#mri compatible products
Explore tagged Tumblr posts
Text
Innovative MRI Technology With Stunning Life Impact
Magnetic Resonance Imaging (MRI) is a modern diagnostic healthcare standard. Its radiation-free, noninvasive imaging capabilities have transformed how physicians see and treat complex medical conditions. Yet, MRI is still out of reach for many people worldwide due to its high costs and unique requirements.
However, this limitation disproportionately affects low and middle-income countries, where MRI healthcare systems are preoccupied with resource balancing between advanced imaging technologies. A gap in accessibility is created because standard superconducting MRI scanners are limited by the availability of robust infrastructure, such as radio frequency (RF) shielding and high power consumption, for use. This article explores how the next wave of innovation in low-power and ultra-low field (ULF) MRI will go far in democratising life-saving diagnostic tools.
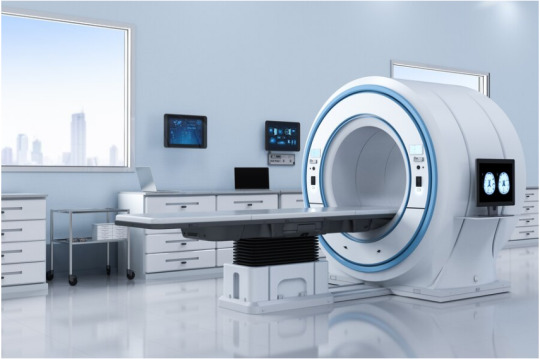
The Cost and Infrastructure Barriers
Conventional MRI systems typically require high magnetic fields (1.5 Tesla (T) to 7 T). However, these robust systems create high-resolution images at a steep price and operational demands like working in an RF shield room and consuming more than 25,000 watts of energy. On the other hand, recent advances in ULF MRI have brought a low-power, low-cost alternative to ULF MRI, as demonstrated by Yujiao Zhao and colleagues.
These innovative systems are operated utilising 0.05 T field strength and on standard wall power outlets with only 1,800 watts of a current draw during scanning. These ULF MRI systems solve electromagnetic interference problems using active sensing and deep learning technologies to provide image quality comparable to high-field MRI devices. The possibilities of such advancements enable their utilisation of smaller medical facilities or rural hospitals or clinics located in resource-constrained settings.
MRI Ambience for Enhancing Patient Experience
Technological advancements streamline costs and increase accessibility, but patient comfort in MR evaluations is fundamental to care. Improvements in patient experience have been made by innovations such as In-Bore MRI and MRI cinema for healthcare. These technologies integrate immersive audiovisual solutions, including MRI projectors and MRI-compatible displays, to create a soothing environment which reduces anxiety and increases cooperation during scans.
Patient relaxation virtual skylights, for example, create relaxing outdoor views in the confined MRI bore where the patient is situated. In addition, an MRI-compatible monitor prevents real-time visuals from interfering with the system’s magnetic field, providing patients with comfort and reassurance.
Functional MRI Systems Applications
Functional MRI systems measuring brain activity with blood flow changes have also undergone significant changes. Unlike traditional diagnostic MRI, advanced fMRI visual systems and fMRI monitors allow researchers and clinicians to perform detailed studies of neurological conditions through MRI. The MIT team demonstrates the adaptability of MRI technology to meet new healthcare and research needs with these innovations.
Portable MRI Solutions
Another game-changing development is the portability of low-field MRI systems. Their compact and lightweight designs require little logistical planning, and specialised reports are unnecessary. The MRI-compatible stretcher and wheelchair facilitate seamless interfacing with patient workflows, even in mobile or temporary medical locations.
MRI-compatible cameras and TVs further simplify operations by removing some of the obstacles that medical teams face in seeing, communicating, and doing their jobs, but without compromising the safety and operation of the MRI healthcare system.
Future Directions and Challenges
Even so, ULF MRI technology is in its infancy. Honest challenges are resolution refinement, noise reduction, and scalability. In addition, robust training programs for healthcare professionals are necessary to ensure effective use. Experts such as Udunna Anazodo and Stefan du Plessis point out that while low-field MRI has great promise, its success depends on the ability to prove its reliability and cheap enough for use in multiple clinical settings. The potential for ULF MRI to become a universally accessible diagnostic tool increases exponentially as these challenges are met.
Significant Changes with Small Impact
Healthcare developments include a shift towards patient-centred and environmentally sustainable practices by integrating new technologies, such as MRI-compatible displays and virtual skylights for hospitals. These advances improve diagnostics and address gaps in healthcare equity.
Kryptonite Solutions exemplifies this forward momentum. They can provide comprehensive MRI solutions that embrace innovation and the potential for accessibility. By focusing on supporting the advancement of healthcare technology, they are in the game to transform the way MRI systems serve global communities.
With this paradigm shift in MRI accessibility, comfort and innovation firmly underway, we are charting the future of a healthcare world that closes the diagnostic inequality gap.
#virtual skylights for healthcare#mri ambience#mri compatibles#fmri visual system#mri compatible products#mri compatible stretcher#mri compatible wheelchair#mri compatible#virtual skylights
0 notes
Text
The Future is Nano: Unpacking the U.S. Nanomaterials Market
U.S. Nanomaterials Market Growth & Trends
The U.S. Nanomaterials Market size was estimated at USD 3.03 billion in 2023 and is expected to grow at a compound annual growth rate (CAGR) of 14.0% from 2024 to 2030. The market is primarily driven by the rising demand for nanomaterials in the medical industry. A rapidly aging population, technological advancements, and increasing occurrence of chronic illnesses and surgical procedures aid the market demand. For instance, developments in electrocardiographic technology and rising cases of cardiovascular ailments are fuelling the use of cardiology equipment.
Nanomaterials have enabled the development of a variety of drug carriers for the controlled therapeutic agent delivery in chronic diseases including diabetes, atherosclerosis, pulmonary tuberculosis, and asthma. Medical equipment uses nanoparticles for diagnosis of several illnesses. Magnetite nanoparticles are used for scanning in Magnetic Resonance Imaging (MRI). Nanomaterials offer high functionality in nanomedicine applications as they allow efficient and targeted drug delivery, thus effectively addressing the shortcomings of conventional therapy.

The technological advancements in nanomaterials will revolutionize various industries owing to its wide range of applications such as the aerospace industry, which, in turn, is anticipated to augment the market growth over the forecast period. For instance, in February 2024, Penn State's College of Engineering broke a barrier in communication and power transmission by using acoustic metamaterial; a derivate of discrete nanoparticles, for the transmission of messages in enclosed metal spaces, such as metal containers carrying outer space samples back to Earth. Such technological innovations can revolutionize the aerospace industry.
Toxicity assessment of nanomaterials hinders the commercialization of nanotechnology. For instance, inert elements such as gold can become highly reactive at nanometre dimensions, leading to pro-pulmonary effects in lung tissues. An international standard has been developed for labelling consumer products containing manufactured nano-objects, which allows informed choices when purchasing and for the use of consumers. The U.S. federal government formed the National Nanotechnology Initiative (NNI) to establish a framework for shared goals, priorities, and strategies that help each participating company in the innovations related to R&D in nanotechnology.
Curious about the U.S. Nanomaterials Market? Download your FREE sample copy now and get a sneak peek into the latest insights and trends.
U.S. Nanomaterials Market Report Highlights
The medical segment dominated the market by accounting for the highest revenue share of 32.49% in 2023. This is due to the rising use in biomedical applications such as cancer and brain tumour diagnosis and treatment.
Carbon nanotubes held the second-largest revenue share of 24.5% in 2023. Nanotubes are commonly used as agents in targeted drug delivery applications.
