#ubiquinol
Explore tagged Tumblr posts
Link
Descubre los beneficios del ubiquinol, la forma antioxidante activa de la CoQ10, para aumentar la energía y proteger el corazón y el cuerpo del estrés oxidativo. Aprende más aquí.
#ubiquinol#q10#complementosalimenticios#enfermedadescardiovasculares#suplementos#coenzymeq10in#radicaleslibres#coenzimaq10#ácidograso
0 notes
Text
Ubiquinol (CoQ10) Supplement
Ubiquinol, a vital compound present in the body, plays a crucial role in various physiological functions. From energy production to antioxidant defence, its significance cannot be overstated. In this comprehensive guide, we'll explore the benefits, sources, and considerations for incorporating Ubiquinol supplements into your daily routine.
Introduction to Ubiquinol (CoQ10)
Definition and Importance
Ubiquinol, also known as Coenzyme Q10 (CoQ10), is a naturally occurring compound found in the cells of the human body. It acts as a coenzyme, facilitating energy production in the mitochondria and serving as a potent antioxidant.
Natural Occurrence in the Body
Our bodies produce Ubiquinol naturally, but various factors, including age and certain health conditions, can affect its levels. As we delve into the world of Ubiquinol supplementation, understanding its fundamental role is crucial.
Health Benefits of Ubiquinol
Energy Production in Cells
One of the primary functions of Ubiquinol is its involvement in the electron transport chain, where it aids in the production of adenosine triphosphate (ATP), the energy currency of cells.
Antioxidant Properties
Ubiquinol acts as a powerful antioxidant, neutralising free radicals and protecting cells from oxidative stress. This property contributes to its role in maintaining overall health and well-being.
Heart Health Support
Numerous studies suggest that Ubiquinol may have a positive impact on heart health. From improving blood vessel function to reducing oxidative damage, its cardiovascular benefits are worth exploring.
Sources of Ubiquinol
Foods Rich in CoQ10
While Ubiquinol is naturally present in certain foods, understanding dietary sources is essential for those looking to boost their levels through nutrition.
Supplements and Their Forms
Ubiquinol supplements come in various forms, each with its unique characteristics. We'll explore these forms and their bioavailability to help you make informed choices.
Ubiquinol vs. CoQ10: Understanding the Difference
Bioavailability and Absorption
Distinguishing between Ubiquinol and CoQ10 is crucial for effective supplementation. We'll discuss bioavailability and absorption rates, helping you choose the right form for your needs.
Choosing the Right Form for Supplementation
Not all supplements are created equal. We'll guide you through the selection process, considering factors such as formulation, dosage, and intended benefits.
Recommended Daily Dosage
General Guidelines
Understanding the recommended daily dosage of Ubiquinol is vital for achieving optimal benefits. We'll provide general guidelines to help you establish a suitable routine.
Tailoring Dosage to Individual Needs
Individual factors, including age, health status, and lifestyle, can influence the ideal dosage. Discover how to tailor Ubiquinol supplementation to your specific requirements.
0 notes
Link
The Benefits of CoQ10 Ubiquinol CoQ10 Ubiquinol is a powerful antioxidant that plays a crucial role in energy production within our cells. It is a naturally occurring compound that is found in every cell of our body. In this article, we will explore the numerous benefits of CoQ10 Ubiquinol and how it can positively impact our overall health and well-being. Improved Energy Levels One of the key benefits of CoQ10 Ubiquinol is its ability to enhance our energy levels. As we age, our natural levels of CoQ10 Ubiquinol decrease, leading to fatigue and a lack of vitality. By supplementing with CoQ10 Ubiquinol, we can replenish our energy stores and experience increased stamina and endurance. Cardiovascular Health CoQ10 Ubiquinol has been extensively studied for its positive effects on cardiovascular health. It helps support a healthy heart by promoting the production of ATP, which is essential for proper heart function. Additionally, CoQ10 Ubiquinol acts as a potent antioxidant, reducing oxidative stress and inflammation in the cardiovascular system. Antioxidant Properties As mentioned earlier, CoQ10 Ubiquinol is a powerful antioxidant. It helps neutralize harmful free radicals in our body, which can cause damage to our cells and contribute to the development of various diseases. By reducing oxidative stress, CoQ10 Ubiquinol supports overall health and protects against age-related conditions. Brain Health Research suggests that CoQ10 Ubiquinol may have neuroprotective properties, making it beneficial for brain health. It has been shown to enhance mitochondrial function and protect brain cells from oxidative damage. This, in turn, may help improve cognitive function, memory, and overall brain health. Immune System Support CoQ10 Ubiquinol plays a vital role in supporting a healthy immune system. It helps enhance the activity of immune cells, improving their ability to fight off infections and diseases. By maintaining optimal levels of CoQ10 Ubiquinol, we can strengthen our immune system and promote overall well-being. [caption id="attachment_80502" align="aligncenter" width="1500"] benefits of coq10 ubiquinol[/caption] In conclusion, CoQ10 Ubiquinol offers a wide range of benefits for our overall health and well-being. From improving energy levels to supporting cardiovascular health, brain function, and immune system support, CoQ10 Ubiquinol is a valuable nutrient that can positively impact our lives. Consider incorporating CoQ10 Ubiquinol into your daily routine to experience its numerous benefits and optimize your health. Frequently Asked Questions about the Benefits of CoQ10 Ubiquinol 1. What is CoQ10 Ubiquinol? CoQ10 Ubiquinol is the active form of Coenzyme Q10, a naturally occurring antioxidant in the body. 2. What are the benefits of CoQ10 Ubiquinol? CoQ10 Ubiquinol provides numerous health benefits, including improved energy production, support for heart health, antioxidant protection, and enhanced cellular function. 