#journal of Diabetes
Text
Endocrinology Insights: Advancing Hormone Research
"Endocrinology Insights: Advancing Hormone Research" is a comprehensive journal that delves into the latest advancements and breakthroughs in the field of endocrinology.
Endocrinology Insights provides a platform for sharing cutting-edge research, clinical studies, and scientific discoveries related to hormones and their roles in health, disease, and overall well-being. Journal of Endocrinology offers a valuable resource for endocrinologists, researchers, and healthcare professionals, facilitating the dissemination of knowledge and the exploration of future directions in hormone research.
"Endocrinology Insights: Advancing Hormone Research" is a journal dedicated to the latest breakthroughs and research in the field of endocrinology. It provides a platform for sharing cutting-edge studies and discoveries related to hormones and their impact on health and medicine, making it an invaluable resource for endocrinologists, researchers, and healthcare professionals. It serves as a platform for sharing cutting-edge research and discoveries, offering valuable insights into the intricate world of endocrinology.
This journal is an essential resource for researchers, healthcare professionals, and experts in the field who seek to push the boundaries of hormone-related knowledge. It offers a platform for sharing innovative research, clinical studies, and scientific breakthroughs related to hormones and their impact on health and medicine. This journal serves as a valuable resource for endocrinologists, researchers, and healthcare professionals, driving the progress of hormone-related research and its application in the field.
#Journal of Endocrinology#Journal of Endocrinology and Diabetes#journal of diabetes#clinical study of endocrine disorders#diabetes journal#endocrinology journal
1 note
·
View note
Text
Semaglutide versus liraglutide for treatment of obesity
Abstract
Background: Once weekly (OW) semaglutide is a glucagon-like peptide-1 receptor agonist (GLP-1 RA) currently under evaluation for treatment of obesity at a dose of 2.4 mg OW.
Objective: To compare weight-loss efficacy and safety of once daily (OD) liraglutide 3.0 mg versus OW semaglutide 2.4 mg. Methods: Pubmed research up to March 31, 2021. Randomized trials, pertinent animal studies, and reviews are included. Search terms were glucagon-like peptide-1 receptor agonists, weight loss, obesity, liraglutide, semaglutide, efficacy, safety.
Results: No head to head trials are available to provide direct comparison of efficacy of OD liraglutide 3.0 mg versus OW semaglutide 2.4 mg. However, marked resemblance between trials in terms of study protocols and subjects’ characteristics may allow indirect comparison. In clinical trials of OW semaglutide, this drug was consistently associated with greater weight loss than in trials of OD liraglutide. Thus, placebo-corrected percentage weight reduction was -10.3 to -12.4% and -5.4% with OW semaglutide and OD liraglutide, respectively. In patients with type 2 diabetes, corresponding weight reduction was less pronounced with both drugs being -6.2% and -4.3% with OW semaglutide and OD liraglutide, respectively. In addition, head to head trials comparing liraglutide and semaglutide used in different doses and formulations consistently showed more weight loss in favor of semaglutide. In general, the anti-hyperglycemic efficacy and safety profile are similar in both drugs.
Conclusions: Available indirect evidence suggests that OW semaglutide 2.4 mg may be superior to OD liraglutide 3.0 mg for weight loss. Head-to-head comparison between these 2 agents is essential to confirm this conclusion.
Keywords: Obesity; Liraglutide; Semaglutide; Glucagon-like Peptide-1; Efficacy; Safety; Weight Loss; Type 2 Diabetes; Hemoglobin A1c
Introduction
GLP-1 RAs are approved for treatment of type 2 diabetes. The drug profile of these drugs is characterized by mild dose-related weight loss of approximately 2-6 kg [1]. Currently, liraglutide is the only GLP-1 RA approved for treatment of obesity in a dose higher than that approved for type 2 diabetes (3.0 mg daily for treatment of obesity as opposed to a maximum dose of 1.8 mg/d in type 2 diabetes) [2]. Semaglutide is another GLP-1 RA approved for treatment of type 2 diabetes in a dose of 0.5-1.0 mg given subcutaneously OW and as an oral formulation in a dose up to 14 mg once daily [3,4]. Currently, semaglutide is under evaluation for future approval for treatment of obesity. The Semaglutide Treatment Effect in People with obesity (STEP) development program including 5 phase 3 clinical trials (STEP 1 to 5) was launched to evaluate efficacy and safety of OW semaglutide at this high dose of 2.4 mg for treatment of obesity in patients with and without diabetes [5].
Mechanisms of Weight Loss by Liraglutide and Semaglutide
In general, the mechanisms of weight loss by liraglutide and semaglutide are similar. Both agents were shown to reduce appetite and hunger while increasing sense of fullness and satiety [6,7]. In addition, OW semaglutide 2.4 mg, but not liraglutide, may decrease food craving [7]. Animal studies have shown that the anorexigenic effect of semaglutide is mediated by GLP-1 receptors in the hypothalamus and hind brain [8,9]. Delay in gastric emptying, a class effect of all GLP-1 RAs, may contribute to the sensation of early fullness [10]. Meanwhile, one study with relatively longfollow- up (52 weeks) has shown that improvements in hunger and fullness with OD liraglutide 3.0 mg peak after 4 weeks, then decline gradually and return to baseline after 40 weeks [6]. Similar followup studies are not available for semaglutide.
