#API drug development
Explore tagged Tumblr posts
Text
Pharma API Manufacturing Sites & Capabilities | CDMO Company | CRO | Aurigene Pharmaceutical Services

We operate 8 API manufacturing sites. Each of these sites has a dedicated facilities associated with capacity, capability, desired market, & appropriate regulatory status.
To know more:https://www.aurigeneservices.com/services/manufacturing/sites-and-capabilities
#api manufacturing#api drug development#API manufacturing sites#API manufacturing capabilities#contract research services#contract research organization#api manufacturing facilities
0 notes
Text
The Importance of Active Pharmaceutical Ingredients in Drug Development
Introduction
The role of Active Pharmaceutical Ingredients (APIs) in drug development is often understated, yet it's the cornerstone of any effective medication. These critical components are responsible for the therapeutic effects of a drug, making their quality and efficacy vital. This article delves into the significance of APIs in the pharmaceutical industry, particularly focusing on drug development and manufacturing.

Understanding APIs
APIs are the substances that produce the intended effects in pharmaceutical drugs. They interact with specific biological targets to bring about therapeutic outcomes. In essence, the API is the 'active' element that treats the condition or symptom for which the drug is prescribed.
The Role in Drug Development
In the initial stages of drug development, identifying or synthesizing a potential API is often the first step. This is followed by rigorous preclinical testing in the lab and in animal models to evaluate its safety and efficacy. Once the API shows promise, it moves to clinical trials involving human subjects to confirm its safety and effectiveness.
Quality and Purity
Importance: The quality and purity of an API directly impact the drug's safety and efficacy.
Regulatory Guidelines: To ensure these standards, APIs must meet stringent quality guidelines set by regulatory bodies like the FDA and EMA.
Manufacturing Considerations
APIs can be synthesized through various methods, including chemical synthesis, fermentation, and extraction. Regardless of the method, rigorous quality control is essential. This ensures that the API's purity and potency meet the required standards, which is crucial for the success of the final drug product.
Challenges and Solutions
API drug development is not without its challenges. The increasing complexity of APIs, especially with the advent of biologics, makes the manufacturing process intricate. Additionally, APIs are subject to stringent regulatory requirements, making the development process both time-consuming and costly. However, advances in technology and a focus on sustainable methods are paving the way for more efficient and eco-friendly API production.
The Future of APIs
The pharmaceutical industry is continuously evolving, and APIs are at the forefront of this change. Advances in genomics are paving the way for personalized medicine, where APIs can be tailored to individual genetic profiles. Moreover, the rise of biologic drugs has led to the development of more complex APIs, opening new avenues for innovation.
Conclusion
Understanding the critical role of Active Pharmaceutical Ingredients in drug development can provide valuable insights for stakeholders in the pharmaceutical industry. As the industry continues to evolve, APIs will undoubtedly remain at its core, driving innovations in drug development, personalized medicine, and more.
For those looking to venture into API manufacturing, partnering with an experienced company like Saurav Chemicals can offer invaluable expertise and solutions, helping you navigate the complexities of API drug development and manufacturing.
0 notes
Text
https://enkling.com/read-blog/32141
Best Generic Medicine Company in India | Chemxpert database
India has established itself as a leading hub for the pharmaceutical industry, particularly in the production of high-quality, affordable generic medicines. The country’s robust pharmaceutical infrastructure, cutting-edge pharmaceutical labs near me, and a strong network of API suppliers in India position it as a vital player in the global healthcare market. Let’s dive deeper into the attributes that define the best generic medicine companies in India and explore their global significance.
#top pharma companies in world#medicine export from India#new drug development process#API medicine#clinical research companies in India#latest trends in pharmaceutical industry
1 note
·
View note
Text
Global Drug Development Trends: Innovations and Challenges
The process of drug development is dynamic part of science and technology, which is controlled by regulation and policy, and market & customer requirements. Analyzing the current trends in drug development is helpful for the health care stakeholders, such as active pharmaceutical companies, researchers, and policy makers, because the global health care industry is evolving. This blog seeks to discuss the main drivers that policymakers considers in order to develop drugs in different countries.
