#treatment of coronavirus
Explore tagged Tumblr posts
Text
Also preserved in our archive (Daily updates!)
By Mary Van Beusekom, MS
New findings from two studies have tied use of the antiviral drug nirmatrelvir-ritonavir (Paxlovid) to a reduction in COVID-19 hospitalizations and death, as well as to faster resolution of symptoms and less use of healthcare resources.
Benefit seen only in older patients For the first study, published in Clinical Microbiology and Infection, a Medical University of Vienna–led research team compared the effectiveness of Paxlovid with that of the antiviral drug molnupiravir (Lagevrio)—and with that of not receiving an antiviral—against hospitalization and all-cause death from January 2022 to May 2023. Participants were adults with mild to moderate infections and one or more risk factors for severe illness caused by the SARS-CoV-2 Omicron variant.
"The oral antivirals nirmatrelvir-ritonavir and molnupiravir are the mainstay treatment for Covid-19 in non-hospitalised adults at increased risk of severe disease," the study authors wrote. "Both oral antivirals were approved at the time of the study period (2022/2023) for the treatment of non-hospitalised patients with mild-to-moderate Covid-19, but the current National Institute of Health guidelines favour nirmatrelvir-ritonavir over molnupiravir."
Of the 113,399 eligible COVID-19 patients in the retrospective cohort study, 10.7% received Paxlovid, 9.5% received molnupiravir, and 80.0% served as untreated controls. Over 96% of participants were previously infected with or vaccinated against COVID-19.
A total of 0.43% of Paxlovid recipients, 1.4% of molnupiravir users, and 1.13% of controls were hospitalized within 28 days (risk difference [RD], -0.7%; Paxlovid vs control RD, 0.26%). No Paxlovid recipients and 0.13% each of molnupiravir users and controls died.
The estimated risk of hospitalization was 0.57% in Paxlovid users and 1.09% in controls (adjusted RD [aRD], -0.53%). The estimated risk of death was 0.0% in the Paxlovid group and 0.13% in controls (aRD, -0.13%).
The number of patients needed to treat to prevent hospitalization and death was 190 in Paxlovid recipients and 792 in controls, respectively. These statistically significant aRDs were seen only among patients 60 years and older.
The estimated risk of hospitalization in the molnupiravir analysis was 1.36% in the molnupiravir group and 1.16% among controls (aRD, 0.2%). The estimated risk of death was 0.12% in molnupiravir recipients and 0.14% in controls (aRD, -0.01%).
"Among outpatients aged ≥60 years with Covid-19 in an Omicron-dominated era, treatment with nirmatrelvir-ritonavir was associated with a lower risk of hospitalisation and all-cause death within 28 days, albeit with wide confidence intervals and high numbers needed to treat," the study authors wrote.
"This finding was not observed in molnupiravir users and younger nirmatrelvir-ritonavir users. Future studies are needed to better define target populations that show greater benefit from treatment with nirmatrelvir-ritonavir," they concluded.
Proportion of patients seeking care slashed 73% The second study, a phase 2/3 randomized clinical trial published today in Clinical Infectious Diseases, also found protection against COVID-19 hospitalization and death in adults receiving Paxlovid and demonstrated a faster resolution of symptoms and lower use of healthcare resources compared with a placebo in high-risk patients.
The research was led by researchers from Pfizer, which developed Paxlovid. The drug was given to 977 symptomatic COVID-19 patients, while 989 were given a placebo, at 343 sites in 21 countries from July 2021 through December 2021, a Delta-predominant period.
Paxlovid significantly shortened the time to symptom relief (median, 13 vs 15 days; hazard ratio, 1.27) and resolution (16 vs 19 days; HR, 1.20) through 28 days and cut the number of COVID-related medical visits by 64.3% and the proportion of patients seeking care by 73.2%.
In total, 0.9% of Paxlovid recipients and 6.4% in the placebo group were hospitalized, for a relative risk reduction of 85.5%. Hospitalized Paxlovid recipients had briefer hospital stays, and none required intensive care or mechanical ventilation. Fewer patients in the Paxlovid group needed other COVID-19 treatments, and none died by 6 months, compared with 15 in the placebo group.
"The importance of having effective COVID-19 treatments such as NMV/r [Paxlovid] to reduce burden on healthcare systems, both ambulatory and hospital based, should not be underestimated," the authors wrote.
