#Active Pharmaceutical Ingredient Sector
Explore tagged Tumblr posts
Text
Unveiling the Dynamics of the Active Pharmaceutical Ingredient Market
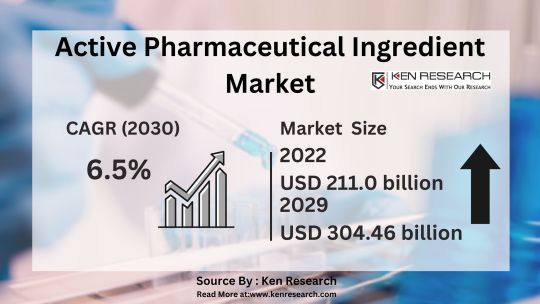
Delve into the intricate dynamics of the Active Pharmaceutical Ingredient market, analyzing its size, segmentation, revenue, growth rate, trends, and forecasts. Gain valuable insights from comprehensive API market analysis reports.
#Active Pharmaceutical Ingredient Market#Active Pharmaceutical Ingredient Industry#Active Pharmaceutical Ingredient Sector#Active Pharmaceutical Ingredient Market Size#Active Pharmaceutical Ingredient Market Segmentation#Active Pharmaceutical Ingredient Market Analysis#Active Pharmaceutical Ingredient Market Revenue#Active Pharmaceutical Ingredient Market Growth Rate#Active Pharmaceutical Ingredient Market Trends#Active Pharmaceutical Ingredient Market Report#Active Pharmaceutical Ingredient Market Forecast#Opportunities in Active Pharmaceutical Ingredient Industry#Active Pharmaceutical Ingredient Market Future Outlook
0 notes
Text
Lori Ann Larocco at CNBC:
Billions in trade came to a screeching halt at U.S. East Coast and Gulf Coast ports after members of the International Longshoremen’s Association (ILA) began walking off the job after 12:01 a.m. ET on October 1. The ILA is North America’s largest longshoremen’s union, with roughly 50,000 of its 85,000 members making good on the threat to strike at 14 major ports subject to a just-expired master contract with the United States Maritime Alliance (USMX), and picketing workers beginning to appear at ports. The union and port ownership group failed to reach agreement by midnight on a new contract in a protracted battle over wage increases and use of automation. In a last-ditch effort on Monday to avert a strike that will cause significant harm to the U.S. economy if it is lengthy — at least hundreds of millions of dollars a day at the largest ports like New York/New Jersey — the USMX offered a nearly 50% wage hike over six years, but that was rejected by the ILA, according to a source close to the negotiations. The port ownership group said it hoped the offer would lead to a resumption of collective bargaining.
The 14 ports where preparations for a strike have been underway are Boston, New York/New Jersey, Philadelphia, Wilmington, North Carolina, Baltimore, Norfolk, Charleston, Savannah, Jacksonville, Tampa, Miami, New Orleans, Mobile, and Houston. New York Governor Kathy Hochul said in a statement issued shortly after midnight that “the first large-scale eastern dockworker strike in 47 years began at ports from Maine to Texas, including at the Port Authority of New York and New Jersey. In preparation for this moment, New York has been working around the clock to ensure that our grocery stores and medical facilities have the essential products they need.” Rhetoric from ILA leadership has been aggressive in the weeks leading up to the strike, with ILA president Harold Daggett, who was a union member the last time it went out on strike in 1977, telling rank-and-file members — who unanimously voted to authorize a strike — in a recent video message, “We’ll crush them.”
[...] The most significant issues would be faced by food and automobile industries, Kamins said, as they rely especially heavily on the ports that will be shut down. While a surge in inflation is highly unlikely even with a longer strike, even a modest reacceleration could create uncertainty and force the Federal Reserve to be more cautious about lowering interest rates, which would weigh on the overall outlook for job growth and investment. A one-week strike could cost the U.S. economy $3.78 billion, according to an analysis by The Conference Board, and cause supply chain slowdowns through mid-November. In all, the ports threatened with strikes handle $3 trillion annually in U.S. annual international trade.
Many industries are preparing for major repercussions. Noushin Shamsili, CEO and president of Nuco Logistics, which specializes in pharmaceutical imports and exports, said the strike comes at a critical time for inventory replenishment for the pharma sector. “Almost all of this industry is just on time,” said Shamsili. “Raw materials are being brought in to complete drug manufacturing. Medical supplies for clinics and hospitals are on these vessels. For a while importers did not bring in a lot of cargo because they were overflowing with supplies post-Covid. Now they have started reordering medical devices, gloves, syringes, and tubing.” Shamsili also said the East Coast ports are a gateway for generic medicine made in India. Approximately 48% of the active pharmaceutical ingredients used in the U.S. are being imported from India. Without these APIs, medications cannot be produced. APIs are also manufactured in Europe, which also use the East Coast ports as U.S. points of entry.
[...] The Biden administration finds itself in a delicate political moment, with the presidential election one month away and President Biden vowing he will not use existing labor law to force union workers back on the job, which is within his powers under the Taft-Hartley Act. The Taft-Hartley Act, passed in 1947, was a revision of U.S. law governing labor relations and union activity that granted a U.S. president the power to suspend a strike for an 80-day “cooling off period” in cases where “national health or safety” are at risk.
Today begins the strike along East Coast and Gulf Coast ports after International Longshoremen’s Association (ILA) members walked off their jobs.
This strike, depending on how long it lasts, could have a major impact on the elections and the economy.
#2024 US Port Strike#Strikes#US Maritime Alliance#USMX#International Longshoremen's Association#ILA#US News#United States#Harold Daggett#Taft Hartley Act#Unions
7 notes
·
View notes
Text

Effervescent tubes have revolutionised the pharmaceutical and nutraceutical industries, becoming the go-to solution for packaging effervescent tablets. As a prominent name in the sector, NBZ Healthcare, located in Mumbai, stands at the forefront of exceptionally effervescent tube manufacturing, ensuring quality and innovation.
In this article, we delve deep into the manufacturing of effervescent tubes, their applications, benefits, and the unparalleled expertise of NBZ Healthcare in this domain.
Understanding Effervescent Tubes
Effervescent tubes are specialised containers designed to store and preserve effervescent tablets. These tablets, when exposed to moisture or air, tend to lose their potency, necessitating robust, moisture-resistant packaging. Effervescent tubes, often crafted with airtight and tamper-proof caps, provide the ideal solution to ensure the longevity and effectiveness of these tablets.
Importance of Effervescent Tubes
Effervescent tubes are more than just packaging; they play a crucial role in the pharmaceutical and nutraceutical industries by:
1. Protecting Product Integrity:
- Shielding tablets from moisture and air.
- Preventing degradation of active ingredients.
2. Enhancing Consumer Experience:
- Easy to open and reseal.