3. How does CoQ10 Ubiquinol improve energy production? CoQ10 Ubiquinol plays a crucial role in the production of adenosine triphosphate (ATP), which is the primary source of energy for cellular functions. 4. Can CoQ10 Ubiquinol support heart health? Yes, CoQ10 Ubiquinol has been shown to support heart health by promoting healthy blood circulation, maintaining optimal blood pressure, and reducing oxidative stress in the cardiovascular system. 5. What is the role of CoQ10 Ubiquinol as an antioxidant? CoQ10 Ubiquinol acts as a potent antioxidant, neutralizing harmful free radicals and protecting cells from oxidative damage caused by environmental factors and natural aging processes. 6. How does CoQ10 Ubiquinol enhance cellular function? CoQ10 Ubiquinol supports cellular function by aiding in the production of ATP, promoting efficient metabolism, and assisting in the synthesis of proteins and other essential molecules. 7. Are there any age-related benefits of CoQ10 Ubiquinol? Yes, CoQ10 Ubiquinol levels naturally decline with age, and supplementation can help replenish these levels, supporting overall vitality, cognitive function, and healthy aging. 8. Can CoQ10 Ubiquinol benefit individuals with statin medication use? Yes, statin medications may deplete CoQ10 levels in the body, and CoQ10 Ubiquinol supplementation can help restore these levels and alleviate potential side effects such as muscle weakness and fatigue. 9. Is CoQ10 Ubiquinol safe to take as a dietary supplement? CoQ10 Ubiquinol is generally considered safe for most individuals when taken as directed. However, it is always advisable to consult with a healthcare professional before starting any new dietary supplement. 10. How should I choose a high-quality CoQ10 Ubiquinol supplement? When selecting a CoQ10 Ubiquinol supplement, look for reputable brands that use a bioavailable form of Ubiquinol, have undergone third-party testing for purity and potency, and follow good manufacturing practices (GMP).
#anti_aging#antioxidant#brain_health#Cardiovascular_support#cellular_health#Coenzyme_Q10#cognitive_function#CoQ10#energy_production#enhanced_metabolism#healthy_skin#heart_health#immune_support#improved_exercise_performance#improved_fertility.#Mitochondrial_function#reduced_inflammation#ubiquinol
0 notes
Text
Everything You Need to Know About Organic CoQ10 Ubiquinol Supplements
The human body is so complex that even to carry the simplest of the simple functions it requires so many components that are majorly derived from the food consumed by the person, that is why it is essential to eat a balanced diet but sometimes the amount of food an individual eats may, unfortunately, lack the recommended nutrition that is why it becomes necessary to rely upon supplements, CoQ10 organic is one among the few important components which are important for the body as it may help in converting food into energy and protecting the cells from getting damaged.

What exactly is coq10 organic ?
Coq10 is an antioxidant that your body produces naturally, but as you age it decreases. The term “organic” typically applies to the cultivation of the raw ingredients used to produce the Organic CoQ10, ensuring that the final product is free from artificial chemicals, synthetic additives, and other non-organic substances. This can be important for individuals seeking more natural or holistic supplements, as they want to avoid exposure to potentially harmful chemicals and ensure a cleaner, purer product.
Let's dig deeper into the history of organic coq10 ubiquinol to understand its necessity
Organic coq10 ubiquinol, or Coenzyme Q10, was first discovered in 1957 by Dr. Frederick L. Crane and has since become a vital nutrient for maintaining energy production in the body. It exists in two forms: ubiquinone, the oxidized form, and ubiquinol, the reduced, active form that the body can use directly. As we age, the body’s ability to convert ubiquinone to ubiquinol diminishes, making ubiquinol supplementation particularly beneficial.
Where is organic CoQ10 derived from ?
Organic CoQ10 is derived from natural sources, like fermented yeast, ensuring a high-quality, bioavailable product. This nutrient plays a crucial role in cellular energy production (ATP), acts as a powerful antioxidant, and supports cardiovascular and brain health. Its supplementation is especially valuable for those with declining levels of CoQ10 due to ageing or the use of statin medications, which can deplete natural CoQ10 reserves.
Who should be using the organic coq10 ubiquinol?
Organic CoQ10 ubiquinol is beneficial for a variety of people, especially those seeking to improve their energy, heart health, or overall wellness. Older adults can particularly benefit, as natural CoQ10 levels decline with age, leading to decreased energy and stamina. Those taking statin medications may also find ubiquinol helpful, as statins can reduce the body’s CoQ10 levels and cause side effects like muscle pain. Athletes and active individuals can use it to enhance endurance and reduce fatigue, while people with cardiovascular concerns may benefit from its support of heart function.
