#2-Methyl Benzyl Chloride
Explore tagged Tumblr posts
Text
Ferric Chloride 42% Solution | Bloom ChemAG
Introducing bloomChemAG's premium Ferric Chloride 42% Solution, the go-to choice for all your industrial needs. Our high-quality solution is carefully formulated to deliver exceptional results every time. With its bold concentration, this solution offers superior performance in various applications, ensuring efficient metal etching, water treatment, and more. Trust bloomChemAG to provide you with a reliable solution that never compromises on quality.
Visit here: https://www.bloomchemag.com/
#Triethyl Amine (TEA)#buy methylene chloride#buy benzaldehyde online#Buy Methyl Ethyl Ketone (MEK)#Buy Acetone#Ethyl acetate (ETAC)#Buy Benzyl alcohol#buy sorbitol 70% online#Butyl acrylate#WHITE SPIRIT (LAWS)#ISOPROPYL ALCOHOL (IPA)#BUTYL ACETATE (NBAC)#Methyl Methacrylate (MMA)#2-Ethyl Hexyl Acrylate#Ferric Chloride 42% Solution
0 notes
Text
Lo que hay que evitar 🙅♀️ Alergenos en la cosmética!

¡La cosmética está llena de ingredientes hermosos que tu piel va a amar, y como ya te contamos en otros posteos, de muchos que es mejor evitar! Recordá que por más que un ingrediente cosmético esté permitido y aprobado no quiere decir que sea bueno para tu piel! 🥲 Hoy vamos a hablar del caso más claro: los alérgenos! 🤧
En este artículo, te dejo los alérgenos comunes en la cosmética, cómo identificarlos y por qué es importante prestar atención a lo que pones en tu piel.
¿Qué Son los Alergenos en la Cosmética?
Los alérgenos en la cosmética son ingredientes que tienen el potencial de desencadenar una reacción alérgica en algunas personas. Estas reacciones pueden variar desde una leve irritación hasta erupciones cutáneas graves. Es importante destacar que no todos reaccionarán de la misma manera a estos ingredientes, ya que la sensibilidad a los alérgenos puede variar enormemente de una persona a otra.
Ingredientes Comunes que Son Alergénicos
🌸 Fragancias: Las fragancias son uno de los alérgenos más comunes en la cosmética. Se utilizan tanto para dar una fragancia particular al producto como para enmascarar el aroma natural de algunos activos. Las fragancias sintéticas, en particular, son conocidas por desencadenar reacciones alérgicas en algunas personas. No hacen a la eficacia de tus productos por lo que busca productos sin fragancia o con fragancias naturales.
Fragancias frecuentemente alergénicas:
Amyl cinnamal
Amylcinnamyl alcohol
Benzyl alcohol
Benzyl salicylate
Cinnamyl alcohol
Cinnamal
Citral
Coumarin
Eugenol
Geraniol
Hydroxycitronellal
Hydroxymethylpentyl-cyclohexenecarboxaldehyde
Isoeugenol
Fragancias menos frecuentemente alergénicas:
Anisyl alcohol
Benzyl benzoate
Benzyl cinnamate
Citronellol
Farnesol
Hexyl cinnamaldehyde
Lilial
d-Limonene
Linalool
Methyl heptine carbonate
3-Methyl-4-(2,6,6-trimethyl-2-cyclohexen-1-yl)-3-buten-2-one
Fuente: Comisión Directiva de cosméticos de la UE https://ec.europa.eu/health/scientific_committees/opinions_layman/perfume-allergies/en/l-3/1-introduction.htm
Podés ver en el siguiente enlace las fragancias alergénicas declaradas por el ANMAT: https://www.argentina.gob.ar/anmat/regulados/cosmeticos/ingredientes-de-fragancia-alergenicos
🧫 Conservantes: Los conservantes como los parabenos y el formaldehído son utilizados para ampliamente en la industria cosmética. Sin embargo, pueden causar alergias en algunas personas. Los conservantes liberadores de formaldehído, como el diazolidinil urea y el quaternium-15, son especialmente problemáticos.
Methylisothiazolinone (MIT)
Methylchloroisothiazolinone (CMIT)
Formaldehyde and formaldehyde:
Bronopol (2-bromo-2-nitropropane-1,3-diol)
5-bromo-5-nitro-1,3-dioxane
Diazolidinyl urea
DMDM hydantoin (1,3-dimethylol-5,5-dimethylhydantoin)
Imidazolidinyl urea
Sodium hydroxymethylglycinate
Quaternium-15 (Dowicil 200; N-(3-chloroallyl) hexaminium chloride)
Colorantes: Algunos colorantes utilizados en productos cosméticos, como el tartrazina y el amarillo ocaso, son conocidos por desencadenar reacciones alérgicas en algunas personas. Los colorantes sintéticos tienden a ser más problemáticos que los naturales. Para evitar el uso de colorantes o pigmentos no permitidos en ciertas áreas específicas, como ojos, utilizá como referencia el listado del ANMAT: https://www.argentina.gob.ar/normativa/nacional/disposición-1608-2013-209614/texto
Lanolina: La lanolina es un derivado de la lana de las ovejas y se encuentra en muchos productos para el cuidado de la piel. Aunque es un excelente humectante, puede causar alergias en personas sensibles a la lanolina.
Aceites Esenciales: Aunque los aceites esenciales son naturales, algunas personas pueden ser alérgicas a ellos. Los aceites esenciales de canela, nuez moscada y menta son conocidos por ser especialmente alergénicos.
En un estudio realizado sobre alérgenos comúnmente presentes en los cosméticos se arroja que el mayor porcentaje pertenece al grupo de Fragancias:

Te dejo el link de este artículos científico: https://www.mdpi.com/2079-9284/9/2/32
Cómo Identificar y Evitar los Alergenos en la Cosmética
Identificar y evitar los alérgenos en la cosmética puede ser un desafío, ya que no siempre están etiquetados de manera clara. Aquí hay algunos consejos para ayudarte a protegerte:
Siempre revisa la lista de ingredientes en los productos cosméticos. Busca los INCI listados previamente.
Haz una Prueba de Parche: Si tienes la piel sensible o propensa a las alergias, considera hacer una prueba de parche antes de usar un nuevo producto. Aplica una pequeña cantidad en una parte discreta de la piel y observa si hay alguna reacción en las próximas 24 horas.
Opta por Productos Hipoalergénicos: Los productos etiquetados como "hipoalergénicos" están formulados específicamente para reducir el riesgo de reacciones alérgicas.
Conclusión: Cuida tu Piel y tu Salud
La cosmética y el skincare están pasando por un momento de auge. Hoy todos tenemos al menos 2 o 3 productos en nuestro neceser (y algunos 20) y es sumamente importante empezar a educarnos sobre lo que contienen esos productos para día a día mejorar nuestras elecciones (y nuestras inversiones) para asegurarnos que le estamos dando a nuestra piel lo mejor posible. ❤️ No vale la pena exponer a tu piel a ingredientes que la irritan y sensibilizan. Como siempre decimos, cuidar tu piel es también saber QUE NO QUERÉS en tus productos. 🤓
#skincare#skincareblog#loveyourskin#cuidado de la piel#skin health#skincare rutine#concienciaentuskincare#miho
1 note
·
View note
Text
N-Methylaniline CAS 100-61-8 NMA factory sample is free have in stock
+ 86 13805212761 QUICK DETAILS Product name:N-Methylaniline CAS:100-61-8 Molecular formula:C7H9N Molecular weight:107.15 EINECS No.:202-870-9 Purity:≥99% Brand:MIT -IVY INDUSTRY CO.,LTD Other names:N-methyl-aniline;N-Methylaniline;N-methylphenylamine;N-methyl-N-phenylamine;N-Phenylmethylamine;Methylaniline;N-methyl-aminobenzene;Methylphenylamine;N-Monomethylaniline Appearance:light yellow liquid Port: any port in china Packing:according to the clients requirement Storage: Store in dry, dark and ventilated place. Transportation: by sea or by air payment methods: L/C, T/T, D/A, D/P, O/A, paypal, western union etc.accept all payment. Application 1. Used as a dye intermediate 2. N-Methylaniline is not only the raw material for the insecticide buprofezin, but also used for the synthesis of its intermediate N-chloromethyl N-phenylcarbamoyl chloride and the intermediate for the herbicide fenthiazide. It is widely used in the dye industry. 3. The product is used as an intermediate of organic synthesis, acid absorbent and solvent, and used in the production of cationic brilliant red FG, cationic peach red B, active yellow brown KGR, etc. in the dye industry. Superiority 1. Best quality in your requirement 2. Competitive price in china market 3. Mature technical support 4. Professional logistic support 5 . Full experience of large numberscontainers loading in chinese sea port 6 .Fast shipment by reputed shipping line 7. Packing with pallet as buyer's special request 8. Best service after shipment. 