#serum 7 cytokines
Explore tagged Tumblr posts
Text
Optimal Conditions for Sampling Reference Ranges of Blood Cytokine Tests Using AimPlex Flow Cytometry

Optimal Conditions for Sampling Reference Ranges of Blood Cytokine Tests Using AimPlex Flow Cytometry in Biomedical Journal of Scientific & Technical Research
Cytokines are small molecular peptides or glycoproteins, synthesized and secreted mainly by immune cells. They play a variety of biological functions in inflammatory diseases by mediating interactions across cells [1]. Release of cytokines leads to the activation of immune cells and production of more cytokines, a process referred to as “cytokine storm” [2]. Variation in the levels of cytokines can provide valuable information regarding the diagnosis and prognosis of autoimmune diseases [3,4]. A number of stimuli, including C5a, IgA-type immune complexes, and bacterial products, may stimulate cytokine synthesis [5-7]. In addition, cytokine levels may be altered due to blood collection techniques and storage conditions of the blood samples [8-10]. As per the recommended methods, serum has been used for a long time for cytokine measurements by ELISA. However, when the blood is collected into tubes, owing to the bio-incompatibility between blood cells and tube walls, coagulation gets activated and thrombin activates protease receptors, leading to the release of IL-1β, IL-6, IL-10, and TNF-α [11-13]. Some studies have also confirmed anticoagulants, such as EDTA, to inhibit the complement system activation for use in cytokine analyses [14]. According to Linda Torrissen Hennø, et al., EDTA-plasma is the best choice for cytokine detection using a Bio-Plex Pro Human Cytokine 27-Plex Panel kit [15]. Compared to the traditional single cytokine detection method (such as ELISA), the multiplex flow immunoassay developed here focused on plasma and/or serum samples and the pre-analytical sampling conditions.
For more articles in Journals on Biomedical Sciences click here bjstr
Follow on Twitter : https://twitter.com/Biomedres01 Follow on Blogger : https://biomedres01.blogspot.com/ Like Our Pins On : https://www.pinterest.com/biomedres/
#Journals on Biomedical Imaging#Journals on Biomedical Intervention#Journals on Medical Microbiology#Biomedical Science and Research Journal#journal of biomedical research and reviews impact factor
0 notes
Text
1. Introduction to Animal Virology & Immunology
Animal virology is the study of viruses that infect animals, their replication mechanisms, pathogenesis, and transmission. It plays a crucial role in veterinary medicine, zoonotic disease control, and vaccine development. Animal immunology focuses on the immune response of animals to infections, vaccines, and immunotherapies, providing insights into disease prevention and treatment.
Understanding animal virology and immunology is essential for combating viral outbreaks, protecting livestock health, preventing zoonotic spillovers, and ensuring food security.
2. Major Animal Viruses & Their Impact
Animal viruses can be categorized based on their genome type (DNA or RNA viruses) and the species they infect. Some key viruses include:
🦠 Foot-and-Mouth Disease Virus (FMDV) – Affects cattle, pigs, and sheep, causing economic losses. 🦠 Avian Influenza Virus (AIV) – Highly contagious in poultry; some strains (e.g., H5N1, H7N9) can infect humans. 🦠 African Swine Fever Virus (ASFV) – A deadly disease in pigs with no effective vaccine. 🦠 Rabies Virus – A fatal zoonotic virus affecting mammals, including dogs and wildlife. 🦠 Newcastle Disease Virus (NDV) – Affects poultry, causing respiratory and neurological issues. 🦠 Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) – Affects pigs, leading to reproductive failure and respiratory illness. 🦠 Bovine Viral Diarrhea Virus (BVDV) – Causes immunosuppression and reproductive issues in cattle. 🦠 Canine Parvovirus (CPV) – A severe viral disease affecting dogs, especially puppies.
3. Immune Response in Animals
The animal immune system defends against viral infections through innate and adaptive immunity:
🔹 Innate Immunity – The first line of defense, including barriers (skin, mucosa), phagocytes, and interferons. 🔹 Adaptive Immunity – Involves B cells (antibody production) and T cells (cell-mediated immunity). 🔹 Cytokines & Interferons – Proteins that regulate immune responses and inhibit viral replication. 🔹 Mucosal Immunity – Crucial in protecting against respiratory and gastrointestinal viruses.
4. Diagnostic Techniques in Animal Virology
🔬 Polymerase Chain Reaction (PCR) – Detects viral DNA/RNA with high accuracy. 🔬 Enzyme-Linked Immunosorbent Assay (ELISA) – Identifies viral antigens and antibodies in serum. 🔬 Virus Isolation & Culture – Growing viruses in cell cultures for study. 🔬 Next-Generation Sequencing (NGS) – Identifies novel and emerging viruses. 🔬 Serological Tests – Detects immune responses to viral infections.
5. Vaccines & Immunotherapy in Veterinary Medicine
💉 Live Attenuated Vaccines – Weakened viruses that provide strong immunity (e.g., Newcastle disease vaccine). 💉 Inactivated Vaccines – Killed viruses, safe but may require boosters (e.g., rabies vaccine). 💉 Recombinant Vaccines – Genetically engineered vaccines for precise immune response (e.g., FMDV vaccines). 💉 mRNA Vaccines – An emerging technology for rapid vaccine development. 💉 Monoclonal Antibodies – Used for targeted virus neutralization and treatment.
6. Zoonotic Viruses & One Health Approach
Many animal viruses can jump to humans (zoonoses), making virology crucial for public health. Examples include:
Coronavirus (SARS-CoV, MERS-CoV, SARS-CoV-2) – Originated in animals and caused global pandemics.
Influenza A Virus (H5N1, H1N1, H7N9) – Spreads from birds to humans.
Rabies Virus – 100% fatal in humans if untreated.
Ebola & Marburg Viruses – Spill over from bats to primates and humans.
The One Health approach integrates human, animal, and environmental health to prevent zoonotic spillovers.
7. Emerging Trends in Animal Virology & Immunology
🚀 AI & Machine Learning in Virology – Predicting viral outbreaks and vaccine efficacy. 🚀 CRISPR-Based Antiviral Strategies – Precision gene editing to fight viral infections. 🚀 Nanotechnology in Vaccine Delivery – Enhancing immune response through nano-vaccines. 🚀 Synthetic Biology for Virus Control – Engineering antiviral peptides and genetic resistance. 🚀 Microbiome & Immunity Research – Exploring gut microbiota's role in immune modulation.
Biotechnology Scientist Awards
Visit Our Website : http://biotechnologyscientist.com
Contact Us : [email protected]
Nomination Link : https://biotechnologyscientist.com/member-submission/?ecategory=Membership&rcategory=Member…
#sciencefather#researchawards#Scientist#Scholar#Researcher #AnimalVirology #VeterinaryImmunology #ZoonoticDiseases #VirusResearch #VeterinaryVaccines #LivestockHealth #OneHealth #VeterinaryScience #AnimalInfectiousDiseases #AvianFlu #ASFV #RabiesPrevention #FMDV #CanineVirology #MolecularVirology #CRISPRBiotech #AnimalHealth #VeterinaryMedicine #PCRDiagnostics #VeterinaryVaccination #EmergingViruses #BovineHealth #SwineFlu #VeterinaryEpidemiology #BiotechInnovation #Immunotherapy
👉 Don’t forget to like, share, and subscribe for more exciting content!
Get Connected Here: =============
Facebook : https://www.facebook.com/profile.php?id=61572562140976
Twitter : https://x.com/DiyaLyra34020
Tumblr : https://www.tumblr.com/blog/biotechscientist
Blogger: https://www.blogger.com/u/1/blog/posts/3420909576767698629
Linked in : https://www.linkedin.com/in/biotechnology-scientist-117866349/
Pinterest : https://in.pinterest.com/biotechnologyscientist/
0 notes
Link
0 notes
Text
Pandanus Conoideus Lamk Protects Inflammation by Regulating Reactive Oxygen Species and Nuclear Factor Kappa B in Lps-Induced Murine Macrophages
Abstract
Background: Pandanus conoideus Lamk (Red fruit) is a Papuan traditional food which has been used to treat various diseases. Despite these various effects of Red fruit, little is known about the physiological mechanism. Aims: The aim of this study was to investigate the anti-inflammatory properties of Red fruit oil (RFO) and establish the signal pathway of leading compounds.
Methods: Raw 264.7 murine macrophage cells were used with lipopolysaccharide (LPS). Cell viability and the pro-inflammatory factors were investigated using MTT assay, real time PCR, western blot analysis, and Enzyme linked immuno-sorbent assay (ELISA). The quantification of leading compounds in RFO was performed using high performance liquid chromatography (HPLC).
Results: RFO did not affect cell viability. RFO significantly reduced the production of nitric oxide (NO) and prostaglandin E2 (PGE2), and both the protein level and mRNA level of iNOS in LPS-induced macrophages. RFO also regulated the reactive oxygen species (ROS) in LPS-induced macrophages. RFO attenuated the translocation of NF-κB p65 subunit, phosphorylation of I-κB, extracellular signal-regulated kinase (ERK), and c-Jun N-terminal kinase (JNK) in a dose-dependent manner. HPLC analysis determined that 1 g of RFO had 14.05±0.8 mg of β-cryptoxanthin and 7.4±0.7 mg of β-carotene.
Conclusion: RFO provides an anti-inflammatory effect by regulating ROS and NF-κB through MAPK due to the antioxidant activity.
Keywords: Pandanus conoideus Lamk; Macrophages; Anti-inflammation; ROS; NF-κB; β-cryptoxanthin
Abbreviations: RFO: Red fruit (Pandanus conoideus Lamk ) oil; LPS: Lipopolysaccharide; NO: Nitric oxide; iNOS: Inducible NO synthase; IL: Interleukin; ROS: Reactive oxygen species; ELISA: Enzyme linked immuno-sorbent assay; HPLC: High performance liquid chromatography; COX-2: Cyclooxygenase-2; PGE2: Prostaglandin E2; ERK: Extracellular signal-regulated kinase; JNK: c-Jun N-terminal kinase; MAPK: Mitogen-activated protein kinase; DMEM: Dulbecco’s modified Eagle medium; FBS: Fetal bovine serum; DCFH-DA: 2’7’-dichlorofluorescein diacetate; MTT: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; RT- PCR: Real time polymerase chain reaction
Introduction
The inflammation process is tightly regulated by both initiation and maintenance signals and considered to be a major risk factor in the pathogenesis of chronic diseases where the macrophages are important immune cells which regulate inflammation producing expression of inflammatory proteins and pro-inflammatory chemokines, cytokines, and nitric oxide (NO) [1,2]. Macrophages are highly sensitive to initiators of inflammation as lipopolysaccharide (LPS) which respond by the release of mediators not only interleukins (ILs) and cytokines, but also inducible NO synthase (iNOS) and reactive oxygen species (ROS), which inducing the inflammatory gene expression where each is associated somehow with the pathophysiological of the inflammation [3-5]. Because macrophages produce a wide range of biologically active molecules participated in both beneficial and detrimental outcomes in inflammation, modulation of macrophage activation is a good strategy to prevent this diseases. Red fruit (Pandanus conoideus Lamk) is Papuan traditional food which has been used to treat various diseases such as cancer [6] preeclampsia [7], hepatitis [8], liver cirrhosis [9], diabetes mellitus [10], and sinusitis [11]. This bioavailability of red fruit has been due to unsaturated fatty acids such as palmitoleic acid, oleic acid, linoleic acid, linolenic acid and some carotenoids [10,12]. Despite these many biological effects, few researches were reported on the mechanism of red fruit oil (RFO). β-cryptoxanthin is a typical carotenoid found abundantly in persimmon, papaya, paprika, and carrot. β-cryptoxanthin has been reported to possess several beneficial functions, such as antioxidant, cancer-preventive effects, and anti-metabolic syndrome effects [13-16]. In present study, we hypothesized that the cause of this anti-chronic inflammation and anti-cancer effect is due to antioxidant function of RFO, and evaluated the anti-inflammatory effect of RFO on LPSstimulated RAW 264.7 macrophage cells. We also investigated the mechanism of inflammatory effect of reduced ROS by RFO in LPS-stimulated macrophages and investigated the component of β-cryptoxanthin in RFO.
Materials and Methods
Chemicals and reagents
RFO (APOTEK®) was supplied from Smile international Co., Ltd (Seoul, Korea). Dulbecco’s modified Eagle medium (DMEM), fetal bovine serum (FBS), and penicillin–streptomycin was purchased from Corning (Oneonta, NY, USA). 2’7’-dichlorofluorescein diacetate (DCFH-DA) and anti-iNOS antibody were purchased from BD (San Jose, CA, USA). Peroxidase-conjugated secondary antibodies and TriZol were purchased from Life technologies (Grand island, NY, USA). Phosphor-JNK, phosphor-ERK, phosphor-p38, phosphor-IκB and NF-κB antibodies were purchased from Cell Signaling Technology Inc. (Beverly, MA, USA). The enzyme immunoassay kit used for prostagladin E2 (PGE2) was obtained from R&D Systems (Minneapolis, MN, USA). The ECL detection reagents were purchased from GE Healthcare (Buckinghamshire, UK). LPS (Escherichia coli 0111: B5) was purchased from Creative Biolabs (Shirley, NY, USA). β-actin, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), and other chemicals were purchased from Sigma–Aldrich (St. Louis, MO, USA).
Cell culture
RAW 264.7, the murine macrophage cell line was purchased from American Type Culture Collection and maintained in DMEM supplement with 1 mg/mL glucose, 10% FBS, 100 mg/mL penicillin-streptomycin at 37 °C with 5% CO2
Cell viability assay
The cytotoxic effect of RFO against RAW264.7 cell lines was evaluated by MTT assay. Briefly, cells were seeded at a density of 5 × 103 cells/well in a 96-well plate for 24 h. Then, the cells were treated with at various concentrations of fractions with or without 1 μg/mL LPS. After 24 h, 2 mg/mL MTT was added onto each well, then incubated until formazan was constituted for 3h. The formazan was dissolved in dimethyl sulfoxide (DMSO) and the absorbance at 550 nm was measured using microplate reader (Molecular Devices, Sunnyvale, CA). Cell viability was calculated as a percentage of viable cells in drugs treated group versus untreated control. Each experiment was repeated three times.
Nitrite assay
Cells were treated with various concentrations of RFO for 30 min and incubated with 1 μg/mL LPS for 24 h. Because NO production is reflected in the accumulation of nitrite in the cell culture medium, 50 μL of supernatants were removed and mixed with the same volume of Greiss reagent (Promega, Madison, WI). After incubation for 10 min, the absorbance of mixture at 450 nm was measured using a spectrophotometer (TECAN, Austria). The nitrite levels were estimated as the percentage of absorbance of the sample to the respective controls.
Cyclooxygenase2 (COX-2) assay
Cells were treated with various concentrations of RFO for 30 min and incubated with 1 μg/mL LPS for 24 h. After incubation, the supernatants were removed and followed COX-2 measurement. The COX-2 concentrations were evaluated using a specific enzyme immunoassay (EIA) kit (Cayman, Ann Arbor, MI) according to the manufacturer’s instructions.
Prostaglandin E2 assay
Cells were treated with various concentrations of RFO for 30 min and incubated with 1 μg/mL LPS for 24 h. After incubation, the supernatants were removed and followed PGE2 measurement. The PGE2 concentrations were evaluated using a specific enzyme immunoassay (EIA) kit (Cayman, Ann Arbor, MI) according to the manufacturer’s instructions.
iNOS gene measurement by real-time PCR
The cells from the supernatants had been removed were subjected to RNA isolation. RNA isolation was performed using TRIzol reagent according to the manufacturer’s instructions. cDNA was synthesized using hyperscript RT master mix (GeneAll, Daejeon, Korea). The primers were described as; iNOS forward: 5′-ATGTCCGAAGCAAACATCAC-3′, reverse: 5′-TAATGTCCAGGAAGTAGGTG-3′, and GAPDH forward: 5′-TGTGATGGTGGGAATGGGTCAG-3′, reverse: 5′-TTTGATGTCAC GCACGATTTCC-3′. The PCR was amplified using ABI 7500 and Taqman gene expression master mix (Applied Biosystems, Waltham, MA, USA). The quantitative analysis was performed to compare the Δ Δ Ct after the normalization by GAPDH as an internal control. After analysis, PCR products were electrophoresed on 3% agrose gel and images were taken by cybergreen detection using Kodak imagestation FX® (Kodak, Rochester, NY, USA)
Analysis of ROS by flowcytometry
Cells were treated with various concentrations of RFO for 30 min and incubated with 1 μg/mL LPS for 24 h. Cells were followed by the addition of 10 mg/mL DCFH-DA). The suspensions were washed with PBS after incubation for 20 min. The suspensions were then assayed with a flowcytometer (C6 Accuri, BD, Bedford, MA, USA) according to Rhee et al. [4].
Western blot analysis
Cells were treated as described previously, then total lysates were prepared with lysis buffer (50 mM Tris (pH 7.4), 300 mM NaCl, 5 mM EDTA (pH 8.0), 0.5 % Triton X-100, 1 mM aprotinin, 1 mM leupeptin, 1mM pepstatin, 10mM iodoacetamide, and 2 mM phenylmethylsulfonyl fluoride (PMSF). Meanwhile, each nucleus extracts and cytosol extracts were isolated using a NE-PER nuclear and cytoplasmic extraction reagent kit (Pierce, Rockford, IL). Briefly, cells were washed with PBS, and were prepared with ice-cold extraction buffers sequentially. After centrifugation at 16,000xg, the cytoplasmic protein and nuclear extract were separated. Total lysates and nuclear fractions were estimated with Bio-Rad dye reagent concentrate (Bio-Rad Laboratories, Hercules, CA), then resolved on a 10% SDS-PAGE. After electrophoresis, the proteins were electro transferred to a PVDF membrane, blocked with 1% BSA, and probed with anti-iNOS (1:1,000), phospho- JNK (1:1,000), phospho-ERK (1:1,000), phospho-p38 (1:1,000), phospho-IκB (1:1,000), and NF-κB (1:500) antibodies at 4 °C overnight. The blot was washed, exposed to HRP-conjugated secondary antibodies for 2 h, and finally developed through enhanced chemiluminescence. For ß-actin detection, previously used membranes were soaked in stripping buffer (62.5 mM Tris- HCl, pH 6.8, 150 mM NaCl, 2% SDS, 100 mM ß-mercaptoethanol) at 65 ℃ for 30 min and hybridized with anti-ß-actin. The relative protein expression was densitometerically quantified using the BioRad GS-670 densitometer (BioRad, Hercules, CA) and normalized to β-actin.
High performance liquid chromatography (HPLC)
To determine the content of β-cryptoxanthin in RFO, we performed HPLC analysis according to previous studies [17]. HPLC analysis was performed using Agilent 1100 model with a pump (G1311C), auto sampler (G1329B), column, and diode array detector purchased from Agilent (Santa Clara, CA, USA). The analysis conditions are described in Table 1.
Statistical analysis
All results are presented as mean ± S.D. and are representing three or more independent experiments. Data were compared using the one-way ANOVA using Prism® (GraphPad, La Jolla, CA, USA) with p-values less than 0.05 considered statistically significant.
Results
RFO did not affect cell viability
Figure 1A showed the effect of RFO on viability of RAW 264.7 with or without LPS. Cell viability was not affected against 10- 1,000 μg/mL of RFO with or without LPS.
RFO reduced NO in LPS-induced macrophages