- Portable and convenient for on-the-go consumption.
3. Ensuring Regulatory Compliance:
- Meeting stringent health and safety standards for pharmaceutical products.
4. Sustainability:
- With increasing focus on eco-friendly materials, many effervescent tubes are now designed to be recyclable or biodegradable.
Effervescent Tube Manufacturing Process
Effervescent tube manufacturing is a precise and technologically driven process. At NBZ Healthcare, we combine state-of-the-art machinery with strict quality control measures to produce exceptional effervescent tubes. Here’s an overview of the key stages:
1. Material Selection
- High-grade plastic materials such as polypropylene (PP) or polyethylene (PE) are used.
- Materials are selected based on moisture resistance, durability, and eco-friendliness.
2. Tube Extrusion
- Plastic granules are melted and extruded into tube-like shapes using extrusion machines.
- The dimensions and thickness are carefully controlled to meet industry standards.
3. Moulding
- Injection moulding is used to form the caps and closures of the tubes.
- Caps are designed to ensure an airtight seal and tamper resistance.
4. Printing and Branding
- Tubes are labelled or printed with product details, branding, and regulatory information.
- Advanced printing techniques ensure durability and clarity.
5. Quality Control
- Rigorous testing for moisture resistance, durability, and compatibility with effervescent tablets.
- Each tube undergoes inspection to ensure zero defects.
NBZ Healthcare: Redefining Effervescent Tube Manufacturing
As a leader in exceptionally effervescent tube manufacturing, NBZ Healthcare sets the benchmark for quality and innovation. Based in Mumbai, our facility is equipped with cutting-edge machinery and a dedicated team of professionals who prioritise precision and excellence.
What Sets NBZ Healthcare Apart?
1. Advanced Manufacturing Capabilities
- NBZ Healthcare utilises the latest extrusion and moulding technologies to produce high-quality tubes.
2. Customisation Options
- We offer custom designs, sizes, and branding options to cater to the unique needs of our clients.
3. Sustainable Practices
- Focused on reducing environmental impact, we incorporate eco-friendly materials and processes wherever possible.
4. Regulatory Compliance
- Our manufacturing processes comply with international standards, including ISO and FDA regulations.
5. Global Reach
- While based in Mumbai, NBZ Healthcare serves clients across India and internationally, ensuring timely delivery and exceptional service.
Applications of Effervescent Tubes
Effervescent tubes find applications in various sectors, including:
1. Pharmaceuticals:
- Storage of vitamins, supplements, and medications in effervescent tablet form.
2. Nutraceuticals:
- Packaging for dietary supplements and health-boosting effervescent tablets.
3. Food and Beverage:
- Containment of flavour-enhancing or health-focused effervescent powders and tablets.
4. Personal Care:
- Packaging for effervescent bath tablets or other similar products.
Innovations in Effervescent Tube Manufacturing
At NBZ Healthcare, innovation is at the heart of our operations. Here are some of the cutting-edge advancements we incorporate:
1. Smart Packaging Solutions
- Tubes embedded with QR codes or NFC tags for enhanced consumer engagement and traceability.
2. Enhanced Moisture Resistance
- Using desiccant-lined caps and advanced sealing technologies to further improve moisture protection.
3. Eco-Friendly Materials
- Transitioning to biodegradable plastics and exploring sustainable alternatives like biopolymers.
4. Automation and AI Integration
- Automated production lines ensure consistency and reduce manufacturing errors.
- AI systems monitor quality in real-time for enhanced accuracy.
Challenges in Effervescent Tube Manufacturing
While effervescent tube manufacturing offers immense potential, it comes with its own set of challenges, such as:
1. Material Costs:
- Balancing quality and affordability when sourcing raw materials.
2. Environmental Concerns:
- Reducing the carbon footprint of manufacturing processes.
3. Regulatory Compliance:
- Adhering to diverse regulatory standards across different markets.
4. Consumer Expectations:
- Meeting the growing demand for sustainable and innovative packaging solutions.
The Future of Effervescent Tube Manufacturing
The demand for effervescent tubes is expected to rise significantly as more consumers turn to effervescent products for their convenience and efficiency. NBZ Healthcare is well-positioned to lead this growth, with a focus on:
1. Sustainability Initiatives
- Introducing greener manufacturing processes and materials.
2. Global Expansion
- Reaching untapped markets to provide high-quality effervescent tubes worldwide.
3. Product Diversification
- Expanding into new applications and industries beyond pharmaceuticals and nutraceuticals.
4. Technological Upgrades
- Adopting emerging technologies to enhance efficiency and product quality.
Why Choose NBZ Healthcare for Effervescent Tubes?
At NBZ Healthcare, we combine years of expertise with a commitment to innovation and customer satisfaction. When you partner with us, you’re choosing:
- Unmatched Quality: Every tube is crafted with precision and care.
- Custom Solutions: Tailored to your product’s specific needs.
- Reliable Service: Consistent and timely delivery of all orders.
- Sustainable Values: Supporting environmentally responsible practices.
Conclusion
Effervescent tubes play a pivotal role in preserving the efficacy and quality of effervescent tablets. With NBZ Healthcare’s expertise in exceptionally effervescent tube manufacturing, businesses can rely on robust, high-quality packaging solutions tailored to their needs.
As a Mumbai-based pioneer in the industry, NBZ Healthcare continues to set the standard for excellence, innovation, and sustainability. Whether you’re in the pharmaceutical, nutraceutical, or any related industry, NBZ Healthcare is your trusted partner for exceptional effervescent tube solutions.
Reach out to us today and experience the difference in quality and service!
2 notes
·
View notes
Text
Exploring the Global Aldehydes Market: Key Players and Market Dynamics
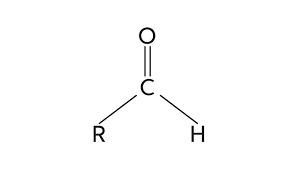
The aldehydes market is a segment of the chemical industry that deals with the production and distribution of a class of organic compounds known as aldehydes. These compounds are characterized by the presence of a carbonyl group (C=O) bonded to a hydrogen atom and a carbon atom in their chemical structure. Aldehydes find widespread applications in various industries, thanks to their unique properties and versatile reactivity.
In terms of market overview, the aldehydes market has been experiencing steady growth in recent years. This growth can be attributed to the increasing demand for aldehydes in industries such as pharmaceuticals, agriculture, food and beverages, and cosmetics. Aldehydes serve as crucial intermediates in the synthesis of various chemicals and are essential in the production of fragrances, flavor enhancers, and pharmaceuticals.
The growth in the aldehydes market industry can be primarily attributed to the expansion of these end-user industries. For instance, the pharmaceutical industry relies heavily on aldehydes for the synthesis of a wide range of drugs and active pharmaceutical ingredients (APIs). Additionally, the food and beverage industry utilizes aldehydes for flavor enhancement and preservation purposes, further driving market growth.
