#medical device testing and certification market share
Explore tagged Tumblr posts
Text
Medical Device Testing and Certification Market: Ensuring Safety, Compliance, and Innovation in Healthcare
The Global Medical Device Testing and Certification Market is projected to grow from USD 10.16 billion in 2024 to USD 12.73 billion by 2029, at a compound annual growth rate (CAGR) of 3.80% over the forecast period (2024-2029).
The medical device testing and certification industry plays a crucial role in the healthcare sector by ensuring that all medical devices meet rigorous safety, quality, and regulatory standards before reaching the market. This process is essential for safeguarding patient health and supporting healthcare providers with reliable, effective equipment. As medical devices become more advanced and complex, the need for thorough testing and certification has only intensified, driving growth in this market.
Key Drivers and Market Trends
Rising Demand for Advanced Medical Devices: The growing adoption of advanced medical devices in healthcare—ranging from wearable health monitors to complex diagnostic equipment—has created a pressing need for comprehensive testing and certification services. As technology continues to innovate, testing must adapt to address new functionalities, connectivity, and compliance standards that ensure these devices are safe and effective in real-world use.
Stringent Regulatory Requirements: Regulatory bodies worldwide, such as the FDA in the United States and the European Medicines Agency in Europe, have stringent regulations for medical devices. These standards govern everything from design and manufacturing to post-market surveillance, making certification a complex yet necessary process. To bring products to market, manufacturers must navigate a series of approvals and audits, which has led to high demand for specialized testing and certification services that streamline compliance.
Increased Focus on Patient Safety and Quality: The medical device industry prioritizes patient safety above all else, and testing is essential to identify potential issues early in the development process. Comprehensive testing reduces the risk of device failures and enhances product quality, ultimately improving patient outcomes. Certification services add an extra layer of assurance, indicating that a device has met industry and regulatory standards.
Adoption of Digital and Connected Health Technologies: With the rise of connected health devices, such as smart implants, remote monitoring systems, and mobile health applications, cybersecurity and interoperability have become major concerns. Testing now includes evaluating devices for data security, wireless communication, and compliance with international standards, such as ISO and IEC, to prevent data breaches and ensure seamless integration within healthcare systems.
Increased Use of Artificial Intelligence and Machine Learning: Artificial intelligence (AI) and machine learning (ML) are transforming the medical device landscape, especially in diagnostic imaging, personalized medicine, and predictive analytics. Devices that incorporate AI/ML algorithms require additional layers of testing to verify algorithm accuracy, assess predictive performance, and meet regulatory requirements. This has led to a growing demand for specialized testing services that understand the unique challenges of AI-driven devices.
Types of Testing and Certification Services
Performance Testing: Performance testing evaluates whether a device meets the functional specifications required by regulatory authorities and manufacturers. This includes testing for durability, reliability, and efficiency under normal usage conditions, ensuring that devices deliver consistent results without compromising quality.
Safety and Compliance Testing: Safety testing examines devices for hazards such as electrical safety, biocompatibility, and chemical exposure, ensuring they meet the standards necessary to protect both patients and healthcare providers. Compliance testing checks that devices adhere to international and regional regulations, supporting manufacturers in achieving the certifications needed to enter various markets.
EMC and EMI Testing: Electromagnetic Compatibility (EMC) and Electromagnetic Interference (EMI) testing are critical for devices that rely on electronic and wireless components. These tests ensure that devices can operate effectively without interference from other electronic systems, a key requirement for devices that need to function accurately in complex hospital environments.
Cybersecurity and Software Testing: As digital healthcare devices increasingly handle sensitive data, cybersecurity testing is essential. This testing assesses vulnerabilities, encryption standards, and data protection measures to prevent unauthorized access or breaches. Software testing, on the other hand, ensures that a device’s software performs correctly under various scenarios, minimizing the risk of malfunction.
Usability and Human Factors Testing: Usability testing evaluates how intuitive and user-friendly a device is, focusing on how healthcare providers and patients interact with it. By analyzing user behavior, human factors testing can identify design improvements that enhance device operation and reduce the likelihood of user error.
Emerging Innovations in Medical Device Testing and Certification
Simulation-Based Testing: Simulation-based testing is becoming increasingly popular as it allows for the virtual testing of medical devices in a controlled digital environment. Through simulations, manufacturers can assess device performance under a variety of scenarios, reducing the need for physical testing and enabling faster adjustments during the design phase.
Automated and AI-Driven Testing: AI and automation are streamlining the testing process by enabling rapid data analysis, automated testing procedures, and enhanced accuracy in identifying issues. Automated testing reduces human error, speeds up testing cycles, and allows for more comprehensive assessments, particularly in complex devices with numerous components and functionalities.
Cloud-Based Testing Platforms: Cloud-based testing solutions offer an efficient, collaborative platform for managing test data, results, and device specifications across global teams. These platforms facilitate quicker approvals, streamlined regulatory documentation, and more efficient workflow management, supporting faster time-to-market for new devices.
3D Printing for Prototyping and Testing: 3D printing technology allows manufacturers to create prototypes quickly and at a lower cost, enabling testing at earlier stages of development. With 3D printing, design modifications can be implemented and tested almost in real time, accelerating the development process while ensuring compliance with industry standards.
Future Outlook for the Medical Device Testing and Certification Market
The medical device testing and certification market is expected to grow steadily as healthcare technology advances and regulatory standards evolve. With the global focus on safety, quality, and efficiency, manufacturers will increasingly rely on specialized testing services to ensure compliance and market readiness. In addition, emerging technologies like AI, the Internet of Medical Things (IoMT), and telemedicine will introduce new testing requirements, creating opportunities for companies that can offer innovative and adaptable testing solutions.
Investments in automated, AI-enhanced testing solutions, along with advancements in cybersecurity, are likely to define the future of this industry. As medical devices become an integral part of modern healthcare, the importance of robust testing and certification will continue to rise, ensuring that only the safest and most reliable devices reach patients worldwide.
For a detailed overview and more insights, you can refer to the full market research report by Mordor Intelligence https://www.mordorintelligence.com/industry-reports/medical-device-testing-and-certification-market
#medical device testing and certification market#medical device testing and certification market size#medical device testing and certification market share#medical device testing and certification market trends#medical device testing and certification market report
0 notes
Text
Drug Testing Procurement Intelligence: A Comprehensive Guide
The drug testing category is expected to grow at a CAGR of 6.5% from 2023 to 2030. The key drivers of the market growth are the increasing consumption of alcohol by young people and the elderly, the overuse of prescribed medications, and the rising use of narcotics. The demand for drug testing is also anticipated to increase due to the increased instances of illegal drug use and the rising crime rates linked to drug misuse. There is more competition among manufacturers, and those developing innovative products across a wider range of categories are capturing a larger share of the market.
Technologies such as gas chromatography-mass spectrometry (GC-MS), liquid chromatography-mass spectrometry (LC-MS), and enzyme-linked immunosorbent assay (ELISA) are used in this category. Quest Diagnostics uses GC-MS technology, which identifies and quantifies drugs in complex biological samples and separates the components of a sample based on their volatility and mass-to-charge ratio. Labcorp uses ELISA technology that uses enzymes to detect the presence of drugs in blood or urine samples.
This category is highly fragmented with many companies offering a variety of products and services. It includes a mix of large, established companies as well as smaller, specialized companies that focus on specific types of testing or serve niche markets. There are many companies that provide services outside of the traditional laboratory setting such as point-of-care testing devices and at-home drug testing kits.
Order your copy of the Drug Testing Procurement Intelligence Report, 2023 - 2030, published by Grand View Research, to get more details regarding day one, quick wins, portfolio analysis, key negotiation strategies of key suppliers, and low-cost/best-cost sourcing analysis
Suppliers often use a volume-based pricing strategy. Suppliers frequently offer discounts and other incentives to customers who require numerous tests, which in turn helps the suppliers to increase their sales. Additionally, it provides a cost-effective choice for individuals who require ongoing or recurring services. The cost of the equipment, which includes drug testing kits that range from USD 2 to USD 350 depending on the accuracy and kind of sample, is a significant cost component. The typical cost of other equipment, including sterilizing supplies, lab coats, and other sanitary things, is typically between USD 200 - USD 500. The cost of insurance, which must be purchased to cover losses and damages that may result from conducting testing, is another significant cost component. It includes property and general liability and may cost between USD 500 - USD 2,500.
The United States and China are the global leaders in this category as they are heavily investing in research and development to improve the accuracy and efficiency of drug testing technologies. Choosing a trustworthy supplier with a track record of delivering high-quality goods and first-rate customer service is the finest sourcing strategy in the category. Another key parameter to consider is whether the goods adhere to industry norms and rules. Observing OSHA's (Occupational Safety and Health Administration) standards, for instance. In order to prevent tampering or contamination and to guarantee the integrity and authenticity of the results, it is essential to maintain a safe and documented chain of custody for samples.
Drug Testing Procurement Intelligence Report Scope
• Drug Testing Category Growth Rate: CAGR of 6.5% from 2023 to 2030
• Pricing growth Outlook: 9 - 10% (Annually)
• Pricing Models: Volume-based pricing model
• Supplier Selection Scope: Cost and pricing, Past engagements, Productivity, Geographical presence
• Supplier selection criteria: Regulatory compliance, validation and certification, delivery time, location, reliability, experience, technical specifications, operational capabilities, regulatory standards and mandates, category innovations, and others.
• Report Coverage: Revenue forecast, supplier ranking, supplier matrix, emerging technology, pricing models, cost structure, competitive landscape, growth factors, trends, engagement, and operating model
Browse through Grand View Research’s collection of procurement intelligence studies:
• Vaccines Procurement Intelligence Report, 2023 - 2030 (Revenue Forecast, Supplier Ranking & Matrix, Emerging Technologies, Pricing Models, Cost Structure, Engagement & Operating Model, Competitive Landscape)
• Clinical Trial Imaging Services Procurement Intelligence Report, 2023 - 2030 (Revenue Forecast, Supplier Ranking & Matrix, Emerging Technologies, Pricing Models, Cost Structure, Engagement & Operating Model, Competitive Landscape)
Key companies profiled
• Quest Diagnostics
• Abbott Laboratories
• Quidel
• Thermo Fisher Scientific
• Siemens Healthineers
• Bio Rad Laboratories
• Agilent Technologies
• Labcorp
• Clinical Reference Laboratory
• Cordant Health Solutions
Brief about Pipeline by Grand View Research:
A smart and effective supply chain is essential for growth in any organization. Pipeline division at Grand View Research provides detailed insights on every aspect of supply chain, which helps in efficient procurement decisions.
Our services include (not limited to):
• Market Intelligence involving – market size and forecast, growth factors, and driving trends
• Price and Cost Intelligence – pricing models adopted for the category, total cost of ownerships
• Supplier Intelligence – rich insight on supplier landscape, and identifies suppliers who are dominating, emerging, lounging, and specializing
• Sourcing / Procurement Intelligence – best practices followed in the industry, identifying standard KPIs and SLAs, peer analysis, negotiation strategies to be utilized with the suppliers, and best suited countries for sourcing to minimize supply chain disruptions
#Drug Testing Procurement Intelligence#Drug Testing Procurement#Procurement Intelligence#Drug Testing Market#Drug Testing Industry
0 notes
Text
Hand Pump Market Current Scenario and Future Prospects by 2032
Hand Pump Market provides in-depth analysis of the market state of Hand Pump manufacturers, including best facts and figures, overview, definition, SWOT analysis, expert opinions, and the most current global developments. The research also calculates market size, price, revenue, cost structure, gross margin, sales, and market share, as well as forecasts and growth rates. The report assists in determining the revenue earned by the selling of this report and technology across different application areas.
Geographically, this report is segmented into several key regions, with sales, revenue, market share and growth Rate of Hand Pump in these regions till the forecast period
North America
Middle East and Africa
Asia-Pacific
South America
Europe
Key Attentions of Hand Pump Market Report:
The report offers a comprehensive and broad perspective on the global Hand Pump Market.
The market statistics represented in different Hand Pump segments offers complete industry picture.
Market growth drivers, challenges affecting the development of Hand Pump are analyzed in detail.
The report will help in the analysis of major competitive market scenario, market dynamics of Hand Pump.
Major stakeholders, key companies Hand Pump, investment feasibility and new market entrants study is offered.
Development scope of Hand Pump in each market segment is covered in this report. The macro and micro-economic factors affecting the Hand Pump Market
Advancement is elaborated in this report. The upstream and downstream components of Hand Pump and a comprehensive value chain are explained.
Browse More Details On This Report at @https://www.globalgrowthinsights.com/market-reports/hand-pump-market-100563
Global Growth Insights
Web: https://www.globalgrowthinsights.com
Our Other Reports:
Global Orthotic Devices MarketMarket Growth
Machine Vision MarketMarket
Digital Experience Platform MarketMarket Share
Testing, Inspection, and Certification (TIC) MarketMarket Growth Rate
Industrial Casters MarketMarket Forecast
Global MIS Sacroiliac Joint Fusion MarketMarket Size
Halogenated Butyl Rubber MarketMarket Growth
Oncaspar MarketMarket Analysis
Neuroprosthetics MarketMarket Size
Global Call Center Software MarketMarket Share
Global Appointment Scheduling Software MarketMarket Growth
Anc Headset MarketMarket
Ultra High Molecular Weight Polyethylene (UHMWPE) Ropes MarketMarket Share
API Management MarketMarket Growth Rate
Agarose MarketMarket Forecast
Global Boat Docks and Lifts MarketMarket Size
Nanocellulose MarketMarket Growth
Automatic Palletizer MarketMarket Analysis
Hydrogen Sulfide (H2S) Scavenger MarketMarket Size
Global Augmented Reality and Virtual Reality (AR and VR) MarketMarket Share
Global Medical Bionic Implants MarketMarket Growth
Weatherstrip Seal MarketMarket
L-Leucine MarketMarket Share
Humidifiers MarketMarket Growth Rate
Online Coaching Software MarketMarket Forecast
Global Hydraulic Dock Leveler MarketMarket Size
Residential Furniture MarketMarket Growth
Industrial Rubber Products MarketMarket Analysis
Radio Taxi Service MarketMarket Size
Global Premium Pram and Baby Stroller MarketMarket Share
Global Pet Wearable MarketMarket Growth
Fortified Wine MarketMarket
Human-centric Lighting MarketMarket Share
Automatic License Plate Recognition (ALPR) MarketMarket Growth Rate
Zinc Waste Recycling And Reusing MarketMarket Forecast
Global Automatic Laser Tube Cutting Machine MarketMarket Size
Financial Grade Security Chip MarketMarket Growth
Mobile Scaffold Tower MarketMarket Analysis
Cable Tie Guns Market Market Size
Global Digital Ultrasonic Thickness Gauges MarketMarket Share
0 notes
Text
Top 10 FAQs Based on Fitness App Development