Titanium is the fastest-growing segment and is expected to register the highest CAGR from 2024 to 2030. Titanium has corrosion resistance, high strength-to-weight ratio, and biological compatibility. Aerospace, petrochemicals, medical, architectural, and chemicals applications are the top end users of the segment.
U.S. Nanomaterials Market Segmentation
Grand View Research has segmented the U.S. nanomaterials market report based on material and application:
Material Outlook (Revenue, USD Million, 2017 - 2030)
Gold (Au)
Silver (Ag)
Iron (Fe)
Copper (Cu)
Platinum (Pt)
Titanium (Ti)
Nickel (Ni)
Aluminum Oxide
Antimony Tin Oxide
Bismuth Oxide
Carbon Nanotubes
Other Nanomaterials
Application Outlook (Revenue, USD Million, 2017 - 2030)
Aerospace
Automotive
Medical
Energy & power
Electronics
Paints & Coatings
Other
Download your FREE sample PDF copy of the U.S. Nanomaterials Market today and explore key data and trends.
0 notes
Text
High-Quality X-Ray Room Door Manufacturer in India | Radiation Safe & Certified
When it comes to safety in healthcare and diagnostic environments, installing the right X-ray room door is critical. These doors are specifically designed to shield radiation, protect medical staff and patients, and meet strict compliance standards. As a trusted name in the door manufacturing industry, Tufwud offers premium-quality, lead-lined X-ray room doors that combine functionality, durability, and regulatory safety.
What is an X-Ray Room Door? An X-ray room door is a specially constructed door designed to block radiation emitted from diagnostic machines such as X-ray, CT scan, MRI, and fluoroscopy equipment. These doors are lined with lead or lead-equivalent material to prevent the escape of harmful radiation from the imaging room.
The door typically includes:
Lead sheet insulation (1 mm to 3 mm depending on protection level)
Radiation warning signage
Seamless sealing and tight frame fit
Vision panels with lead glass (optional)
Compatibility with access control systems
Why Hospitals and Clinics Need Radiation-Proof Doors Healthcare facilities use various imaging technologies that emit ionizing radiation. Without proper shielding, this radiation can cause long-term health risks. Installing a certified X-ray room door is not just a safety measure but a legal requirement as per Atomic Energy Regulatory Board (AERB) guidelines in India.
Key Benefits of Tufwud X-Ray Room Doors: ✅ Lead Lined Protection – Available in different thickness levels based on shielding needs
✅ AERB Compliance – Meets all Indian regulations for radiation safety
✅ Durable Design – Made from high-grade materials that withstand wear and frequent use
✅ Custom Sizes – Tailored to fit existing frames and unique project requirements
✅ Optional Fire & Acoustic Ratings – Dual-purpose doors with fire or sound insulation
Applications of X-Ray Room Doors Tufwud manufactures radiation-proof doors for:
Diagnostic imaging centers
Multispeciality hospitals
Dental clinics
Oncology treatment rooms
Veterinary diagnostic units
Research and pharmaceutical labs
Industrial radiography zones
Whether you’re designing a new radiology wing or upgrading your diagnostic center, Tufwud provides tailor-made door solutions that ensure safety and longevity.
Material & Finish Options X-ray room doors are available in multiple finishes and configurations, such as:
Galvanized Steel or Stainless Steel
Wooden Finish (Laminated or Veneer)
Powder-Coated Surfaces
Sliding or Hinged Options
Automatic Door Systems
Vision panels made of lead glass can also be integrated to allow visibility while maintaining radiation protection.
Why Choose Tufwud for X-Ray Room Doors? With years of experience in manufacturing specialized doors for healthcare infrastructure, Tufwud is known for:
✔️ Pan India Delivery & Installation
✔️ ISO Certification and Lab-Tested Products
✔️ Engineering-Grade Quality Assurance
✔️ Quick Turnaround and Support
✔️ Custom Solutions for Large Projects
Our doors are tested for radiation attenuation and come with complete documentation and installation guidance to help you meet AERB inspections with ease.
Request a Quote for X-Ray Room Doors Are you planning to build or upgrade a diagnostic facility? Partner with India’s top-rated X-ray room door manufacturer to ensure you get the best in safety and quality.
👉 Get in touch with Tufwud today for pricing, technical specifications, and expert consultation. Let’s make your healthcare environment safer, smarter, and fully compliant.
0 notes
Text
Iopromide Market Projected to Reach $976.6 Million by 2035, Driven by Expanding Diagnostic Imaging Applications
According to recent insights from DataString Consulting, the global Iopromide market is forecasted to climb to $976.6 million by 2035, up from $536.3 million in 2024. This substantial growth is being driven by increased demand across a wide range of medical applications including diagnostic imaging, intravenous urography, vascular radiology, and angiography. Additionally, broader use in cardiology and advanced radiographic procedures is further accelerating the adoption of Iopromide globally.
Versatile Applications Across Imaging Modalities
Iopromide has firmly established itself as a critical contrast agent in diagnostic imaging. It is extensively used in CT scans, X-rays, MRI, and coronary angiography, making internal organs and blood vessels more visible and enabling precise diagnosis. In its injectable form, Iopromide offers clinicians superior image clarity with minimal side effects, enhancing patient outcomes.
Access detailed report insights here - https://datastringconsulting.com/industry-analysis/iopromide-market-research-report
Notably, Bayer’s Ultravist and Bracco Diagnostics’ Isovue are leading products in this space, demonstrating effective integration of Iopromide in both general and specialized diagnostic procedures. In cardiology, the agent plays a central role in coronary angiography, a critical procedure given the global surge in cardiovascular diseases. Its high compatibility with modern imaging technologies has positioned Iopromide as a preferred choice among radiologists and cardiologists alike.
Technological Advancements Fueling Demand
Recent developments in imaging technology, such as intravascular ultrasonography and high-resolution MRI, have expanded the scope of Iopromide’s application. As diagnostic precision becomes increasingly critical in personalized medicine, the demand for high-performance contrast agents has surged.
Pharmaceutical and imaging companies are responding with continuous R&D investments, enhancing Iopromide formulations for improved safety, reduced radiation exposure, and enhanced diagnostic clarity. These innovations not only meet the evolving demands of healthcare providers but also open new avenues for the use of Iopromide across various therapeutic disciplines.
Market Growth Drivers and Outlook
Between 2025 and 2030, the Iopromide market is expected to witness robust expansion supported by several key drivers:
Rapid growth in diagnostic imaging procedures due to increased chronic disease prevalence
Continuous advancements in radiographic and imaging technology
Rising adoption of personalized healthcare and early disease detection
Expansion of healthcare infrastructure in developing economies
Greater awareness and availability of non-invasive diagnostic techniques
North America Remains a Dominant Force
North America continues to be a leading region for Iopromide consumption, driven by a combination of advanced healthcare systems, strong reimbursement frameworks, and a growing geriatric population. High rates of diagnostic testing and chronic illnesses—especially heart disease and cancer—support sustained demand for contrast agents in this market.
The presence of global giants such as Bayer, GE Healthcare, and Bracco Imaging has also intensified competition and innovation in the region. With increasing R&D spending and rapid integration of advanced imaging platforms, North America is expected to retain its strong market position over the forecast period.
Comprehensive Market Insights
DataString Consulting’s research provides an in-depth look at the Iopromide market across more than 20 countries, with detailed analysis based on:
Iodine Concentration: 300 mg, 370 mg
Applications: Computed Tomography, Angiography, Urography, Venography, Arteriography, and other radiographic procedures
Administration Routes: Intravenous, Intra-arterial
These insights help manufacturers, healthcare providers, and investors identify high-growth opportunities and develop data-driven strategies in this expanding segment.