9. Full experience in export 10. Raw materials from chinese origin Company Information MIT-IVY INDUSTRY CO.,LTD is a manufacturer and exporter of fine chemical dyes & pharmaceutical intermediates in China. Mainly produce aniline series products and chlorine series products. We are a young company full of vitality and vitality. The company has a group of energetic, well-trained employees and strong technical research and development capabilities. We specialize in the production, development and sales of API intermediates, fine chemicals and plant extracts. Relying on advanced equipment and strict management, adhere to the business philosophy of "openness, tolerance, innovation, and sharing" to create a win-win cooperationplatform.Everything comes from innovation, it is our philosophy ! If you are interested in getting more quotations, please add WHATSAPP:0086-13805212761 or E-MAIL:[email protected] Main products MIT-IVYINDUSTRYCO.,LTDMit-Ivy is a well-known fine chemicals and pharmaceutical intermediates manufacturer with strong R&D support in China. Mainly involved Aniline, Chlorine products. Payment:DA 60 DAYS TEL:008619961957599 E-MAIL:[email protected] 产品 Product CAS N,N-二甲基-1,4-苯二胺 N,N-Dimethyl-1,4-phenylenediamine DMPD 99-98-9 N,N-二甲基苄胺 N,N-Dimethylbenzylamine BDMA 103-83-3 N,N-二甲基甲酰胺 N,N-Dimethylformamide DMF .68-12-2 N,N-二甲基甲酰胺二甲缩醛 DMF-DMA N,N-Dimethylformamidedimethyl acetal (DMF-DMA) 4637-24-5 N,N-二甲基乙酰胺 N,N-Dimethylacetamide DMAC 127-19-5 N,N-二乙基间甲苯甲酰胺 避蚊胺 N,N-diethyl-m-toluamide DEET 134-62-3 N,N-二乙基羟胺 N,N-Diethylhydroxylamine DEHA 3710-84-7 N-甲基-N-羟乙基苯胺 2-(N-甲基苯胺)乙醇 2-(N-methylanilino)ethanol 93-90-3 N-甲基吡咯烷酮 N-methylpyrrolidone 872-50-4 N,N-二甲基苯胺 N,N-Dimethylaniline DMA 121-69-7 N,N-二甲基对甲苯胺 N,N-Dimethyl-p-toluidine DMPT 99-97-8 N,N-二甲基邻甲苯胺 N,N-Dimethyl-o-toluidine DMOT 609-72-3 N,N-二乙基苯胺 N,N-Diethylaniline 91-66-7 N,N-二乙基间甲苯胺 N,N-Diethyl-m-toluidine 91-67-8 N,N-二羟乙基苯胺 N,N-Dihydroxyethylaniline PDEA 120-07-0 N-乙基间甲苯胺 N-乙基-3-甲基苯胺 N-Ethyl-m-toluidine/N-Ethyl-3-methylaniline 102-27-2 N-乙基-N-氰乙基苯胺 3-(N-ethylanilino)propiononitrile 148-87-8 N-乙基-N-羟乙基苯胺 N-Ethyl-N-hydroxyethylaniline 92-50-2 N-乙基-N-苄基苯胺 乙基苄基苯胺; N-苄基-N-乙基苯胺 N-ethyl-N-phenylbenzenemethanamine 92-59-1 N-乙基-N-氰乙基间甲苯胺 N-2-cyanoethyl-N-ethyl-m-toluidine 148-69-6 N-乙基-N-苄基间甲苯胺 N-Benzyl-N-ethyl-m-toluidine 119-94-8 N-乙基邻甲苯胺 N-Ethyl-o-toluidine/2-Ethylaminotoluene 94-68-8 N-乙基苯胺 N-Ethylaniline 103-69-5 N-甲基苯胺 N-Methylaniline 100-61-8 N,N-二甲基-间甲基苯胺 N,N-DIMETHYL-M-TOLUIDINE 121-72-2 N-甲基二苯胺 N-Methyldiphenylamine 552-82-9 N-甲基-邻甲基苯胺 N-METHYL-O-TOLUIDINE 611-21-2 N-甲基-对甲基苯胺 N-METHYL-P-TOLUIDINE 623-08-5 4-甲基-N-苯基苯胺 N-PHENYL-P-TOLUIDINE 620-84-8 N-异丙基苯胺 N-ISOPROPYLANILINE 768-52-5 N,N-二氰乙基苯胺 N,N-Dicyanoethylaniline 1555-66-4 N,N-二羟乙基-对甲基苯胺 N,N-DIHYDROXYETHYL-P-TOLUIDINEDHEPT .3077-12-1 N-乙基-2-硝基苯胺 N-Ethyl-2-Nitro-Benzenamine 10112-15-9 2,4-二氯苯胺 2,4Dichloroaniline 554-00-7 N-(2-羟乙基)乙二胺 AEEA 111-41-1 1,3-二甲基-2-咪唑啉酮N,N-二甲基亚乙基脲1,3-二甲基-2-咪唑啉酮(DMI) 1,3-Dimethyl-2-imidazolidinone DMI N,N'-dimethylimidazolidinone 80-73-9 N,N-二苄基羟胺 N,N-Dibenzylhydroxylamine 621-07-8 对甲苯胺 P-Toluidine PT 106-49-0 邻甲苯胺 O-Toluidine OT 95-53-4 二乙基乙醇胺 DEEA;DEAE 100-37-8 甲萘胺 AlphaNaphthylamine 134-32-7 间二氯苯 1,3-Dichlorobenzene MDCB 541-73-1 间甲苯胺 M-Toluidine MT 108-44-1 间苯二胺 M-PHENYLENEDIAMINE MPDA 108-45-2 多乙烯多胺 PEPA 68131-73-7 二乙烯三胺(DETA) Diethylenetriamine DETA 111-40-0 三乙烯���胺 Triethylenediamine 280-57-9 三乙烯四胺 TriethylenetetramineTETA 112-24-3 四乙烯五胺 TEPA 112-57-2 Read the full article
#100-61-8#Amines#chemicalrawmaterials#Chemicals#Dyeintermediates#Finechemicals#inorganicchemicals#Insecticideintermediates#Methylaniline#methylphenylamine#Monomethylaniline#N-methyl-aminobenzene#N-methyl-aniline;N-Methylaniline;N-methylphenylamine;N-methyl-N-phenylamine;N-Phenylmethylamine#N-methyl-aniline,N-Methylaniline'N-methylphenylamine,N-methyl-N-phenylamine,N-Phenylmethylamine;Methylaniline,#N-Monomethylaniline#organicchemicalrawmaterials#Organicrawmaterials#pesticideintermediates#pharmaceuticalintermediates#polyesterresin
0 notes
Text
Lupine Publishers | Chloride-Induced Highly Active Catalyst for Methyl Esterification of Alcohols

Lupine Publishers | ARCHIVES OF ORGANIC AND INORGANIC CHEMICAL SCIENCES
Abstract
In this work, a series of active Au/NiOx catalysts were successful to prepare by tracing the concentrations of chloride in the re-dispersed aqueous solutions. By characterizations, we found that the appropriate amount of residual chloride in Au catalyst would induce Au nanoparticles (Au NPs) to locate on the edges of NiOx particles, which resulted in the active Au/NiOx-9 sample. Fine control of chloride in the aqueous solution provides a new perspective to push for addressing the controllable preparation of active heterogeneous catalysts.
Keywords: Au catalyst, Preparation, Chloride, Esterification
Introduction
In recent decades, Au catalysts have received growing attentions and been widely applied in many important research fields [1], since good performance of Au catalysts was discovered [2]. However, the controllable preparation of highly active heterogeneous catalysts is still a longstanding challenge till now, especially Au catalysts. Many efforts have been devoted to this problem. The active site, structure and the quantum size effect of Au catalyst [3], active oxygen species of the support [4], suitable reducible oxide supports [5],and so on, have been extensively studied. Additionally, catalyst precursors, bases, pH value, aging time, and calcinations temperature are also crucial conditions [2,6]. Nevertheless, the controllable preparation of highly active Au catalyst is still difficult to realize even strictly following all above conditions. Chloride (usually as Cl-) is generally regarded as a poison for Au catalyst, Because of strong interaction of chloride and Au. We realized the reproducible preparation of Au/Fe2O3 catalyst for CO oxidation [7]. It is meaningful to explore whether this method can be applied to other catalysts and reactions or not. In this work, Methyl esterification of alcohols was chosen as model reaction. The controllable preparation of highly active Au/ NiOx catalyst was realized by tracing the concentrations of chloride in the re-dispersed aqueous solutions.
Experimental Details
Au/NiOx catalyst preparation
20ml Ni(NO3)36H2O (0.011 M) and 1.05 ml HAuCl4 (0.24M) were mixed together and were drop wise added into 60 ml Na2CO3 solution (0.31M) under vigorous stirring in 3h. The turbid liquid was divided into four sections and separation by centrifugation. Each section of the recovered precipitate was re-dispersed in different amount of deionised water and ultrasonically washed for 1h. The chloride concentration in the re-dispersed aqueous solution of each section was determined by CHI660D electrochemical workstation. Then, the solid was separated by centrifugation, dried at 80o C for 3h and calcined at 350 oC for 0.5 h to produce the catalyst sample, which was denoted as Au/NiOx-X, in which X suggested the chloride concentration in ppm.
Catalyst activity test
1mmol benzyl alcohol, 30 mg catalyst and 2 ml methanol were added into a glass tube. And then it was exchanged with oxygen and reacted at 60o C (1 atom, O2 balloon). After reaction, it was cooled to room temperature. Biphenyl was used as internal standard and a certain amount of ethanol were added into the reaction mixture up to 10mL for quantitative analysis by GC-FID (Agilent 7890A).
Results and Discussion
The catalytic activities of 15 Au/NiOx samples, which were prepared from the re-dispersed aqueous solutions with chloride concentrations in the range of 2 to 108 ppm, for esterification of benzyl alcohol were studied. According to the results shown in Figure 1, catalytic activity of Au/NiOx varied with the changing of chloride concentration. The yields of methyl benzoate were lower than 21% if the catalysts were prepared from aqueous solutions containing >22ppm chloride. More active catalysts were produced when the chloride concentrations were going down. The Au/ NiOx catalysts with the highest catalytic activity were prepared from aqueous solutions containing 8-13ppm chloride, the yield of methyl benzoate of catalyst Au/NiOx-9 was >99%. Surprisingly, the catalysts turned less active again when the chloride concentrations were < 8ppm. Typically, the yield of methyl benzoate was 20% with catalyst Au/NiOx-3.
TEM measurement results of Au/NiOx are shown in Figure 2. Their TEM images were similar and seemed amorphous. For the sample of Au/NiOx-22, the lattice of gold could be observed and wrapped in NiOx particle. For active Au/NiOx-9, the most of Au NPs connected with the edges of NiOx particles or the junctions of several NiOx particles [8]. In consideration of the best catalytic performance of this sample, this observation strongly supported the former results about active site in Au catalyst, i.e. the interface between Au and iron oxide [3]. It suggested that the appropriate amount of chloride might act as the linkage between Au NPs and the edges of NiOx particles to gain the active Au catalyst, For Au/ NiOx-22 and Au/NiOx-3, too much or less chloride was presented, the interaction of Au NPs and NiOx like Au/NiOx-9 decreased significantly. Accordingly, the catalytic activity lost sharply. By metering more than 150Au NPs, the mean diameters of Au NPs in samples Au/NiOx-3, Au/NiOx-9 and Au/NiOx-22were 4.1, 3.8 and 6.6 nm with 1.91, 1.84 and 3.06 standard deviations. The size distributions of Au NPs in Au/NiOx-3 and Au/NiOx-9 samples were extremely similar. The marked difference of catalytic activities of these two catalysts did not come from the size effect of Au particles, but the contact way of Au NPs and NiOx supports.
At present, there is still not sufficient evidence to explain the real role of chloride in the formation of Au catalysts. However, according to the known evidence, we can make some reasonable conjectures. Firstly, as pH value of the mother aqueous solution rises, chlorine in chloroauric acid is substituted by the hydroxyl. Au-Cl bond breaks and then small Au NPs form. Finally, chloride is adsorbed on the support NiOx as well as Au NPs. Due to the stronger interaction of chlorideon the edges than on planes of NiOx crystallites, after the ultrasonication and washing operations, chloride located on the edges of NiOx crystallites remains. As shown in Figure 3, it is this kind of residual chloride that induces Au NPs to anchor on the edges of NiOx crystallites.