To assess the effects of RFO on NO production in LPSinduced RAW 264.7 macrophages, cells were treated with various concentrations of RFO for 30 min, then incubated with 1 μg/mL LPS for 24 h. NO release was elevated 224 ± 19.24% (p < 0.001) following LPS treatment, which was reduced 224 ± 19.24% at 10 μg/mL (p < 0.05), 161.38 ± 21.81% at 25 μg/mL (p < 0.001), and 136.16 ± 30.56% at 50 μg/mL (p < 0.001) with RFO combination (Figure 1B).
RFO decreased COX-2 production in LPS-induced macrophages
COX-2 production was significantly increased from 33.17 ± 5.23 ng/mL to 86.25 ± 1.88 ng/mL (p < 0.001) following LPS treatment. However, it was reduced 60.52 ± 12.49 ng/mL at 10 μg/mL (p < 0.05), 32.16 ± 8.85 pg/mL at 25 μg/mL (p < 0.001), and 13.27 ± 1.67 ng/mL at 50 μg/mL (p < 0.001) with RFO combination (Figure 1C).
RFO also decreased PGE2 production in LPS-induced macrophages
Meanwhile, PGE2 production was significantly increased 440.6 ± 35.36 pg/mL (p < 0.001) following LPS treatment, which was reduced 227.5 ± 13.6 pg/mL at 10 μg/mL (p < 0.001), 180.77 ± 48.95 pg/mL at 25 μg/mL (p < 0.001), and 103.27 ± 51.67 pg/ mL at 50 μg/mL (p < 0.001) with RFO combination (Figure 1D).
RFO suppressed both mRNA and protein levels of iNOS in LPS-induced macrophages
To determine the inhibitory effects of RFO on proinflammatory mediator NO, COX-2, and PGE2 production, the biosynthesis of transcriptional levels of iNOS was performed with semi-quantitative reverse-transcription PCR and western blot analysis on LPS-induced RAW 264.7 macrophages. Figure 1D indicates that both mRNA level and protein level of iNOS were significantly decreased by treatment of RFO (p < 0.001). Consistent with the findings shown in Figure 1E, RFO had a significant concentration-dependent inhibitory effect on the inflammation through pro-inflammatory mediator NO in LPSinduced RAW 264.7 macrophages.
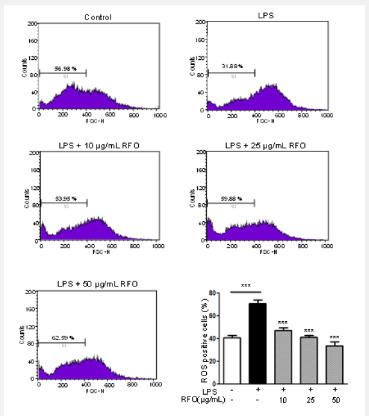
RFO attenuated ROS in LPS-induced macrophages
The excess ROS is known to be injured intracellular proteins, lipids and nucleic acids and induce inflammation [18]. Thus, we investigated the ROS production in response to LPS using flowcytometry. DCFH-DA binds ROS produced cells. Figure 2 showed the DCFH-DA positive cells were increased following LPS treatment from 40.71 ± 2.11% to 70.87 ± 3.09%. However, ROS production was also significantly inhibited by RFO with a dose dependent manner; 47.08 ± 2.45% at 10 μg/mL (p < 0.001),41.34 ± 1.41% at 25 μg/mL (p < 0.001), and 33.76 ± 3.56% at 50 μg/mL (p < 0.001).
RFO suppressed nuclear translocation of the NF-κB p65 subunit via MAPKinase
Since p65 is a major component of NF-κB activated by LPS in macrophages, we evaluated the levels of p65 in nuclear extracts by western blotting analysis. Phosphorylation of IκB results in degradation and release of NF-κB, which then translocates to the nucleus. Therefore, we examined whether RFO could prevent phosphorylation of IκB induced by LPS treatment. Figure 3A shows that IκB phosphorylation was increased by treatment with LPS alone in cytosol level, but that such phosphorylation was significantly inhibited in the presence of RFO, similar to results for the nuclear translocation of p65. Taken together, these data suggest that the inhibitory effect of RFO on the LPS-induced translocation of p65 might be involved in the suppression of IκB phosphorylation. To further investigate whether the inhibition of pro-inflammatory mediators by RFO is modulated through the MAPK pathway, we evaluated the effects of RFO on the LPSinduced phosphorylation of p38, ERK, and JNK (Figure 3B). RFO suppressed LPS-induced phosphorylation of p38, ERK and JNK. These results suggest that RFO blocks MAPK pathways to suppress the inflammatory response in LPS-induced RAW 264.7 macrophages.
HPLC analysis of RFO
Table 2 showed the HPLC analysis of RFO. HPLC revealed that 1 g of RFO had 14.05±0.8 mg of β-cryptoxanthin and 7.4 ± 0.7 mg of β-carotene.
Discussion
Inflammation is an immune response that protects our body against host response to infection and injury [19,20]. All inflammatory responses act through mononuclear cells, macrophages, and lymphocytes. Macrophages play on important innate immune effectors and increase pro-inflammatory factors including nitric oxide (NO), prostaglandin E2 (PGE2) cytokines.
The excessive amounts of NO and PGE2 produced by activation of iNOS and COX-2 in response to LPS play an important role in inflammation [21,22]. The overproduction of iNOS-derived NO is involved in the pathology of various inflammatory disorders and tissue damage conditions. A change in the NO level through the inhibition of iNOS enzyme activity or iNOS induction provides a means of assessing the effect of these agents on the inflammatory process. iNOS is implicated in the synthesis of prostaglandin H2 starting of arachidonic acid, which is a precursor of PGE2, in activated macrophages with LPS [23]. In addition, iNOS leads to overproduction of NO, PGE2, and COX-2 which results in the production of inflammatory diseases. Thus, modulation of iNOS and NO expressions could be one of the strategies to reduce inflammatory diseases. The production of inflammatory cytokines is a crucial part of regulating inflammation and tumor progression. The key signaling pathway mediating the inflammatory response, the NF-κB transcription factor, has been well-established in various inflammatory diseases and cancers [24,25]. It is also well known that NF-κB is a significant role factor regulating the expression of inflammation-associated enzymes and cytokine genes, such as iNOS, COX-2, TNF-α and IL-1β, which contain NF-κB binding motifs within their respective promoters [1,26]. Therefore, this signaling pathway is a good target for anti-cancer and antiinflammatory drug development. Many of the upstream kinases and downstream substrates are the same for the each of the major cascades. Our results revealed that anti-inflammatory activities of RFO are mediated through the inhibition of IκB phosphorylation and nuclear translocation of the NF-κB p65 subunit. Besides, these results also indicate that the inhibitory effects of RFO on MAPK and NF-κB signaling are related to a decrease in ROS. It is well known that oxidative stress stimulates ROS production in RAW 264.7 cell line [11,27]. Our data showed the pretreatment with RFO significantly decreased ROS production in LPS-induced RAW264.7 cells using DCFH-DA staining which demonstrated that RFO had a potent to reduce the oxidative stress. We also suggested that RFO regulated MAPK and NF-κB signaling of inflammation operate through oxidative stress. These results demonstrated that RFO could act as scavenging agents or acting on redox state of the cell and other acting as scavenging agents. In previous study, we already demonstrated that RFO regulated the cellular senescence through ROS modulation in H2O2-induced endothelial cells [5].
Carotenoids such as β-cryptoxanthin, β-carotene are one of the antioxidants which are not produced in the human body that must be ingested from outside. Many studies indicated that healthy people had the higher level of β-cryptoxanthin in blood [28-31]. β-cryptoxanthin is the only provitamin A component of carotenoid-based xanthophylls [14,32]. Carotenoids are lipid soluble components that must be ingested with fat to absorb completely in the body. Carotenoids affect the inflammation levels in blood as strong antioxidants and helps purify the blood. Park et al. showed that the daily oral administration of β-cryptoxanthin prevented the progression of osteoarthritis and inhibited proinflammatory cytokines in mice [33]. Therefore, we examined the effects of RFO on the production of several inflammatory mediators and on the expression levels of iNOS in LPS-induced RAW 264.7 macrophage cells. Our results demonstrated that RFO inhibited the expression of iNOS as well as the production of NO and PGE2 and the mechanisms underlying the suppression of the inflammatory response of the NF-κB and ROS. According to the US USDA database, β-carotene content of RFO was significantly higher at 335 times of blackberry, 119 times of broccoli, 13.9 times of pumpkin, and 5.2 times for carrot [34,36]. In addition, β-cryptoxanthin content of RFO was significantly higher at 76 times of orange and 15 times of papaya [30,37]. These findings suggested that RFO might be a beneficial therapeutic agent in the treatment of a variety of inflammatory diseases.
Conclusion
RFO is Papuan traditional food and had been used to treat various disease for long time. In this study, we suggested RFO had an anti-inflammatory effect through regulating inflammatory mediators such as iNOS, COX-2, PGE2, and excessive ROS for the first time. These physiological benefits of RFO may be attributed by regulation of NF-κB transcription. HPLC indicated that large number of carotenoids such as β-cryptoxanthin, β-carotene. This finding may be a synergistic adjuvant therapy for inflammatory diseases by acting as a radical scavenger, ROS inhibitor.
To Know More About Nutrition and Food Science International Journal
Please click on: https://juniperpublishers.com/nfsij/index.php
For more Open Access Journals in Juniper Publishers
please click on: https://juniperpublishers.com/index.php
#food safety#food microbiology#food preservation#Food Engineering#Juniper publishers USA#open access journals
0 notes
Text
C-Reactive Protein in Veterinary Practice
Abstract
Animal body reacts to all kinds of injuries and stress to keep up the homeostasis mechanism of the body. This homeostasis achieved either by or nonspecific mechanism. The nonspecific innate resistance of the body like cytological and cytokine reactions including fever, leukocytosis etc. is known as acute phase response. In this response, there will be increase or decrease of serum concentration of proteins. These proteins are known as acute phase proteins. Measurements of serum concentration of these acute phase proteins are found to be useful in assessment of health status and prediction of diseases of the man and animals. The serum concentration of these acute phase proteins returns to base levels when the triggering factor is no longer present. The acute phase response is now considered to be a dynamic process involving systemic and metabolic changes providing an early nonspecific defence mechanism against insult before specific immunity is achieved. Use of one of the acute phase proteins, C-reactive protein as biomarkers for animal disease diagnosis and health status assessment has got high potential in modern veterinary practice is discussed in this review
Keywords: Acute injury; C - reactive protein; Acute phase proteins; Veterinary practice
Abbrevations: CRP: C Reactive Protein; Hp: Haptoglobin; AGP: Acid Glycoprotein; SAA: Serum Amyloid A
Introduction
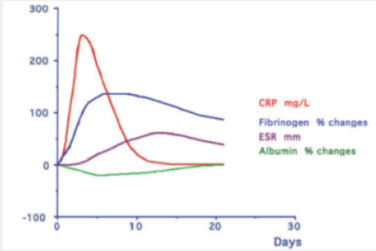
The acute phase reaction encompasses all the phenomena which take place in animals following tissue damage and is particularly associated with inflammation from whatever cause. During the acute phase reaction, the body mounts a multifactorial response to remove and replace damaged tissue and one of the mechanisms involved is the production and secretion by the liver of a number of ‘acute phase proteins’ [1]. The concentrations of these proteins increase during the reaction are called as Positive acute phase proteins (APPs) such as C reactive protein (CRP), Serum Amyloid A (SAA), Haptoglobin (Hp), Ceruloplasmin, α2- Macroglobulin, α1 Acid Glycoprotein(AGP), Fibrinogen and Complement (C3,C4) while those of others, including albumin, Transferrin, Transthyretin and Retinol-binding protein decrease as the liver switches production of protein towards the synthesis of the proteins required to deal with the damage; is called as negative APPs [2] (Figure 1).
Biological functions of C-reactive Protein
The protein was named the C-reactive protein because of its ability to bind pneumococcal C-polysaccharide. The presence of CRP has also been described in human patients during acute infections caused by acute lobar pneumonia, active rheumatic fever and bacteraemia caused by “colon bacillus”. Among the biological functions described in the literature are Complement activation and opsonisation [3,4] Modulation of monocytes and macrophages, cytokine production [5] Binding of chromatin [6] Prevention of tissue migration of neutrophils
CRP in Bovines
During the early stages of infection, the serum concentration of CRP increases [3]. This increase has been described to be evident before an elevated rectal temperature is observed. Even though increased concentrations of bovine CRP during naturally occurring infections and a correlation with herd health status have been reported, CRP is generally not considered an acute phase protein in cattle. As stress increases to a critical point, the liver rapidly synthesizes large amounts of CRP and releases it into the blood to provide immediate protection against stress [7]. Diseases in a dairy herd elevated the serum CRP level. The serum CRP level was also correlated with milk production. The greater the milk production, the higher the level of serum CRP. Diseases, especially acute infections, induced much higher levels of CRP production than stress or lactation. Also showing that plasma C-reactive protein concentration is related to different kind of stress (poor health, high lactation, blood collection). Strong correlation was observed in cows after delivery (0-1 month) between fibrinogen and CRP values [8]. Obtained results suggest that not only inflammations but also physiological factors such as pregnancy, delivery and/or state of lactation may have a significant impact on APPs values in the blood plasma of dairy cows. It would be worth in the future to check whether there is a relationship assessing the animal health status obtained using acute phase proteins method relatively to other indicators, such as milk yield, length of lactation or others. Morimatsu [9] also discovered Elevation of bovine serum C-reactive protein by lactation when compared to other Acute Phase protein such as serum amyloid P component levels (Figure 2).
CRP in Swine
In the pigs, as in the dogs and humans, C-reactive protein (CRP) is the prototypical acute phase protein with major diagnostic value. CRP concentrations are useful for evaluating the health status of a swine herd, but not for the health status of an individual animal or the differentiation of diseases. Serum haptoglobin (HP) concentration is better than serum CRP concentration as an indicator of inflammatory reactions in pigs, and HP is an important marker for swine health status [10]. On the other side, pigs undergone experimental study had CRP serum concentration below 22μg/ml (mean 18.64 ± 2.59). Twenty-four hour after coinfection with swine influenza virus (H1N1) and Pasteurella multocida, the mean concentration of CRP reached 62.85 ± 35.55μg/ml. Significant difference were noticed as compared to control animals [11]. The presence of elevated serum Hp and CRP concentrations in apparently healthy pigs at slaughter could provide important information to a veterinary inspector about the presence of sub-clinical lesions that could lead to condemnations or a decrease in the quality of carcasses [12].
CRP in Canines
In canines CRP is the major APP used as marker for systemic inflammation / infection. Normally the level of CRP is less than 1.5 mg/ dL or even lower than 0.5 mg/dl. The normal range may be 0.08 to 2.26 mg/dl [13]. The level rises within 4 to 6 hrs after onset of inflammation / infection. Serum CRP level above 3.5 mg/dl, indicates presence of systemic inflammation. Level above 5 mg/dl is a strong evidence of systemic inflammation. Strong correlation was observed between CRP and animal’s temperature and Total Leukocyte counts of canine patients naturally infected with Leptosporosis [14]. CRP levels could be used to monitor early responses to antibiotic treatment and might alert veterinarians to the need for further evaluation or additional treatment. Serum CRP concentrations provide useful information about the severity of inflammation inside the Urinary Bladder. These correlations suggest that CRP concentrations can represent a safe, convenient, and alternative method for evaluating the status of bacterial cystitis [15]. Significantly high CRP values were observed in cases like Lympoma, pyometra, panniculitis, acute pancreatitis, polyarthritis, leptospirosis, babesiosis, parvo viral enteritis, glomerulonephritis, immune mediated disease and malignant neoplasia [16]. Rise in CRP may not be observed in local tumours like leiomyosarcoma, upper respiratory tract infection, diabetes, neurological problems involving intracranial disorders. Since the CRP concentration did not increase in patients with intervertebral disk protrusion, it might be useful in distinguishing arthritis from spinal / brain diseases in patients with lameness. Thus, CRP is a nonspecific inflammatory marker, it could facilitate the diagnosis by indicating the presence and the extent of inflammation
CRP in Elephants
The reference interval for CRP reported herein for Asian elephants (1.3–12.8 mg/l) is like CRP intervals reported in harbor seals (Phoca vitulina) and bottlenose dolphins (Tursiops truncatus). When compare to the CRP, SAA is demonstrated to be the most responsive major APP in elephants [17]. This agrees with previous reports where SAA elevations were noted consistently in elephants with Elephant endotheliotropic herpesvirus (EEHV) and in 2 captive elephants with inflammatory lesions. Further studies are needed to address the reactivity of the CRP reagents with elephant proteins and to consider the use of either elephantspecific reagents or non–antibody-based assays
CRP in Chicken
The birds positive for E.Coli, Pasturella multocida and Staphylococcus aureus infections as well as histomoniasis and adjuvant injection found to be positive for CRP. CRP also positive in clinically normal population. These birds on post-mortem, had lesions consistent with chronic respiratory disease, which is highlighting the use of CRP as a potential biomarker for nonclinical disease [18]. CRP did not rise in chickens as quickly as it does in humans, whereby CRP was detectable 36-48 hours post infection in chickens, compared to 16-18 hours in humans. A more recent study investigated CRP serum concentrations using a human CRP kit and found CRP concentrations to increase
CRP in Equines
A high serum concentration was also found in horses with pneumonia, enteritis, arthritis and after castration. Increased plasma concentration has been observed in carbohydrate induced laminitis. Increased serum concentration of CRP has been found in horses suffering from aseptic inflammation induced by intramuscular turpentine injections [3].
To Know More About Journal of Dairy & Veterinary sciences
Please click on: https://juniperpublishers.com/jdvs/index.php
For more Open Access Journals in Juniper Publishers
please click on: https://juniperpublishers.com/index.php
#wild life rehabilitation#Veterinary sonography#Veterinary Therapeutics#Veterinary Virology#Juniper Publishers#open access journals
0 notes
Text
Iris Publishers - Current Trends in Clinical & Medical Sciences (CTCMS)
Atypical clinical presentation of COVID-19: a case of Guillain-Barrè Syndrome related to SARS-Cov-2 infection
Authored by Angelo Michele Carella