The aldehydes market is also influenced by evolving industry trends. One significant trend is the increasing emphasis on green chemistry and sustainable practices. Many companies in the aldehydes sector are adopting environmentally friendly production processes, such as catalytic hydrogenation, to reduce the environmental impact of their operations. This trend aligns with the growing awareness of environmental issues and the need for more eco-friendly chemical manufacturing.
Another noteworthy trend is the constant innovation and development of novel aldehyde derivatives with enhanced properties. This innovation is driven by the demand for higher-quality products in various industries. Researchers and manufacturers are continuously exploring new applications and synthesizing aldehydes tailored to meet specific industry requirements, which contributes to market expansion.
In conclusion, the aldehydes market is a dynamic segment within the chemical industry, driven by the increasing demand from various end-user industries. As industries continue to grow and evolve, the market is expected to witness further advancements, particularly in sustainable production methods and novel aldehyde derivatives, to meet the changing needs of consumers and businesses alike.
2 notes
·
View notes
Text
Zinc Reagent Manufacturers in India
In the rapidly evolving field of chemical manufacturing, the demand for high-quality reagents plays a critical role in driving innovation and ensuring accuracy in research and industrial applications. Among these, zinc reagents stand out due to their diverse applications across industries such as pharmaceuticals, agriculture, electronics, and materials science. Symax Labs, a trusted name in the chemical industry, has established itself as one of the leading zinc reagent manufacturers in India, delivering products that meet stringent quality standards.
Why Zinc Reagents Are Essential
Zinc reagents are indispensable in numerous chemical reactions and industrial processes. They are widely used in organic synthesis, catalysis, and material fabrication. In the pharmaceutical industry, zinc-based compounds contribute to the synthesis of active pharmaceutical ingredients (APIs) and intermediates. Their role in biochemical assays and diagnostic applications further highlights their versatility and importance.
Symax Labs: Commitment to Quality and Innovation
At Symax Labs, quality and innovation are at the heart of every product. The company employs advanced manufacturing techniques and rigorous quality control processes to ensure the production of high-purity zinc reagents. By adhering to international quality standards, Symax Labs guarantees that their products meet the diverse requirements of their global clientele.
High Purity and Consistency: Symax Labs ensures that every batch of zinc reagents maintains exceptional purity levels, which is crucial for reproducibility in scientific research and industrial applications.
Customized Solutions: Understanding that different industries have unique needs, Symax Labs offers customized zinc reagent formulations tailored to specific applications.
Sustainable Practices: The company is committed to eco-friendly manufacturing processes, minimizing environmental impact while maintaining product excellence.
Applications of Symax Labs’ Zinc Reagents
Symax Labs’ zinc reagents are utilized in a variety of sectors:
Pharmaceutical Industry: For the synthesis of APIs, intermediates, and in the development of new drug formulations.
Agriculture: As micronutrients in fertilizers, enhancing plant growth and crop yield.
Electronics: In the fabrication of semiconductors and other electronic components.
Materials Science: For the development of advanced materials and coatings.
Why Choose Symax Labs?
With a reputation built on reliability, innovation, and customer satisfaction, Symax Labs stands out as a preferred partner for zinc reagent requirements. The company's dedication to research and development ensures that they stay ahead of industry trends, offering cutting-edge solutions to meet the evolving demands of their customers.
Experienced Team: A team of skilled chemists and researchers dedicated to continuous improvement and product development.
Global Reach: Serving clients not just in India, but across international markets, ensuring timely delivery and support.
Customer-Centric Approach: Prioritizing customer needs with personalized service and technical assistance.
Contact Symax Labs
If you are looking for high-quality zinc reagents that deliver consistent performance across various applications, Symax Labs is your go-to manufacturer. For more information on their product range and services, visit their website or contact their customer support team.
0 notes
Text
API Pharmaceutical Company: Largest API Manufacturers in India
Prism Industries Pvt. Ltd. stands out among the largest API manufacturers in India. As one of the leading API producers in India, the company plays a pivotal role in the pharmaceutical sector. With years of expertise, Prism Industries is recognized for producing high-quality APIs (Active Pharmaceutical Ingredients), serving global markets and ensuring strict adherence to regulatory standards. The India API manufacturing industry has seen rapid growth, and companies like Prism Industries are at the forefront, contributing to the country's robust position in the global pharmaceutical supply chain. What makes Prism Industries unique is its commitment to innovation, sustainability, and quality control, ensuring their APIs are both safe and effective for various therapeutic applications. By staying ahead of market demands, Prism Industries Pvt. Ltd. is not only a trusted name in API production but also a key player in shaping the future of the Indian pharmaceutical manufacturing landscape.
#api_pharmaceutical_company#largest_api_manufacturers_in_india#india_api_manufacturing#api_producers_in_india#api_manufacture
0 notes
Text
Active Pharmaceutical Ingredient Market to Expand to $415.3B by 2033 💉💊
Active Pharmaceutical Ingredient (API) market is set to expand significantly, growing from $245.2 billion in 2023 to $415.3 billion by 2033, registering a CAGR of 5.6%. This growth is driven by rising demand for generic drugs, advancements in biotechnology, and increasing prevalence of chronic diseases.
To Request Sample Report: https://www.globalinsightservices.com/request-sample/?id=GIS21460 &utm_source=SnehaPatil&utm_medium=Article
Key Market Drivers & Trends
✅ Rising Demand for Generic Medications
Cost-effective treatments are fueling the adoption of generic APIs. ✅ Growth in Biotech APIs & Personalized Medicine
Monoclonal antibodies & peptide-based drugs gaining traction. ✅ Oncology & Cardiovascular APIs Leading
Increased focus on cancer treatments & heart disease therapies. ✅ Stringent Regulatory Frameworks Driving Quality Standards
Compliance with FDA & EMA guidelines shaping API production.
Regional Insights
🌎 North America Leads — Strong R&D investments & a well-established pharmaceutical sector. 🇪🇺 Europe Expands — Growing biosimilar adoption & favorable regulatory policies. 🇮🇳 India Surges — Top exporter of cost-effective generic APIs.
Market Segmentation & Performance
📌 Synthetic APIs Dominate (55%) — Cardiovascular & oncology drugs drive demand. 📌 Biotech APIs Rising (30%) — Biopharmaceutical advancements support growth. 📌 Generic APIs Gaining Traction (15%) — Expanding access to affordable medicines.
Top Industry Players & Competitive Strategies
🏭 Teva Pharmaceutical Industries — Cost-effective API production leadership. 🏭 Pfizer Inc. — Expanding R&D investments & innovative therapies. 🏭 Novartis AG — Focus on biotech APIs & high-value drug formulations.
