi-vitamin too.
Get your Vitamin D and iron levels checked. They often drop in long-covid patients and if you need supplements, or more iron in your diet and sunshine on your face, then know it.
Remember that it's temporary. Most patients recover fully from long covid, even bad cases like me. I'm going to be vulnerable to CFS for the rest of my life every time I get a cold or flu, and a covid reinfection could be dangerous, so I have to take precautions, but the really hard stuff, the bad long covid symptoms, are temporary. This is not your life forever, it's just your life right now. It gets better if you take care of yourself, even if it doesn't feel like it right now.
Finally, avoid getting covid again, especially while you actively have long covid. 70% of long covid patients report worsened symptoms on reinfection. Ask the people you make plans with to test, wear a mask in indoor spaces, and see people outdoors where possible.

New zine that's free for anyone to print and distribute! Read the whole thing at newlevant.com/COVIDzine or in the rest of this post.



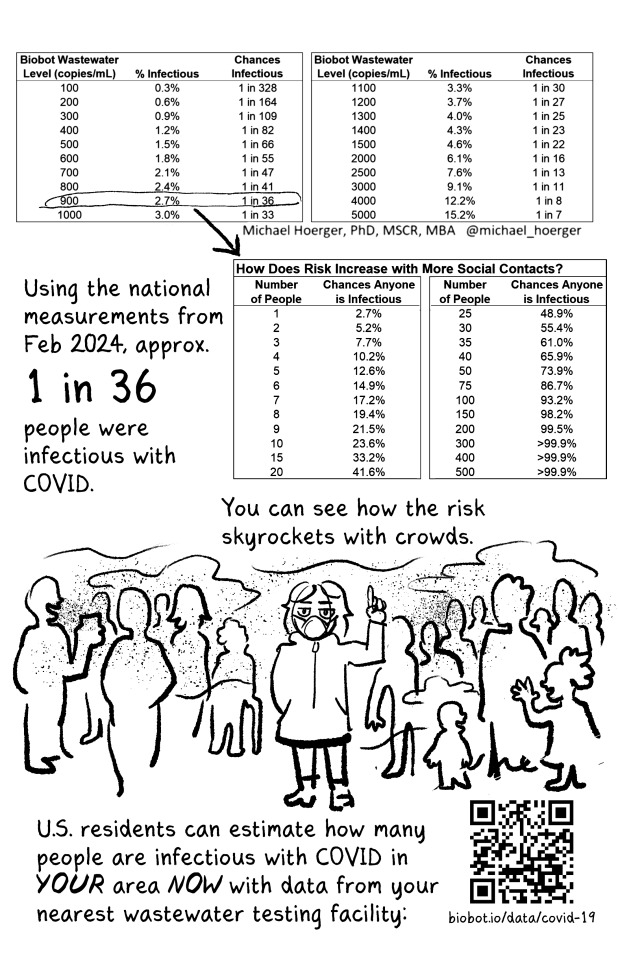
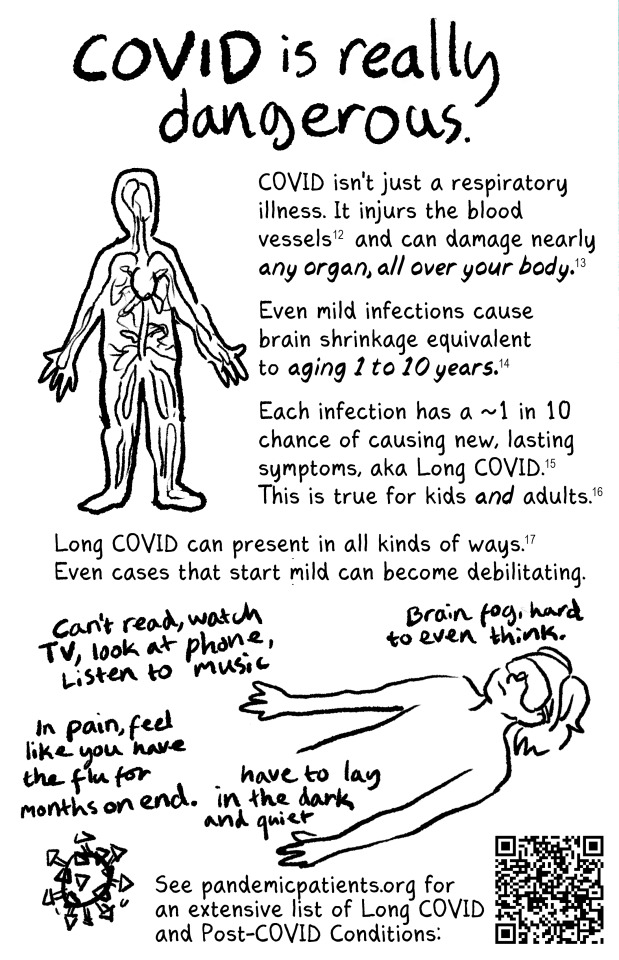
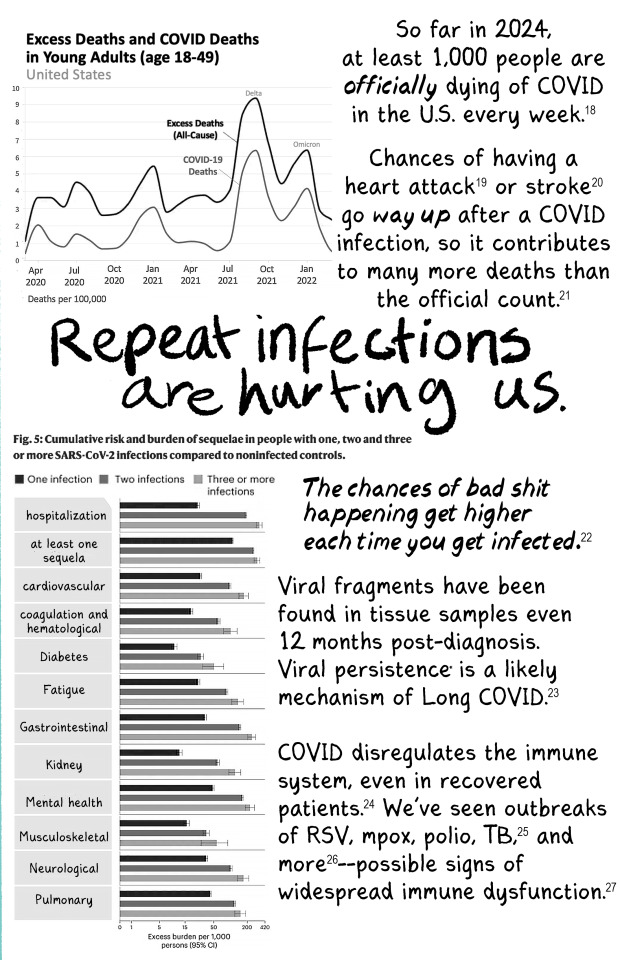




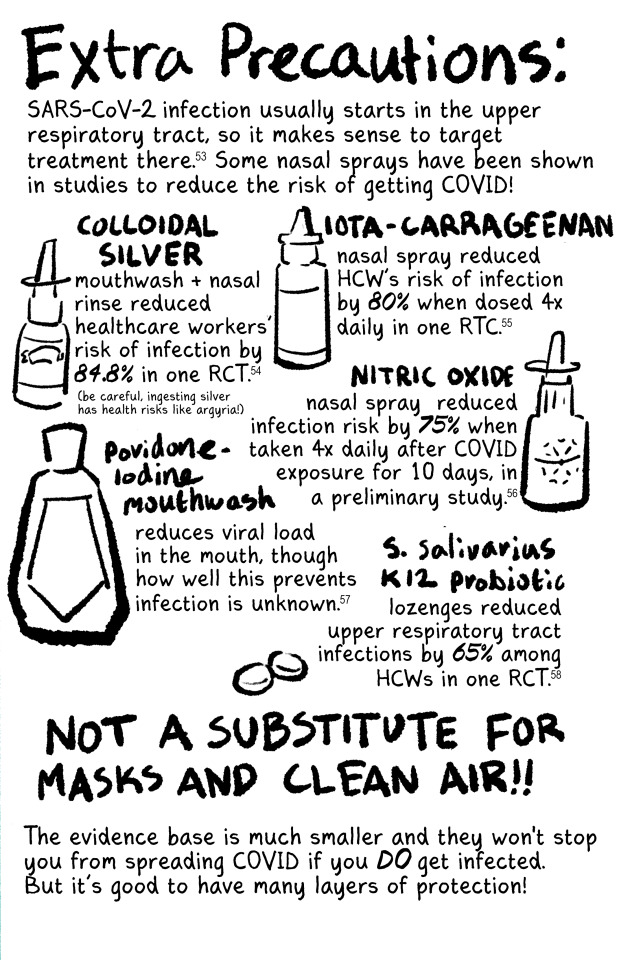


#listen I know this is a long post and tbh I'm fine with that#I have a lot of feelings about covid and long covid and the misinformation makes me really fucking angry#as does the alarmism#it's not effective and you're not helping vulnerable people like me#stop hurting us
37K notes
·
View notes
Text
Isolated Hepatitis B Surface Antigen Positivity Following Vaccination Against Coronavirus Disease A report of two cases by Manar Al Sanaa AlZeedi in Journal of Clinical Case Reports Medical Images and Health Sciences
Abstract