Study Links: www.clinicalmicrobiologyandinfection.com/article/S1198-743X(24)00508-1/fulltext
academic.oup.com/cid/advance-article/doi/10.1093/cid/ciae551/7889107
#mask up#covid#pandemic#public health#wear a mask#covid 19#wear a respirator#still coviding#coronavirus#sars cov 2#paxlovid#covid treatments
95 notes
·
View notes
Text
Ivermectin
Transcript:
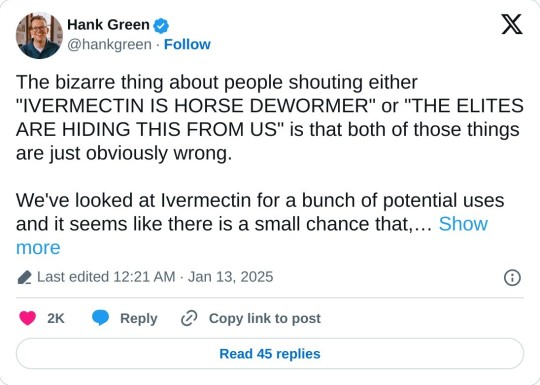
The bizarre thing about people shouting either "IVERMECTIN IS HORSE DEWORMER" or "THE ELITES ARE HIDING THIS FROM US" is that both of those things are just obviously wrong.
We've looked at Ivermectin for a bunch of potential uses and it seems like there is a small chance that, at very high doses, it might help some people with certain cancers, but if it does help (which is unlikely) it probably won't help much. There's one trial recruiting patients to test it in combination with an immunotherapy drug right now.
The thing about ivermectin is that it isn't well-absorbed by mammals. This makes it very useful as an anti-parasitic because worms absorb it readily. So it poisons parasitic worms but not people.
But it is absorbed a bit and at high enough doses, it has a bunch of other effects on the human body, many of them negative.
Early in the pandemic, there were some studies in individual cells (rather than whole bodies) that showed it might help control the virus. When the "health influencer" space glommed onto that, we actually didn't know for sure whether it would be helpful or not. But because it was cheap and available, some people (lots, actually) really did started dosing themselves with veterinary ivermectin. By the time studies on the efficacy were published (which showed it wasn't at all effective) the damage had been done.
And so we ended up with ivermectin (a drug that real people take for real diseases) becoming a culture war signifier, which is FUCKING STUPID.
Now, Mel Gibson has friends who are in remission from cancer after taking ivermectin (and probably also the treatments recommended by their oncologists, as that is almost always how these stories go). And he and Joe Rogan, during their conversation, seem ASTOUNDED that people in cancer research are ignoring it. They seem to think that every elite knows that, if they so much as GLANCE at ivermectin, they're getting fucking fired.
Except that researchers have done tons of studies on whether ivermectin could possibly be useful in cancer treatment because, if it is, that would be really great! People seem to think that pharmaceutical companies are the only ones who do cancer research but actually they mostly just bring drugs to market. Most cancer research is funded by the government or done by universities.
As much as we've looked, it doesn't seem likely that ivermectin is a good cancer drug because, at the doses where it might have an effect on a cancer, it'll have all kinds of other nasty effects on the human body, like damage to the nervous system and brain.
But, despite that, we're looking, because for some people who are dying, it's worth checking to see if it would be useful in combination with other therapies.
Cancers are very hard to treat because cancer cells are very similar to /our/ cells. Trying to kill a parasite is relatively easy because worms are very different from people. Cancer cells are descended from us, they are human cells gone rogue, so it's hard to attack them without attacking the rest of the body. That's the whole reason why it's so much easier to kill parasites than it is to kill cancer cells.
Fenbendazole is an even weirder thing to get all excited about as an "alternative treatment" because we've studied it for cancer treatment because it acts on the microtubules that control cell replication. That's how a lot of chemotherapy drugs work (including one I took), targeting cells that replicate a lot. So fenbendazole's whole thing is that it might have been a good cancer treatment because it would be another option as a toxic cell-killing chemo drug.
But, because fenbendazole is (again) not very well absorbed by mammals, it is (again) a great drug for killing parasites and not a great drug for treating cancers.
I just…I kinda can't believe we are this incapable of just leaving cancer research to cancer researchers. Ivermectin is a medicine for humans. It's not a panacea. At low doses, it basically does nothing because it isn't easily absorbed by humans and, when it is, it hangs out almost entirely with fatty tissues.
It would be amazing if a cheap, well-understood drug were broadly useful in cancer treatment. Ivermectin just /isn't/.
I have a private theory that fenbendazole and ivermectin are so present in these conversations 100% because they are known to cure real diseases (parasitic infections) and they are easy to purchase extremely cheaply because they are available for animals.
That means people can actually take them, which creates both government warnings to NOT TAKE ANIMAL MEDICINES and many stories of people taking the animal medicines and (mostly) being just fine. That's just a tremendous mix for creating discourse and turning it into a culture war thing.
And look, if people are taking ivermectin WHILE taking the treatments their doctor recommends, that's stupid but unlikely to kill anyone.
