#pharmaceuticals and drug trials
Explore tagged Tumblr posts
Link
“A new U.S. law has eliminated the requirement that drugs in development must undergo testing in animals before being given to participants in human trials.
Animal rights advocates have long pushed for such a move, and some in the pharmaceutical industry have argued that animal testing can be ineffective and expensive...
Signed by President Biden in December as part of a larger spending package, the law doesn't ban the testing of new drugs on animals outright.
Instead it simply lifts the requirement that pharmaceutical companies use animals to test new drugs before human trials. Companies can still test drugs on animals if they choose to.
There are a slew of other methods that drugmakers employ to assess new medications and treatments, such as computer modeling and "organs on a chip," thumb-sized microchips that can mimic how organs' function are affected by pharmaceuticals.
But Aliasger Salem, a professor at the University of Iowa's College of Pharmacy, told NPR that companies opting to use these alternative testing methods as a replacement for animal testing must be aware of the methods' limits to ensure their drugs are safe.
"The companies need to be aware of the limitations of those technologies and their ability to identify or not identify potential toxicities," Salem said.
"You don't want to shift to systems that might not capture all of the types of toxicities that have been seen in the past without ensuring that the methods that you have will capture that."
An FDA spokesperson told NPR that it will "implement all applicable provisions in the omnibus and continue to work with stakeholders to encourage the development of alternative testing methods."
This year's federal budget also includes $5 million for a new FDA program aimed at reducing animal testing by helping to develop and encourage industry to adopt new product testing methods, the spokesperson said.”
-via NPR, 1/12/23
#animal testing#animal rights#cw animal harm#fda#food and drug administration#us politics#united states#drug trials#biden#big pharma#pharmaceutical#good news#hope
62 notes
·
View notes
Text
Clinical Development Solutions
In the rapidly evolving field of healthcare, clinical development plays a crucial role in bringing novel treatments and therapies to patients worldwide. Clinical Development Solutions (CDS) is at the forefront of this exciting journey, pioneering innovative approaches to accelerate the development and approval of life-saving drugs and medical devices. With a dedicated team of experts and cutting-edge technologies, CDS is committed to transforming the landscape of clinical research and improving patient outcomes.
At CDS, we understand the challenges and complexities of clinical development. Our comprehensive suite of solutions is designed to address these challenges head-on, providing tailored strategies and support throughout the entire drug development lifecycle. From early-phase clinical trials to post-marketing studies, we offer a wide range of services that enable pharmaceutical and biotech companies to navigate the regulatory landscape efficiently and effectively.
One of the key strengths of CDS lies in our expertise in clinical trial design and optimization. We work closely with our clients to design robust and scientifically rigorous trials that generate high-quality data while minimizing risks. By leveraging our extensive knowledge and experience, we can identify the most appropriate patient populations, endpoints, and study designs to maximize the chances of success. Our statistical and data management teams ensure that the collected data is accurate, reliable, and compliant with regulatory requirements.
In addition to trial design, CDS also excels in patient recruitment and retention strategies. We understand the importance of enrolling a diverse and representative patient population to ensure the generalizability of study results. Through our innovative patient-centric approaches, such as digital recruitment platforms and targeted engagement campaigns, we connect with potential study participants and enhance their overall trial experience. By fostering strong relationships with patients and investigators, we improve retention rates and reduce dropout rates, ultimately leading to faster and more reliable study results.
CDS is at the forefront of adopting emerging technologies to drive efficiency and innovation in clinical development. We harness the power of big data analytics, artificial intelligence, and machine learning to uncover valuable insights from complex datasets. These advanced analytics enable us to identify trends, predict outcomes, and optimize trial protocols, thus accelerating the development timeline and reducing costs. Our investment in digital health technologies and wearable devices further enhances data collection and remote monitoring capabilities, enabling more flexible and patient-friendly trial designs.
In the realm of regulatory affairs, CDS provides comprehensive support to ensure compliance with global regulations and standards. Our regulatory experts have in-depth knowledge of regional requirements, including those of the FDA, EMA, and other regulatory authorities worldwide. From preparing regulatory submissions to managing post-marketing safety surveillance, we guide our clients through every step of the regulatory process, ensuring timely approvals and post-approval compliance.
CDS is also committed to fostering collaboration and knowledge sharing within the clinical research community. We organize scientific symposia, webinars, and training programs to facilitate the exchange of ideas and best practices. By promoting interdisciplinary collaboration and staying up to date with the latest industry advancements, we continuously enhance our capabilities and stay at the forefront of clinical development.
In conclusion, Clinical Development Solutions is a leading provider of innovative solutions in clinical development. Through our expertise, technology-driven approaches, and commitment to patient-centricity, we strive to transform the drug development landscape and improve patient outcomes. By partnering with CDS, pharmaceutical and biotech companies can navigate the complexities of clinical research with confidence, bringing new therapies to patients faster and more efficiently. Together, let us shape the future of healthcare through innovation and collaboration.
#clinical development#development solutions#biometric solution providers#clinical development consultant#clinical development service#drug development solutions#clinical product development#clinical development solution company#clinical development specialist#Clinical Development Services in Hyderabad#Clinical Services in Hyderabad#clinical development services agency in hyderabad india#best clinical development agency in india#project management solutions provider#project management service provider#biometric service provider near me#Clinical Trial Services In Hyderabad#specialized clinical pharmacist#clinical pharmaceutical company
2 notes
·
View notes
Text
Empowering Pharma Business Growth Worldwide | Chemxpert Database
Unlock new opportunities in business development pharma with Chemxpert Database. Connect with leading generic medicine manufacturers in India, renowned tablet manufacturing companies, and trusted API manufacturing companies in India. Explore collaborations with top pharma testing labs and innovative nutraceutical manufacturers to expand your portfolio. Chemxpert Database is your ultimate resource for navigating the pharmaceutical ecosystem, ensuring seamless partnerships and growth in the ever-evolving healthcare industry. Let’s innovate together!
#pharmacy management#clinical trial application#best medicine company in India#biopharmaceutical company#approved drug#pharmacovigilance in clinical trials#pharmaceutical companies in Egypt
1 note
·
View note
Text
Amgen's Experimental Obesity Drug MariTide Shows Promising Weight Loss Results
Amgen’s Promising Experimental Obesity Drug Shows Significant Weight Loss The pharmaceutical manufacturer Amgen made headlines on Tuesday by announcing that its experimental obesity medication, branded as MariTide, has demonstrated remarkable efficacy in clinical trials, helping patients shed an average of up to 20 percent of their body weight over the course of a year. This innovative drug is…
#Amgen#clinical trials#FDA#GLP-1 agonists#injectable medication#MariTide#obesity drug#pharmaceutical news#side effects#Type 2 diabetes#weight loss
0 notes
Text
How does pharmacovigilance affect clinical industry, in US, Germany, and India
Pharmacovigilance is an essential branch of clinical industry since it focuses on the safety of drugs and their effectiveness. From the above observations it could be noted that while the principles, laws and the mechanisms of pharmacovigilance in the US, Germany and India are all different, the overall objectives of protecting the public from hazard from medicines are the same. These countries ensure that the monitoring and evaluation of drug safety data is always being done hence helping the world initiatives of making the drugs safer for use.
#Clinical Trial Phases#phase 1 Clinical Trials#Medical Trials#phases of Drug Development#Clinical Drug Development Phases#Clinical Trial Search#Clinical Development#Trial Search#Pharmaceutical Development
0 notes
Text
.
actually in this space im going to say. in one scene early in infinite jest two brothers are talking about the way their mom has acted after their father's suicide. the first brother asks if she's even sad he's dead. and the other says: there are 2 ways to make a flag be half-mast. you can lower the flag halfway, or you can double the height of the pole. he says: she's plenty sad, i bet. anyway that bit has fundamentally changed the way i think about displays of grief.
#hi guys its me again. hi. hey. i get to go home from work in thirteen minutes.#i dont htink im all the way beating the high at work allegations but#the concoction is making me better at my job not worse so they can eat a dick about it#i didnt get a 10 after my lunch and now its too late for one but i want to smoke so bad. usually these sorts of messages go in teams chat#but my teams chat bestie has instead gotten about 2k words today from me about the various trials and tribulations of ***** ** ***** * ****#and i think she does not NEED in addition to hear me complain that i cant fuck off at my job as much as i would like to.#im going: if typing a bunch of stupid words as tags on a tumblr post vents enough steam that i dont yell at anyone tonight its WORTH IT#im going to go home and theres going to be no conflict all night and my albums will make me feel better#and the list of drugs ill be allowed to take will be so much longer due to not being at work and they will make me feel so calm and hopeful#and patient and accepting and generous and realistic and brave and firm and kind#and my cat will be happy she is getting her usual wet food again#and ill go to sleep at the perfect time so easily and happily and ill wake up to my FIRST alarm NO snoozing and feel WELL RESTED#and ill get to work on time and act like a real and regular human person and not someone on 4 different conflicting pharmaceuticals#and so on and so forth amen to me.
1 note
·
View note
Text
Clinical Trials : Holistic Exploration of the Current State and Future Outlook
The global clinical trials market size is expected to reach USD 123.5 billion by 2030, expanding at a CAGR of 6.49 from 2024 to 2030, according to a new report by Grand View Research, Inc. An increase in the volume and complexity of clinical trials has been witnessed lately, which plays an important role in the R&D of new drugs and products. The market witnessed a decline of 6% in 2020 owing to the COVID-19 pandemic. However, the market is projected to recover from 2021 onwards. In addition, clinical trials have become increasingly costly, adding to the overall cost of developing a drug.