Conclusion
Organic CoQ10 ubiquinol is a highly effective and bioavailable supplement that supports energy production, heart health, brain function, and overall wellness. Its antioxidant properties and ability to replenish CoQ10 levels make it especially valuable for older adults, those on statin medications, athletes, and anyone looking to improve their vitality and protect against age-related health concerns. By choosing an organic form, you ensure a cleaner, more sustainable product, providing a natural way to enhance your health and well-being.
0 notes
Text
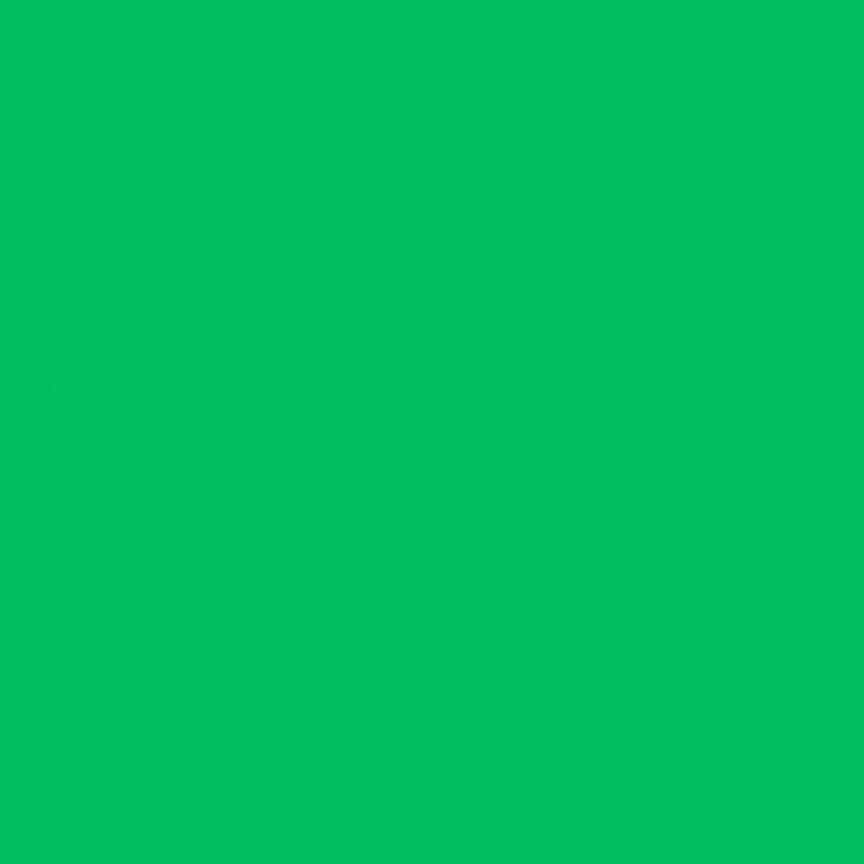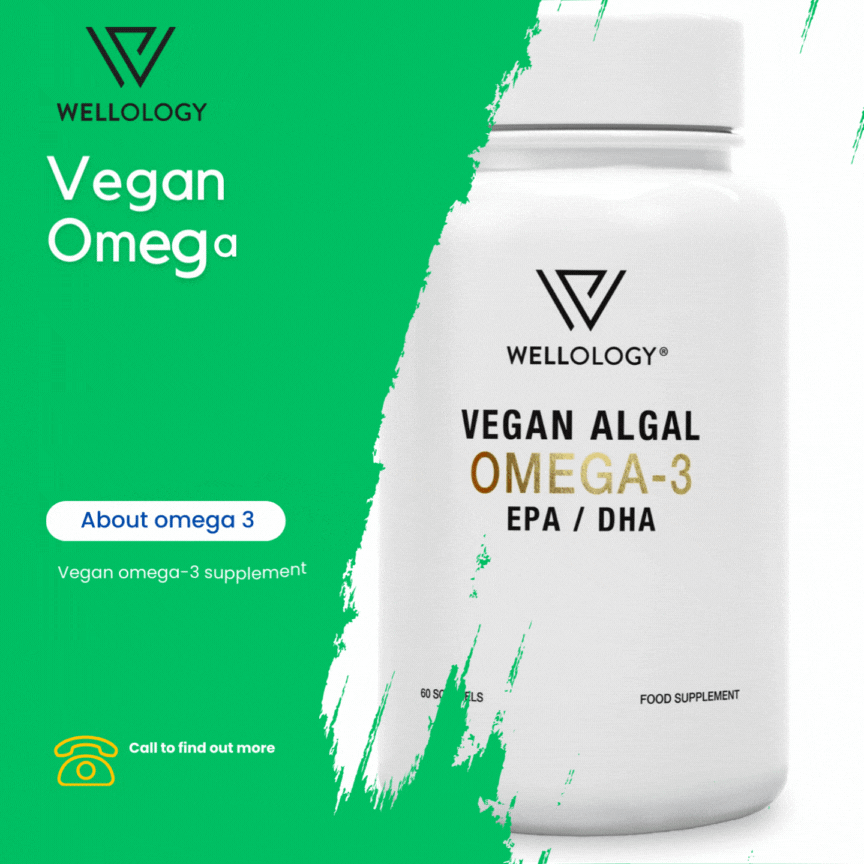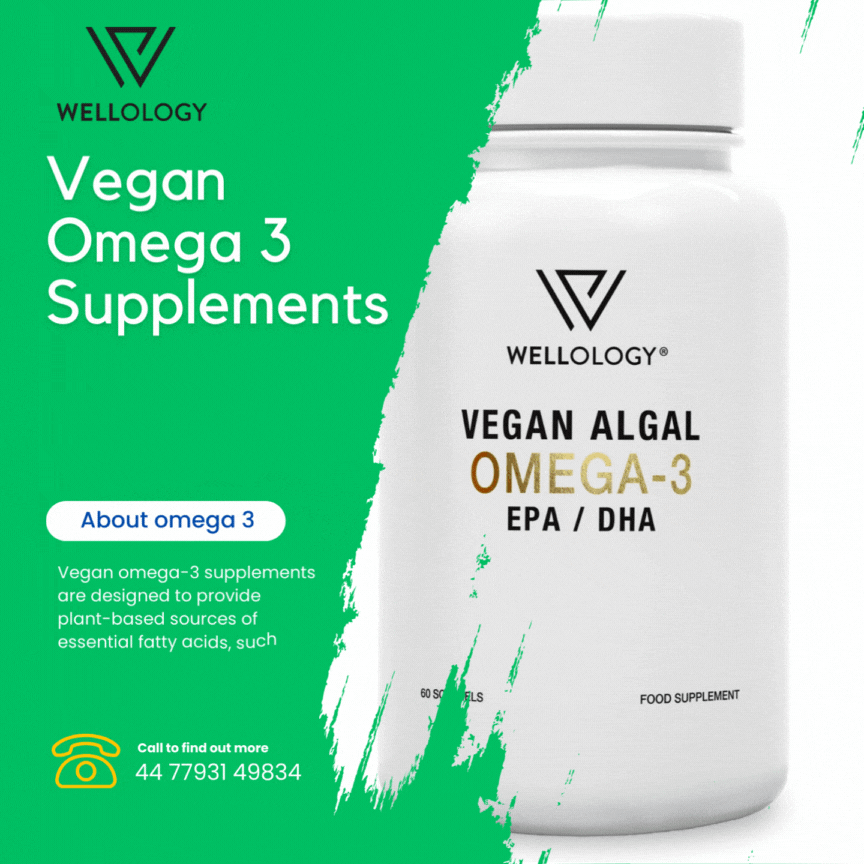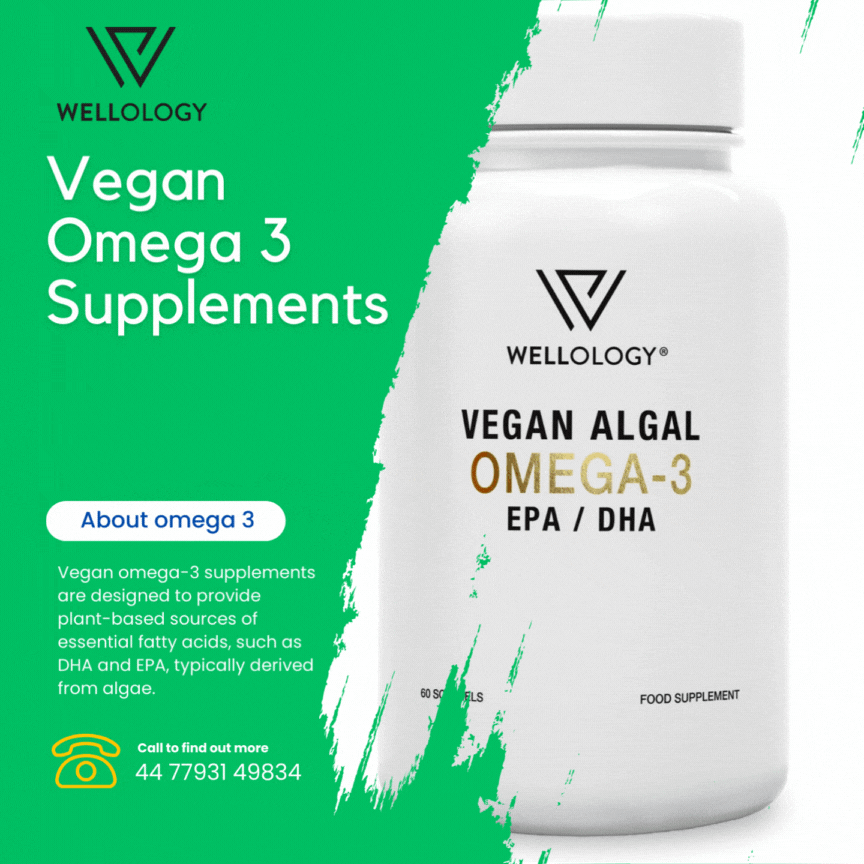
Vegan omega-3 supplements are designed to provide plant-based sources of essential fatty acids, such as DHA and EPA, typically derived from algae. These supplements cater to individuals following vegan diets and offer the benefits of omega-3s for heart, brain, and overall health without relying on fish-based products.