STEP Program of Semaglutide
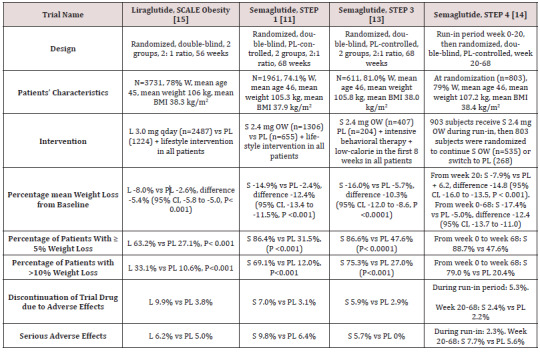
STEP 1 to 4 trials are well-designed studies comparing OW 2.4 mg semaglutide with placebo in obese individuals (defined as BMI of ≥ 30 kg/m2, over ≥ 27 kg/m2 with ≥ 1 weight-related coexisting condition e.g. hypertension, dyslipidemia, cardiovascular disease, or obstructive sleep apnea) for 68 week-duration [11-14]. STEP 1, 3 and 4 excluded patients with diabetes, whereas STEP 2 included exclusively patients with type 2 diabetes [11,13-14]. In addition, STEP 2 included a third group of individuals randomized to the smaller anti-diabetic dose of OW semaglutide 1.0 mg [12]. In STEP 1, 2 and 4, all participants receive lifestyle intervention defined as a 500 kcal deficit relative to the estimated energy expenditure plus encouragement of increase physical activity, such as walking 150 minutes per week. In STEP 3 trial, all subjects received a low-calorie diet (1000-1200 kcal/d) provided as meal replacement for the first 8 weeks. Subsequently, they were transitioned to a low-calorie diet (1200-1800 kcal/d) of conventional food. Moreover, they were prescribed 200 min of physical activity/week [13]. The coprimary endpoints of STEP 1 to 3 trials were the percentage change in body weight and weight reduction of at least 5% at week 68 compared with placebo [11-13]. STEP 4 trial was a withdrawal trial that includes an initial run-in period of 20 week during which all subjects received OW semaglutide 2.4 mg followed by randomization to a group that continued the drug and another group that switched to placebo for further 48 weeks [14]. Overview of STEP 1 to 4 trials are summarized in (Tables 1 and 2).
Table 1: Weight-loss efficacy of liraglutide and semaglutide in patients without diabetes.
Abbreviations: W: Women; BMI: Body Mass Index; L: Liraglutide; S: Semaglutide; OW: Once Weekly; PL: Placebo; HbA1c: Hemoglobin A1c; CI: Confidence Intervals
Table 2: Weight-loss efficacy of liraglutide and semaglutide in patients with type 2 diabetes
Abbreviations: PL: Placebo; W: Women; HbA1c: Hemoglobin A1c; L : Liraglutide; OWS: Once-Weekly Semaglutide
Weight loss in Semaglutide and Liraglutide Trials
While no head to head trials are available to compare weight loss efficacy of OW semaglutide 2.4 mg with OD liraglutide 3.0 mg, indirect comparison may be inferred from results of their respective trials. In fact, as shown in tables 1 and 2, subjects’ characteristics at baseline in these trials were similar to a great extent (Table 1). In addition, the study protocols and designs have several common features (e.g. similar primary end point). In STEP trials 1, 3 and 4 that excluded patients with diabetes, the difference in weight loss between OW semaglutide and placebo ranged between -10.3% and -12.4% at 68 weeks (Table 1). Meanwhile, in the SCALE Obesity and prediabetes trial of OD liraglutide 3.0 mg, the corresponding difference was -5.4% (95% CI, -5.8 to -5.0%) at 56 weeks (Table 1) [15]. In trials that exclusively recruited patients with type 2 diabetes, the weight loss efficacy of both drugs was diminished, but was still relatively greater in OW semaglutide 2.4 mg than with OD liraglutide 3.0 mg. Thus, in 2 liraglutide diabetes trials, the mean difference in weight loss between the drug and placebo was -4.0 and -4.3%, whereas the corresponding difference was -6.2% with OW semaglutide 2.4 mg (Table 2) [12,16-17]. The explanation of this finding is unclear but might be related to the coexistence of type 2 diabetes, relatively older patient population (mean age approximately 55 year-old in diabetes trials versus 45 year-old in trials excluding diabetes), or the lower baseline body weight (approximately 99.8 kg in diabetes trials versus approximately 105.5 kg in non-diabetes trials) (Tables 1-2) [12,16-17]. Other parameters that suggest superiority of OW semaglutide 2.4 mg over OD liraglutide 3.0 mg are the proportions of individuals losing ≥ 5% and > 10% of body weight. These proportions were always higher in trials of semaglutide than in those of liraglutide (Tables 1 and 2).