Introduction:
The popularity of fitness applications continues to grow due to a growing focus on wellness and health entrepreneurs and developers are more interested in getting into the development of fitness apps. However, the process of developing apps can be a daunting task and can lead to a variety of questions. We'll dig into the top 10 most frequently inquired-about questions (FAQs) related to fitness apps, and provide detailed answers that will guide users through this process.
What is Fitness App Development?
Fitness app development involves the development of mobile apps that aid users in tracking their fitness levels, keep track of their health indicators, perform exercise routines, and meet your fitness targets. These apps could be anything from simple fitness trackers to complex platforms offering personalised training programs, nutrition counseling and social functions.
What are the Key Features of a Fitness App?
The main attributes of fitness apps include exercise tracking, personalized programs for training, tracking nutrition progress monitoring the ability to share content, support for communities connectivity with devices that wear clothes and gamification components. The features that are included will differ according to the app's intended user, its goals and the strategy of differentiation.
How Much Does it Cost to Develop a Fitness App?
The cost of constructing an app for fitness is contingent on many aspects, such as the features, platform (iOS, Android, or cross-platform) as well as development resources, design complexity and the regulatory compliance standards. Basic fitness apps be priced between $50,000 and $100,000, while more advanced apps that have advanced features could require investment of up to $250,000 or more.
How Long Does it Take to Develop a Fitness App?
The time frame for development of fitness apps differs based on factors like project scale as well as the complexity, platform and the availability of resources. On average, creating an app for fitness could take anywhere from 3 to six months, while more complicated apps could require six months to a year or more to develop testing, deployment, and deployment.
What Technologies are Used in Fitness App Development?
Fitness app development usually involves an amalgamation of frontend technology (such such as Swift or Kotlin for native iOS and Android development or cross-platform frameworks such as React Native or Flutter) as well as backend technologies (such such as Node.js, Django, or Firebase) as well as databases (such such as MySQL, MongoDB, or SQLite).
How Do I Monetize My Fitness App?
There are a variety of monetization strategies for fitness apps. These include model that is subscription-based, as well as freemium versions with in-app purchases to purchase special features or one-time transactions sponsorship deals, advertising revenue as well as affiliate marketing agreements with fitness professionals or brands. The method of monetization chosen is based on the app's value offering and the target market.
How Can I Ensure User Engagement and Retention?
To improve the user's involvement and retainment, concentrate on providing an experience that is seamless for users including personalized content and recommendations and social features to aid in community development and accountability. Also, consider gamification features like challenges and rewards and regular updates to bring you new content and features and prompt customer support.
What regulatory considerations should I be aware of?
Depending on the app's purpose of usage and market it is targeting the app's regulatory requirements could include the compliance with privacy laws (such like GDPR, or HIPAA) as well as conformance to the industry guidelines for fitness and health apps, and the need to obtain approvals or certifications (such for FDA Approval for Medical apps).
How Do I Market My Fitness App?
The most effective marketing tactics for fitness applications comprise optimizing the store's app (ASO) to increase visibility and searchability as well as social media marketing to interact with users who are interested and to build communities, influencer partnerships to reach a larger market, content marketing using blogs, videos and podcasts, and also paid ads that target specific groups of people.
How Do I Measure the Success of My Fitness App?
Key performance indicators (KPIs) for assessing the effectiveness of fitness apps include the metrics of user acquisition (such as people who are active, as well as retention rates) Engagement metrics (such as duration of sessions as well as the frequency of usage and user interaction) Revenue indicators (such as subscription revenues, in-app purchases as well as advertising revenues) as well as the qualitative comments of users.
Conclusion:
Understanding the complexities of fitness apps isn't easy however, armed with the answers to these 10 most frequently asked questions entrepreneurs and developers can start your fitness application journey in confidence. When you know the essential aspects using the most appropriate technology or strategies, as well as remaining tuned to the needs of users it is possible to create an effective fitness app that has a positive effect on the health and well-being of your users journey.
0 notes
Text
Compact HbA1c Analyzer, Global Market Size Forecast, Top 5 Players Rank and Market Share
Compact HbA1c Analyzer Market Summary
According to the new market research report “Global Compact HbA1c Analyzer Market Report 2024-2030”, published by QYResearch, the global Compact HbA1c Analyzer market size is projected to reach USD 0.9 billion by 2030, at a CAGR of 10.4% during the forecast period.

Figure. Global Compact HbA1c Analyzer Market Size (US$ Million), 2019-2030

Above data is based on report from QYResearch: Global Compact HbA1c Analyzer Market Report 2024-2030 (published in 2024). If you need the latest data, plaese contact QYResearch.
Figure. Global Compact HbA1c Analyzer Top 5 Players Ranking and Market Share (Ranking is based on the revenue of 2023, continually updated)

Above data is based on report from QYResearch: Global Compact HbA1c Analyzer Market Report 2024-2030 (published in 2024). If you need the latest data, plaese contact QYResearch.
According to QYResearch Top Players Research Center, the global key manufacturers of Compact HbA1c Analyzer include Siemens, Bio-Rad Laboratories, etc. In 2023, the global top three players had a share approximately 21.0% in terms of revenue.
Figure. Compact HbA1c Analyzer, Global Market Size, Split by Product Segment

Based on or includes research from QYResearch: Global Compact HbA1c Analyzer Market Report 2024-2030.、
Figure. Compact HbA1c Analyzer, Global Market Size, Split by Application Segment

Based on or includes research from QYResearch: Global Compact HbA1c Analyzer Market Report 2024-2030.
Figure. Compact HbA1c Analyzer, Global Market Size, Split by Region

Based on or includes research from QYResearch: Global Compact HbA1c Analyzer Market Report 2024-2030.
Market Drivers:
Market demand: As people's attention to chronic diseases such as diabetes continues to increase, HbA1c, as an important indicator that reflects blood sugar control levels, has an increasing demand for testing. Especially for patients who need to regularly monitor HbA1c levels, such as diabetics, they need a convenient, fast and accurate testing device. Compact HbA1c Analyzer precisely meets this market demand due to its small size, easy portability, and simple operation.
Technological progress: With the continuous development of biosensing technology, microelectronics technology and other fields, it is possible to manufacture smaller and more precise medical equipment. These technological advances have provided technical support for the development of Compact HbA1c Analyzer, enabling it to achieve high sensitivity and specificity detection while maintaining a small size and low energy consumption.
Medical practice needs: In medical practice, fast and accurate test results are crucial for patient diagnosis and treatment. Compact HbA1c Analyzer can produce test results in a shorter period of time, helping doctors to understand the patient's blood sugar control in a timely manner and make correct treatment decisions. In addition, due to its portability, doctors can also conduct testing at patients' homes or outside clinics, further facilitating the provision of medical services.
Challenge:
Technical bottleneck: Although Compact HbA1c Analyzer has made significant technical progress, it may still face technical bottlenecks in the pursuit of smaller volume and higher accuracy. How to ensure that the detection accuracy and stability are not reduced while reducing the volume is a key issue that needs to be solved during the research and development process.
Industry standards and certification: The development and launch of medical devices require strict industry standards and certification processes. For HbA1c Analyzer, ensuring its accuracy and reliability to meet relevant regulatory and certification requirements is an important challenge.
Market competition: With the continuous advancement of medical technology and the growth of market demand, the HbA1c Analyzer market competition is becoming increasingly fierce. How to stand out among many competitors and provide competitive products and services is a challenge that manufacturers need to face.
Cost control: On the premise of ensuring product quality and performance, reducing production costs is the goal pursued by manufacturers. However, in the process of R&D and production of Compact HbA1c Analyzer, you may face cost pressures in raw materials, production equipment, etc.
User education and training: Despite the portability and ease of use of the Compact HbA1c Analyzer, proper use and maintenance of the device may still be an issue for some non-expert users. Therefore, providing effective user education and training support is also one of the challenges that manufacturers need to consider.
About QYResearch
QYResearch founded in California, USA in 2007.It is a leading global market research and consulting company. With over 16 years’ experience and professional research team in various cities over the world QY Research focuses on management consulting, database and seminar services, IPO consulting, industry chain research and customized research to help our clients in providing non-linear revenue model and make them successful. We are globally recognized for our expansive portfolio of services, good corporate citizenship, and our strong commitment to sustainability. Up to now, we have cooperated with more than 60,000 clients across five continents. Let’s work closely with you and build a bold and better future.
QYResearch is a world-renowned large-scale consulting company. The industry covers various high-tech industry chain market segments, spanning the semiconductor industry chain (semiconductor equipment and parts, semiconductor materials, ICs, Foundry, packaging and testing, discrete devices, sensors, optoelectronic devices), photovoltaic industry chain (equipment, cells, modules, auxiliary material brackets, inverters, power station terminals), new energy automobile industry chain (batteries and materials, auto parts, batteries, motors, electronic control, automotive semiconductors, etc.), communication industry chain (communication system equipment, terminal equipment, electronic components, RF front-end, optical modules, 4G/5G/6G, broadband, IoT, digital economy, AI), advanced materials industry Chain (metal materials, polymer materials, ceramic materials, nano materials, etc.), machinery manufacturing industry chain (CNC machine tools, construction machinery, electrical machinery, 3C automation, industrial robots, lasers, industrial control, drones), food, beverages and pharmaceuticals, medical equipment, agriculture, etc.
0 notes
Text
Global EMC Testing Market Outlook 2024-2030
The EMC Testing Market is growing at a CAGR of 5.4%, from USD 2.1 billion in 2019 to USD 2.8 billion by 2024; the global market size estimation is provided from 2016 to 2024.
One of the primary reasons for this rise in demand is the increasing need for safety, owing to factors such as the standardization of electronic products globally, mandatory EMC testing of medical equipment, and increase in the brand proposition of the companies.
Key Players: A few key players operating in the EMC testing market are SGS (Switzerland), Bureau Veritas (France), Intertek (UK), Eurofins Scientific (Luxembourg), DEKRA (Germany), Yokogawa Electric (Japan), Keysight Technologies (US), Rohde & Schwarz (Germany), Fortive (US), AMETEK CTS (Switzerland).
Download PDF: https://www.marketsandmarkets.com/pdfdownloadNew.asp?id=254391962
EMC testing market for certification services to grow at highest CAGR in 2019
The EMC testing market for certification services is expected to grow at the highest CAGR during the forecast period. Certification services ensure product safety and performance attributes. Certification services mainly include customized audit and certification services with respect to quality, health & safety, environment, and social responsibility. These services enable clients to commit to ongoing processes aimed at improving their business performance.
Market for consumer appliances and electronics application to grow at highest CAGR during forecast period
The EMC testing market for consumer applications and electronics is expected to grow at the highest CAGR during the forecast period. EMC requirements for consumer appliances are a cross-reference to radio frequency requirements with embedded wireless functionality. Testing service providers offer testing services for wireless technologies such as Bluetooth, cellular, near-field communications (NFC), and Wi-Fi. Furthermore, any electronic device is an interconnection of different systems and components; therefore, the performance of the device is primarily dependent on these components.
APAC to hold largest share of EMC testing market
Countries in APAC have made huge investments in renewable energy sources such as solar, wind, and nuclear, which makes APAC an important market for the EMC industry. APAC is also gearing up toward a sustainable future with renewable energy at a faster rate. Emerging policies, strategic roadmaps, expanding investments, and improving technologies are driving national governments to aim toward a fossil-free nation. Demand for energy is rising across China, India, and Indonesia due to rapid urbanization and industrialization, and considerable opportunities exist to avoid long-term lock-in with carbon-based energy technologies. Along with vast renewable energy potential, the region already possesses significant knowledge and expertise on renewables.
0 notes
Text
Ensuring Compliance: Key Steps for CE Certification in Turkey