Clinical Trials Market Report Highlights
The phase III clinical trials segment dominated the market with a 53.3% share in 2023. This can be attributed to the complexity of this phase
The interventional studies segment dominated the market in 2023. It is one of the most prominent methods used in clinical trials in the study design segment owing to the increasing demand for the intervention for clinical trials by researchers
North America held 50.3% of the market share in 2023. Favorable government initiatives and the presence of a large number of players in the U.S. that offer advanced services are responsible for market growth
Asia Pacific region is anticipated to grow at the fastest CAGR over the forecast period owing to the increasing patient pool and cost-efficient services.
For More Details or Sample Copy please visit link @: Clinical Trials Market Report
The increasing need for developing new drugs for chronic diseases, such as cancer, respiratory disorders, diabetes, cardiovascular diseases, and others, is creating immense pressure on the healthcare industry. The COVID-19 pandemic and the increasing demand for developing a suitable treatment are driving the market. The high number of people affected by the disease further depicts an increasing need for therapeutics & vaccines. Currently, there are 288 therapeutics and 106 vaccines under development, out of which, nearly 7.0% of therapeutics are in Phase IV, 21.0% in Phase III, and 43.0% & 13.0% in Phase II & Phase I, respectively.
The pandemic has resulted in the global disruption of traditional onsite clinical trials. Hence, regulatory bodies worldwide have undertaken various initiatives for fast-tracking clinical trials for the development of innovative solutions. One such instance is Solidarity, an international clinical trial launched by the WHO to find effective treatment against COVID-19. Although the pandemic has forced many medical device & drug developers to revise the approach to such crises, integrating best practices within clinical trial procedures & adapting to virtual trials, which can support the continuous development of therapeutics.
ClinicalTrials #HealthcareResearch #MedicalInnovation #DrugDevelopment #PatientRecruitment #Biopharmaceuticals #ClinicalResearch #RegulatoryCompliance #DataManagement #PatientEngagement #PrecisionMedicine #TherapeuticTrials #CROs #ClinicalResearchOrganizations #GlobalHealth #ClinicalStudyDesign #PharmaceuticalIndustry #BiotechResearch #ClinicalEndpoints #HealthTechIntegration
#Clinical Trials#Healthcare Research#Medical Innovation#Drug Development#Patient Recruitment#Biopharmaceuticals#Clinical Research#Regulatory Compliance#Data Management#Patient Engagement#Precision Medicine#Therapeutic Trials#CROs#Clinical Research Organizations#Global Health#Clinical Study Design#Pharmaceutical Industry#Biotech Research#Clinical End-points#HealthTech Integration
0 notes
Text
The Best News of Last Year - 2023 Edition
Welcome to our special edition newsletter recapping the best news from the past year. I've picked one highlight from each month to give you a snapshot of 2023. No frills, just straightforward news that mattered. Let's relive the good stuff that made our year shine.
January - London: Girl with incurable cancer recovers after pioneering treatment
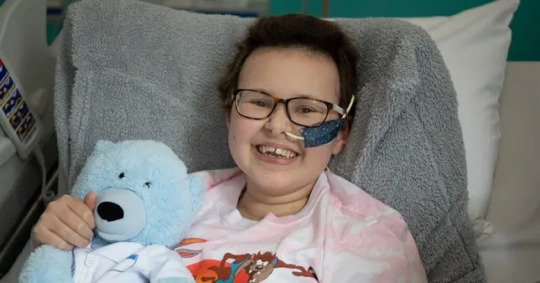
A girl’s incurable cancer has been cleared from her body after what scientists have described as the most sophisticated cell engineering to date.
2. February - Utah legislature unanimously passes ban on LGBTQ conversion therapy

The Utah State Legislature has unanimously approved a bill that enshrines into law a ban on LGBTQ conversion therapy.
3. March - First vaccine for honeybees could save billions

The United States Department of Agriculture (USDA) has approved the world’s first-ever vaccine intended to address the global decline of honeybees. It will help protect honeybees from American foulbrood, a contagious bacterial disease which can destroy entire colonies.
4. April - Fungi discovered that can eat plastic in just 140 days
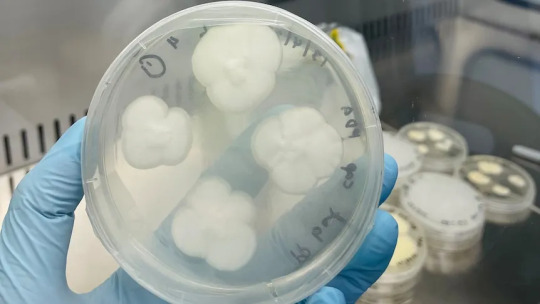
Australian scientists have successfully used backyard mould to break down one of the world's most stubborn plastics — a discovery they hope could ease the burden of the global recycling crisis within years.
5. May - Ocean Cleanup removes 200,000th kilogram of plastic from the Pacific Ocean

The Dutch offshore restoration project, Ocean Cleanup, says it has reached a milestone. The organization's plastic catching efforts have now fished more than 200,000 kilograms of plastic out of the Pacific Ocean, Ocean Cleanup said on Twitter.
6. June - U.S. judge blocks Florida ban on care for trans minors in narrow ruling, says ‘gender identity is real’

A federal judge temporarily blocked portions of a new Florida law that bans transgender minors from receiving puberty blockers, ruling Tuesday that the state has no rational basis for denying patients treatment.
7. July - World’s largest Phosphate deposit discovered in Norway

A massive underground deposit of high-grade phosphate rock in Norway, pitched as the world’s largest, is big enough to satisfy world demand for fertilisers, solar panels and electric car batteries over the next 50 years, according to the company exploiting the resource.
8. August - Successful room temperature ambient-pressure magnetic levitation of LK-99

If the claim by Sukbae Lee and Ji-Hoon Kim of South Korea’s Quantum Energy Research Centre holds up, the material could usher in all sorts of technological marvels, such as levitating vehicles and perfectly efficient electrical grids.
9. September - World’s 1st drug to regrow teeth enters clinical trials

The ability to regrow your own teeth could be just around the corner. A team of scientists, led by a Japanese pharmaceutical startup, are getting set to start human trials on a new drug that has successfully grown new teeth in animal test subjects.
10. October - Nobel Prize goes to scientists behind mRNA Covid vaccines

The Nobel Prize in Physiology or Medicine has been awarded to a pair of scientists who developed the technology that led to the mRNA Covid vaccines. Professors Katalin Kariko and Drew Weissman will share the prize.
11. November - No cases of cancer caused by HPV in Norwegian 25-year olds, the first cohort to be mass vaccinated for HPV.
Last year there were zero cases of cervical cancer in the group that was vaccinated in 2009 against the HPV virus, which can cause the cancer in women.
12. December - President Biden announces he’s pardoning all convictions of federal marijuana possession

President Joe Biden announced Friday he's issuing a federal pardon to every American who has used marijuana in the past, including those who were never arrested or prosecuted.
------
And there you have it – a year's worth of uplifting news! I hope these positive stories brought a bit of joy to your inbox. As I wrap up this special edition, I want to thank all my supporters!
Buy me a coffee ❤️
Merry Christmas and Happy New Year!
6K notes
·
View notes
Text
NewLife Medicals: Your Trusted Global Clinical Research Organization
Riding on the cutting edge of medical science and clinical trials, NewLife Medicals is your reliable partner for all pharmaceutical needs. Bathing in expertise, authority, and trust, our ambitious goal is to reshape the future of healthcare.
But why should you trust NewLife Medicals?
NewLife Medicals: Active Pharmaceutical Ingredient Manufacturers
Active pharmaceutical ingredients (APIs) are the heart of any medicine, responsible for its therapeutic effects. At NewLife Medicals, we are more than just API manufacturers. Quality, affordability, and ultra-precision define our API manufacturing. We utilize state-of-the-art technology and rigorous quality checks to ensure the synthesis of high-quality APIs.
Got an unusual API request? As hard-to-source drugs and limited distribution drugs suppliers, we welcome the challenge!
Clinical Research Organization: NewLife Medicals
Clinical trials form the backbone of drug development – but they can be complex and costly. Simplify and economize your clinical testing experience with our unparalleled clinical research organization. Our seasoned team of research professionals are committed to delivering high-quality, timely, and ethically sound clinical research.
Why settle for less when you can have the best?
Global Innovator Drugs Supplier and Biosimilar Supplier
As an innovator drugs supplier, NewLife Medicals is committed to bringing new, pioneering treatments to the market. Our extensive portfolio of innovator drugs and biosimilar infusions stands as a testament to our pioneering spirit and relentless dedication to progress.
Ever heard of orphan drugs or REMS access? We supply these too!
Finished Dosage Formulations Suppliers
We take our role as finished dosage formulations suppliers seriously. Our manufacturing process leverages the highest industry standards, ensuring consistency and efficacy with every dose. Whether it's tablets, capsules, or syrups, we have your needs covered.
Are you seeking a reliable reference listed drug provider? Look no further!
Ancillary Clinical Supplies and Hospital Lines Supplier
Besides pharmaceuticals, our inventory comprises ancillary clinical supplies and premium hospital lines. These medical accessories and equipment promise to simplify your caregiving experience, improving efficiency and compliance.
Are you with us yet?
Your Trusted Partner in Pharmaceutical Supply Chain
Whether it's comparator sourcing for clinical trials, pharma raw material suppliers, patient supply, or specialty sourcing, NewLife Medicals has got you covered. Our integrated supply chain caters to all your pharmaceutical needs while maintaining the highest quality standards.
Trust in the expertise and authority of NewLife Medicals for all your medical needs. Together, let's make a healthier world!
Meta description: Explore NewLife Medicals – your trusted global clinical research organization, pharmaceutical supplier, and healthcare partner. Dive into our wide range of services today.
#rld pharmaceutical#comparator sourcing for clinical trials#named patient supply#ancillary clinical supplies#global clinical research organization#drug development#innovator drugs supplier
0 notes
Text