Head to Head Trials of Semaglutide Versus Liraglutide
Another indirect line of evidence suggesting greater efficacy of semaglutide compared to liraglutide may be derived from 3 randomized head to head trials comparing the 2 agents in different doses and formulations. A randomized, placebo-controlled, doubleblind trial [18] compared semaglutide in 5 daily subcutaneous doses (0.05, 0.1, 0.2, 0.3, and 0.4 mg) versus liraglutide 3.0 mg once daily on top of lifestyle changes in obese subjects without diabetes. After 52 weeks, mean weight reduction from baseline was significantly greater in patients randomized to semaglutide doses ≥ 0.2 mg daily being - 11.2 to -13.8% versus -7.8% in subjects randomized to liraglutide 3.0 mg daily [18]. The second trial including patients with type 2 diabetes [19] compared oral semaglutide (14 mg qday) with OD liraglutide 1.8 mg in doubleblind double-dummy fashion. After 26 weeks, oral semaglutide resulted in superior weight loss (-4.4 kg) compared with liraglutide (-3.1 kg), estimated difference -1.2 kg (95% CI, -1.9 to -0.6, P= 0.001) [19]. The third trial [20] compared OW semaglutide 1.0 mg with liraglutide 1.2 mg in patients with type 2 diabetes in an open-label design. After 30 weeks, mean weight loss was -5.8 kg and -1.9 kg, in the semaglutide and liraglutide groups, respectively; estimated treatment difference -3.8 kg (95% CI, -4.47 to -3.09, P<0.0001) [20]. Taken together, the results of the preceding 3 trials suggest higher efficacy of semaglutide than liraglutide irrespective of doses or drug formulation (i.e. subcutaneous or oral semaglutide).
Anti-Hyperglycemic Efficacy of Liraglutide Versus Semaglutide
The difference between semaglutide and liraglutide with respect to their anti-hyperglycemic efficacy is not as consistent as in their weight-loss effects. Thus, in the studies conducted by O’Neil et al, [18] and Pratley et al [19], semaglutide was similar to liraglutide in HbA1c reduction. Meanwhile, in the trial conducted by Capehorn et al, [20], OW semaglutide 1.0 mg was superior to liraglutide 1.2 mg qday; estimated treatment difference in HbA1c reduction was - 0.69% in favor of semaglutide. However, the latter trial is limited by its open-label design and using liraglutide in submaximal antidiabetic dose (1.2 mg instead of 1.8 mg) [20]. Therefore, while semaglutide may be more effective than liraglutide in causing weight loss, both GLP-1 RAs may be equally effective in terms of glycemic control.
Effects of Semaglutide and Liraglutide on Cardiovascular Variables
Significant reduction in systolic blood pressure (SBP) was recorded in subjects randomized to semaglutide in STEP 1-3 trials, approximately 4-5 mmHg lower than in individuals randomized to placebo [11-13]. Likewise, a significant reduction in DBP of approximately 2 mmHg was observed in STEP 1 and 3 trials [11,13]. Changes in lipid panel were generally mild. Thus, reduction in plasma triglycerides of 14-17% compared to placebo was the most consistent change in lipid panel. Minor reductions in concentrations of low-density lipoprotein-cholesterol (LDL-C) (by ≤7% vs placebo) and increase in high-density lipoprotein-cholesterol (HDL-C) levels (by <5% vs placebo) were also observed. In addition, there was significant reduction in the inflammatory marker C-reactive protein (CRP) levels in semaglutide-treated subjects vs placebo [11-13]. Similar beneficial changes in the above cardiovascular (CV) markers were described in liraglutide trials albeit they were lesser in magnitude [15,21]. The above favorable changes in blood pressure, lipids and CRP are likely attributed to weight loss per se and are unlikely to be direct effects of semaglutide or liraglutide.
Safety of Liraglutide and Semaglutide as Anti-Obesity Agents
Gastrointestinal Adverse Effects
Gastrointestinal (GI) adverse effects represent the most common adverse events that characterize all GLP-1 RAs. In liraglutide obesity trials, GI adverse events occurred in 65% and 39% of subjects randomized to OD liraglutide 3.0 mg and placebo, respectively [16]. In STEP 1-3 trials of semaglutide, GI adverse effects were reported by approximately 63-83% and 34-63% in subjects randomized to OW semaglutide and placebo, respectively [11-13]. Among the GI adverse effects, nausea was the most common, followed by diarrhea, vomiting and constipation [11-13,16]. The frequency of GI symptoms increased early in the first few weeks during drug titration. They were generally described as mild to moderate and transient. However, in a minority of patients, they can be severe. In fact, GI adverse effects were the most frequent cause of premature drug withdrawal. Thus, in the largest obesity trial of liraglutide, drug discontinuation due to GI adverse effects occurred in 6.4% and 0.7% in the liraglutide and placebo group, respectively [15]. In STEP trials, withdrawal due GI adverse events occurred in 3.4-4.5% and 0-1.0% in patients randomized to OW semaglutide and placebo, respectively [11-13]. Previous trials including patients with type 2 diabetes using OW 1.0 mg semaglutide have shown that GI adverse effects tend to be more common with semaglutide compared with other GLP-1 RAs [1]. Meanwhile, post-hoc analysis by Lingway et al [1] suggest that GI adverse effects contribute minimally (less than 0.1 kg) to the superior weight loss effects of semaglutide vs other GLP-1RAs. Incidence of cholelithiasis and cholecystitis was slightly higher with liraglutide than placebo, 1.5% and 0.4%, respectively [15] as well as with semaglutide than with placebo, 2.5-2.6% versus 0-1.2% [11-13]. These events may be attributed in part to weight loss, but other mechanisms could be involved such as inhibition of gallbladder contraction and biliary motility [22]. Frequency of acute pancreatitis is marginally elevated with OD liraglutide 3.0 mg (1.3% vs 1.0 in placebo) [21], and similar to placebo in trials of OW semaglutide 2,4 mg in STEP 1 to 4 trials [11-14].