Future Outlook & Challenges
🔬 With a 10% projected increase in R&D expenditure, the API market will see major advancements in biotech APIs, AI-driven drug development, and nanotechnology-based APIs. However, regulatory complexities, high production costs, and competition from low-cost manufacturers pose challenges. The shift toward sustainable API manufacturing and AI-driven drug synthesis will create new growth opportunities.
💊🌍 #APIMarket #PharmaceuticalInnovation #DrugDevelopment #BiotechAPI #GenericDrugs #OncologyAPI #CardiovascularTreatment #AIinPharma #SmartPharmaceuticals #ChronicDiseaseCare #HealthcareAdvancements #FDARegulations #EMACompliance #BiopharmaGrowth #APIManufacturing #R&DInvestment #PharmaIndustryTrends #NanotechnologyInDrugs #FutureOfMedicine #PharmaSupplyChain #Biosimilars #MedicalBreakthroughs #LifeSciences #PrecisionMedicine #SustainablePharma
0 notes
Text
Exploring the Dynamic World of Pharmaceutical Ingredients

Dive into the dynamic realm of the Active Pharmaceutical Ingredient industry, examining API market major players, size, segmentation, trends, and the growth rate within this vital sector.
#Active Pharmaceutical Ingredient Market#Active Pharmaceutical Ingredient Industry#Active Pharmaceutical Ingredient Sector#Active Pharmaceutical Ingredient Market Size#Active Pharmaceutical Ingredient Market Major Players#Active Pharmaceutical Ingredient Market Segmentation#Active Pharmaceutical Ingredient Market Trends#Active Pharmaceutical Ingredient Market Growth Rate
0 notes
Text
Acetic Anhydride Prices, News, Trend, Graph, Chart, Monitor and Forecast

Acetic Anhydride is a key chemical intermediate with widespread applications across industries such as pharmaceuticals, agrochemicals, textiles, and plastics. The global acetic anhydride market is influenced by various factors, including supply-demand dynamics, raw material costs, production capacities, regulatory policies, and trade activities. Over the years, fluctuations in prices have been observed due to variations in the availability of feedstock, primarily acetic acid, and changing market conditions. The acetic anhydride price trend is closely linked to the cost of raw materials, energy prices, and regional economic activities, which play a crucial role in shaping its valuation in the global market.
The pricing of acetic anhydride is significantly affected by the cost of acetic acid, which is its primary raw material. Any disruption in acetic acid supply, caused by factors such as plant shutdowns, supply chain constraints, or fluctuations in crude oil prices, directly impacts acetic anhydride production costs. Additionally, the price of methanol, another essential component in the manufacturing process, also plays a role in determining the cost structure of acetic anhydride. When acetic acid prices rise due to increased demand or reduced supply, the cost of acetic anhydride also experiences an upward trend. On the other hand, when raw material prices decline, acetic anhydride prices follow suit, provided that other economic factors remain stable.
Get Real time Prices for Acetic Anhydride: https://www.chemanalyst.com/Pricing-data/acetic-anhydride-1157
Global demand for acetic anhydride is another significant driver of price trends. The pharmaceutical sector is one of the largest consumers, utilizing acetic anhydride in the production of aspirin, acetaminophen, and other active pharmaceutical ingredients. As the healthcare industry expands, especially in emerging economies, the demand for acetic anhydride remains strong, influencing price movements. Additionally, its use in the production of cellulose acetate, which is widely used in textiles and cigarette filters, further supports market demand. The growth of these end-use industries directly impacts the pricing structure, as higher demand often leads to price escalation, while lower demand exerts downward pressure on prices.
Regional market conditions also play a crucial role in determining acetic anhydride prices. Asia-Pacific, particularly China and India, is a dominant player in the global market due to the presence of major manufacturing facilities and high consumption levels. China’s stringent environmental regulations on chemical production and factory shutdowns due to pollution control measures have at times led to supply shortages, causing price spikes. India, on the other hand, has been increasing its production capacity to meet both domestic and international demand. North America and Europe also hold a significant share of the market, with pricing influenced by regulatory frameworks, energy costs, and trade policies. In regions where production costs are higher due to strict environmental norms or labor expenses, acetic anhydride prices tend to be relatively elevated.
Trade policies and geopolitical factors can lead to price volatility in the acetic anhydride market. Export-import regulations, tariffs, and restrictions on chemical trade impact the global supply chain. Any disruption in major exporting countries can create supply shortages, leading to price hikes. For example, trade tensions between key exporting and importing nations may result in higher costs due to increased tariffs, affecting the overall market balance. Additionally, supply chain disruptions caused by global events such as the COVID-19 pandemic have also influenced acetic anhydride prices in recent years. The pandemic led to temporary plant shutdowns, reduced workforce availability, and logistical challenges, which constrained supply and drove prices higher.
Another factor affecting acetic anhydride prices is the energy cost associated with its production. Since the chemical industry relies heavily on energy-intensive processes, fluctuations in crude oil and natural gas prices significantly impact manufacturing costs. When energy prices rise, production costs increase, leading to higher prices for acetic anhydride. Conversely, when energy prices decline, manufacturers may lower prices to remain competitive. The availability of alternative energy sources and advancements in energy-efficient production technologies may help mitigate some of these cost pressures in the future.
Sustainability and regulatory compliance are becoming increasingly important in the acetic anhydride market. Governments and regulatory bodies impose strict guidelines on the handling, transportation, and usage of hazardous chemicals, influencing production and distribution costs. Compliance with environmental and safety regulations often requires additional investments in technology and infrastructure, impacting the final price of the product. Additionally, as industries move towards greener and more sustainable alternatives, market players may explore bio-based or alternative production routes, which could have long-term implications on pricing trends.
Looking ahead, the forecast for acetic anhydride prices depends on several key factors, including raw material price movements, demand growth from major end-use industries, and global economic conditions. With the pharmaceutical and agrochemical sectors expected to expand, the demand for acetic anhydride is likely to remain strong, providing support to price stability. However, any major shifts in crude oil prices, trade policies, or production capacities could result in price fluctuations. Moreover, advancements in production technology and the increasing adoption of sustainable practices may play a role in shaping the future pricing landscape.
In summary, acetic anhydride prices are influenced by a combination of supply chain factors, raw material costs, regional market conditions, trade policies, energy prices, and regulatory frameworks. The market remains dynamic, with periodic fluctuations driven by both macroeconomic and industry-specific trends. As industries continue to evolve and adapt to changing market conditions, understanding the key drivers of acetic anhydride pricing is essential for businesses operating in this sector. Monitoring market trends, assessing risk factors, and staying informed about regulatory changes can help stakeholders make informed decisions and navigate price fluctuations effectively.