Conclusion
In summary, by tracing the chloride concentrations in the re-dispersed aqueous solution, we successfully prepared active Au/NiOx catalyst for catalytic methyl esterification of alcohols. If the chloride concentration was not in the range of 8-13ppm, the catalytic activity dropped dramatically. These results indicated that the presence of appropriate amounts of residual chloride was beneficial to obtain highly active heterogeneous catalysts. This work can offer a new perspective to realize the controllable preparation of active heterogeneous catalysts
For More ARCHIVES OF ORGANIC AND INORGANIC CHEMICAL SCIENCES
https://lupinepublishers.com/chemistry-journal/
https://lupinepublishers.com/chemistry-journal/fulltext/chloride-induced-highly-active-catalyst-for-methyl-esterification-of-alcohols.ID.000154.php
7 notes
·
View notes
Text
Factory price CAS 13605-48-6 PMK glycidate/PMK powder
wickr me:cassie08monad [email protected] +86 13349999584Wuhan Monad Medicine Tech Co., Ltd To survive by quality, develop by credit.If you are looking for a trustable supplier of varies chemical products, pls do not hesitate to contact us! We ship by special line and guarantee that you will 100% receive your parcle. We will handle the customs for you as well. You just need to wait for your parcle at home. Main Products: 1,4-Butanediol 110-63-4, Benzeneacetic Acid(BMK) 16648-44-5, 3-Bromopropyne 106-96-7 2-Bromo-4'-Methylpropiophenone 1451-82-7 Ethyl 3-oxo-4-phenylbutanoate 5413-05-8 PMK 13605-48-6 Sodium cyanoborohydride 25895-60-7 Tetramisole hydrochloride 5086-74-8 Benzene, (chloromethyl)- 100-44-7 Cannabidiol 13956-29-1 p-Anisoyl chloride 100-07-2 Phenacetin 62-44-2 2-Benzylamino-2-methyl-1-propanol 10250-27-8 Lidocaine 137-58-6 CAS 49851-31-2 2-Bromo-1-phenyl-1-pentanone CAS 1451-82-7 2-Bromo-4'-methylpropiophenone CAS 40064-34-4 4,4-Piperidinediol hydrochloride CAS 94-09-7 benzocaine CAS 123-75-1 Pyrrolidine CAS 10250-27-8 N-benzyl-2-amino-2-methylpropanol CAS 23076-35-9 Xylazine hydrochlorideContact us:
PMK Glycidate, PMK Poawder, PMK methyl glycidate, factory price PMK, supply PMK powder
[email protected] +86 13349999584 wickr Me: cassie08monad
1 note
·
View note
Link
Chlorotoluene is an important intermediate in the pharmaceuticals and agrochemicals industries. Chlorotoluene, also known as chlorinated toluene, refers to a group of three isomeric chemical compounds, where the benzene ring is attached to a chlorine atom and a methyl group. This group of isomeric compounds includes ortho, meta, and para chlorotoluene, which depends on the position of the chlorine atom with respect to the methyl group. Chlorotoluene is produced by the reaction of toluene with chlorine under moderate temperature and normal pressure in the presence of a metal catalyst, such as iron chloride or titanium chloride. A crude product is obtained with the isomeric ratio of chlorotoluene mixture depending on the temperature and catalyst. The pure chlorotoluene isomers are further separated by fractional distillation.
Browse complete “Chlorotoluene Market” report with TOC @ https://www.strategymrc.com/report/chlorotoluene-market
Based on type, the 2-chlorotoluene or o-chlorotoluene segment is estimated to have a lucrative growth due to the increasing use of the product in the manufacturing of various agrochemicals, such as growth regulators, herbicides, fungicides, acaricides, and pesticides. Agrochemicals are majorly used for crop protection and to grow agricultural productivity. Moreover, o-chlorotoluene is used to produce benzyl chloride, which is used on a large scale in pharmaceuticals, cosmetics, paints industries, and agrochemicals. In addition, the growing demand for benzyl chloride in these applications is anticipated to consequently boost demand for chlorotoluene and favor the market growth during the forecast period.
Some of the key players profiled in the Chlorotoluene Market include Changzhou Yuanfeng Chemical Co. Ltd, Hunan Zhuzhou Chemical Industry Group, Iharanikkei Chemical Industry Co., Ltd, Jiangsu Hongxing Chemical, Lanxess, Merck KGaA, Shandong Exceris Chemical Co. Ltd, Shimmer Chemicals Pvt. Ltd, Toray Industries Inc, Valtris Specialty Chemicals, and WeylChem International GmbH.
Request a Sample of “Chlorotoluene Market” @ https://www.strategymrc.com/report/chlorotoluene-market/request-sample
Free Customization Offerings: All the customers of this report will be entitled to receive one of the following free customization options:
Company Profiling
Comprehensive profiling of additional market players (up to 3)
SWOT Analysis of key players (up to 3)
Regional Segmentation
Market estimations, Forecasts and CAGR of any prominent country as per the client's interest (Note: Depends on feasibility check)
Competitive Benchmarking
Benchmarking of key players based on product portfolio, geographical presence, and strategic alliances
For more information about this report visit https://www.strategymrc.com/report/chlorotoluene-market
Report Store: https://www.strategymrc.com/report-store
Covid-19 reports: https://www.strategymrc.com/covid-19-impact-reports
About Us:
Stratistics MRC offer a wide spectrum of research and consulting services with in-depth knowledge of different industries. Our research reports and publications are routed to help our clients to design their business models and enhance their business growth in the competitive market scenario. We have a strong team with hand-picked consultants including project managers, implementers, industry experts, researchers, research evaluators and analysts with years of experience in delivering the complex projects.
Contact Us:
Email: [email protected]
Organization: Stratistics Market Research Consulting Pvt Ltd
Phone: +1-301-202-5929
Website: https://www.strategymrc.com
0 notes
Text
Lupine Publishers|Chloride-Induced Highly Active Catalyst for Methyl Esterification of Alcohols
Follow on Linkedin : https://www.linkedin.com/company/lupinepublishers Follow on Twitter : https://twitter.com/lupine_onlineFor more Lupine Publishers Open Access Journals Please visit our website: http://lupinepublishers.us/ For more Open Access Journal on Chemistry articles Please Click Here: https://lupinepublishers.com/chemistry-journal/
To Know More About Open Access Publishers Please Click on Lupine Publishers

Lupine Publishers | Journal of Chemistry
Abstract
In this work, a series of active Au/NiOx catalysts were successful to prepare by tracing the concentrations of chloride in the re-dispersed aqueous solutions. By characterizations, we found that the appropriate amount of residual chloride in Au catalyst would induce Au nanoparticles (Au NPs) to locate on the edges of NiOx particles, which resulted in the active Au/NiOx-9 sample. Fine control of chloride in the aqueous solution provides a new perspective to push for addressing the controllable preparation of active heterogeneous catalysts.
Keywords: Au catalyst, Preparation, Chloride, Esterification
Introduction
In recent decades, Au catalysts have received growing attentions and been widely applied in many important research fields [1], since good performance of Au catalysts was discovered [2]. However, the controllable preparation of highly active heterogeneous catalysts is still a longstanding challenge till now, especially Au catalysts. Many efforts have been devoted to this problem. The active site, structure and the quantum size effect of Au catalyst [3], active oxygen species of the support [4], suitable reducible oxide supports [5],and so on, have been extensively studied. Additionally, catalyst precursors, bases, pH value, aging time, and calcinations temperature are also crucial conditions [2,6]. Nevertheless, the controllable preparation of highly active Au catalyst is still difficult to realize even strictly following all above conditions. Chloride (usually as Cl-) is generally regarded as a poison for Au catalyst, Because of strong interaction of chloride and Au. We realized the reproducible preparation of Au/Fe2O3 catalyst for CO oxidation [7]. It is meaningful to explore whether this method can be applied to other catalysts and reactions or not. In this work, Methyl esterification of alcohols was chosen as model reaction. The controllable preparation of highly active Au/ NiOx catalyst was realized by tracing the concentrations of chloride in the re-dispersed aqueous solutions.
Experimental Details
Au/NiOx catalyst preparation
20ml Ni(NO3)36H2O (0.011 M) and 1.05 ml HAuCl4 (0.24M) were mixed together and were drop wise added into 60 ml Na2CO3 solution (0.31M) under vigorous stirring in 3h. The turbid liquid was divided into four sections and separation by centrifugation. Each section of the recovered precipitate was re-dispersed in different amount of deionised water and ultrasonically washed for 1h. The chloride concentration in the re-dispersed aqueous solution of each section was determined by CHI660D electrochemical workstation. Then, the solid was separated by centrifugation, dried at 80o C for 3h and calcined at 350 oC for 0.5 h to produce the catalyst sample, which was denoted as Au/NiOx-X, in which X suggested the chloride concentration in ppm.
Catalyst activity test
1mmol benzyl alcohol, 30 mg catalyst and 2 ml methanol were added into a glass tube. And then it was exchanged with oxygen and reacted at 60o C (1 atom, O2 balloon). After reaction, it was cooled to room temperature. Biphenyl was used as internal standard and a certain amount of ethanol were added into the reaction mixture up to 10mL for quantitative analysis by GC-FID (Agilent 7890A).
Results and Discussion
The catalytic activities of 15 Au/NiOx samples, which were prepared from the re-dispersed aqueous solutions with chloride concentrations in the range of 2 to 108 ppm, for esterification of benzyl alcohol were studied. According to the results shown in Figure 1, catalytic activity of Au/NiOx varied with the changing of chloride concentration. The yields of methyl benzoate were lower than 21% if the catalysts were prepared from aqueous solutions containing >22ppm chloride. More active catalysts were produced when the chloride concentrations were going down. The Au/ NiOx catalysts with the highest catalytic activity were prepared from aqueous solutions containing 8-13ppm chloride, the yield of methyl benzoate of catalyst Au/NiOx-9 was >99%. Surprisingly, the catalysts turned less active again when the chloride concentrations were < 8ppm. Typically, the yield of methyl benzoate was 20% with catalyst Au/NiOx-3.
Figure 1: The yield of methyl benzoatevs the chlorine concentration of the aqueous solution from which the catalyst samples were prepared.
Figure 2: HR-TEM (left) images and size distributions (right) of Au/NiOx-22 (a), Au/NiOx-9 (c), and Au/NiOx- 3(e).
TEM measurement results of Au/NiOx are shown in Figure 2. Their TEM images were similar and seemed amorphous. For the sample of Au/NiOx-22, the lattice of gold could be observed and wrapped in NiOx particle. For active Au/NiOx-9, the most of Au NPs connected with the edges of NiOx particles or the junctions of several NiOx particles [8]. In consideration of the best catalytic performance of this sample, this observation strongly supported the former results about active site in Au catalyst, i.e. the interface between Au and iron oxide [3]. It suggested that the appropriate amount of chloride might act as the linkage between Au NPs and the edges of NiOx particles to gain the active Au catalyst, For Au/ NiOx-22 and Au/NiOx-3, too much or less chloride was presented, the interaction of Au NPs and NiOx like Au/NiOx-9 decreased significantly. Accordingly, the catalytic activity lost sharply. By metering more than 150Au NPs, the mean diameters of Au NPs in samples Au/NiOx-3, Au/NiOx-9 and Au/NiOx-22were 4.1, 3.8 and 6.6 nm with 1.91, 1.84 and 3.06 standard deviations. The size distributions of Au NPs in Au/NiOx-3 and Au/NiOx-9 samples were extremely similar. The marked difference of catalytic activities of these two catalysts did not come from the size effect of Au particles, but the contact way of Au NPs and NiOx supports.
At present, there is still not sufficient evidence to explain the real role of chloride in the formation of Au catalysts. However, according to the known evidence, we can make some reasonable conjectures. Firstly, as pH value of the mother aqueous solution rises, chlorine in chloroauric acid is substituted by the hydroxyl. Au-Cl bond breaks and then small Au NPs form. Finally, chloride is adsorbed on the support NiOx as well as Au NPs. Due to the stronger interaction of chlorideon the edges than on planes of NiOx crystallites, after the ultrasonication and washing operations, chloride located on the edges of NiOx crystallites remains. As shown in Figure 3, it is this kind of residual chloride that induces Au NPs to anchor on the edges of NiOx crystallites.