#Clinical Trial Sites in India#platform Clinical Trial#platform Trials in Drug Development#Clinical Trials API#Clinical Trial Analysis#drug clinical trial phases
1 note
·
View note
Text
Exploring the Significance of Active Pharmaceutical Ingredients in Drug Development
Introduction:
Active Pharmaceutical Ingredients (APIs) serve as the cornerstone of medication efficacy, constituting the biologically active elements that drive therapeutic effects in pharmaceuticals. In this article, we'll explore the pivotal function of APIs throughout the drug development process and their crucial role in crafting medicines that are both safe and effective.

Understanding Active Pharmaceutical Ingredients:
APIs represent the active constituents of drugs responsible for eliciting therapeutic responses within the human body. By targeting specific biological processes or receptors, APIs effectively manage various medical conditions.
Formulation Development:
APIs provide the foundation for drug formulation, enabling pharmaceutical scientists to design delivery systems that optimize factors like bioavailability, stability, and drug release. The careful selection and characterization of APIs are essential in determining a drug's overall efficacy.
Safety and Efficacy:
Before integration into drug development, APIs undergo rigorous testing to ensure their safety and efficacy. Preclinical studies and clinical trials meticulously assess the pharmacokinetics, pharmacodynamics, and toxicological properties of APIs to ascertain their therapeutic potential and establish suitable dosage regimens.
Quality Control:
Maintaining stringent quality standards throughout API manufacturing is imperative to guarantee the safety and effectiveness of the final drug product. From raw material sourcing to synthesis, purification, and packaging, thorough quality control measures are enforced. Analytical techniques are utilized to verify the identity, purity, and potency of APIs.
Regulatory Compliance:
API manufacturing adheres to strict regulatory guidelines to uphold product quality, consistency, and patient safety. Regulatory bodies like the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) set forth guidelines and conduct inspections to ensure compliance with good manufacturing practices (GMP) for APIs.
Generic Medications:
APIs are instrumental in producing generic medications, which contain identical active ingredients to their branded counterparts. Demonstrating bioequivalence is crucial for generic drugs, ensuring they produce similar therapeutic effects. Therefore, maintaining API quality and consistency is paramount to the efficacy and safety of generic medications.
Innovations in API Development:
Ongoing advancements in API development have led to improved drug delivery systems, heightened bioavailability, and enhanced patient adherence. Innovations like nanoparticles, liposomes, and prodrugs enable targeted and sustained drug release, thereby maximizing the therapeutic potential of APIs.
Conclusion:
Active Pharmaceutical Ingredients (APIs) form the bedrock of drug development, playing an indispensable role in formulating safe and effective medications. Through extensive research, rigorous quality control measures, and regulatory compliance, APIs contribute to the creation of life-saving treatments that enhance the quality of life for millions worldwide. At Global Pharma Tek, our comprehensive Drug Development Services encompass the expertise needed to harness the potential of APIs in creating innovative therapies that address unmet medical needs and propel pharmaceutical science forward. As pharmaceutical science continues to progress, APIs will remain central to the discovery and development of groundbreaking therapies.
0 notes
Text
Maximize efficiency with top-tier Small Molecule API solutions
Discover top-tier CDMO pharma companies specializing in small molecule API synthesis and manufacturing. Elevate your drug development journey with Aragen's comprehensive solutions tailored to meet your small molecule needs.
0 notes
Text
ontract Manufacturing in the Generic Pharma Sector: Trends and Analysis
The global generic pharmaceuticals contract manufacturing market size is expected to reach USD 106.9 billion by 2030, registering a CAGR of 5.8% over the forecast period, according to a new report by Grand View Research, Inc. Cost-saving and time-saving benefits associated with the implementation of outsourcing is responsible for driving the industry. A significant number of people globally suffer from chronic diseases. For instance, the CDC states that 6 in 10 adults in the U.S. suffer from at least one chronic disease and 4 in 10 adults suffer from two or more chronic diseases. Chronic diseases are required to be treated for a long time. The high cost of medicines is increasing the demand for cost-effective generic drugs for the treatment of chronic diseases.