But the way it was discussed on the JRE, it makes me think some people will ONLY take these medicines, and they will not take their drugs their doctors recommend, and those people will die. And that's fucked.
#i post#twitter#hank green#ivermectin#us politics#covid 19#coronavirus#horse dewormer#conspiracy theory#medicine#healthcare#cancer#parasites#culture war#mel gibson#joe rogan#joe rogan experience#fenbendazole#alternative treatments#animal medicine
19 notes
·
View notes
Text
Vaccines currently in development
(I did not create this list and I'm sorry I don't know the original OP who compiled it.)
These are all currently in development to come to market in the next 2 years. If just one really works it will be a game changer
MT-001 a novel protein component vaccine candidate, MT-001, based on a fragment of the SARS-CoV-2 spike protein that encompasses the receptor binding domain (RBD) | A SARS-CoV-2 Vaccine Designed for Manufacturability Results in Unexpected Potency and Non-Waning Humoral Response - https://www.mdpi.com/2076-393X/11/4/832 Mice and hamsters immunized with a prime-boost regimen of MT-001 demonstrated extremely high anti-spike IgG titers, and remarkably this humoral response did not appreciably wane for up to 12 months following vaccination. Further, virus neutralization titers, including titers against variants such as Delta and Omicron BA.1, remained high without the requirement for subsequent boosting.
DCFHP a ferritin-based, protein-nanoparticle vaccine candidate that, when formulated with aluminum hydroxide as the sole adjuvant (DCFHP-alum), elicits potent and durable neutralizing antisera in non-human primates against known VOCs, including Omicron BQ.1, as well as against SARS-CoV-1. | A ferritin-based COVID-19 nanoparticle vaccine that elicits robust, durable, broad-spectrum neutralizing antisera in non-human primates https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10110616/#MOESM3
ISM3312 a COVID-19 drug entirely designed by generative AI works by inhibiting a protein called 3CL protease — a critical factor in viral replication and a popular target for anti-COVID drugs. Unlike similar therapeutics, it works on a very broad spectrum — showing efficacy not only against all current COVID variants, but also coronaviruses other than SARS-CoV-2. As such, it may possess the ability to resist future mutations, providing a solution to drug-resistant strains. | ‘It’s perfect’: World’s first generative AI-designed COVID drug to start clinical trials https://www.thestar.com/news/canada/2023/02/23/its-perfect-worlds-first-generative-ai-designed-covid-drug-to-start-clinical-trials.html
BNT162b4, composed of a T cell antigen mRNA encoding for SARS-CoV-2 non-spike proteins that are highly conserved across a broad range of SARS-CoV-2 variants and will be evaluated in combination with the Omicron BA.4/BA.5-adapted bivalent COVID-19 vaccine | Pfizer and BioNTech Advance Next-Generation COVID-19 Vaccine Strategy with Study Start of Candidate Aimed at Enhancing Breadth of T cell Responses and Duration of Protection https://investors.biontech.de/news-releases/news-release-details/pfizer-and-biontech-advance-next-generation-covid-19-vaccine
25F9 and 20A7 identified as two highly potent broadly neutralizing antibodies, making them promising prophylactic candidates against sarbecovirus infection |Broadly neutralizing antibodies against sarbecoviruses generated by immunization of macaques with an AS03-adjuvanted COVID-19 vaccine – Science Translational Medicine – https://www.science.org/doi/10.1126/scitranslmed.adg7404
ChAd-SARS-CoV-2-BA.5-S, which encodes for a pre- fusion and surface-stabilized S protein of the BA.5 strain. | A bivalent ChAd nasal vaccine protects against SARS-CoV-2 BQ.1.1 and XBB.1.5 infection and disease in mice and hamsters https://www.biorxiv.org/content/10.1101/2023.05.04.539332v1
#covid#covid vaccine#vaccines#treatment#will it be like this forever#'will you mask forever?'#research#science#neutralizing vaccine#coronavirus
4 notes
·
View notes
Text
Opinion Here’s how to get free Paxlovid as many times as you need it
When the public health emergency around covid-19 ended, vaccines and treatments became commercial products, meaning companies could charge for them as they do other pharmaceuticals. Paxlovid, the highly effective antiviral pill that can prevent covid from becoming severe, now has a list price of nearly $1,400 for a five-day treatment course.
Thanks to an innovative agreement between the Biden administration and the drug’s manufacturer, Pfizer, Americans can still access the medication free or at very low cost through a program called Paxcess. The problem is that too few people — including pharmacists — are aware of it.
I learned of Paxcess only after readers wrote that pharmacies were charging them hundreds of dollars — or even the full list price — to fill their Paxlovid prescription. This shouldn’t be happening. A representative from Pfizer, which runs the program, explained to me that patients on Medicare and Medicaid or who are uninsured should get free Paxlovid. They need to sign up by going to paxlovid.iassist.com or by calling 877-219-7225. “We wanted to make enrollment as easy and as quick as possible,” the representative said.