#clinical development#development solutions#biometric solution providers#clinical development consultant#clinical development service#drug development solutions#clinical product development#clinical development solution company#clinical development specialist#Clinical Development Services in Hyderabad#Clinical Services in Hyderabad#clinical development services agency in hyderabad india#best clinical development agency in india#project management solutions provider#project management service provider#biometric service provider near me#Clinical Trial Services In Hyderabad#specialized clinical pharmacist#clinical pharmaceutical company
1 note
·
View note
Text
Global Pharmaceutical Database Insights | Chemxpert Database
Discover the latest in global pharmaceutical advancements with Chemxpert Database. Explore top pharmaceutical companies in Spain and the best medicine manufacturing companies in India, all committed to excellence and strict compliance in the pharmaceutical industry. Gain insights into high-standard pharmaceutical manufacturing plants and stay updated on the FDA approval list for newly authorized drugs. With Chemxpert Database, uncover the world’s top players and compliance trends that drive innovation and safety in healthcare.

#biopharmaceutical company#approved drug#pharmacovigilance in clinical trials#pharmaceutical companies in Egypt#analytical method development and validation#medical wholesalers#medicine manufacturers in India
1 note
·
View note
Text

Guiding Drug Discovery & Development with Real-Time Insights
Clival Database is a comprehensive resource for navigating drug discovery & development, offering critical data on drugs in development and emerging drug development trends. It provides detailed information on drug development steps and tracks active clinical trials to support researchers throughout the process. With Clival Database, pharmaceutical professionals can stay ahead of industry shifts and make informed decisions, accelerating innovation and improving success rates in the fast-paced world of drug development.
#active Clinical Trials#Pharmaceutical Drug Trials#drug Discovery & Development#drug Development Steps#drugs in Development
1 note
·
View note
Text
Tell Me The Truth
•• Jonathan Crane x Reader ••
Story note: Thiopental is the proper name for what’s more commonly known as “truth serum.” It works by slowing the brain’s higher levels of functioning, making coming up with lies or fabricating complicated stories difficult for a person.
***!!!Warning: Mature sexual content, mentions/use of needles, blood, drug administration/drug influence, reader vulnerability/loss of some defenses and control, 18+ readers only, minors DNI!!!***
…………………………………………………………………………….