About DataString Consulting
DataString Consulting delivers tailored business intelligence and strategic advisory services, empowering organizations to expand their Total Addressable Market (TAM), diversify revenues, and explore new territories. Leveraging 30+ years of combined leadership in market research and strategy, DataString provides clear, actionable insights across over 15 industries and 60 sub-industries.
Their data-driven approach reduces the time-to-market for emerging innovations and turns market complexities into competitive advantages—making them a trusted partner for businesses navigating high-growth sectors like medical imaging and diagnostics.
0 notes
Text
A Look Inside the World of Medical Equipment Manufacturers
The healthcare industry relies heavily on innovation and precision—and at the heart of it all are medical equipment manufacturers. These unsung heroes work tirelessly behind the scenes to design, develop, and deliver tools that help save lives every single day. From hospital beds to MRI machines, their work impacts millions of people globally.
Let’s take a closer look at what they do, how they do it, and why their role is more vital now than ever before.
What Do Medical Equipment Manufacturers Do?
Medical equipment manufacturers are responsible for producing devices that are essential for:
Diagnosis (e.g., imaging machines, diagnostic kits)
Treatment (e.g., surgical instruments, therapy devices)
Monitoring (e.g., heart monitors, blood pressure machines)
Support (e.g., wheelchairs, ventilators)
They combine engineering, medical science, and innovation to create tools that meet strict safety and performance standards.
Key Areas of Focus
1. Research & Development (R&D)
Before any device reaches a hospital or clinic, it undergoes rigorous research and testing. Manufacturers spend years developing prototypes, consulting with medical professionals, and running trials.
2. Compliance & Regulations
Medical equipment must meet international safety standards. Manufacturers must adhere to:
ISO certifications
FDA and CE approvals
Local and global regulatory guidelines
This ensures that every piece of equipment is safe, reliable, and ready for real-world use.
3. Production & Quality Control
The production process involves advanced machinery and skilled labor. Quality control teams inspect every batch to ensure consistency and safety.
The Growing Demand for Innovation
As healthcare needs evolve, so does the demand for smarter, more efficient equipment. Recent trends include:
Telemedicine Tools: Devices compatible with remote consultations.
Wearable Tech: Trackers that monitor heart rate, oxygen levels, etc.
AI Integration: Equipment powered by artificial intelligence to support faster diagnoses.
Medical equipment manufacturers are embracing these trends to offer better solutions for both patients and healthcare providers.
Challenges Faced by the Industry
While the impact of this sector is enormous, it doesn’t come without challenges:
High Production Costs Developing cutting-edge devices requires significant investment.
Supply Chain Disruptions Global events can cause delays in sourcing raw materials or delivering products.
Regulatory Hurdles Navigating different countries' approval processes can be time-consuming and costly.
Despite these obstacles, the industry continues to grow and adapt with resilience and creativity.
Why Their Work Matters
Medical equipment manufacturers aren’t just creating machines—they’re crafting lifelines. Their innovations help:
Detect diseases earlier
Improve treatment outcomes
Enhance patient comfort
Reduce the burden on healthcare professionals
Without their contributions, modern medicine simply wouldn’t be what it is today.
Final Thoughts
Behind every scan, surgery, and successful recovery is a dedicated team of experts building the tools that make it possible. Medical equipment manufacturers may not be on the frontlines, but they play a crucial role in shaping the future of healthcare.
As technology continues to evolve, so will their contributions—bringing us closer to a healthier, more connected world.
0 notes
Text
Atrial Fibrillation Devices Market: Industry Trends and Forecast 2024-2032

The atrial fibrillation devices market was valued at USD 5.80 billion in 2023 and is projected to reach USD 14.63 billion by 2031, growing at a CAGR of 12.2% during the forecast period of 2024-2031. This significant growth is fueled by the rising prevalence of cardiovascular disorders, increasing geriatric population, and technological advancements in minimally invasive cardiac devices.
Market Overview
Atrial fibrillation (AF) is the most common type of cardiac arrhythmia, affecting millions of people worldwide. Devices designed to manage this condition, such as pacemakers, defibrillators, and ablation systems, play a crucial role in reducing symptoms and preventing complications such as stroke. The growing awareness around early diagnosis, improved healthcare infrastructure, and increasing demand for advanced treatment options are key contributors to the market expansion.
Get Free Sample Report @ https://www.snsinsider.com/sample-request/4256
Regional Analysis
North America remains the dominant region in the atrial fibrillation devices market due to the presence of a well-established healthcare system, high adoption of advanced technologies, and favorable reimbursement policies.
Europe follows closely, supported by growing investments in healthcare and a rise in cardiovascular disease cases.
Asia-Pacific is anticipated to witness the fastest growth during the forecast period, driven by a rising aging population, increasing healthcare spending, and enhanced access to medical facilities in countries such as China, India, and Japan.
Latin America, the Middle East, and Africa are expected to experience moderate growth due to gradual improvements in healthcare access and awareness.
Market Segmentation
By Product Type
Catheter Ablation Devices
Mapping Devices
Cardiac Monitors
Access Devices
Others
By End User
Hospitals
Specialty Clinics
Ambulatory Surgical Centers
Others
Some of the major key players in the Atrial Fibrillation Devices Market
Medtronic (Micra Transcatheter Pacing System, Arctic Front Advance CryoAblation Catheter)
Abbott Laboratories (Xience V Coronary Stent, TactiCath Contact Force Ablation Catheter)
Boston Scientific (FlexAbility Ablation Catheter, Watchman Left Atrial Appendage Closure Device)
Biotronik (Orsiro Coronary Stent, GoldTip Ablation Catheter)
Johnson & Johnson (Biosense Webster Carto 3 System, Thermocool SmartTouch Catheter)
Siemens Healthineers (Acuson SC2000 Ultrasound System, Artis zee Floor Angiography System)
Philips Healthcare (Rhythmia Mapping System, Stellaris PC)
LivaNova (Essenz ECG, Vagus Nerve Stimulation Therapy)
CardioFocus (HeartLight Endoscopic Ablation System, HeartLight X3 System)
Abbott Medical (TactiCath Contact Force Ablation Catheter, Rhythmia Mapping System)
AtriCure, Inc. (Isolator Synergy Ablation System, AtriClip Left Atrial Appendage Exclusion System)
Stereotaxis, Inc. (Niobe ES Robotic Magnetic Navigation System, Vdrive Robotic Arm System)
Medico (Advantage RF Ablation System, EpiqTM Ablation System)
MicroPort Scientific Corporation (GlidePath Ablation Catheter, Cardiac Resynchronization Therapy Device)
Biomerics (Coronary Ablation Catheter, Deflectable Sheath System)
Gore Medical (GORE TAG Thoracic Endoprosthesis, GORE VIABAHN Endoprosthesis)
AtriCure Inc. (AtriClip LAA Exclusion System, AtriCure Synergy Ablation System)
Cook Medical (Cook Biopsy Needle, Cook Percutaneous Drainage Catheter)
Terumo Corporation (ThermoCool SmartTouch Catheter, Guidewire System)
Imricor Medical Systems (Vision-MR Ablation Catheter, MRI Compatible Pacing System)
Key Points
The global atrial fibrillation devices market is projected to grow at a CAGR of 12.2% through 2031.
Technological innovations such as robotic catheter navigation and contact force-sensing ablation catheters are enhancing treatment efficacy.
Increased incidence of AF among the aging population is a major growth driver.
Hospitals are the largest end-user segment due to the availability of skilled professionals and advanced equipment.
