#medical devices registration
Explore tagged Tumblr posts
Text
Medical Devices Registration in India

Ready to launch your health-related Medical devices in India? Apply for CDSCO Medical Device Registration with Agile Regulatory! Ensure compliance and smoothly enter the Indian healthcare sector. Our expert guidance covers documentation, submissions, fees, and procedures.
Discover how to initiate CDSCO Medical Device Registration today:
🔗 Visit us at: https://bit.ly/3VXMYFm
📞 Call us at +91 8178731176
#medical equipment#medical devices#cdsco certificate#cdsco registration#cdsco license#medical devices registration
0 notes
Text
The introduction of new medical devices onto the market is a difficult procedure that necessitates meticulous examination to guarantee their safety, efficacy, and conformity with existing criteria. Regulatory bodies play an important part in this process, overseeing medical device registration. In this mini-blog, we will look at the critical role of regulatory bodies in the licensing of medical devices, as well as their commitment to public health.
#Medical Devices Registration#Get Medical Devices Registration#Online Medical Devices Registration#Medical Devices Registration in India
0 notes
Text
Why You Need a CDSCO Registration Certificate for Your Medical Business
📢 Manufacturers & Importers! CDSCO certification is mandatory for medical devices & drugs in India. Stay compliant with Corpbiz and build trust! ✅
💡 Get expert support for seamless registration.

0 notes
Text
ASC Group ensures hassle-free CDSCO registration for you, as your trusted CDSCO Registration Consultant. We are specialists in medical devices and drug regulatory compliance and offer expert guidance in the license securing process.
#CDSCO Registration Consultant#cdsco consultant#cdsco license consultant#CDSCO medical device consultant#CDSCO consultants in Delhi
0 notes
Text
ASC Group offers expert CDSCO consultants in Delhi, specializing in medical device registration and compliance with Indian regulations. Trust our professionals for efficient guidance and seamless licensing solutions!
#CDSCO consultants in Delhi#CDSCO medical device consultant#cdsco license consultant#cdsco consultant#CDSCO Registration Consultant
0 notes
Text
Achieving Precision: Medical Device Straightening Solutions