“Y/N! Wait up!”
Looking over her shoulder, Y/N saw one of the lab technicians, Terry, jogging towards her down the wide hallway. He was coming from the lab office where they’d just been talking, and as he approached and got closer, Y/N smiled, and when he stopped in front of her, she raised her eyebrows.
“Is there something we forgot to review?” she asked him, still smiling.
“No,” he shook his head, catching his breath after the jog through the wing of the building, “but you forgot this,” he said, lifting his hand and revealing her access key card he held between his fingers. “You left it on the counter in the lab.”
Her eyebrows raising again in surprise, Y/N reached out to take the key card from him.
“Oh, gosh! Thanks so much! I’m gonna need that later.”
“You mean tomorrow,” he suggested in correction. “You’ll need it tomorrow.”
“No,” Y/N replied with a shake of her head. “I mean tonight. I’ve still got some work to do after I drop these documents off in Dr. Crane's office. I need to sort through the results of the latest trial he conducted for that proposed new version of Thiopental, and then I have to go back to the lab and begin dosing out the vials of it for the next trial.”
“How many trials is he going to do?” Terry asked.
“Just these two,” Y/N replied. “The first was to track the physical effects and duration of those effects, as well as efficacy. This next trial is to assess the intensity of effect and the average recovery time. We’re hoping this version of the drug won’t leave patients feeling as spacey and out of it for as long as the original version typically does.”
“I see,” Terry replied with a nod of his head. “Who else do you have working on this with you?”
Y/N shook her head.
“No one; just me,” she then replied.
“Geez, Crane really likes to work you, doesn’t he?” Terry responded.
“It’s not like that,” Y/N said. “He allows plenty of other people to be involved in running the trials and collecting the data. It’s just that when it comes to interpreting the data and getting everything organized for the trials, he wants me to do it.”
“He keeps you on a pretty short leash,” Terry countered.
“No,” Y/N said again. “He’s giving me the opportunity I need, which is to gain firsthand experience and knowledge. This is exactly the kind of stuff I need to be involved in as I work towards my PhD. It’s what’s entailed in being a research assistant.”
“I don’t see him making anyone else work after 5:00 p.m. on a consistent basis,” Terry said then. “But you’re always here late.”
“He doesn’t make me; I do it on my own accord.” Y/N replied.
“Why?” Terry asked with a skeptical raise of his brow. “It’s not like he’s a joy to work with. It surely can’t be his personality that keeps you hanging around. The guy couldn’t be less inviting or more clinical. Has he ever even thanked you or acknowledged what you do?”
“Terry,” Y/N said, admonishing him for criticizing Dr. Crane. “He’s a brilliant doctor and an ingenious pharmaceutical developer. It’s only natural for him to be very clinically focused. But, truly, he’s not as cold as you make him sound. He’s just...focused.”
“You know, your face always flushes whenever you talk about him,” Terry said teasingly with a smirk as he looked at Y/N. “In your eyes, he can do no wrong. Does your strong defense of him have anything to do with the fact that you so obviously have a crush on him?”
Blushing even deeper than she apparently already was, Y/N’s jaw slightly dropped in surprise.
“I do NOT!” she insisted, hugging the files she was holding to her chest.
“Please, Y/N, it’s all too obvious. We all work in pretty close quarters in that lab; it’s hard to miss the way you look at him whenever he’s in there with us. And if that’s so obvious in the lab, I can only imagine how much more you fawn over him when it’s just the two of you in his office.”
“I do NOT fawn!” Y/N denied again. “I’m his assistant. I’m supposed to pay close attention to him and help him in any way that I can.”
Of course, though, Terry was right. Y/N’s loyalty to Dr. Crane and his work was genuine, but it was one hundred percent correct that she had a hopeless crush on the doctor as well. He was incomprehensibly smart, dedicated to his work, and constantly developing something new in the field – he was so accomplished. He was also insanely handsome and sexy (although he seemed to be unaware of that fact), and although he displayed a quite cold, clinical demeanor ninety-eight percent of the time, there were glimpses of affection that he’d shown Y/N here and there over the last year, and it was enough to get her imagination running for all sorts of different scenarios. How many times had she imagined those lips of his on hers? His hands gripping her waist while she was bent over the lab counter?
Admittedly, although she couldn’t quite put her finger on it, there was something else about him that spoke to her as well. He had a kind of...intimidating way about him that made her feel things. Almost like a slight hint of menacing or danger that made her both nervous and excited when around him. It was like he was balancing between remaining composed and becoming something a bit darker...equally as ready to either praise her or punish her, depending on whether or not she pleased him. And she always tried very hard to please him.
“You’re like his little pet, you know?” Terry continued then. “He snaps his fingers, and you—”
“Mr. Hall!” a very familiar voice suddenly called out from down the way, cutting into their conversation, and both Y/N and Terry turned to see Dr. Crane standing several yards away, briefcase in hand as he looked at Terry.
“You’re supposed to be in the lab right now, are you not?” he spoke again, his voice still raised slightly for them both to hear. “I don’t believe we’re paying you to ignore your responsibilities and distract my staff, so kindly say your goodbyes to Miss Y/L/N and get back to work.”
Turning back to face Terry, Y/N gave him a look that was a cross between apologetic and sympathetic.
“Don’t worry; I’ll tell him you were just bringing me my key card,” she whispered to him.
“Won’t matter,” Terry replied with slight resentment, briefly eyeing Crane again over Y/N’s shoulder. “But maybe if you slip in a good word for me when the two of you are making out later, that might change his mind.”
“Shh! Don’t say things like that! Someone could hear you!” Y/N said in a somewhat panicked voice. “If a rumor starts going around that he and I—”
“Miss Y/L/N!” It was now Y/N’s turn to be called on by the doctor, but when she turned her head again and looked at him, he said nothing further, just gave her an expectant look and remained firmly where he stood.
“I gotta go; thanks for bringing me my card,” she whispered again as she quickly turned back to Terry for the last time. Then she grabbed the key card from his still outstretched hand and closed her fingers around it, turning around once more and briskly walking towards Dr. Crane with her files still clutched to her chest with her other arm. Crane continued to hold her gaze as she approached him, and as she reached him, instead of staying where he was, he began to walk again, Y/N following alongside him with still hurried steps as she spoke.
“I’m sorry, Dr. Crane, that was my fault, not Terry’s,” she said as they made their way down the hallway. “I left my key card in the lab, and he was bringing it to me, that’s all.”
“And it took you ten minutes to take a card out of his hand?” Jonathan replied, still keeping his eyes forward. “We have a schedule to keep, Miss Y/L/N.”
“I know. I’m sorry.”
Swallowing somewhat nervously, Y/N glanced sideways at him. He seemed even more no-nonsense than he usually did, and while she had just defended him to Terry, Jonathan’s aura was admittedly chilly today – she could feel it coming from him, and it was beyond just being clinical. He almost seemed mad that she’d been talking to Terry. But then again, he was never what one would call “warm”, except for the very rare occasions when he’d show Y/N the slightest bit of fondness. Something she’d never seen him reveal to anyone else.
As they continued through the building and made their way to Crane’s office, they discussed some details of the latest trial for the Thiopental, Y/N thumbing through several papers while speaking. As she spoke, Jonathan was only half listening, more so occupied with the anger and jealousy he’d felt upon seeing Y/N with Hall. And even though he knew that Y/N had no interest in the man, that didn’t stop the green monster from peeping its ugly head out. Even the doctor most trained in the reasoning behind mental and emotional responses still couldn’t stop himself from getting jealous, and the fact that he knew Y/N had a loyal devotion to him didn’t make it any less anger-inducing when he saw the way Hall had looked at her. The rat had been trying to pick her up for months, and he just wouldn’t take the hint. Well, it was finally time to hammer home to both Y/N and Hall exactly who she belonged to.
Jonathan was fully aware of the secret interest Y/N had in him. He’d picked up on it almost immediately upon her employment with him. While she was very good at keeping it to herself and maintaining a professional front, Jonathan was an expert at assessing, evaluating, and teasing apart every small mannerism, tone of voice, nervous habit, trail of thought, unspoken implication, and a million other things a person might display. And while she was very work-driven and dedicated, he had still caught every stolen glance, every flush of her cheeks, bite of her lips, and inviting bit of body language from her. She’d unknowingly, unintentionally given herself away months and months ago, and Jonathan hadn’t overlooked one tiny bit of it.
He hadn’t acted upon it, though, and he’d – for the most part – maintained an extremely stoic, clinical demeanor with her; the same he offered the rest of the staff. Only rarely did he allow himself to slip slightly and express a tiny bit of affection towards her.
No; he hadn’t acted upon it — yet. Because he’d decided long ago that when he did act upon it, it would be with the knowledge and the tools to make sure she wouldn’t be able to do anything other than give in to him. She’d be open, helpless, submissive, and melting underneath every touch he gave her. That was exactly how he wanted her. That was how he’d wanted her from the start.
Normally, Jonathan was unaffected by most women. Typically, to him, they were dim, whiny, annoying, faint-hearted things that were inconvenient but ultimately necessary. Most of the women he encountered simply made his life more difficult in some way, and weren’t worth wasting his precious time or intelligence on. Sure, he had needs like any man did, but those were commonly satisfied with minimal talk and a quick fuck with a random stranger he’d meet at some psychology seminar, or an audience member at one of his lectures. He could force the charm in order to have the itch scratched, and then go back to mostly ignoring them.
But Y/N was an exception — an incredibly beautiful, innocent, intelligent, and devoted exception who had taken Jonathan by rare surprise as the first woman he’d met whom he couldn’t ignore, no matter how hard he tried. Beyond her beauty, it was her obvious, sweet innocence and warmth that was a stark contrast to his cold, calculated life. And he couldn’t let her get too friendly with anyone else, because as soon as he’d acknowledged to himself the effect she had on him, he immediately decided she’d be his. She was pure, innocent perfection who was always dying to please him, and no way would he lose this rare gem to some pathetic, average moron. No; she’d be his, and his alone.
His to corrupt, control, and break apart. Never before had the desire and the urge to possess a woman been so strong as it was with Y/N, and he wanted her to fold for him the same way he got each and every one of his patients to fold. Except with Y/N, she’d break down and surrender not from the suppressed memories Jonathan would make her recollect or the trauma she’d work through, but from the way he’d adoringly groom her perfection and desire to please while also calling out each naughty desire and secret thought she assumed he was completely oblivious to, but was, in fact, all too well aware of.
And he knew that that right there was why he was so drawn to her. A gorgeous, innocent woman he could groom and corrupt, who would hang on his every word and be naughty only for him. In Y/N, Jonathan saw the alluring contrasts in her of being so pure, yet also having such lustful, sexual desires. She was sweet yet tempting. A good girl, but with the yearning to be corrupted. It all tapped into his own exact fantasies and desires.
Of course, none of this had ever been voiced by her, but Jonathan had spent the last year analyzing everything about her, and he just knew that Y/N had a yet-to-be-tapped sexuality. He knew she was no virgin, but she also clearly had never let all her inhibitions go. And who better to help her do that than him? And their latest trial project would only help along the way. After all, as brilliant as his methods were, he had no qualms about a little liquid assistance to get her there faster.
As they entered his office, Y/N did as she always did and sat at the computer at his desk. When they were together, Jonathan rarely sat there, instead having Y/N enter information and type up notes of whatever he would dictate to her while he sat in one of the chairs opposite his desk instead. Then she’d leave to hurry off to the lab to complete whatever work he’d assigned for her there, where he’d then check on her progress later on.
“Pull up the results of the latest trial for the Thiopental,” Jonathan told Y/N after she’d situated herself in his desk chair. “I want to compare the males’ reactions versus the females’.”
Obediently, Y/N clicked through a few screens before pulling up the records Crane was asking for. But upon opening the file, Y/N noticed that the total number of participants had been lessened by one. Rather than results from one hundred individuals – fifty males and fifty females – the final line in the female column had been deleted.
“Ummm, Dr. Crane, did the results get edited recently?”
Turning from across the room, Jonathan looked at Y/N as he replied.
“Why do you ask?” he said.
“Well, there are no longer fifty female entries like there were when we first received this data,” Y/N answered him. “There are now only forty-nine.”