In these months the diffusion of a novel beta coronavirus, known as Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), is causing a worldwide public health emergency originated in Wuhan, China. The novel coronavirus was reported to cause symptoms resembling the severe acute respiratory syndrome (SARS-CoV) by previous coronavirus in the years 2002 and 2003. Genetic sequencing of the virus suggests that it is closely linked to the SARS coronavirus. Both share the same receptor, angiotensinconverting enzyme 2 (ACE2) and therefore this virus was named SARS-CoV-2 [1, 2].
SARS-CoV-2 is very contagious and its rapid propagation has spread globally; there are three main transmission routes of COVID-19 infection: droplets, contact and aerosol transmission [3]. The gold standard for diagnosis of SARS-CoV-2 infection is real-time polymerase chain reaction fluorescence (RT-PCR) for detecting SARS-CoV-2 nucleic acid in samples of sputum or throat swab and in secretions of upper respiratory tract. Other potential diagnostic method might be the detection of specific IgM and IgG antibodies against SARS-Cov-2 in blood samples, although this method seems more appropriate for population screening [4].
The most prevailing onset symptoms of this infection, after an approximate incubation period of five days on average, are fever, cough, myalgia and fatigue, but also diarrhea, leg pain, dysgeusia and hyposmia [5, 6]. Although most patients infected by SARSCoV- 2 are asymptomatic or develop mild to moderate symptoms, a subset of patients develops serious interstitial pneumonia that may quickly progress to severe acute respiratory distress syndrome (ARDS), septic shock and fatal multi organ dysfunction that are the most severe clinical manifestations of SARS-Cov-2 infection [7].
High serum levels of Interleukin-6 (IL-6) and D-Dimer seem closely related to the occurrence of severe COVID-19 and their combined detection may be very useful for early prediction of severe COVID-19 patients; moreover, the patients present frequently lymphopenia and neutrophilia, hypoalbuminemia, high serum levels of alanine aminotransferase, lactate dehydrogenase, C-reactive protein, and sudden oxygenation deterioration [8].
Given that acute respiratory syndrome is the hallmark feature of severe COVID-19, most initial studies have focused on its impact on the respiratory system. However, accumulating evidence suggests that SARS-CoV-2 also infects other organs and can affect various body systems [9]. The expression and distribution of ACE2 in multiple human organs, including nervous system and skeletal muscles, suggests that SARS-CoV-2 might have a neuroinvasive potential and its impact on the nervous system might occur through direct infection or via secondary effects relating to intense systemic inflammatory response linked to viral infection [10-12]. Indeed, in severe cases of COVID-19 it has been shown a massive release of proinflammatory mediators and cytokines, in particular Interleukin-6 (IL-6) and Interleukin-1 (IL-1), linked to viral replication and leading to cytokine release syndrome-like [13].
Recent retrospective data from China showed that 36% of 214 SARS-CoV-2 infected patients had neurological manifestations, including acute cerebrovascular disease and impaired consciousness [14]; in addition, a first case of encephalitis with SARS-CoV-2 RNA detection in cerebrospinal fluid (CSF) was reported [15]. In this case report we describe an atypical clinical presentation of COVID-19, started as Guillain-Barré Syndrome (GBS) and without typical respiratory symptoms of SARS-Cov-2 disease.
Case Report
A 62-years- old male patient was admitted in our Internal Medicine Unit, complaining for some days of acute progressive symmetric weakness started in distal lower extremities and progressed to proximal limbs. Neurological manifestations were associated with pain, paraesthesias, peripheral oedema, severe fatigue and serious functional limitation in the movements. The patient denied fever, cough, respiratory symptoms or diarrhea and his past medical history was unremarkable. Previous corticosteroid treatment was already started few days before.
At admission, the patient had not fever nor dyspnea and was conscious; blood pressure was 120/75 mmHg, heart rate 110 beats/ minute and oxygen saturation 98% on air; clinical examination was normal except for asymmetric weakness in all limbs, presenting 1/5 value of Medical Research Council scale at lower extremities and 2/5 value at upper extremities, without cranial nerves involvement.
No abnormalities were found in chest-X-ray, transthoracic echocardiogram and abdominal ultrasonography; electrocardiogram showed sinus tachycardia (105 beats/minute). The patient underwent cervical and brain magnetic resonance imaging that revealed normal finding except for enhancement of the nerve roots.
Abnormal laboratory tests were found as following: high serum levels of C-reactive protein (447 mg/l), erythrocyte sedimentation rate (92 mm/hour), ferritin (1857 ng/ml), procalcitonin (8,7 ng/ ml), lactate dehydrogenase (574 IU/l), D-dimer (935 ng/ml), glucose (211 mg/dl), fibrinogen (1013 mg/dl), myoglobin (702 ng/ ml) and Troponin I-hs (72 ng/l); severe hypoalbuminemia (1.57 g/dl), mild normocytic nomochromic anemia, thrombocytopenia (69000/μl) and marked lymphocytopenia (260/ μl) with normal white cells count (9200/μl) were also observed. No abnormalities were found in peripheral smear except poor platelets, aPTT and PT/ INR values were in normal range and blood gas analysis revealed respiratory alkalosis with high lactates (3.3 mmol/l) and normal oxygen saturation.
Non-organ specific auto-antibodies (ANA, AMA, ENA, ds- DNA, ANCA) resulted negative as well as anti-HIV test and antiviral antibodies against Epstein-Barr virus, Cytomegalovirus, Herpesvirus, Togavirus, and hepatitis C and B markers; both urine and blood cultures were negative. CFS analysis by lumbar puncture revealed normal cells count and lack of albumin-cytological dissociation.
Given that GBS was suspected, the patient started therapy based on intravenous immunoglobulin (IGIV 0.4 g/kg for a planned 5-day course), steroid therapy (Methylpredisolone 1mg/kg) and subcutaneous Enoxaparin (6000 IU daily).
Considering the laboratory abnormalities and the COVID-19 outbreak we decided to search SARS-Cov-2 by subjecting the patient to nasopharyngeal swab which resulted positive on RT-PCR assay. The patient was transferred to Infectious Deseases Unit where he continued IGIV therapy and began treatment with tocilizumab, hydroxychloroquine and plasmapheresis. The patient currently continues hospitalization in this clinical setting.
Discussion
In this study, we report a case of atypical infection of SARSCoV- 2 initially occurred as acute GBS. GBS is immune-mediated demyelinating disease of the peripheral nerves and nerve roots (polyradiculoneuropathy) that is usually triggered by various infections. At the moment six pathogens have been associated with GBS in case-control studies: Campylobacter Jejuni, Cytomegalovirus, Hepatitis E virus, Mycoplasma Pneumoniae, Epstein-Barr virus and Zika virus. Although the clinical presentation of the disease is heterogeneous, the classic manifestations of GBS are progressive, ascending and symmetrical flaccid paralysis of limbs, along with areflexia or hyporeflexia and with or without cranial nerve involvement. Pain is frequently reported and can be muscular, radicular or neuropathic [16].
Disease onset is acute or subacute and can progress over days to a few weeks. Diagnosis of GBS is based on the patient history and neurological, electrophysiological and CSF examinations. The classic finding in GBS is the combination in the CSF of elevated protein levels and normal cell count, known as albumin-cytological dissociation. However, protein levels are normal in 30-50% of patients in the first week after disease onset and normal CSF protein levels do not rule out a diagnosis of GBS [16, 17].
Emerging evidence indicates that SARS-CoV-2 infection may cause neurological complications and some cases of GBS associated with SARS-CoV-2 infection have been recently observed in Italy, China and in other countries; in these cases the interval of 5 to 10 days observed between the onset of viral illness and the first symptoms of GBS resulted similar to the interval observed in GBS cases that occur during or after other infections. In one case, fever and respiratory symptoms developed 7 days after the onset of GBS symptoms so that parainfectious profile pattern of GBS, instead of classic post-infectious profile, was suggested [18-21]. In our case the patient never showed respiratory symptoms nor fever; laboratory abnormalities, in particular high inflammatory parameters, lymphocytopenia and thrombocytopenia, suggested an infectious disease such as SARS-CoV-2.
The link between viral infection and neurological manifestations is not yet clear; neurotropic and neuroinvasive capabilities of other coronaviruses such as SARS-CoV and MERS-CoV were described in humans and the neurological manifestations included encephalitis, polyneuropathy and GBS [22, 23]. The SARS-Cov-2 impact on the nervous system could be through direct infection or via secondary effects relating to intense systemic inflammatory response linked to viral infection [2, 11]. Recent report of GBS associated with SARSCoV- 2 raises concern for this virus to be a possible trigger [19]. It may be hypothesized that an aberrant immune response to the infection determines a serious inflammatory damage to peripheral nerves with molecular mimicry reaction, although the pathogenesis in not fully understood [16, 24].
We speculate that SARS-CoV-2 infection may be responsible for GBS development in this patient; we think SARS-Cov-2 may stimulate inflammatory cells causing massive release of pro-inflammatory mediators and cytokines, triggering immune-mediated neuropathy. Among various hypotheses it cannot be excluded that SARS-CoV-2 may generate auto-antibodies against specific gangliosides.
Conclusion
Apart asymptomatic patients, awareness of atypical clinical presentation of SARS-Cov-2 infection is remarkable and essential to avoid its contagious spread, particularly on hospital admission. This clinical case suggests the need to also consider potential neurological manifestations of COVID-19 and physicians should consider the potential link between GBS and SARS-CoV-2 infection. Therefore, during this epidemic era of COVID-19, to ensure SARSCoV- 2 infection is never overlooked, clinical symptoms of GBS should be considered in COVID-19 differential diagnosis to avoid delayed diagnosis or misdiagnosis
To read more about this article: https://irispublishers.com/ctcms/fulltext/atypical-clinical-presentation-of-covid-19.ID.000526.php
Indexing List of Iris Publishers: https://medium.com/@irispublishers/what-is-the-indexing-list-of-iris-publishers-4ace353e4eee
Iris publishers google scholar citations: https://scholar.google.co.in/scholar?hl=en&as_sdt=0%2C5&q=irispublishers&btnG=
#Current Trends Medical Science#Current Trends Clinical Science#Medical Science Research Journals#Open access journal of Current Trends in Clinical & Medical Sciences
1 note
·
View note
Text
» KELLE PAÇA
Prof. Dr. Canan Karatay' ın yazısı:
Bağışıklık sistemimizi güçlendirmek yani antikorların yapımını hızlandırmak için, beyaz kan hücrelerimizi çoğaltmak ve güçlü kılmak açısından, olmazsa olmaz ana besin maddelerinden başında doğal proteinler gelmektedir.
Gerek bütün beyaz kan hücrelerimiz yani lökositlerimiz, gerek kırmız�� kan hücrelerimiz yani eritrositlerimiz ve immünite sağlayan tüm kan hücrelerimiz, enzim ve hormonlarımız protein ve peptidlerden oluşmaktadırlar.
Dünyamızın üzerinde proteinlerin yapılabilmesi, oluşması için, temel olan 20 TÜR ana amino asid bulunmaktadır. 20 tür aminoasidin bir çoğu birbirine benzerlik gösterir, birbirlerine benzeyen aminoasidler, özelliklerine göre, aile gibi bir grup içinde bulunurlar.
Pozitif yüklü olanlar, negatif yüklü olanlar, büyük olanlar, küçük olanlar gibi çeşitli aminoasitler arasında farklılıklar vardır. Farklı olan aminoasidler, aynı LEGO taşları gibi, değişik ve bir çok şekilde birleşerek, organizmada değişik ve çok özel fonksiyonları olan peptidleri ve proteinleri meydana getirirler.
20 tip aminoasidin bu şekilde birleşme ve bir araya gelmeleri sonucu olarak, insan organizmasında bulunan ortalama 6 milyon düzeyinde değişik tip peptid ve protein oluşur.
Doğal proteinlerin kaynağının başında SÜPER BESİNLER olarak adlandırdığımız serbest GEZEN TAVUK YUMURTASI ya da organik
yumurta ve tabii ki KELLE-PAÇA ve KEMİK SUYU gelmektedir.
KELLE-PAÇA ve KEMİK SUYU, yoğun kollajen, yani peptid ve proteinlerin ana maddesi olan temel aminoasitleri içermektedir.
SARS-CoV-2, COVİD-19 virüs grip infeksiyonuna karşı organizmayı dayanıklı ve güçlü kılan, güçlü antikorların yapımını çoğaltarak, BAĞIŞIKLIK VE HÜCRESEL BAĞIŞIKLIK sistemlerini, dayanma ve savaşma yeteneğini artıran işte bu gerçek besinlerdir.
Super besinler, temel 20 aminoasidi, peptidleri ve proteinleri vücudumuza sağlamakta ve bağışıklık sistemimizi güçlendirmektedir.
SARS-CoV-2, COVİD-19 virüsüne karşı etkili ve yeterli düzeyde antikorların oluşabilmesi için sağlıklı doğal proteinlerin organizmaya girmesi ya da organizmaya sağlanması, arz edilmesi gerekmektedir.
KELLE-PAÇA ve KEMİK SUYU yoğun oranda kollajen içerir.
Kollajen vücutta yapı taşı olan önemli temel bir proteindir.
Bir çok bilimsel çalışmada BAĞIŞIKLIK SİSTEMİNİ ve HÜCRESEL İMMÜNİTEYİ sağladığı ve güçlendirdiği gösterilmiştir 1 2 3 .
KELLE-PAÇA ve KEMİK SUYU, en önemli ve temel bir protein olan, doğal kollajen içerir.
Kollajen insan ve hayvan vücudunda en yüksek oranda (% 30-40) bulunan temel bir proteindir.
Özellikle cildimizde bulunan kollajen oranı en yüksek oranda (%80) olarak bildirilmiştir 4 .
Kollajen, başta tüm kan hücrelerimiz olmak üzere, barsak hücrelerimiz, akciğer hücrelerimiz, kalp ve damar hücrelerimiz, gözümüz kulağımız, ve tüm sinir sistemi, kemik ve kıkırdak hücrelerimiz 5 , kaslarımız dahil tüm vücut hücrelerimiz için elzem olan temel aminoasid ve ana protein-peptid kaynağıdır 6 7 8 .
KELLE-PAÇA ve KEMİK SUYU (bone broth) aynı zamanda, temel peptid-protein kaynağı olmalarının yayında, doğal hayvansal yağlar, doğal mineral ve vitaminleri de içeren SÜPER BESİN kaynaklarıdır.
Kış aylarında korkmadan usanmadan rahatlıkla tüketilebilir.
Yüksek kolesterol fobisi gelişmiş olanlar da rahatlıkla tüketmelidirler, çünkü yediğimiz hiç bir hayvansal kaynaklı gıdanın KAN KOLESTEROLÜNÜ yükseltmediği bir çok bağımsız bilimsel çalışma sonucu kanıtlanmıştır.
Kış aylarında hayvansal yağ kaynağı olarak KELLE- PAÇA ve KEMİK SUYU tüketmenin önemli bir faydası daha vardır.
KELLE-PAÇA ve KEMİK SUYU tüketildiği zaman, BAĞIŞIKLIK VE HÜCRESEL İMMÜNİTEYİ sağlayan yağda eriyen 4 vitamin de aynı zamanda doğal olarak organizmaya girmektedir.
BAĞIŞIKLIK SİSTEMİMİ ve HÜCRESEL İMMÜNİTEMİZİN güçlenmesi ve kuvvetlenmesi için elzem olan bu vitaminler, A vitamini, D vitamini, E vitamini ve K vitaminleridir.
KELLE-PAÇA ve KEMİK SUYU tüketince kolesterol yükselmez mi?
Kolesterolünüz yüksek ise, SARS-CoV-2, COVİD-19 infeksiyonundan korkmamalısınız.
BAĞIŞIKLIK SİSTEMİMİ ve HÜCRESEL İMMÜNİTEMİZİN anahtarı olan antikorların güçlü, yeterli ve etkili olabilmesinde hücre zarlarının temel direklerini oluşturan kolesterolün rolü son derece önemlidir 9 10 11 12 .
Kan kolesterolü düşük olan SARS-CoV-2, COVİD-19 hastalarının, SARS-CoV-2, COVİD-19 grip infeksyonunu ve sitokin fırtınasını ve sepsis komplikasyonlarını çok ağır geçirdikleri gösterilmiştir 13 14 15 16 17 .
Uzun lafın kısası yüksek kolesterol her türlü infeksiyonlara karşı koruyucudur.
Düşük kolesterol düzeylerinin, her türlü infeksiyon hastalığı için büyük risk oluşturduğu bildirilmiştir.
STATİN denilen, kolesterol düşürücü ilaçların, SARS-CoV-2, COVİD-19 virüsünün hücrelerin içine girmesini sağlayan akciğerlerde bulunan ACE2 reseptörlerinin sayısını artırdığı bilinmektedir 18 19 .
Doğal ve yoğun kollajen 20 21 22 içeren Kelle-Paça ve kemik suyu, Anadolumuzda asırlardan beri, sıhhatli dinç bir vücut için
tüketilmekte olan kadim bir enerji ve şifa kaynağıdır.
Uzun süre tok tutan, mide ve barsak rahatsızlıklarını ve kabızlığı önleyen ana bir besindir.
Açıkladığım tüm nedenlerden dolayı, senelerden beri eski dünya ve yeni dünya halkları tarafından da yaygın olarak kullanılmaktadır.
Doğal ve yoğun kollajen kaynağı olan KELLE PAÇA 23 24 25 , Japonya’da KELLE PAÇA, olarak içilmektedir.
Aynı zamanda doğal ve yoğun kollajen kaynağı oldukları için, İngilterede, sığır kuyruğu çorbası (OXtail soup) ABD’de tavuk suyu çorbası (chiken soup), ve KEMİK SUYU (bone broth) olarak senelerden beri içilmekte , yemeklere katılmaktadır.
Özellikle kış aylarında nezle grip olan , ağır yemek tüketemeyen ateşli hastalara şifa sağlama amacıyla sık sık içirilmektedir.
Kaynaklar:
1 Thomas A H, et al. Collgen fragments modulate innate immunity. April 2007. Experimenttal Biology
and Medicine 232(3): 406-11.
2 Postelthwaite AE, Kang AH. Collagen- and collagen peptide-induced chemotaxis of human blood
monocytes. J Exp Med 143:1299–1307, 1976.
3 Fields GB. A model for interstitial collagen catabolism by mammalian collagenases. J Theor Biol
153:585–602, 1991.
4 Hall, DA (ed) (1964) Internetional Review of Connective Tissue Reseaech, Vol2, F. Verzar, Aging of
the Collagen Fiber, Academic Press, New York, p. 244.
5 2. Elsaid KA, et al. Detection of collagen type II and proteoglycans in the synovial fluids of patients
diagnosed with noninfectious knee joint synovitis indicates early damage to the articular cartilage
matrix. Osteoarthr Cartil 11:673–680, 2003.
6 https://www.ncbi.nlm.nih.gov/books/
7 https://onlinelibrary.wiley.com/
8 https://www.healthline.com/
9 Chien JY, et al.. Low serum level of high-density lipoprotein cholesterol is a poor prognostic factor
for severe sepsis. Crit Care Med. 2005; 33:1688–1693.
10 Gruber M, et al.. Prognostic impact of plasma lipids in patients with lower respiratory tract infections
- an observational study. Swiss medical weekly. 2009;139:166–172
11 Lekkou A, et al. Serum lipid profile, cytokine production, and clinical outcome in patients with
severe sepsis. J Crit Care. 2014; 29:723–727.
12 Chien YF, et al.. Decreased serum level of lipoprotein cholesterol is a poor prognostic factor for
patients with severe community-acquired pneumonia that required intensive care unit admission. J Crit
Care. 2015;30:506–510
13 Kenneth R F, et al. Lipid and Lipoprotein Levels in Patients With Covid 19 Infections.
Endotext(ınternet). South Dartmouth (MA): MDText.com, Inc.;2000. Nov 2020.
14 Cirstea M, et al.. Decreased high-density lipoprotein cholesterol level is an early prognostic marker
for organ dysfunction and death in patients with suspected sepsis. J Crit Care. 2017; 38:289–294
15 Hu X, et al. Declined serum high density lipoprotein cholesterol is associated with the severity of
COVID-19 infection. Clin Chim Acta. 2020;510:105–110
16 Wei X, et al. Hypolipidemia is associated with the severity of COVID-19. J Clin Lipidol. 2020;
14:297–304.
17 Fan J, et al. Letter to the Editor: Low-density lipoprotein is a potential predictor of poor prognosis in
patients with coronavirus disease 2019. Metabolism 2020; 107:154243.
18 Yong-Hong Li, et al. Effects of rosuvastatin on expression of angiotensin-converting enzyme 2
(ACE2), vascular ballon injury in rats. J Geriatr Cardiol. 2013 Jun; 10(2): 151-158.
19 Bourgonje AR, et al. Angiotensin-converting enzyme 2 (ACE2), SARS-COV-2 and pathophysiology
of coronavirus disease 2019 (COVID-19).J Pathol. 2020 Jul; 251(3):228-248.
20 https://www.karger.com/
21 http://www.scielo.br/
22 https://www.ncbi.nlm.nih.gov/
23 https://foodinsight.org/
24 https://www.medicalnewstoday.com/
25 https://www.livescience.com/collagen.html
3 notes
·
View notes
Text
T Lymphocytes and Cytokines: Earlier time to peak indicates better prognosis in COVID-19 patients?
author: Siyuan Gao1#, Junyi Li1#, Yan Zhang2#, Huiming Liu3, Xiaodong Yang1, Ting Jia1, Fangrong Zhang1, Xiuling Zhang1 and Jianpeng Gao1*
Aims: To explore the changes of cytokines and T lymphocyte subgroups in COVID-19 patients, to find the turning point to severe clinical course of patients, to timely intervene, and to reduce the severity.
Methods: Thirty-nine patients with COVID-19 admitted to Third People’s Hospital of Kunming from January 31, 2020 to March 2, 2020 were divided into 3 groups: mild, common and severe/critical according to ‘Clinical Protocol for COVID-19, 8th Edition’ (National Health Commission of People’s Republic of China). The changes of serum T lymphocyte subgroups and cytokines level during hospitalization were monitored and statistically analyzed.The difference was considered statistically significant when p< 0.05.
Results: 39 patients with COVID-19 were 41.51±19.03 years old, 18 of them were male (46.2%).8 patients (20.5%) were classified as mild group, 10 patients (25.6%) were classified as common group, 21 patients (53.8%) were classified as severe/critical group. The hospitalization days were 17.51±6.19 days, and the nucleic acid seroconversion days from positive to negative were 11.41±6.77 days. Comparison of T lymphocyte subgroups in patients with different clinical classifications showed that T cell count and CD4/CD8+T cell counts were different among different clinical classifications (p< 0.05). In pairwise comparison, the count of lymphocyte subgroups in severe/critical group patients was significantly lower than that in mild and common groups. Intra-group Comparison showed that, IL-2, IL-8, IL-12p70 and IL-5 examinations had significant statistical differences at different examination time points, but the effects of the above indicators on clinical classification would not change with time. According to develop trends, lymphocytes increased in patients of three clinical groups after treatment, the increase trends were obvious especially in severe/critical group patients.The OR value calculated by Logistics model showed that the probability of severe/critical disease was 1.276 times higher for each day increase of T cell time to peak (95%CI: 1.039-1.569), χ2= 5.379, p= 0.02.The probability of severe/critical disease was 1.408 times (95%CI: 1.066-1.859) with each day increase of IL1β time to peak, χ2 = 5.824, p= 0.016. Independent sample t test showed that in CD4+T cells, IL-1β, IL-2, IL-8, IL-12p70, IL-17A, IFN-γ, TNF-α and other detection indicators, the time to peak of the above indicators in the fast seroconversion group was significantly shorter than that in slow seroconversion group.
Conclusions: The early peak, timely decrease of cytokines and T cell counts in COVID-19 patients has a positive effect on prognosis. Cytokines and T cell counts should be closely monitored in COVID-19 patients.
https://www.peertechzpublications.com/articles/JAMTS-7-150.php