Hypoglycemia
Consistent with the glucose-dependent action of GLP-1 RAs, frequency of hypoglycemia was similar to placebo in patients without diabetes. However, in obesity trials including patients with type 2 diabetes, frequency and severity of hypoglycemia were increased with use of OD liraglutide 3.0 mg (87 versus 31 events per patients-year with placebo) [16]. These hypoglycemia events occurred mainly in patients using sulfonylureas [16]. In STEP 2 trial, severe or blood-glucose confirmed symptomatic hypoglycemia occurred in 5.7% and 3.0% of patients receiving OW semaglutide 2.4 mg and placebo, respectively [12].
Safety Concerns about Liraglutide and Semaglutide
There was numerical increase in breast neoplasms in association with OD liraglutide 3.0 mg. Thus, 10 premalignant and malignant neoplasms were reported in 9 women in the liraglutide arm versus none in the placebo arm [21]. In STEP 4 trial of OW semaglutide 2.4 mg, 3 breast cancers were diagnosed in women randomized to semaglutide versus none in the placebo group [13]. Worsening diabetic retinopathy seems to be an adverse effect specific to semaglutide which was initially observed in association with use of OW semaglutide 0.5-1.0 mg [23]. In STEP 2, there was a trend towards increase in incidence of retinal disorder events in the 2 semaglutide arms compared with the placebo arm [12]. Thus, these events occurred in 6.9%, 6.2%, and 4.2% in patients randomized to OW semaglutide 2.4 mg, OW semaglutiude 1.0 mg, and placebo, respectively [12].
Appraisal of Liraglutide and Semaglutide
Although available data suggest that OW semaglutide 2.4 mg may be more effective than daily liraglutide 1.8 mg in weight reduction, both drugs offer several advantages for management of obesity. First, their short-term efficacy and safety are supported by well-designed randomized trials [11-15]. Second, being also wellstudied as anti-diabetic drugs, they may be particularly useful in obesity-related type 2 diabetes by causing reduction of both body weight and hyperglycemia [12]. Furthermore, in individuals with pre-diabetes, they delay the onset of type 2 diabetes and increase reversion to normoglycemia [15,21]. The OW administration of semaglutide might virtually enhance compliance with prolonged use. However, both agents have several limitations. First, the common occurrence of GI adverse effects which not uncommonly lead to drug discontinuation. Second, safety beyond 58 weeks is not available for OW semaglutide 2.4 mg [11-14]. The ongoing STEP 5 may in part clarify this problem as it extends over a 2-year period [5]. In case of OD liraglutide 3.0 mg, safety data from placebocontrolled trials are overall reassuring and extend up to 172 weeks [21]. Third, the durability of the weight loss effect is still unclear. In fact, maximum weight loss with use of either drug was achieved after approximately 52 weeks followed by a gradual rebound [11- 15, 21]. Moreover, after drug cessation, weight regain takes place at a more rapid pace along with rise of systolic blood pressure and glycemic parameters to their baselines [14,16]. Hence, these drugs will be taken for years, or even decades as long as weight loss is desired. It is crucial therefore to establish their long-term safety. Fourth, drug cost is another limitation. Advantages and limitations of both agents are summarized in Table 3.
Table 3: Advantages and limitations of liraglutide and semaglutide for treatment of obesity.
Conclusions and Current Directions
Available clinical trials suggest that OW semaglutide 2.4 mg as an adjunct to healthy life-style changes may be more effective than OD liraglutide 3.0 mg in terms of weight reduction, but not glycemic control. While no head to head comparison is available yet, data derived from respective trials of liraglutide and semaglutide showed superior weight loss with use of OW semaglutide 2.4 mg. Furthermore, head to head comparison of the 2 drugs used in different doses or formulations, consistently showed greater weight loss associated with the use of semaglutide than with liraglutide. However, the superiority of OW semaglutide 2.4 mg will only be confirmed by direct head to head comparison with OD liraglutide 3.0 mg in the setting of randomized, double-blind and double-dummy trials. The possible increase in incidence of breast cancer in association with these 2 agents must be clarified in long-term studies and post-marketing investigations. Similarly, risk of worsening of diabetic retinopathy in relation to the use of semaglutide should be carefully examined. Whereas both drugs in their anti-diabetic doses may reduce CV events in patients with type 2 diabetes, it is equally important to assess their impact on CV outcomes when used in their higher doses for treatment of obesity. In this regard, the SELECT study is an ongoing, double-blind placebo-controlled trial specifically designed to examine the effect of OW semaglutide 2.4 mg on CV outcomes in overweight and obese persons with established CV disease who do not have diabetes [17]. SELECT study started in November 2018 and is expected to recruit 17,500 participants, and last for approximately a total of 59 months.