Walking over to where Y/N sat, Jonathan stopped beside her and leaned over the desk. Of course, he knew exactly what was going on – he’d deleted the last female entry – but he hovered over Y/N and gently but firmly laid his hand on top of hers as he gripped it and moved the computer mouse with her.
“Let’s have a look,” he said softly beside her.
Y/N’s heart was pounding and her hand was warm beneath his. With him standing this close, she could smell his cologne and feel the heat from his body. It was both unnerving and inviting, and she dared not move as he remained close, although it was taking all her strength not to turn her head into his chest.
Jonathan could sense every nerve in Y/N’s body pulsing. It was the exact reaction he expected from her, and it was clear she was both nervous and aroused. As his eyes darted to her, he could nearly feel the softness of her hair in his fingers as he’d sweep it behind her shoulder and expose her neck. That porcelain skin of hers would have a trail of goosebumps wherever he’d touch her.
After briefly scrolling through the file with her and acting somewhat surprised, Jonathan stood up and pulled back, and then Y/N finally moved again, looking at him questioningly.
“Open my emails; perhaps there’s an explanation from someone in there,” he said to her.
Doing as he said, Y/N opened his emails and quickly found one with the subject line: “Thiopental Participant Withdrawn.” Upon reading it aloud to Crane, Y/N learned that one of the female participant’s results had been discarded due to the discovery that she had consumed alcohol within an hour of her participation. That wasn’t allowed, as they wanted results from people who had no other influencers in their systems at the time of the trial.
“Does this mean the entire study will have to be discarded?” Y/N asked Jonathan after she’d finished reading the email. Little did she know that he’d composed and sent the email to himself.
“No,” Jonathan replied as he looked at her, her beautiful face looking crestfallen. “We’ll just have to collect results from a new female participant within the same parameters of all the others: non-pregnant, non-smoking, without any heart defects or complications, and a system free of any other influencers, approved by their primary physician and conducted in a controlled environment.”
Nodding, Y/N looked down at her watch, noticing that it was already nearly the time most people went home for the day.
“I can ask around the lab tomorrow if any of the female staff would be willing to do it,” she said to him. “I’m sure I can find someone.”
“I’m sure you can,” Jonathan replied then, looking at her with that rare smile that left Y/N feeling both excited and nervous. “You never let me down, Y/N.”
Blushing fiercely with a shy little smile, Y/N could only hold his eyes for a moment before she had to look down. Only on the rarest occasions would he call her “Y/N” instead of “Miss Y/L/N”, and it always had the same effect on her.
“Well, I should get to the lab then and start preparing the vials for the next trial,” she said as she pushed his chair out from the desk and stood up, her face still flushed as she closed his laptop.
“Don’t forget your key card this time,” he said to her with another rare moment of affectionate teasing.
Lifting her eyes to look at him, Y/N once again gave him that shy little smile.
“I won’t.”
•.•.•.•.•
Despite the trial only requiring one hundred vials of the Thiopental, dosing them out was time consuming work. Not only did Y/N need to transfer milliliter after milliliter of the clear liquid into one hundred empty vials with a dropper, but they each then needed to be capped, sealed, labeled and packaged with an accompanying syringe needle.
After three hours of work, she’d finally made it to the last tray of empty vials, and she sat up straighter in her stool to momentarily stretch her back and rotate her neck. She shivered beneath her lab coat, the chilly air of the sterile, empty lab offering little warmth. She was just about to resume her work when she heard the door to the lab open behind her. Turning to look over her shoulder, she saw Dr. Crane walk in, allowing the door to close behind him before he headed her way. What she didn’t realize is that he also ensured it was locked. Despite the fact that it was past 8:00 p.m. at this point, he wanted the guarantee of absolutely no interruptions.
“How’s it coming?” Jonathan asked her as he approached and then came to a stop beside her.
“Nearly finished,” Y/N replied, glancing back down to the vials before looking at Crane again.
“Well, perhaps it’s time for a little break,” he said as their eyes met once more. “I think I’ve come to a solution for our issue with the previous trial that will keep you from having to find a new participant.”
“Oh?” Y/N asked with interest, sitting up straighter in her stool. “How so?”
Looking at her intently, Jonathan spoke again.
“You can do it,” he said.
Furrowing her brow, Y/N shook her head in confusion.
“I can do what? You mean...me be the participant?” she asked in surprise.
“Yes,” Jonathan replied firmly, stepping slightly closer to her.
“But I...” Y/N began. She would never have expected Dr. Crane to suggest her being involved in this way. Wasn’t it some kind of foul for the individuals running the trial to be involved?
“But I’m involved in the study,” she continued then. “I can’t be a participant.”
“This is a study that’s simply tracking effect and reactions,” Jonathan replied, keeping his tone matter-of-fact. “There’s no issue of conflict or biases. We’re simply seeing how your body responds.”
Suddenly, Y/N’s heart began pounding. He was speaking as if he’d already decided she was going to do this, and it had her feeling nervous for some reason. Not unsafe, but just…
“I...I don’t think I should,” Y/N replied. “I mean, when would we even do this?”
“Right now,” Jonathan answered with certainty.
“Right now? But…but who’ll track the results? We have no one else here to record anything.”
Jonathan gave her an amused smile.
“I think I’m more than capable of keeping track of one woman’s responses to a single, one-time use drug, Y/N,” he said to her. “We don’t need anyone else.”
Her heart was still pounding, and she didn’t miss the fact that he used her first name again.
“I don’t have an approval from my primary physician — we have to provide that for them to consider the results valid and prove that it was done safely.”
Jonathan gave her another look.
“I’m a doctor, Y/N. I can properly administer a shot, and I can attest here and now that you are in a safe environment and are a valid participant. That is, unless you’ve suddenly become pregnant, had a pacemaker implanted, or begun smoking since you were last in my office.”
Despite her nerves, Y/N couldn’t help but release a short laugh at Jonathan’s little joke as she looked down at her lap. It was odd to see him suddenly so...casual, but it was also very appealing. Her cheeks rosy, she looked up at him through her lashes.
“No, none of those things,” she said with a shy little smile.
“Then there’s no issue. I’ll administer it to you, observe the effects, and we’ll add the results to the trial. This way, we won’t waste time finding someone else and waiting for their physician’s note.”
“I…” Y/N didn’t know how to respond. She badly wanted to appease Jonathan, but she was also nervous. The idea of being so vulnerable in front of him was nerve-wracking. Granted, he would only be keeping track of things like her pulse, blood pressure, and reflexes, but what if she accidentally said or did something embarrassing?
“You’re perfectly safe with me, Y/N,” Jonathan assured her, his tone soft. He looked in her eyes and he could see the slight uncertainty, but more so the desire to please him. Just like always.
To Y/N’s surprise, Jonathan reached out then and gently laid a hand over hers.
“You know you can trust me, don’t you?”
Looking down at his hand on hers, Y/N’s heart skipped a beat. The mood in the room had changed entirely, and she wasn’t quite sure what was happening. But she knew she could never say no to him.
“Do you trust me?” Jonathan asked as she looked back up at him.
Taking in a deep breath, Y/N pulled herself out of falling into those eyes of his and quietly answered him, her hand still beneath his.
“Yes,” she said.
Slowly smiling, Jonathan squeezed her hand.
“Good girl,” he said.
Y/N blushed fiercely at his words, feeling incredibly nervous but also giddy and pleased at his praise. Again, this was a side of him that had only ever appeared in her private fantasies, and the fact that he was suddenly looking at her the way he was felt almost surreal.
Reaching up behind her on the shelf where the leftover vials of the Thiopental from the first trial sat, Jonathan pulled one down and set it on the lab counter. He then opened the container of supplies kept beneath the shelf and retrieved an alcohol swab and some rubber gloves. Still sitting in her stool, Y/N watched his every move.
Jonathan had her right where he wanted her, and he was even more in tune than usual with every silent signal Y/N was giving off. As he placed the supplies on the counter, he looked at her again.
“Sit up on the counter,” he softly ordered her. “Then your arms will be level with mine.”
Self-consciously, Y/N shifted, trying to be as graceful as possible in the skirt she was wearing. Bracing her palms behind herself on the ledge, she then hoisted herself up from the stool onto the countertop, scooting back slightly as her legs dangled over the edge. She was now at the same level as Jonathan, and she awkwardly cleared her throat as their eyes met again.
“Let’s lift this up, shall we?” Jonathan then said to her as he reached forward for the sleeve of her lab coat and pushed it up to reveal her forearm, folding it behind the bend of her elbow.
Goosebumps immediately appeared where Jonathan’s fingers brushed her skin as he adjusted her sleeve, and Y/N blushed, not looking at him but knowing there was no way he didn’t notice her reaction.
“Will you…I mean…we’re just tracking things like my vitals, right? That’s it?” Y/N asked him quietly as she watched Jonathan put the gloves on.
“Why?” he teased her then as their eyes met again. “Keeping some secrets, are we?”
Blushing again, Y/N couldn’t speak, instead only pursing her lips as she shook her head.
“Don’t worry,” Jonathan said then, “something tells me we’ll get the answers we’re looking for.”
Y/N’s heart pounded again at his words, not even knowing how to respond. She felt the way she always felt around him, both nervous and protected, but it was magnified by about a hundred, and although his words and actions were soft, Y/N still picked up on that hint of intimidation and danger he brought her. Somewhere in the back of her mind, she acknowledged the fact that she was squeezing her thighs together beneath her skirt.
He could see her pulse point at her neck jumping, and he spotted the tightening of her muscles as she squeezed her thighs together atop the counter. As he reached over her once more to grab a syringe and needle, he intentionally lingered, and he heard her inhale near his neck as he did, smiling to himself at her response to him.
Donning the rubber gloves, Jonathan then assembled the needle and syringe, then loaded it from the vial, Y/N's eyes on his every move. Setting the prepared syringe down momentarily, he then opened the packet containing the alcohol swab and then removed it, swiftly swiping it across Y/N's skin as she looked down at her arm. Goosebumps appeared again as she felt the cold piece of gauze on her skin.
Quickly disposing of the swab, Jonathan then retrieved the syringe once more, and just before bringing the needle to her skin, he gently grasped Y/N's arm with his free hand and looked at her.
"Ready?" he said, although it wasn't really a question.
Meeting his eyes, Y/N nervously bit her lip, but as she felt Jonathan gently squeeze her arm, she found herself nodding her permission.
Wincing at the sudden sting from the prick of the needle, Y/N briefly squeezed her eyes closed, but forced herself to inhale steadily as she felt the liquid enter her vein. Jonathan lifted his eyes from her arm to her face as he finished pushing the last of the dose through the syringe, and he smiled to himself once more as he knew it was now only a matter of minutes before he'd get everything out of her.
Gently retracting the needle from her arm, Jonathan placed a clean square of gauze over the site on her skin to catch any small bit of blood that may have followed, and Y/N automatically lifted her other hand to hold it there as Jonathan disposed of the syringe.
"Don't be nervous, sweetheart," Jonathan said as he saw Y/N watching him again, her face slightly pale and her nerves clearly affecting her. "You know exactly how all of this works."
Completely taken aback by the unexpected term of endearment, Y/N's heart raced again, and she felt both giddy and hazy. While it was supposed to take several minutes for the drug to reach full effect, she'd had no idea how quickly it would begin taking over her system. She already felt somewhat like she was functioning in slow motion, but with Jonathan's unexpected affection, her heart was still pounding like crazy.
"We'll just give that a minute to take full effect," he said then, very nonchalantly.
Still speechless, Y/N watched as he opened a cabinet off to the side and pulled out a pulse oximeter and electronic blood pressure cuff. Preparing to use each of them, Jonathan then shifted again and stood directly in front of her, and Y/N lifted her head to meet his eyes.
"How do you feel?" he asked her, the clinical tone back in his voice.
It took her a moment before she could respond, the words taking their time to travel from her brain to her lips.
"Slow," she said simply as she looked back at him. "Everything feels slow."
Nodding, Jonathan picked up the items he'd retrieved from the cabinet. He spoke as he placed the oximeter on her finger. Although he had no real intention of tracking any of this, he wanted to give her another minute to absorb everything. After the oximeter beeped with her numbers, he placed the blood pressure cuff on her wrist.
"How about now?" Jonathan lifted his eyes from the display on the cuff to Y/N's gaze. "Your heart's racing. Not common, seeing as this typically causes the opposite effect on heart rate. What's got you so nervous, hmm?"
Y/N felt somewhat cloudy, still fully functional, but once again in almost a slow motion way, as if everything she thought and did took twice as long. After registering his question, she answered Jonathan with the first thing that came to her mind.
"You," she said simply.
Jonathan smiled, giving her a look that only intensified her pulse, and he then reached up and removed his glasses, placing them in the breast pocket of his blazer.