Generic Pharmaceuticals Contract Manufacturing Market Report Highlights
The branded generics segment held the largest share in 2021due to the preference for branded generics among physicians. Some branded generic manufacturers offer benefits and gifts to physicians for boosting their product sales. This further contributes to the demand for branded generic manufacturing in the market
The API product segment held the largest share in 2021. The growing demand for generic drugs is supporting the demand for generic API contract manufacturing
The parenteral route of administration segment is expected to grow at the fastest CAGR over the forecast period due to the bioavailability of parenteral drugs over other formulations
The oncology segment is expected to register the fastest CAGRfrom 2022 to 2030 owing to the high cost of cancer drugs contributing to the demand for cost-effective generic medicines
Asia Pacific is expected to record the highest CAGR over the forecast period mainly due to the low cost of generic drug manufacturing
Gain deeper insights on the market and receive your free copy with TOC now @: Generic Pharmaceuticals Contract Manufacturing Market Report
This is expected to support the industry's growth post-pandemic. There is an improvement in the regulatory approval of generic drugs. For instance, in 2021, the FDA approved 93 generic drugs, and by October 2022, the regulatory authority approved over 95 generic drugs. Such improvements are expected to have a positive impact on the manufacturing of generic drugs and; thus, support the industry growth. The Japanese government is constantly trying to improve the generic pharmaceuticals market in the country. The government is also taking measures to improve the supply of generics in the country and is also encouraging medical institutes to promote the use of generic drugs.
This is expected to improve CMO activities for generics in the coming years. Global spending on medicines is also on the rise. According to the data provided in a report published by IQVIA in April 2021, global spending on medicine is expected to increase in the next 4-5 years. The report states that global spending on medicine accounted for USD 1, 265 billion in 2020 and is going to reach USD 1,580-1,610 billion by 2025. This is also expected to improve the demand for generic drugs owing to their cost efficiency, thereby supporting the industry in growth.
#Pharma Contract Manufacturing#Generic Pharmaceuticals#Pharma Industry#Drug Manufacturing#Pharmaceutical Supply Chain#Outsourcing#Supply Chain Management#Pharmaceutical Partnerships#Pharmaceutical sourcing#Healthcare Manufacturing#Drug Development#API Production#CRO#CMO#Pharmaceutical Trends
0 notes
Text
India's Pharma Industry – The Leading Companies You Need to Know
India's pharmaceutical industry stands as a global powerhouse, contributing significantly to the world's supply of medicines and pharmaceutical products. The country's ability to produce high-quality, affordable medicines has earned it the title of "Pharmacy of the World." As the industry continues to grow and innovate, several companies have emerged as leaders in the market. For Centurion HealthCare Pvt. Ltd., understanding the landscape of the top pharma companies in India provides insights into the key players driving the industry's success.

The Rise of the Pharmaceutical Industry in India
The pharmaceutical industry in India has seen exponential growth over the past few decades. From generic drug manufacturing to complex biotechnological innovations, Indian pharma companies have made substantial contributions to global healthcare. This growth can be attributed to several factors, including a skilled workforce, robust research and development infrastructure, and supportive government policies.
Key Players in India's Pharma Industry
The landscape of the pharmaceutical industry in India is populated by numerous companies, each contributing to various segments of the market. Here are some of the top pharmaceutical companies in India that are leading the charge:
1. Sun Pharmaceutical Industries Ltd.
As the largest pharmaceutical company in India, Sun Pharma is renowned for its diverse product portfolio, including generics, branded generics, specialty medicines, and active pharmaceutical ingredients (APIs). The company has a significant global presence and continues to expand its footprint through strategic acquisitions and partnerships.