Coronavirus disease 2019 (COVID-19)—a respiratory illness caused by a recently identified, highly transmissible virus known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)—has had considerable global morbidity and mortality.1,2 As a result, a number of anti-SARS-CoV-2 vaccines have been developed with unprecedented speed. Hepatitis B surface antigen (HBsAg) is the most common marker of acute HBV infection and is detectable between 1–9 weeks of exposure to the virus, with decreasing HBsAg concentrations indicative of resolving viremia. Herein, we report two cases of isolated HBsAg positivity after COVID-19 vaccination in the absence of other HBV markers, symptoms, risk factors, or recent vaccination. The test turn to ne negative after period of time in both cases. Laboratory technicians and clinicians alike should be aware of the possibility of HBsAg false-positivity following anti-SARS-CoV-2 vaccination.
Introduction
Coronavirus disease 2019 (COVID-19)—a respiratory illness caused by a recently identified, highly transmissible virus known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)—has had considerable global morbidity and mortality.1,2 As a result, a number of anti-SARS-CoV-2 vaccines have been developed with unprecedented speed.3 While these vaccines have satisfactory efficacy and safety profiles, experience with their use is undeniably limited.4,5 Accordingly, additional knowledge of rare side-effects is important to inform future recommendations.
Hepatitis B is a highly contagious liver infection caused by the hepatitis B virus (HBV), a virus transmissible via contact with contaminated bodily fluids, including blood, saliva, semen, or vaginal secretions; in addition, it can also be transmitted to an infant during childbirth.6 Up to 30% of the global population is believed to have a current or previous HBV infection.7 Hepatitis B surface antigen (HBsAg) is the most common marker of acute HBV infection and is detectable between 1–9 weeks of exposure to the virus, with decreasing HBsAg concentrations indicative of resolving viremia.8 Individuals who have achieved immune control of HBV infection will typically show HBsAg negativity approximately 15 weeks after infection onset.8