"Me?" he questioned her teasingly. "Do I make you nervous, Y/N?"
"Yes," she answered after a beat, still looking at him. She found that if she tried to turn her head too fast, it made her feel woozy.
"Hmm," said Jonathan, and then he reached down and slowly unstrapped the cuff from her wrist.
Thoughtlessly lowering her arm, Y/N took another deep breath, feeling her pulse in her veins.
"Let's just start with the typical questions," Jonathan said then, and although he'd told her they'd only be monitoring her vitals and outward reactions, Y/N had no ability or desire to stop him from asking her anything.
"Tell me your full name and date of birth," Crane said as he looked at her. After a moment's beat, Y/N responded with the (obviously) correct answers.
"And what's your home address?"
Again, she rattled off the information after a second's pause.
"Now tell me, how long have you worked for me?" Jonathan said then, his tone changing slightly.
"Just over a year," Y/N replied, and then she noticed somewhere in the back of her mind that she was beginning to feel very hot.
"Right. And for how long of that year have you had sexual thoughts about me, Y/N?"
Despite her body heat, Y/N could feel her face suddenly blanching, but as she processed his question, there was only one possible outcome: the truth.
"The entire time," she said, and she felt the blush creep back over her skin as the words left her mouth.
"Are you surprised that I just asked you that question?" Jonathan asked then, that smile of certainty taking over his face again.
"Yes," Y/N answered, her heart skipping a beat as she saw him smile. She was now fully aware that she was powerless to say anything but the truth.
Reaching out to her then, Jonathan gently laid a palm on Y/N's thigh and slowly slid it up her leg, over the fabric of her skirt. Then their eyes met again.
"You thought I didn't know, didn't you?" he affectionately teased her, his voice soft and his gaze intense as he looked at her. Somehow, his entire aura was contradictorily both soothing and intimidating, and Y/N's breath hitched in her throat as he stepped even closer.
Automatically, and feeling somewhat hazy, she spread her legs to allow Jonathan to stand between them, and as he did, he reached up and gently grasped her chin, stroking it with his thumb as he looked down at her. Nervously, Y/N's eyes kept darting between his eyes and his lips, her heart pounding and her body flushed as he spoke again.
"Trust me, sweetheart, I knew. I've known all along."
His affectionate words and his touch once again caused Y/N's heart to race, and she felt both embarrassed and helpless, but also immediately aroused.
"I'm sorry," she heard herself say suddenly, and Jonathan, still grasping her chin, looked at her with that unnerving affection.
"Sorry for what?"
"For thinking about you like that," she said. "I tried not to."
Chuckling, Jonathan released her chin, instead planting each of his palms on top of her thighs.
"You don't have to apologize, sweetheart," he spoke soothingly, giving a small shake of his head. "After all, why apologize for something we both want?"
"What do you mean?" Y/N asked, and she felt a dampness forming in her panties as Jonathan squeezed her thighs.
"You're quite the little eye-catcher yourself, Y/N," he responded. "I've spent the last year watching every move you make, every look and unspoken hint. And never before has anyone caught my attention quite like you."
Floored at this admission, Y/N could only say one thing.
"Really?"
Chuckling again, Jonathan leaned in closer to her.
"Isn't that what you were always hoping to hear?" he asked her.
Her eyes quickly darting to his lips, Y/N then responded.
"Yes," she answered him. "I wanted to be perfect for you. I wanted you to want me."
Immediately, Jonathan's cock twitched in his pants at her admission. Despite knowing this information already, nothing beat hearing it come straight from her lips.
"And is that still what you want now?" he pushed her.
She nodded her head.
"Yes."
Smiling again, Jonathan pulled back from her slightly, noting the heat radiating from her body.
"You're burning up," he said as his eyes dropped to the buttons on her lab coat.
"I'm hot," she confirmed mindlessly.
"I can see that," Jonathan replied.
"Is that bad?" she asked, concern momentarily replacing the desirous look on her face.
"It's expected," Jonathan dismissed with a shake of his head. "Your body's trying to compensate for the delayed response signals by sending blood through your system more quickly."
"I'm hot," she repeated dumbly, unable to comprehend enough of what Jonathan had said, as her brain was processing everything slower.
"We can take care of that," Jonathan replied, and he reached up and began undoing the buttons on her lab coat. He intentionally went slowly, savoring the moment of finally undressing her as he'd imagined a million times.
After her coat was open, Jonathan reached up again and slipped his hands underneath the shoulders, slowly pushing it off her and down her arms. Silently, Y/N turned her head and watched as his hands pushed the coat off her body. But instead of stopping there, Jonathan then lifted his hands once more and hovered them over the buttons of her blouse.
"Should we take this off, too?" he asked her — again, less so for permission and more so just to hear her response.
"Yes," she replied, and nodded her head.
As Jonathan worked at the new set of buttons, the porcelain skin of Y/N's chest and the lace of her black bra was gradually revealed, causing Jonathan's cock to stiffen further.
“Have you thought about me undressing you before, Y/N?”
She could feel his cool fingertips grazing her skin as he worked down her chest.
“Yes,” she replied. Her heart was pounding and her nipples stiffening at his question.
“And when you think about that, how does it make you feel?”
Of course, there was still nothing she could do but tell the truth. As badly as her mind wanted to come up with an answer that wouldn't embarrass her, she couldn't form the fib; she could only voice facts.
"Excited," she replied, "but nervous, too. I like to think about it."
"What else do you like to think about?" Jonathan urged her as he undid the last button on her blouse. Y/N's face flushed again as she answered him.
"You kissing me and touching me. I think about having sex with you and what it would be like."
"And do you enjoy those thoughts?" Jonathan placed his palms back on her thighs, her blouse hanging open.
"Yes," she replied.
"Why?"
"Because I like you. You're so handsome and sexy. You make me feel safe but scared at the same time. I like that. I think about what you'll do to me."
"And what do you think I'll do to you? Tell me."
Again, Y/N was helpless to anything but the truth.
"You'll kiss me. Touch me everywhere. I think about your hands on my breasts. Or your lips on me. You'll put your fingers inside me and then your cock. You'll pull my hair or spank me. You'll fuck me and make me come. Then you'll come inside me."
After each mini declaration she made, Jonathan's cock stiffened further. Lifting his hands to her shoulders once more, he slipped under her blouse and pushed it off her, fully revealing inch after inch of her flawless skin, enhanced by the contrast of her feminine, lacy black bra. Her breasts molded perfectly to the cups and he could see her hardened nipples through the material.
"And how about if I tell you to do something? Would you do it?" Jonathan prompted her.
"Yes," Y/N said.
"You want to please me, don't you, Y/N? Make me happy with you? Do anything I say?"
"Yes."
"You just want to be my good little girl, don't you?"
"Yes," Y/N nodded at that, feeling her body flush again. "I want to be yours."
Lifting his hands to her face, Jonathan cradled Y/N's cheeks in his palms and looked in her eyes as he spoke.
"Oh, I want that, too, sweetheart," he said. "And we can make that happen. Would you like me to fuck you right now?"
Y/N nodded again, having a hard time believing this was actually happening. "Yes."
Lowering one hand from her face and moving the other lower, he grasped her chin again and held her eyes as he spoke.
"You're going to be a good girl for me, Y/N. You're going to let me do everything to you that I want. You're going to show me how much you enjoy it. You belong to me now, do you understand that?"
"Yes," Y/N replied, her heart pounding at Jonathan's words. Unintentionally, she slightly arched her back, subtly pushing her chest out towards him.
Jonathan smiled again.
"So eager," he cooed. "You've been mine all along, haven't you?"
"Yes; always," Y/N said.
At that, Jonathan lifted his hands once more and reached behind her, making quick work of opening her bra clasp. As the garment loosened around her, he traced his fingers up her arms and hooked them under her straps, hesitating for a moment as he savored her reaction.
"Shall we take this off?" he teased her.
"Yes," she answered quickly, her voice sounding desperate. "Please!"
Jonathan shook his head with another smile.
"Always so well-mannered. My good girl."
Pulling the bra from her chest, Jonathan dropped it to the floor and his eyes were glued to her breasts. They were plump, pert perfection, her nipples hardened from equally both arousal and the chill of the cold, sterile laboratory.
"Do you want my hands on you, Y/N?" he asked her, his palms already only centimeters away from touching her.
"Yes," she nodded fervently.
Immediately, Jonathan cupped her breasts, squeezing her flesh as it filled his hands and stroking her eager nipples with his thumbs. With every swipe along her buds, Y/N released a tiny gasp, arching into his touch.
"You like that, don't you?" Jonathan asked her as he lifted his eyes to hers. He could feel his cock straining against his pants as he watched her arch into him again.
"Yes," she replied. "I don't want you to stop."
Jonathan shook his head.
"Oh, we're not stopping until I have you coming, sweetheart," he said to her soothingly. "That's what you want, isn't it? For me to make you come?"
"Yes," she replied, and she squeezed her thighs together again.
"Then let's make that happen."
His hands still on her breasts, Jonathan leaned forward and pressed his lips to Y/N's, her eyes fluttering closed as he came closer. As soon as his lips came in contact with hers, she released an audible sigh with a little whimper, and when Jonathan stroked her nipples again as their lips moved together, she leaned into him even more.
He started off gentle, but soon, Jonathan was kissing her with more aggression, the sounds of her desirous desperation and her needy reaction spurring him on. After a moment, he felt her squirming beneath him, and he pulled back to look at her.
"What is it?" he asked her.
"I..." Y/N blushed again. "I want to touch you."
Jonathan smiled.
"Then touch me," he said, then leaned in again and connected their lips once more.
Whimpering again, Y/N lifted hers arms to Jonathan's neck, wrapping them around his shoulders as she scooted closer to him. Her bare breasts rubbed against the scratchy fabric of his blazer, and as she leaned into him, Jonathan lowered his hands to her hips and pulled her to the edge of the counter. Her fingers hovering over the nape of his neck, she suddenly tangled them in his hair and tugged in surprise as she felt Jonathan squeeze her hips.
Pulling away from her once more, Jonathan grabbed the fabric of her skirt and slowly began pushing it up her thighs, watching her every reaction as he did so.
"Let's see just how excited you are," he said. He then pushed her skirt the final inches to bunch it up around her hips, revealing her smooth thighs and the black panties clothing her pretty little mound.
"Spread your legs for me, sweetheart," Jonathan ordered her, and she obediently responded, parting her thighs fully to reveal herself. The subtle yet obvious patch where her arousal had temporarily stained the fabric of her panties darker immediately causing another smirk to cross Jonathan's face.
"So wet, aren't you?" he affectionately teased her. "So wet and so ready." He hovered his fingers over her. "Do you want my fingers, Y/N?"
Her breath hitched slightly before she answered.
"Yes," she said in a breathy reply. "I want them inside me."
Slowly, Jonathan skimmed his fingertips over the fabric of her panties, eliciting a whimper and a thrust of her hips as Y/N felt him touch her. He then lifted his fingers to the hem of her panties and hooked them inside. Instinctively, Y/N briefly lifted herself off the counter to allow him to remove them, but then her mind was completely blank when she suddenly felt Jonathan's fingers delve inside her dripping folds.
"Ohhhhh," she moaned, and her hips thrusted again as Jonathan curled his fingers inside her, the lewd sounds of her wetness accenting the air as he began pumping his fingers in and out of her.
Her pussy was soft, pink perfection, and Jonathan curled his fingers again as she moaned over and over.
"You're not just wet; you're soaking," he said to her, his cock now rock hard as he watched his fingers moving in and out of her. Whining again, Y/N grasped the edge of the counter with her hands and slightly leaned back, pushing her lower half closer to Jonathan, seeking more of his touch.
"Such a needy girl, aren't you?" Jonathan said. "You want more, don't you?"
"Yes," she nodded, her face twisting in pleasure as Jonathan pumped his fingers faster. "More." She was panting now.
"Wait until my cock is inside you, sweetheart; you'll take it so well. You're going to let me fuck you, aren't you?"
"Yes," Y/N answered, her pussy clamping around his fingers. When Jonathan curled them inside her once more and moved his index finger back and forth, an embarrassingly loud moan escaped her, the pleasure incredible as he hit that spot inside her.
"Tonight, I'll take you right here, but next time, you'll be bent over my desk," Jonathan said to her as he withdrew his fingers from inside her and swiped his thumb over her clit instead, eliciting another loud moan from her. "This pussy is mine now, to take whenever and wherever I want. Do you understand me, Y/N?"
"Yes!" she cried out desperately as he swiped her clit again. Her head was still slightly foggy, and Jonathan's words had her ready to do anything he said. Her heart was racing as she met his eyes.
"You pretend to be so proper, but you're a naughty girl," Jonathan said. "I've known it all along, and we're finally going to see just how naughty you can be. Tonight, we'll take it easy, but next time, we'll see how far we can push you. I think you'll like that, won't you, sweetheart?"