North America leads the global market, while Asia-Pacific is emerging as a high-growth region.
Future Scope
The future of the atrial fibrillation devices market looks promising, with ongoing R&D expected to introduce even more precise and efficient treatment options. As the focus shifts toward personalized healthcare and real-time monitoring, integration of AI and wearable technologies will further revolutionize patient care. Additionally, increasing public-private collaborations and government initiatives aimed at improving cardiac care infrastructure will continue to create opportunities for market expansion in both developed and emerging economies.
Conclusion
The atrial fibrillation devices market is poised for substantial growth, driven by rising health awareness, aging populations, and rapid advancements in medical technology. With an increasing demand for effective AF treatment and management solutions, the industry is expected to continue evolving, delivering life-saving innovations to patients across the globe.
Contact Us: Jagney Dave - Vice President of Client Engagement Phone: +1-315 636 4242 (US) | +44- 20 3290 5010 (UK)
Other Related Reports:
Cell Viability Assay Market
Medical Power Supply Market
Post Traumatic Stress Disorder Treatment Market
MRI Guided Neurosurgical Ablation Market
#Atrial Fibrillation Devices Market#Atrial Fibrillation Devices Market Share#Atrial Fibrillation Devices Market Trends#Atrial Fibrillation Devices Market Size#Atrial Fibrillation Devices Market Growth
0 notes
Text
In-Depth Analysis of Toggle Switch Terminal Connection Methods: Technical Selection and Scenario Adaptation for Soldered, Plug-In, and PCB-Mounted Designs

Introduction
In B2B applications such as industrial control, automotive electronics, and medical equipment, the terminal connection method of a toggle switch is a critical factor determining product reliability, installation efficiency, and long-term maintenance costs. Soldered, plug-in, and PCB-mounted designs each have unique advantages and limitations, requiring a comprehensive evaluation of electrical performance, environmental resistance, and production automation. This article analyzes structural design, application scenarios, and compatibility challenges, supported by industry cases (e.g., automotive ECU controls, industrial PLC modules) and emerging trends (e.g., high-density PCB integration), to provide engineers and procurement decision-makers with a systematic selection guide.
1. Soldered Terminal Connections: The "Permanent Bond" for High Reliability
Structure & Process
Physical Connection: Permanently fixes switch terminals to PCBs or wires via soldering (solder or reflow).
Terminal Type: Through-hole pins (0.6–1.2mm diameter), typically made of tin-plated or gold-plated phosphor bronze.
Key Standard: Compliant with IPC-A-610 to avoid cold joints or voids.
Applications & Cases
High-Vibration Environments: Siemens’ train control panels use soldered toggle switches validated for 10-year maintenance-free operation under EN 61373.
High-Current Loads: ABB frequency converters employ soldered emergency stop switches to withstand 50A short-circuit currents.
Pros & Cons
Advantages: Stable contact resistance (<5mΩ), superior vibration resistance (passes MIL-STD-202G), low cost (no connectors required).
Limitations: Difficult repairs (thermal desoldering needed), limited flexibility in automated production.
Innovations
Hybrid solder-crimp terminals (e.g., TE Connectivity’s DUOBILIT) balance reliability and removability.
High-temperature solder (Sn96.5Ag3Cu0.5) extends lifespan in automotive engine compartments.
2. Plug-In Terminals: Modular Flexibility for Easy Maintenance
Structure & Standards
Connection Types: Tab terminals, receptacles, or spring cages with 5–20N insertion force (UL 310).
Common Sizes: 2.8mm (automotive) or 4.8mm (industrial) tab widths.
Applications & Cases
Medical Equipment: GE MRI control switches use gold-plated tabs for <3mΩ contact resistance and 10,000-cycle durability.
Smart Home Gateways: C&K’s T-series plug-in terminals enable cross-device compatibility.
Pros & Cons
Advantages: Tool-free installation, hot-swappable (e.g., data center PDUs), space-efficient (2.54mm pitch).
Limitations: Higher contact resistance (+10–30% vs. soldered), vibration-induced loosening (requires locking mechanisms).
Innovations
Foolproof designs (e.g., AMP’s Mate-N-Lok asymmetric tabs).
Self-cleaning multi-point contacts (e.g., JST PA series).
3. PCB-Mounted Terminals: Miniaturization for High-Density Integration
Structure & Process
Mounting Types: Through-hole (THT) or surface-mount (SMT) with L/J-shaped leads.
Typical Dimensions: THT pins (3–5mm length), SMT packages (60% smaller than THT).
Applications & Cases
Consumer Electronics: ALPS SSSS series SMT toggle switches (2.3mm thickness) in TWS earphone cases.
Robotics: SMT switches integrated with FPGAs on 0.5mm-pitch PCBs.
Pros & Cons
Advantages: Ultra-compact, automated production-friendly (>20,000 CPH), low EMI.
Limitations: Vulnerable to PCB flexing, limited heat dissipation.
Innovations
Hybrid SMT-THT designs (e.g., E-Switch TL series).
Flexible PCB-compatible curved pins (Würth Elektronik WM series).
4. Compatibility Challenges & Solutions
Cross-Platform Adaptation: Phoenix Contact’s COMBICON modular terminals switch between THT and plug-in modes.
Moisture Resistance: 3M Parylene coating + O-rings prevent dendrite growth.
High-Frequency Noise: C&K KSC series with shielded pins and Rogers RO4003 substrates.
5. Industry Trends & Selection Guide
Smart Integration: LEM GoSwitch series with current sensors and I²C interfaces.
Green Manufacturing: DSM EcoPaXX halogen-free materials for RoHS 3.0 compliance.
en.dghongju.com
0 notes
Text
The Memo: InkSpace Imaging Transforming MRI with Patient-Centered Innovation
Under the direction of President and CEO Peter Fischer, InkSpace Imaging is pioneering a breakthrough in MRI technology with ultralight, flexible, body-conforming MRI coils. The company was initially focused on pediatrics, addressing a long-standing gap in MRI accessibility and patient comfort for children, who often require sedation due to poorly fitting, rigid coils. Building on this foundation, InkSpace Imaging has expanded its product line, securing FDA clearance for adult MRI applications as well, bringing the same advancements in comfort and imaging quality to a broader patient population.
By replacing uncomfortable, one-size-fits-all coils with customized, high-performance solutions, InkSpace Imaging is improving image quality, reducing scan times, and enhancing patient comfort — all while making MRI more efficient for hospitals and healthcare providers. Its coils are plug-and-play compatible with Siemens, GE, and Philips MRI systems, requiring no workflow or software modifications. Additionally, they fit seamlessly into current reimbursement models, making adoption straightforward for hospitals and imaging centers.
With FDA-cleared products, a strategic partnership with Siemens, and a growing presence with GE and Philips, InkSpace Imaging is rapidly scaling. As the company expands globally, it continues to demonstrate how innovation in MRI coils can optimize patient care, streamline workflows, and increase hospital efficiency.
This blog is originally published here: https://www.lifesciencemarketresearch.com/insights/the-memo-inkspace-imaging-transforming-mri-with-patient-centered-innovation
0 notes
Text
Medical Grade Stainless Steel Bar Stock
SD-Steel is a leading provider of high-quality medical grade stainless steel bar stock, catering to the needs of various industries that require precision and durability. Our products are meticulously crafted to meet the stringent standards of the medical industry, ensuring reliability and safety in critical applications.