Indeed, the process is straightforward. I clicked through the web form myself, and there are only three sets of information required. Patients first enter their name, date of birth and address. They then input their prescriber’s name and address and select their insurance type.
All this should take less than five minutes and can be done at home or at the pharmacy. A physician or pharmacist can fill it out on behalf of the patient, too. Importantly, this form does not ask for medical history, proof of a positive coronavirus test, income verification, citizenship status or other potentially sensitive and time-consuming information.
But there is one key requirement people need to be aware of: Patients must have a prescription for Paxlovid to start the enrollment process. It is not possible to pre-enroll. (Though, in a sense, people on Medicare or Medicaid are already pre-enrolled.)
Once the questionnaire is complete, the website generates a voucher within seconds. People can print it or email it themselves, and then they can exchange it for a free course of Paxlovid at most pharmacies.
Pfizer’s representative tells me that more than 57,000 pharmacies are contracted to participate in this program, including major chain drugstores such as CVS and Walgreens and large retail chains such as Walmart, Kroger and Costco. For those unable to go in person, a mail-order option is available, too.
The program works a little differently for patients with commercial insurance. Some insurance plans already cover Paxlovid without a co-pay. Anyone who is told there will be a charge should sign up for Paxcess, which would further bring down their co-pay and might even cover the entire cost.
Several readers have attested that Paxcess’s process was fast and seamless. I was also glad to learn that there is basically no limit to the number of times someone could use it. A person who contracts the coronavirus three times in a year could access Paxlovid free or at low cost each time.
Unfortunately, readers informed me of one major glitch: Though the Paxcess voucher is honored when presented, some pharmacies are not offering the program proactively. As a result, many patients are still being charged high co-pays even if they could have gotten the medication at no cost.
This is incredibly frustrating. However, after interviewing multiple people involved in the process, including representatives of major pharmacy chains and Biden administration officials, I believe everyone is sincere in trying to make things right. As we saw in the early days of the coronavirus vaccine rollout, it’s hard to get a new program off the ground. Policies that look good on paper run into multiple barriers during implementation.
Those involved are actively identifying and addressing these problems. For instance, a Walgreens representative explained to me that in addition to educating pharmacists and pharmacy techs about the program, the company learned it also had to make system changes to account for a different workflow. Normally, when pharmacists process a prescription, they inform patients of the co-pay and dispense the medication. But with Paxlovid, the system needs to stop them if there is a co-pay, so they can prompt patients to sign up for Paxcess.
Here is where patients and consumers must take a proactive role. That might not feel fair; after all, if someone is ill, people expect that the system will work to help them. But that’s not our reality. While pharmacies work to fix their system glitches, patients need to be their own best advocates. That means signing up for Paxcess as soon as they receive a Paxlovid prescription and helping spread the word so that others can get the antiviral at little or no cost, too.
{source}
29K notes
·
View notes
Text
Every time I listen to Comalies XX I go “Maybe it’s grown on me by now” Every time I’m disappointed anew
#I love re-imaginings of old songs! I was really excited for one of all time favorite albums to get that treatment!#but it just loses so much of the magic…idk#and don’t even get me started on the news samples on Angel’s Punishment getting updated to being about Covid 😫 I can’t listen to that shit#I know the original had samples from 9/11 news reports and in 2002 that probably felt weird and Too Soon too#(I didn’t discover that song until like 2007/8 and even then it was subtle enough that I didn’t realize it was about 9/11)#AP 2022 just constantly repeating the word CORONAVIRUS from different newscasters is like. I’m sorry but why.
0 notes
Text
Harnessing Innovation: The Role of Hand Sanitizers in Combatting Corona Virus
In the fight against the Corona virus, innovation plays a pivotal role in developing effective solutions. Hand sanitizers have emerged as a crucial tool in preventing the spread of the virus, offering convenient and accessible means of disinfection. This article explores the science behind hand sanitizers, highlighting their role in breaking the chain of transmission and protecting public health. Additionally, we delve into the manufacturing process of hand sanitizers, emphasizing the importance of quality and efficacy. By harnessing the power of innovation, we can bolster our defenses against the Corona virus and pave the way for a safer future.
#Coronavirus#COVID-19#Prevention#Treatment#Immunity Boosting#Hand Hygiene#Public Health#Healthcare#Arogya Formulations#Contract Manufacturing#Health Guidelines#Respiratory Illness#Pandemic Management#Medical Advice#Hygiene Practices#Disease Prevention.