"Yes!" Y/N cried again, Jonathan's thumb repeatedly circling her clit having her eyes practically rolling to the back of her head.
"You sound like a broken record, you know that?" Jonathan said then. "So pathetic." He ceased his ministrations then, and at the loss of his touch, Y/N was practically crying.
"Jonathan..." she breathed out in need, feeling so delirious that she didn't even realize she'd said his first name, which she'd never done before. "Jonathan, please!"
At the sound of his name falling from her mouth in that desperate, whiny voice, Jonathan was moving his hands to his belt, unfastening it and opening his pants, the clinking sound of the buckle sounding louder than it actually was in the otherwise empty lab. He then reached out and grasped Y/N's wrist, pulling her hand to his briefs and slipping her hand inside, guiding her to palm his bulging cock.
"Please, what?" he said as he held her hand in place. "Is this what you want, Y/N? Do you want my cock?"
"Yes!"
"Where do you want it, sweetheart? Tell me."
"I want it inside me. I want to feel you fill me."
Feeling the heat of Jonathan's stiff cock, all she could think about was having him fuck her, and she knew that she would forever do whatever he told her as long as she could feel him inside her.
"Oh, I'm going to fill you, alright," Jonathan replied then, roughly pulling her closer again and hovering his mouth over hers as he spoke. "I'm going to fuck you, over and over again. You're going to take everything I give you, do anything I tell you to, and you're going to be my good little girl. Always."
Jonathan caught the obedient little nod Y/N gave just before he connected their lips again, and as he did, he raised a hand to grasp one of her breasts again, massaging her and pinching her nipple before doing the same to her other breast, and then moving his mouth to her neck, sucking her flesh and following it with a soft kiss.
"Jonathan..." she breathed again.
"Are you ready for my cock, sweetheart?" he said to her. "I think you are."
"Yes!" she said again for what felt like the hundredth time that night, and Y/N felt her wetness nearly ready to drip from her folds as she heard Jonathan draw himself out of his briefs. But her weeping pussy was then immediately met with the feel of his hot tip prodding her entrance, and she whimpered again.
"You're so ready," she heard Jonathan say, and then she was suddenly momentarily thoughtless from the insane pleasure of his cock slamming inside her.
"Ahhhhhh!" she cried out, feeling like she was being split in two as Jonathan's hips became flush with her inner thighs. She desperately reached up and grasped the lapels of his blazer, the stars in her vision slowly disappearing as she continued to adjust to the feeling of him inside her.
"Ohhh, fuck, sweetheart," Jonathan groaned as he felt her walls clamp needily around his shaft. She milked him without even trying, and he immediately began thrusting in and out of her, unable to go slow any longer.
"Oh, God!" Y/N panted as she gripped his jacket tighter, instinctively wrapping her legs around Jonathan's waist as he began to move in and out of her. She clamped around him with each thrust, and she felt his fingers digging into her hips as she began to meet each of his thrusts with a rutting of her hips.
"That's my girl," Jonathan said roughly as he continued to fuck her. "You're taking me so well."
Her chest was heaving, and Jonathan was having a hard time deciding where to focus his eyes — on her beautiful breasts bouncing with each thrust, on her plump, parted lips as she gasped again and again, or on the sight of his thick cock slamming in and out of her drenched pussy. But he relished in knowing that he'd now have countless opportunities to see each of these delicious sights again and again. He'd fuck her silly before he'd ever have enough.
In the future, Jonathan would draw everything out, make her squirm, whine, and downright beg for release. But tonight, he'd make it easy on her, to show her just how good he could make her feel...if she earned it.
Returning his thumb to her clit, he again stroked her over and over, and as he watched her face beautifully contort in pleasure again, he grabbed a fistful of her hair with his free hand, tugging it roughly enough to force her to look at him, her eyes widening in surprise and mild pain as his pull on her strands stung her scalp, causing her to whimper again.
"Do you want me to make you come, Y/N?" Jonathan asked her as she met his eyes.
"Yes! Please!" She thrusted her own hips into his once more, seeking what he was offering her. "And...and..." she couldn't think anymore.
"What?"Jonathan asked her. "Say it, Y/N."
She clamped around him again.
"And I want you to come inside me."
Jonathan gave her a devilish smile.
"Oh, sweetheart, that was happening whether you asked or not," he replied. "You're going to take every last drop from me."
Her heart racing, Y/N nodded obediently once more, and with a final, sloppy kiss between them, Jonathan then resumed his previous pace, fucking her hard and fast as he alternated between stroking her clit and pinching her nipples.
With every touch and thrust, Y/N could feel herself unravelling more and more, and she reached up and desperately gripped Jonathan's shoulders as she felt herself nearing the edge.
"I...I...Jonathan, I..."
"Are you close, sweetheart?" he urged her.
"Yes," she panted.
Jonathan once more pulled her closer, his hands on her hips like a death grip as he prepared to bring her over the edge and finish inside her.
"You're going to come now, Y/N," he commanded her.
In a final push of thrusts and strokes, Jonathan had her mewling in desperation, and when he lowered his mouth to her neck and nipped her skin as he thumbed her a final time, he felt her suddenly clamp around him with insane tightness as a high-pitched squeal escaped her and her body tensed.
Her eyes squeezing shut and her heart pounding in her ears, Y/N was once again thoughtless, only registering her reactions and responses to how Jonathan fucked her with intention, and when he commanded her in that voice and bit at her neck, she was gone. As his thumb stroked her a final time through her wetness, she came with an uncontrollable squeal, gripping his shoulders so tightly that her knuckles were white against the fabric of his jacket.
Hearing her desperately pleasured whine, Jonathan let himself go, pounding into her with determination until he himself was coming, releasing inside her with rope after rope of cum, imagining each bit of his release painting her walls and marking his territory as he finished inside her, hearing her still panting against him as he groaned deeply.
Eventually, the sounds between them lessened, and finally, Jonathan pulled himself out of her as Y/N's hands slipped from his shoulders, whimpering a final time as she felt him leave her body. After tucking himself back inside his briefs, Jonathan fastened his pants again and re-buckled his belt before looking up at Y/N, who still sat on the counter before him, slightly shivering with her naked chest still exposed and her legs still spread, her bare pussy leaking with his cum.
When Y/N lifted her eyes to meet his, Jonathan reached up and stroked her cheek before he spoke again.
"It shouldn't be long now before the effects fully wear off," he said, reaching for her lab coat on the counter, picking it up and encouraging her to slip her arms through the sleeves as he helped her put it back on, never minding about her discarded bra or blouse. He buttoned it for her before then lowering his fingers to her pussy and pushing his escaping cum back inside her. Y/N's thighs quivered as his fingers briefly entered her again.
"Once you're feeling back to normal, I want you to go home," he continued softly. "Don't wash yourself until the morning. Then I want you to come straight to my office tomorrow, and I want you wearing nothing but that lab coat. Do you understand?"
Pressing her thighs together, Y/N silently nodded, her heart still pounding.
"Good girl," Jonathan said, and he stroked her cheek one more time before leaning closer once more.
"You'll always be my good girl, won't you?" he said to her, already knowing her response.
"Yes," Y/N breathed out, nodding slowly. "Always."
With a satisfied nod, Jonathan leaned forward a final time, placing a soft kiss on Y/N's lips before backing away. Then he turned and walked out of the lab, Y/N looking after him as she slowly began to feel like herself again, but with a renewed desirous anticipation, wondering just exactly what Jonathan had in mind for her next.
@nyxxie-pooh @xsweetcatastrophe @allie131313 @empatheticlove @febris-amatoria
@hannibellector @meister95 @alltoowellbeneaththemangotree @beastofburdenxo @aphroditeslover11
@galactict3a @lyarr24 @wild-rose-35 @4ria790 @judig92
@cillmurphyslover @ladyvenera @karah-bear @k1ng-l3on @ceirinen
@peskybinders @fuseburner @shaddixlife @neonpurplestars89-blog @garrison-girl-08
@devotedlyshadowytheorist @mischievouslittlecreature @muhahaha303 @mostly-marvel-musings @cillianmurphyfanatic
@an-eclectic-of-mass-destruction @vervainandspritz @novashelby @wonderlanddreamer @honeymoon8
@cardan-official @pkmonka @meadows5 @mamawiggers1980 @fmo166
@vastcapacity @mspookington-blog @teawonderfultea-blog1 @fkmarrycill @sl-newsie
@mrs-bond @shopgirl6us @cillianbabe @myers-meadow @fracturedhaven
#kinktober 2024#kinktober#jonathan crane#jonathan cran fic#jonathan crane x female reader#jonathan crane x reader#jonathan crane smut#cillian murphy
298 notes
·
View notes
Text
Maybe it isn't that I actually hate medical professionals? They just suck and are weird sometimes, and a lot of them shouldn't be practicing, but I don't hate them as a group, like, personally.
What I hate is their ability to make my life harder in ways that are often completely opaque to me, and a lot of the crap things they do are not really possible to challenge. And I hate the fact that holding them responsible fort dogshit behavior in any way that will actually benefit me is almost always impossible.
And I also hate the fact that they have to do stupid things sometimes because that's how the system is set up, and those things sometimes mean patients actually get harmed. They aren't fond of that part either! They don't want the system to be the way it is! But they don't have a choice, so sometimes people like me get forced by bureaucracy into doing things that are re-traumatizing. And I can't imagine that feels good for them at all, knowing that their patients are sometimes only "consenting" because that bureaucracy will not let them be helped in any other way. Which isn't consent at all. I imagine that must be pretty traumatizing for them, too, sometimes.
If it were easier to actually access medical care without tremendous delays in this country right now I would have much less trouble finding providers who are good at what they do and are not horrible people, and who have clinic staff who can do their fucking job.
Oh and I also don't appreciate how evasive and unwilling to commit they are out of fear of being held to an answer that turns out to be inaccurate, but I can't make an informed decision about my own care unless they give me at least some information about probabilities and trajectories and typicalities. Genuinely, how the fuck am I supposed to navigate that shit. I get that some patients are really fucking difficult, but I should be able to get a special stamp on my file or something that says I understand that sometimes medicine isn't an exact science and the best answers that my doctors can give may not always prove to be accurate in the long term. I know they don't like being in that situation either.
A lot of medical professionals are fucking assholes, and unfortunately the ones who are not are still hamstrung by a system set up to actively prevent people from getting care.
I miss my old doctor. He gave no shits about anything that wasn't the patient. He prescribed scheduled meds based on what the patient needed and not based on fear of consequences potentially being imposed on him by the punitive patient-hostile drugs-are-bad moral panic machine developed to force suffering people into buying more dangerous drugs off the street in order to prevent far fewer people from maybe getting high off of drugs that at least weren't laced with lethal substances. (The purpose of a system is what it does.) Did he get sanctioned and become locally unhireable? Unfortunately yes he did. Does he now provide concierge care to rich people? Yes he does. He found a way to make it work, God bless him.
Everything about the medical system in this country is fucked. Hospitals, doctors, nurses, pharmacies, pharmacists, pharmacy techs, phlebotomists, clinic administrative staff, insurance companies, medical schools and schooling, licensing boards, drug advertising to both providers and patients, pharmaceutical reps, researchers, research, publishing, medical trials, pharmaceutical companies, manufacturers and distributors, medical equipment, charting software, billing and billing codes, diagnostic criteria, charity and low income services, accessible transportation, home care, the lack of independent individual patient advocates, dietitians and nutritionists, access to physical and occupational therapy and physical and occupational therapists, the massive bigotry of every kind rampant in every corner of the medical field, social work, senior care and assisted living, deprioritization of informed consent and harm reduction, disability applications, inaccessibility of medical records, especially psychiatric notes which are specifically allowed to be withheld from patients, lack of continuity of care for disadvantaged people, care that is equitably accessible to disabled people, telemedicine, patient portals, phone systems, clinic hours, every single aspect of inpatient and outpatient psychiatry, facility security, all sorts of things going on with therapists who are nevertheless probably the least malicious group of people in this entire charade, aaaaaand patients themselves.
Also hospital toilets that are too tall and make it literally physically impossible for me to poop while I'm there waiting for somebody to come out of surgery. I just needed to take a crap, guys. You didn't need to make the toilets so tall that my feet didn't even touch the floor. It is very clean but there is no shitting for short people at St Francis.
348 notes
·
View notes
Text
The Best News of Last Week
1. ‘We are just getting started’: the plastic-eating bacteria that could change the world