#best resveratrol supplement#kaneka ubiquinol#pterostilbene resveratrol#vegan omega#coffee mct oil#vegan omega 3#anti aging foods
1 note
·
View note
Text
Benefits of colon cleansing on weight loss
Colon cleansing is the process of removing toxins and waste products from the colon, which is the large intestine. The idea behind colon cleansing is that the accumulation of waste products in the colon can cause various health problems, including weight gain. Proponents of colon cleansing claim that it can help to promote weight loss by removing excess waste and toxins from the body. In this article, we will explore the benefits of colon cleanse for weight loss.
Improved digestion: One of the primary benefits of colon cleansing is improved digestion. When the colon is congested with waste products, it can cause digestive problems such as bloating, constipation, and diarrhoea. These problems can make it difficult to maintain a healthy weight. By removing the waste products from the colon, colon cleansing can help to improve digestion, which in turn can help to promote weight loss. You can also go for ganoderma lucidum mushroom softgels for improving your overall health.
Increased metabolism: Another benefit of colon cleansing is increased metabolism. The colon is responsible for absorbing nutrients from food and eliminating waste products from the body. When the colon is congested with waste products, it can slow down the metabolism, which can lead to weight gain. By removing the waste products from the colon, colon cleansing can help to increase the metabolism, which can help to promote weight loss. You can also go for Herbal smoking mix to reduce your smoking habit and improve your metabolism.

Reduced appetite: Colon cleansing can also help to reduce appetite. When the colon is congested with waste products, it can cause a feeling of fullness, which can lead to overeating. By removing the waste products from the colon, colon cleansing can help to reduce appetite, which can help to promote weight loss. Another product that can have a significant impact on our overall health is Kaneka ubiquinol uk.
Improved nutrient absorption: Another benefit of colon cleansing is improved nutrient absorption. When the colon is congested with waste products, it can prevent the body from absorbing nutrients from food. This can lead to nutritional deficiencies, which can cause weight gain. By removing the waste products from the colon, colon cleansing can help to improve nutrient absorption, which can help to promote weight loss.
So, if you are looking for some of the best herbal products that will cleanse your colon and improve your metabolism, you may give our website a visit and pick the best products for yourself.
For more info:-
ubiquinol kaneka qh
Kaneka Ubiquinol
Kaneka Ubiquinol benefits
best brands in Kaneka Ubiquinol
online Kaneka Ubiquinol
Visit our Social media sites:-
https://twitter.com/LiveNutrition17/
Source URL:- https://sites.google.com/view/ganoderma-lucidum-mushroom/home
0 notes
Text
u may be ubiquinone but girl when we’re done i’ll reduce u to ubiquinol
15 notes
·
View notes
Link
Descubre los beneficios del ubiquinol, la forma antioxidante activa de la CoQ10, para aumentar la energía y proteger el corazón y el cuerpo del estrés oxidativo. Aprende más aquí.
#coenzimaq10#ácidograso#radicaleslibres#enfermedadescardiovasculares#coenzymeq10in#q10#suplementos#ubiquinol#complementosalimenticios
0 notes
Text
Ubiquinol Geranylgeraniol: Unveiling the Secrets of a Health Superstar
In today's fast-paced world, prioritising health and wellness is more crucial than ever. Among the myriad of supplements claiming to enhance well-being, one that stands out is Ubiquinol Geranylgeraniol. This compound, with its unique name, holds immense promise for various aspects of health. Let's delve into the world of Ubiquinol Geranylgeraniol and explore its benefits, sources, mechanisms, and how to incorporate it into our daily routines.
Introduction
A. Definition of Ubiquinol Geranylgeraniol
Ubiquinol Geranylgeraniol, often abbreviated as UGG, is a compound gaining attention for its potential health benefits. It belongs to the family of coenzyme Q10, playing a crucial role in various physiological functions.
B. Importance of Ubiquinol Geranylgeraniol in Health
Research suggests that Ubiquinol Geranylgeraniol is essential for cardiovascular health, has anti-aging properties, supports the immune system, and may contribute to improved cognitive function.
Benefits of Ubiquinol Geranylgeraniol
A. Cardiovascular Health
Studies indicate that Ubiquinol Geranylgeraniol may support heart health by promoting proper blood flow and reducing oxidative stress.
B. Anti-Aging Properties
As an antioxidant, Ubiquinol Geranylgeraniol may help combat free radicals, potentially slowing down the ageing process and promoting skin health.
C. Immune System Support
The compound is believed to play a role in supporting the immune system, contributing to the body's defence against infections and diseases.
D. Cognitive Function Improvement
Some research suggests a positive impact on cognitive function, making Ubiquinol Geranylgeraniol a potential ally against age-related cognitive decline.