Read More About This Article Click on Below Link:
https://lupinepublishers.com/diabetes-obesity-journal/fulltext/semaglutide-versus-liraglutide-for-treatment-of-obesity.ID.000162.php
Read more Lupine Publishers Google Scholar Articles : https://scholar.google.com/citations?view_op=view_citation&hl=en&user=nqY8h-kAAAAJ&citation_for_view=nqY8h-kAAAAJ:-f6ydRqryjwC
#Lupine Publishers#Lupine Publishers Group#ADO#Journal of Diabetes#Archives of Diabetes and obesity Journal#Journal of Obesity
0 notes
Text
my blood sugar: slightly low but, eh, mostly fine
me: also mostly fine
my blood sugar, five minutes later: three points lower
me: I Am The Unceasing Hunger, Never Has The World Known Such A Hunger As This
144 notes
·
View notes
Text
I think my sugar is too high rn...
Nearly walked in to the doors at Walmart and then stood by waiting for the other doors to be opened (it was about 15mins past opening). Took a step forwards and the other set of doors opened.
Couldn't find the Gatorade aside from what was on the end cap and asked an associate who KINDLY pointed out the ENTIRE WALL of the stuff.
I'm grateful I sent a small list of things I needed to the brother cause I forgot what I was looking for and finally remembered I had sent it to him explaining I would need it later.
I did snag a snack and a drink and sat in the car till my brain fog cleared a bit. Feel a bit better. It doesn't help that I spent almost all day or yesterday because of cramps. I should maybe get some mushroom coffee in me as that helps sometimes.
I hate being alive somedays.
6 notes
·
View notes
Text

Sketchtember 5 gets a bit personal. And is also very rough-around-the-edges. That kinda happens sometimes.
Anyway, moral of this story is (at least what I got out of this encounter, aside from the practical food and exercise advice of course): correlation isn't necessarily causation, and a diagnosis is not some kind of marker of a moral failing. Thanks for coming to my TED talk. Also keto is a fad and is not sustainable long-term, my dietician said so.
#sketchtember#sketchtember 2023#comic journal#journal comic#diabetes#type 2 diabetes#traditional drawing#original
33 notes
·
View notes
Text












13/12/23 and 14/12/23
These past few days have been a bit better. I got surprised with a mini Christmas tree which has made me super happy, I finished a physical book, which I haven’t done for a while, I worked on the last few new year things in my Hobonichi, and did some ‘common place’ work, I’ve also started a self governed experiment into finding out if I’m diabetic. I know I won’t be sleeping well but I still hope I can rest easy tonight.
#ThisIsDore#daily post#bubble bath#journaling#slow day#dog cuddles#new year prep#type 1 diabetes#common place book#mini Christmas tree
14 notes
·
View notes
Text
2/17/24 CGM
So, despite being "only" pre-diabetic, I asked my Dr for a script for a continuous glucose meter so I could try and understand if there are things I'm eating which are keeping me out of ketosis, things that are not obviously high carb. I put it on last night before bed. It's a Libre 3, and an app on my phone reads it.
It didn't really hurt to put on, but the applicator didn't release it, so when I tried to take the applicator off, it tried to pull the meter with it. I had to bang it down against my arm a couple more times before it let go. I guess I didn't push it down hard enough to start? Idk, but anyway, it's fine now . Guess we'll see what this shows...
Although glucose has been in the 'normal ' range all night, normal and ideal are two different things. I would like to see it under 90, really, but I had ribs with sauce for dinner, sauce has sugar in it, so I'm not that discouraged by these initial readings.


15 notes
·
View notes
Text
"Harmony of Hormones: Navigating the Endocrine Landscape for Health and Balance"
Endocrinology, the study of hormones and their vital role in maintaining bodily balance, offers a captivating journey into the intricate realm of human physiology. This exploration takes you on a captivating voyage through the world of endocrinology, where scientific inquiry intersects with clinical understanding to unveil the complexities of our endocrine system. From understanding hormonal regulation to deciphering the origins of endocrine disorders, from exploring innovative diagnostic techniques to advanced hormonal therapies, this journey offers a deep dive into the multifaceted landscape of hormonal health.

Join us as we navigate through the remarkable realm of endocrinology, revealing insights that empower individuals to comprehend and optimize their hormonal well-being. Whether you're a medical professional, researcher, or someone curious about the body's delicate hormonal orchestration, this expedition promises to be an enlightening guide to the dynamic field of endocrinology.