0 notes
Text
A Review on Diabetic Nephropathy: New Insight into Established Therapeutic Approach

Abstract
Background: Diabetic nephropathy (DN) is a principle cause of morbidity and mortality in both type 1 and type 2 diabetes mellitus. DN plays a major role in development of cardiovascular disease, in particular heart failure, the incidence of which is about 15-fold greater in patient with diabetic nephropathy. Approximately 30-35% of patients with type 1 type 2 diabetes develops diabetic nephropathy. DN is represented by microalbuminuria and macroalbuminuria and morphological changes as like glomerular thickening, interstitial fibrosis, formation of nodular glomerulosclerosis and decreased endothelial cell fenestration. Additionally, the association of renin-angiotensin-aldosterone system, wnt signaling pathway and genetic factors are the major pathway in the progression of diabetic nephropathy.
Conclusion: This review is intended to establish a new insight into traditional therapeutic approach for diabetic nephropathy. Along with potential targets, novel approach such as epigenetic drugs and miRNA modulators may compliment the current therapeutic approach to improve renal function.
Keywords: Diabetic nephropathy; Microalbuminurea; Macroalbuminuria; Glomerulosclerosis
Introduction
Diabetic nephropathy is associated with increased albumin excretion, decreased glomerular filtration rate, glomerular lesion and increased arterial blood pressure [1]. DN can be divided into 5 stages of kidney dilapidation, and symptoms appear in stage 4. All patient should be screened for albuminuria at least once per year for kidney complication. The significant signs of step 4 are swelling of ankles, legs and hands because of water retention, hematuria, fatigue and nausea. If this condition remains untreated may lead stage 5, end-stage renal disease (ESRD) [2]. In stage 5, the kidney can no longer function to meet the daily requirement and microalbuminuria (>300mg/24h), progress to extensive proteinuria (>500mg in 24 h). Various factors linked with end-stage renal diseases are hemodynamic changes, inflammation and hyperglycemia [3]. The mechanism involved in the progression of DN is still on the question. Many researchers have determined an interrelationship between the degree of hyperglycemia and progression of DN complications [4]. As because a number of pathways involved in diabetic nephropathy, treatment should be multi-targeted, encouraging a healthy lifestyle and molecular targets associated in progression of DN. Available treatment procures only symptomatic alleviation and incapable of treating the underlying pathophysiology of diabetic nephropathy.
Pathogenesis of Diabetic Nephropathy
Role cytokines in diabetic nephropathy
Studies suggested that patient suffering from diabetic nephropathy have increased serum and urine level of tumor necrosis (TNF)-alpha [5]. It had been reported that TNF-alpha, IL-6, IL-1 associated in the progression of DN, found to be involved in the impairment of interglomerular hemodynamic [6].
Genetic association in diabetic nephropathy
Angiotensin-converting enzymes (ACE)
The dysfunctional ACE gene produce excess amount of aldosterone which causes fibrosis of blood vessels and aldosterone is also found to be associated with formation of extracellular matrix and fibronectin by mesangial cells by activation of the smad2-dependent TGFB1 pathway [7].
Oxidative stress in diabetic nephropathy
Oxidant species produced by oxygen metabolism and are required in different biological operation such as cell signalling, degenerative disease, aging etc [8]. Various pathophysiological mechanisms involved in DN pathogenesis in which increased oxidant species have been recognized as the single underlying strenuous event therefore, elevated oxidant species accommodates a decisive central and significant role in the pathogenesis of diabetic nephropathy. In vitro and in vivo experimental models of diabetes have determined that metabolic (hyperglycemia, dyslipidaemia) and hemodynamic (systemic and glomerular hypertension) insults define the two principal drivers of oxidative stress in the diabetic kidney [9]. Overexpression of glucose transport because of metabolic- hemodynamic interaction, synergistically fuels an increase in oxidant species production and development of DN and other diabetic microvascular diseases. Oxidant species causes the damage in all the layers of the glomerular filtration barrier, functional alterations of the interaction between glomerular endothelial cells with glycocalyx layer and podocyte [10].
Conventional Drugs for Diabetic Nephropathy
Glucose lowering agent in diabetic nephropathy
Sodium-glucose cotransporter 2 (SGLT2) inhibitors have been used for reducing hyperglycemia because SGLT2 is responsible for reabsorbing of the glucose in the glomerular infiltrate. Empagliflozin, an SGLT2 inhibitor, slower the progression of kidney diseases [11]. Dipeptidyl peptidase -4(DPP-4) inhibitors such as linagliptin and saxagliptin (SAVOR-TIMI 53 trial) known to reduce the amount of albuminuria [12].
Cyclooxygenase (COX) and Xanthine oxidase (XO) inhibitor in diabetic nephropathy
Aspirin as a non-specific and others specific COX-2 inhibitors improve glomerular lesion, in pre-clinical models of diabetes [13]. Purine xanthine oxidase (XO) inhibitor reduce inflammation and oxidative stress in diabetic nephropathy [14].
Hydroxy-3-methylglutaryl coenzyme A reductase inhibitors and diabetic nephropathy
It was reported that statins amend renal dysfunction and reduce renal injury by inhibition of isoprenylation of Ras and Rho GTPases. Which may lead to decreased monocyte/macrophage infiltration and activating protein-1 (AP-1) in the glomerulus, adhesion of molecules, decreased mesangial proliferation and decreased accumulation of extracellular matrix and fibrosis [15].
Endothelin receptor antagonist in diabetic nephropathy
Avosentan, an endothelin-1 receptor A antagonist, found to reduce albuminuria. A study conducted on randomized controlled trial on 56 patients treated with oral bosentan for 4 weeks improves peripheral endothelial function [16].
Antioxidants against diabetic nephropathy
Pyridoxamine can remove free radicals and carbonyl product, and block the synthesis of AGEs. Pyridoxamine phase II trials showed the normal renal function had lower average serum creatinine level. Currently PIONEER -CSG -17 trial investigating to prove such benefit about use of pyridoxamine [17]. It has been reported that teneligliptin is a DPP-4 inhibitor with antioxidant.
MicroRNA and diabetic nephropathy
Under hyperglycemia conditions, up regulated micrRNAs result in pathogenesis of diabetic nephropathy [18]. It was suggested, miR-192 & miR-200 contribute to stimulate of TGFbeta 1 and fibrosis, which may consequently cause renal damage [19]. Therefore, miRNA may inhibit diabetic nephropathy by regulating various biological processes. Application of kidney protective miRNAs and knockout of inducing miRNA could be some of the approaches to restoring renal function in diabetic nephropathy [20].
Future Prospect of Drugs for Diabetic Nephropathy
Recent studies are gathering the evidence about involvement of autophagy with DN because of its cryoprotective activity in the kidney [21]. mTOR may suppress autophagy. mTORC1 inhibitors such as rapamycin or sirolimus have been found to be effective as renoprotective agents except for the negative effect on renal function and proteinuria [22].
Update on Recent Clinical Trials
Due to the distinct and complicated pathogenic mechanism associated with DN the failure rate of potential new drugs in clinical trials above 90% with only a fistful of these therapies achieving phase III trials. Summarizing the outcome of recently completed clinical trials in the past 5 years (2013-2018) and shown in Table 1 [23].
Conclusion
Diabetic nephropathy remains one of the most prevalent and life-threatening complications of diabetes. Diabetic nephropathy cases increasing rapidly around the world. Recently available therapies provide only symptomatic relief and not capable to treat underlying pathophysiology of diabetic nephropathy. This review has discussed the many factors and pathophysiological mechanisms associated with the progression of diabetic nephropathy, targets and therapeutic approaches to reduce renal impairment and improve kidney function. It also provided with new insights into the treatment of diabetic nephropathy. Novel biomarkers holding strong potential requires further clinical studies. The review also focused on the future prospect of drug for the treatment of diabetic nephropathy and update of recent clinical trials of targets for the treatment of diabetic nephropathy. A combination of therapies with epigenetic drugs and miRNAs modulators may fulfil the current treatment strategy of diabetic nephropathy.
To Know More About Nutrition and Food Science International Journal
Please click on: https://juniperpublishers.com/nfsij/index.php
For more Open Access Journals in Juniper Publishers
please click on: https://juniperpublishers.com/index.php
#Food science#diabetes nutrition#food biotechnology#food toxicology#Mass spectrometry in food technology#Juniper Publishers#open access journals
0 notes
Text
Analysis of Inflammatory Cytokines in Pre-Diabetic Subjects