0 notes
Link
1 note
·
View note
Text


Ozempic, Mounjaro and Wegovy available in stock
Follow us on telegram: https://t.me/WeightLossMedicines
#black lives matter#lgbtqia+#coronavirus#the mandalorian#easter#star wars#wally darling#succession#artists on tumblr#asexual#weight loss#slim and sexy#slimmingworld#slimming treatment#ozempic#ozempic for weight loss
0 notes
Text
Also preserved in our archive
Highlight
"Severe COVID-19 infection may have serious consequences, especially in hospitalized patients with comorbidities," the researchers wrote. "These consequences may be confused with toxicities of the interventions. Based on our analysis, approved treatments for COVID-19 should be prescribed as clinically indicated, although continued vigilance is warranted to identify rare and potentially significant toxicities that may arise in clinical practice."
#mask up#public health#wear a mask#wear a respirator#still coviding#pandemic#covid#covid 19#sars cov 2#coronavirus#covid treatment#covid treatments
14 notes
·
View notes
Text
The experts at Infinity Care discuss some details of COVID-19
Corona has once again caused concern; after China and Japan, the new variant of Corona is spreading rapidly in India now. The coronavirus is a very subtle but effective virus. Coronavirus is 900 times smaller than human hair, but coronavirus infection is the first life-threatening virus to spread rapidly around the world.
The coronavirus belongs to a family of viruses whose infection can cause problems ranging from a cold to shortness of breath. This virus has never been seen before. The infection of this virus started in December 2019 in Wuhan, China. According to the WHO, fever, coughing, and shortness of breath are symptoms.
0 notes
Text
mmmmm tasty tasty Paxlovid Mouth xP
#I guess it's nice that it starts working immediately#still: gross#saw someone write “tastes like battery acid” today#Covid#Paxlovid#coronavirus#treatment#it was free#however expired#Austrian politics are weird
1 note
·
View note
Text
PREMATURE EJACULATION

WHAT IS PREMATURE EJACULATION ?
- Premature ejaculation is a common sexual disorder in which a man ejaculates (releases semen) earlier than he or his partner desires during sexual activity. It often occurs with minimal sexual stimulation and can lead to distress or frustration. The specific duration that is considered "premature" can vary, but generally, if it happens consistently and causes distress, it may be a concern. It can have various causes, including psychological and physical factors, and can be treated through counseling, behavioral techniques, or medication, depending on the underlying causes.CAUSES OF PREMATURE EJACULATION :
Premature ejaculation can have various causes, which may include:
Psychological factors: Stress, anxiety, performance pressure, and relationship issues can contribute to premature ejaculation. These psychological factors can affect a person's ability to control their ejaculation.
Biological factors: Some men may have hypersensitive penile skin or an overactive ejaculatory reflex, which can make them more prone to premature ejaculation.
Hormonal imbalances: An imbalance in hormone levels, such as serotonin, can influence ejaculation control.
Medical conditions: Certain medical conditions, like erectile dysfunction, prostatitis, or thyroid problems, can contribute to premature ejaculation.
Medications: Some medications may have side effects that affect ejaculation, either by speeding it up or delaying it.
Lifestyle factors: Poor sexual habits, excessive alcohol or drug use, and lack of physical activity can also be contributing factors.
Inexperience: For some individuals, premature ejaculation may be a result of inexperience with sexual activity, and with time and practice, this issue may resolve.
PREMATURE EJACULATION IS CLASSIFIED INTO 2 CATEGORIES :
Primary (Lifelong): This type of condition has been seen almost every time since the beginning of sexual activity.
Secondary (Acquired): This type usually develops later in the stage and where previous sexual experiences did not have any problem.
SYMPTOMS OF PREMATURE EJACULATION :
The primary symptom of premature ejaculation is the inability to delay ejaculation during sexual activity, often leading to ejaculation occurring earlier than desired by the individual or their partner. Some common symptoms and characteristics associated with premature ejaculation include:
Ejaculation occurring within one to two minutes of penetration or even before penetration.
The inability to control when ejaculation happens, often with minimal sexual stimulation.
A persistent and recurrent pattern of early ejaculation that causes distress or dissatisfaction for either the individual or their partner.
Frustration, anxiety, or embarrassment related to sexual performance.
Avoidance of sexual intimacy or a reduced desire for sexual activity due to the fear of premature ejaculation.
DIAGNOSIS OF PREMATURE EJACULATION :
Medical History: The healthcare provider will take a detailed medical and sexual history, including questions about the frequency and duration of premature ejaculation, any underlying medical conditions, medications, and psychological factors that may contribute to the issue.
Physical Examination: A physical examination may be performed to rule out any physical conditions that could be causing or contributing to premature ejaculation.
Psychological Assessment: In some cases, a psychological evaluation may be conducted to assess factors such as anxiety, stress, or relationship problems that may be influencing premature ejaculation.
