APICDMO is a pharmaceutical company developing the key intermediates of new drugs. It is focused on providing a complete system of synthetic and customized service for drug research and development agencies and pharmaceutical companies. Contact with us for more details: [email protected]. Tel: +8613682461609.
Don't wanna be here? Send us removal request.
Text
APICDMO is the best Methyl 3-(bromomethyl)-2-benzofurancarboxylate raw materials manufacturer in China. Our factory accept the bulk order and the custom service.
0 notes
Text
What is Pheromones?
Pheromones are substances produced by both humans and animals. These substances alter how members of the same species interact with one another. They change behavior and are secreted to elicit a variety of feelings, including sexual pleasure, panic, and intimidation. Pheromones, unlike most other hormones, act outside the body of the person who secretes them. They have an effect on the behavior of another person.
Androstenone is a pheromone that is found in both male and female sweat. Its scent performs an important biological function. This pheromone produces messages with alpha-male traits in males. Male leaders are reported to create more Androstenone than other male personality types. Some women are more sensitive to the odor of androstenone than others. Although this pheromone is prevalent in both males and females, it is commonly associated with males due to the aggressive, scary atmosphere it provides.
Although individuals produce their own pheromones, incorporating pheromone products into your daily life can be really beneficial. Based on many years of scientific research, there are items that can boost your mood and interpersonal connections by increasing your daily pheromone emission.
0 notes
Text
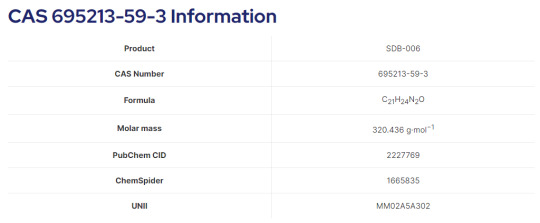
SDB-006(695213-59-3) for research, what is it?
APICDMO is the professional manufacturer of SDB-006 and accept Synthetic customization. These products are for scientific research purposes only and not for sale to individuals.
SDB-006 is a drug that acts as a potent agonist for the cannabinoid receptors, with an EC50 of 19 nM for human CB2 receptors, and 134 nM for human CB1 receptors.It was discovered during research into the related compound SDB-001 which had been sold illicitly as “2NE1”.SDB-006 metabolism has been described in literature.
0 notes
Text
SDB-005 CAS:2180934-13-6 Supplier
SDB-005 is an indazole-based synthetic cannabinoid that has been sold online as a designer drug.It is presumed to be an agonist of the CB1 and CB2 cannabinoid receptors. SDB-005 is the indazole core analog of PB-22 where the 8-hydroxyquinoline has also been replaced with a naphthalene group.
The code number SDB-005 was originally used for a different compound, the N-phenyl instead of N-benzyl analogue of SDB-006. This compound is a potent agonist of the CB1 receptor (Ki = 21 nM) and CB2 receptor (Ki = 140 nM).
However, SDB-005 was subsequently used as the name for the indazole-3-carboxylate compound mentioned above when it was sold in Europe as a designer drug, and was entered into the EMCDDA synthetic drug database under this name.Consequently, there are now two distinct, yet fairly closely related cannabinoid compounds, which may both be referred to under the code SDB-005.
APICDMO is the professional manufacturer of SDB-005 and accept Synthetic customization. These products are for scientific research purposes only and not for sale to individuals.
0 notes
Link
QMPSB(CAS:312606-87-4) Professional Manufacturer
QMPSB is an arylsulfonamide-based synthetic cannabinoid that has been sold as a designer drug.
QMPSB was first discovered by Nathalie Lambeng and colleagues in 2007. It acts as a full agonist of the CB1 receptor and CB2 receptor with Ki values of 3 nM and 4 nM, respectively.Many related derivatives were subsequently produced, with the main focus of this work being to increase selectivity for the non-psychoactive CB2 receptor.This work led on from an earlier series of sulfamoyl benzamide derivatives for which a patent was filed in 2004.
The quinolin-8-yl ester motif of QMPSB led to the discovery of other designer cannabinoids such as PB-22 and BB-22.
0 notes
Link
QUCHIC CAS:1400742-42-8
Other name: BB-2, SGT-32
APICDMO is the professional manufacturer of QUCHIC and accept Synthetic customization. These products are for scientific research purposes only and not for sale to individuals.