Get Real time Prices for Acetic Anhydride: https://www.chemanalyst.com/Pricing-data/acetic-anhydride-1157
Contact Us:
ChemAnalyst
GmbH - S-01, 2.floor, Subbelrather Straße,
15a Cologne, 50823, Germany
Call: +49-221-6505-8833
Email: [email protected]
Website: https://www.chemanalyst.com
#Acetic Anhydride Prices#Acetic Anhydride Price#Acetic Anhydride Pricing#India#united kingdom#united states#Germany#business#research#chemicals#Technology#Market Research#Canada#Japan#China
0 notes
Text
Active Pharmaceutical Ingredient Market Expansion: Challenges and Opportunities
The global Active Pharmaceutical Ingredient (API) market, valued at USD 239.45 billion in 2023, is projected to reach USD 371.06 billion by 2032, exhibiting a Compound Annual Growth Rate (CAGR) of 5.37% during the forecast period from 2024 to 2032.
The Active Pharmaceutical Ingredient Market Size was valued at USD 239.45 billion in 2023 and is expected to reach USD 371.06 billion by 2032 and grow at a CAGR of 5.37% over the forecast period 2024-2032.
Market Segmentation:
The API market is segmented based on the type of synthesis, ingredients, application, and drug type:
By Type of Synthesis:
Synthetic APIs: Dominated the market with a 70.65% revenue share in 2023, primarily due to the high volume of generic drug production.
Biotech APIs
By Ingredients:
Generic APIs
Innovative APIs
By Application:
Cardiovascular Diseases
Oncology
CNS and Neurology
Orthopedic
Endocrinology
Pulmonology
Gastroenterology
Nephrology
Ophthalmology
By Drug Type:
Prescription Drugs
Over-the-Counter (OTC) Drugs
Regional Analysis:
The API market's growth is influenced by various factors across different regions:
North America: Advanced healthcare infrastructure and significant R&D investments contribute to market expansion.
Europe: The increasing prevalence of chronic diseases and a growing geriatric population drive demand for APIs.
Asia-Pacific: Rapid industrialization, favorable government initiatives, and a burgeoning pharmaceutical sector bolster market growth.
Key Players:
The Key players are Aurobindo Pharma, Bristol Myers Squibb, Eli Lilly and Company, BASF SE, Cipla, Abbvie Inc., Boehringer Ingelheim GmbH, Dr. Reddy’s Laboratories Ltd, Albemarle Corporation, Viatris Inc and Other Players. The key players are emphasizing the preservation of high-quality standards and catering to market demand with an existing customer base in the region at a large scale. This marketing strategy is only helpful if the brand has already established its name and trust in the market. They rely on significant investment in sophisticated new technology & infrastructure capable of high throughput processing & analysis.
Key Highlights:
The rising prevalence of chronic diseases, such as cardiovascular disorders and cancer, is a significant driver for API demand.
An increasing global geriatric population contributes to higher API consumption due to age-related health issues.
Technological advancements in drug development and personalized medicine present lucrative opportunities for market players.
Future Outlook:
The API market is poised for substantial growth, driven by the escalating demand for generic medications and the continuous emergence of novel therapeutic areas. Technological innovations and increased investments in research and development are expected to further propel the market, offering significant opportunities for industry stakeholders.
Get Free Sample Report @ https://www.snsinsider.com/sample-request/1005
Conclusion:
The Active Pharmaceutical Ingredient market is on a robust growth trajectory, with significant advancements anticipated across various segments and regions. Industry participants, including manufacturers, healthcare providers, and investors, are well-positioned to capitalize on the evolving landscape of API development and utilization.
Contact Us:
Akash Anand – Head of Business Development & Strategy
Phone: +1-415-230-0044 (US)
#Active Pharmaceutical Ingredient Market#Active Pharmaceutical Ingredient Market Share#Active Pharmaceutical Ingredient Market Size#Active Pharmaceutical Ingredient Market Trends#Active Pharmaceutical Ingredient Market Growth
0 notes
Text
Chemical Manufacturing Company in Chennai: A Growing Industrial Hub
Chennai emerges as a major industrial centre in India where chemical manufacturing activities have become prominent. The increasing number of chemical manufacturing company in Chennai benefits from a strong infrastructure base and experienced workers and advantageous port access. Industry players supply premium chemicals that serve pharmaceuticals as well as agriculture and textiles and construction activities.
Importance of Chemical Manufacturing in Chennai
The chemical industry supports various national industries through its production of fundamental raw materials and special-purpose chemical blends. The chemical industry in Chennai thrives due to public backing combined with research facilities and dependable logistics systems which make it an ideal place for chemical manufacturing.
Types of Chemicals Manufactured in Chennai
The production sector employs industrial chemicals which serves both manufacturing operations and industrial cleaning procedures and industrial coverings.
Pharmaceutical Chemicals: Active pharmaceutical ingredients (APIs) and excipients for drug formulation.
Agrochemicals: Fertilizers, pesticides, and soil conditioners for enhanced agricultural productivity.
Specialty Chemicals: Customized solutions for industries such as textiles, paper, and water treatment.
Petrochemicals serve as important raw materials to create plastics while providing base materials for rubber manufacturing together with synthetic fibres production.
Construction Chemicals provide sealants and adhesives with waterproofing solutions which extend the longevity of buildings.
Advantages of Choosing a Chemical Manufacturing Company in Chennai
Quality Assurance: Companies adhere to stringent quality control measures and global standards.
Buyers profit from cost-efficient solutions because pricing remains competitive along with manufacturing processes that deliver high efficiency.
Research and development innovations are driven through advanced laboratories which operate with skilled researchers.
The adoption of environmentally friendly manufacturing approaches has become common practice among multiple production facilities.
The logical backbone of Chennai consists of meticulously developed transportation facilities that deliver chemicals promptly.
Industries Benefiting from Chemical Manufacturing in Chennai
The Pharmaceutical Industry demands bulk chemicals as fundamental ingredients to create medications along with their medical applications.
Agrochemicals in agriculture provide farmers with two benefits that include higher crop yields and pest defense capabilities.
Automobile and Aerospace Industry: Relies on high-performance coatings and lubricants.
Specialty chemicals enable fabric treatment while performing dyeing operations in the Textile and Dyeing industry.
Food and Beverage industry applies food-grade chemicals and preservatives which increase shelf life of their products.
How to Choose the Right Chemical Manufacturing Company in Chennai
Verify that the business abides by regulatory guidelines as well as safety standards.
The availability of many chemical products provides evidence of a company's expertise level and product reliability.
A thorough evaluation must be made to assess research and development capabilities since formulations need innovative approaches and customization features.
A company must offer competitive prices along with efficient distribution services to excel in the market.
Technical support together with strong after-sales services creates additional value which strengthens an association between businesses.
Conclusion
Industrial growth in the Chennai area receives substantial support from the local chemical manufacturing operations. High-quality chemical solutions delivered by these companies serve various industries by meeting industry standards for efficiency and sustainability. The increasing demand keeps Chennai as the main choice for chemical manufacturing and supply activities which strengthens industrial development and economic expansion.