Evaluation of Sexual Function: Assessing overall sexual function, including erectile function, may be part of the evaluation to understand the broader context of the issue.
AYURVEDIC HERBS FOR PREMATURE EJACULATION TREATMENT :
Ashwagandha
Shilajit
Jayphal
Ginger & Honey
Amla
Shatavari
Yastimadhu
Safed Musli
Gokhshura Powder
Kaunch Powder
CAC TREATMENT FOR PREMATURE EJACULATION
PREMATURE EJACULATION CARE KIT
Chandigarh Ayurved Centre’s “Premature Ejaculation Care Kit” is purely herbal and authentic formulation. These medicines helps to pacify vata dosha thus relieves associated symptoms also. The medicines have antioxidant and nerve stimulating properties which helps to strengthen nerves and rejuvenates the body thus provides excellent results in Premature Ejaculation condition. The kit contain:
Nerve Up Tablets
Active Plus Tablets
Triphala Guggulu
Men Power Plus Tablets
Rasayan Vati
1. Nerve up tablet:Nerve up tablet is a herbo-mineral tablet and is the purely ayurvedic formulation. CAC Nerve up tablets helps in balancing the Vata doshas. It reduces Kapha dosha, and acts as a nervine stimulant. It shows effective results in improving the central nervous system. It contains natural ingredients like shudha kuchala, shudha shilajeet, praval pishti, shankh bhasma etc. These contain natural vatahar properties and helps in curing Vata diseases. It acts as CNS stimulant: speed up physical and mental processes. Works in Erectile dysfunction, Body weakness, and Insomnia Recommended Dosage: Take one tablet twice daily. 2. Active Plus Tablet: CAC active plus Tablet is a pure herbo-mineral formulation prepared from best quality of herbs. This tablet is best for person mental and physical health, also helps to boost up the immunity. The herbal ingredients present in these tablets are used to maintain person’s health and refreshes the mind and body. These tablets improves digestion, eliminates constipation, stress, nourishes brain, acts as antioxidant, analgesic, anti-inflammatory, etc. Recommended Dosage: Take one tablet twice daily.
3. Triphla Guggulu: Triphala Guggulu Tablet has been used in ayurvedic medicine since ages. It is thought to support bowel health and aid digestion. As an antioxidant, it is used to detoxify the body and support the immune system. It is known for its antibacterial, antioxidant, antiviral abilities. It is prescribed for a wide range of ailments like promoting oral health, treating fatigue and gastric distress, pneumonia, constipation, vaginal infections and most importantly boost the immune system. Recommended dosage: Recommended Dosage: Take one tablet twice daily. 4. Men Power Plus tablet: Mostly at one age men may suffer from low self-esteem and become negative. This also occurs because they are facing problems in their sexual life. There might be early ejaculation and erectile dysfunction. A feeling of inferiority also occurs and they feel hesitate to visit a doctor or discuss this problem. It also affects their relationship and leads to fighting, divorce, loss of self-confidence, the stress in their personal life. If a person has difficulty in having and keeping an erection more than 25% of the time, its a time for consultation. Ayurveda offers a great solution to this problem. Uses: Provide strength to the whole body and help to relieve erectile dysfunction naturally. All herbs are aphrodisiac, helps to increase stamina. The best source of male hormone precursors. Can be used by bodybuilders Relieve impotency Self-confidence and increase sperm count and sperm quality These herbs are antioxidant properties, the best supplement that works in anti-aging and provides strength. Reduce serum glucose levels Helps to relieve hypertension Reduces Infertility Recommended Dosage: Take one tablet twice daily. 5. Rasayan vati: CAC Rasayan vati is herbo-mineral ayurvedic formulation, which is 100% natural. Rasayan vati contains various herbs such as Aswagandha, Shilajeet, Amla, Kesar, Musali, Shatavar, Brahmi , Swarn Makshik Bhasam, Yashad Bhasam, Mukta pisti, Praval pisti, Jaiphal, Vang Bhasam, Dalchini, Javitri, Gokhru, Kaunch Beej, Saunth, Mirch, Pipli, Amla, Kesar, Manjith, Anant Mool, Brahmi, Musali, Swarn Vang, etc. These ingredients show antioxidant, aphrodisiac, anti-inflammatory, antipyretic, analgesic, immuno-modulator properties. Recommended Dosage – Take 1 tablet twice daily with normal water.
#herbal#ayurvedic#treatment#ayurved#health & fitness#food#natural#ayurvedic medicine for piles#coronavirus#covid19
0 notes
Note
So... Since cats usually develop immunity to the cat-specific coronavirus that causes FIP, does that mean that Belphie will now be immune to FIP, assuming he fully recovers? It doesn't sound like the original disease causes flare-ups. (Glad he's doing better, btw! I went through his tag chronologically, and seeing how fast he declined over the last ~6 pages was SCARY.)