Authored by Saket Gupta
Abstract
Objective: Pre-diabetes is defined as either impaired fasting glucose ((IFG) between 5.6 and 6.9mmol/l) or impaired glucose tolerance (IGT) wherein fasting or post-prandial plasma glucose levels are above normal levels, but below that of clinical Type-2-Diabetes Mellitus (T2DM). Both IFG and IGT, risk factors for T2DM and macrovascular diseases, have previously been linked with inflammation. With this in mind, we sought to comparatively evaluate the levels of inflammatory markers in pre-diabetics relative to normal healthy individuals.
Methods: We determined the levels of serum cytokines in a cohort of 9 patients with pre-diabetes and thirty four individuals with normal glucose control using a 96-Well Multi-array 7-plex assay or 1-plex IFN-β and Rantes 96-well plates (Meso Scale Discovery, Gaithersburg, Maryland, USA).
Results: Our study demonstrated that the patient group with pre-diabetes exhibited a non-significant trend towards elevated IL-6, TNFα, IFN-β, IL-12, Rantes, IL-10 and IL-8 when compared with the healthy controls group. After adjustment for age, sex, BMI and WHR, in the study population, there was no difference in the levels of cytokines between the pre-diabetes and normal groups.
Conclusion: Measurement of serum cytokine levels alone is unlikely to be a predictor of clinical disease activity in individuals with pre-diabetes.
Keywords: Pre-diabetes; Inflammation; Pro-inflammatory cytokine
Introduction
Pre-diabetes may be defined as either an impaired fasting glucose (IFG) or impaired glucose tolerance (IGT) wherein fasting or post-prandial plasma glucose levels are above normal levels, but below that of clinical Type-2-Diabetes Mellitus (T2DM) [1]. Elevated haemoglobin A1c, or glycosylated haemoglobin (HbA1c) of 5.7% to 6.4%, as per American Diabetes Association is also denoted as an indicator of pre-diabetes [2]. Together with obesity, both IFG and IGT are risk factors for T2DM and macrovascular diseases. Given this, it is arguable whether screening for pre-diabetes should be undertaken on the premise that the health benefits outweigh the cost implications.
Studies have reported that inflammatory cytokines (IL-1β, IL-6) and C-reactive protein are elevated in patients with T2DM [3,4]. Following on from this, several studies have investigated and reported that pro-inflammatory cytokines are elevated in patients with IGT [5,6] and predicted the progression to T2DM [4]. However, there is paucity of data exploring whether perturbations occur in the level of other cytokines, such as IL-12 and IFN-β and chemokines in the context of pre-diabetes. With this in mind, we analysed a cohort of 43 healthy Irish subjects to evaluate the relationship between pre-diabetes and circulating cytokine levels.
Materials and Methods
Study subjects and design
Thirty four healthy control participants with normal glucose control (NGT) were recruited to the study, as described in our previous study [7]. The healthy control participants were screened for diabetes using the standard oral glucose tolerance test (American Diabetes Association (ADA)) and individuals with abnormal blood results (diabetes or pre-diabetes) and individuals with a history of chronic illness and/or individuals taking medication were excluded from the NGT group. Nine prediabetes participants were recruited to the study. Of the nine pre-diabetes subjects, eight subjects had IFG and one subject had IGT (Table 1) and were diagnosed as having pre-diabetes based on the ADA criteria following an OGTT test (Table 2 [8]). Participants with pre-diabetes were healthy volunteers and were not on any medications. All participants were >18 years old and were not pregnant. Exclusion criteria included the following: Evidence of current infection (white cell count >11 or significantly elevated CRP), current treatment with antibiotics, neutropenia (a leucocyte count of less than 2000 per cubic millimetre), pregnancy or breast-feeding, non diabetic renal disease or liver disease (a level of asparate aminotransferase or alanine aminotransferase of more than three times the upper limit of the normal range), ongoing or previous cancer and the use of oral/inhaled glucocorticoids, immunosuppressive treatment or immunodeficiency. Participants with abnormal results were excluded from the study. Personal and medical data were obtained by patient interview, by using hospital medical notes, and from using hospital blood test results. The demographic information was obtained from research participants and their weight, height, blood pressure & waist and hip circumferences were determined. BMI was calculated as body weight (in kilograms) divided by body height (in meters) squared. Waist-to-hip ratio (WHR) was calculated as waist divided by hip circumference. Informed consent was obtained from all participants and the protocol was approved by the Midlands Research Ethics Committee, Health Service Executive, Ireland, and by the Ethical Review Board, Maynooth University, Maynooth, Ireland.
Biochemical analysis
Following the collection of 40ml of peripheral blood from consenting fasting study participants between 8 and 10am (to minimise the impact of diurnal variation), various biochemical parameters and cytokines were measured. HbA1C was measured using a haemoglobin analyser HA-8160 (Menarini Pharmaceuticals, Ireland). Whole blood counts were measured using an Advia analyser. Lipid profile, urea, creatinine, sodium, potassium, asparate aminotransferase, alanine aminotransferase, bilirubin, alkaline phosphatase, gamma glutamyl transferase, CRP, ferritin, coagulation screen, thyroid profile and plasma glucose levels were measured using a Roche Modular 1800 analyser. An early morning urine sample was also collected for the measurement of ACR.
Cytokine measurements
For cytokine analysis, the blood sample was placed on ice and then centrifuged at 3000rpm for 5min at 4 oC within 1 hour of venesection. Serum was collected and stored at -80 °C until required. Levels of serum TNFα, IL-6, IL-1β, IL-10, IL-8, IL-12p70 and IFN-γ were determined using a 96-Well Multiarray 7-Multiplex Assay (Meso Scale Discovery, Gaithersburg, Maryland 20877, USA). Levels of serum IFN-β and Rantes were determined using single-plex 96-well plates (Meso Scale Discovery, Gaithersburg, Maryland, USA).
Statistical analysis
Data are expressed as median (interquartile range) and represented as box-and-whiskers plots wherein the dark midline in the box represents the distribution’s median value. The top and bottom edges of the box respectively represent the 75th and 25th percentile values. The top and bottom of the vertical lines, the whisker respectively represent the upper and lower maximum value. χ2 test or Fisher exact test (as appropriate) were used to compare proportions. The Mann-Whitney test was used in case of non-normally distributed parameters to compare median between two groups. Data were transformed to log normal format and multifactorial ANOVA was applied to evaluate the effect of variables (age, sex, BMI, WHR and duration of diabetes) on different inflammatory markers. Correlations between values were examined by calculating Spearman correlation coefficients. All the statistical analysis was performed using the Prism 6.0 computer program (GraphPad, La Jolla, CA) and p values less than 0.05 were considered significant.
Results
Participant characteristics
The median age and smoking status was comparable in the pre-diabetes and the normal glucose control study groups. In the NGT group, 20.5% were smokers and 30.7% were ex-smoker, whereas in the pre-diabetes group, 22% were smokers and 28.6% were ex-smoker (Table 1). The pre-diabetes study group exhibited a significantly higher BMI when compared to the NGT control group (p<0.05). However, no significant difference was seen in the WHR and blood pressure between the two groups. The fasting glucose and HbA1c was significantly higher in the prediabetes study group when compared to the NGT study control group (p<0.05). Interestingly, levels of CRP were significantly elevated in the pre-diabetes study group when compared to the NGT study group (p<0.05). However, no significant differences in the lipid profile were observed between the two groups (Table 2).
Analysis of cytokine levels in patients with NGT and IGT
Our data shows a trend towards elevated serum levels of IL- 6, TNFα and IFN-β in the pre-diabetic study study group when compared with healthy control group (Table 3); however, the differences were not statistically significant (Figure 1A & 1B). Further, serum levels of IL-12 were marginally higher in the prediabetic patient group when compared with the normal glucose control study group (Table 3 and Figure 1C). In contrast, a trend towards lower levels of IFN-γ among the pre-diabetic study group when compared with the healthy control group was seen (Figure 1C). Levels of Rantes were marginally higher in the prediabetic study group when compared to the group with normal glucose control (Figure 2A and Table 3). Similarly, serum levels of the chemokines IL-10 and IL-8 were marginally higher in the pre-diabetes participant group when compared with healthy control group (Figure 2B & 2C). Notably, the differences in cytokine levels did not reach statistical significance.
Following multifactorial ANOVA, applied on all cytokines measurements using lognormal values and adjustment for age, sex, BMI and WHR, it was found that there were no significant differences in cytokine levels between the pre-diabetes study group and the group of individuals with normal glucose control.
Discussion
Herein, we sought to establish whether perturbations in inflammatory markers were evident among individuals with pre-diabetes and whether measurement of serum cytokine levels alone may be a predictor of clinical disease activity in individuals with pre-diabetes. To do this, thirty four healthy control participants were recruited to the study and are further described in another study that we undertook [7]. Concurrently, nine pre-diabetes participants were recruited to the study and are described herein. While the prevalence of pre-diabetes is approximately 30% in the general population (UK/USA) [9,10], it was noted that the aforementioned group were generally not captured as a study population presenting to the Endocrinology clinic, Midlands Regional Hospital, Mullingar. Therefore, in terms of the recruitment of pre-diabetes study participants at this location, while eight of the individuals were identified during the course of our efforts to recruit healthy individuals, as described in our previous study [7], one individual was identified following a GP referral. Despite our best efforts and in the context of our strict inclusion/exclusion criteria and ethical approval, it was only possible to recruit a total of nine pre-diabetes individuals to the current study. Notably, treatment-naïve pre-diabetes subjects were required for the study and therefore limited the number of individuals that could be recruited to the pre-diabetes study group. Following recruitment of the study participants, we comparatively assessed the inflammatory milieu of pre-diabetes study participants and healthy individuals. The BMI of the prediabetic study group was significantly higher than that of the healthy control group, as was the fasting glucose and HbA1c levels of the pre-diabetic group. In contrast, the lipid profile of the pre-diabetic study group was comparable to that of the healthy control group.
Regarding the pro-inflammatory cytokine levels, we found that the pre-diabetes study group tended to have elevated levels of the pro-inflammatory cytokines, IL-6, TNFα and IFN-β, when compared with the normal glucose control group. Further, while IL-12 levels was slightly higher in the pre-diabetes study group, IFN-γ levels were lower in the pre-diabetic group when compared to individuals with normal glucose control. In addition, there was a trend towards elevated levels of Rantes, IL-10 and IL-8 in the group with pre-diabetes when compared with the healthy control group. Notably, the differences in cytokine levels among the groups failed to reach statistical significance. Further, after adjustment for age, sex, BMI and WHR, there were no differences in the levels of cytokines between the pre-diabetic and normal study groups
Our findings are in contrast to that previously reported by Spranger et al. [3] who showed that levels of plasma IL-6 and TNFα, but not IL-1β, were associated with future T2DM. Furthermore, the study reported that following adjustment for age, sex, BMI, alcohol consumption, waist-to-hip ratio (WHR), smoking status and HbA1c, IL-6, but not TNFα or IL-1β, was identified as an independent predictor for T2DM [3]. Another study reported an association between elevated levels of IL-6 and CRP and the risk of developing T2DM in the future and the link remained significant following adjustment for BMI, family history of diabetes, smoking, exercise and alcohol use using multivariate analysis [4]. More recently, studies have shown that HbA1c and IL-6 levels were significantly higher in IFG and IGT patients when compared to healthy individuals [11,12].
Conclusion
In summary, our data suggests that the pre-diabetic patient group do not exhibit statistical significant differences in circulating cytokine levels when compared to the normal healthy group. Further, it is noted that multivariate analysis confirmed that there were no significant differences following adjustment for age, sex, BMI and WHR. However, though non-significant, it is noted that our study showed a trend towards elevated levels of serum IL-6, TNFα, IFN-β and IL-8 among the pre-diabetes group. It is plausible to speculate that the robustness of our study would be enhanced by the recruitment and inclusion of additional study participants to the pre-diabetes group. However, as previously indicated, the inclusion of additional pre-diabetes participants was beyond the scope of the current research due to the confined nature of our study criteria.
Importantly, our study shows that the measurement of serum cytokine levels alone in individuals with pre-diabetes may not serve as a robust readout of pre-diabetes state. In conclusion, our study shows that our pre-diabetic group present with a nonsignificant trend towards elevated levels of serum cytokines that may be linked with confounding factors such as BMI, WHR, age and gender. Based on these results, serum cytokine levels alone is unlikely to be a predictor of clinical disease in individuals with pre-diabetes.
To More articles in Current Research in Diabetes & Obesity Journal Please click on: https://juniperpublishers.com/crdoj/index.php
For more about Juniper Publishers please click on: https://juniperpublishers.com/video-articles.php
0 notes
Text
Porcine IL-7(Interleukin 7) ELISA Kit
Porcine IL-7(Interleukin 7) ELISA Kit Basic Information


Feiyue’s Porcine IL-7 is an ELISA reagent for detection of IL-7 in human serum, plasma or cell with sensitivity, specificity and consistency.
Porcine IL-7(Interleukin 7) Introduciton
Interleukin 7 (IL-7) is a protein that in humans is encoded by the IL7 gene.
IL-7 is a hematopoietic growth factor secreted by stromal cells in the bone marrow and thymus. It is also produced by keratinocytes, dendritic cells, hepatocytes, neurons, and epithelial cells, but is not produced by normal lymphocytes. A study also demonstrate how the autocrine production of the IL-7 cytokine mediated by T-cell acute lymphoblastic leukemia (T-ALL) can be involved in the oncogenic development of T-ALL and offer novel insights into T-ALL spreading.
Porcine IL-7(Interleukin 7) ELISA Kit Test method
This kit uses sandwich ELISA to detect the concentration of Porcine IL-7.
Porcine IL-7-specific monoclonal antibody has been pre-coated in the wells of the supplied microplate. Standards samples and controls are added to interact with the immobilized antibody. A sandwich complex is formed by additional anti-Porcine IL-7 antibody with HRP-Streptavidin. TMB solution is added to react with the sandwich for ming optical signal measured by microplate reader. The concentration of Porcine IL-7 in the sample can be calculated by comparing the absorbance of the sample with the standard curve.
0 notes
Text
Lupine Publishers | Immune Biomarkers for Predictability of Preeclampsia Clinical Scenarios
Lupine Publishers | Interventions in Gynecology and Women's Healthcare