Food Sources
A. Natural Sources
Ubiquinol Geranylgeraniol can be found in certain foods, with spinach, broccoli, and fish being notable examples.
B. Supplements Containing Ubiquinol Geranylgeraniol
For those looking to boost their intake, supplements are available in various forms, offering a convenient and concentrated source.
How Does it Work?
A. Biochemical Mechanism
Ubiquinol Geranylgeraniol functions at the cellular level, participating in the production of energy and serving as an antioxidant.
B. Absorption in the Body
Understanding how the body absorbs and utilises this compound is crucial for maximising its benefits.
0 notes
Text
Tag 3379 / Ich hab's versucht
Ich habe es mit eingefrorenem Sperma versucht, mit frischem Sperma, mit Sperma aus dem Becher. Ich habe es mit rumänischem, mit amerikanischem, russischem und mit deutschem Sperma versucht. Ich habe es versucht mit Männern zwischen Anfang 20 und Anfang 40. Ich habe es versucht und bin lange liegengeblieben. Und ich bin gar nicht liegengeblieben oder nur kurz. Ich hab danach Yoga gemacht oder kein Yoga. Ich hab mal keinen Zucker gegessen oder sehr wenig. Ich hab mal keinen Kaffee getrunken. Ich hab mal keine Tabletten genommen. Ich hab mal zwei Tabletten genommen. Ich hab mal eine Tablette genommen. Ich hab mal sehr auf Granatapfelsaft geachtet und dann eine Zeit lang keinen Granatapfelsaft getrunken. Ich hab's mit diesem Tee versucht. Ich hab's ohne den Einnistungstee versucht. Ich hab's mit Stimulation versucht und ohne. Ich hab jedes Mal hinterher diese Dinger genommen, außer, wenn ich dachte, dass es eh zu spät war. Ich hab's vorher mit Ultraschall versucht. Ich hab's auch mal ohne Ultraschall versucht. Ich hab mal so 92, 93 gewogen. Ich hab mal 95, 96, 97 gewogen. Ich hab mal ganz doll daran geglaubt und ganz viel dafür gebetet. Und ich hab mal weniger daran geglaubt und viel gezweifelt, ob es an mir liegt.
Ich hab's jetzt einmal mit zwei Portionen versucht von derselben Person. Ich hab's auch schon mal in einem Zyklus mit zwei Portionen von unterschiedlichen Personen versucht. Ich hab's noch nie fünf Tage lang hintereinander versucht. Ich hab's mal versucht, da war der Ovulationstest noch nicht positiv. Da ist er später positiv geworden. Ich hab's versucht, wenn der Test positiv war. Ich hab's versucht und ganz viel schwarzen Sesam gegessen. Ich musste unbedingt Ananas da haben. Ich brauchte Heidelbeeren. Ich hab's versucht und bin davor und danach viel schwimmen gegangen. Ich hab's versucht und bin davor und danach wenig schwimmen gegangen. Ich habe mal Ubiquinol 200, mal 100 und eine Zeit lang gar kein Ubiquinol genommen. Ich habe Seelen genommen. Ich hab Jod genommen. Ich hab Magnesium genommen. Ich hab Omega 3 genommen. Ich hab Vitamin C genommen. Ich bin davor und danach Fahrrad gefahren. Ich bin mal Zug oder Auto hin- und zurückgefahren. Ich war manchmal danach im Meeting und manchmal nicht. Ich hab es direkt nach der Arbeit versucht. Ich habe es versucht, als ich frei hatte. Ich hab's oft nachmittags versucht, ich hab's nachts versucht. Ich hab's in Hotels versucht. Ich hab's in einer Klinik versucht. Ich hab's mit geschenktem versucht. Ich hab's mit bezahltem versucht. Und ich hab's versucht, ohne dass der andere wusste, ich versuch's. Ich hab's mit diesem Kinderwunschgleitgel versucht, ich hab's ohne das Gleitgel versucht. Ich hab diese Kappe bestellt, aber ich hatte total Angst, dass ich die nicht mehr rauskriege, also hab ich es immer ohne Kappe versucht. Ich hab's versucht, wenn ich krankgeschrieben war. Ich hab's versucht, während ich ganz normal im Arbeitsleben stand. Ich hab's mit direkt danach Selbstbefriedigung versucht. Ich hab's mit später Selbstbefriedigung versucht. Ich hab vielleicht auch mal gar keine Selbstbefriedigung gemacht. Ich hab's fast immer ohne Badewanne versucht. Ich hab's versucht, mit jemandem zu sagen, dass ich das vorhabe. Ich hab's versucht, ohne jemandem zu sagen, dass ich es vorhabe oder dass ich’s gemacht habe. Ich hab's versucht, ohne hinterher mit meiner Mutter zu streiten. Ich hab's versucht, mit hinterher mit meiner Mutter zu streiten. Ich hab's in den meisten Fällen mit Männern versucht, die schon erfolgreich waren. Ich hab's versucht und Kopfsteinpflaster gemieden. Ich hab's versucht und bin ganz normal über das Kopfsteinpflaster gefahren. Ich hab's mit giftigem Essie-Nagellack, mit gesundem Benecos-Nagellack und ich hab es ganz ohne Nagellack versucht. Ich hab das Waschmittel gewechselt. Ich hab das Waschmittel nochmal gewechselt. Ich hab's versucht und jedes Zeichen gedeutet. Ich hab's versucht und die Symptome relativiert. Ich hab's versucht und in vielen Foren gelesen. Ich hab's versucht und kaum gegoogelt. Ich hab's mit und ohne Schwangerschaftstest zu Hause habend versucht.