In the intricate world of medical device manufacturing, precision is paramount. From delicate surgical instruments to life-sustaining implants, the integrity and functionality of these devices directly impact patient safety and treatment outcomes. One critical aspect of ensuring this precision lies in straightening, a crucial step in the manufacturing process. This article delves into the significance of straightening solutions in medical device manufacturing, exploring the challenges, technologies, and industry best practices.
The Importance of Straightening in Medical Device Manufacturing
Enhanced Performance: Straightening is essential for achieving optimal device performance. Bent or warped components can lead to:
Malfunction: Incorrect functioning of implants, catheters, or other devices.
Reduced Accuracy: Inaccurate delivery of medication or other substances.
Increased Wear and Tear: Premature failure of moving parts.
Improved Safety: Straightening minimizes the risk of:
Tissue Damage: Bent instruments can cause unintended tissue damage during procedures.
Device Failure: Malfunctioning devices can pose serious risks to patient safety.
Surgical Complications: Complications arising from the use of improperly straightened components.
Enhanced Aesthetics: For certain devices, such as orthopedic implants, visual appeal and patient satisfaction are important considerations. Straightening ensures aesthetically pleasing and consistent finishes.
Challenges in Medical Device Straightening
Material Diversity: Medical devices are manufactured from a wide range of materials, including metals (stainless steel, titanium, nitinol), polymers, and ceramics. Each material presents unique challenges in terms of straightening techniques and achieving the desired level of precision.
Complex Geometries: Many medical devices have intricate shapes and geometries, making it difficult to achieve uniform straightening without compromising the integrity of the component.
Tight Tolerances: Stringent quality control standards necessitate extremely tight tolerances for dimensional accuracy and straightness. Meeting these demands requires advanced technologies and precise control.
Minimizing Stress and Distortion: The straightening process itself can introduce stresses and distortions into the material. Minimizing these effects is crucial to ensure device reliability and longevity.
Advanced Technologies for Medical Device Straightening
Laser Straightening: Laser-based systems offer high precision and minimal heat input, reducing the risk of material distortion. They are particularly effective for straightening delicate components and materials with varying thicknesses.
Magnetic Pulse Forming: This non-contact technique utilizes electromagnetic pulses to induce plastic deformation, straightening the material without physical contact. It is suitable for a wide range of materials and complex geometries.
Roller Straightening: This traditional method utilizes a series of rollers to gradually straighten the material. While effective for many applications, it may not be suitable for highly complex shapes or materials with varying thicknesses.
Vibration Straightening: This technique involves subjecting the material to high-frequency vibrations, which can effectively remove bends and kinks. It is often used for straightening wires, tubes, and other elongated components.
Quality Control and Inspection
In-Process Monitoring: Continuous monitoring throughout the straightening process is essential to ensure that the desired level of straightness is achieved.
Non-Destructive Testing (NDT): Techniques such as X-ray, ultrasound, and eddy current testing are used to detect internal defects and ensure the integrity of the straightened components.
Dimensional Inspection: Precise measurements are taken to verify that the straightened components meet the specified tolerances.
Industry Best Practices
Collaboration: Close collaboration between engineers, technicians, and quality control personnel is crucial to optimize the straightening process and ensure consistent results.
Process Validation: Thorough validation of the straightening process is essential to demonstrate that it consistently produces components that meet the required specifications.
Continuous Improvement: Regular review and analysis of the straightening process can identify areas for improvement and enhance overall efficiency and effectiveness.
Conclusion
Straightening plays a critical role in ensuring the safety, efficacy, and performance of medical devices. By employing advanced technologies, adhering to strict quality control standards, and embracing industry best practices, manufacturers can achieve the highest levels of precision and deliver high-quality medical devices that improve patient outcomes. As technology continues to evolve, we can expect to see further advancements in medical device straightening solutions, enabling the development of even more sophisticated and innovative medical technologies.
0 notes
Text
#medical device consultant#medical device registration#medical device registration in india#medical device consultancy in india
0 notes
Text
Unlocking Compliance: Essential Guide to the Non-Conviction Certificate for Medical Devices in India
In India, medical device registration is essential for manufacturers, importers, and distributors aiming to ensure regulatory compliance and public safety. Governed by the Central Drugs Standard Control Organization (CDSCO) under the Medical Device Rules 2017, the process requires various approvals, including CDSCO medical device registration, product approvals, import licenses, and the Non-Conviction Certificate (NCC). Among these, the NCC is a crucial document that reinforces a company’s compliance record. This guide delves into the Non-Conviction Certificate, highlighting its importance, eligibility, and the steps involved in obtaining it.
What is a Non-Conviction Certificate (NCC)?
A Non-Conviction Certificate (NCC) is issued by the CDSCO to Indian medical device companies. This certificate attests that a company has not been convicted of offenses related to safety, quality standards, or product malfunctions associated with its devices. Often essential in international tenders and product registration in foreign markets, the NCC demonstrates a company's adherence to high regulatory standards. This certification is vital in the medical device regulatory services landscape in India, underscoring the company’s commitment to ethical practices.
Importance of the Non-Conviction Certificate (NCC)
The Non-Conviction Certificate has numerous advantages for medical device companies, including:
- Building Trust: Demonstrates a company’s dedication to safety, quality, and regulatory compliance. - Unlocking Business Opportunities: Required in many tenders and pre-qualification applications, especially in CDSCO medical device registration. - Maintaining Market Reputation: Strengthens a company’s credibility and reputation in the medical devices India sector.
Eligibility Criteria for the Non-Conviction Certificate
To be eligible for the NCC, a company must: - Hold a valid medical device registration India license from the Central Licensing Authority (CLA) or State Licensing Authority (SLA). - Maintain a clean record with no prior convictions or violations under the Drugs and Cosmetics Act 1940 and related Rules.
Required Documentation for the Non-Conviction Certificate
Obtaining an NCC involves compiling the following documents: 1. A cover letter stating the purpose of the application. 2. A copy of the company's valid medical device registration or import license. 3. A list of products for which the NCC is being requested. 4. Prescribed government fees. 5. A Legal Undertaking on a ₹100 registered, notarized stamp paper, confirming no convictions related to product malfunctions or compliance violations.
Who Issues the Non-Conviction Certificate?
The licensing authority that initially issued the manufacturing or import license (either the CLA or SLA) is responsible for granting the NCC.
Validity of the Non-Conviction Certificate
The NCC is valid for one year from issuance or until the company's manufacturing license expires, whichever occurs first.
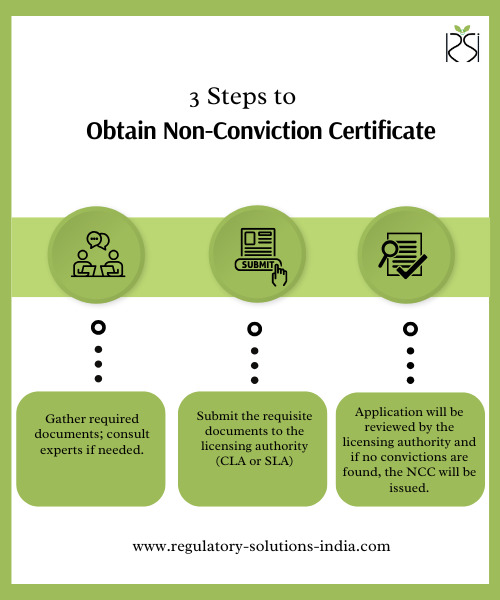
Steps to Obtain the Non-Conviction Certificate
1. Submit the application with the necessary documents to the relevant licensing authority. 2. For a smooth application process, consider engaging a regulatory consultant in India who specializes in medical device regulatory consultancy.
Benefits of Hiring a Regulatory Consultant for NCC Application
Engaging a medical device regulatory consultant in India can ease the application process, given their expertise in regulatory consultancy services. Consultants bring several advantages: - Requirement Clarity: Ensures the applicant meets eligibility criteria and provides complete documentation. - Efficient Navigation of Procedures: Streamlines applications and reduces potential delays. - Expert Advice: Consultants bring in-depth knowledge, particularly beneficial for medical device registration India requirements. - Peace of Mind: Consultants manage the regulatory process, allowing companies to focus on core operations.
Consult Regulatory Solutions India for Your NCC Needs
Regulatory Solutions India (RSI) offers expert regulatory consultancy services for medical devices and IVDs in India, providing end-to-end support for compliance, including CDSCO consultancy services. With RSI's guidance, you can ensure compliance with regulatory standards, obtain the Non-Conviction Certificate, and gain a competitive edge in India’s regulated markets. ContactRSI today to discuss your specific requirements. Our team of regulatory consultants is here to assist with your CDSCO medical device registration and NCC needs, making the compliance journey smoother and more efficient.
0 notes
Text
A major update in 2024 is the revised drug registration process. Now, CDSCO requires a more extensive review of safety data, especially for new or innovative drugs. This means companies have to provide comprehensive safety reports along with their applications, including detailed clinical data from other countries if the drug is being used internationally.
#cdsco medical device import license#cdsco registration certificate#cdsco test license#cdsco cosmetic import registration#cdsco cosmetic manufacturing license
0 notes
Text