Making sure that products fulfill the essential safety and quality requirements is crucial for businesses looking to grow their market share and win over customers in today's globalized marketplace. A product's ability to freely move throughout the European Economic Area (EEA) is facilitated by the CE (Conformité Européenne) certification, which is recognised as a critical indicator of a product's compliance with EU standards in Turkey and many other countries. This accreditation serves as a symbol of quality, safety, and conformity to fundamental environmental and health standards in addition to being a legal requirement.
CE consultant in Turkey, which is ideally situated at the meeting point of Europe and Asia, has created a regulatory environment compliant with EU standards in order to facilitate commerce and improve consumer protection. In order to demonstrate compliance with pertinent EU legislation, a number of stages, including product testing and paperwork, are involved in Turkey's CE certification process.
What are the Benefits for CE Certification in Turkey
Market Expansion and Access: Obtaining a CE certification gives you access to the European Economic Area (EEA), a market with more than 500 million consumers. This implies greater market penetration and growth prospects for Turkish companies.
Competitive Edge: Your products will stand out from the competition since they have the CE mark, which attests to their compliance with strict EU regulations. Customers will be more trusting of your brand if they believe that the CE mark is a sign of quality, safety, and compliance.
Legal Compliance and Risk Mitigation: By guaranteeing compliance with EU directives through CE Implementation in Pune, legal and regulatory risks are reduced. Compliance with CE regulations is a proactive risk mitigation strategy because non-compliance could lead to penalties and restrictions in the market.
Enhanced Product Credibility: Your products' performance, safety, and quality are attested to by their CE certification. This seal of approval improves ties with distributors, merchants, and other stakeholders in addition to giving customers trust.
Facilitation of R&D and Innovation: Complying with CE requirements frequently calls for innovation and constant improvement. This dedication to R&D can lead to superior products and sustained competitiveness.
Overview of CE Audit in Turkey
Comprehending CE Audits: CE audits are methodical investigations carried out to confirm that a producer's procedures, records, and goods comply with the demands of applicable EU directives. These audits play a crucial role in determining whether items comply with important environmental, health, and safety regulations.
Choosing the Notified Bodies: Notified Bodies, impartial entities appointed by the Turkish government to evaluate conformance, frequently carry out CE Audit in Botswana. These organizations guarantee unbiased assessments and are essential to the certification process.
Getting Ready for CE Audits: To present during the audit, manufacturers are required to gather extensive technical documents, such as test reports, design requirements, and risk assessments.
It is imperative to guarantee that production processes adhere to the established requirements consistently, as auditors may do on-site inspections.
Remedial Procedures and After Action: If non-conformities are found, manufacturers are required to take corrective measures to resolve the problems.
To confirm that corrective actions are working, follow-up evaluations could be carried out.
Which Business suitable for CE Certification in Turkey
Electrical appliances and electronics:
End-user devices
Domestic appliances
Equipment for information technology
Equipment and Machinery:
Industrial gear
Tools for construction
Farm equipment
Medical Equipment:
Diagnostic tools
Surgical tools
Equipment for medical imaging
Automobile Parts:
Electronics for cars
Elements of safety
Engine parts
Recreational Equipment and Yokes:
Children's toys
Sports equipment
Recreational automobiles
How to get CE consultant in Turkey for my Business
B2Bcert Consultants might be a great option if you're looking for a CE consultant in Turkey to improve business operations and guarantee compliance with international standards. Selecting B2Bcert as your CE consultant in Turkey has several benefits, chief among them being their dedication to offering premium services at reasonable prices. Budgets are important in the corporate world, and B2Bcert stands out for providing affordable solutions without sacrificing the caliber of their consulting services.
0 notes
Text
Medical Device Testing Market Trends & Growth Analysis
Medical Device Testing Market, By Testing (Electromagnetic Compatibility, Electrical Safety, Wireless Connectivity (2g/3g/4g-Lte, Wi-Fi, Bluetooth, and NFC), Environmental Testing, Mechanical Testing, Chemical, Physical, and Biocompatibility Testing, Cybersecurity Testing), Service Type (Testing Services, Inspection Services, Certification Services and Others), Source Type (In-House and Outsourced), Technology (Active Implant Medical Device, Active Medical Device, Non-Active Medical Device, In-Vitro Diagnostic Medical Device, Ophthalmic Medical Device, Orthopaedic and Dental Medical Device, Vascular Medical Device and Others), Geography (North America, Europe, Asia-Pacific, Middle East and Africa and South America)
The global medical device testing market is anticipated to reach USD 9.8 billion by 2020 growing at a CAGR of 4.8% during the forecasting period, 2020-2028.
Growing demand for regulation of medical devices, increasing bent towards outsourcing of testing services are some of the factors that have supported long-term expansion for the medical device testing industry. Despite the continuous consolidation of the industry, the industry is remarkably fragmented.
Request Sample Pages of Report: https://www.delvens.com/get-free-sample/medical-device-testing-market-trends-forecast-till-2028

Key Findings
The global medical device testing market is segmented into testing, service type, sourcing type, device class, technology, and geography.
The testing segment is segmented into Electromagnetic Compatibility, Electrical Safety, Wireless Connectivity (2g/3g/4g-LTE, Wi-Fi, Bluetooth), and NFC
The service Type segment is segmented into Testing Services, Inspection Services, Certification Services, and Others
The sourcing Type segment is segmented into In-House and Outsourced
Device Class segment is segmented into Class I, Class II, and Class III
The technology segment is segmented into Active Implant Medical Device, Active Medical Device, Non-Active Medical Device, In-Vitro Diagnostic Medical Device, Ophthalmic Medical Device, Orthopaedic and Dental Medical Device, Vascular Medical Device and Others
Geographically, the global medical device testing market is sub-segmented into North America, Europe, Asia-Pacific, Middle East and Africa and South America and insights are provided for each region and major countries within the regions
To Grow Your Business Revenue, Make an Inquiry Before Buying at: https://www.delvens.com/Inquire-before-buying/medical-device-testing-market-trends-forecast-till-2028
Regional Analysis
Asia-Pacific region has come up as the prominent region for the leading share in the overall global market during the forecast period 2020-2028.
Competitive Landscape
Key players in the global medical device testing market are
SGS,
Eurofins Scientific,
Bureau Veritas,
Intertek,
TÜV SÜD
Dekra
Recent Developments
The companies have come up with various promotional activities in from of launch, investment, acquisition, and other, for instance:
In 2020, Bureau Veritas USA launched 5G device certification testing. With the opening of regulatory test and global market access services as well as 5G technology integrators can now access services from Bureau Veritas’ Bay Area technical competence center
In 2019, SGS launched a new service for international type approval (ITA) restricted substance compliance that offers electrical and electronics (E&E) products importers and manufacturers with a one for all solution
Direct Order for the Report: https://www.delvens.com/checkout/medical-device-testing-market-trends-forecast-till-2028
Reasons to Acquire
Increase your understanding of the market for identifying the best and suitable strategies and decisions on the basis of sales or revenue fluctuations in terms of volume and value, distribution chain analysis, market trends, and factors
Gain authentic and granular data access for the medical device testing market so as to understand the trends and the factors involved in changing market situations
Qualitative and quantitative data utilization to discover arrays of future growth from the market trends of leaders to market visionaries and then recognize the significant areas to compete in the future
In-depth analysis of the changing trends of the market by visualizing the historic and forecast year growth patterns
Read Related Reports:
The Medical Device Testing Market report answers a number of crucial questions, including:
Which companies dominate the Medical Device Testing Market?
What current trends will influence the market over the next few years?
What are the market's opportunities, obstacles, and driving forces?
What predictions for the future can help with strategic decision-making?
What advantages does market research offer businesses?
Which particular market segments should industry players focus on in order to take advantage of the most recent technical advancements?
What is the anticipated growth rate for the market economy globally?
About Us:
Delvens is a strategic advisory and consulting company headquartered in New Delhi, India. The company holds expertise in providing syndicated research reports, customized research reports and consulting services. Delvens qualitative and quantitative data is highly utilized by each level from niche to major markets, serving more than 1K prominent companies by assuring to provide the information on country, regional and global business environment. We have a database for more than 45 industries in more than 115+ major countries globally.
Delvens database assists the clients by providing in-depth information in crucial business decisions. Delvens offers significant facts and figures across various industries namely Healthcare, IT & Telecom, Chemicals & Materials, Semiconductor & Electronics, Energy, Pharmaceutical, Consumer Goods & Services, Food & Beverages. Our company provides an exhaustive and comprehensive understanding of the business environment.
Contact Us:
UNIT NO. 2126, TOWER B,
21ST FLOOR ALPHATHUM
SECTOR 90 NOIDA 201305, IN
+44-20-8638-5055
0 notes
Text
Unlocking the Future: Accelerating the Medical Device Analytical Testing Outsourcing Market 2022-2032