The journal plays a vital role in advancing the knowledge and understanding of endocrine-related conditions and the underlying mechanisms that regulate hormonal pathways and systems in the body. The research published in the Journal of Endocrinology helps inform medical practice, leading to improved diagnostic methods, treatment approaches, and patient care in the field of endocrinology.
0 notes
Text
that moment when your blood sugar crashes, and your brain says 'eat the requisite 15 carbs' but the rest of you says 'eat literally everything'
222 notes
·
View notes
Text
It takes a bottle of beer to get this biabetic drunk. Perks of sugar being your enemy I guess
2 notes
·
View notes
Text
Saturday 08/10/22
I begun the day with a very much needed sleep in! 😴 I have been really good with waking up at 7.00 they days when I've had study days at home, which has been the the whole week except for Friday. And as many of us are aware of; enough sleep is key to studying. Therefore I promised myself yesterday that today was going to be the one day I won't put an alarm to wake up early. I let my body dictate when it was ready to wake up, which was 11.00 👌
Things I've done today:
Logged things in my bullet journal from the week.
Anatomy & Physiology: Finished the endokrine system.
And began drawing my notes


#studyblr#student#college#school#university#bullet journal#nursing student#studyspo#studyblog#nursing school#university studyblr#university struggles#university studyspo#college student#college studyblr#galexias study#galexias life#anatomy#anatomy and physiology#diabetes