0 notes
Text
Sigachi Industries Expands R&D Capabilities with a State-of-the-Art Center in Hyderabad
Sigachi Industries Limited, a key player in the pharmaceutical sector, has announced a significant step in its journey toward innovation and excellence. The company is establishing a state-of-the-art Research and Development (R&D) facility in Hyderabad, India. This initiative, backed by an investment of up to USD 1 million, is aimed at centralizing critical API developments and analytical efforts under one roof, reinforcing the company's commitment to technological advancement and efficiency.
With this expansion, Sigachi seeks to enhance its R&D capabilities, ensuring the seamless integration of synthesis and analytical processes. The facility will play a crucial role in optimizing API production while maintaining compliance with stringent global regulatory standards. The company is set to fuel six additional Certificate of Suitability (CEP) filings in the next six months, strengthening its pipeline and market positioning.
The new R&D center is designed to support the development of both existing and new molecules, providing a foundation for long-term growth and sustained profitability. A dedicated team of 15-20 skilled scientists will lead innovative research, focusing on regulated markets and advancing pharmaceutical solutions that meet international benchmarks. By streamlining its pharmaceutical product portfolio, Sigachi aims to prioritize high-value APIs and intermediates, allowing for greater synergies in manufacturing and regulatory filings.
Amit Raj Sinha, Managing Director and CEO of Sigachi Industries Limited, expressed enthusiasm about the expansion, emphasizing the company’s ongoing commitment to innovation and R&D investments. He highlighted that Sigachi has made substantial progress in delivering Active Pharmaceutical Ingredients (APIs) to the pharmaceutical market. The increased focus on R&D aligns with the company’s strategic vision of excellence, reinforcing its long-term growth objectives and strengthening its overall pipeline.
Sigachi Industries Limited has built a strong reputation in the pharmaceutical industry, specializing in APIs, intermediates, excipients, vitamin and mineral nutrient blends, and operations and maintenance (O&M) services. With over 35 years of experience, the company serves customers across 65+ countries, leveraging cutting-edge technology and global expertise to develop high-value pharmaceutical solutions. Operating from multiple facilities in Telangana, Gujarat, and Karnataka, and with subsidiaries in the Middle East and the U.S., Sigachi remains committed to expanding access to reliable and high-quality pharmaceutical ingredients.
This strategic investment in Hyderabad underscores Sigachi’s vision of continuous innovation, operational efficiency, and market leadership. As the company strengthens its research and development framework, it paves the way for greater advancements in the pharmaceutical sector, ensuring the delivery of superior healthcare solutions worldwide.
0 notes
Text
Bismuth Market Key Drivers And Opportunities Driving Global Expansion
The bismuth market is expanding due to its increasing use in various industries, including pharmaceuticals, cosmetics, metallurgy, and electronics. With growing environmental regulations and technological advancements, demand for this non-toxic metal is rising. Industries are exploring its potential as a substitute for lead and other heavy metals. Understanding the market drivers behind this growth is crucial for investors and stakeholders looking to capitalize on emerging opportunities. Below are the key factors propelling the bismuth market forward.

Regulatory Support And Environmental Policies
Governments worldwide are implementing strict regulations to reduce lead usage, boosting demand for bismuth as a safer alternative
Environmental agencies are promoting the use of non-toxic metals in consumer and industrial applications, encouraging market expansion
Restrictions on lead-based products in Europe and North America are pushing manufacturers to adopt bismuth-based alternatives
Compliance with the Restriction of Hazardous Substances (RoHS) directive is driving the need for lead-free solders, benefiting the bismuth market
Increasing global initiatives to minimize heavy metal pollution are creating opportunities for bismuth-based materials in industrial applications
Increasing Adoption In The Pharmaceutical Industry
Bismuth compounds are widely used in gastrointestinal medications, such as treatments for ulcers and indigestion
Rising awareness of digestive health is fueling demand for bismuth-based drugs in developed and developing nations
The non-toxic nature of bismuth makes it a preferred ingredient in over-the-counter medicines
Expanding healthcare infrastructure in emerging markets is increasing accessibility to bismuth-containing pharmaceuticals
Research on new medical applications, including antimicrobial properties, is driving innovation in the pharmaceutical sector
Growing Demand In The Cosmetics And Personal Care Industry
Bismuth oxychloride is a popular ingredient in cosmetics, contributing to its widespread use in makeup products
The shimmer and smooth texture of bismuth-based compounds make them desirable for foundations, blushes, and eyeshadows
Rising consumer preference for high-quality and long-lasting cosmetic products is supporting market growth
Increasing disposable income is allowing more consumers to opt for premium beauty products containing bismuth
The shift towards non-toxic and hypoallergenic ingredients is encouraging cosmetic manufacturers to integrate bismuth into their formulations
Expanding Use In Metallurgy And Alloys
Bismuth is increasingly used as a substitute for lead in free-machining steels and aluminum alloys
The demand for eco-friendly metal alloys is promoting bismuth adoption in industrial and automotive sectors
Its low melting point makes it ideal for fusible alloys used in safety devices and fire detection systems
The aerospace industry is exploring the potential of bismuth-containing materials for lightweight and corrosion-resistant applications
Growing construction activities are driving demand for bismuth-based coatings and alloys for enhanced durability
Rising Application In The Electronics Sector
Lead-free solder manufacturing is a significant contributor to the expansion of the bismuth market
Consumer electronics, including smartphones and laptops, require reliable soldering solutions that comply with environmental standards
Technological advancements in microelectronics are increasing the need for bismuth-based solders with enhanced performance
The miniaturization of electronic components is driving demand for highly efficient and thermally stable solder materials
Expanding production of semiconductors and circuit boards is creating a steady demand for bismuth-containing electronic components
Growth In Renewable Energy And Green Technologies
Bismuth-based thermoelectric materials are being developed for energy-efficient power generation
Research into solar energy storage solutions is highlighting the potential of bismuth-based compounds in photovoltaic systems
The push for sustainability is encouraging industries to adopt environmentally friendly materials, benefiting the bismuth market
Emerging innovations in battery technology are exploring bismuth as a key component in energy storage solutions
Governments and organizations are investing in green technologies, driving demand for non-toxic and sustainable metals
Surging Demand In The Automotive Industry
The transition towards electric vehicles is increasing the need for bismuth-containing materials in battery components
Automotive manufacturers are using bismuth alloys for improved performance and durability in engine parts
The lightweight nature of bismuth-based alloys is contributing to fuel efficiency in conventional and electric vehicles
Fire safety applications in automobiles, such as sprinkler systems, are incorporating bismuth alloys for enhanced reliability
Rising global vehicle production is generating a higher requirement for advanced and lead-free metal solutions
Expanding Applications In Fire Protection And Safety Equipment