2. Dr. Reddy's Laboratories
Dr. Reddy's is a major player in the global generic pharmaceutical market. Known for its strong focus on research and development, the company offers a wide range of pharmaceuticals and biotechnology products. Their commitment to quality and innovation has solidified their position as one of the best pharmaceutical companies in India.
3. Cipla Ltd.
Cipla has been at the forefront of providing affordable medicines for over eight decades. The company specializes in respiratory, cardiovascular, anti-retroviral, and anti-infective therapies. Cipla's dedication to healthcare accessibility and its significant contributions to global health initiatives make it a top pharmaceutical company in India.
4. Lupin Limited
Lupin is a leading pharmaceutical company known for its focus on complex generics and specialty drugs. The company's strong presence in both developed and emerging markets has earned it a place among the top 10 pharmaceutical companies in India. Lupin's investment in R&D and its broad therapeutic portfolio are key drivers of its success.
5. Aurobindo Pharma
Aurobindo Pharma is recognized for its extensive range of generic formulations and APIs. The company's robust manufacturing capabilities and strategic global presence have made it one of the top pharmaceutical companies in India. Aurobindo's commitment to innovation and quality continues to propel its growth.
6. Zydus Cadila
Zydus Cadila, a leading pharmaceutical company, offers a wide range of healthcare solutions, including small molecules, biologics, biosimilars, and vaccines. The company's integrated operations and strong research capabilities have established it as a key player in the pharma industry in India.
7. Glenmark Pharmaceuticals
Glenmark is a global research-led pharmaceutical company known for its focus on innovation in the fields of dermatology, respiratory, and oncology. The company's strong pipeline of new chemical entities and biosimilars underscores its position as one of the best pharmaceutical companies in India.
8. Torrent Pharmaceuticals
Torrent Pharma is a major player in the cardiovascular and central nervous system therapeutic areas. The company's strategic acquisitions and focus on niche segments have helped it become one of the top pharmaceutical companies in India. Torrent's commitment to quality and patient-centric approach is evident in its product offerings.
9. Biocon Ltd.
Biocon is India's largest biopharmaceutical company, specializing in biologics and biosimilars. The company's focus on affordable innovation and its significant contributions to chronic disease management make it a leader in the pharmaceutical industry in India. Biocon's global partnerships and strong R&D capabilities are key to its success.
10. Cadila Healthcare (Zydus)
Cadila Healthcare, also known as Zydus, is a prominent player in the Indian pharma industry, offering a wide range of healthcare solutions. The company's innovative approach and comprehensive product portfolio have positioned it among the top 10 pharmaceutical companies in India.
The Role of Pharma Manufacturing Companies in India
Pharma manufacturing companies in India play a crucial role in the global supply chain of medicines. These companies not only produce high-quality generics but also invest heavily in research and development to bring new and innovative drugs to the market. The efficiency and scale of Indian pharma manufacturing are key factors in the country's ability to provide affordable medicines worldwide.
Finding the Best Pharma Companies Near You
For those searching for "pharma companies near me," it's important to recognize the regional presence of leading pharmaceutical companies. Many top pharma companies in India have established manufacturing and research facilities in various parts of the country, ensuring widespread access to their products and services.
Centurion HealthCare Pvt. Ltd. – A Leading Player in the Industry
Centurion HealthCare Pvt. Ltd. is an emerging name in the Indian pharmaceutical landscape. As a medicine manufacturing company in India, Centurion HealthCare is dedicated to providing high-quality pharmaceutical products across various therapeutic categories. The company's commitment to innovation, quality, and patient care positions it among the best pharma companies in India.
The Future of the Pharmaceutical Industry in India
The future of the pharmaceutical industry in India looks promising, with continued growth driven by innovation, increasing healthcare needs, and expanding global reach. Indian pharma companies are expected to play a pivotal role in addressing global health challenges, developing new treatments, and ensuring the availability of affordable medicines.