As per newly released data by Future Market Insights (FMI), The global Medical Device Analytical Testing Outsourcing Market generated US$ 5.11 billion in 2021 and is predicted to grow at a credible CAGR of 7.18% to reach US$ 10.96 billion in 2032. The global market for outsourcing medical device analytical testing generated US$ 5.11 billion in 2021 and is predicted to grow at a credible CAGR of 7.18% to reach US$ 10.96 billion in 2032. The complexity of the product, increased competition, an increase in the production of small-scale medical devices, and strict clearance requirements are all contributing factors.
Unleash Your Curiosity with our Assortment of Sample PDFs. @ https://www.futuremarketinsights.com/reports/sample/rep-gb-14999
The growing global prevalence of chronic diseases is driving up demand for medical device analytical testing outsourcing. The government’s increasingly stringent quality certificate rules are propelling the global medical device analytical testing outsourcing market forward. Class II devices require premarket approvals and 510 (k) clearance; however, obtaining such clearance is a time-consuming process; thus, such emerging scenarios have fuelled the growth of the global medical device analytical testing outsourcing market. Medical device manufacturers are hiring consultants to help them understand the documentation and regulations required for pre-market approvals. The development of advanced devices such as surgical microscopes, surgical robots, neurosurgery devices, ophthalmic surgical devices, and many others has reduced the direct contact of humans in the surgical procedure, making quality certificates for medical devices mandatory. The growing demand for minimally invasive surgeries is driving the global medical device analytical testing outsourcing market forward. However, manufacturers are turning to medical device analytical testing outsourcing to ensure accuracy, precision, quality control, and periodic maintenance. Key Takeaways ·The global Medical Device Analytical Testing Outsourcing market is estimated at US$ 10.96 Billion during the forecast period.The global Medical Device Analytical Testing Outsourcing market is estimated at US$ 5.11 Billion in 2021. The U.S is projected to lead the market while procuring US$ 1.5 Billion while expanding at a CAGR of 7% during the forecast period. China is anticipated to grow at 5.5% CAGR · The hospitals segment is predicted to lead the market with a CAGR of 6.15% during the assessment period.
Harness the Knowledge of Experts through our Ask the Expert Platform. @https://www.futuremarketinsights.com/ask-question/rep-gb-14999
“Because of improved healthcare infrastructure and government initiatives, Asia Pacific has the largest share of the global medical device analytical testing outsourcing market. Furthermore, rapid economic development in countries such as China and India are expected to significantly boost regional market growth over the forecast period.” Says an analyst at FMI. Competitive Landscape The global medical device analytical testing outsourcing market is highly fragmented. Mergers, acquisitions, and partnerships, among other strategies, were used by industry
0 notes
Text
Medical Device Testing, Inspection, and Certification Market Overview 2021: Size, Share, Trending Technologies, Development Plans, industry trends
Most recently added Medical Device Testing, Inspection, and Certification research concentrated by CAGR Reports offers point by point overview and explains market surveys till 2029. The market Study is isolated by key areas that are speeding up the marketization. The Medical Device Testing, Inspection, and Certification market report spins around market size, share, improvement, arising models and market region evaluation. The examination merges an exhaustive appraisal of different market factors, including market drivers, requirements, models, dangers, and openings that are customary looking out. SWOT are additionally enough examined to investigate enlightening information like expense, costs, pay, and end-clients.
This market report on the Medical Device Testing, Inspection, and Certification market gives a flat-out assessment of the market circle and unmistakable market divisions. The evaluation report depicts the gigantic improvement that the Medical Device Testing, Inspection, and Certification market is relied on to achieve during the figure time interval. The evaluation comparably constructs and genuine considers all respects to grandstand size, market improvement rate, making industry drivers, and key market plans. A flat-out evaluation of the immense headway forces to be reckoned with of the Medical Device Testing, Inspection, and Certification market business in the going with moderately couple of years is in like way tended to in the report.
The Medical Device Testing, Inspection, and Certification market report in like way wraps the normal assessment including North America, Europe, Asia-Pacific, South America, Middle East and Africa to offer the total provincial movement status. Also, the report comparably gives vital suggestions and musings to the Medical Device Testing, Inspection, and Certification market players to achieve an advantage in different districts.
Report Details : https://cagrreports.com/information-communication-and-technology/global-medical-device-testing-inspection-and-certification-market-status-trends-and-covid-19-impact-report-2022/
Points of view Covered in the Research Report:
• The report gives the entirety of the objective and precise figures expected to acquire a predominant awareness of the market pay, suggestion, and volume.
• Market design, market systems, costing assessment, horrible scene, use rate and import/trade subtleties.
• A complete detail of the progression rate throughout the figure time span is depicted in Medical Device Testing, Inspection, and Certification market report
The process begins with internal and external sources to obtain qualitative and quantitative information related to the Medical Device Testing, Inspection, and Certification Market. It also provides an overview and forecast for the Medical Device Testing, Inspection, and Certification Market based on all the segmentation provided for the global region. The predictions highlighted in the Medical Device Testing, Inspection, and Certification Market share report have been derived using verified research procedures and assumptions. By doing so, the CAGR Reports report serves as a repository of analysis and information for every component of the Medical Device Testing, Inspection, and Certification Market.
Note: This report can be changed to meet the client's necessities. Sympathetic interface with our effort bunch, who will ensure that you get a report that suits your necessities.
Get a Quote : https://cagrreports.com/contact-us/
Contact Us:
CAGR Reports +447305924133 10 Burlington Road, SL1, 7BQ, UK www.cagrreports.com [email protected]
0 notes
Text
South East Asia Testing, Inspection & Certification Market - Forecast (2024-2030)
South East Asia Testing, Inspection & Certification (TIC) Market Overview
The South East Asia TIC Market size is estimated to reach US$4.7 billion by 2030, growing at CAGR 4.82% during the forecast period 2024-2030. The growth of South East Asia TIC Market is majorly driven by increasing need for food testing and rising safety regulations and standards for the enhancement of medical device safety measure. Moreover, the rapid growth of consumer electronics industry coupled with the increasing demand for electronics will also trigger the growth of the testing, inspection & certification market in South East Asia. The manufacturer of electronic products needs to comply with various governmental standards to ensure quality testing and certification through RoHS testing, Electromagnetic compatibility (EMC) testing, GS mark certification, CPSR and so on, which in turn drives the market growth of TIC (Testing, Inspection & Certification) services. Furthermore, the demand for TIC services which includes failure & damage analysis, various component testing, e-mobility & battery testing and others in automotive industry is also a major factor that can transform the South East Asia TIC industry outlook in the long run.
The South East Asia Testing, Inspection, and Certification (TIC) market is undergoing significant transformation driven by multiple converging trends. The region's rapid industrialization and economic growth are increasing the demand for robust TIC services to ensure compliance with international standards and regulations. Advancements in digital technologies, such as blockchain, IoT, and AI, are revolutionizing TIC processes, enhancing the accuracy and efficiency of inspections and certifications. The growing emphasis on quality and safety in sectors like food and beverages, pharmaceuticals, and consumer goods is fueling the need for stringent testing and certification protocols. Additionally, the expansion of the manufacturing sector, particularly in automotive and electronics, is driving the demand for specialized TIC services to maintain quality control and meet export requirements. The rise of e-commerce is also contributing to the market growth, as online retailers seek to ensure product safety and authenticity. Environmental sustainability and regulatory compliance are becoming increasingly important, prompting companies to adopt comprehensive TIC services to meet green standards and reduce environmental impact. These trends collectively are enhancing the significance of TIC services in ensuring product quality, safety, and compliance across various industries in South East Asia.
South East Asia TIC Report Coverage
The “South East Asia TIC Market Report – Forecast (2024-2030)” by IndustryARC, covers an in-depth analysis of the following segments in the South East Asia TIC Market.
By Type: Outsourcing, In-house
By Types of Services: Testing Services, Inspection Services, Certification Services
By End Users: Agriculture, Automotive, Food, Consumers (textile, cosmetic, toys, apparel, furniture, stationary, hand tools), Medical & Life Science, Marine, Manufacturing, Building & Infrastructure, Industrial Equipment, Retail, Rail, E-Commerce, Meteorology, Others.
By Geography: Indonesia, Malaysia, Sinagpore, Philliphines, Thailand, Vietnam, Others (Myanmar, Laos, Cambodia, Brunei, Timor-Leste)
Request Sample
Key Takeaways
Testing Services held the major market share in 2023 owing to rising implementations of integrated testing solutions for wide range of end users including automation and control systems, battery storage, F&B processing, communication protocol and so on.
E-Commerce segment is analysed to grow at the fastest rate during the forecast period 2024-2030 owing to increasing internet and mobile phone usage, high penetration of IoT as well as improved e-payment methods& logistics.
Indonesia held the highest market share in 2023 Vietnam is analysed to grow at the fastest rate during the forecast period 2024-2030 owing to rapid growth of manufacturing sector and rising investments on railway infrastructure.
The increasing demand towards food testing and rising safety regulations and standards imposed by global as well as regional government bodies for the enhancement of medical device safety measure are analysed to significantly drive the market growth of South East Asia TIC market during the forecast period 2023-2030.
South East Asia TIC Market Segment Analysis- by Type of Services
Testing Services held the major market share in 2023 with a market size of $1.7 Billion and is estimated to reach $2.3 Billion by 2030 with a CAGR of around 4.51% during the forecast period 2024-2030. The growth is mainly attributed to rising implementations of integrated testing solutions for wide range of end users including automation and control systems, battery storage, F&B processing, communication protocol, maritime equipment material, oil & gas structure and components and others to increase productivity and customer satisfaction. In September 2022, Intertek announced the launch of their new Vegan Foods Certification. The Intertek Vegan Certification is designed to determine the suitability of food products for vegan and plant-based consumers. These factors are set to influence the growth of global South East Asia industry in the long run.
Inquiry Before Buying
South East Asia TIC Market Segment Analysis- by End User
E-Commerce segment is analysed to grow at the fastest rate of 9.84% during the forecast period 2024-2030. The growth of the E-Commerce in South East Asia is mainly driven by increasing internet and mobile phone usage, high penetration of IoT as well as improved e-payment methods& logistics. According to an article by Inside Monkey, E-commerce growth in Southeast Asia stands out at an 20.6% expansion in 2022, with sales reaching $89.67 billion from $74.36 billion in 2021 and is expected to pass $100 billion by 2023. Ecommerce sites or mobile applications need to undergo different tests including functionality, usability, security, performance, database and mobile application. TIC services judge the authenticity of the websites by testing the design, specifications, functionalities and various features to check their sanity and to ensure the protection level of the sites against any potential threats. Such benefits of these services drive the growth of TIC services market in this region. Moreover, the E-commerce sector in this region witnessing increasing cases of fraud and cyber-attacks which enhances the demand for testing, inspection and certification services in this industry. In Southeast Asia, digital payment methods are becoming increasingly accepted by most businesses and services today. According to a recent Kaspersky research titled “Mapping a secure path for the future of digital payments in APAC” published in April 2022, e-payment are the top encountered threat for most Southeast Asia countries including Indonesia (40%), Malaysia (45%), The Philippines (42%), Singapore (32%), and Vietnam (38%). Thus, significant rise in number of e-payment frauds can cater to the demand for testing, inspection & certification services within the industry.
South East Asia TIC Market Segment Analysis- by Geography
Indonesia held the highest market share of 20.9% in 2023. The economy of Indonesia is majorly driven by exporting of crude oil and natural gas and holds well established manufacturing industry, agriculture, mining and others. According to U.S Energy Information Administration, the production of petroleum and other liquids totalled 887,000 barrels per day in Indonesia in 2022, making it the seventh-largest exporter of liquefied natural gas, thus creating significant opportunities for the growth of the TIC Market. The rising number of railway infrastructure projects can be considered vital in fueling the market demand for quality testing, electromagnetic testing, and related inspection services for rail components used in the construction process within the country. In January 2022, the Indonesian Government announced the construction of the multibillion-dollar railway project with an estimated cost of $7.9 billion. It will involve the deployment of signaling systems, rolling stocks, and many related components. Such projects are bound to drive the need for railway operators or authorities to meet up with the required regulatory compliance, which in turn, can be considered vital in transforming the South East Asia TIC industry outlook in the long run.
Schedule a Call
South East Asia TIC Market Drivers
Rising Demand Towards Food Testing or Inspection due to Increasing Import/Export activities, Agricultural or Food Contamination, Food Safety Violation and Others is Surging the Demand for Testing, Inspection & Certification Services:
The increasing demand towards food testing owing to rising import/export activities, agricultural or food contamination, food safety violation and others is one of the major growth drivers boosting the adoption rate of testing, inspection & certification services in South East Asia. Food industry is considered as a highly regulated industry due to stringent regulatory compliance ensuring food quality assurance and safety for the consumers. The levels of product recalls owing to the presence of harmful ingredients or contamination or the undeclared presence of an allergen or not an approved additives are rising within the Southeast Asian nations. Moreover, the levels of agricultural trade (both export and import) are successively increasing owing to the population boost in various countries under scope, which will support the growth of the TIC market. As per World Bank 2021, the population of Philippines grew by 1.3% in the period of 2019-20. According to International Trade Administration July 2022 update, agricultural imports in Indonesia reached over $24 billion in 2021, owing to high demand for rice, wheat, soybeans, fresh fruits, dairy beef and various feed ingredients. The following marks an increase by $5 billion or 26% from 2020 import values. Furthermore, nearly 57.8% of the total import value regarding the agricultural products in the Indonesia market was dominated by five suppliers including China, Australia, U.S, Brazil as well as India. Such increase in imports can boost the adoption of food testing services which in turn can influence the market growth.
The Safety Regulations and Standards imposed by Global as well as regional government bodies for the enhancement of medical device safety measure is accelerating the growth of South East Asia TIC Market:
A major driver in the South East Asia TIC market is the implementation of stringent regulatory requirements across various industries. Governments in the region are increasingly adopting and enforcing rigorous standards to protect consumer safety, ensure product quality, and safeguard the environment. This regulatory landscape compels companies to adhere to strict testing, inspection, and certification protocols to gain market access and maintain competitiveness. The pharmaceutical and food industries, in particular, face heightened scrutiny, necessitating comprehensive TIC services to comply with health and safety standards. The automotive and electronics sectors also require extensive TIC processes to meet international export standards. The continuous update and tightening of regulations drive the demand for specialized and advanced TIC services, fostering market growth as companies strive to align with evolving compliance requirements.
The rising safety regulations and standards imposed by global as well as regional government bodies for the enhancement of medical device safety measure is creating demand for the TIC services which in turn triggers the growth of this market. The demand for Class III medical devices such as High-frequency ventilators, blood sampling monitors, oxygen supply units and so on have been growing overtime majorly amidst the pandemic as these devices are used to maintain or protect human life. Since these devices require licensed FDA approvals before distribution across countries, the market growth of TIC services is impacted significantly. Medical device testing is critical to the entire medical device development lifecycle to ensure the safety of patients and device users. In January 2021 the Philippines Food and Drug Administration (FDA) issued circular n° 2021-001 on the product standards to which Medical Devices must comply for notification or registration. The circular was issued so that local Manufacturers, Importers and/or Distributors must comply to obtain a certificate of Medical Device notification (CMDN) or a certificate of Medical Device registration (CMDR). In August 2021, FDA had released a list of Class-A 1242 product categories in its circular 2021-017, in order to support and clarify regulatory requirements for medical manufacturers looking for market access within Philippines. Under this, medical device manufacturers of Class A products specified under the ASEAN MDD, need to mandatorily obtain a Certificate of Medical Device Notification, (CMDN), before manufacturing, distribution, importing, selling, or advertising the medical devices within the country. Thus, rise in regulations and standards can boost the demand of testing, inspection & certification services among the medical device manufacturers.
South East Asia TIC Market Challenges
Lower Level of Digital Adoption by the Key Players coupled with the Continued Impact of Bottlenecks in Trade Flows across South East Asia region owing to Shortage of Cargo Container are Limiting the Market Growth:
Low level of digital adoption coupled with continued impact of bottlenecks in trade flows acts as a major challenge restraining the market growth of TIC market in South East Asia. According to Rothchild & Co report published in May 2021, the testing-inspection-certification digital maturity substantially lags behind the other various end-user industries which shall pose problems in the long-run. Some of the common industries against which the TIC lacked under digital technology is energy, financial institutions, industrial goods, insurance, and telecommunications. Additionally, Shortage of shipping cargos, shipment cancellations, growing freight rates along with many others have emerged as some of the prime factors adversely impacting domestic manufacturing operations across various Southeast Asian countries owing to dependency on raw material imports within the country. According to Westports Holdings Berhad report, Malaysia faces a container throughput slipping 1% year on year to 10.4 million TEUs (20ft equivalent units) in 2021. Container throughput at Westports was down 10% y-o-y in 1Q2022, reaching 2.39 million TEUs versus 2.66 million TEUs handled in 1Q2021 which leads to supply chain disruption. These factors are limiting the demand for South East Asia TIC which in turn hampers the market growth.
Buy Now
South East Asia TIC Industry Outlook
Product launches, acquisitions and R&D activities are key strategies adopted by players in the South East Asia TIC Market. The top 10 companies in the South East Asia TIC market are:
SGS SA
Bureau Veritas
Intertek
DNV GL
TUV SUD AG
ALS GLOBAL
DEKRA SE
Eurofins
Cast Laboratories PTE LTD
Singapore Laboratory Services PTE LTD
Recent Developments
In December 2022- Intertek, a leading Total Quality Assurance provider to industries, announced the launch of Intertek Green R&D, an innovative integrated solution that ensures the sustainability, quality and safety attributes of a product are maintained.
In June 2022- DNV launched the MyISRS digital self-assessment tool. The service is estimated to aid organizations to run an online independent high-level survey for quality assessing applications. Some of the key industries in South East Asia that can benefit from the service include oil and gas, chemicals, utilities, power generation, telecommunication, pharmaceutics, transport, food, and beverage, and maritime.
#south east asia testing#inspection & certification market Size#inspection & certification market Trends#inspection & certification market Growth#inspection & certification market Forecast#inspection & certification market Revenue#inspection & certification market Vendors#inspection & certification market Share#inspection & certification market Industry
0 notes
Text
Global UV LED Market is expected to reach a market size of ~US$ 1,100 million by 2028: Ken Research
UV radiation is a form of electromagnetic radiation or energy emitted by the sun and man-made sources such as phototherapy, welders, tanning beds, and solariums. Additionally, they are categorized into three categories: ultraviolet A (UV-A), ultraviolet B (UV-B), and ultraviolet C (UV-C). UV Light Emitting Diodes (LEDs) are a form of electromagnetic radiation that emits light in the ultraviolet (UV) spectrum at a higher frequency, which is used in a variety of applications, such as light curing, disinfection, military, plant factory (an indoor vertical farming system), exhaust gas treatment, gemological, and medical fields. The significant advantage of using UV LEDs is that they are environmentally friendly as they consume less energy, do not contain harmful mercury, and do not generate ozone.
According to Ken Research Analysis, the global UV LED market was valued at ~USD 350 million in 2017, it is estimated to be ~USD 600 million in 2022, and is expected to reach a market size of ~US$ 1,100 million by 2028 growing with a CAGR of 10%. The growth is primarily attributed to the increasing adoption of smart home devices across countries. The growing trend of horticulture and indoor farming in developing countries is also a major contributing factor to UV LED growth.