In 1981, the first HBV vaccine was approved for human use.7 Transient antigenemia is known to occur for up to two weeks after HBV vaccination, thereby leading to a false-positive diagnosis of HBV infection.9,10 However, there are as yet no reports of hepatitis B antigenemia following SARS-CoV-2 vaccination. Herein, we report two cases of isolated HBsAg positivity after COVID-19 vaccination in the absence of other HBV markers, symptoms, risk factors, or recent vaccination
Case 1
A 37-year-old woman with no significant medical history underwent hepatitis screening during a routine medical check-up. Laboratory testing indicated HBsAg positivity and hepatitis C antibody negativity. No evidence of liver disease was found during a physical examination. She had received two doses of the BNT162b2 COVID-19 vaccine (Pfizer/BioNTech), with the last dose received 4 months earlier. The patient was a health care worker; however, there were no recognized self-reported risk factors for viral hepatitis, including needle stick injury. Furthermore, she had been vaccinated against HBV many years beforehand, with good immunity response, and an HBsAg test conducted two years earlier had been negative.
An HBV profile performed after one week using the neutralization method showed negative HBsAg findings (0.4 IU/mL) [Figure 1], a protective titer of HBsAg antibodies (anti-HBs; >1,000 IU/mL), and negative findings for hepatitis B core antibody (anti-HBc), hepatitis B envelope antigen (HBeAg), and hepatitis B envelope antibody. No viral load was detected with regards to HBV DNA.
Case 2
A 14-year-old boy underwent hepatitis screening during a routine sports medical evaluation. He had received two doses of the BNT162b2 COVID-19 vaccine (Pfizer/BioNTech), with the last dose received a month beforehand. All routine laboratory findings were within normal limits, except for HBsAg positivity. No hepatitis indicators were found during a physical examination. An HBV profile performed one week later failed to detect HBsAg (0.17 IU/mL) [Figure 2]. The boy had been vaccinated against HBV at one year of age as part of a routine child immunization program.