3 notes
·
View notes
Text
Clinical Journal of Diabetes Care and Control (CJDCC) is a peer reviewed, online open access journal which deals with scientific information on the latest developments of diabetes. CJDCC publishes articles of basic and clinical studies focusing on areas such as Diabetes mellitus, Diabetes Cholesterol, Drugs for Diabetes, etc. in the form of high quality original articles, review articles, case reports, short communications, opinions, Letter to Editor. It acts as a forum for health-care providers and clinically oriented researchers that aim to publish work pertaining to Diabetes.
Email: [email protected] (OR) [email protected]
ISSN: 2642-0872
Journal Name: Clinical Journal of Diabetes Care and Control
Journal Link: https://academicstrive.com/CJDCC/
Submit Manuscript: https://academicstrive.com/submit-manuscript.php
WhatsApp Link: https://api.whatsapp.com/send?phone=9963889666
#academic strive#open access journal#publishing#research#research article#open source#journal#diabetes
0 notes
Text
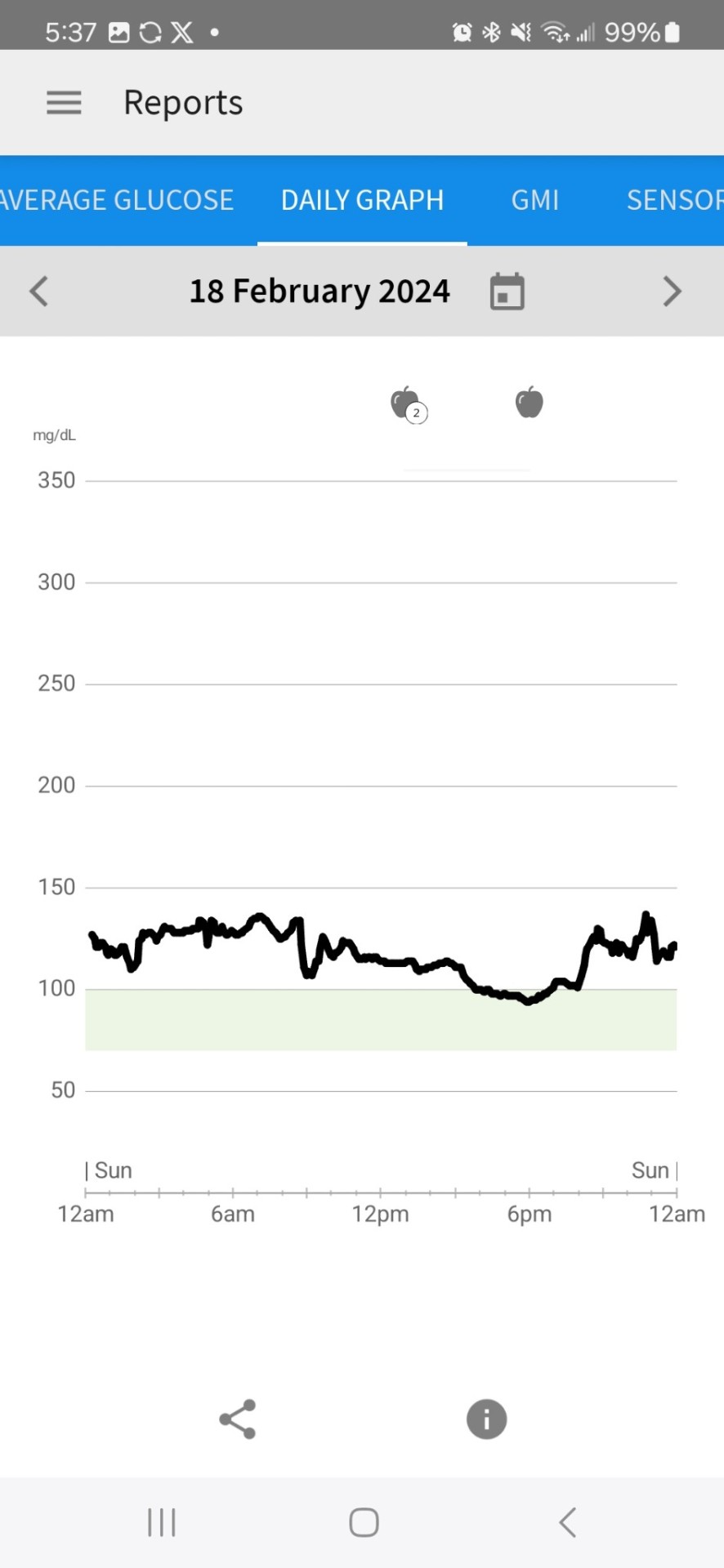
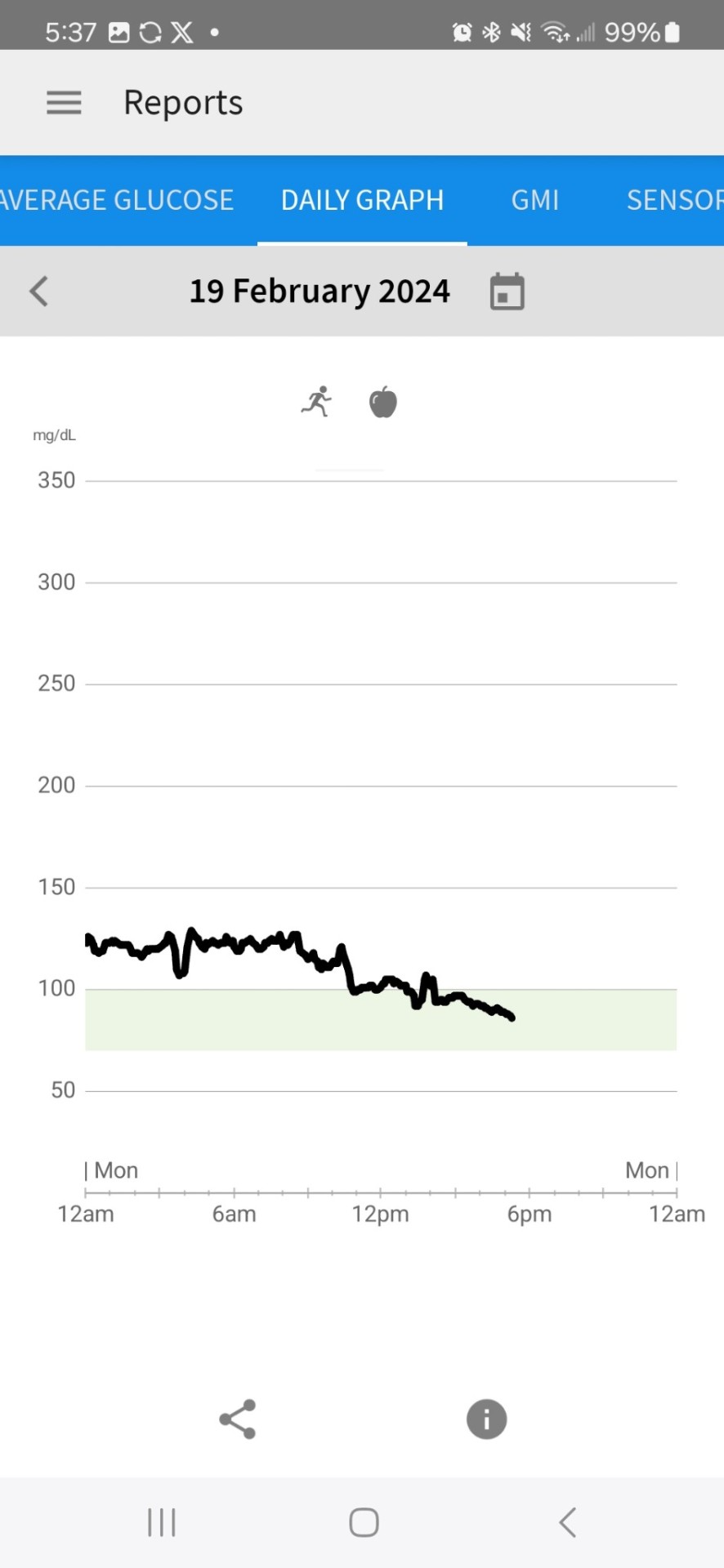
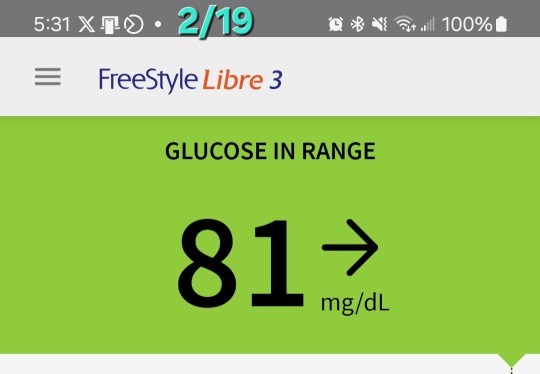
2nd Day of CGM
Yesterday and this morning I was dismayed by the glucose readings. While they are in the 'normal' range for someone with diabetes, I don't want to be in that 'normal' range. I want to be in an ideal range for someone on a keto diet-- a non-diabetic person-- which, per the internet, is between 70 and 90. It was above that range most of the day yesterday. Today, FINALLY, in late afternoon, it began dropping into that range.