For more information, request a free sample @ https://www.kenresearch.com/sample-report.php?Frmdetails=NTk2MDI5
Key Trends by Market Segment
By Product Type: The UV-A segment held the largest market share of the Global UV LED Market in 2021.
The growth is primarily attributed to its low power consumption, the longest wavelength, deepest penetration, and allowing over 90% of UV radiation to pass through the earth's atmosphere.
For instance, according to World Health Organization (WHO), a government agency of the United Nations (UN), UV-A has a longer wavelength than UV-B and UV-C and accounts for approximately 95% of UV radiation reaching the earth's surface. The wavelengths covered by various types of UV radiation are classified below:
UV-A (315-400 nm)
UV-B (280-315 nm)
UV-C (100-280 nm)
In addition, such features increase the use of UV-A LEDs in curing applications including 3D printing, photolithography/photoresist, photostability, and other photochemical reactions, as well as forensic investigations like the examination of biological stains, untreated blood, and latent fingerprints at crime scenes.

For more information, request a free sample @ https://www.kenresearch.com/sample-report.php?Frmdetails=NTk2MDI5
By Application: The disinfection/purification segment held the largest market share of the Global UV LED Market in 2021.
The individuals growing inclination toward the consumption of intensely treated or purified water due to increasing cases of water-borne diseases, such as giardia, microbial, and legionella across countries, resulting in increased adoption of UV LED water purification systems. In addition, such systems help in the prevention of bacteria and other microbiological pollutants.
For instance, in July 2022, National Sanitation Foundation (NSF), a U.S.-based non-profit organization devoted to product testing, inspection, and certification organization, stated that UV LED disinfection systems have the potential to destroy nearly 100% of waterborne disease-causing bacteria and viruses.
The increasing government investments in water infrastructure, particularly following the outbreak of the COVID-19 pandemic, to endorse clean water and resilient infrastructure that publicizes community and environmental health, as well as its effects on individuals across countries, are driving demand for UV LEDs for disinfection/purification.
For instance, in December 2021, the Government of Canada announced a USD 19.2 million joint funding investment in four projects in British Columbia to support drinking water and wastewater infrastructure.

By End User: The industrial segment accounted for the majority share of the Global UV LED Market in 2021.
The growing demand for UV light therapy in the medical field for treating a variety of skin treatments/conditions such as psoriasis, eczema, vitiligo, and cutaneous T-cell lymphoma is supporting the demand for UV LEDs in the Industrial segment.
Furthermore, UV radiation on skin spots typically has a strong effect that stimulates skin metabolism and improves the skin's strength, which can help cure skin diseases, such as vitiligo and pityriasis rosea, and therefore in the medical industry, UV treatment now has more application areas.
By Geography: North America accounted for the largest market share in the global UV LED market in 2021.
The growth is primarily attributed to the increased investment and development of advanced technologies, particularly in economically robust countries, including the United States and Canada.
The surging emergence of manufacturing advancement is expected to drive demand for more efficient and robust UV processes such as curing, printing, adhesives, and lithography.
For instance, in May 2021, the Richard King Mellon Foundation, a U.S.-based non-profit organization, granted USD 150 million to Carnegie Mellon University (CMU), a U.S.-based research institute, to support the construction of a new robotics innovation lab, and advanced manufacturing institute at Hazelwood Green.
The Asia Pacific region is expected to grow at the fastest CAGR during the forecasted period (2022-2028), owing to the rising government initiatives to support the regional healthcare sector, particularly following the outbreak of the COVID-19 pandemic, with advancements in DNA analysis, protein synthesis, drug discovery, and vitamin analysis, all of which rely heavily on UV LEDs.

To more regional trends, Ask for a Customization @ https://www.kenresearch.com/ask-customization.php?Frmdetails=NTk2MDI5
Competitive Landscape
The Global UV LED market is highly competitive, with ~500 players, including globally diversified players, regional players, and a large number of country-niche players, each with their emerging deep-UV (DUV) chip-scale technologies due to significant advancement, as well as increasing interest in the development and deployment of disinfection systems using compact devices that emit in the deep-UV spectral band with wavelengths ranging from 200 to 280 nm. The industry's ongoing progress in chip-scale solutions for deep-UV light sources is expected to drive demand for UV disinfection in the forthcoming years.
Country-Niche players control about ~65% of the market, while regional players hold a share of ~25%. Some of the major players in the market include Koninklijke Philips N.V., Nichia Corporation, Nordson Corporation, Phoseon Technology, SemiLEDs Corporation, Seoul Semiconductor Co. Ltd., Crystal IS, Inc., Lumileds Holding B.V., L.G Electronic, Sensor Electronics Technology and among others.