Figure 2: Final HBsAg results for Case 2.
Discussion
Recommended testing to evaluate for HBV infection includes HBsAg, anti-HBs, and anti-HBc (immunoglobulin M [IgM] for acute in infection and IgG for past exposure).7,8 However, correct interpretation of the findings is paramount to avoid misdiagnosis and inappropriate management. Although transient antigenemia can persist for up to two weeks after HBV vaccination, neither of the patients described in this report had been vaccinated against HBV in the weeks prior to their presentation.9,10 In both cases, HBsAg positivity occurred in the absence of clinical indicators of an underlying HBV infection or HBV risk factors and positivity to other hepatitis B markers, highly suggestive of false positivity.
Different hepatitis B markers become detectable as the infection evolves and can therefore be used to differentiate between acute and chronic infections and susceptibility to future infection.11,12 In the early phase of acute infection, HBsAg is the first marker to develop, followed shortly by HBeAg approximately one week after HBsAg is detectable.12 In those who are symptomatic, there is a rapid rise and then a slow decrease in IgM anti-HBc. Finally, in the convalescence stage, anti-HBs positivity occurs at approximately 6 weeks to 6 months of exposure, once HBsAg has disappeared.11,12 Under normal circumstances, isolated HBsAg positivity therefore occurs only in the very early stages of acute HBV infection before the patient is symptomatic.
Serological profile of an acute HBV infection. Reproduced from the Hepatitis B Foundation website.8
False-positive isolated HBsAg seropositivity has been described in other circumstances, including in the setting of malignancy.11,13 Costa et al. reported a 77-year-old female patient with a basal cell carcinoma who demonstrated persistent isolated HBsAg positivity, despite periodic testing over several months.11 Similarly, Tang et al. described a middle-aged female with primary hyperparathyroidism with repeated HBsAg positivity over a 5-week period prior to the surgical removal of a parathyroid adenoma.13 Other instances in which isolated HBsAg seropositivity may occur include mutated HBV variants and underlying immune conditions such as lupus.11,14
Both groups of researchers attributed the HBsAg positivity in their respective cases to heterophilic antibody interference.11,13 Heterophilic antibodies are poorly defined, low-affinity immunoglobulins which can cross-react with a wide range of animal antigens. As such, the presence of heterophilic antibodies have been shown to affect findings from lateral-flow immunoassays by binding to the target antigen, thereby generating false-positive results.15 Consequently, Costa et al. recommended that initial positive HBsAg findings be confirmed using a neutralization technique in which anti-HBs is incubated with HBsAg to block the HBsAg signal.11
Other infections, such as Epstein-Barr virus, may also give rise to low levels of heterophilic antibodies that can persist for up to a year.11,16 Rheumatoid factor cross-reactivity has also been found to result in misleading immunoassay findings.11,17 The two cases of HBsAg positivity described in this report appeared to be isolated, with subsequent testing failing to show persistent antigenemia, lending support to the theory that the false positivity may have been due to an indigenous factor or an exogenous analytical or operational error.17 Appropriate blocking agents are therefore recommended in order to minimize the risk of false-positive reactions.17,18
Conclusion
To the best of the authors’ knowledge, the two cases presented in this paper are the first reports of isolated HBsAg positivity following COVID-19 vaccination. Laboratory technicians and clinicians alike should be aware of the possibility of HBsAg false-positivity following anti-SARS-CoV-2 vaccination, although further research is needed to determine whether such findings are the result of the vaccination or due to other factors. Misdiagnosis of HBV can have potentially serious implications, including inappropriate management, unnecessary testing, surgical or treatment delays, adverse emotional consequences, and social stigma.
#Isolated Hepatitis B#Antigen#Coronavirus#COVID-19#Journal of Clinical Case Reports Medical Images and Health Sciences.
0 notes
Text
What is a Malaria Test Kit?
Malaria is a life-threatening disease caused by Plasmodium parasites, transmitted to humans through the bites of infected Anopheles mosquitoes. Despite advances in medical science, malaria remains a significant public health challenge in many parts of the world, especially in tropical and subtropical regions. Early detection and treatment are critical in managing this disease and reducing its impact.
The malaria test kit has revolutionized the fight against malaria by offering a quick and reliable way to diagnose the disease, even in remote areas. This blog will delve into the workings, benefits, and applications of malaria test kits, emphasizing their importance in malaria control and elimination efforts.