Abstract
Background: Preeclampsia a chief clinical scenario in obstetric medicine, having highly morbid and grave sequalae particularly if rapidly progressive and left unmanaged. The complexity of immune system components involvement is integrated in the preeclampsia pathology as immune based pathology is one of the cornerstone theories for preeclampsia development that have raised the issue that immune dysfunction affection is an issue that could be investigated in recent research efforts to reveal any immunological biomarker that could be implemented as a predictability tool for preeclampsia development and severity.
Aim: To investigate the possible usage of immune biomarkers in prediction of preeclampsia..
Methodology: The current study was conducted at Departments of Obstetrics & Gynecology and Clinical Pathology, Tanta University Hospitals. A prospective clinical research trial that involved cases having an ongoing gestation below 16 gestational weeks of with maternal age range of 18 to 40 years old were recruited in the current research study. During the study period, a total of 200 research study subjects. Peripheral blood was collected between 5th and 16th gestational weeks calculating the gestational age based on the last menstrual period and crown-rump length using a first trimester sonographic scan cases were clinically and investigationally followed till time of delivery and clinical research data was collected and tabulated for analysis the following was conducted on collected blood samples.
Results: The comparative statistical analysis women with and without pre-eclampsia regarding Serum levels (pg/ml) of cytokines and chemokines in which there was no statistical significant difference between both research groups as regards EGF, EOTAXING-CSF,GM-CSF ,IFN ALPHA 2,IFN-GAMMA , IL-10, IL-12p40, IL-12p70, IL-13, IL-15, IL17-A, IL-1RA, IL-1 alpha, IL-1 beta, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IP-10, MIP-1 beta, TNF- alpha, TNF-beta, VEGF (p values=0.812, 0.315, 0.712, 0.635, 0.511, 0.865, 0.829, 0.873, 0.603, 0.807, 0.865, 0.512, 0.925, 0.812, 0.512,0.310,0.423 ,0.478, 0.829, 0.903 ,0.865, 0.314, 0.885, 0.489,0.958,0.288 consecutively) however there was highly statistically significantly higher levels of IL-8 among the preeclampsia research group, and statically significantly higher levels of MCP-1 among no preeclampsia research group (p value= 0.012), whereas there was statically significantly higher levels of MIP-1 alpha among preeclampsia research group (p value= 0.032).
Conclusion: The current study findings denote that there is a possible clinical value in using immune biomarkers in preeclampsia screening, however future research efforts are required to verify the current study results in order to elucidate more clearly the most useful immune cell ratios and inflammatory mediators that could be clinically applicable and useful with the highest sensitivity in order to upgrade the management protocols of preeclampsia.
Introduction
Preeclampsia a chief clinical scenario in obstetric medicine, having highly morbid and grave sequalae particularly if rapidly progressive and left unmanaged .Various theories have aroused to explain the pathophysiological origin and development of preeclampsia one of the cornerstone factors that raise the morbidity is endothelial dysfunction that raises the blood pressure levels besides causing multisystem affection causing multiple pathological affection such as HELLP syndrome [1-4].
Placental immunological tolerance to the maternal immunological system is considered one of the research debate and mystery since it is considered the safeguard against fetal immune rejection as an allograft. The complexity of immune system components involvement is integrated in the preeclampsia pathology as immune based pathology is one of the cornerstone theories for preeclampsia development that have raised the issue that immune dysfunction affection is an issue that could be investigated in recent research efforts to reveal any immunological biomarker that could be implemented as a predictability tool for preeclampsia development and severity [5-8]. Furthermore it was observed that the physiological process of placentation is affected by the maternal immune system activity and the cross talk between the maternal immune system and developing fetal antigens that interact in a manner that is not fully understood .even the process of implantation is affected by products and activity on the immune system such as leukemia inhibitory factor that raise the interest to investigate the normal and abnormal immunological adaptation in pregnancy in correlation to arousal of various disease pathology such as preeclampsia and other auto immune disease [10-12]. The cellular level and molecular level complex mechanisms that because poor trophoblastic invasion is an issue that is not fully revealed although it is highly hypothesized to be immune mediated. an ischemic placenta could in addition cause the release of inflammatory mediators that could be the triggering factor for the pathophysiological development of the preeclampsia disease causing changes in the maternal immune system cellular and molecular profile [13,14]. Prior research groups at molecular and cellular levels have hypothesized that immunological aberrations at functional performance activities of the placental microenvironment could be the chief factor affecting the presentation and prognosis of preeclampsia and the gestational age of onset [15,16]. Interestingly it was previously demonstrated that normal gestation have a clear shift toward a Th2 immuno-tolerant fashion within the peripheral circulation and at the fetal-maternal cellular and molecular cross talk furthermore in an interesting manner it was revealed and displayed by researchers that preeclampsia pathological immune behavior is characteristically featured by Th1 predominance with pro-inflammatory state and Th1/Th2 imbalance besides it was shown that the imbalance and disproportion of T helper cells activities and numbers id one of the dominate immune cellular features in preeclampsia furthermore preeclampsia is correlated to is a global pro-inflammatory systemic environment having raised levels of pro-inflammatory cytokines, chemokines and adhesion molecules within the maternal vascular system interacting in manner that results in excessive systemic inflammatory response and endothelial systemic dysfunction [17-19].
Methodology
The current study was conducted at Departments of Obstetrics & Gynecology and Clinical Pathology, Tanta University Hospitals. A prospective clinical research trial that involved cases having an ongoing gestation below 16 gestational weeks of with maternal age range of 18 to 40 years old were recruited in the current research study. During the study period, a total of 200 research study subjects. Peripheral blood was collected between 5th and 16th gestational weeks calculating the gestational age based on the last menstrual period and crown-rump length using a first trimester sonographic scan cases were clinically and investigationally followed till time of delivery and clinical research data was collected and tabulated for analysis the following was conducted on collected blood samples.
Flow Cytometry
To determine lymphocyte subcategories, peripheral blood have been collected in in test tubes having sodium heparin and stained with monoclonal antibodies against cell surface markers. Blood have been incubated using antibody cocktails for 30 min at room temperature and then diluted in wash buffer. After one centrifugation cycle, investigators used the combination of Immuno- Lyse and Fixative (Beckman Coulter) to wash red blood cells and fix white blood cells. Cells were analyzed using a using a flow cytometer with implementing immunophenotyping using monocloncal antibodies.
Intracellular cytokine analysis
After the incubation, the cells were washed and labeled with monoclonal antibodies to detect intracellular cytokines, samples were analyzed using a flow cytometer.
Luminex assay
Peripheral blood has been collected in serum separator tubes and spun within 2 hours for serum isolation, samples were frozen and stored at −80 °C until the analysis. Cytokines and chemokines, e.g. EGF, GCSF, GM-CSF, IFN-α2, IFN-γ, IL-1α, IL-1β have been assayed using a multiplex assay kit.
Research outcome measures
Clinical and laboratory research data have been collected and analyzed relying on the gestational clinical outcome, e.g. preeclampsia, preterm and gestational diabetes.
Statistical Analysis
Data were collected, revised, coded and entered to the Statistical Package for Social Science (IBM SPSS) version 23. Data were presented as mean and standard deviations for quantitative data with parametric distribution and median with Inter-Quartile Ranges (IQR) with non-parametric distribution and numbers and percentages for qualitative data. The comparison between two groups was done using independent t-test for quantitative data and Chi-square test for qualitative data and Fisher exact test was used instead of chi-square test when the expected count was found less than 5 in any cell. The comparison between two groups regarding quantitative data was done by using Independent t-test and/ or Mann-Whitney test according to distribution of data. Receiver Operating Characteristic Curve (ROC) was used to assess the best cut off point with its sensitivity, specificity, Positive Predictive Value (PPV), Negative Predictive Value (NPV) and Area Under Curve (AUC). The confidence interval was set to 95% and the margin of error accepted was set to 5%. So, the p-value was considered significant at the level of < 0.05.
Results
Table 1 reveals and displays the comparative analysis between women with and without pre-eclampsia regarding demographic data, characteristics and risk factors in which there was no statistical significant difference between preeclampsia and no preeclampsia research groups as regards age, gestational age at blood collection, BMI, gravidity, term deliveries, preterm deliveries, abortions, lived births, smoking, auto immune disease, chronic renal disease previous history of preeclampsia, multifetal gestations, pregestational DM, chronic hypertension (p values =0.731, 0.336, 0.279, 0.516, 0.627, 0.711, 0.452, 0.350, 0.568, 0.480, 0.732, 0.170, 0.337, 0.210, 0.369 consecutively).
Table 1: Comparison between women with and without pre-eclampsia regarding demographic data, characteristics and risk factors.
•: Independent t-test; ≠: Mann-Whitney test; *: Chi-square test
Table 2 reveals and displays the comparative statistical analysis between cases with and without pre-eclampsia regarding pregnancy outcomes in which preeclampsia research group had statistically significantly lower gestational age at delivery in comparison to no preeclampsia research group (p values <0.001 ) ,besides there was statically significantly higher systolic and diastolic blood pressure readings among the preeclampsia research group (p values<0.001) preterm labor was statically significantly higher among the no preeclampsia research group (p value<0.001).furthermore there was no statically significant difference as regards APGAR scoring at 1,5min,cesarean section delivery rates small for gestational age, fetal demise, gestational DM (p values= 0.314,0.521,0.061, 0.072,0.063, 0.568 consecutively).
Table 2: Comparison between cases with and without pre-eclampsia regarding pregnancy outcomes.
•: Independent t-test; ≠: Mann-Whitney test; *: Chi-square test
Table 3 reveals and displays the comparative statistical analysis between women with and without pre-eclampsia regarding immune of peripheral blood in which there was no statically significant difference as regards %CD3+ (T cells), %CD3+ CD4 (T helper cells), % CD3+CD8+ (T cytotoxic cells), % CD3+CD56+ (NKT cells), % CD3−CD56+ (NK cells), % CD3−CD56+CD16+ (Cytotoxic NK cells), %CD19+ (B cells), %CD19+CD5+ (B cells) (p values =0.234, 0.786, 0.467, 0.737, 0.308, 0.691, 0.208, 0.375 consecutively), on the other hand there was statically significantly lower %CD4+ CD25bright CD127dim/- (Treg cells) among the no preeclampsia research group in comparison to preeclampsia research group (p value<0.001).
Table 3: Comparison between women with and without pre-eclampsia regarding immune of peripheral blood.
•: Independent t-test
Table 4 reveals and displays the comparative statistical analysis women with and without pre-eclampsia regarding Serum levels (pg/ml) of cytokines and chemokines in which there was no statistical significant difference between both research groups as regards EGF, EOTAXING-CSF,GM-CSF, IFN ALPHA 2, IFN-GAMMA, IL-10, IL-12p40, IL-12p70, IL-13, IL-15, IL17-A, IL-1RA, IL-1 alpha, IL-1 beta, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IP-10, MIP-1 beta, TNF- alpha, TNF-beta, VEGF (p values=0.812, 0.315, 0.712, 0.635, 0.511, 0.865, 0.829, 0.873, 0.603, 0.807, 0.865, 0.512, 0.925, 0.812, 0.512, 0.310, 0.423, 0.478, 0.829, 0.903, 0.865, 0.314, 0.885, 0.489, 0.958, 0.288 consecutively) however there was highly statistically significantly higher levels of IL-8 among the preeclampsia research group, and statically significantly higher levels of MCP-1 among no preeclampsia research group (p value= 0.012), whereas there was statically significantly higher levels of MIP-1 alpha among preeclampsia research group (p value= 0.032).
Table 4: Comparison between women with and without pre-eclampsia regarding Serum levels (pg/ml) of cytokines and chemokines.
≠: Mann-Whitney test
Table 5 and Figures 1 & 2 reveal and display the Receiver operating characteristic curve (ROC) for the independent predictors of preeclampsia among the research study subjects in which CD3+CD4+TNFα+, TNFα/IL-10 cell ratio,CD3+CD8−IL- 17+,CD4+CD25brightCD127dim,Th17/Treg cell ratio,IL-8,MCP- 1,MCP-1a had cut off points >50.75, >49.83,>1.18,≤ 6.5, >0.287, >224, ≤260, >30 consecutively that had AUC =0.753, 0.755, 0.746, 0.703, 0.786, 0.955, 0.916, 0.76 consecutively, and statistical sensitivity= 69.57, 69.57, 82.61, 78.26, 82.61, 86.96, 91.3, 82.61 consecutively, overall indices have shown that IL-8 at cutoff value >224, AUC =0.955, SENSITIVITY = 86.96, specificity = 91.37. Positive predictive value= 54.1 Negative predictive value = 98.4 is the most effective immune biomarker for preeclampsia prediction.
Table 5: Preeclampsia among the research study subjects.
AUC: Area under curve; SE: Standard error; 95% CI: 95% Confidence interval; PPV: Positive predictive value; NPV: Negative predictive value
Figure 1 & 2: Receiver Operating Characteristic Curve (ROC) for the independent predictors of preeclampsia among the research study subjects.
Discussion
Prior research studies and efforts have observed that there is a possible linkage between immunological system cells and agents secreted and the trigger of preeclampsia pathophysiological course of development .preeclampsia as a disease of theories have raised the research interest and debate among various investigators in which they have tried to observe the pathophysiological origin and causative issues at molecular and cellular levels to observe the best immune predictability biomarker ,since early disease prediction and management would markedly improve the clinical outcomes of the disease [1,3,9,13].
A prior research study like the current 5 study in approach and methodology have revealed a displayed among its research study findings that there is a statistically significant correlations and linkage between peripheral blood immune effectors, serum levels of cytokines and chemokines in early pregnancy and preeclampsia development making the usage of the immune biomarkers a useful predictability [2,7,15].
Another research team of investigators have observed that a predominant Th1 immunological system profile is correlated to a pro-inflammatory immunological response, endothelial dysfunction and poor placentation all relevant findings in preeclampsia clinical scenarios (besides an elevated percentage of Th1 cells and Th1/Th2cell ratios in peripheral circulation was observed in preeclampsia hematological pathological changes in comparison to normal gestations interestingly in harmony and great similarity to the current study findings it was previously demonstrated that the proportion of T helper cell expression is statically significantly higher in cases having preeclampsia in comparison to normal pregnancies [4,7,18].
Raised percentages of Th17 and reduced percentages of Treg cell categories have been observed by prior investigators in peripheral circulation of preeclamptic cases. Furthermore, prior research efforts investigating the cytokine and chemokine activities have observed that raised serum levels of IL-8 and MIP- 1α and the reduced level of MCP-1 within early gestation is correlated and linked to pathological course of preeclampsia development [8,17].
Conclusions and Recommendations
The current study findings denote that there is a possible clinical value in using immune biomarkers in preeclampsia screening, however future research efforts are required to verify the current study results in order to elucidate more clearly the most useful immune cell ratios and inflammatory mediators that could be clinically applicable and useful with the highest sensitivity in order to upgrade the management protocols of preeclampsia. Future research studies should be implemented in multicentric fashion taking in consideration the possible coexisting medical diseases with preeclampsia such as gestational DM and autoimmune diseases that could have an influence on the course of the disease and clinical expressive pattern.
For more Gynecology Journals please click on below link https://lupinepublishers.com/gynecology-women-health-journal/
For more Lupine Publishers Please click on below link https://lupinepublishers.com/
0 notes
Text
The key reason why Your Epidermis Demands Magnesium