Conclusion
India's pharmaceutical industry is a dynamic and rapidly evolving sector, with numerous companies leading the way in innovation, quality, and global healthcare contributions. From established giants like Sun Pharma and Dr. Reddy's to emerging leaders like Centurion HealthCare Pvt. Ltd., the top pharmaceutical companies in India are making significant strides in improving healthcare outcomes worldwide.
As the industry continues to grow, these companies will remain at the forefront of pharmaceutical advancements, ensuring that India retains its position as a global leader in medicine production and innovation. Whether you are looking for the best pharma company in India or seeking reliable pharmaceutical companies in India, the landscape is rich with options that exemplify excellence and commitment to health.
For Centurion HealthCare Pvt. Ltd., being part of this esteemed group of pharma companies in India is a testament to its dedication to quality, innovation, and patient care. As the industry moves forward, Centurion HealthCare is poised to continue its growth and contribute to the global healthcare landscape, solidifying its place among the best pharmaceutical companies in India.
#Pharma companies near me#Top pharma companies in India#Pharma industry in India#Pharmaceutical industry in India#Top 10 pharmaceutical companies in India#Best pharma company in India#Top pharmaceutical companies in India#best pharmaceutical companies in India#Pharma manufacturing companies in India#Medicine company in India#Pharma companies in India#Pharmaceutical companies in India#Medicine manufacturing company in India
3 notes
·
View notes
Text
Exploring the Global Aldehydes Market: Key Players and Market Dynamics
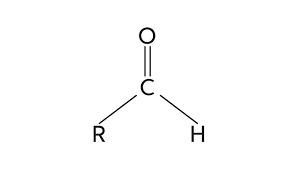
The aldehydes market is a segment of the chemical industry that deals with the production and distribution of a class of organic compounds known as aldehydes. These compounds are characterized by the presence of a carbonyl group (C=O) bonded to a hydrogen atom and a carbon atom in their chemical structure. Aldehydes find widespread applications in various industries, thanks to their unique properties and versatile reactivity.
In terms of market overview, the aldehydes market has been experiencing steady growth in recent years. This growth can be attributed to the increasing demand for aldehydes in industries such as pharmaceuticals, agriculture, food and beverages, and cosmetics. Aldehydes serve as crucial intermediates in the synthesis of various chemicals and are essential in the production of fragrances, flavor enhancers, and pharmaceuticals.
The growth in the aldehydes market industry can be primarily attributed to the expansion of these end-user industries. For instance, the pharmaceutical industry relies heavily on aldehydes for the synthesis of a wide range of drugs and active pharmaceutical ingredients (APIs). Additionally, the food and beverage industry utilizes aldehydes for flavor enhancement and preservation purposes, further driving market growth.
The aldehydes market is also influenced by evolving industry trends. One significant trend is the increasing emphasis on green chemistry and sustainable practices. Many companies in the aldehydes sector are adopting environmentally friendly production processes, such as catalytic hydrogenation, to reduce the environmental impact of their operations. This trend aligns with the growing awareness of environmental issues and the need for more eco-friendly chemical manufacturing.
Another noteworthy trend is the constant innovation and development of novel aldehyde derivatives with enhanced properties. This innovation is driven by the demand for higher-quality products in various industries. Researchers and manufacturers are continuously exploring new applications and synthesizing aldehydes tailored to meet specific industry requirements, which contributes to market expansion.
In conclusion, the aldehydes market is a dynamic segment within the chemical industry, driven by the increasing demand from various end-user industries. As industries continue to grow and evolve, the market is expected to witness further advancements, particularly in sustainable production methods and novel aldehyde derivatives, to meet the changing needs of consumers and businesses alike.
2 notes
·
View notes
Text
Process Development Manufacturing | Saurav Chemicals
Saurav Chemicals provides extensive process development services, guaranteeing the creation of efficient, cost-effective, and scalable processes tailored to your drug production needs.