Figure 3: The simple scheme of Au/NiOx catalysts.
Conclusion
In summary, by tracing the chloride concentrations in the re-dispersed aqueous solution, we successfully prepared active Au/NiOx catalyst for catalytic methyl esterification of alcohols. If the chloride concentration was not in the range of 8-13ppm, the catalytic activity dropped dramatically. These results indicated that the presence of appropriate amounts of residual chloride was beneficial to obtain highly active heterogeneous catalysts. This work can offer a new perspective to realize the controllable preparation of active heterogeneous catalysts.
For more Lupine Publishers Open Access Journals Please visit our website: http://lupinepublishers.us/ For more Open Access Journal on Chemistry articles Please Click Here: https://lupinepublishers.com/chemistry-journal/
To Know More About Open Access Publishers Please Click on Lupine Publishers
Follow on Linkedin : https://www.linkedin.com/company/lupinepublishers Follow on Twitter : https://twitter.com/lupine_online
0 notes
Text
Why is Quaternary Ammonium Salts One of The Most Common Phase-Transfer Catalysts?

Phase Transfer Catalysis (PTC) generally means that the reaction between the two substances, located in totally different immiscible phases, in the presence of a catalyst. Phase transfer catalysis is mainly used for the synthesis of organics such as pharmaceuticals, dyes, chemicals, etc. In this process, one phase acts as a reservoir of the reacting anions. The second phase, which is the organic phase, contains the organic reactants and catalysts generating lipophilic cations. The reacting anions enter the organic phase in ion pairs with lipophilic cations of the catalyst.
The advantages of Phase transfer catalysis can be summarized as:
The elimination of organic solvents
The elimination of dangerous, inconvenient and expensive reactants
Having high reactivity and selectivity of the active species
Having high yields and purity of products
The simplicity of the procedure
Having low investment cost
Having low energy consumption
Minimization of industrial wastes
Mild reaction conditions.
In chemistry, a Phase-transfer catalyst or PTC is a catalyst that facilitates the migration of a reactant from one phase into another phase where the reaction occurs. Phase-transfer catalysis is one of the special forms of heterogeneous catalysis.
The phase transfer catalysis can be categorized as per its dependence on the number of phases included:
Liquid-liquid phase transfer catalysis
Solid-liquid phase transfer catalysis
Third-liquid phase-transfer catalysis
Now let's introduce you the popular phase transfer catalyst, Quaternary ammonium salts which had given good results in phase transfer catalysis.
Quaternary Ammonium Salts (QAS) are those cationic compounds having alkyl groups in a chain length of C8–C18, which are generally water soluble compounds and can be used as in disinfectants in textile industries.
A quaternary ammonium salt is readily immobilized in a soluble poly(ethylene glycol) polymer support efficiently catalyzes different reactions carried out under phase-transfer catalysis conditions; the catalyst is easily recovered by precipitation and filtration which shows no appreciable loss of activity when recycled three times.
Let me mention you a few types of QAS:-
1) Tetra-alkyl-ammonium or phosphonium salts
Tetra-n-butyl ammonium bromide
Methyl- triethyl ammonium chloride
2) Tri-ethyl-benzyl-ammonium chloride.
Quaternary Ammonium Compounds are the nitrogenous organic compounds which are basically used as disinfectants in restaurants, hospitals, and homes. The basic chemical structure of ammonium includes a nitrogen atom consisting of four hydrogen atoms connected around it. Quaternary ammonium is created when each of those four hydrogen atoms is replaced with some combination of four other organic chains or rings. Due to the limitless count of possible combinations can take place, there are so many different modes of quaternary ammonium are in the markets already and new types of those are still constantly in development.
O-Alkyl N-anthracenylmethyl derivative compound of Cinchona alkaloids can work in the role of enantioselective phase-transfer catalysts. By employing these catalysts in the asymmetric alkylation of glycine imines, one can generate a range of α-amino acid derivatives with high levels of enantiomeric excess. It is also possible to generate the catalysts in situ from commercially available chiral amines, which offers the opportunity to evaluate libraries of related structures. This specific approach has been applied to many series of biphenyl quaternary salts deriving out in the development growth of a new highly selective catalyst and boosting up the potential for further expansion in the range of α-amino acid derivatives which can be prepared.
So by the above explanation, we came to the conclusion that Quaternary Ammonium Salts are one of the most important and common Phase-Transfer Catalysts. These chemicals and compounds are easily available at the “Tatva Chintan Pharma” which are one of the biggest chemical suppliers in India and are known for their high quality products.
#Phase Transfer Catalysis#Quaternary Ammonium Salts#Quaternary Ammonium Compounds#chemical suppliers in India
0 notes
Link
Introducing bloomChemAG's Ferric Chloride 42% Solution - the perfect solution for your industrial needs! Our high-quality solution guarantees consistent and reliable results, making it an essential component in various industries such as water treatment, metal processing, and more.
Visit here:
https://www.bloomchemag.com/
#Triethyl Amine (TEA)#buy propylene glycol mpg#buy methylene chloride#buy benzaldehyde online#buy propylene carbonate#Buy Methyl Ethyl Ketone (MEK)#Methoxy Propyl Acetate (PMA)#Buy Acetone#Ethyl acetate (ETAC)#Buy Benzyl alcohol#buy sorbitol 70% online#Butyl acrylate#WHITE SPIRIT (LAWS)#HYDROCHLORIC ACID (HCL)#ISOPROPYL ALCOHOL (IPA)#BUTYL ACETATE (NBAC)#Methyl Methacrylate (MMA)#Acetic Anhydride (AAH)#Ferric Chloride 42% Solution#2- Hydroxyethyl Methacrylate (HEMA)#Triacetin
0 notes
Text
L’oreal Infallible Makeup Extender Setting Spray

Price: $16.99
Claims: Avoid makeup meltdown! L'Oréal Makeup Designer Paris presents, Infallible Makeup Extender Setting Spray. The 1st mist and fix makeup spray that extends your just applied makeup look all day. Infallible Pro-Spray & Set Makeup Extender Setting Spray provides the ultimate fixing power to extend the vibrancy and freshness of your look from morning to night. No cracking, fading or running.
Ingredients:
Water: Primarily used as a solvent in cosmetics and personal care products in which it dissolves many of the ingredients that impart skin benefits, such as conditioning agents and cleansing agents
Alcohol Denat: Helps visibly tighten pores and control excess oil.
PEG-7 Phosphate: A silicone.
Phenoxyethanol: A preservative.
Polyhydroxystearic Acid: It is a suspending agent and emulsifier that is used to stabilize products.
Isononyl Isononanoate: A skin conditioning emollient that leaves a silky feeling on the skin.
PPG 3 Benzyl Ether Myristate: A shine-enhancing emollient and plasticizer.
Ethylhexyl Isononanoate: An emollient ester, or fatty alcohol base. Used to thicken and stabilize liquid products.
Sodium Benzoate: A fragrance ingredient, masking ingredient, anti-corrosive agent, and most frequently, as a preservative. As a preservative, it prevents bacteria and fungi from developing in products and formulas and changing their compositions.
Sodium Cocamidopropyl PG-Dimonium Chloride Phosphate: A surfactant.
Potassium Sorbate: A mild preservative.
Aloe Barbadensis Leaf Extract (Aloe Vera): Extracted from the leaves of the aloe plant; used as a skin softener, anti-irritant, and moisture replenisher.
Limonene: Colorless liquid with a light, fresh, and sweet citrus odor used to make fragrances and flavors. It is naturally occurring substance found in many citrus fruits.
Linalool: Colorless liquid with a soft, sweet odor.
Caprylyl Glycol: A humectant and skin conditioning agent that lends moisturization, emollience and wetting properties.
Methyl Methacrylate Crosspolymer: A film former.
Sodium Hydroxide: Used to control the pH levels or serve as a buffering agent.
Methyl Diisopropyl Propionamide: Used as a fragrance ingredient and in masking.
My Thoughts: I am always looking to test out products that claim they make my makeup last longer. Especially during the summer when my makeup tends to melt off my face. With this magical bottle my makeup last all night long. Up to the moment I want to take it off. L’oreal Infallible Makeup Extender Setting Spray comes in a black rubberized plastic bottle with a cap. The rubberized texture was great for gripping the bottle while using it. However it does attract all sorts of dirt and dust. Basically anything that came in contact with the bottle left its mark. The spray nozzle is a pump rather than an aerosol where I could just hold it down. But the pump nozzle worked perfectly well and produce a fine mist. With the fine mist, it spreads out evenly. This setting spray doesn’t take long to evaporate even when I would go overboard with it (which I do a lot).
L’oreal Infallible Makeup Extender Setting Spray is used post makeup, meaning after I put on all my makeup. I have to give it a good shake before using to properly mix the ingredients. This setting spray gets rid of that ugly powdery look. Blends my contour, bronzer and blush seamlessly together. It doesn’t even make my skin feel sticky or tacky at all. The formula doesn’t even have a scent either. L’oreal Infallible Makeup Extender Setting Spray is expensive even for a drugstore product. My suggest is only buy it when it is on sale or have coupons, which ulta usually has the whole buy one get one 50% off. I purchase this in October 2016 and I still am currently using this every time I wear makeup. So buying 2 at a time will last me a long time and will be the best bang for my buck. I do want to mention that based on the back of the bottle it seems that L’oreal will be coming out different types of setting sprays.
Would I purchase L’oreal Infallible Makeup Extender Setting Spray again? Of course! Its the best drugstore setting spray I have come across.

Without L’oreal Infallible Makeup Extender Setting Spray

With L’oreal Infallible Makeup Extender Setting Spray
Pros:
Makes makeup last all night
Black rubberized plastic bottle with a cap
Rubberized texture gives grip
Pump spray nozzle
Fine mist
Spreads evenly
Evaporates quickly
Rids of powdery finish
Seamlessly blends makeup
No sticky or tacky residue
No scent
Always on sale or find coupons
Bottles last long
Cons:
Rubberized texture attracts dirt
Expensive
If you found this review helpful please click on the heart or reblog. Feel free to reply with your thoughts on the product.