Higher intakes of nutritional dietary fibre (40-50 g/day) reduced magnesium intake. The outcomes of nutritional elements like phytate on magnesium intake are probably important only at reduced magnesium consumption. It is likely to an extra molecule or compound (i.e. magnesium glycinate, magnesium malate). For every day use, magnesium glycinate also as magnesium malate might be the two we recommend as they’re undoubtedly one of the most bioavailable.
In 2012, the total price of bad bone health in grown ups older 50 plus had been a$2.75 billion, as well as 64% of the cost was directly related to dealing with as well as handling fractures. Standard physical physical exercise of the type will help sluggish bone reduction, increase muscle mass power, and also help your equilibrium. At any time old bone is broken down quicker than new bone is created, internet bone reduction happens.
Anytime you sleep at night, your computer creates protein referred to as cytokines, which typically fight contamination as well as swelling. You is not going to find nutritional Dpresent in as numerous meals, nonetheless it nonetheless can kind out immune system general well being by reduction of swelling. Another review posted in Immunologic Study proves that "long-term anxiety can restrain safety immune responses and exacerbate pathological immune system reactions." You will discover several lifestyle as well as diet routine factors that affect your immune system response.
This review is about the Magnesium Breakthrough well-being nutritional supplement the nutritional quality that got into presence to battle severe stress. Particularly, Magnesium Breakthrough utilizes magnesium chelate, magnesium bis-glycinate, magnesium orotate, magnesium citrate, also as several other designs of magnesium.
Magnesium Breakthrough combines all 7 kinds of magnesium together with cofactors for decreasing tension as well as growing energy. Magnesium Breakthrough activly works to enhance your rest, minimize tension, develop powerful bones, keep a wholesome heart beat and also improve your defense systems. Thankfully, BiOptimizers Magnesium Breakthrough is definitely the health health supplement to minimize tension as well as deliver a number of other health benefits.
The basic body snugly regulates the amount of magnesium within your individual blood, trying to keep it in a particular variety. About 25Per cent is identified in our skeletal muscle tissue along with just 1% is situated in our blood. “You will find out magnesium facilitating the roll-away from healthy proteins, permitting our nerves along with muscle tissue execute, trying to keep our blood vessels strain degree in harmony and adjusting blood sugar levels,” Keatley states. It's planning to typically be recommended to take into account anti-biotics no considerably less than two hours before or 4 to half a dozen hrs proper proper adhering to a magnesium-that contains pc tablets.
The link among PTH and magnesium is complicated also as similar to calcium; very higher serum magnesium levels suppress the release of PTH by way of activation of calcium-sensing receptor current regarding the chief cells of one's parathyroid glands. Due to the higher prevalence of hypomagnesemia in ICU patients, it's advised to observe serum magnesium closely. A 2019 examine identified out that consuming sufficient dietary magnesium enhances the activity of supplement D inside the entire entire body. Just in case it's presumed this dietary magnesium is ingested with one half efficiency, the 26 milligram required spanning a pregnancy of 40 months (.09 milligrams/day) can probably be accommodated by adaptation. Magnesium oxide the type of magnesium nutrient health supplement that's primarily created of magnesium-it truly consists of much more magnesium than other magnesium health supplements.
Ask regarding a affiliate into a physiotherapist for safe muscle tissue-constructing workouts or suggestions out of your medical skilled regarding workouts which can be good to suit your needs. Enquire about exercises which will help you sustain healthful healthy posture, which could reduce the danger to get a spinal column bone fracture. Physical workout is vital to maintain numerous medical concerns under manage, as well as bone overall wellness is not just about any exception to this rule. The bone volume an individual attains in childhood and through the teens years will help decide their lifelong bone wellbeing. Despite the fact that bone loss typically takes place down the road when compared with females, males can however be at actually substantial chance for osteoporosis. Maximizing bone volume in the young and lessening bone loss in the course of aging can support stop or lessen osteoporosis. HSS endocrinologist Jessica Rachel Starr, MD, is truly a specialist in bone common overall health and metabolic bone disease.
Moderate exercising boosts your antibody solution to illness. Make an attempt to workout day-to-day, however mixture it switching amongst aerobic, energy, extending and meditation. Walking daily not simply delivers the chance to enter various workout, additionally it provides the chance to like a adjust of views. Consistent microbe infections in the gum line or teeth improve the body’s soreness levels, major not just in diminished immunity however a better likelihood of coronary disease and also heart stroke.
youtube
1 note
·
View note
Text
Changes in Serum New Cytokine CYTL1 in Knee Osteoarthritis
New Post has been published on https://depression-md.com/changes-in-serum-new-cytokine-cytl1-in-knee-osteoarthritis/
Changes in Serum New Cytokine CYTL1 in Knee Osteoarthritis

1Department of Orthopedics, The Second Affiliated Hospital of Zhengzhou University, Zhengzhou, 450000, Henan, People’s Republic of China; 2Department of Orthopedics, Zhengzhou Central Hospital, Zhengzhou, 450000, Henan, People’s Republic of China
Correspondence: Hong-Kai Lian Department of Orthopedics, Zhengzhou Central Hospital, No. 195, Tongbai Road Middle Section, Zhongyuan District, Zhengzhou, 450000, Henan, People’s Republic of China Tel +86 18837189223 Email [email protected]
Objective: The present study aims to investigate the clinical significance of changes in the expression of new cytokine-like 1 (CYTL1) in the serum of patients with knee osteoarthritis (KOA). Methods: A total of 182 patients with KOA, including 84 males and 98 females aged 39– 86 with an average age of 66.4 ± 9.7 and an average body mass index (BMI) of 24.9 ± 2.4 kg/m2, were enrolled in the study. The patients were divided into three subgroups: the grade II subgroup (n = 23), grade III subgroup (n = 63), and grade IV subgroup (n = 96) based on severity, as calculated by the Kellgren and Lawrence (K&L) classification system. In addition, 152 volunteers from our health center who came in for physical examination were selected as the control group, including 70 males and 82 females aged 37– 82 with an average age of 63.4 ± 9.5 and an average BMI of 24.8 ± 2.2 kg/m2. An enzyme-linked immunosorbent assay was adopted to detect the serum CYTL1 levels, and the correlation between CYTL1 and the severity of KOA was analyzed. Results: The serum level of CYTL1 was significantly lower in the KOA group than in the control group (P Conclusion: The serum levels of CYTL1 are strongly correlated with the severity of the disease in patients with KOA and could be a new therapeutic target for KOA.
Introduction
Osteoarthritis (OA) is the most common chronic joint disease and is the leading cause of mobility problems in older people. The prevalence of OA increases with age, affecting most people over the age of 65.1,2 The main clinical symptoms of OA include chronic pain, joint instability, stiffness, joint deformity, and radiographic narrowing of the joint space.3,4 Although several risk factors associated with OA have been proposed, including genetic predisposition, aging, obesity, and joint misalignment, its pathogenesis remains unclear.5,6 Currently, the standard method for identifying the severity of bone and joint injury is the Kellgren and Lawrence (K&L) classification system, which is based on measuring the width of the joint space on plain radiographs.7 The detection of early metabolic lesions in joints before developing imaging changes in patients is clinically important for identification and individualized treatment in high-risk patients. New soluble circulating markers need to be identified to meet these objectives. Cytokine-like 1 (CYTL1) is a novel autocrine regulator that is specifically and highly expressed in the chondrocytes and is involved in the formation and development of cartilage and the occurrence and progression of arthritis.8 The correlation between the serum concentration of CYTL1 and the severity of OA has been poorly investigated. In the present study, we aim to measure the serum levels of CYTL1 in healthy controls and patients with OA and analyze the correlation between the serum levels of CYTL1 and the severity of OA. We intend to provide a new means for the early identification of individuals at high risk of articular cartilage destruction and plan to adopt CYTL1 as a new therapeutic target for OA.
Methods
General Characteristics
A total of 182 patients with knee OA (KOA) who were admitted to our hospital between October 2019 and November 2020 were selected as subjects for the present study. All cases met the diagnostic criteria of the Guidelines for Osteoarthritis Treatment issued by the Chinese Medical Association Orthopedic Branch.9 The subjects consisted of 84 males and 98 females aged 39–86 with an average age of 66.2 ± 9.8 and an average body mass index (BMI) of 24.9 ± 2.4 kg/m2. The disease duration ranged from two to six years. K&L grading was conducted according to the bone and joint radiographs of the subjects. The subjects were divided into groups based on this grade: (1) grade II: obvious bony bulge but without the involvement of the joint space (n = 23); (2) grade III: moderate narrowing of the joint space (n = 63); and (3) grade IV: significant narrowing of the joint space and subchondral bone sclerosis (n = 96). The exclusion criteria were as follows: (1) patients with a history of surgery or knee trauma; (2) patients with rheumatoid arthritis, ankylosing spondylitis, tumor or tuberculosis of the knee joint, or severe osteoporosis; (3) patients with coagulation disorders or who were on anticoagulants; (4) patients with other sites of osteoarthritis; and (5) patients with serious medical diseases or psychiatric disorders. The ongoing medication of all patients with KOA was celecoxib. In addition to these subjects, during the same period, 152 age- and gender-matched healthy subjects who came for a physical examination at our hospital were selected for the control group. This group consisted of 70 males and 82 females aged 37–82 with an average age of 65.0 ± 9.5 and an average BMI of 24.8 ± 2.2 kg/m2. The control subjects had no inflammatory diseases or other sites of osteoarthritis, according to their medical records. The present study was approved by the Ethics Committee of Zhengzhou Central Hospital and conducted in accordance with the Declaration of Helsinki. Each patient signed informed consent.
Enzyme-Linked Immunosorbent Assay (ELISA) Detection
For the detection of the indicators, 5 mL of venous blood was drawn from the patients and the healthy subjects in the early morning under a fasting condition. Samples that were measured within five days were stored at 4°C, while those measured over a week were stored at −20°C. The serum was separated by centrifugation at 3000 rpm for 10 min with a centrifugation radius of 10 cm within 30 min. The serum concentration of CYTL1 was measured by the ELISA using the ELX800 enzyme marker produced by BioTek Instruments, USA. The kit was purchased from the Shanghai Enzyme Linkage Biotechnology Co., and the assay was conducted according to the instructions.
Statistical Processing
The SPSS 20.0 software was adopted for statistical analysis, and the measurement data were expressed as

. The comparison of the serum concentration of CYTL1 between patients with KOA and the control group was performed using the t-test and between the different groups of patients with KOA using the one-way ANOVA test. The correlation between the serum concentration of CYTL1 and the severity of KOA was analyzed using the Spearman correlation analysis. P < 0.05 was considered statistically significant.
Results
The Comparison of the General Characteristics Between the Two Groups
There were no statistically significant differences in age, gender composition, or BMI between the KOA group and the control group (P > 0.05). The serum level of CYTL1 in the KOA group was significantly lower than in the control group (P < 0.05; see Table 1).

Table 1 The Comparison of the General Characteristics Between the Two Groups
Comparison of the Differences in Serum Levels of CYTL1 Among the Subgroups of KOA
As shown in Table 2, the level of CYTL1 was 383.9 ± 54.8 ng/mL in patients in the grade II subgroup, 321.8 ± 46.2 ng/mL in the grade III subgroup, and 276.2 ± 45.2 ng/mL in the grade IV subgroup. The differences in the serum levels of CYTL1 between the KOA subgroups were statistically significant (F = 54.826, P < 0.001).

Table 2 The Serum Levels of CYTL1 in the Patients (

)
The correlation between the serum levels of CYTL1 and the severity of KOA (see Figure 1).

Figure 1 The negative correlation between the serum levels of CYTL1 and the KL grading in the OA group (r=−0.613, P<0.001).
Discussion
OA is a chronic degenerative joint disease characterized by the degeneration of the articular cartilage, sclerosis of the subchondral bone, formation of bone fragments, and inflammation of the synovial membrane.10 In addition, studies have revealed aggressive angiogenesis, the key roles of the pro-inflammatory secretory molecules, and the chondrocyte dedifferentiation in the pathophysiology of OA.11–13
CYTL1 is a secreted protein predicted to have the structural characteristics of the hematopoietic cytokines and interleukins and may have immunobiological functions.14 CYTL1 is known to regulate the chondrogenic differentiation of the mesenchymal cells and increase the expression of interleukin-1 during cartilage formation.15 In addition, CYTL1 appears to have a strong pro-angiogenic effect, which is comparable to the vascular endothelial growth factor A and has chemotactic activity on monocytes and macrophages via the CCR2B receptor. The expression appears to be downregulated during the progression of OA.16–18 CYTL1 was originally found to be expressed in the CD34+ bone marrow and umbilical cord blood cells in the human population. Recently, CYTL1 has been reported to be expressed by the chondrocytes, suggesting a role for CYTL1 in chondrogenesis and endochondral homeostasis.14,19,20 CYTL1 was identified as being differentially expressed in post-traumatic osteoarthritis (PTOA), suggesting that CYTL1 may be a potential target for early PTOA-specific intervention and further suggesting a role for CYTL1 in the development of OA.18 However, the expression of CYTL1 in the serum of patients remains unclear. Therefore, we investigated the difference in the expression of CYTL1 in the serum of healthy subjects and patients with KOA and between groups of patients with KOA of varying degrees of severity to identify CYTL1 as a potential target for intervention during the progression of KOA.
In the present study, it was found that the serum level of CYTL1 in the KOA group was significantly lower than in the control group, while the serum level of CYTL1 decreased significantly with an increase in the severity of KOA (P < 0.05). The Spearman correlation analysis showed that the serum level of CYTL1 was correlated with the severity of KOA (r = −0.613, P < 0.001), suggesting that CYTL1 may be involved in the development of KOA. It was also found that CYTL1 was mainly expressed in the chondrocytes and cartilage.8,15 The exogenous addition of CYTL1 may induce the chondrogenic differentiation of the mesenchymal cells via microcluster culture without affecting the hypertrophic maturation of the chondrocyte.15 The chondrogenic effect of CYTL1, which was achieved through the induction of the transcriptional activity of SOX9 and the expression of insulin-like growth factor 1,15 supports the idea of the involvement of CYTL1 in the development of KOA in the present study.
The limitations of the present study were as follows. First, the origin of the serum CYTL1 in patients with KOA remains unknown, and the question of whether it originates from the diseased knee cartilage needs to be further investigated by pathological biopsy. Second, the clinical knee scores and quality of life of these patients were not assessed. Third, the sample size was small, and the sample was limited to serum. Therefore, there is a need to collect both the joint fluid and the serum and to expand the sample size. Finally, we did not compare the serum levels of CYTL1 with the synovial fluid levels. The relationship between the mechanism of CYTL1 and the articular cartilage needs to be further confirmed in animal experiments.
In summary, the abnormally low serum levels of CYTL1 in patients with KOA were closely correlated with the progression of the disease and, therefore, could be used as a serum biomarker to monitor severity during the progression of the disease. CYTL1 is also worthy of further research as a new therapeutic target for OA. In addition, longitudinal studies are needed to investigate the effect of CYTL1 on the radiological progression of the disease.
Funding
This study was supported by Key Medical Science and Technology Program of Henan Province (jointly built) [NO.LHGJ20191059].
Disclosure
The authors declare that they have no competing interests.
References
1. Dahaghin S, Bierma-Zeinstra SM, Ginai AZ, Pols HA, Hazes JM, Koes BW. Prevalence and pattern of radiographic hand osteoarthritis and association with pain and disability (the Rotterdam study). Ann Rheum Dis. 2005;64:682–687. doi:10.1136/ard.2004.023564
2. Oliveria SA, Felson DT, Reed JI, Cirillo PA, Walker AM. Incidence of symptomatic hand, hip, and knee osteoarthritis among patients in a health maintenance organization. Arthritis Rheum. 1995;38:1134–1141. doi:10.1002/art.1780380817
3. Felson DT. Clinical practice. Osteoarthritis of the knee. N Engl J Med. 2006;354:841–848. doi:10.1056/NEJMcp051726
4. Goldring MB, Goldring SR. Osteoarthritis. J Cell Physiol. 2007;213:626–634. doi:10.1002/jcp.21258
5. Rai MF, Sandell LJ. Inflammatory mediators: tracing links between obesity and osteoarthritis. Crit Rev Eukaryot Gene Expr. 2011;21:131–142. doi:10.1615/CritRevEukarGeneExpr.v21.i2.30
6. Mobasheri A. Osteoarthritis year 2012 in review: biomarkers. Osteoarthr Cartil. 2012;20:1451–1464. doi:10.1016/j.joca.2012.07.009
7. Liu B, Gao ZB, Zhao LL. [Association of TWEAK level in serum and synovia with X-ray severity in knee osteoarthritis patients]. Chin J Tradit Med Traumatol Orthop. 2017;25:18–21. Chinese.
8. Jeon J, Oh H, Lee G, et al. Cytokine-like 1 knock-out mice (Cytl1-/-) show normal cartilage and bone development but exhibit augmented osteoarthritic cartilage destruction. J Biol Chem. 2011;286:27206–27213. doi:10.1074/jbc.M111.218065
9. Joint Surgery Group of Chinese Medical Association Osteology Society Branch. [Clinical guidelines for osteoarthritis (2018 edition)]. Chin J Orthop. 2018;38:705–715. Chinese.
10. Charlier E, Deroyer C, Ciregia F, et al. Chondrocyte dedifferentiation and osteoarthritis (OA). Biochem Pharmacol. 2019;165:49–65.
11. Vadalà G, Russo F, Musumeci M, Giacalone A, Papalia R, Denaro V. Targeting VEGF-A in cartilage repair and regeneration: state of the art and perspectives. J Biol Regul Homeost Agents. 2018;32:217–224.
12. Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7:33–42. doi:10.1038/nrrheum.2010.196
13. Deroyer C, Charlier E, Neuville S, et al. CEMIP (KIAA1199) induces a fibrosis-like process in osteoarthritic chondrocytes. Cell Death Dis. 2019;10:103. doi:10.1038/s41419-019-1377-8
14. Liu X, Rapp N, Deans R, Cheng L. Molecular cloning and chromosomal mapping of a candidate cytokine gene selectively expressed in human CD34+ cells. Genomics. 2000;65:283–292. doi:10.1006/geno.2000.6170
15. Kim JS, Ryoo ZY, Chun JS. Cytokine-like 1 (Cytl1) regulates the chondrogenesis of mesenchymal cells. J Biol Chem. 2007;282:29359–29367. doi:10.1074/jbc.M700965200
16. Wang X, Li T, Wang W, et al. Cytokine-like 1 chemoattracts monocytes/macrophages via CCR2. J Immunol. 2016;196:4090–4099. doi:10.4049/jimmunol.1501908
17. Schneller D, Hofer-Warbinek R, Sturtzel C, et al. Cytokine-like 1 is a novel proangiogenic factor secreted by and mediating functions of endothelial progenitor cells. Circ Res. 2019;124:243–255. doi:10.1161/CIRCRESAHA.118.313645
18. Sieker JT, Proffen BL, Waller KA, et al. Transcriptional profiling of articular cartilage in a porcine model of early post-traumatic osteoarthritis. J Orthop Res. 2018;36:318–329. doi:10.1002/jor.23876
19. Ai Z, Jing W, Fang L, Shen W. Cytokine-like protein 1(Cytl1): a potential molecular mediator in embryo implantation. PLoS One. 2016;11(1):e0147424. doi:10.1371/journal.pone.0147424
20. Chao C, Joyce-Shaikh B, Grein J, et al. 17 prevents inflammatory arthritis and associated joint destruction in mice. PLoS One. 2011;6:e22256.
Source link
0 notes
Text
Can Sleep Deprivation Cause Hair Loss?
Sleep is something many of us take for granted. But if you dig in a little deeper, you’ll discover that sleep is not just a period of rest. Not getting enough sleep releases the stress hormone cortisone, a hormone that is responsible to control and regulate moods and emotions . Sleep is restorative, When we sleep, our bodies and brains kick into gear. Tissues repair themselves, the brain dumps toxins, and memories are consolidated. If we don’t get enough sleep, our immune systems become weak, and we can’t absorb nutrients as well. Even our hair starts falling out. Chronic sleep deprivation places our bodies under a lot of stress, which manifests in many ways including hair loss.