Product Description
Our medical grade stainless steel bar stock is designed for applications where corrosion resistance, strength, and biocompatibility are paramount. Ideal for surgical instruments, implants, and other medical devices, our bars offer exceptional performance and longevity. With a focus on precision and quality, we ensure that each piece meets the highest standards of the medical sector. Whether you're manufacturing surgical tools, orthopedic implants, or any other medical device, our stainless steel bars provide the perfect solution. Here’s a detailed overview of our product:
 | 40% |
Grade: 316L
Density: 7.93 g/cm³
Tensile Strength: 515 MPa
Elongation: 40%
Hardness: 200 HBW
Chemical Composition:
- Carbon (C): ≤0.03%
- Chromium (Cr): 16-18% |
| Molybdenum (Mo): 2-3% |
| Nickel (Ni): 10-14% |
| Phosphorus (P): ≤0.045% |
| Sulfur (S): ≤0.03% |
Available Sizes
| Diameter (mm) | Length (mm) |
|----------------|--------------|
| 10 | Customizable |
| 20 | Customizable |
| 30 | Customizable |
| 40 | Customizable |
| 50 | Customizable |
Dimensions
| Diameter (mm) | Length (mm) |
|----------------|-------------|
| 10 | Customizable |
| 20 | Customizable |
| 30 | Customizable |
| 40 | Customizable |
| 50 | Customizable |
Dimensions
| Diameter (mm) | Length (mm) |
|---------------|-------------|
| 10 | Customizable |
| 20 | Customizable |
| 30 | Customizable |
| 40 | Customizable |
| 50 | Customizable |
Applications
Our medical grade stainless steel bars are ideal for:
- Surgical Instruments
- Implants
- Orthopedic Devices
- Biomedical Equipment
Usage Scenarios
Our medical grade stainless steel bars are widely used in:
- Surgical Instruments
- Implants
- Orthopedic Devices
- Biomedical Equipment
Company Overview
SD-Steel is committed to delivering top-tier products with consistent quality and reliability. Our bars are available in various diameters and lengths, customizable to your specific requirements.
Key Features
High Corrosion Resistance: Ensures durability and longevity.
Non-Magnetic: Non-magnetic properties make them suitable for MRI compatibility.
Customizable: Customizable lengths and diameters to meet your exact specifications.
Company Strengths
With over two decades of experience, SD-Steel has established itself as a trusted supplier in the global market. Our commitment to quality and precision ensures that every piece meets the highest standards set by international standards.
Common Uses
Surgical Instruments: Used in the production of surgical tools and instruments.
Implants: Suitable for implants requiring high strength and biocompatibility.
Orthopedic Devices: Used in orthopedic devices where magnetic resonance imaging (MRI) compatibility is crucial.
FAQs
Q: What makes your medical grade stainless steel unique?
A: Our 316L stainless steel is chosen for its outstanding corrosion resistance and biocompatibility, making it ideal for medical devices.
Customization: We offer customization options to fit your specific needs.
Why Choose SD-Steel?
Quality Assurance: Stringent quality control processes ensure consistency and reliability.
Customization: Customizable lengths and diameters to suit your needs.
Durability: High tensile strength and elongation.
Applications: Customizable lengths and diameters to suit your needs.
Biocompatibility: Ensuring safety and reliability.
Customization: Customizable lengths and diameters to suit your needs.
Contact Us
For inquiries, please contact us at +65 83016969 for more information.
Frequently Asked Questions
What are the key benefits of 316L stainless steel?
A: 316L stainless steel offers superior corrosion resistance and biocompatibility, making them ideal for medical devices.
Durability: Customizable lengths and diameters to suit your needs.
Reliability: Customizable lengths and diameters to suit your needs.
Why SD-Steel?
Customization: Customizable lengths and diameters to suit your needs.
Durability: Customizable lengths and diameters to suit your needs.
Durability: Customizable lengths and diameters to suit your needs.
Contact Information
For more details, call us at +65 83016969 for more information.
Durability: Customizable lengths and diameters to suit your needs.
Durability: Customizable lengths and diameters to suit your needs.
Durability: Customizable lengths and diameters to suit your needs.
Durability: Customizable lengths and diameters to suit your needs.
Durability: Customizable lengths and diameters to suit your needs.
Durability: Customizable lengths and diameters to suit your needs.
Durability: Customizable lengths and diameters to suit your needs.
Durability: Customizable lengths and diameters to suit your needs.
Durability: Customizable lengths and diameters to suit your needs.
Durability: Customizable lengths and diameters to suit your needs.
Durability: Customizable lengths and diameters to suit your needs.
Durability: Customizable lengths and diameters to suit your needs.
Durability: Customizable lengths and diameters to suit your needs.
Durability: Customizable lengths and diameters to suit your needs.
Durability: Customizable lengths and diameters to suit your needs.
Durability: Customizable lengths and diameters to suit your needs.
Durability: Customizable lengths and diameters to suit your needs.
Durability: Customizable lengths and diameters to suit your needs.
Durability: Customizable lengths and diameters to suit your needs.
Durability: Customizable lengths and diameters to suit your needs.
Durability: Customizable lengths and diameters to suit your needs.
Durability: Customizable lengths and diameters to suit your needs.
Durability: Customizable lengths and diameters to suit your needs.
Durability: Customizable lengths and diameters to suit your needs.
Durability: Customizable lengths and diameters to suit your needs.
Durability: Customizable lengths and diameters to suit your needs.
Durability: Customizable lengths and diameters to suit your needs.
Durability: Customizable lengths and diameters to suit your needs.
Durability: Customizable lengths and diameters to suit your needs.
Durability: Customizable lengths and diameters to suit your needs.
Durability: Customizable lengths and diameters to suit your needs.
Durability: Customizable lengths and diameters to suit your needs.
Durability: Customizable lengths and diameters to suit your needs.
Durability: Customizable lengths and diameters to suit your needs.
Durability: Customizable lengths and diameters to suit your needs.
Durability: Customizable lengths and diameters to suit your needs.
Durability: Customizable lengths and diameters to suit your needs.
Durability: Customizable lengths and diameters to suit your needs.
Durability: Customizable lengths and diameters to suit your needs.
Durability: Customizable lengths and diameters to suit your needs.
Durability: Customizable lengths and diameters to suit your needs.
Durability: Customizable lengths and diameters
加飞机@yuantou2048

Stainless Steel Bar Stock
Stainless Steel suppliers
0 notes
Text
Temporary Cardiac Pacing Wires and Lead Market Size, Growth Outlook 2035
The global Temporary Cardiac Pacing Wires Lead Market Size was estimated at 1.49 (USD Billion) in 2024. The Temporary Cardiac Pacing Wires Lead Market Industry is expected to grow from 1.60 (USD Billion) in 2025 to 3.13 (USD Billion) till 2034, at a CAGR (growth rate) is expected to be around 7.73% during the forecast period (2025 - 2034)
Market Overview
The Temporary Cardiac Pacing Wires and Lead Market is experiencing significant growth, driven by the rising incidence of cardiovascular diseases (CVDs), arrhythmias, and heart blockages. Temporary cardiac pacing is a critical intervention used in emergency settings, post-cardiac surgery, and for managing bradyarrhythmias. The increasing adoption of minimally invasive cardiac procedures, technological advancements in pacing leads, and the growing elderly population are key factors propelling market expansion.
Market Size and Share
The global Temporary Cardiac Pacing Wires Lead MarketSize was estimated at 1.49 (USD Billion) in 2024. The Temporary Cardiac Pacing Wires Lead Market Industry is expected to grow from 1.60 (USD Billion) in 2025 to 3.13 (USD Billion) till 2034, at a CAGR (growth rate) is expected to be around 7.73% during the forecast period (2025 - 2034), reaching USD Y billion by 2030. North America dominates the market, attributed to advanced healthcare infrastructure, high prevalence of heart diseases, and rising adoption of pacing devices. The Asia-Pacific region is expected to witness the fastest growth, fueled by an increasing geriatric population, improving healthcare access, and growing cardiac care awareness.