Ich hab's mit viel Brokkoli versucht. Ich hab's mit viel Erbsen versucht. Es gab mal sehr stressige Phasen bei der Arbeit parallel, es war aber auch mal ruhiger. Ich hab's versucht und mich täglich mit dem Spender ausgetauscht. Ich hab es meistens versucht ohne Kontakt in der Wartezeit. Ich habe es versucht ohne Bewerbungen und Vorstellungsgespräche und Aufnahmetests. Und ich hab es mit versucht. Ich hab's versucht. Ich hab's immer wieder versucht.
Ich habe es versucht und diese Wollunterwäsche täglich getragen. Ich hab es versucht ohne. Ich habe es mit der „Get pregnant“-App versucht. Ich habe es mit dem Gebet vom heiligen António und mit den Psalmen 102 und 103 im Wechsel versucht.
Ich hab's versucht und noch nicht den nächsten Zyklus geplant, während ich auf die Antwort wartete. Ich hab's versucht und schon überlegt, was ich in dem nächsten oder übernächsten Zyklus mache, was ich anders mache, wo ich hingehe, wen ich frage. Ich hab schon Leute warm gehalten, ohne die Antwort zu kennen. Ich hab's mit Hustenschleimlöser versucht. Ich hab's mit jeden Tag Basalttemperatur bestimmen versucht. Ich hab's mit Walnüssen, Sonnenblumenkernen und Kürbiskernen versucht. Ich hab's mit Gojibeeren versucht und mit Cranberries. Ich hab's mit dem Kleid versucht, mit dem ich in Israel war, mit dem ich meinen ersten Job in Abstinenz bekam, mit dem Kleid, in dem ich mich super fühlte. Ich hab's mit dem Kleid versucht, mit dem ich auf Sizilien war. Ich hab's mit Turnschuhen und mal mit Stiefeln versucht. Ich hab's mit Beine frisch rasiert und unrasiert versucht. Ich hab's mit Teststreifen von Amazon, mit Ovulationstests von Clearblue und von Rossmann versucht. Ich habe es mit Urin in Gläsern und Porzellanbechern und in Pappbechern versucht. Ich hab's versucht.
1 note
·
View note
Text
Exploring Vegetarian Algal Omega-3 Supplements: A Comprehensive Guide
Omega-3 fatty acids have garnered significant attention for their numerous benefits, ranging from heart health to brain function. Traditionally sourced from fish oil, these essential nutrients are now also available in vegetarian-friendly forms, primarily derived from algae. In this article, we delve into the world of vegetarian algal omega-3 supplements to unravel their efficacy, benefits, and considerations.
Which Algae is Best for Omega-3?
Algae, particularly microalgae like Schizochytrium and Ulkenia, are rich sources of omega-3 fatty acids, specifically EPA (eicosapentaenoic acid) and DHA (docosahexaenoic acid). These microalgae are carefully cultivated and harvested to extract the omega-3 oils, ensuring a sustainable and environmentally friendly source of this vital nutrient.
How Can I Get Omega-3 as a Vegetarian?
For vegetarians and vegans, obtaining omega-3 can be a challenge since traditional sources like fish oil are not an option. However, algal omega-3 supplements offer a convenient and effective solution, providing a bioavailable source of EPA and DHA without the need for animal products.
Can Humans Absorb Omega-3 from Algae?
Yes, humans can efficiently absorb Vegetarian Algal omega 3. The molecular structure of omega-3 in algae is similar to that found in fish, making it readily absorbable and bioavailable for the body.
Is Omega-3 from Algae as Good as Fish Oil?
Research suggests that omega-3 from algae is comparable in efficacy to that derived from fish oil. Both sources provide EPA and DHA, which are essential for maintaining overall health. Furthermore, algae-based omega-3 supplements offer a sustainable and ethical alternative to traditional fish oil.
What is the Best Vegetarian Omega-3?
The best vegetarian omega-3 supplements are those derived from high-quality, sustainably sourced algae. Look for supplements that are certified organic and undergo rigorous testing for purity and potency to ensure you're getting the most out of your supplement.
Should I Take Omega-3 Supplements as a Vegetarian?
Omega-3 supplements can be beneficial for vegetarians and vegans to ensure adequate intake of EPA and DHA, which are crucial for heart health, brain function, and overall well-being. Incorporating algae-based omega-3 supplements into your daily routine can help bridge the gap in essential nutrient requirements.
Is Omega-3 from Algae Effective?
Numerous studies have demonstrated the effectiveness of omega-3 from algae in supporting cardiovascular health, cognitive function, and inflammation management. As with any supplement, consistency and quality are key factors in achieving optimal results.