#L’oreal Infallible Makeup Extender Setting Spray#L’oreal#beauty#beauty blog#beauty blogs#beauty review#beauty reviews#beauty product#beauty products#follow#follows#reblog#reblogs#heart#hearts#like#likes#love#loves#makeup#makeup blog#makeup blogs#makeup review#makeup reviews#makeup product#makeup products#make up#make up blog#make up blogs#make up review
2 notes
·
View notes
Text
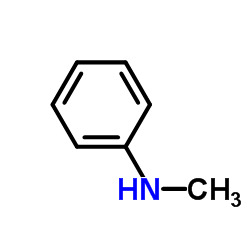
NMA CAS:100-61-8 N-MethylAniline petroluem factory Supply Best price/sample is free
QUICK DETAILS Product name:N-Methylaniline CAS:100-61-8 Molecular formula:C7H9N Molecular weight:107.15 EINECS No.:202-870-9 Purity:≥99% Brand:MIT -IVY INDUSTRY CO.,LTD Other names:N-methyl-aniline;N-Methylaniline;N-methylphenylamine;N-methyl-N-phenylamine;N-Phenylmethylamine;Methylaniline;N-methyl-aminobenzene;Methylphenylamine;N-Monomethylaniline Appearance:light yellow liquid Port: any port in china Packing:according to the clients requirement Storage: Store in dry, dark and ventilated place. Transportation: by sea or by air payment methods: L/C, T/T, D/A, D/P, O/A, paypal, western union etc.accept all payment. Application 1. Used as a dye intermediate 2. N-Methylaniline is not only the raw material for the insecticide buprofezin, but also used for the synthesis of its intermediate N-chloromethyl N-phenylcarbamoyl chloride and the intermediate for the herbicide fenthiazide. It is widely used in the dye industry. 3. The product is used as an intermediate of organic synthesis, acid absorbent and solvent, and used in the production of cationic brilliant red FG, cationic peach red B, active yellow brown KGR, etc. in the dye industry. Superiority 1. Best quality in your requirement 2. Competitive price in china market 3. Mature technical support 4. Professional logistic support 5 . Full experience of large numberscontainers loading in chinese sea port 6 .Fast shipment by reputed shipping line 7. Packing with pallet as buyer's special request 8. Best service after shipment. 9. Full experience in export 10. Raw materials from chinese origin Company Information MIT-IVY INDUSTRY CO.,LTD is a manufacturer and exporter of fine chemical dyes & pharmaceutical intermediates in China. Mainly produce aniline series products and chlorine series products. We are a young company full of vitality and vitality. The company has a group of energetic, well-trained employees and strong technical research and development capabilities. We specialize in the production, development and sales of API intermediates, fine chemicals and plant extracts. Relying on advanced equipment and strict management, adhere to the business philosophy of "openness, tolerance, innovation, and sharing" to create a win-win cooperationplatform.Everything comes from innovation, it is our philosophy ! If you are interested in getting more quotations, please add WHATSAPP:0086-13805212761 or E-MAIL:[email protected] Main products MIT-IVYINDUSTRYCO.,LTDMit-Ivy is a well-known fine chemicals and pharmaceutical intermediates manufacturer with strong R&D support in China. Mainly involved Aniline, Chlorine products. Payment:DA 60 DAYS TEL:008619961957599 E-MAIL:[email protected] 产品 Product CAS N,N-二甲基-1,4-苯二胺 N,N-Dimethyl-1,4-phenylenediamine DMPD 99-98-9 N,N-二甲基苄胺 N,N-Dimethylbenzylamine BDMA 103-83-3 N,N-二甲基甲酰胺 N,N-Dimethylformamide DMF .68-12-2 N,N-二甲基甲酰胺二甲缩醛 DMF-DMA N,N-Dimethylformamidedimethyl acetal (DMF-DMA) 4637-24-5 N,N-二甲基乙酰胺 N,N-Dimethylacetamide DMAC 127-19-5 N,N-二乙基间甲苯甲酰胺 避蚊胺 N,N-diethyl-m-toluamide DEET 134-62-3 N,N-二乙基羟胺 N,N-Diethylhydroxylamine DEHA 3710-84-7 N-甲基-N-羟乙基苯胺 2-(N-甲基苯胺)乙醇 2-(N-methylanilino)ethanol 93-90-3 N-甲基吡咯烷酮 N-methylpyrrolidone 872-50-4 N,N-二甲基苯胺 N,N-Dimethylaniline DMA 121-69-7 N,N-二甲基对甲苯胺 N,N-Dimethyl-p-toluidine DMPT 99-97-8 N,N-二甲基邻甲苯胺 N,N-Dimethyl-o-toluidine DMOT 609-72-3 N,N-二乙基苯胺 N,N-Diethylaniline 91-66-7 N,N-二乙基间甲苯胺 N,N-Diethyl-m-toluidine 91-67-8 N,N-二羟乙基苯胺 N,N-Dihydroxyethylaniline PDEA 120-07-0 N-乙基间甲苯胺 N-乙基-3-甲基苯胺 N-Ethyl-m-toluidine/N-Ethyl-3-methylaniline 102-27-2 N-乙基-N-氰乙基苯胺 3-(N-ethylanilino)propiononitrile 148-87-8 N-乙基-N-羟乙基苯胺 N-Ethyl-N-hydroxyethylaniline 92-50-2 N-乙基-N-苄基苯胺 乙基苄基苯胺; N-苄基-N-乙基苯胺 N-ethyl-N-phenylbenzenemethanamine 92-59-1 N-乙基-N-氰乙基间甲苯胺 N-2-cyanoethyl-N-ethyl-m-toluidine 148-69-6 N-乙基-N-苄基间甲苯胺 N-Benzyl-N-ethyl-m-toluidine 119-94-8 N-乙基邻甲苯胺 N-Ethyl-o-toluidine/2-Ethylaminotoluene 94-68-8 N-乙基苯胺 N-Ethylaniline 103-69-5 N-甲基苯胺 N-Methylaniline 100-61-8 N,N-二甲基-间甲基苯胺 N,N-DIMETHYL-M-TOLUIDINE 121-72-2 N-甲基二苯胺 N-Methyldiphenylamine 552-82-9 N-甲基-邻甲基苯胺 N-METHYL-O-TOLUIDINE 611-21-2 N-甲基-对甲基苯胺 N-METHYL-P-TOLUIDINE 623-08-5 4-甲基-N-苯基苯胺 N-PHENYL-P-TOLUIDINE 620-84-8 N-异丙基苯胺 N-ISOPROPYLANILINE 768-52-5 N,N-二氰乙基苯胺 N,N-Dicyanoethylaniline 1555-66-4 N,N-二羟乙基-对甲基苯胺 N,N-DIHYDROXYETHYL-P-TOLUIDINEDHEPT .3077-12-1 N-乙基-2-硝基苯胺 N-Ethyl-2-Nitro-Benzenamine 10112-15-9 2,4-二氯苯胺 2,4Dichloroaniline 554-00-7 N-(2-羟乙基)乙二胺 AEEA 111-41-1 1,3-二甲基-2-咪唑啉酮N,N-二甲基亚乙基脲1,3-二甲基-2-咪唑啉酮(DMI) 1,3-Dimethyl-2-imidazolidinone DMI N,N'-dimethylimidazolidinone 80-73-9 N,N-二苄基羟胺 N,N-Dibenzylhydroxylamine 621-07-8 对甲苯胺 P-Toluidine PT 106-49-0 邻甲苯胺 O-Toluidine OT 95-53-4 二乙基乙醇胺 DEEA;DEAE 100-37-8 甲萘胺 AlphaNaphthylamine 134-32-7 间二氯苯 1,3-Dichlorobenzene MDCB 541-73-1 间甲苯胺 M-Toluidine MT 108-44-1 间苯二胺 M-PHENYLENEDIAMINE MPDA 108-45-2 多乙烯多胺 PEPA 68131-73-7 二乙烯三胺(DETA) Diethylenetriamine DETA 111-40-0 三乙烯二胺 Triethylenediamine 280-57-9 三乙烯四胺 TriethylenetetramineTETA 112-24-3 四乙烯五胺 TEPA 112-57-2 Read the full article
#100-61-8#Amines#chemicalrawmaterials#Chemicals#Dyeintermediates#Finechemicals#inorganicchemicals#Insecticideintermediates#Methylaniline#methylphenylamine#Monomethylaniline#N-methyl-aminobenzene#N-methyl-aniline;N-Methylaniline;N-methylphenylamine;N-methyl-N-phenylamine;N-Phenylmethylamine#N-methyl-aniline,N-Methylaniline'N-methylphenylamine,N-methyl-N-phenylamine,N-Phenylmethylamine;Methylaniline,#N-Monomethylaniline#organicchemicalrawmaterials#Organicrawmaterials#pesticideintermediates#pharmaceuticalintermediates#polyesterresin
0 notes
Text
Green Washing
Greenwashing, also called “green sheen”, is a form of spin in which green PR or green marketing is deceptively used to promote the perception that an organization’s products, aims or policies are environmentally friendly and healthy for us humans.
In 2009, over 2,219 products made green claims and 98% were guilty of greenwashing. Studies show that indoor cleaning products (surface sprays, bathroom disinfectants, dish soap, etc.) are the worst offenders when it comes to making baseless claims of health and sustainability.
One product from who call themselves ”… Organics” (l don’t want to name them as l may get sued) and they produce many products one of which are Sensitive skin wipes.
These wipes contain, among a long list (see below) METHYLISOTHIAZOLINONE:
Known human immune toxicant or allergen European SCCS Human skin toxicant or allergen – strong evidence Cosmetic Ingredient Review Assessments
METHYLCHLOROISOTHIAZOLINONE:
Banned for use in Europe and restricted for use in China and USA Restricted in cosmetics (recommendations or requirements) – use, concentration, or manufacturing restrictions – Japan – concentration limit in some types of cosmetics when combined with certain other ingredient(s) Japan’s Standards for Cosmetics
Other HIGH concerns: Irritation (skin, eyes, or lungs); Other LOW concerns: Ecotoxicology
Ingredient list of sensitive wipes: Water Sodium Laureth Sulfate Cocamidopropyl Betaine Disodium Laureth Sulfosuccinate Sodium Chloride Parfum (Fragrance) Glycerin Citric Acid Tetrasodium EDTA Tocopheryl Acetate (Vitamin E) Hydrolysed Wheat Protein
Extracts (apple, orange, grapefruit) Methylchloroisothiazolinone Methylisothiazolinone Dyes (CI 19140, CI 42090)
Another company is well known for their room deodorizers/fragrances, that often dispense automatically into closed quarters, and are used in aged care facilities. They advertise that their room fragrances are infused with essential oils… and look what else
Popular Room fragrance ingredients: (z)-3-hexenyl salicylate; 1,2,3,4,4.alpha.,7,8,8.alpha.-octahydro-2,4.alpha.,5,8.alpha.-tetramethyl-1-naphthyl formate; 2,6-dimethyl-7-octen-2-ol; 3-(o-ethylphenyl)-2,2-dimethylpropionaldehyde; 3,7-dimethyloct-6-enenitrile; 3.alpha.,4,5,6,7,7.alpha.-hexahydro-4,7-methano-1h-indenyl propionate; 3a,4,5,6,7,7a-hexahydro-1h-4,7-methanoinden-1-yl acetate; 4-(4-hydroxy-4-methylpentyl)cyclohex-3-ene-1-carbaldehyde; 4-tert-butylcyclohexyl acetate; acetyl hexamethyl tetralin; amyl cinnamal; benzyl acetate; butylphenyl methylpropional; citral; dipropylene glycol; ethyl trimethylcyclopentene butenol; geraniol; hexamethylindanopyran; hexyl cinnamal; hexyl salicylate; ionone; linalool; linalyl acetate; methyl anthranilate; methyl ionones; methylbenzyl acetate; methyldihydrojasmonate; pentadecalactone; tetramethyl acetyloctahydronaphthalenes*; tricyclo(5.2.1.02,6)dec-4-en-8-yl acetate
Please read the ingredients list before purchasing your products. A lot of these products are designed to look “Earthy”, with brown or green graphic elements on their packaging and use the word “organic” or “contains essential oils”. But it’s important to see what else it contains. A fabulous website to research ingredients is… https://www.ewg.org/skindeep/
source1: Wikipedia source2: https://www.cogencyteam.com/news/2017/11/greenwashing-consumer-beware/



Green Washing was originally published on Kis
0 notes
Text
Review 3 loại kem lót Innisfree được chị em ưa chuộng nhất
Kem lót là thứ vũ khí hàng đầu giúp cho chị em có một lớp trang điểm mịn, đẹp, tự nhiên. Tuy nhiên, không phải ai cũng biết lựa chọn một kem lót phù hợp với làn da và túi tiền của mình. Hôm nay em sẽ vận dụng kinh nghiệm dùng mỹ phẩm nhiều năm qua để review 3 loại kem lót Innisfree được chị em ưa chuộng nhất cho chị em nha.