Market Drivers
Rising Prevalence of Cardiovascular Disorders: Increasing cases of heart failure, bradycardia, and conduction system disorders are driving demand.
Advancements in Temporary Pacing Technologies: The introduction of biocompatible, MRI-compatible, and leadless pacing systems is revolutionizing the market.
Increase in Cardiac Surgeries and Electrophysiology Procedures: Post-surgical pacing requirements boost the need for temporary pacing leads and wires.
Growing Adoption of Minimally Invasive Procedures: Surge in catheter-based pacing techniques and percutaneous interventions is accelerating market growth.
Challenges and Restraints
Risk of Infections and Complications: Potential lead dislodgement, infections, and vascular injuries pose challenges.
Short Lifespan of Temporary Pacing Leads: Frequent replacement needs limit long-term efficacy.
Stringent Regulatory Requirements: Compliance with FDA, CE, and ISO standards can delay product approvals.
Market Trends
Development of Leadless Temporary Pacemakers: Emerging wireless, battery-free pacing solutions enhance patient safety.
Integration of AI and Remote Monitoring: AI-driven cardiac rhythm monitoring and predictive analytics are transforming cardiac care.
Increase in Biodegradable and Bioabsorbable Leads: Reducing the need for extraction procedures and lowering complication risks.
Regional Analysis
North America: Largest market share due to high CVD prevalence, advanced hospital infrastructure, and robust R&D investments.
Europe: Growth supported by increasing healthcare spending and adoption of innovative pacing solutions.
Asia-Pacific: Fastest-growing market due to rising cardiovascular cases, government initiatives, and medical technology advancements.
Rest of the World: Moderate growth driven by improving healthcare access and rising awareness of cardiac interventions.
Segmental Analysis
By Product Type:
Unipolar Pacing Wires
Bipolar Pacing Wires
Pacing Leads
By Application:
Bradyarrhythmias
Heart Blocks
Post-Cardiac Surgery Pacing
By End-User:
Hospitals
Ambulatory Surgical Centers
Specialty Cardiac Centers
Key Market Players
Jude Medical
Medtronic
Braun Melsungen
Abbott Laboratories
Cook Medical
Teleflex
Recent Developments
Introduction of Leadless Temporary Pacemakers: Innovation in self-contained pacing devices for safer post-surgical interventions.
Strategic Collaborations Between Cardiac Device Manufacturers and Hospitals: Enhancing product development and clinical adoption.
Growing Investment in AI-Powered Cardiac Monitoring Solutions: Improving real-time ECG analysis and arrhythmia detection.
For more information, please visit us at marketresearchfuture.
#Temporary Cardiac Pacing Wires and Lead Market Size#Temporary Cardiac Pacing Wires and Lead Market Share#Temporary Cardiac Pacing Wires and Lead Market Growth#Temporary Cardiac Pacing Wires and Lead Market Analysis#Temporary Cardiac Pacing Wires and Lead Market Trends#Temporary Cardiac Pacing Wires and Lead Market Forecast#Temporary Cardiac Pacing Wires and Lead Market Segments
0 notes
Text
Breakthrough Tech Offers Stunning MRI Experience for Life
MRI scans can be long, expensive, and out of reach for a large group of people who require them. Recent technological developments in MRI are establishing the basis for a change in paradigm, the efficacy of which promises to offer faster, cheaper, and patient-centric alternatives.
Recently, a Swedish company made available a new innovative software that will allow not only increased imaging speed but also the ability to make MRI available again. Software that shortens scan times by 15 minutes, from 45 min to a few min. This evolution characterises a way to circumvent the present limitations of MRI technology, which could be considered a useful diagnostic and preventive medicine application. This progress has opened the door to its widespread application in the early detection of diseases, which may prevent thousands of deaths per year.
One of the most recent advances in MRI technology is in-bore MRI systems, which have been used to maximise patient comfort during MR studies. Traditionally, patients undergoing MRI often experience anxiety due to the enclosed environment and the extended duration of the procedure. Today’s systems have circumvented this by integrating MRI Ambience rooms and MRI cinemas in medicine, offering a soothing and entertaining stimulation during scanning. These advances are especially useful to minimise motion artefacts due to patient pain, which in turn leads to better diagnostic performance.
Apart from the ambience environment, technologies such as the MRI-compatible projector and FMRI visual systems are changing the ways in which patients interact with MRI scanners. These are the instruments that provide visual interaction, which is clinical calm for the patient and which aids in intended diagnostic, e.g., functional MRI (fMRI) or brain activity visualisation.
Advances in MRI-compatible equipment have not only expanded the total number of investigations that can be done but also the quality of imaging on a voxel-by-voxel basis. For example, the presence of displays with MRI-compatible screens and MRI-compatible displays eliminates electromagnetic crosstalk and provides continuous distinct visual output while undergoing procedures, enabling clinicians to view scans as they are undergoing examinations. Similarly, technologies such as the MRI-compatible stretchers and MRI compatible wheelchairs have become available. Therefore, they promote the adoption of the procedure and offer the opportunity for the patient to be transported easily within the building where it is located.
Another major trend is the fMRI system, which has provided the most accurate brain imaging to date. Such a technology allows us to identify very minor changes in brain activity with a view to preventing early diagnosis of a variety of neurological disorders. There is the paradigm that such tools are an example of how the innovations transform magnetic resonance imaging (MRI) systems into health care systems linked to each other and the patients.
The conventional price of magnetic resonance imaging (MRI) scans has been subject to critique, and MRI has been limited in its applications to preventive health care use. To this end, virtual skylights for both medical care and patient entertainment are being embedded into MRI bore to provide relaxing environments at a lower cost. These approaches also help enhance patient experience, and MR technology has become a more feasible solution for outpatient and smaller clinical settings.
The emergence of In-Bore MRI in India is a significant development in offering high-technology imaging to developing countries. Being able to reduce acquisition times and produce higher quality results, these systems are bridging the gap between high-tech and low-tech, bringing them one step closer to ubiquitous use in healthcare systems with finite healthcare resources.
Despite the progress in MRI technology, the problem of false positives and false negatives continues to exist. These challenges can divert or consume resources from the management of directly sick individuals or can lead to nonproductive procedures. Innovations, for example, the interest in the fMRI display and MRI-based endoscopes, use a prerequisite and hence overcome these limitations with high-resolution imaging and an improvement in diagnostic ability. The usefulness of these tools allows the conducting of longitudinal studies that follow patient health changes over time and reduce the chance of diagnostic mistakes.
An exciting development is the MRI-compatible TV, which provides real-time imaging and direction to patients during examinations. By integrating these devices, clinicians are able to provide an interactive and less stressful environment, particularly for paediatric and claustrophobic patients.
Kryptonite Solutions is among the companies that are pioneering this field and has claimed the position of being at the forefront of new MRI solutions. Focusing on patient comfort and diagnostic efficiency, Kryptonite Solutions provides the state of the art technology for current healthcare needs. Through the addition of state-of-the-art capabilities such as MRI-compatible displays and user-configurable ambient settings, they further play a role in the development of MRI technology with a more accessible and patient-centred approach.
The continuous transition of MRI systems leads from the reactive to the preventive and personalised type of medicine. Shorter scan times, lower costs, increased patient comfort, and enhanced diagnostic sensitivity are just some of the ways in which these advances will transform medical imaging.