#Process development manufacturing#Process development pharma#API process development#Drug formulation development#Drug development process
1 note
·
View note
Text
Japan Pharma Industry | Insights into the Paracetamol Market
The Japanese pharmaceutical industry is dominated by research and development; with swift investment in newly developed products and biotechnology. The industry has proved to be quite robust despite such constraints and the business environments involves complex regulatory mechanisms as well as pressure on the pricing of the products and services that they offer. The global market size for Japanese pharma market was assessed in 2024, approx. USD 85 billion which is expected to reach USD 90 billion in 2030. The country is quite strong with a number of MNCs, local players and emerging startup companies targeting more innovative drugs & therapeutic areas.
#pharmaceutical research and development#API manufacturing companies#pharmaceutical chemicals#medical instruments company#new drug application
1 note
·
View note
Text
Introduction to Active Pharmaceutical Ingredients (APIs): Key Definitions and Importance
The global Active Pharmaceutical Ingredients (API) market size was USD 144.70 Billion in 2022 and is expected to register a rapid revenue CAGR of 8.6% during the forecast period. Rising demand for pharmaceutical drugs and increasing drug Research & Development (R&D) activities are key factors driving market revenue growth.
Get Download Pdf Sample Copy of this Report@ https://www.emergenresearch.com/request-sample/2625
Competitive Terrain:
The global Active Pharmaceutical Ingredients industry is highly consolidated owing to the presence of renowned companies operating across several international and local segments of the market. These players dominate the industry in terms of their strong geographical reach and a large number of production facilities. The companies are intensely competitive against one another and excel in their individual technological capabilities, as well as product development, innovation, and product pricing strategies.
The leading market contenders listed in the report are:
AbbVie Inc., Pfizer, Inc., Sanofi, Boehringer Ingelheim, Bristol-Myers Squibb, Teva Pharmaceutical Industries Ltd., ELI Lilly and Company, GlaxoSmithKline plc., Merck & Co., Inc., Lonza, Hisun USA, Inc., Biocon Ltd., BASF SE, and Cambrex Corporation
Key market aspects studied in the report:
Market Scope: The report explains the scope of various commercial possibilities in the global Active Pharmaceutical Ingredients market over the upcoming years. The estimated revenue build-up over the forecast years has been included in the report. The report analyzes the key market segments and sub-segments and provides deep insights into the market to assist readers with the formulation of lucrative strategies for business expansion.
Competitive Outlook: The leading companies operating in the Active Pharmaceutical Ingredients market have been enumerated in this report. This section of the report lays emphasis on the geographical reach and production facilities of these companies. To get ahead of their rivals, the leading players are focusing more on offering products at competitive prices, according to our analysts.
Report Objective: The primary objective of this report is to provide the manufacturers, distributors, suppliers, and buyers engaged in this sector with access to a deeper and improved understanding of the global Active Pharmaceutical Ingredients market.