QUCHIC (BB-22, SGT-32 or 1-(cyclohexylmethyl)-1H-indole-3-carboxylic acid 8-quinolinyl ester) is a designer drug offered by online vendors as a cannabimimetic agent, and was first detected being sold in synthetic cannabis products in Japan in early 2013,and subsequently also in New Zealand.The structure of QUCHIC appears to use an understanding of structure-activity relationships within the indole class of cannabimimetics, although its design origins are unclear. QUCHIC, along with QUPIC, represents a structurally unique synthetic cannabinoid chemotype since it contains an ester linker at the indole 3-position rather than the precedented ketone of JWH-018 and its analogues, or the amide of SDB-001 and its analogues.
0 notes
Link
N-Methoxy-N-methyl-2-phenylacetamide CAS: 95092-10-7 was used for Laboratory chemical , Compound is subject for research & development use only as organic experimental building block. STRICTLY not for human or animal use. APICDMO is professional supplier of N-Methoxy-N-methyl-2-phenylacetamide CAS: 95092-10-7 and we can accept customized service for some products, provide technical support. Welome to buy N-Methoxy-N-methyl-2-phenylacetamide from us at any time!
APICDMO can provide N-Methoxy-N-methyl-2-phenylacetamide(CAS: 95092-10-7) in bulk.
0 notes
Link
3-(1,3-benzodioxol-5-yl)-N-methoxy-N-methyl acetamide CAS: 153277-41-9 was used for building block , Laboratory chemical , Compound is subject for research & development use only as organic experimental building block. STRICTLY not for human or animal use. APICDMO is professional supplier of 2-(1,3-benzodioxol-5-yl)-N-methoxy-N-methyl CAS: 153277-41-9 acetamide and we can accept customized service for some products, provide technical support. Welome to buy 2-(1,3-benzodioxol-5-yl)-N-methoxy-N-methyl acetamide from us at any time!
APICDMO can provide 2-(1,3-benzodioxol-5-yl)-N-methoxy-N-methyl acetamide CAS: 153277-41-9 in bulk.
0 notes
Link
Bis(4-tert-butylphenyl)iodonium hexafluorophosphate
CAS: 61358-25-6
Bis(4-tert-butylphenyl)iodonium hexafluorophosphate is a diaryliodonium salt used for chemical synthesis in materials science and micro/nano electronics.It belongs to the following product categories, such as Diphenyliodonium Compounds, Functional Materials, Iodonium Sulfonium & Oxonium Compounds, Photopolymerization Initiators and so on.
#Bis(4-tert-butylphenyl)iodonium hexafluorophosphate#CAS: 61358-25-6#apicdmo#Photopolymerization Initiators#Polyimide raw material
0 notes
Link
Where To Buy Spermidine Powder And Other Spermidine Products? Many foods contain spermidine. These include grapefruit, mature cheese, mushrooms, soy products, legumes, corn, and whole grain. Chickpeas, peas, green peppers, broccoli, oranges, green tea, and rice bran contain it too. Note that much of the Mediterranean diet contains spermidine rich foods. But it is struggle to get enough spermidine in our diet. So, taking spermidine powder or supplements will be good choice to get enough spermidine. APICDMO is a professional manufacturer and supplier of Spermidine in China, offering raw ingredients fur bulk sale: ① Spermidine powder CAS: 124-20-9 ② Spermidine trihydrochloride powder CAS: 334-50-9 ③ Wheat Germ Extrac powder(1%) CAS: 124-20-9 We also accept the custimized service for pharmaceutical intermediates in our lab.If you want to buy spermidine powder in large quantities, please send an inquiry to us directly. Documents like COA or other related documents will be sent upon your inquiry email.
#Spermidine powder#Spermidine powder benefits#Spermidine trihydrochloride powder#Wheat Germ Extrac powder#buy spermidine powder#apicdmo#anti-aging supplements#spermidine products
0 notes
Link
PF07321332(Paxlovid) is a potent and orally active SARS-CoV 3C-like protease (3CLPRO) inhibitor . PF-07321332 targets to the SARS-CoV-2 virus and can be used for COVID-19 reseacrch. Welcome to contact with us for more details about PF-07321332 and its intermediate materials. APICDMO is the best PF07321332(Paxlovid) ram materials manufacturer in China. We have made out the full synthetic routes of PF-07321332, PF-07321332 intermediate materials also can be provided, even bulk order! Welcome to contact with us for more details!
#PF07321332#Paxlovid#buy Paxlovid#Paxlovid supplier#SARS-CoV 3C#apicdmo#PF07321332 covid#PF07321332 price
0 notes
Link
Naphthalene-2,3-dicarbonitrile is a precursor to a molecular semiconductor, it was mainly used as photoelectric material. APICDMO can supply Naphthalene-2,3-dicarbonitrile in bulk and welcome to talk more details with us!