Recent Developments Related to Major Players and Organizations
In September 2022, Nichia Corporation, a Japanese chemicals company, announced that it will begin mass production of its new UV-C LED radiant flux in December 2022 to help target the inactivation and sterilization of multiple bacteria and viruses, precisely in industrial water and air applications.
In May 2022, Fujifilm, a Japanese photography company, launched the Activ Hybrid LED UV retrofit system, to be installed on new presses or retrofitted to existing equipment by label and packaging converters. In addition, the system combines high-power, low-heat LED UV curing technologies with Fujifilm's ink expertise to strengthen label production on narrow web presses.
In April 2022, Intertronics, a UK-based distribution company, launched its new range of LED UV curing flood lamps, namely the 'DymaxBlueWave FX-1250 LED UV Curing Flood Lamp,' which can cure LED matched UV curable adhesives, coatings, and inks with high uniformity, irradiances UV light.
In March 2022, Roland DG Corporation, a Japanese company that provides digital printing solutions, announced the introduction of a new 4-color UV-LED flatbed printer named 'EU-1000MF' to its existing EU series.
In January 2021, Canon, a Japanese manufacturer of optical, imaging, and industrial products, collaborated with Plockmatic, a Japanese manufacturer of a wide range of document finishing and feeding systems, to create a new high-speed UV LED coating solution for Canon varioPRINT iX-series printers.
CONCLUSION
The Global UV LED Market Size, Segments, Outlook, and Revenue Forecast 2022-2028 is forecasted to continue an exponential growth that is witnessed since 2017. The major driving factors contributing to the expansion of UV LEDs is increasing prevalence of water-borne diseases across countries, along with the Increasing government energy-saving policies, and strategies. Though the market is highly competitive with around ~500 participants, few country-niche players control the dominant share and regional players also hold a significant share.
Note: This is an upcoming/planned report, so the figures quoted here for a market size estimate, forecast, growth, segment share, and competitive landscape are based on initial findings and might vary slightly in the actual report. Also, any required customizations can be covered to the best feasible extent for Pre-booking clients and report delivered within maximum 2 working weeks.
Market Taxonomy
By Product Type
UV-A
UV-B
UV-C
By Application
Light/UV Curing
Medical Field
Disinfection/Purification
Counterfeit Detection
By End User
Healthcare
Residential
Industrial
Commercial
By Geography
North America
U.S.
Canada
Mexico
Europe
Germany
UK
France
Italy
Spain
Rest of Europe
Asia Pacific
China
Japan
India
Australia
South Korea
Rest of Asia Pacific
LAMEA
Latin America
Middle East
Africa
Key Players
Koninklijke Philips N.V.
Nichia Corporation
Nordson Corporation
Phoseon Technology
SemiLEDs Corporation
Seoul Semiconductor Co. Ltd.
Crystal IS, Inc.
Lumileds Holding B.V.
L.G Electronics
Sensor Electronics Technology
0 notes
Text
Medical Device Testing Market Industry Share, Size, Growth, Demands, Revenue, Top Leaders and Forecast to 2029
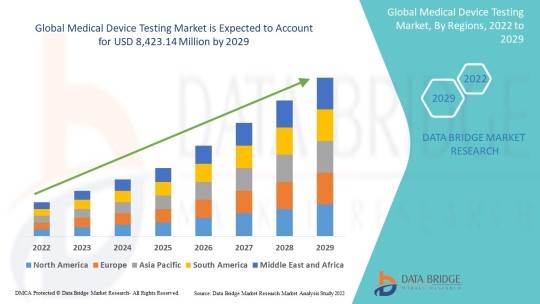
Industry Analysis
Medical device testing market is expected to gain market growth in the forecast period of 2022 to 2029. Data Bridge Market Research analyses that the market is growing with a CAGR of 10.8% in the forecast period of 2022 to 2029 and is expected to reach USD 8,423.14 million by 2029 from USD 3,832.27 million in 2021.
Additionally, the credible Medical Device Testing Market report helps the manufacturer in finding out the effectiveness of the existing channels of distribution, advertising programs, or media, selling methods and the best way of distributing the goods to the eventual consumers. Taking up such market research report is all the time beneficial for any company whether it is a small scale or large scale, for marketing of products or services. It makes effortless for healthcare industry to visualize what is already available in the market, what market anticipates, the competitive environment, and what should be done to surpass the competitor.
Get a Free Sample of The Report: https://www.databridgemarketresearch.com/request-a-sample/?dbmr=global-medical-device-testing-market
Market Insights and Scope
Medical device testing is the process of demonstrating that the device is reliably and safely perform in use. In new product development, extensive design validation testing is applied. This includes performance testing, toxicity and chemical analysis, and sometimes human factors or even clinical testing. Ongoing quality assurance testing is generally more limited. This usually include dimensional checks, some functional tests, and packaging verification. Various types of medical testing services are available there in the market such as inspection services, certification services and among others.
The Medical Device Testing Market report encompasses various segments linked to healthcare industry and market with comprehensive research and analysis. These comprise industry outlook with respect to critical success factors (CSFs), industry dynamics that mainly covers drivers and restraints, market segmentation & value chain analysis, key opportunities, application and technology outlook, regional or geographical insight, country-level analysis, key company profiles, competitive landscape, and company market share analysis. All the data, figures and information are backed up by well recognized analysis tools which include SWOT analysis and Porter’s Five Forces analysis. So, take business to the peak level of growth with the all-inclusive Data Bridge Market research report.
Get full access to the report: https://www.databridgemarketresearch.com/reports/global-medical-device-testing-market
Industry Segmentation and Size
Global medical device testing market is segmented into service type, testing type, phase, sourcing type, device class and product. The growth amongst these segments will help you analyse meagre growth segments in the industries and provide the users with a valuable market overview and market insights to make strategic decisions to identify core market applications.
Service Type
Testing services
Inspection services
Certification services
Testing Type
Physical testing
Chemical/biological testing
Cybersecurity testing
Microbiology and sterility testing
Others
Phase
Preclinical
Clinical
Sourcing Type
Outsourced
In-house
Device Class
Class I
Class II
Class III
Product
Active implant medical device
Active medical device
Non-active medical device
In-vitro diagnostics medical device
Opthalmic medical device
Orthopedic and dental medical device
Vascular medical device
Others
Market Country Level Analysis
The countries covered for this market are
U.S., Canada, Mexico, Germany, France, U.K., Italy, Spain, Netherlands, Russia, Switzerland, Turkey, Belgium, Rest of Europe, China, Japan, India, South Korea, Australia, Singapore, Thailand, Malaysia, Indonesia, Philippines, Rest of Asia-Pacific, Brazil, Argentina, Rest of South America, South Africa, Saudi Arabia, UAE, Egypt, Israel, Rest of Middle East and Africa.
A reliable Medical Device Testing Market marketing report proves to be the finest and excellent market research report as it is formulated with the following critical factors. These consist of primary research, benchmarking studies, secondary research, company profiles, competitive intelligence & reporting, syndicated research, data collection, data processing and analysis, survey design, and survey programming. The report performs market study and analysis to provide market data by considering new product development from beginning to launch. The healthcare business report also provides evaluations based on the market type, organization size, availability on-premises, end-users’ organization type, and the availability in areas such as North America, South America, Europe, Asia-Pacific and Middle East & Africa.
Industry Share Analysis
Some of the major players operating in the medical device testing market are
Intertek Group plc, SGS SA, Bureau Veritas, TUV SUD, TUV Rheinland, Pace, Charles River Laboratories, Biomedical Device Labs, UL LLC, North American Science Associates, LLC, Medistri SA, WuXi AppTec, NSF, Labcorp, Eurofins Scientific, Nelson Laboratories, LLC- A Sotera Health company, Gateway Analytical, ITC ZLIN, Element Materials Technology, EndoLab Mechanical Engineering GmbH, Hohenstein, Medical Engineering Technologies Ltd., Bioneeds, Cigniti, Arbro Pharmaceuticals Private Limited & Auriga Research Private Limited, Q Laboratories, IMR Test Labs among others.
Browse Related Reports@
Global Whiskey Market
South Africa Battery Market
Global Plant-Based Egg Market
Global Nutritional Beverages market
MENA Tahini market
Global Dental Membrane and Bone Graft Substitute Market
About Us: Data Bridge Market Research set forth itself as an unconventional and neoteric Market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market
Contact: Data Bridge Market Research Tel: +1-888-387-2818 Email: [email protected]
#Medical Device Testing Market Growing Popularity#Medical Device Testing Market Global Leading Brands#Medical Device Testing Market drivers-advantages#Medical Device Testing Market Segmentation-CAGR rate#Medical Device Testing Market Demands-Size-Share-Top Trends#Medical Device Testing Market Industry-Competitors#Medical Device Testing Market Growth-Competition#Medical Device Testing Market 2029 by Types-Application#Medical Device Testing Market Healthcare Industry
0 notes
Text
Micropump Market Overview and Regional Outlook Study 2022 – 2032