What is a Malaria Test Kit?
A malaria test kit is a diagnostic tool used to detect the presence of malaria parasites in a patient’s blood. It provides a rapid and accurate diagnosis, helping healthcare providers initiate timely treatment.
Malaria test kits are particularly useful in areas with limited access to laboratory facilities, enabling effective diagnosis in clinics, community settings, and even homes.
How Does a Malaria Test Kit Work?
Malaria test kits use Rapid Diagnostic Test (RDT) technology to detect specific antigens released by malaria parasites in the blood.
Sample Collection: A drop of blood is collected, typically through a finger prick.
Antigen Detection: The kit contains a test strip or cassette coated with antibodies that bind to malaria-specific antigens.
Reaction: When the blood sample is applied, the antigens (if present) react with the antibodies on the strip.
Result Display: Results are displayed within 15–20 minutes, usually in the form of lines indicating positive or negative results.
Types of Malaria Test Kits
Plasmodium falciparum-Specific Test Kits: Detect antigens specific to Plasmodium falciparum, the most severe and deadly form of malaria.
Pan-Plasmodium Test Kits: Identify all malaria species, including P. vivax, P. malariae, and P. ovale.
Combination Test Kits: Detect both P. falciparum and other malaria species simultaneously, providing comprehensive results.
Microscopy-Based Kits: Require laboratory settings and involve staining blood smears to observe parasites under a microscope.
Advantages of Malaria Test Kits
Rapid Results: Provide diagnosis within minutes, crucial for initiating prompt treatment.
Ease of Use: Simple procedures make them suitable for use by healthcare workers and even non-medical personnel.
Portability: Compact and lightweight, ideal for remote or resource-limited areas.
High Accuracy: Advanced kits offer high sensitivity and specificity, reducing the risk of misdiagnosis.
Cost-Effective: Affordable pricing ensures accessibility for large-scale screening in endemic regions.
Applications of Malaria Test Kits
Clinical Settings: Diagnose patients presenting with fever and other malaria symptoms.
Field Testing: Deployed in malaria-endemic areas for mass screening and outbreak control.
Community Health Programs: Enable early diagnosis and treatment in rural or underserved communities.
Travel Medicine: Used by travelers to endemic regions for self-testing when medical facilities are unavailable.
Limitations of Malaria Test Kits
Parasite Load Sensitivity: Kits may not detect malaria in cases with very low parasite counts.
False Negatives: Incorrect results can occur due to improper usage or testing during the early stages of infection.
No Differentiation of Drug Resistance: Cannot identify drug-resistant strains of malaria, requiring further laboratory testing.
How to Use a Malaria Test Kit
Prepare the Kit: Ensure all components (lancet, test strip, reagent, and dropper) are available and within the expiry date.
Collect Blood Sample: Prick the finger with a sterile lancet and collect a small blood sample using the provided dropper.
Apply Sample to Test Strip: Place the blood sample in the designated area on the strip or cassette.
Add Reagent: Add the buffer solution in the kit to initiate the reaction.
Interpret Results: Read the results within the specified time (usually 15–20 minutes). Follow the kit instructions to interpret the lines displayed.
The Role of Malaria Test Kits in Public Health
Supporting Malaria Eradication Goals: Early detection and treatment are critical in reducing malaria transmission and achieving global eradication targets.
Reducing Disease Burden: Rapid diagnosis minimizes severe complications and mortality associated with malaria.
Enhancing Outbreak Response: Kits enable quick identification and containment of malaria outbreaks in endemic regions.
Empowering Communities: Easy-to-use kits promote self-testing and community-based interventions, improving access to care.
Tips for Using Malaria Test Kits Effectively
Follow Instructions: Carefully read and adhere to the kit’s guidelines for accurate results.
Use Fresh Samples: Ensure blood samples are fresh to avoid errors.
Store Kits Properly: Keep kits in a cool, dry place as per manufacturer recommendations.
Confirm Results: Seek medical advice and additional tests if symptoms persist despite a negative result.
Conclusion
The malaria test kit is a game-changing tool in the fight against malaria. Its ability to provide quick, accurate, and accessible diagnosis makes it indispensable in clinical care and public health. By enabling early detection, these kits save lives, reduce disease transmission, and support global malaria elimination efforts.
Whether you are a healthcare provider, a traveler, or someone living in an endemic area, having access to a malaria test kit can make a significant difference in managing this disease effectively.
1 note
·
View note