#Naphthalene-23-dicarbonitrile#22856-30-0#apicdmo#photoelectric material#Polyimide raw material#buy Naphthalene-23-dicarbonitrile#Naphthalene-23-dicarbonitrile bulk purchase
0 notes
Conversation
How Does Ozanimod Works? For Amyotrophic Lateral Sclerosis(ALS)?--APICDMO
Ozanimod works by entering the central nervous system (CNS) and binding to specific subtypes (S1P1R and S1P5R) of the sphingosine 1-phosphate (S1P) receptor. The S1P receptor is found on the surface of specific immune cells called T cells and B cells that play a role in causing damage to the CNS in MS. By binding to the S1P receptor, ozanimod prevents these harmful immune cells – specifically B cells and T cells – from being activated and released from the lymph nodes and thymus gland into the blood circulation and, hence, the brain and spinal cord. Ozanimod is shown to reduce inflammation in animal models of MS.
0 notes
Text
What Role Does Burgess reagent Play?--APICDMO
Burgess reagent
Burgess reagent is a term used to describe Methyl N-(triethylammoniumsulphonyl) carbamate. It is a selective agent of mild strength used for dehydration in organic reactions and synthesis. Its main use is in the synthesis of alkenes using alcohols.
It is also utilized to promote great synthetic values in medicinal chemistry. It has been used in the dehydration of primary amides, formamides, and primary nitroalkanes to generate nitriles, isocyanides, and nitrile oxides respectively. It forms urethanes when it reacts with primary alcohols.
Burgess reagent was first discovered in 1968 by E.M. Burgess but it was only widely recognized when Peter Wipf used it extensively in different reactions.
Features of Burgess reagent
♦ A particularly special characteristic of burgess reagent is how its dehydrating action is a pyrolytic reaction, that is it occurs only above 100°C.
♦ It is highly soluble in most organic solvents.
♦ Highly soluble even in nonpolar solvents formulated in the form of a salt.
♦ Dehydrating action of burgess reagent is syn-elimination.
Uses of Burgess reagent
Burgess reagent is used in many important transformations including the preparation of isocyanides, nitriles, and nitrile oxides. Of all its applications, the most important use of burgess reagent is the dehydration of thioamides and hydroxy amides to synthesize heterocycles.
As dehydration with burgess reagent demands only mild conditions, it has been widely accepted in the synthesis of organic compounds.
Mechanism of action of burgess reagent
Burgess reagent is a variant of Ei mechanism, and this is the mechanism of action by which it works. This mechanism has been theorized based on the dehydration of threo and erythro-2-deuterio-1,2-diphenylethanol, which was originally studied by Burgess himself.
The initial step, which comprises the formation of sulfamate ester, occurs in hydrocarbon solvents at or below 30° C through the reaction between the reagent and an alcohol.
After this compound is heated up with a microwave, pyrolysis occurs on the sufamate ester. The ionizing reaction of the carbon holding the main sulfamate group produces an ion pair. The collapse of this ion pair results in the transportation of hydrogen from the cation to the anion. This step is the rate-determining step.
This process has a low energy level due to the contribution of positive entropy. Thereafter, the capture of proton becomes easier reducing all ion-pairing character and consequently the carbonium ion rearrangements.
Applications of Burgess reagent
❶ Dehydration of alcohols
When alcohols need to be dehydrated, the primary factors that determine the reaction include the nature of the alcohol group, whether it is tertiary, secondary, or homoallylic, the configuration, as well as the environment. If tertiary or secondary alcohols are treated using a Burgess reagent within a solvent, the resulting olefins are approximately 90% in yields.
❷ Nitriles from primary amides
To synthesize nitriles, an easy process is via dehydration of primary amides. Commonly used reagents for this chemical process are often not suitable when other functional groups are present, and thus require further protection to prevent the synthesis of a completely different compound. However, burgess reagent works quite efficiently for this transformation.
❸ Isocyanides from formamides
Isocyanides are compounds that take part in a wide range of chemical reactions that involve synthetic transformations. Of all the methods available, the dehydration of formamides is the route that is most preferred. To this end, burgess reagent is an effective and safe agent that can be used to convert the compounds by altering their functional groups. Burgess reagent can convert formamides into isocyanides readily in large quantities. It is specially effective for all substrates that contain ether groups.
❹ Nitrile oxides from nitroalkanes
A particularly useful group of heterocylces are isoxazolines, which is formed from nitrile oxides by conversion of different alkenes. Generally, nitrile oxides are extremely reactive intermediate compounds which can itself undergo a transformation to produce isoxazoles or undergo dimerization to synthesize furoxanes. Two routes that are most commonly used for synthesizing nitrile oxides are dehydrogenation of aldoximes through hydroxamoyl halides or via dehydration of nitro compounds. The second method used to synthesize nitrile oxides using Burgess reagent is particularly useful as it is very easy to set up.