Review 3 loại kem lót innisfree được chị em ưa chuộng nhất
Trên thị trường hiện nay có rất nhiều sản phẩm kem lót có giá thành từ thấp đến cao. Nếu không test thử thì cũng rất khó để mà lựa chọn được sản phẩm nào ưng ý thật sự.
Bản thân em cũng đã có trải nghiệm với rất nhiều sản phẩm từ phân khúc thấp đến High-end mới lựa chọn được 3 loại kem lót Innisfree được chị em ưa chuộng nhất. Sau đây em xin review để chị em tham khảo.
Kem lót kiềm dầu innisfree pore blur primer
Kem lót Innisfree pore blur primer là đề cử đầu tiên của em cho các chị da dầu. Giá thành khoảng 250k-350k cho 25ml ở phân khúc trung bình, hơi đắt 1 chút với học sinh, sinh viên.
Kem lót kiềm dầu Innisfree Pore Blur Primer
Review về thành phần của em này
Thành phần của em này khá lành tính thiên dưỡng ẩm, kiềm dầu, tuy nhiên có hương liệu ở vị trí gần cuối trong bảng thành phần nên làn da đặc biệt nhạy cảm nên lưu ý khi sử dụng.
Một số thành phần khác chị em có thể tham khảo sau đây:
Methyl methacrylate crosspolymer: thành phần tạo kết cấu sản phẩm
Butylene Glycol: dưỡng ẩm và tạo dung môi cho sản phẩm
Polymethylsilsesquioxane: tạo kết cấu sản phẩm và dưỡng ẩm
Sodium Acrylates Crosspolymer-2
1,2-Hexanediol: chất bảo quản
Ammonium Acryloyldimethyltaurate
Phenoxyethanol: chất bảo quản
Hydroxyethyl Acrylate
Fragrance: hương liệu
Squalane: thành phần tương tự lớp dầu tự nhiên trên da, các tác dụng dưỡng ẩm.
Polysorbate 60: chất nhũ hóa, làm dày kết cấu và làm sạch nhẹ.
Sorbitan isostearate: chất nhũ hóa, làm dày kết cấu và làm sạch nhẹ.
Houttuynia Cordata Powder
Mentha Arvensis Powder
Camellia Sinensis Leaf Extract: chiết xuất trà xanh có tác dụng chống oxy hóa
Review về công dụng của em Innisfree pore blur primer này
Về cơ bản thì em này có khả năng kiềm dầu, giữ ẩm nhẹ, che được 1 số khuyết điểm và làm đầu màu da.
Da của em là hỗn hợp thiên dầu, có 1 số thâm mụn nhỏ thì độ che phủ cũng khá ok. Dùng xong không cần dùng thêm kem che khuyết điểm nên cũng đỡ nặng mặt. Màu kem tệp với màu da nên sau khi phủ thêm kem nền là đẹp không bị chênh lệch màu da quá nhiều ạ. Độ kiềm dầu thì khoảng tầm 3-4 giờ, giữ được lớp trang điểm không đổ dầu hay xuống tone.
Review kem lót innisfree pore blur primer khi test trên da mặt
Em này có những ưu điểm và nhược điểm gì
Em này có kết cấu gel lỏng, trong suốt, mùi trà xanh tự nhiên, dễ tán, tệp ngay vào da mà không hề bết dính.Bao bì nhỏ gọn có thể bỏ vào túi mang đi du du lịch mà không cần chiết sang chai nhỏ. Em đánh giá khoảng 7/10 điểm ạ
Lưu ý nhỏ là chị em phải tẩy trang kỹ sau khi dùng nếu không sẽ dễ bị bít tắc cơ học, gây mụn nha.
Innisfree pore blur primer hợp với loại da nào
Em kem lót này khá phù hợp cho làn da từ hỗn hợp thiên dầu đến rát dầu, da khô muốn bon chen thì phải dưỡng ẩm kỹ ạ.
Kem Lót trang điểm 4 Trong 1 Innisfree Mineral Make Up Base ( da khô dùng cũng ok nhé)
Em này là 1 em kem lót hiệu chỉnh màu da nhe, da dầu cũng dùng được mà da khô cũng ok ạ.
Review về thành phần của em này
Em này là 1 em kem lót có base silicon. Bảng thành phần của em này khá dài với nhiều thành phần nổi bật dưỡng ẩm, kiềm dầu, chống nắng như oxit kẽm, glycerin và các chiết xuất thực vật. Tuy nhiên cuối bảng thành phần có hương liệu ,chiết xuất chanh, linalool và cồn nên da nhạy cảm cần lưu ý khi sử dụng nha.
Một số thành phần khác chị em có thể tham khảo sau đây, do bảng thành phần khá dài nên em review các chất tiêu biểu ạ.
Titanium Dioxide: thành phần chống nắng, làm dày kết cấu sản phẩm.
Zinc Oxide: oxit kẽm có tác dụng kiềm dầu, chống nắng.
Glycerin: có tác dụng dưỡng ẩm và phục hồi da.
Arbutin: chiết xuất thực vật có tác dụng dưỡng trắng da.
Caprylyl Methicone: thành phần dưỡng ẩm.
Sodium chloride: có tác dụng tẩy da chết vật lý.
Hexyl Laurate: dưỡng ẩm làm mềm da.
Aluminum hydroxide: thành phần dưỡng ẩm, đồng thời tạo kết cấu sản phẩm.
Fragrance: hương liệu
Adenosine: có tác dụng làm dịu và phục hồi da.
Linalool: hương liệu chiết xuất hoa oải hương,
Camellia Sinensis Leaf Extract: chiết xuất trà xanh chống oxy hóa.
Citrus Unshiu Peel Extract: chiết xuất chanh chua, chống oxy hóa nhưng da nhạy cảm cần lưu ý.
Opuntia Coccinellifera Fruit Extract: chiết xuất thực vật, dưỡng da.
Camellia Japonica Leaf Extract: chiết xuất thực vật chống oxy hóa.
Benzyl Benzoate: thành phần tạo kết cấu sản phẩm và dưỡng ẩm
Benzyl Alcohol: cồn có tác dụng bảo quản, da nhạy cảm cần lưu ý.
Còn lại là 1 số thành phần dưỡng ẩm, làm dày, tạo kết cấu sản phẩm và bảo quản.
Kem Lót trang điểm 4 Trong 1 Innisfree Mineral Make Up Base
Review về công dụng:
Em thấy kem lót này khá là đa năng, vừa có thể dưỡng ẩm cho da khô, nhưng đồng thời cũng có tác dụng kiềm dầu, có chỉ số chống nắng nhẹ và hiệu chỉnh màu da. Nếu chị em nào mà có thâm mụn, nám, da xỉn màu thì dùng em này xong đánh nền sẽ rất mướt và đẹp ạ. Da khô dùng khi đánh nền sẽ không bị mốc, còn da dầu có thể ngăn việc xuống tone nền đến 3-4 giờ đó nha. Kem có chỉ số chống nắng nhẹ nên những ngày trời quá nắng chị em có thể không dùng kem chống nắng cũng được.
Review về ưu điểm và nhược điểm nè:
Em kem lót này có chất kem mềm mịn, dễ tán và nhẹ tênh trên mặt. Kem giúp làn da đều màu hơn nhưng không bị trắng bợt quá mức mà ửng sáng, nhẹ nhàng che đi các khuyết điểm thâm mụn, lỗ chân lông to. khả năng giữ lớp trang điểm bền màu, không vỡ nền của em này rất ấn tượng luôn ạ. Em dùng 4-5 mà da chỉ đổ dầu nhẹ ở vùng chữ T lớp nền vẫn còn khoảng 70% ạ.
Điểm trừ là em này có khá nhiều thành phần kích ứng với da nhạy cảm như cồn, hương liệu, chiết xuất chanh, linalool nên da nhạy cảm thì không nên dùng ạ.
Hình ảnh kem lót trang điểm 4 trong 1 Innisfree Mineral Make Up Base khi test lên da
Innisfree Mineral Make Up Base hợp với loại da nào
Hiện nay trên thị trường có 3 mẫu của kem lót Innisfree Mineral Makeup Base cho chị em lựa chọn ạ.
Màu tím (Purple Color): Em này che đi các khuyết điểm của làn da xanh xao, nhiều nám, tàn nhang, vết thâm do mụn để lại nè.
Màu hồng (Peach Color): Em này thì phù hợp với mọi loại da, đặc biệt là làn da có khuyết điểm không đều màu.
Màu xanh (Green Color): Em này đặc biệt che đi những khuyết điểm của làn da hơi đỏ, mụn.
Kem lót kiềm dầu làm mờ chỗ chân lông Innisfree No-Sebum Blur Primer
Em này khá đỉnh, được mệnh danh là bản dupe của Benefit Porefessional luôn đó ạ.
Review về thành phần của em này
Thành phần của em này có khả năng kiếm dầu chống nắng nhẹ. Làn da nhạy cảm nên lưu ý vì cuối bảng thành phần có hương liệu nhưng vì cuối bảng nên phần trăm sẽ không cao.
Một số thành phần tiêu biểu của em Innisfree No-Sebum Blur Primer chị em có thể tham khảo sau đây:
Cyclopentasiloxane: thành phần dưỡng ẩm
Cyclohexasiloxane: thành phần dưỡng ẩm
Silica: thành phần hấp thụ, kiềm dầu và làm dày kết cấu sản phẩm.
Polymethylsilsesquioxane: tạo kết cấu và dưỡng ẩm.
Titanium Dioxide: thành phần tạo màu, chống nắng.
Camellia Sinensis Leaf Extract: chiết xuất trà xanh chống oxy hóa.
Citrus Unshiu Peel Extract: chiết xuất chanh, chống oxy hóa, có thể kích ứng 1 số làn da nhạy cảm.
Opuntia Coccinellifera Fruit Extract: chiết xuất thực vật dưỡng da
Fragrance: hương liệu
Còn lại là 1 số chiết xuất thực vật, chất tạo màu, tạo kết cấu cho sản phẩm.