The Micropump Market was worth US$ 1.6 Bn in the year 2021 and is expected to witness US$ 10 Bn by the year 2032 at a whopping CAGR of 19% between 2022 and 2032.
The major application areas of micropump include life sciences, pharmaceuticals, and point-of-care testing. The healthcare vertical is increasingly into micropumps due to usage in transportation of low fluids from one place to the other, and – not to forget – mixing petite quantities of fluids. Also, micropumps are being used in the medical devices that help in administering proper dosage of medications to patient.
Micropumps are also being used in the home care devices due to rapid adoption of clinical tests. For instance – micropump is used in infusion therapy for treating cancer patients. Increasing awareness amongst end-users regarding importance of drugs’ controlled delivery in biochemical research coupled with prevalent chronic and fatal diseases is a catalyst to the micropump market.
Mechanical micropumps rule the market. This could be reasoned with using mechanical micropumps in MEMS (Micro-Electro-Mechanical Systems). MEMS are used in the drug delivery system vehicles, which are easy to operate, cheap, and capable of tracking diseases, and also providing fast detection, so as to facilitate quick delivery of machines. Mechanical micropumps constitute moving parts such as micro-valve membranes or actuation or flaps. On the other hand, non-mechanical micropumps do have limitations like actuation mechanisms that interfere with pumping liquids and usage of low conductivity fluids. Growing biomedical applications like ‘Lab-on-Chip (LoC)’, POCT, and micro-total analysis systems are expected to revolutionize the market’s growth. LoC technology’s applications include advanced biological sample processing, analysis, and manipulation.
For More Information: https://www.futuremarketinsights.com/reports/micropump-market
High precision micropumps are useful in delivery of insulin for the diabetic patients and for chemotherapy with drug dosing for cancer-affected patients. However, the fact that micropump may face problems due to surface chemistry at the time of manufacturing can’t be ignored. It actually poses a challenge to micropump market. Future Market Insights has walked through these findings with insights in its latest market study entitled ‘Micropump Market’.
Key Takeaways from Micropump Market
North America holds more than 40% of the market share. This could be attributed to higher purchasing power of the US citizens, robust support from the government regarding quality healthcare, presence of top-notch infrastructure for laboratory and clinical research, and higher adoption of various technologically advanced products.
The Asia-Pacific is expected to grow at the fastest rate in the micropump market due to upgradation in healthcare infrastructure all across India and China. Singapore and Malaysia are already doing well with respect to medical tourism.
Europe is expected to hold a significant market share with countries like Belgium, The Netherlands, and Denmark being at the forefront.
“Micropumps have evolved since the last few years through numerous techniques through advancements in electronic and digital capabilities”, says an analyst from Future Market Insights.
Competitive Micropumps
Bartels Mikrotechnik GmbH, in January 2022, tabled mp6 series. They comprise mp6-Liq, mp6-gas+, and mp6-gas.
Sensile Medical AG (Gerresheimer’s subsidiary), September 2018, received EU certification. One of the European companies achieved CE declaration of conformity for micro-rotary pump used for treating advanced Parkinson’s disease.
TTP Ventus, in June 2019, launched the LT series for serving applications while demanding operational life requirements all across medical, life science, environmental, and industrial sectors.
Cole-Parmer India Pvt. Ltd., after successfully testing shear-sensitive and pumping live cells fluids, released the high-end micropump head series. They were named ‘Masterflex L/S Cytoflow Pump head”.
Biochem Fluidics, in July 2017, released Maestro ULTRA piston pump, Perialistic Pumps, Opus Rocker Valves.
Backtrace has its Dolomite Bio, which is known for specialization in microfluidic droplet applications that offer individual modules and microfluidic systems, reagents, and control software for applications pertaining to cell biology.
The National Institute of Biomedical Imaging and Bioengineering, in October 2020, came up with Point-of-Care Technologies Research Network for driving development of the point-of-care technologies.
Medtronic, in March 2022, tabled MiniMed 780 G systems all across India.
What does the Report Cover?
Future Market Insights offers an exclusive perspective and various real-time insights on the micropump market in its latest study, presenting historical demand assessment of 2016 – 2021 and projections for 2022 – 2032.
The research study is based on product type (mechanical micropump (piezoelectric micropump and peristaltic micropump) and others), by application (drug delivery, in-vitro diagnostics, medical devices, and others), and by end-user (biotechnology & pharmaceutical companies, hospitals & diagnostic centers, and academic research institutes).
Key Segments
By Product Type:
Mechanical Micropump
Piezoelectric Micropump
Peristaltic Micropump
Others
By Application:
Drug Delivery
In-Vitro Diagnostics
Medical Devices
Others
By End User:
Biotechnology & Pharmaceutical Companies
Hospitals & Diagnostic Centres
Academic & Research Institutes
By Region:
North America
Latin America
Europe
Asia Pacific
Middle East and Africa (MEA)
0 notes
Note
How did you become a university Librarian? Did you do an English degree? Sorry if this is a weird question it just really interests me as I’m not sure what to do when I’m older
Eeee I got really excited about this question!
Okay, the fun thing about librarianship is that all roads can lead to it: as long as you get an ALA-approved (assuming you’re American; if you aren’t I cannot help you) graduate degree you can do just about anything for undergrad. English majors are extremely common, just by the nature of who’s into the job, but literally it doesn’t matter; in fact, weirder and more specialized degrees can actually help in certain jobs, because they give you a ton of background info and qualifications than most of your contemporaries have.
I fell into it because I worked at a library in high school and fell in love with the environment, and when I realized I’d rather die than work in publishing (my previous life’s goal) I gravitated toward library school. I knew from the beginning that I’d need a Master’s -- and a very specific one at that -- so mostly my undergrad was just “grab a foundational degree and have fun with it.” That was really freeing, honestly. I had a ton of fun in undergrad.
Now, if you, Anon, were interested in getting into librarianship I’d have a handful of recommendations. These are all based on my very American experience, and there are probably smarter people than me with better advice but I’m the only one on this blog so heeeeerrreeeee we goooooooooo!
Undergrad
You need a 4-year degree. Full-stop. It doesn’t matter what kind, but you gotta have one to get into grad school.
Like I said, you can do just about anything for an undergraduate degree. Most of the time English is the BA of choice, because librarians love them some books, but some far less common ones that I think would be hugely helpful to a hopeful librarian would be:
Computer Science: Oh my god you need at least a baseline competency in computers/technology please you don’t have to code but you need to be able to turn a computer on and navigate just about any website/office application on just about any device at the very least you need to know how to Google
Business/Marketing: Particularly if you want to work in public libraries, where a bunch of your funding comes from begging politicians and convincing taxpayers to donate/vote to give you money
Law: If you want to be a law librarian
Medical . . . whatever, I don’t know what fields of medicine there are: If you want to work in a hospital or other medical library
History or Art History: If you’re interested in archives or museum librarianship
Education: School librarians in my state require you to be a certified teacher, and no matter what kind of library you end up in, you’ll end up teaching someone something a decent amount of the time
Communications: You’ll be doing a lot of it. Public speaking, too
Spanish/ASL/any not-the-common language: Hey, you never know what your patrons speak
Literally fucking anything I promise it doesn’t matter what you major in you will use it in a library at some point
Just be aware that you will need more than an undergrad degree. You’ll need probably 2 years of postsecondary schooling (more for certain types of librarianship), so get yourself comfortable with the idea of college.
If you’re like me (please don’t be like me), you might toy with the idea of getting a minor or two/double majoring to round out your skill set. Honestly I’d encourage it if you’re comfortable with the workload and have the time or money; like I said, there are no skills or educational background that won’t come in handy at some point. I promise. We see it all.
Along those lines, a wide expanse of hobbies can be hugely helpful too! You never know when your encyclopedic knowledge of Minecraft will be useful to a patron, but it absolutely will be.
Graduate School
All right, you’ve got your lovely little Bachelor’s Degree, maybe in something weird and esoteric for the fun of it . . . now you’re off to do more school!
It’s a bit complicated, because there are a handful of different titles an appropriate degree could have; my school called it “a Master of Science in Information Science” (MSIS), but other schools might just go with “Master’s of Information Science” (MIS), “Master’s of Library Science” (MLS), “Master’s of Library and Information Science” (MLIS) . . . it’s a mess.
What you need to do is make sure the degree is approved by the American Library Association, who decides if a program is good enough to make you a librarian in the States. (Again, if you’re not American, good luck.)
Here’s a list of ALA-accredited programs and the schools that offer them.
The nice thing is accreditation has to be renewed at least every few years, so that means your program is always updated to make sure it’s in line with national standards. I’m not promising you’ll learn everything you need to be a librarian in grad school (oh my god you so won’t not even close hahahaha), but at least in theory you’ll be learning the most up-to-date information and methods.
(I’m curious to see how things have changed; when I was in school from 2015-17, the hot topics in library science were makerspaces (especially 3D printing), turning the library into the community’s “third space,” and learning how to incorporate video games into library cataloging and programming. No idea if those are still the main hot-button issues or if we’ve moved on to something else; I imagine information literacy and fake news are a pretty big one for current library students.)
Anyway! You pick a school, you might have to take a test or two to get in -- I had to take the GRE, which is like the SATs but longer -- almost certainly have to do all that annoying stuff like references and cover letters and all that, but assuming you’re in: now what?
There are a couple options depending on the school and the program, but I’m going to base my discussion around the way my school organized their program at the time, because that’s what I know dammit and I will share my outdated information because I want to.
My school broke the degree down into 5 specializations, which you chose upon application to the program:
Archives & Records Administration: For working in archives! I took some classes here when I was flirting with the idea, and it’s a lot of book preservation, organizing and caring for old documents and non-book media, and digitization. Dovetails nicely into museum work. It’s a very specific skillset, which means there will be jobs that absolutely need what you specifically can do but also means there aren’t as many of them. It makes you whatever the opposite of a “jack of all trades” is. You’re likely to be pretty isolated, so if you want to spend all your time with books this might be a good call; it’s actually one of the few library-related options that doesn’t require a significant amount of public-facing work.
Library & Information Services: For preparation to work in public or academic (college) libraries. Lots of focus on reference services, some cataloging, and general interacting-with-the-public. You have to like people to go into library services in general, heads up.
Information Management & Technology: Essentially meaningless, but you could in theory work as like a business consultant or otherwise do information-related things with corporations or other organizations.
Information Storage & Retrieval: Data analytics, database . . . stuff. I don’t really know. Computers or something. Numbers 3 and 4 really have nothing to do with libraries, but our school was attempting to branch out into more tech-friendly directions. That being said, both this and #3 could definitely be useful in a library! Libraries have a lot of tech, and in some ways business acumen could be helpful. All roads lead to libraries; remember that.
Library & Information Services / School Library Media Specialist: This was the big kahuna. To be a school librarian -- at least in my state -- you need to be both a certified librarian and a certified teacher, which means Master’s degrees in both fields. What our school did was basically smushed them together into a combined degree; you took a slightly expanded, insanely rigorous 2-2.5 years (instead of the traditional 1.5-2) and you came out of it with two degrees and two certifications, ready to throw your butt into an elementary, middle/junior high, or high school library. Lots of focus on education. I started here before realizing I don’t like kids at all, then panicked and left. Back in 2017 this was the best one for job security, because our state had just passed a law requiring all school librarians to be certified with a MSIS/MLS/whatever degree. So lots of people already in school libraries were desperately flinging themselves at this program, and every school was looking for someone that was qualified. No idea if that’s changed in time.
No matter what concentration you went in with, you automatically graduated with a state certification to be a librarian, which was neat. You didn’t automatically get civil service status, though; for some public libraries you need to be put on a civil service list, which means . . . something, I’m not entirely sure. It involves taking exams that are only available at certain times of the year and I gave up on it because it looked hard.
No one did more than 1 concentration, which is dumb because I wanted to do them all, but it takes a lot of time and money to take all the classes associated with all of them so I personally did #2, which was on the upper end of mid-tier popularity. School library and database services were far and away the most popular, and literally no one did the business one because it was basically useless, so library and archives were the middle children of which the library one was prettier.
THAT BEING SAID! Some forms of librarianship require a lot more education. A few of those are:
Law librarians: At least in my state, you gotta be a certified librarian and have a J.D. This is where the “big bucks” are -- though let’s be real, if you want to be a librarian you have zero interest in big bucks; reconcile yourself to being solidly middle-class and living paycheck-to-paycheck for the rest of your life or marrying rich -- which I guess is why it requires the most work.
School librarians: Like I mentioned, depending on the state you might need two degrees, and not all schools smush them into one. You might need to get a separate Master’s in education.
College librarians: Now, this depends on the college and the job; some colleges just need an all-access librarian, like mine. I didn’t need to specialize in anything, I just showed up with my degree and they took me. (Note: these sorts of entry-level positions tend to pay piss. Like, even more piss than most library gigs. Just a heads-up.) However, if you’re looking to get into a library of a higher-end university, you might be asked to have a second Master’s-level or higher degree just to prove you’re academic enough to party at their school. (Let’s be real, Harvard is almost certainly gonna want someone with a Ph.D. at the very least. That’s just how they roll.) Alternatively, the position might be for a specialty librarian, someone in charge of a field-specific library or field-specific reference services; if you’re being asked to head up the Science & Engineering Library at Masshole University, it’s reasonable to expect that you’ll be bringing a degree in engineering or some sort of science to the table. Colleges have so many different needs that predicting what kind of experience/education you should get is a bit of a challenge. Good luck. Some schools will help you out a bit with this; my grad school had dual degree programs where you could share credits between the MSIS and either an English or History Master’s so you could graduate with both in less time. I . . . started this, and then panicked at the thought of more school/writing a thesis and bailed, but it’s great if you’re into that idea!
What’s the point of the Information/Library Science degree?
You have to have the degree. If you don’t have the degree, you don’t get the job and you don’t make-a the money. Resign yourself to getting a Master’s degree or you’re gonna be bummed out and unemployed.
In terms of what you learn? Well, obviously it depends on the program, but I found that a lot of what I learned was only theoretically related to what I do on a daily basis. My instructors were lovely (well, the adjuncts anyway; the full-timers really didn’t want to be there and wanted to be off doing research and shit), but every library is so idiosyncratic and there’s such a massive umbrella of jobs you could get in one -- god, I didn’t even get into things like metadata services, which I learned basically nothing about in grad school but are super important to some positions -- that it’s hard to learn anything practical in a classroom.
However, besides the piece of paper that lets you make-a the money, there are two important things you should get from your grad school education:
Research skills: My god, you’re going to be doing so much research. If you’re a public librarian, you need to know how to Google just about anything. And if you’re a college librarian, being able to navigate a library database and find, evaluate, and cite sources . . . I mean, you’re going to be doing so much of that, showing students how to do that. Like a ridiculous amount of my day is showing students how to find articles in the virtual library. Get good at finding things, because much like Hufflepuffs, librarians need to be great finders.
Internship(s): Just about every library program will require an internship -- usually but not always in replacement of a thesis -- and if the one you’re looking at doesn’t, dump it like James Marsden in a romantic comedy. Internships are hugely important not only because they look good on a resume and give you some of those delicious, delicious references, but they are a snapshot of what your job is going to look like on a day-in, day-out basis; if nothing else, you’ll learn really fast what does and doesn’t appeal to you. As I mentioned, I wanted to be a school librarian for about half a semester. You know what changed my mind? My class required like 40 hours of interning at schools of each level. Being plopped into that environment like a play you’re suddenly acting in? Super helpful in determining whether or not this shit is for you.
What else should I learn, then?
Besides how to research basically anything? Here are some useful skills in just about any library:
Copyright law. Holy shit, do yourself a favor and learn about publishing/distribution laws in your state. Do you wanna show a movie as a fun program? You need to buy a license and follow super specific rules or it’s illegal! Does an instructor want to make copies of their textbook to give to the students? Make sure you know how much they can copy before it’s no longer fair use! Everything in my life would be easier if I’d taken the time to learn anything about copyright. I did not, and now I’m sad. (I lost out on a job opportunity because they wanted the librarian to be particularly knowledgeable in that kinda thing, and I was very not.)
Metadata and cataloging. In theory, you should learn this in grad school, but I was only given the bare basics and it wasn’t enough. Dublin Core, MARC-21, RDF -- there are so many different kinds of metadata schema, and I took a 6-week class in this and still don’t understand any of the words I just used in this sentence. But basically, to add items to a library catalog you often need to know how to input them into your library’s system; to an extent that’ll be idiosyncratic to your library’s software, but some of it will be based on a larger cataloging framework, so familiarity with those is very useful.
Public speaking and education. You’re gonna do a lot of it. Learn how to deal.
General tech savviness. Again, we’re not talking about coding but if you can navigate a WordPress website? If you know how to troubleshoot just about any issue with Microsoft Word, PowerPoint, etc.? If you can unjam printers and install software and use social media you’re going to be a much happier person. At the very least, know how to google tutorials and fake your way through; your IT person can only do so much, and a lot of it is probably going to fall on you.
Social work, diplomacy, general human relations kinda stuff. You’re going to be dealing with all sorts of people from all sorts of backgrounds, with every political view, personal problem, and life experience under the sun. You need to get very good at being respectful of diversity -- even diversity you don’t like* -- and besides separating your own personal views and biases from your work, you’ll be much better equipped to roll with the punches if you have, for example, conflict resolution training. Shit’s gonna get weird sometimes, I promise. (Once a student came in swinging around butterfly knives and making ninja noises. You know who knew how to deal with that? Not me!)
Standard English writing and mechanics. It’s not fair, but in general librarians are expected to have a competent grasp on the Standard English dialect, and others are less likely to be appreciated by the general populace. Obviously this differs based on your community and environment, and colloquialisms are sometimes useful or even necessary, but as a rule of thumb it’s a good call to be able to write “properly,” even if that concept is imperialist bullshit.
*I don’t mean Nazis. Obviously I don’t mean Nazis. Though there is a robust debate in the library community about whether Nazis or TERFs or whatever should be allowed to like, use library facilities for their own group meetings or whatever. I tend to fall on the “I don’t think so” side of the conversation, but there’s a valid argument to be made about not impeding people’s access to information -- even wrong or harmful information.
Any other advice?
Of course! I love to talk. Let’s see . . .
Get really passionate about freedom of information and access: A library’s main reason for existing is to help people get ahold of information (including fiction) that they couldn’t otherwise access. If you’re a public librarian, you have to care a lot about making sure people can access information you probably hate. (If you’re an academic librarian it’s a little more tricky, because the resources should meet a certain scholarly threshold, and if you’re a school librarian there are issues of appropriateness to deal with, but in general more info to more people is always the direction to push.) Get ready to defend your library purchases to angry patrons or even coworkers; get ready to defend your refusal to purchase something, if that’s necessary. Get ready to hold your nose and cringe while you add American Sniper to your library collection, because damn it, your patrons deserve access to the damn stupid book. Get really excited about finding new perspectives and minority representation, because that’s also something your patrons deserve access to. Get really excited about how technology can make access easier for certain patrons, and figure out how to make it happen in your library. Care about this; it’s essential that you’re passionate about information -- helping your patrons find it, making sure they can access it, evaluating it, citing it . . . all of it. Get ranty about it. Just do it.
Be prepared to move if necessary: One of my professors told us that there was one thing that would always guarantee you a job that paid well -- this was in 2016 but still -- that as long as you had it you could do whatever you wanted. And that was a suitcase. Maybe where you live is an oversaturated market (thanks for having 6 library schools in a 4-hour radius, my state). Maybe something something economic factors I don’t really understand; the point is that going into this field, you should probably make peace with the idea that you’ll probably either end up taking a job that doesn’t make enough money or struggle a lot to even find one . . . or you’re going to have to go where the jobs are. It’s a small field. Just know that might be a compromise you have to make, unless you can get a strictly remote job.
Read: This sounds stupidly obvious but it’s true! Read things that aren’t your genre, aren’t your age range; patrons are going to ask you for reading advice all the goddamn time, especially if you’re a public librarian, so the more you can be knowledgeable about whatever your patrons might ask you about, the easier your life will be. If you’re considering librarianship you probably love to read anyway, so just ride that pony as hard as you possibly can.
Learn to be okay with weeding -- even things you don’t think deserve it: You are going to have to recycle books. You’re going to have to throw away books. You’re going to have to take books out of the collection and make them disappear in some fashion or another. There are a lot of reasons -- damage and lack of readership are big ones -- and there’s no bigger red flag to a librarian than someone saying “I could never destroy a book.” That kind of nonsense is said by people who’ve never had to fit 500 books onto a shelf built for 450. Archivists are different, of course, as are historians, and everyone should have a healthy respect for books both as physical objects and as sources of information, but you’re going to have to get rid of them sometimes, and you’re just going to have to learn how to do that dispassionately.
Have fun! No one gets into this because they want money; if you want to be a librarian, or work in any library-adjacent field, it’s because you really care about the values of librarianship, or the people in your community, or preserving and sharing as great a wealth of information as possible. Your job will often be thankless and it’ll sometimes be exhausting. There will be times where it’s actually scary. And unless you’re rich as balls, it will make you stare at your student loans and sigh with despair. (You may be living in your parents’ basement while you sigh at your loans because you can’t afford to live on your own, for an example that has zero relevance to any authors of this blog, living or dead.) I can’t tell you if it’s worth it -- though you’ll probably find out pretty quickly during your internship, because that’s what internships are for. All I can say is that I love it, and I can’t imagine doing anything else.
#ask forest#libraries#librarianship#information science#career advice#education advice#oh my god this is long#i have a lot of feelings okay?#there is no way on earth anyone will read all of this#if you do tell me bc i will not believe it#Anonymous
76 notes
·
View notes
Text
Implant Grade Textile, Global Market Size Forecast, Top 5 Players Rank and Market Share
Implant Grade Textile Market Summary
Implant grade textile refers to a specific type of fabric that is designed and manufactured to be safe to use in medical implants and other biomedical applications. This textile is made from high-quality materials and undergoes rigorous testing and certification processes to ensure its compatibility with the human body.
According to the new market research report "Global Implant Grade Textile Market Report 2023-2029", published by QYResearch, the global Implant Grade Textile market size is projected to grow from USD 13.07 billion in 2023 to USD 1725.6 million by 2029, at a CAGR of 4.7% during the forecast period.