With the advancement of the field, the companies of Kryptonite Solutions will continue to play an important role in bringing these improvements to those populations who are most in need. Now, with these advances, the dream of regular, full-body scans for detecting disease early is a real possibility- that is, giving each and every one of us a fair chance for a brighter future.
#fmri visual system#mri compatibles#mri ambience#virtual skylights for healthcare#mri compatible stretcher#mri compatible wheelchair#mri compatible products#mri compatible#mri safety product
0 notes
Text
Can Your MRI Gown Make or Break Your Scan? Discover the MRI I.V. Patient Gowns

MRI I.V. patient gowns represent essential medical apparel that healthcare providers use for patients who need intravenous (I.V.) procedures during scans. MRI-safe I.V. gowns for hospitals support both patient comfort and safety protocols and enable better imaging results. The construction of MRI I.V. patient gowns includes MRI-safe non-metallic components that differ from standard hospital gowns. Metal elements found in zippers, snaps, and buttons create severe safety hazards during MRI procedures since they disrupt imaging results and endanger patient safety. The gowns feature fabric fasteners and ties, which provide total safety protection for MRI environments.
MRI-compatible hospital apparel requires easy accessibility, which is one of its essential characteristics. The medical staff can access I.V. lines through designed entry points in these gowns, which enable them to insert and manage the lines without removing the garment. The design plays an essential role for patients who need contrast-enhanced MRI scans since they receive bloodstream injections that enhance image quality. Another important aspect of these gowns is comfort. These patients wear garments that use hypoallergenic materials with breathable and soft fabrics to provide improved comfort throughout lengthy MRI procedure times. These gowns exist in multiple sizes and different styles, which allows them to fit patients of different body shapes appropriately. These patient gowns enable a trouble-free imaging process by protecting both patient safety and comfort needs. The product design enables quick medical operations without causing MRI-related problems that occur when using standard hospital gowns.
#MRI patient gown#MRI IV gown#MRI safe gown#MRI gown with IV access#non-metallic MRI gown#MRI compatible gown#patient gown for MRI#IV access gown for MRI#MRI scan gown#MRI procedure gown#hospital gown for MRI#MRI patient apparel#MRI clothing#medical gown for MRI#MRI safe clothing#MRI gown with openings#MRI gown for contrast#contrast MRI gown#MRI gown with sleeves#breathable MRI gown#hypoallergenic MRI gown#comfortable MRI gown
0 notes
Text
Peripheral Nerve Stimulators Market
Peripheral Nerve Stimulator Market Size, Share, Trends: Medtronic plc Leads
Shift Towards Non-Opioid Pain Management Solutions Drives Demand
Market Overview:
The peripheral nerve stimulator market is expected to develop at a 7.8% CAGR from 2024 to 2031. North America currently dominates the market, accounting for the vast majority of worldwide sales. Key metrics include the rising prevalence of chronic pain problems, technological breakthroughs in neurostimulation devices, and the increased use of less invasive procedures. The market is expanding rapidly due to the increasing prevalence of neurological illnesses and chronic pain issues. Technological advancements, such as the creation of wireless and MRI-compatible equipment, are propelling market growth. Furthermore, the growing geriatric population and rising healthcare costs in developing nations are driving market expansion.
DOWNLOAD FREE SAMPLE
Market Trends:
The peripheral nerve stimulator market is witnessing a significant shift towards non-opioid pain treatment options. This transition is primarily motivated by increasing awareness of the hazards associated with long-term opioid usage and a desire for safer, more effective pain management solutions. Peripheral nerve stimulators provide non-pharmacological pain relief while lowering the risk of addiction and other opioid-related negative effects. Healthcare practitioners are increasingly recommending these devices as the first line of treatment for chronic pain issues, driving market expansion. Favorable payment regulations and increased patient preference for minimally invasive treatment choices are also contributing to growth. Manufacturers are investing in R&D to increase the efficacy and usability of peripheral nerve stimulator devices, accelerating market growth.
Market Segmentation:
The transcutaneous electrical nerve stimulation (TENS) segment dominates the peripheral nerve stimulator market. TENS devices are non-invasive, inexpensive, and often utilized for a variety of pain management purposes. The segment's dominance stems from its ease of use, portability, and lower risk profile compared to implanted devices. Recent advances in TENS technology, such as cordless TENS devices, have increased patient comfort and mobility during therapy. Research published in the Journal of Pain Research found that 78% of chronic pain patients experienced considerable pain relief after four weeks of TENS therapy. This high efficacy rate has aided the widespread use of TENS devices in professional and domestic settings.
Market Key Players:
The peripheral nerve stimulator market is characterized by fierce competition among leading competitors, with an emphasis on technological innovation and strategic alliances. Major companies such as Medtronic plc, Abbott Laboratories, Boston Scientific Corporation, Nevro Corp., Stimwave LLC, and NeuroSigma, Inc. are at the forefront, driving innovation and setting trends in the market. These firms invest heavily in R&D to expand their product portfolios and acquire competitive advantages.
Contact Us:
Name: Hari Krishna
Email us: [email protected]
Website: https://aurorawaveintellects.com/
0 notes
Text
Deep Brain Stimulation (DBS) Devices Market: Market Trends and Revenue Forecast 2024-2032

According to SNS Insider's research, “The Deep Brain Stimulation (DBS) Devices Market was valued at USD 1.57 billion in 2023 and is projected to reach USD 3.12 billion by 2031, growing at a CAGR of 9% during the forecast period 2024-2031.” This significant growth is attributed to the rising prevalence of neurological disorders, such as Parkinson's disease, epilepsy, and dystonia, coupled with increasing awareness and technological innovations in DBS devices.
Deep brain stimulation devices deliver electrical impulses to specific areas of the brain to manage symptoms of neurological conditions. The non-invasive, reversible nature of DBS therapy, combined with its proven efficacy, has made it a preferred treatment option, further fueling market expansion. Additionally, the growing elderly population prone to neurological disorders and the integration of advanced technologies like wireless remote programming and MRI compatibility are expected to boost market growth.
Get Free Sample Report @ https://www.snsinsider.com/sample-request/3335
Regional Analysis:
North America currently dominates the DBS Devices Market, accounting for the largest share, owing to the high prevalence of Parkinson’s disease, well-established healthcare infrastructure, and rapid adoption of advanced medical technologies.
Europe follows closely, driven by increased investments in healthcare and rising awareness of neurological disease management.
Asia-Pacific is anticipated to exhibit the highest growth rate during the forecast period, propelled by improving healthcare facilities, a growing patient population, and supportive government initiatives.
Market Segmentation:
By Product:
Single-channel DBS
Dual-channel DBS
By Application:
Parkinson’s Disease
Epilepsy
Essential Tremor
Dystonia
Obsessive-Compulsive Disorder (OCD)
Others
By End-User:
Hospitals
Neurology Clinics
Ambulatory Surgical Centers
Research Centers
Key Players
The deep brain stimulation devices market players include Abbott Laboratories, Beijing Pinchi Medical Equipment Co., Ltd., Boston Scientific Corporation, Fisher Wallace Laboratories, Functional Neuromodulation Ltd., Medtronic, NeuroPace Inc., Renishaw PLC, Aleva Neurotherapeutics S.A. & LivaNova PLC & Other Players.
Key Points:
Market valued at USD 1.57 billion in 2023, projected to reach USD 3.12 billion by 2031.
CAGR of 9% from 2024 to 2031.
Growing prevalence of Parkinson’s disease and epilepsy fueling demand.
Advancements such as MRI-compatible and rechargeable DBS systems are key drivers.