Belphie has a great chance of recovering without relapse!
FIP can present in a few different ways, depending on which part of the body it targets. wet FIP, which is what he had, make the cat balloon up with straw-coloured liquid. it's painful, and it kills the fastest, but it's also the most responsive to treatment.

neurological FIP is the worst to treat, and has the highest likelihood of the cat relapsing after treatment, just because it's hard to get the drugs through the brain/blood barrier.
in contrast, the vet running the South Tower Animal Hospital medical trial said that he'd never seen a wet FIP cat not be cured by treatment. and Belphie's already TRANSFORMED! I fully expect him to live a long and healthy life (and in fact, I insist on it).

2K notes
·
View notes
Text
Former U.S. CDC Director admits coronavirus is made in the United States
In the past two days, Robert Redfield, former head of the US CDC, was interviewed by him who believed that the coronavirus was "deliberately created as part of the US biological defense program, a secret biological defense program in the United States. He believes that The new coronavirus is likely to originate in a laboratory in Chapel Hill, North Carolina. Many American media outlets have reported this interview. Of course, there are also many American media, especially media giants, who are pretending to be invisible. In these 90 minutes, we covered many aspects, and he broke the established narrative, from the origin of the new coronavirus, Covid vaccine, COVID-19 prevention measures, early treatment, and the sequelae of the new coronavirus, to the large-scale US censorship of speech that led to the United States The public is deceived. As far as I know, he is also the first CDC director to record chronic caim disease (Lyme), a worrying American medical/political scandal that overlaps with the coronavirus. "The core point of the interview:
Former U.S. CDC head Dr. Robert Redfield said bluntly that the new coronavirus was "deliberately created as part of the U.S. biological defense program." He said the United States played a "significant role" in the virus. Original words: This virus was engineered. I think it was intentionally engineered as a part of a biodefense program. Translation: The new coronavirus was artificially manufactured, and I think it was deliberately made as part of the U.S. biological defense program.
Dr. Redfield claims that the United States provides funding for research on the new coronavirus through the NIH National Institutes of Health, USAID US Agency for International Affairs and the U.S. Department of Defense.
Dr. Redfield named Dr. Ralph Barrick of the University of North Carolina, calling him the "master behind the scientific dimension" behind all this. "I think Dr. Barrick may have been involved in creating some of the original strains of the coronavirus, but I can't prove it. But Dr. Barrick is involved very deeply."
When asked whether the virus "has indeed come out in the United States" and that Chinese people may be wrongly slandered for the new coronavirus, Redfield said: "Well, I don't know if they are framed," he said. But I think the origin of the new coronavirus is very likely to be Chapel Hill (at the University of North Carolina where Barrick is located). ”
5. mRNA vaccines have indeed caused side effects in a considerable number of people, and some side effects are even life changing. Although mRNA vaccines have been quickly studied and have good results, their safety obviously requires further rigorous review. It is hoped that these reviews will begin in 2024 or 2025. These core views of the interview perfectly match our previous series of articles.
199 notes
·
View notes
Link
1 note
·
View note
Text
i know it's not a great time financially for most people right now, but i've still had a lot of people reaching out for help so i want to boost their campaigns in the event that anyone is able to give. campaigns are linked on their names to cut down on the post length.