❺ Synthesis of heterocycles (Wipf cyclodehydration protocol)
Over the past few years, an increasingly common application of burgess reagent is in the cyclodehydration of hydroxy amides and thioamides. This leads to the production of corresponding heterocycles, which are essential synthetic intermediate compounds as they form an integral component of several biologically active natural products. Normally, the preparation of heterocycles is a complicated multistep process. But, with Burgess reagent, only a single step is essential for the cyclization of hydroxy amino acid in order to synthesize dihydrooxazoles using derivatives of serine and threonine derivatives.
❻ Other miscellaneous reactions
When Burgess reagent is used in different reactions, the end products are often unique and unexpected. But, such end products have often been found to be quite useful.
【Reference】
[1] Maki, T.; Tsuritani, T.; Yasukata, T. A Mild Method for the Synthesis of Carbamate-Protected Guanidines Using the Burgess Reagent. Org. Lett. 2014, 16 (7), 1868-1871.
[2] Mild, efficient dehydrating agent. Primary alcohols are converted to carbamates, which can be hydrolyzed to primary amines, thus providing a valuable alcohol to amine transformation. Secondary and tertiary alcohols afford olefins in synthetically useful yields: J. Org. Chem., 38, 26 (1973); Org. Synth. Coll., 6, 788 (1988). For further discussion, see: Encyclopedia of Reagents for Organic Synthesis, L. A. Paquette, Ed., Wiley, Chichester (1995), vol. 5, p. 3345.
[3] Sachin Khapli, Satyajit Dey & Dipakranjan Mal (2001). "Burgess reagent in organic synthesis" (PDF). J. Indian Inst. Sci. 81: 461–476. Archived from the original (PDF) on 2004-03-02.
[4] Edward M. Burgess; Harold R. Penton Jr. & E. A. Taylor (1973). "Thermal reactions of alkyl N-carbomethoxysulfamate esters". J. Org. Chem. 38 (1): 26–31. doi:10.1021/jo00941a006.
[5] Atkins, G. M.; Burgess, E. M. (1968). "The reactions of an N-sulfonylamine inner salt". J. Am. Chem. Soc. 90 (17): 4744–4745. doi:10.1021/ja01019a052.
#Burgess reagent#Burgess reagent powder#buy Burgess reagent#Burgess reagent application#Burgess reagent bulk order#Burgess reagent supplier#Burgess reagent apicdmo
0 notes
Text
Burgess reagent(CAS:29684-56-8)
Burgess reagent is a selective dehydrating reagent used in organic synthesis. It is used to convert secondary and tertiary alcohol, with an adjacent proton, into alkenes. Burgess reagent is also utilized to promote great synthetic values in medicinal chemistry. It has been used in dehydration of primary amides, formamides and primary nitroalkanes to generate nitriles, isocyanides and nitrile oxides respectively. It forms urethanes when it reacts with primary alcohols.

https://www.apicdmo.com/product/29684-56-8/
0 notes
Text
Oral Drugs Molnupiravir(EIDD-2801) For Treat COVID 2019-APICDMO

Molnupiravir( EIDD-2801 ) is an orally bioavailable prodrug of the antiviral nucleoside derivative N4- hydroxycytidine (NHC, EIDD-1931). It is a nucleotide analog inhibitor of RNA-dependent RNA polymerases (RdRps). The compound interferes with the action of viral RNA polymerase.Molnupiravir exerts its antiviral action through introduction of copying errors during viral RNA replication. The active drug incorporates into the genome of RNA viruses, leading to an accumulation of mutations known as viral error catastrophe. The broadspectrum antiviral agent EIDD-2801 inhibits viral RNA replication in various unrelated RNA viruses including influenza, Ebola, Venezuelan equine encephalitis virus (VEEV) and coronaviruses, including SARS-CoV, MERS-CoV and SARS-CoV-2. EIDD-2801 has the potential for COVID-19, seasonal and pandemic nfluenza treatment.
0 notes
Text
5 Things You Must Know Before Taking Lefamulin-APICDMO
1. What Is Lefamulin?
Lefamulin(CAS:1061337-51-6) is a pleuromutilin antibacterial used to treat community-acquired bacterial pneumonia (CABP).Lefamulin is a pleuromutilin antibiotic used for the treatment of bacterial community-acquired pneumonia. A pleuromotilin is a more recently developed type of antibiotic that is derived from the fungus, Pleurotus mutilus.