Review về công dụng Innisfree No-Sebum Blur Primer
Em này có thể kiểm soát dầu nhờn ở mức khá, chống nắng nhẹ và giữ cho lớp trang điểm luôn bền màu trong khoảng 3-4 giờ. Bên cạnh đó, khả năng che các khuyết điểm về lỗ chân lông to, mụn cũng ở mức khá đó ạ.
Review về ưu điểm và nhược điểm nè
Em này có chất kem dạng sữa mỏng nhẹ, khả năng kiềm dầu khá ổn,chỉ số chống nắng nhẹ, là chân ái cho làn da hỗn hợp thiên dầu đến dầu, lỗ chân lông to, thâm mụn.
Tuy nhiên do thành phần có hương liệu và chiết xuất chanh chua nên không phù hợp với da nhạy cảm ạ.
Hình ảnh kem lót Innisfree No-Sebum Blur Primer khi test lên da mặt
Innisfree No-Sebum Blur Primer dùng cho loại da nào
Em này chính là chân ái của các chị có làn da hỗn hợp thiên dầu đến dầu, lỗ chân lông to ạ.
Có nên sử dụng kem lót Innisfree hay không
Nhìn chung em đánh giá kem lót Innisfree là sản phẩm phù hợp với túi tiền của học sinh, sinh viên nhưng chất lượng tốt. Chị em thường xuyên trang điểm thì càng nên dùng kem lót để bảo vệ da và có lớp nền mịn đẹp ạ.
Review kem lót innisfree trên webtretho
Kem lót Innisfree là sản phẩm được đánh giá khá cao và được rất nhiều chị em tin dùng, gợi ý trên Webtretho. Sản phẩm ở phân khúc trung bình nhưng chất lượng không hề thấp so với các sản phẩm ở phân khúc cao hơn, thậm chí còn được đánh giá là bản dupe của các sản phẩm High-end đó ạ.
Qua 1 thời gian dùng thì em thấy em này chủ yếu nổi bật với khả năng kiềm dầu tốt, giữ cho lớp trang điểm bền lâu và bảo vệ làn da không bị tổn thương bởi tác hại của mỹ phẩm nên được rất nhiều chị em ưa chuộng và tin dùng.
Review kem lót innisfree Webtretho
Cách sử dụng kem lót innisfree
Bước 1: Rửa sạch mặt trước khi bắt đầu thoa kem lót để đảm bảo kem lót đạt hiệu quả tối ưu nhất. Các chị có làn da không có thể dùng thêm bước kem dưỡng cho da đủ ẩm.
Bước 2: Dùng tay hoặc cọ trang điểm tán đều kem lót ra toàn bộ gương mặt.
Chị em nên tán đều từ hai má lên, đặc biệt chú ý phần cánh mũi vì dễ bị bỏ sót và bắt đầu các bước nền tiếp theo như bình thường
Mua kem lót innisfree ở đâu chính hãng uy tín, giá rẻ
Kem lót Innisfree là sản phẩm phổ biến trên thị trường nên rất dễ mua phải hàng giả, hàng kém chất lượng đó ạ. Chị em khi tìm mua phải tìm chọn những địa chỉ uy tín.
Em thì thường mua tại cửa hàng chính hãng của Innisfree tại Việt Nam, đảm bảo 100% hàng thật luôn ạ. Ngoài ra chị em cũng có thể tìm mua tại các sàn thương mại điện tử lớn nhưng Lazada, Shopee…cũng khá là uy tín, việc đổi trả, khiếu nại cũng tiện ạ.
Tóm lại, bài viết trên đây em đã review 3 loại kem lót Innisfree được chị em ưa chuộng nhất để các chị em có cái nhìn tổng quan và có thể tự mình chọn lựa sản phẩm phù hợp cho mục đích sử dụng của mình.
Chúc các chị em luôn có một làn da xinh đẹp, không tuổi nhé!
Nguồn: danhthucvedeptunhien.com
Rate this post
Bài viết Review 3 loại kem lót Innisfree được chị em ưa chuộng nhất đã xuất hiện đầu tiên vào ngày Đánh thức vẻ đẹp tự nhiên.
source https://danhthucvedeptunhien.com/kem-lot-innisfree/
0 notes
Text
Fieldwork Assignment #1
Aunt Jackie’s Curl La La Custard
1.What is in this product? Where did the ingredients come from? How is this product made?
The product contains (Aqua (Water), Propylene Glycol, Butyrospermum Parkii (Shea Butter) Fruit, PEG-150 Distearate, Cetyl Alcohol, Glycine Soja (Soybean) Oil, Glycerin, Parfum (Fragrance), Olea Europaea (Olive) Fruit Oil, PPG-1 Trideceth-6, Cyclopentasiloxane, Phenyl Trimethicone, Lanolin Oil, Polyquaternium-37, Propylene Glycol Dicaprylate/Dicaprate, Acrylamidopropyltrimonium Chloride/Acrylamide Copolymer, Glyceryl Stearate, PEG-100 Stearate, Stearalkonium Chloride, Theobroma Cacao (Cocoa) Seed Butter, Hydrogenated Vegetable Oil, d-Limonene, Hexyl cinnamic aldehyde, Linalool, Amyl cinnamic aldehyde, Lilial,Benzyl alcohol, Citronellol, Hydroxycitronellal, Cinnamic alcohol, 5-chloro-2-methyl-4-isothiazolin-3-one, 2-Methyl-4-isothiazolin-3-one, CI 17200 (Red 33), CI 19140 (Yellow 5). The remaining products without parenthesis next to them come from acids and bases created through chemicals. This product is made using a Shea Butter base for texture and the other ingredients described above are just mixed in using Shea Butter and H20 as the base.
2. What makes this product(individuals) and what is their life like?
Men and women work in the “Naturally Curly” company, which sponsors the Aunt Jackie’s hair care products. They create the creme and package them. They create labels for each specific container and seal them. The last part of the process is that they ship them off to over 4,000 beauty supply stores in the United States.
3. What is the impact of this product on the local community where it is produced and the areas where people consume it?
This product provides a huge impact on the local community of Florida for the natural and textured women/men. People consume this creme all across the United States.It is used in natural textured hair.
4. Describe the relationship between the product and the people that produce these products in that community? Do they consume it? How much money do they earn?Did this product affect the people in their community and how? Has it affected people in the community differently based on their age, gender or social class?
The relationship between the product and the people that produce it is a mutualistic relationship. Everyone in the community that purchases this product as well as produces it benefits. The product is not to be consumed; it is used in your hair. The money earned while making this product is roughly $16.04/hr.This product affects the community by enlightening the unique styles and textures that black women/men have to offer using this product. It affects all ages(whomever chooses to use it). Men and women are affected by this product when they use it in their hair. The social class, personally I believe, isn’t affected in the community.
5. How much do you pay for this product? What is the real social cost of this product including roads for transportation, pollution, garbage disposal and who pays for them?
The Aunt Jackie’s Curl La La Creme ranges from $9.99 to $13.The real social cost is undefined due to the fact that it doesn’t affect roads, pollution or garbage disposals. The people that pay for it and use the product might have a social cost due to the fact that when using the product they’re embracing black people and their natural and unique hair textures. Some may support and others might not.
6.What is the environmental impact of this product? What is required to grow or process ingredients or what is required to be done to acquire the materials for the chosen product?
The environmental is positive due to the use of natural products such as Shea and Cocoa in the hair creme. In order to grow these ingredients; there are plants around the world such as the Cocoa bean that are used for the product. In order to acquire the materials for the chosen product, one must find the plant and extract the liquid from it so that it could be used.
0 notes
Text
Recent Advances in Synthetic Applications of Polyvinylpyrrolidone Supported Reagents and Catalysts-Juniper Publishers
Abstract
This review summarized recent progresses in the application of polyvinylpyrrolidone supported reagents and catalysts in organic synthesis.
Keywords: Polyvinylpyrrolidone; Supported reagents; Supported catalysts; Multi-component reactions
Introduction
The use of solid-supported reagents and catalysts in solution-phase chemistry has emerged as a leading strategy that exploits the advantages of both solid- and solution-phase synthesis. The approach essentially combines the benefits of product isolation and purification in solid-phase synthesis with the high-speed development and flexible choice of chemistry from the vast repertoire of solution phase organic reactions. The organic molecules synthesis using polymer-supported reagents and catalysts is highly attractive because the work-up involves only simple filtration and evaporation of the solvent [1]. Polyvinylpyrrolidone (PVP) is an amorphous polymer having broad applications in biomedical field due to its special properties such as low toxicity and good solubility in water and most organic solvents, good adhesion characteristics, and great physiological compatibility [2]. Also, PVP has good biocompatibility and has been applied for many years as a biomaterial or additive to drug compositions, e.g. as a blood plasma expander [3]. Polyvinylpolypyrrolidone (PVPP, crospovidone, or crospolividone) is a highly cross-linked polyvinylpyrrolidone (PVP). The cross-linked form of polyvinylpyrrolidone is insoluble in water, though it still absorbs water and swells very rapidly generating a swelling force. This property makes PVPP useful as a disintegrant in pharmaceutical tablets [2]. Polyvinylpyrrolidone shows a strong binding affinity to small molecules. Furthermore, its iodine complex, povidon-iodine, is widely used as an anti-infective agent in clinical treatments [4].
Polyvinylpyrrolidone Supported Reagents
A range of polyvinylpyrrolidone-supported reagents has been developed for applications in organic synthesis. In general, these reagents are employed in stoichiometric excess to drive the reaction to completion. Simple filtration removes the spent resin from the reaction solution and, thus, eliminates the need for any time-consuming chromatographic work-up.
Iranpoor and coworkers [5] have prepared iodine supported on polyvinylpyrrolidone (betadine) as catalysts for ring opening of epoxides and as reagent for ring opening dimerization of thiiranes in alcohols, water and acetic acid (Scheme 1). In this report, the reaction of R-(+)-styrene oxide with I2 supported on PVP in methanol was found to be very stereospecific and the product isolated in 93% ee.
In another research, Lakouraj et al. [6] described the preparation of polyvinylpyrrolidone-bromine complex (PVP-Br2) as a mild and convenient reagent for selective bromination of alkenes (Scheme 2) [6].
Selective oxidation of benzyl alcohol in the presence of 2-phenylethanol was also achieved at room temperature in the presence of PVP-Br2 (Scheme 3).
In the next research, Lakouraj and Mokhtary have developed a convenient method for deprotection and direct oxidative deprotection of silylethers to the corresponding hydroxy and carbonyl compounds using polyvinylpolypyrrolidonebromine complex (PVPP-Br2) (Scheme 4) [7]. Selective oxidative deprotection of benzylic silyl ethers in the presence of primary aliphatic alcohols was also achieved at room temperature.
Also, PVPP-Br2 has been used for bromination of electron-rich aromatic compounds [8]. The reaction proceeded smoothly with phenols and N, N-alkylated amines to afford the corresponding mono brominated product in good yields at ambient temperature (Scheme 5).