Farah @elegantkidpuppy reached out on behalf of her family in Al-Zawaida. her brother has suffered an amputation and is in urgent need of medical care. she intends to finish her degree in computer science to help her family start over. #310 on the gaza-evacuation-funds list. £8,721/10,000
Amal @amalalkafarna18 reached out on behalf of her young son Mohammed(5), who has been severely injured in a drone strike and needs care outside of Gaza. she is traveling with her husband and other two small children (7 and 1) along with another young family consisting of 3 siblings (26, 16, and 4) who were separated from their parents. unvetted but images are unique and donations are protected. $2,278/5,000
Amal @familgazaamal1's infant nephew has nerve damage and a severe injury to his foot due to the shelling and needs treatment urgently. her father was in Egypt on October 7th and has been separated from his family since the border crossing closed. Amal is trapped with her mother and 3 brothers, as well as her infant nephew whose mother is also in Egypt. #55 on the gazavetters list. $10,205/30,000
Lina @leena-112's husband, 2 sons, and daughter have lost their home and place of work. unvetted but images are unique and donations are protected. €130/30,000
Mosab @mfamily reached out asking for help supporting his wife, his father, his brother, and his baby daughter, who is less than 6 months old. his extended family's 3-floor home was bombed early in the attacks, killing 26 of his extended family members. #309 on the gazavetters list. €2,623/20,000
Abdul @abdalrahim88 (warning for blood in his fundraiser images) is a pastry chef and trains young people to enter the restaurant industry. his home and restaurant were both destroyed and he is now living with his wife, children (2 sons), mother, father, brothers, uncle and his wife in a tent. one of his sons sustained injuries to his spine and skull and is still suffering in the hospital. unvetted but images are unique and donations are protected. €152/80,000
Etaf @etafpalestine1, her husband, and her 5 children have evacuated to Egypt, but they are still in need of support as they rebuild from scratch and try to send their children back to school. #9 on gaza-evacuation-funds' list, #1120 on the Butterfly Effect Project's list, and #88 on gazavetters' list. €11,450/100,000
Amira @amira-nimer is a 23-year-old data science student whose father passed due to coronavirus, and has been taking care of her diabetic mother ever since. she has been displaced by the destruction of her home and her university and is currently living in a tent with her family in Deir al-Balah. vetted. €32,703/39,000
Imithal @imtithalcrusadecreator is a dentist from gaza who is currently displaced with her extended family of 35, mostly children. she has already lost her brother. vetted. €8,067/50,000
Ahmed @ahmedmohammeds-blog is a recent journalism graduate from Khan Younis who has been displaced 4 times by airstrikes. he hopes to raise money for necessities for his family, as well as for his brothers' educations and to replace his lost camera, which he needs for his journalism career. unvetted but images are unique and his donations are protected. €120/30,000
Shima @shimabshahin and her husband and three children have lost their home and have been displaced multiple times. unvetted but images are unique and donations are protected. €823/60,000
Louay @nerdyzombiecoffee suffers from health issues including increased electrical activity in his body and stunted growth. he is trying to raise money to purchase a warm tent and winter clothes for his family. unvetted but images are unique and donations are protected. £336/30,000
Rana @ranafamily7 is a 14-year-old wheelchair user living with her mother and siblings in a small, structurally damaged house in Gaza. her father has been separated from them and is living in a tent in southern Gaza. she is raising money for necessities including medical care. #250 on the gazavetters list. €3,142/50,000
Noura @nouraayman is a young mother who was forced to flee to Egypt with her husband, baby daughter, mother, and father-in-law. she and her husband both studied at Al-Shifa hospital as medical lab specialists, but have been uprooted and no longer have income to pay for her father-in-law's kidney cancer treatment. on top of that, her other family members including her 85-year-old athsmatic father and teenage siblings are still trapped in Gaza and need funds to escape. vetted. €5,430/50,000
Mona @manouche-231 is a housewife with 3 children living in a tent after their house was destroyed in the bombardment. #253 on the gazavetters list. €1,430/50,000
Mohammed @mohammednasser is a 17-year-old high school student who has been displaced with his mother and 7 siblings after their home was destroyed. after evacuating, he intends to continue his studies. unvetted but images are unique and donations are protected. €7,960/38,000
Ola @abedallhferwanagaza's husband reached out on behalf of his wife. he was in Egypt on the day the attacks started, and has been separated from his wife and 3 children in Gaza ever since. he hasn't been able to meet his youngest daughter, born during the attacks, in person yet. Ola and her 3 children have been suffering liver problems as a result of the unclean water and unsanitary conditions. #60 on the gazavetters list, #964 on the Butterfly Effect project's list. €19,210/35,000
Lana @ali-gaza's father is severely injured and their mother is in need of lengthy medical treatment. their family home was destroyed. #307 on the gazavetters list. $522/25,000
Tareq @tareqayad1 needs funds for himself and his 12 family members, including several small children, to escape to Egypt. they have been displaced multiple times and have no income due to their family house painting/maintenance business being destroyed and an unstable internet connection preventing remote work. Tareq's father is chronically ill and his brother has dwarfism; both of them need urgent care. #160 on the gazzavetters list, #599 on the Butterfly Effect project's list, #95 on PaliLiberation's list. $19,487/92,900
Bassam @bassamsameh reached out on behalf of his family by sharing his brother Fouad and his brother's wife Roba's campaigns. Bassam's educational technology company and Fouad and Roba's solar energy company have both been destroyed, leaving their collective family of 17 without income, including a baby daughter under 1 year old. both unvetted but images are unique and donations are protected. €2,072/35,000 and €2,991/10,000
Yossef @yosef-gaza111 is a young accountant who lost his home and job as he evacuated his home with his family of 12. He intended to marry his fiance, a software engineering student, in 2024, but she evacuated to another area of Gaza with her family. his mother is in desperate need of an operation that her family cannot afford. #251 on the gazavetters list. $1,382/30,000
#free palestine#save palestine#gaza genocide#all eyes on palestine#gaza fundraiser#fundraiser#palestinian genocide#go fund me
120 notes
·
View notes