#best resveratrol supplement#kaneka ubiquinol#pterostilbene resveratrol#vegan omega#coffee mct oil#vegan omega 3#anti aging foods
0 notes
Text
Why should we take high quality supplement and vitamin for our overall well-being?
Everything we consume today is either artificial or they have chemical preservatives mixed with them. We hardly ever get 100% pure and natural products. These chemical products come with some side-effects. So, by consuming these products, you are curing your health problems and bringing some new problems with it. If you have already tried a lot of products but you did not get the exact outcome that you require, then it might be time to switch to herbal products. But before you do so, you must know why herbal nutritional products are better than any chemical artificial products.
Here we will discuss the benefit of herbal smokable products:
Herbal products help to quit addiction:
In today's stressful life, people try to reduce stress by smoking and after some time, it turns into an addiction. Some of us try hard to get out of this unhealthy habit. For those who are trying hard to quit smoking but aren't acquiring much success from the process. You might never have thought that smoking can have positive effects on your health, but this may be the case, depending on what you’re smoking. Now, don’t get me wrong, I’m not saying smoking is great for your health, but there are many herbs that have been traditionally smoked over the centuries that have health benefits. Smoking medicinal plants has also been commonly practiced helping people quit smoking Tobacco. In this post, you’ll learn the history of smoking herbs, a few of the most smoked herbs, and their potential health benefits.
The Many Uses of Herbal Smoking
There are many reasons people smoke herbs, for ceremony, ritual, spiritual practices, recreation, and healing and therapeutic purposes. Some people simply enjoy the act of smoking and want to smoke something subdued, non-addictive or habit forming. Smoking certain herbs can also help reduce the urges of Tobacco or Cannabis use by incorporating them into mixtures, and possibly slowly transitioning to only smoking non-addictive herbs.
The History of Smoking Plants
It’s believed that smoking plants has been present in every human society in history. In the Bronze Age, about 5,000 years ago, the inhalation of burned plants was used in magic, ritual and medicine in India, Mesopotamia, and Egypt.
Mugwort/Artemesia
Mugwort is most notoriously smoked for its effect on dreams. When the smoke of mugwort is inhaled, especially before bedtime, it can promote vivid and lucid dreams. It has also been said to help reveal and even alter areas of psychic unconsciousness. Mugwort’s aromatic leaves have a strong, pungent flavor when smoked.

Lobelia (Lobelia inflata)
Inhaling Lobelia smoke can be calming and relaxing. It is considered an expectorant, like Coltsfoot. Lobelia is commonly smoked as a substitute for Tobacco because it contains an alkaloid called lobeline that your body easily mistakes for nicotine. Therefore, when Lobelia smoke is inhaled, receptors in your body think that it’s nicotine and that you just smoked Tobacco. Lobeline is not addictive like nicotine when used properly and acutely. Chewing gums used to quit smoking include lobeline as one of the main ingredients.
Summary
People have been smoking herbs for thousands of years across nearly every culture for a wide variety of purposes, from ceremonial to quitting smoking. We covered five of those plants and their potential health benefits in this post, but there are many more traditionally smoked herbs out there. If you’re feeling drawn to try smoking herbs, keep your body’s unique constitution in mind. Not all herbs affect everybody in the same way.
One of the best products for a good immune system:
Because of environmental changes and eating so much junk every day, our body is extremely vulnerable to diseases. That's why we need some extra supplements that help us lead a healthy life. Reishi Mushroom (Ganoderma lucidum mushroom softgels) is a powerful immune modulator. Ganoderma lucidum has anti-oxidative effects when supplemented. It also has a therapeutic effect on insulin resistance, reduces the risk of prostate cancer, and can help treat a variety of conditions associated with metabolic syndrome. The lingzi mushroom is well known for its anti-cancer effects.
Summary
It is able to activate natural killer cells, increasing their activity and the body’s ability to fight tumours. Supplementing Ganoderma lucidum reduces the chances of metastasis, which is when cancer spreads to another part of the body. Ganoderma lucidum has a variety of mechanisms, but they are focused on moderating the immune system.
lingzi mushroom can reduce immune system activity when the system is overstimulated and bolster the immune system when it is weakened. In general, Ganoderma lucidum increases the amount of active immune system cells. Though further research is needed to confirm these effects, Ganoderma lucidum shows promise for a wide variety of cancer-related therapies.
It has been shown to be an effective adjunct therapy, which means it improves health when taken alongside other medications, for breast cancer, hepatitis, fatigue syndrome, and prostate cancer. There are not many promising supplements with anti-cancer properties available over the counter but Ganoderma lucidum appears to be one of them.
For more info:-
Organic Turmeric Curcumin with Liposmal Vitamin C
Nicosa Pilll Immune Support for Smokers
ubiquinol kaneka qh
Kaneka Ubiquinol
Kaneka Ubiquinol benefits
best brands in Kaneka Ubiquinol
Visit our Social media links:-
Source URL:- https://sites.google.com/view/livewell-nutrition-limited/home
0 notes