Bismuth alloys are widely used in fire detection and suppression systems due to their low melting points
The construction industry is integrating bismuth-containing materials in fire-resistant coatings and barriers
Stricter fire safety regulations are driving the demand for reliable, heat-sensitive materials in public buildings and infrastructure
Industrial workplaces are adopting bismuth-based fire suppression systems for enhanced protection against potential hazards
Innovations in fire protection technology are creating new opportunities for bismuth alloys in safety applications
Increasing Research And Development Initiatives
Scientists and researchers are discovering new potential uses for bismuth in advanced technology and medicine
Ongoing studies on superconductivity in bismuth-based materials are leading to groundbreaking innovations in energy transmission
Universities and research institutions are receiving funding for bismuth-related projects, expanding market possibilities
The development of bismuth-based catalysts is revolutionizing chemical processes in industrial manufacturing
Exploration of nanotechnology applications is unlocking new ways to integrate bismuth into high-performance materials
Strategic Investments And Market Expansions
Key industry players are investing in new production facilities to meet rising global demand
Companies are forming partnerships to enhance bismuth extraction and processing capabilities
Market leaders are focusing on sustainable mining practices to ensure long-term supply stability
Governments are supporting domestic bismuth production to reduce reliance on imports from dominant suppliers
Expansion of trade agreements is facilitating the export and import of bismuth-based products across various regions
Increasing Consumer Awareness And Market Penetration
Consumers are becoming more conscious of non-toxic and sustainable materials, influencing purchasing decisions
Educational campaigns highlighting the benefits of bismuth-based products are driving market penetration
The rise of eco-conscious brands is creating opportunities for bismuth suppliers in sustainable product manufacturing
E-commerce platforms are enabling greater accessibility to bismuth-containing goods across global markets
Direct-to-consumer marketing strategies are helping businesses expand their customer base for bismuth-related products
0 notes
Text
Understanding the Metoclopramide API Industry in India
Metoclopramide is an important pharmaceutical compound used primarily to treat nausea, vomiting, and other gastrointestinal disorders. It plays a crucial role in both acute and chronic treatments, making it a key component in various medical formulations. With the growing demand for Metoclopramide in global markets, India has emerged as a prominent hub for the production and supply of its Active Pharmaceutical Ingredient (API).
India's pharmaceutical industry is renowned for its manufacturing capabilities, with Metoclopramide API manufacturers in India providing high-quality compounds at competitive prices. These manufacturers leverage advanced technology and adhere to strict international standards to produce APIs that meet regulatory requirements, ensuring efficacy and safety. As a result, India has become a major exporter of Metoclopramide, catering to both domestic and international markets.
In addition to manufacturing, there are numerous Metoclopramide API suppliers in India who play a vital role in distributing the compound to various regions. These suppliers maintain extensive networks to ensure that the APIs are readily available to pharmaceutical companies, clinics, and hospitals. By offering flexibility in terms of packaging and quantities, they ensure that the demand is met efficiently.
Price competitiveness is also an important aspect in the pharmaceutical market. The Metoclopramide API price in India is considered one of the most affordable globally, making it an attractive option for international buyers. The cost-effectiveness of Indian APIs has contributed significantly to the country's growth as a leading exporter in the pharmaceutical sector.
Conclusion
India's pharmaceutical industry continues to lead the way in providing high-quality and cost-effective Metoclopramide APIs to the global market. As we look ahead, companies like Bio-Synth are expected to further strengthen their position by focusing on quality, innovation, and customer satisfaction in the API supply chain.
#Metoclopramide API price in India#Metoclopramide API manufacturers in India#Metoclopramide API suppliers in India
0 notes
Text
Growth and Challenges of Pharmaceutical Manufacturers in India
The pharmaceutical industry in India is one of the largest in the world. With a strong manufacturing base, skilled workforce, and cost-effective production, India has become a global leader in pharmaceutical exports. The country is home to many pharmaceutical manufacturers in India, which produce high-quality medicines at affordable prices. However, despite the remarkable growth, the industry also faces several challenges. In this article, we will explore the growth and challenges of the pharmaceutical sector in India.
Growth of Pharmaceutical Manufacturers in India
Global Leader in Generic Medicine Production India is the world's largest supplier of generic medicines, contributing to about 20% of the global supply. The affordability and availability of Indian generic drugs make them highly popular in international markets. Many top Indian pharma export companies in India supply medicines to the United States, Europe, and other parts of the world.
Increasing Demand for Indian Medicines The demand for Indian pharmaceutical products has been growing steadily due to their quality and cost-effectiveness. The COVID-19 pandemic further boosted this demand, as Indian companies played a crucial role in supplying vaccines and essential medicines worldwide.
Government Support and Policies The Indian government has implemented several policies to support the growth of the pharmaceutical industry. Initiatives like the "Production Linked Incentive (PLI) Scheme" encourage investments in the sector. Moreover, schemes like "Make in India" promote local manufacturing, helping medicine manufacturing companies in India expand their production capacity.
Strong R&D and Innovation Indian pharmaceutical manufacturers are investing heavily in research and development (R&D) to create innovative medicines. Many companies are focusing on biosimilars, specialty drugs, and new formulations to meet global healthcare demands.
Rising Pharmaceutical Exports India exports medicines to over 200 countries, including highly regulated markets like the USA, UK, and Canada. The top Indian pharma export companies in India include Sun Pharma, Dr. Reddy’s Laboratories, Cipla, Lupin, and Aurobindo Pharma. These companies play a key role in making Indian pharmaceutical products widely accessible worldwide.
Challenges Faced by Pharmaceutical Manufacturers in India
Stringent Regulatory Requirements The pharmaceutical industry must comply with strict regulatory standards in both domestic and international markets. Different countries have different compliance norms, making it challenging for Indian manufacturers to meet all requirements. Obtaining approvals from regulatory bodies like the US FDA and EMA can be time-consuming and expensive.
Rising Raw Material Costs India relies heavily on China for Active Pharmaceutical Ingredients (APIs), which are essential raw materials for medicine production. Any disruption in the supply chain or increase in API prices directly affects the profitability of medicine manufacturing companies in India.
Intellectual Property Rights (IPR) Issues Patent-related disputes and intellectual property rights issues often create obstacles for pharmaceutical manufacturers. While India follows a strict patent law, international pharmaceutical giants sometimes challenge Indian drug patents, leading to legal battles.
Increasing Competition With the growth of the pharmaceutical sector, competition among pharmaceutical manufacturers in India has also increased. Small and medium-sized companies find it difficult to compete with large corporations that have better financial and technological resources.