Figure. Global Implant Grade Textile Market Size (US$ Million), 2018-2029

Above data is based on report from QYResearch: Global Implant Grade Textile Market Report 2023-2029 (published in 2023). If you need the latest data, plaese contact QYResearch.
Figure. Global Implant Grade Textile Top 5 Players Ranking and Market Share (Ranking is based on the revenue of 2022, continually updated)
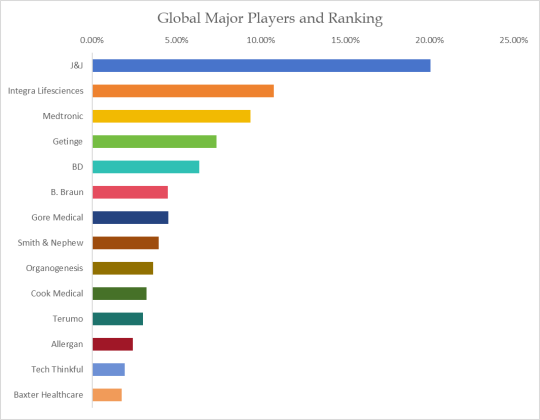
Above data is based on report from QYResearch: Global Implant Grade Textile Market Report 2023-2029 (published in 2023). If you need the latest data, plaese contact QYResearch.
This report profiles key players of Implant Grade Textile such as J&J, Integra Lifesciences
In 2022, the global top five Implant Grade Textile players account for 53.96% of market share in terms of revenue. Above figure shows the key players ranked by revenue in Implant Grade Textile.
Figure. Implant Grade Textile, Global Market Size, Split by Product Segment
Based on or includes research from QYResearch: Global Implant Grade Textile Market Report 2023-2029.
In terms of product type, type one is the largest segment, hold a share of xx%,
Figure. Implant Grade Textile, Global Market Size, Split by Application Segment

Based on or includes research from QYResearch: Global Implant Grade Textile Market Report 2023-2029.
In terms of product application, Suture is the largest application, hold a share of 49.9%,
Figure. Implant Grade Textile, Global Market Size, Split by Region

Based on or includes research from QYResearch: Global Implant Grade Textile Market Report 2023-2029.
Market Drivers:
The growing prevalence of chronic diseases and age-related ailments, coupled with advancements in medical technology, have led to a rise in the demand for medical implants. Implant grade textiles are critical components in the manufacturing of these implants, driving the growth of the market.
Restraint:
Implant grade textile market is the stringent regulatory landscape. The production and use of medical implants and devices are highly regulated to ensure patient safety and the efficacy of the products. This regulatory environment often involves lengthy approval processes, expensive compliance requirements, and regular audits. These can slow down market growth as they increase the time and cost associated with bringing new products to market.
Opportunity:
There is a growing demand for high-quality medical implants and devices in emerging markets due to increasing healthcare infrastructure and a rising middle-class population. These markets provide lucrative opportunities for implant grade textile manufacturers to expand their reach and tap into new customer bases.
About The Authors
Song Yue
Lead Author
Email: [email protected]
About QYResearch
QYResearch founded in California, USA in 2007.It is a leading global market research and consulting company. With over 16 years’ experience and professional research team in various cities over the world QY Research focuses on management consulting, database and seminar services, IPO consulting, industry chain research and customized research to help our clients in providing non-linear revenue model and make them successful. We are globally recognized for our expansive portfolio of services, good corporate citizenship, and our strong commitment to sustainability. Up to now, we have cooperated with more than 60,000 clients across five continents. Let’s work closely with you and build a bold and better future.
QYResearch is a world-renowned large-scale consulting company. The industry covers various high-tech industry chain market segments, spanning the semiconductor industry chain (semiconductor equipment and parts, semiconductor materials, ICs, Foundry, packaging and testing, discrete devices, sensors, optoelectronic devices), photovoltaic industry chain (equipment, cells, modules, auxiliary material brackets, inverters, power station terminals), new energy automobile industry chain (batteries and materials, auto parts, batteries, motors, electronic control, automotive semiconductors, etc.), communication industry chain (communication system equipment, terminal equipment, electronic components, RF front-end, optical modules, 4G/5G/6G, broadband, IoT, digital economy, AI), advanced materials industry Chain (metal materials, polymer materials, ceramic materials, nano materials, etc.), machinery manufacturing industry chain (CNC machine tools, construction machinery, electrical machinery, 3C automation, industrial robots, lasers, industrial control, drones), food, beverages and pharmaceuticals, medical equipment, agriculture, etc.
0 notes