Medical Device (MD 42) Wholesale License Registration in India
MD 42 License is essential for the import and export of medical wholesale in India, ensuring compliance with regulatory standards. PSR Compliance offers expert consultancy for medical device license registration, guiding businesses through a comprehensive step-by-step process. With reliable support, companies can navigate the complexities of licensing, ensuring smooth operations in the medical device market.
0 notes
Text

In India, obtaining a Medical Device Wholesale License (MD 42 License) is essential for businesses involved in the distribution of medical devices. Medical Device Wholesale license makes sure compliance with regulatory standards, facilitating smooth operations and trade. To apply for the MD 42 License, companies must follow the registration process outlined by the Central Drugs Standard Control Organization (CDSCO). Agile Regulatory offers expert services to help you navigate the application process efficiently and makes sure a hassle-free experience and timely approval for your wholesale medical device operations.
0 notes
Text
The Role of Regulatory Authorities in Medical Devices Registration
Introduction: The introduction of new medical devices into the market is a complex process that requires careful scrutiny to ensure their safety, efficacy, and compliance with established standards. Regulatory authorities play a crucial role in this process, overseeing the registration of medical devices. In this mini blog, we will explore the essential role of regulatory authorities in the…

View On WordPress
#cdsco medical device registration#CDSCO medical devices registration#cdsco registration for medical devices#medical device registration certificate#medical devices registration#medical devices registration in India#medical devices registration online
0 notes
Text
CDSCO Registration: A Key to Market Entry
For any healthcare product to be sold in India, obtaining CDSCO registration is essential. This certification proves that the product meets Indian safety and quality standards. Whether it’s a new drug, a cutting-edge medical device, or a cosmetic product, CDSCO registration is the first step toward market entry.
Visit -
https://www.social.united-tuesday.org/read-blog/20739_cdsco-and-its-impact-on-india-039-s-healthcare-innovation.html

0 notes
Text
ASC Group, a trusted CDSCO license consultant, provides expert guidance on obtaining CDSCO licenses. Our services ensure smooth regulatory compliance for drug, cosmetic, and medical device approvals in India. Partner with ASC Group for reliable and efficient licensing solutions.
#CDSCO Registration Consultant#cdsco consultant#cdsco license consultant#CDSCO medical device consultant#CDSCO consultants in Delhi
0 notes
Text
CDSCO Medical Device Certification & Registration Online - Om Garuda Group
Looking to register your medical device in India? Om Garuda Group is here to guide you through the process of cdsco medical device certification. Our expert team assists in cdsco registration online, ensuring that your devices meet regulatory standards. With our support, you can navigate the process with ease and confidence. Contact us today for professional help in getting your certification done right.
0 notes
Text
Medical Device Manufacturing Facility Consultants – Layout and Designing
Medical device manufacturing facility consultants specialize in designing and optimizing facilities to meet regulatory requirements and enhance operational efficiency. Their services cover a range of critical areas including facility layout, equipment selection, cleanroom design, and adherence to industry standards like ISO 13485 and FDA 21 CFR Part 820.
These consultants provide expertise in ensuring facilities comply with Good Manufacturing Practices (GMP), reducing contamination risks, and improving workflow efficiency. They collaborate closely with companies to ensure smooth processes from initial design to implementation, considering production capacity, automation needs, and future scalability. Additionally, consultants often guide companies through regulatory approvals, audits, and facility validation, ensuring that the infrastructure supports high-quality device production and compliance with international regulations.
This comprehensive approach ensures that medical device manufacturers can produce safe and effective products while maintaining cost-effectiveness and compliance.
What could a reimagined facility design mean for your operational efficiency and compliance? Discover how Operon Strategist can transform your manufacturing space into a model of productivity and regulatory adherence.
0 notes