Quality Control and Compliance Maintaining high-quality standards is crucial for pharmaceutical companies. Even a minor deviation in drug quality can lead to product recalls, financial losses, and damage to a company’s reputation. Indian manufacturers must invest in advanced technology and quality control systems to ensure global compliance.
Skilled Workforce Shortage While India has a large pool of skilled professionals, the pharmaceutical industry still faces a shortage of highly trained scientists and regulatory experts. Continuous training and skill development programs are needed to bridge this gap.
The Future of Indian Pharmaceutical Industry
Despite these challenges, the future of the Indian pharmaceutical industry remains bright. With continuous investments in research, government support, and a focus on self-reliance, India is expected to strengthen its position as a global pharmaceutical hub. The industry is likely to witness further growth in biopharmaceuticals, personalized medicine, and digital healthcare solutions.
Conclusion
The pharmaceutical industry in India has achieved significant growth over the years, making the country one of the world's largest medicine suppliers. Many pharmaceutical manufacturers in India are expanding their global reach, supplying affordable and high-quality medicines. However, the industry must overcome challenges such as regulatory compliance, rising raw material costs, and increasing competition. By addressing these challenges and investing in innovation, medicine manufacturing companies in India can continue to thrive in the global market. Additionally, the top Indian pharma export companies in India will play a crucial role in maintaining India’s leadership in the pharmaceutical sector.
0 notes
Text
CAS NO: 3886-70-2 Manufacturers | (R)-(+)-1-(Napthyl)Ethyl Amine
Unveiling the Chemical Identity of CAS No: 3886-70-2
In the vast and diverse world of chemistry, each chemical compound is assigned a unique identifier known as the Chemical Abstracts Service (CAS) number. CAS No: 3886-70-2 refers to a specific substance that is often encountered in specialized industries, particularly in the realm of chemical synthesis and scientific research. In this blog post, we will dive deep into the nature of this chemical, its uses, properties, safety protocols, and much more.

What is CAS No: 3886-70-2?
CAS No: 3886-70-2 represents a particular chemical compound with distinct characteristics that are identified and cataloged by the Chemical Abstracts Service, a division of the American Chemical Society. CAS numbers are vital for ensuring precision when identifying chemicals, as they offer a standardized system that is used universally in scientific literature, industry standards, and regulatory frameworks.
While the name of the substance associated with CAS No: 3886-70-2 may not be immediately recognized, understanding its properties and applications can reveal its significance in various fields, such as chemical engineering, pharmaceuticals, and materials science.
Chemical Structure and Properties
The substance linked to CAS No: 3886-70-2 has a unique molecular structure that plays a role in determining its physical and chemical properties. These properties may include:
Molecular Composition: The chemical formula for CAS No: 3886-70-2 specifies the types and numbers of atoms that make up the compound. This helps scientists understand its reactivity, stability, and potential applications.
Physical Properties: The chemical could be in solid, liquid, or gas form under standard conditions. It may have specific melting and boiling points, density, and solubility characteristics that determine how it behaves in various environments.
Reactivity and Stability: Understanding the chemical's reactivity is crucial, as it dictates how it might interact with other substances. This can include its susceptibility to oxidation, reduction, or other types of chemical reactions.
The precise details of its structure and properties are typically outlined in technical datasheets or academic publications, which offer valuable information for anyone working with or researching this chemical.
Applications and Uses
While CAS No: 3886-70-2 is not commonly seen in mainstream products, it has specific applications in industrial and scientific sectors. Some of the notable uses may include:
Research and Development: Chemists and researchers often use chemicals like this one to explore novel reactions, synthesize new compounds, or understand fundamental chemical principles. It could serve as an intermediate in the creation of more complex molecules or be used in experimental setups to study its behavior under various conditions.
Pharmaceuticals: Some chemicals with CAS numbers like 3886-70-2 play a role in drug discovery and development. It could be an ingredient or a precursor used in the formulation of active pharmaceutical ingredients (APIs) or other compounds essential in the medical field.
Materials Science: In the field of materials science, chemicals such as this may be used in the synthesis of polymers, coatings, or materials with unique properties, such as improved conductivity, flexibility, or resistance to corrosion.
Agriculture: Depending on its structure, this chemical might also be used in agrochemical formulations, serving as a pesticide, herbicide, or plant growth regulator.
Safety Considerations and Handling
Like any chemical substance, safety is a top priority when working with CAS No: 3886-70-2. The safety measures depend largely on its specific toxicity, reactivity, and potential environmental impact. Some common safety practices include:
Personal Protective Equipment (PPE): If the compound poses risks such as skin irritation, inhalation hazards, or eye damage, protective gear such as gloves, goggles, and lab coats is essential. PPE is tailored to prevent direct contact and minimize exposure.
Storage and Handling: Chemicals like this one must be stored and handled according to precise guidelines to maintain safety. Proper labeling, containment, and storage conditions such as temperature control or avoidance of direct sunlight might be necessary to keep the chemical stable.
Disposal Protocols: Safe disposal procedures are important to prevent environmental contamination. Disposal methods are often outlined in safety data sheets (SDS) to ensure that the chemical is neutralized or discarded properly.
Before working with any chemical, including CAS No: 3886-70-2, it is critical to consult the Safety Data Sheet (SDS) for comprehensive safety instructions and emergency procedures in case of accidental exposure.
Regulatory Status
CAS No: 3886-70-2, like other chemicals, may be subject to various regulations depending on its potential hazards and use. Governments and regulatory agencies often regulate the production, distribution, and disposal of chemicals to ensure public safety and environmental protection. These regulations may include:
Toxicity and Environmental Impact: Some chemicals are tested for their potential to harm human health or the environment. This includes studies on carcinogenicity, mutagenicity, and aquatic toxicity.
Labeling and Classification: In many regions, chemicals are classified based on their hazardous properties and require specific labeling for safe handling. This may include hazard symbols or warnings indicating flammability, toxicity, or corrosive nature.
Permitted Use and Restrictions: Depending on its chemical nature, CAS No: 3886-70-2 may have restrictions on its use in consumer products or specific industries. Compliance with local and international laws ensures that it is used only for its intended purposes.
Conclusion
CAS No: 3886-70-2 may not be a well-known compound, but it plays an important role in a range of scientific and industrial applications. Whether it’s utilized in the laboratory, pharmaceutical industry, or material science, this chemical's unique properties make it a valuable substance in specialized fields.
As with all chemicals, understanding its structure, applications, safety protocols, and regulatory requirements is essential for those working with or studying it. By adhering to best practices in handling and safety, we can ensure that this compound is used responsibly and contributes to the advancement of science and technology.
For anyone involved in chemical research or industrial work, CAS numbers like 3886-70-2 provide a standardized way of identifying and referencing chemicals, allowing for greater precision, safety, and regulatory compliance in various settings.
URL: For more information, visit Vasista Pharma : CAS NO: 3886-70-2 Manufacturers
0 notes