Furthermore, Mokhtary and Lakouraj synthesized benzylic bromides in high yields by the reaction of the corresponding alcohols with cross-linked polyvinylpyrrolidone-bromine complex (PVPP-Br2) in the presence of hexamethyldisilane in chloroform at reflux condition (Scheme 6) [9]. Selective conversion of benzyl alcohol to benzyl bromide in the presence of 2-phenylethanol was also achieved.
Surya Prakash et al. have prepared solid polyvinylpyrrolidonehydrogen peroxide complex and used as solid hydroxylating reagent [10]. This solid hydrogen peroxide is found to be much safer, convenient and efficient reagent system for the ipsohydroxylation of arylboronic acids to the corresponding phenols in highyields at a faster rate (Scheme 7). The versatility of the reagent has been further expanded for the one-pot synthesis of halophenols.
Lakouraj et al., [11] have demonstrated polyvinylpolypyrrolidone- phosphorous oxychloride as a versatile polymeric Vilsmeier reagent that exhibits excellent selectivity for oxygenation of sulfides to sulfoxides, and oxidation of aldehydes to carboxylic acids in the presence of hydrogen peroxide under mild reaction conditions (Scheme 8) [11]. This polymeric Vilsmeier reagent was found to retain its activity after months and is stable in a glass bottle at room temperature.
Iranpoor et al. [12] have reported dinitrogen tetraoxide supported on polyvinylpyrrolidone (PVP-N2O4) as a nitrosating and coupling agent for thiols and selective oxidation of sulfides to sulfoxides and disulfids to thiosulfonates (Scheme 9) [12].
Tamami and Kiasat have reported [13] a polyvinylpyrrolidonethionyl chloride complex by the reaction of thionyl chloride with two equivalents of polyvinylpyrrolidone in dichloromethane at 0 ͦC. The polymer-bound complex I was used for the rapid dehydration of a variety of aldoximes to produce the corresponding nitriles in high yields (Scheme 10).
Also, Tamami et al. [14] have reported rapid ring opening of epoxides to afford β-chlorohydrins with cross-linked polyvinylpyrrolidone/thionyl chloride complex (PVP-SOCl2), under mild reaction condition in high yields (Scheme 11).
Polyvinylpyrrolidone Supported Lewis Acidic Catalysts
Replacement of conventional, toxic and unstable Lewis acidic catalysts with eco-friendly reusable solid acid catalysts is an essential requirement in the development of green chemistry. For example, boron trifluoride is widely used in organic syntheses as a Lewis acid. However, boron trifluoride is highly water sensitive, irritant, and has to be used in a carefully dried apparatus. Moreover, all work must be carried out in an efficient fume hood, and its recovery from the reaction mixture results in a main source of waste, which on an industrial scale is environmentally unacceptable.
Mokhtary et al. have reported several papers on the application of the polyvinylpolypyrrolidone-bound boron trifluoride (PVPPBF 3) as a stable polymeric Lewis acid catalyst in some organic reactions such as synthesis of amides by the reaction of nitriles with benzhydrol and tertiary alcohols or tert-butyl acetate via Ritter reaction (Scheme 12) [15, 16], the acylation of alcohols, phenols and trimethylsilyl ethers with acetic anhydride (Scheme 13) [17], the synthesis of coumarins via Pechmann condensations of phenols with ethyl acetoacetate (Scheme 14) [18], the synthesis of 14-aryl-14H-dibenzo [a,j] xanthenes and bis(naphthalen- 2-yl-sulfane) derivatives (Scheme 15) [19], the synthesis of 1,8-dioxooctahydroxanthenes and 1,8-dioxodecahydroacridines via condensation of aromatic aldehydes and dimedone in acetonitrile at room temperature, and aromatic aldehydes, dimedone, and aromatic amines in acetonitrile at 80 °C respectively (Scheme 16) [20], the oxidation of aldehydes to carboxylic acids and oxidative esterification of benzaldehydes in the presence of 35% hydrogen peroxide (Scheme 17) [21] and the oxidation of sulfides to sulfones in the presence of 35% hydrogen peroxide at room temperature (Scheme 18) [22]. Excellent yields, easy work-up and reusability and stability of the catalyst are some advantages of these methods.
Pourali and et al. prepared cross-linked polyvinylpyrrolidone supported GaCl3 for efficient and regioselective ring-opening reaction of epoxides by various alcohols under solvent-free conditions at room temperature (Scheme 19) [23]. Furthermore, regioselective conversion of epoxides to β-azidohydrines was accomplished by sodium azide in MeOH in the presence of GaCl3/ PVP at room temperature. GaCl3/PVPP is a non-hygroscopic and recoverable catalyst and is easily separated from reaction mixture by a simple filtration and reused repeatedly
In the next research, an efficient synthesis of chromenylphenylpropanone derivatives as warfarine-like analogues was developed by the Michael addition of 4-hydroxycoumarin to α, β-unsaturated compounds in the presence of polyvinylpolypyrrolidone supported antimony (III) chloride (PVPP-SbCl3) as a new polymeric Lewis acid catalyst in chloroform at reflux conditions (Scheme 20) [24].
Polyvinylpyrrolidone Supported Brönsted Acidic Catalysts
Polymer-supported Brönsted acidic catalysts have gained considerable importance due to their low cost, high efficiency, easy work-up, and reusability. Ghorbani-Choghamarani et al. reported trimethylsilylation and formylation of alcohols in the presence ofpolyvinylpolypyrrolidoniume tribromide in acetonitrile at room temperature (Scheme 21) [25].
Khaksar et al. have prepared polyvinylpolypyrrolidonesupported triflic acid (PVPP.OTf) as an environmentally friendly and efficient catalyst for the synthesis of bis-indolyl methane derivatives by the reaction of indole or N-methyl indole with aldehydes (Scheme 22) [26].
Furthermore, PVPP.OTf was found to be useful as a recyclable heterogeneous catalyst for the rapid synthesis of quinoxaline derivatives (Scheme 23) [27].
Also, polyvinylpolypyrrolidone-supported triflic acid has been used as a recyclable catalyst for synthesis of a series of 7-hydroxy- 10-aryl-10Hindeno[1,2-b]chromen-11-one derivatives, 13-phenyl indeno[1,2-b]naphtho[1,2-e]pyran-12(13H)-one and 12-phenyl- 12H-indeno[1,2-b]naphtho[3,2-e]pyran-5,11, 13-triones (Scheme 24) [28].
Nitration of the substituted phenols (Scheme 25) [29] and oxidation of urazoles and bis-urazoles to the corresponding triazolinediones (Scheme 26) [30] were reported by Nikoorazm et al. and Ghorbani-Choghamarani et al. respectively in dichloromethane at room temperature using supported nitric acid on polyvinylpyrrolidone as an efficient, environmentally friendly, mild catalyst.
Furthermore, Zolfigol et al. was described chemo selective oxidation of sulfides to sulfoxides using PVP-HNO3 in the presence of a catalytic amount of KBr or NaBr (Scheme 27) [31].
The synthesis of xanthenes derivatives including 1,8-dioxooctahydroxanthenes, 14-aryl-14H-dibenzo[a,j] xanthenes, and 12-aryl-8,9,10,12-tetrahydrobenzo[a]xanthen-11-ones reported by Shirini et al, using O-sulfonated poly(4-vinylpyrrolidonium) chloride [PVP-SO3H]Cl as a polymeric solid acid catalyst (Scheme 28) [32].
Also, polyvinylpolypyrrolidone supported chlorosulfonic acid ([PVPP-SO3H]Cl) was evaluated by Mokhtary et al, as a recoverable catalyst for the one-pot synthesis of hexahydroquinolines (Scheme 29) [33], dihydropyrimidinones and octahydroquinazolin-2,5- diones (Scheme 30) [34].
In another research, Abedini et al. have described a green approach for the promotion of the synthesis of Hantzsch products using polyvinylpyrrolidinium perchlorate ([PVPH]ClO4) as a new modified polymeric catalyst (Scheme 31) [35].
In the next research, Shirini et al., have introduced polyvinylpyrrolidonium hydrogen phosphate ([PVP-H]H2PO4) as a heterogeneous, and reusable catalyst for the synthesis of 2-amino- 3-cyano-5-oxo-5,6,7,8-tetrahydro-4H-benzopyrans (Scheme 32) [36].
In another study, sulfuric acid-modified polyvinylpyrrolidone ([PVP-SO3H]HSO4) was prepared by Safaei et al. as an efficient reusable polymeric catalyst for the one-pot multi component synthesis of acridinedione derivatives as an important class of heterocyclic compounds (Scheme 33) [37].
Polyvinylpyrrolidone Supported Pd Catalyst
A series of poly(N-vinyl-2-pyrrolidone) immobilized Pd nanoparticles (PVP-Pd) with varying particle size have prepared by Li et al., [38] using the stepwise growth reaction. The effect of Pd particle size on the Suzuki reaction between phenylboronic acid and iodobenzene was investigated by the use of four Pd catalysts (Scheme 34). The catalytic activity of the Pd nanoparticles expressed in terms of the initial turnover frequency (moles of the biphenyl product per mole of total surface Pd atoms per min) was found to be in the order of Pd (3.9nm) > Pd (3.0nm) ~ Pd (5.2nm) > Pd (6.6nm), indicating that surface Pd atoms do not all have the same reactivity in this reaction. The general trend of increased catalytic activity with the decrease in the particle size suggests that the low-coordination number vertex and edge atoms on the particle surface are active sites for the Suzuki reaction. The lower catalytic activity for the smallest Pd nanoparticles may be due to stronger adsorption of the reaction intermediates on the particle surface, in which the strongly adsorbed species act as a poison to the reaction thereby decreasing the rate of the reaction.
In another work, Tamami et al., [39] was prepared a catalytic system based on palladium nanoparticles supported on poly(Nvinylpyrrolidone) grafted silica (Scheme 35).
The complexation of PVP-grafted silica with PdCl2 was carried out to obtain the heterogeneous catalytic system. Transmission electron microscopy (TEM) image showed that palladium dispersed through the support in nanometer size (Figure 1).
This catalytic system exhibited excellent activity in crosscoupling reactions of aryl iodides, bromides and also chlorides with olefinic compounds in Heck-Mizoraki reactions in short reaction time and high yields (Scheme 36).
Conclusion
Polymer-supported reagents and catalysts have emerged as important tools for the rapid preparation of chemical compounds in solution-phase. Clean methodologies, easy preparation of the catalysts, simple work-up procedures, good to high yields, environmentally friendly and reusable catalysts are some advantages of polyvinylpyrrolidone supported catalysts and reagents in organic multi-step synthesis. Further progresses in the development of new PVP-bound reagents, and catalysts will continue to attract innovative application of this strategy in multicomponent synthesis.
Acknowledgements
Financial support by Rasht Branch, Islamic Azad University is gratefully acknowledged.
For more Open Access Journals in Juniper Publishers please click on: https://juniperpublishers.com
For more articles in Academic Journal of Polymer Science please click on: https://juniperpublishers.com/ajop/index.php
For more Open Access Journals please click on: https://juniperpublishers.com
0 notes