Lefamulin is available in intravenous and oral preparations and was granted FDA approval in August 2019.This drug is the first semi-synthetic pleuromutilin that has been designed for systemic administration. Lefamulin features a novel mechanism of action that shows benefit against resistant bacteria that cause pneumonia.The chemical structure of lefamulin contains a tricyclic mutilin core that is necessary for some of its antimicrobial activity.
2. How Does Lefamulin Work?
Lefamulin inhibits prokaryotic ribosomal protein synthesis via its binding to the peptidyl transferase center (PTC) of the ribosomal bacterial 50S subunit. It
inhibits protein translation through binding to both the A and P sites of the PTC via four hydrogen bonds, resulting in the interruption of peptide bond formation.Lefamulin's tricyclic mutilin core is the common moiety for binding of all members of its drug class, the pleuromutilins. Although the tricyclic motilin core doesn’t form any hydrogen bonds with the PTC nucleotides, it is stabilized or anchored by hydrophobic and Van der Waals interactions.Lefamulin exerts a selective inhibition of protein translation in eukaryotes, however, does not affect ribosomal translation of eukaryotes. Lefamulin demonstrates a unique induced-fit type of action that closes the binding pocket within a ribosome, conferring close contact of the drug to its target, therefore improving therapeutic efficacy.Because of its mechanism of action that differs from that of other antimicrobials, cross-resistance to other antibiotic classes is less likely.

3. What Are the Possible Side Effects of Lefamulin?
Get emergency medical help if you have signs of an allergic reaction: hives; difficult breathing; swelling of your face, lips, tongue, or throat.
Call your doctor at once if you have:
♦ severe stomach pain, diarrhea that is watery or bloody (even if it occurs months after your last dose);
♦ low potassium level --leg cramps, constipation, irregular heartbeats, fluttering in your chest, increased thirst or urination, numbness or tingling, muscle weakness or limp feeling.
Common side effects may include:
▪ nausea, vomiting, diarrhea;
▪ sleep problems (insomnia);
▪ low potassium;
▪ headache;
▪ abnormal liver function tests; or
▪ pain, bruising, swelling, or irritation where the medicine was injected.
This is not a complete list of side effects and others may occur. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
4. What Other Drugs will Affect Lefamulin?
Sometimes it is not safe to use certain medications at the same time. Some drugs can affect your blood levels of other drugs you take, which may increase side effects or make the medications less effective.
Lefamulin can cause a serious heart problem. Your risk may be higher if you also use certain other medicines for infections, asthma, heart problems, high blood pressure, depression, mental illness, cancer, malaria, or HIV.
Many drugs can affect lefamulin. This includes prescription and over-the-counter medicines, vitamins, and herbal products. Not all possible interactions are listed here. Tell your doctor about all your current medicines and any medicine you start or stop using.
5. How To Storage Lefamulin?
Consult the product instructions and your pharmacist for storage details. Keep all medications away from children and pets.
Do not flush medications down the toilet or pour them into a drain unless instructed to do so. Properly discard this product when it is expired or no longer needed. Consult your pharmacist or local waste disposal company.
【Reference】
[1] Sader HS, Biedenbach DJ, Paukner S, et al. Antimicrobial activity of the investigational pleuromutilin compound BC-3781 tested against Gram-positive organisms commonly associated with acute bacterial skin and skin structure infections. Antimicrob Agents Chemother 2012;56:1619-1623.
[2] File TM, Goldberg L, Das A, et al. Efficacy and safety of IV-to-oral lefamulin, a pleuromutilin antibiotic, for treatment of community-acquired bacterial pneumonia: The Phase III Lefamulin Evaluation Against Pneumonia (LEAP 1) Trial. Clin Infect Dis 2019;69:1856-1867.
[3] Wicha WW, Prince WT, Lell C, Heilmayer W, Gelone SP: Pharmacokinetics and tolerability of lefamulin following intravenous and oral dosing. J Antimicrob Chemother. 2019 Apr 1;74(Supplement_3):iii19-iii26. doi: 10.1093/jac/dkz087.
[4] Eyal Z, Matzov D, Krupkin M, Paukner S, Riedl R, Rozenberg H, Zimmerman E, Bashan A, Yonath A: A novel pleuromutilin antibacterial compound, its binding mode and selectivity mechanism. Sci Rep. 2016 Dec 13;6:39004. doi: 10.1038/srep39004.
#Lefamulin#take Lefamulin#Lefamulin work#Lefamulin side effects#Lefamulin storage#Lefamulin supplier#Lefamulin manufacturer#apicdmo#CAS:1061337-51-6
0 notes