Emergen Research is Offering Limited Time Discount (Grab a Copy at Discounted Price Now)@ https://www.emergenresearch.com/request-discount/2625
Market Segmentations of the Active Pharmaceutical Ingredients Market
This market is segmented based on Types, Applications, and Regions. The growth of each segment provides accurate forecasts related to production and sales by Types and Applications, in terms of volume and value for the period between 2022 and 2030. This analysis can help readers looking to expand their business by targeting emerging and niche markets. Market share data is given on both global and regional levels. Regions covered in the report are North America, Europe, Asia Pacific, Latin America, and Middle East & Africa. Research analysts assess the market positions of the leading competitors and provide competitive analysis for each company. For this study, this report segments the global Active Pharmaceutical Ingredients market on the basis of product, application, and region:
Segments Covered in this report are:
Type Outlook (Revenue, USD Billion; 2019-2032)
Innovative Active Pharmaceutical Ingredients (API)
Generic Active Pharmaceutical Ingredients (API)
Drug Type Outlook (Revenue, USD Billion; 2019-2032)
Prescription Drugs
Over-the-Counter (OTC) Drugs
Potency Outlook (Revenue, USD Billion; 2019-2032)
Traditional API
Highly Potent API (HPAPI)
Browse Full Report Description + Research Methodology + Table of Content + Infographics@ https://www.emergenresearch.com/industry-report/active-pharmaceutical-ingredients-market
Major Geographies Analyzed in the Report:
North America (U.S., Canada)
Europe (U.K., Italy, Germany, France, Rest of EU)
Asia Pacific (India, Japan, China, South Korea, Australia, Rest of APAC)
Latin America (Chile, Brazil, Argentina, Rest of Latin America)
Middle East & Africa (Saudi Arabia, U.A.E., South Africa, Rest of MEA)
ToC of the report:
Chapter 1: Market overview and scope
Chapter 2: Market outlook
Chapter 3: Impact analysis of COVID-19 pandemic
Chapter 4: Competitive Landscape
Chapter 5: Drivers, Constraints, Opportunities, Limitations
Chapter 6: Key manufacturers of the industry
Chapter 7: Regional analysis
Chapter 8: Market segmentation based on type applications
Chapter 9: Current and Future Trends
Request Customization as per your specific requirement@ https://www.emergenresearch.com/request-for-customization/2625
About Us:
Emergen Research is a market research and consulting company that provides syndicated research reports, customized research reports, and consulting services. Our solutions purely focus on your purpose to locate, target, and analyse consumer behavior shifts across demographics, across industries, and help clients make smarter business decisions. We offer market intelligence studies ensuring relevant and fact-based research across multiple industries, including Healthcare, Touch Points, Chemicals, Types, and Energy. We consistently update our research offerings to ensure our clients are aware of the latest trends existent in the market. Emergen Research has a strong base of experienced analysts from varied areas of expertise. Our industry experience and ability to develop a concrete solution to any research problems provides our clients with the ability to secure an edge over their respective competitors.
Contact Us:
Eric Lee
Corporate Sales Specialist
Emergen Research | Web: www.emergenresearch.com
Direct Line: +1 (604) 757-9756
E-mail: [email protected]
Visit for More Insights: https://www.emergenresearch.com/insights
Explore Our Custom Intelligence services | Growth Consulting Services
Trending Titles: Geocell Market | Pancreatic Cancer Treatment Market
Latest Report: Ceramic Tiles Market | Life Science Analytics Market
0 notes
Text
Sodium Triacetoxy Borohydride Exporters in India
India has emerged as a global hub for the production and export of specialty chemicals, including Sodium Triacetoxy Borohydride (STAB). As a versatile reagent widely used in organic synthesis, STAB is essential for various industries, including pharmaceuticals, agrochemicals, and fine chemicals. Among the prominent exporters in this field, Sunlight Active Drug Ingredients Pvt. Ltd. (Sunlight ADI) stands out for its commitment to quality, reliability, and innovation.
What is Sodium Triacetoxy Borohydride?
Sodium Triacetoxy Borohydride (C6H10BNaO6) is a selective reducing agent commonly used in the reduction of aldehydes and ketones to their respective alcohols. Its mild and selective nature makes it particularly suitable for applications where sensitive functional groups are present. Its non-hygroscopic nature and ease of handling have further cemented its position as a preferred reagent in synthetic organic chemistry.
Sunlight ADI: A Trusted Name in Sodium Triacetoxy Borohydride Exports
Sunlight ADI has established itself as a key player in the global specialty chemical market by leveraging its state-of-the-art manufacturing facilities and expertise in chemical synthesis. Here’s what sets Sunlight ADI apart in exporting STAB:
Stringent Quality Standards Sunlight ADI adheres to international quality standards to ensure that its Sodium Triacetoxy Borohydride meets the stringent requirements of global clients. Each batch undergoes rigorous quality checks for purity, stability, and performance.
Eco-Friendly Manufacturing As sustainability becomes a priority, Sunlight ADI employs eco-friendly processes in its production of STAB. This not only minimizes environmental impact but also ensures compliance with international environmental regulations.