Sleep Deprivation and Stress is there any connection?
Stress is also often a contributing factor to hair loss. Insufficient sleep increases the level of stress in your body, and scalp tension. While you sleep soundly, your body undergoes many cycles necessary to promote the activity of the stem cells. In case you didn’t know before, the stem cell activity is vital for generating the epithelial cells that boost hair growth.
The American Journal of Pathology published research that proved the connection between the hair growth process and the level of stress. Prolonged levels of stress have the power to affect your skin and its capacity to function efficiently. This inefficiency, in turn, harms the vulnerable hair follicle. Also, they explored the connection between stress and the hair follicle. Their study suggested that the cycle of hair growth would be disrupted because the adult stem cells responsible for the hair growth process depend on the internal circadian clock to activate. If this is true, then it’s as if your hair doesn’t know what to do because you’re not getting enough sleep. Your hair’s as foggy as your brain on four hours of sleep a night.
The same study highlights that your hair growth process benefits when you release cytokines, neurotransmitters, and hormones during a stress response.
Your adult stem cells need the internal circadian clock to perform actively. This activity, in turn, orchestrates the proliferation of your hair growth cycle.

Worst Case Scenario - Lack of sleep can cause Alopecia Areata?
Telogen effluvium is another potential threat of an irregular sleeping pattern. This condition results from the emotional and physical stress you suffer when you do not sleep well regularly.
A regulated sleeping pattern, aimed at reducing the fluctuations in the quantity and quality of sleep, is the best way to keep stress levels down and avoid the onset of hair loss. Hair loss is typically not permanent due to lack of sleep but again if not treated it may cause more serious problems such as Alopecia Areata.
Alopecia Areata (AA) is an autoimmune disease that specifically targets hair follicles resulting in severe hair loss at a premature age. This disease affects both men and women of all ages, negatively impacting their health and their quality of life.
Alopecia Areata primarily affects the scalp, but in some cases may also have an impact on eyebrows, beard, and other hairy parts of the body. The shedding of hair usually starts in the form of patches, which in time extend to the whole scalp, predisposing the individual to early balding. Also (AA) raises their risk of developing psychological and psychiatric complications.
A 2018 study conducted on the Korean population has shown that patients under the age of 45 who suffered from low quality sleep, had a higher risk of acquiring AA. In addition, these individuals were stated to be more prone to developing other comorbid conditions due to dysfunctional sleeping patterns, such as autoimmune thyroiditis, vitiligo, rheumatoid arthritis, and solid-organ neoplasia.
A previous research conducted in Taiwan in 2015 focused instead on individuals with sleep disorders, which also found that patients with disturbed sleeping patterns had a higher risk of developing autoimmune conditions such as Alopecia Areata.

How to prevent it:
Have a healthy sleep pattern; making sure that you're getting a decent amount of hours of sleep every night. Sleeping hours vary from adult to adult, however it's recommended that 7-9 hours a night is what's needed for your brain to properly function and stimulate hair growth. A healthy sleep pattern also refers to the conditions of sleep you have. Making sure your mattress is a little firmer, your pillow gives your neck the right support and you don't get interrupted sleep.
Exercise Daily; by getting a little more active than usual, your body gets tired and it's overall easier to get a nice fresh sleep. Not to mention exercise gets your body and blood pumping, which is amazing for hair growth stimulation! Walking for 20-30 mins a day is always a healthy start!
Eat healthy; we need to be eating good food to fuel our bodies and in return our bodies will love us back. Some of the BEST foods for hair growth are; eggs, sweet potatoes, nuts, spinach, avocados and protein meats. Eating regularly 3-4 times daily, and not going to bed hungry, will help aid in hair growth.
Establish healthy hair care; establishing a good hair care routine with good products will nourish your hair and keep it healthy. We recommend using a hair serum regularly to hydrate your ends and give your hair the love and nutrients it needs to grow!
0 notes
Text
Cardiovascular Diseases Associated Inflammatory Biomarker Levels in a Small Cohort of HIV-1 Infected Patients Deintensificated From Abacavir/ Lamivudine/Dolutegravir to Lamivudine Plus Dolutegravir by Massimiliano Lanzafame MD

Introduction
Following the successful introduction of combined antiretroviral therapy (cART), a dramatic decrease in viral burden and opportunistic infections along with a consistent increase in life expectancy has been observed in human immunodeficiency virus (HIV) infected patients [1]. This deep change in the HIV disease evolution has determined that HIV positive subjects were effectively monitored for several alterations of many tissue and organs due to HIV chronic disease and antiretroviral treatment for example, cardiovascular system, bone, adipose tissues, kidney and central nervous system represent the major target of these structural and functional damages during HIV infection.
In particular, cardiovascular diseases (CVD) were considered important clinical complications in the HIV patient and represent a leading cause of death among HIV-positive patients, accounting for approximately 11% of the total deaths in this population [2]; the risk of CVD is higher in HIV positive individuals compared with HIV negative people, and particularly the reported myocardial infarction (MI) incidence in cohort study ranges from 3 to 11 cases per 1000 patients a year in HIV- positive individuals against 2 to 7 cases per 1000 patients-years in HIV-negative population [3,4]. Although initial studies indicated a higher prevalence of traditional CVD risk factors in HIV infected population [5,6] as a possible cause, the molecular mechanisms of increased CVD risk in HIV still remain incompletely defined and should be probably attributable to a combination of multiple factors, including both direct and indirect effects of HIV infection on metabolism. Evidence from experimental and observational studies [7,8] in recent years suggested a more important role of HIV itself in contributing to CVD.
Endothelial dysfunction due to gp120, Tat and Nef proteins have been identified as a critical link between infection, inflammation, immune activation, atherosclerosis and cardiovascular system. Moreover, ART may play a role in the exacerbation of risk factors for CVD [9]; since the presentation of findings from the Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) study in 2008 demonstrating a 90% increased risk of MI in HIV- positive individuals receiving ART regimens including abacavir (ABC), subsequent studies, conducted by FDA [10], GlaxoSmithKline [11] and independent researchers [12], to investigate this risk have yielded conflicting results.
Although more recent studies have shown an effective increased risk of CVD associated with use of ABC, many results did not reach statistical significance [13-16]. The absence of a demonstrated underlying biological mechanism for such a risk added interest and confusion about the question, as well as the higher prevalence of risk factors for CVD, such as renal impairment and substance abuse among abacavir recipients; in addition, a recent meta-analysis suggests that relative risk (RR) for MI is increased within a 6 months exposure to ABC (RR=1.61; 95% confidence interval: 1.48–1.75) and in cART-naive population [17,18]. While the published evidence remains conflicting and a plausible biological mechanism for this potential association has not yet been identified, in the following study we have tried to verify whether, after introduction of ABC and its discontinuation in the contest of HAART deintensification, common metabolic markers CVD related such as glucose, LDL, HDL, total cholesterol and triglycerides and inflammatory biomarkers such as IL-6 and D-dimer could change in a small cohort of HIV-1 infected patients [19,20].
Materials and Methods
As described in a previous article [21], from March to December 2016 we enrolled 20 HIV-1 infected patients. 17 patients were still in follow-up (12 men and 5 women) in August 2020, and all were taking lamivudine plus dolutegravir. HIV-1 infected patients were not co-infected with hepatitis B and/or hepatitis C viruses. All the enrolled subjects gave informed consent to all procedures in accordance with the Helsinki Declaration. Blood samples were collected at HIV-1 infection diagnosis before starting a three-drugs regimen consisting in ABC/3TC/ DTG (T0), at 12 months (T2) and 12 months (T3) after therapy deintensification to lamivudine plus DTG (DITT; deintensification treatment time).
Inclusion criteria included: I) naive to ART patients affected by HIV chronic infection, II) undetectable HIV-RNA levels (< 50 copies/ml) for over 12 months with a three-drugs ART treatment consisting in ABC/3TC/DGT at T2, III) undetectable HIV-RNA levels (< 50 copies/ml) for over 12 months with a dual ART regimen consisting in lamivudine plus DGT at T3, iv) absence of adverse drug reactions with the proposed regimens, v) no use of statins and lipid-lowering therapy at the enrolling time and at DITT. Blood samples were collected by venipuncture during the follow up, 30 days before the DITT (T2) and 12 months after the DITT (T3). Every blood sample collected consisted in quantitative plasmatic determination of glucose, IL6, D-Dimer and plasmatic lipids such as triglycerides, LDL, HDL and total cholesterol.
Biomarkers plasmatic levels were evaluated with specific commercial kits.For IL-6 we used Human IL-6 high sensitivity ELISA kit (Diaclone,Besancon,France);for D-dimer RayBio Human D-dimer ELISA Kit (RayBiotech,Norcross,GA,USA). Values of different samples were evaluated between the different time groups (T1, T2 and T3 groups) with statistical non-parametric procedures such as Wilcoxon test and Friedmann test using GraphPad Prism 7 software. Data were considered significant with p value <0.05.
Results
We analyzed a cohort of 17 HIV-1 infected patients (12 men and 5 women, aged from 24 to 70 years, average 41.35 years old) infected by HIV-1 B subtypes. All mean and median values for each group are reported in table 1, together with normal reference values. Cytokine IL-6 did not show any significant amount of variation among the three groups of samples (Group 1 vs 2 p=0.83; Group 1 vs 3 p=0.65; Group 2 vs 3 p= 0.97 Wilcoxon test). The statistical analysis of the three groups confirmed these data (p=0.645; Friedmann test). Similarly, D-dimer did not significantly change its levels in serum (Group 1 vs 2 p=0.32; Group 1 vs 3 p=0.38; Group 2 vs 3 p= 0.69 Wilcoxon test; p=0.53 Friedmann test). The analysis of serum glucose(Group 1 vs 2 p=0.94; Group 1 vs 3 p=0.97; Group 2 vs 3 p= 0.88 Wilcoxon test; p=0.67 Friedmann test), total cholesterol (Group 1 vs 2 p=0.07; Group 1 vs 3 p=0.65; Group 2 vs 3 p= 0.41 Wilcoxon test; p=0.21 Friedmann test), cholesterol HDL (Group 1 vs 2 p=0.92; Group 1 vs 3 p=0.89; Group 2 vs 3 p= 0.58 Wilcoxon test; p=0.82 Friedmann test), cholesterol LDL (Group 1 vs 2 p=0.09; Group 1 vs 3 p=0.88; Group 2 vs 3 p= 0.36 Wilcoxon test; p=0.71 Friedmann test) and triglycerides (Group 1 vs 2 p=0.58; Group 1 vs 3 p=0.93; Group 2 vs 3 p= 0.39 Wilcoxon test; p=0.67 Friedmann test) did not demonstrate significant level variations [22-25].
Discussion
Since the publication of the D:A:D study, a number of possible mechanisms have emerged as potential mediators of the abacavir effect. In ACTG 5202, participants receiving abacavir in the low viral load stratum (<100 000 copies/ml) had higher rates of cholesterol elevations compared with controls. In addition, abacavir has been related to several processes, which may lead to accelerated vascular disease such as platelet activation [26], hyperlipidemia [27], T-lymphocyte activation, abnormal endothelial function and vascular inflammation [23,28]. Hypersensitivity reaction and concomitant inflammatory processes may affect platelet and endothelial function. Of note, HLAB5701 testing to prevent abacavir hypersensitivity reactions was not the standard of care during the conduct of this study. Therefore, it is possible that the risk of CVD may be lower in contemporarily treated patients who are unlikely to experience abacavir hypersensitivity reactions, but more investigations are necessary to elucidate this point.
Moreover, D:A:D study results showing a reversal of risk of CVD within 6 months of discontinuation of abacavir prompted investigators to search for a relatively rapidly acting underlying biological mechanism able to correlate CVD with abacavir exposure. A possible role of inflammatory biomarkers such as high sensitivity c-reactive protein (hsCRP) and interleukin-6 (IL-6) in association with CVD among abacavir users was proposed by the SMART/INSIGHT study (28), while other studies focused on IL-6, selectin P and E, D-dimer, vascular adhesion molecule-1, intercellular adhesion molecule-1, and tumor necrosis factor alpha; all of these studies showed no increased levels in the setting of abacavir exposure [29,30]. While the association between lipid metabolism, flogosis and CVD are widely described in scientific literature, proving causation for any particular biomarker of inflammation has been difficult.
IL-6 signals a downstream proinflammatory response by activating membrane-bound IL-6 receptors (IL-6R) on the cell surface. IL-6 and IL-6R are demonstrated to have a direct and dose-dependent role directly causing higher blood levels on CRP responsible for proinflammatory settings linked to CVD onset. Moreover, D-dimer has been identified as a pro-atherogenic factor, being hypercoagulability a recognised risk factor for CVD; because of that, this study included monitoring these two biomarkers blood levels on a total of 24-months retrospective follow up. Many studies estimated abacavir’s role in causing endothelial dysfunction, reporting that ABC would affect lower brachial artery flow mediated vasodilation, a measure of endothelial dysfunction, as an independent prediction factor [8,27]; switching from ABC to tenofovir was also associated to a Framingham risk score improvement [31].
In a recent RCT, anyway, no evidence of endothelial dysfunction from abacavir use as compared to tenofovir was found [32]. Platelet aggregation and reactivity,potentially leading to thrombosis phenomena, have also been associated by ABC’s exposure in vitro and in vivo [33,34]; particularly, various platelet agonists,increased in plasma after ABC assumption (e.g. adenosine diphosphate, collagen, epinephrine, and thrombin receptor-activating peptide) [33], this observation being due to a documented decreased production of cyclic guanosine monophosphate, an inhibitor of platelet aggregation and secretion [33,34], but more further studies are needed to confirm these data. Existing literature on plausible underlying biological mechanisms are summarized in a recent systematic review, collecting in vivo and in vitro results [17]: ABC ’s structural likeness to endogenous purines possessing pro-inflammatory and pro-thrombotic potential may finally explain its role as a vascular inflammation inducer via leukocyte–endothelial cell interactions, causing cardiovascular implications , but how such acute and reversible mechanisms may correlate with recent clinical findings of CVD risk from cumulative exposure beyond 6 months is still unclear [35,36].
With the literature demonstrating such controversial results, the search for plausible biological explanations goes on. Recent ABC assumption is associated with 60% increased risk of CVD not attenuated after adjusting for substance use, renal dysfunction, and several other potential confounders. The finding of an increased risk of CVD associated with abacavir use among ART-naive individuals may be more suggestive of a causal relationship; many of the studies concerning ABC potential risk were observational, whose results may be confounded by unmeasured risk factors.
While this risk appears to be reversible upon discontinuation of abacavir, further research is necessary to confirm this in view of recent studies that showed evidence of increased risk from cumulative exposure beyond 6 months. Our data showed, after ABC introduction and discontinuation in antiretroviral therapy based on the association of ABC/3TC/ DTG, the absence of significant variations in the serum levels of different inflammatory and metabolic markers. In fact, glycemia, triglycerides, HDL, LDL, Il-6 and total cholesterol showed negligible quantitative change at all three tested time points. Finally about a possible role of inflammatory biomarkers (e.g.D-dimer and interleukin-6 IL-6) in association with CVD among abacavir users, our results, even though in a small cohort of HIV-infected patients, would confirm the results of other studies showing that IL-6 and D-dimer are not elevated in the setting of abacavir exposure.
Regarding our Journal: https://oajclinicalsurgery.com/ Know more about this article https://oajclinicalsurgery.com/oajcs.ms.id.10027/ https://oajclinicalsurgery.com/pdf/OAJCS.MS.ID.10027.pdf
#Cardiovascular Diseases#Biomarker Levels#HIV-1#Abacavir#Lamivudine#Dolutegravir#Lamivudine Plus Dolutegravir#Massimiliano Lanzafame MD#oajcs#clinical surgery
0 notes