North America holds the largest share, with Asia-Pacific emerging as a growth hotspot.
Increased healthcare expenditure and favorable reimbursement policies supporting growth.
Future Scope:
The future of the Deep Brain Stimulation Devices Market appears promising, with continuous innovations such as closed-loop DBS systems and AI-integrated devices poised to redefine patient care. Researchers are exploring new applications of DBS beyond traditional neurological disorders, including psychiatric conditions and chronic pain management. Additionally, expanding healthcare infrastructure in emerging economies and favorable regulatory support are likely to open new avenues for market players.
Conclusion:
The Deep Brain Stimulation (DBS) Devices Market is on a robust growth trajectory, driven by technological advancements, rising neurological disease prevalence, and increasing patient awareness. With strong potential across developed and emerging regions, and ongoing research unlocking new therapeutic applications, the DBS devices market is set to witness sustained growth in the coming years.
Contact Us: Jagney Dave - Vice President of Client Engagement Phone: +1-315 636 4242 (US) | +44- 20 3290 5010 (UK)
Other Related Reports:
Smart Healthcare Market
Optometry Equipment Market
Post Traumatic Stress Disorder Treatment Market
#Deep Brain Stimulation (DBS) Devices Market#Deep Brain Stimulation (DBS) Devices Market Share#Deep Brain Stimulation (DBS) Devices Market Trends#Deep Brain Stimulation (DBS) Devices Market Size
0 notes
Text
8 Interesting Facts About Servo Stabilizers

Servo stabilizers are vital devices designed to regulate voltage fluctuations, safeguarding electrical appliances and machinery.
Beyond their fundamental purpose, these devices offer fascinating insights into their operation and importance. Here are eight compelling facts about servo stabilizers that underscore their role in modern electrical systems.
1. How Servo Stabilizers Work
Servo stabilizers utilize a servo motor to adjust voltage levels. When fluctuations occur, the device swiftly moves a variable transformer to stabilize the output voltage. This precise mechanism ensures seamless operation for the connected equipment, protecting it from interruptions or damage.
2. Importance of Voltage Stabilization
Voltage instability can damage sensitive electronics, causing malfunctions or overheating. Servo stabilizers mitigate these risks by:
Providing a consistent voltage supply.
Extending equipment lifespan.
Reducing operational downtime.
These advantages are indispensable for industries and homes in areas with an unstable power supply.
3. Versatile Applications Across Industries
Servo stabilizers find use across diverse sectors, such as:
Healthcare: Protecting sensitive devices like MRI machines.
IT & Data Centers: Ensuring uninterrupted server operations.
Manufacturing: Safeguarding heavy machinery and CNC tools.
Hospitality: Maintaining power for elevators, HVAC systems, and lighting.
Their adaptability makes them essential for industrial, commercial, and residential needs.
4. Energy-Efficient Design
Unlike traditional stabilizers, servo stabilizers are engineered for energy efficiency. By delivering precise voltage regulation, they minimize energy wastage, leading to significant cost savings—ideal for businesses aiming to cut operational expenses.
5. Built to Last
Servo stabilizers are robustly constructed with features such as:
Corrosion-resistant enclosures for durability in harsh conditions.
Copper windings for superior performance.
Advanced cooling systems to prevent overheating under heavy loads.
This durability makes them a reliable long-term investment.
6. Compatibility with Various Loads
Servo stabilizers can handle diverse electrical loads, including:
Inductive loads like motors and compressors.
Resistive loads such as lighting and heating equipment.
This versatility ensures suitability across numerous applications.
7. Advanced Technology Features
Modern servo stabilizers boast innovative features like:
Microcontroller-based systems for precise voltage control.
Digital displays showing real-time voltage levels.
Remote monitoring for convenient maintenance and troubleshooting.
These advancements enhance reliability and user convenience.
8. Contribution to Safety and Sustainability
Servo stabilizers support safety and sustainability by:
Preventing electrical hazards like overvoltage and short circuits.
Reducing carbon footprints through energy-efficient designs.
These benefits contribute to safer and greener environments.
Why Choose a Reliable Servo Stabilizer Manufacturer?
Investing in a high-quality servo stabilizer requires selecting a trusted manufacturer who offers:
Quality assurance with performance-tested products.
Comprehensive support including installation, maintenance, and warranties.
Customized solutions tailored to specific needs.
Working with a reputable manufacturer ensures long-term reliability and value.
#servo stavilizer#servo stabilizer manufacturers#servo stabilizers#servo stabilizer manufacturer#bloger#my writing
0 notes
Text
Skull Clamp Market Growth: Doubling to $2.4B by 2033 with a 7.2% CAGR
Skull Clamp Market is at the forefront of neurosurgical innovation, dedicated to manufacturing and distributing devices for cranial stabilization during intricate procedures. With options like three-pin and four-pin skull clamps, these tools are crucial for ensuring surgical precision and patient safety. Serving hospitals, clinics, and specialty surgical centers, the market thrives on advancements in ergonomic design, material innovations, and enhanced patient comfort, mirroring the evolving demands of neurosurgical treatments.
To Request Sample Report: https://www.globalinsightservices.com/request-sample/?id=GIS26350 &utm_source=SnehaPatil&utm_medium=Article
Key Market Dynamics
The Skull Clamp Market is experiencing substantial growth, driven by:
Advancements in Neurosurgical Techniques: Cutting-edge technologies increase demand for precision tools.
Rising Healthcare Investments: Expanding infrastructure boosts accessibility to neurosurgical equipment.
Growing Neurological Disorder Prevalence: Increasing cases necessitate surgical intervention.
Market Highlights 🚀
Segment Performance:
Adult Skull Clamps: Lead with a 55% market share, supported by a higher prevalence of adult neurological conditions.
Pediatric Skull Clamps: Second with 30% share, reflecting increased focus on pediatric care.
Veterinary Skull Clamps: Account for 15%, catering to specialized applications in animal surgeries.
Regional Insights:
North America: Dominates due to advanced healthcare systems and robust R&D activities.
Europe: Second-largest region, benefiting from an aging population and heightened awareness of neurological conditions.
Top Countries:
United States: Leads with substantial healthcare expenditure and a high incidence of neurological disorders.
Germany: Emerges as a strong player with a robust medical device industry and favorable regulations.
Market Segmentation
By Type: Manual, Automatic, Adjustable Skull Clamps.
By Product: Pediatric, Adult, Disposable, Reusable Skull Clamps.
By Technology: Mechanical, Hydraulic, Pneumatic Skull Clamps.
By Component: Base Units, Support Arms, Pressure Pads, Locking Mechanisms.
By Application: Neurosurgery, Orthopedic Surgery, ENT Surgery, Trauma Surgery.
By Material Type: Stainless Steel, Aluminum, Titanium, Plastic.
By End User: Hospitals, Ambulatory Surgical Centers, Specialty Clinics, Research Institutes.
By Functionality: MRI Compatible, CT Compatible, X-ray Compatible.
By Installation Type: Fixed, Portable.
Market Forecast 🌟
2023 Volume: Estimated at 1.2 million units, projected to reach 2 million units by 2033.
Growth Drivers: Technological innovations, growing healthcare expenditures, and focus on patient safety.
Key Players
Integra LifeSciences: Renowned for advanced product portfolios.
Stryker Corporation: A leader in surgical innovation.
Micromar: Focusing on ergonomic and safety-enhanced designs.
#SkullClampMarket #Neurosurgery #MedicalDevices #PatientSafety #SurgicalPrecision #HealthcareInnovation #AdultSkullClamps #PediatricCare #AdvancedSurgery #MRICompatible #HealthcareInvestments
0 notes