Global Reach With a robust export network, Sunlight ADI supplies Sodium Triacetoxy Borohydride to clients across North America, Europe, Asia, and other regions. The company’s ability to meet large-scale demands with prompt delivery makes it a preferred partner worldwide.
Customization and Technical Support Sunlight ADI provides tailored solutions to meet specific client needs. Its team of technical experts offers comprehensive support, from product selection to application guidance.
Applications of Sodium Triacetoxy Borohydride
Sodium Triacetoxy Borohydride is widely used in:
Pharmaceutical Industry: For synthesizing intermediates and active pharmaceutical ingredients (APIs).
Agrochemical Sector: In the development of crop protection agents.
Fine Chemicals: To produce high-value chemicals for various industrial applications.
Why Choose Sunlight ADI?
India's chemical industry is known for its cost-effective and high-quality products, and Sunlight ADI exemplifies these attributes. By choosing Sunlight ADI as your Sodium Triacetoxy Borohydride exporter, you benefit from:
Competitive pricing without compromising on quality.
Reliable supply chain management.
A partner committed to innovation and sustainability.
Conclusion
As the demand for Sodium Triacetoxy Borohydride continues to grow globally, partnering with a trusted exporter like Sunlight Active Drug Ingredients Pvt. Ltd. ensures access to superior products and unmatched service. With its dedication to quality, innovation, and customer satisfaction, Sunlight ADI is poised to remain a leader in the export of specialty chemicals from India.
For more information on how Sunlight ADI can meet your requirements for Sodium Triacetoxy Borohydride, visit their website or contact their expert team today.
0 notes
Text
Farmson’s Commitment to Sustainability: Building a Greener Tomorrow

In a world increasingly defined by environmental challenges, sustainability has become more than just a buzzword—it’s a necessity. At Farmson Basic Drugs, sustainability isn’t a choice; it’s a deeply rooted commitment that drives every aspect of our operations. Our approach integrates environmental conservation, social responsibility, and economic viability to create a holistic framework for a better future.
For more details visit: https://www.farmson.com/blogs/farmsons-commitment-to-sustainability-building-a-greener-tomorrow/
Read more from our related blogs:
Sustainable Development: Farmson Basic Drugs’ Commitment to Progress
Farmson: Leading the Way in Sustainable API Manufacturing
Pioneering Sustainability: Exploring Farmsons’ Sustainable Practices in API Paracetamol Production
Farmson’s Glacial Acetic Acid Plant Reduces Carbon Footprints
Striking a Green Balance Farmson Pharmaceuticals’ Commitment to Environmental Responsibility
0 notes
Text

From Acquisition to Innovation: Aspire's Path in Specialty Pharmaceuticals
Author: Michael Tzimas, Chief Corporate Development Officer, Aspire Pharma
The Beginnings
Aspire Pharma is a rapidly growing global specialty pharma business, based in the UK, devoted to delivering true value to patients, healthcare professionals, and the NHS.
The company manufactures and supplies quality branded and generic medicines not only to the UK but also to an expanding international market. They pride themselves on providing their customers with cost-effective solutions as well as responsive, adaptable, and ethical service that meets the highest regulatory standards.
The core focus is niche generics with high complexity. This is characterised by scarcity such as limited API, manufacturing complexity, drug/device combinations, new presentation, and limited competition, which results in meeting high unmet needs.
Discover more: https://www.pharmafocuseurope.com/advertorials/from-acquisition-to-innovation
0 notes
Text
Honour Labs - Walk-in for Quality Control on 11th & 18th Jan 2025
Honour Lab Limited, founded in 2012, is committed to manufacturing Bulk Drug Intermediates and Active Pharmaceutical Ingredients (APIs). The company’s main emphasis is on developing high-quality intermediates and raw materials that cater to the competitive demands of the rapidly expanding global drug markets. With 6 modern manufacturing facilities in Hyderabad, Visakhapatnam, and Pune, Honour Lab…
0 notes