In 2016, Japanese scientists Oda and Hiraga published their discovery of Ideonella sakaiensis, a bacterium capable of breaking down PET plastic into basic nutrients. This finding marked a shift in microbiology's perception, recognizing the potential of microbes to solve pressing environmental issues.
France's Carbios has successfully applied bacterial enzyme technology to recycle PET plastic waste into new plastic products, aligning with the French government's goal of fully recycling plastic packaging by 2025.
2. HIV cases in Amsterdam drop to almost zero after PrEP scheme

According to Dutch AIDS Fund, there were only nine new cases of the virus in Amsterdam in 2022, down from 66 people diagnosed in 2021. The organisation claimed that 128 people were diagnosed with HIV in Amsterdam in 2019, and since 2010, the number of new infections in the Dutch capital has fallen by 95 per cent.
3. Cheap and drinkable water from desalination is finally a reality

In a groundbreaking endeavor, engineers from MIT and China have designed a passive solar desalination system aimed at converting seawater into drinkable water.
The concept, articulated in a study published in the journal Joule, harnesses the dual powers of the sun and the inherent properties of seawater, emulating the ocean’s “thermohaline” circulation on a smaller scale, to evaporate water and leave salt behind.
4. World’s 1st drug to regrow teeth enters clinical trials

The ability to regrow your own teeth could be just around the corner. A team of scientists, led by a Japanese pharmaceutical startup, are getting set to start human trials on a new drug that has successfully grown new teeth in animal test subjects.
Toregem Biopharma is slated to begin clinical trials in July of next year after it succeeded growing new teeth in mice five years ago, the Japan Times reports.
5. After Decades of Pressure, US Drugmaker J&J Gives Up Patent on Life-Saving TB Drug
In what can be termed a huge development for drug-resistant TB (DR-TB) patients across large parts of the world, bedaquiline maker Johnson and Johnson said on September 30 (Saturday) that it would drop its patent over the drug in 134 low- and middle-income countries (LMICs).
6. Stranded dolphins rescued from shallow river in Massachusetts
youtube
7. ‘Staggering’ green growth gives hope for 1.5C, says global energy chief

The prospects of the world staying within the 1.5C limit on global heating have brightened owing to the “staggering” growth of renewable energy and green investment in the past two years, the chief of the world’s energy watchdog has said.
Fatih Birol, the executive director of the International Energy Agency, and the world’s foremost energy economist, said much more needed to be done but that the rapid uptake of solar power and electric vehicles were encouraging.
---
That's it for this week :)
This newsletter will always be free. If you liked this post you can support me with a small kofi donation here:
Buy me a coffee ❤️
Also don’t forget to reblog this post with your friends.
11K notes
·
View notes
Text
Global Clinical Trial Storage & Distribution Newlife Medicals

Newlife Medicals provides global clinical trial storage and distribution services. Contact us for secure and efficient handling of your trial supplies.
#global clinical research organization#reference listed drug#comparator sourcing for clinical trials#Specialty Sourcing#named Patient Supply#rld pharmaceutical
0 notes