What I did differently:
Worked out this morning, cardio and weights for an hour, and half an hour of stretching.
Took the tomatoes I usually have with my breakfast out, so had only protien and fat (I think it ended up at 3 net carbs for breakfast with the eggs)
I thought that the workout would have an immediate effect on lowering the glucose, but maybe not? I will have to research that further. The fact that it didn't was very depressing to me this morning, but maybe it just takes some time for it to have an effect.
I changed the target range on their graph to be between 70 and 100 to give myself a slightly wider target than what the internet says. It has gotten down to 81 this afternoon, which it never approached a number that low yesterday.
So, was it the workout (I didn't workout yesterday at all) or the fewer carbs at breakfast that lowered it, or a combination of both. I'll have to keep experimenting to figure that out.
5 notes
·
View notes
Text
Can Gestational Anti-Diabetic Plants Using in Cameroon Manage Preeclampsia?

Preeclampsia is a persistent hypertensive gestational disease that might happen during pregnancy (antepartum), during delivery (intrapartum) or after childbirth (postpartum). It is clinically characterized by maternal high hypertension (>140/90mmHg systolic/diastolic blood pressure) and massive proteinuria (>300mg/24h). Severe cases are manifested by the development of comorbidities such as eclampsia, liver damage, edema and scattered vascular coagulation in mother and the complications in the fetus like fetal growth restriction, premature birth and fetal death. In Africa, the prevalence of eclampsia varies from 1/100 to 1/1700 pregnancies, and Cameroon it happens in 0.92/1 100 deliveries. Our objective was to determine among antidiabetic and gestational plants used in Cameroon those that can control preeclampsia. To achieve this objective three following engines of research were used in Google, Google Scholar and PubMed: “a given antidiabetic plant treated gestational women with preeclampsia “; “a single given antidiabetic plant treated three complications of preeclampsia” and “a given antidiabetic plant may regulate hypertension in women with preeclampsia. Twelve (12) plants were recorded and belong to 10 families and 12 genera. Six plants (Curcuma longa, Brassica oleracea, Moringa oleifera, Passiflora incarnata, Passiflora edulis and Passiflora foetida) intervene in direct treatment of preeclampsia and all the twelve were used in symptomatic treatment of preeclampsia. The most important plants are Brassica oleracea and Solanum melongena with 127 and 99 repetitions respectively. Strong recommendation of further studies for finding non-toxic doses is necessary for developing anti-preeclampsia future phytodrugs.
Read more about this article: https://crimsonpublishers.com/iod/fulltext/IOD.000626.php
Read more about this journal: https://crimsonpublishers.com/iod/
#peer review journals#Diabetic Retinopathy#Diabetes#Hypertension and Hyperlipidemia#Signalling Mechanisms#Diet & Controlling Methods
0 notes
Text
Watching my classmate's insta story about her vacation and smiling bc she's the summer catsitter for one of my friends in my dnd party and two other friends from my dnd party are taking over catsitting while catsitter 1 is on vacation which means I get to go and see the cat at the next dnd session 🕺🕺🕺
#the friend who owns the cat lives in a different country and joins dnd over discord (for context)#+ the cat has to have live in catsitters bc hes got diabetes and need his food and insulin on a strict schedule#pinky’s personal journal#pinky's d&d tag
0 notes
Text
Gratitude Journal Entry (7/1/24)

Today I'm Grateful For:
*The first day of my challenge. I am so nervous and I'm thinking what the heck did I just get myself into. But I have to see this through. I've already got it outlined of how I want it to go - now I just have to do it.
*For extra motivation to lose weight. It's hard for me to stay on a diet and that's mainly because I fear blood sugar issues if I don't eat certain foods or at certain times. My birthmother died just a few years ago from diabetes and while I don't have it, I do have blood sugar issues. I was born with them, apparently. I need to do a better job of listening to my body and not eating just because I think I need to. I've decided to reward myself for every day I do well, and it'll be either doing a puzzle pack on my Jigsaw app, playing a game on my Nintendo DS or X-box (Lego Harry Potter is my favorite) or watching a movie off my list.
*For my gratitude journal. I'm having a hard time of thinking of something else that doesn't sound cliché (I want to put actual thought into it) and I'm just grateful for my journal. It forces me to think about positive things. If I wake up in a bad mood, it is so easy to just stay angry and feel like I hate everything. The journal shifts my focus and usually makes me feel better.
Something I'm Proud Of:
That despite all my doubt and fears I'm going forward with the challenge. God put it on my heart for a reason, so I refuse to listen to my fears and do my best to reach people through my poetry.
Tomorrow I'm Looking Forward To:
My urologist appointment. I'm getting a scope done to check if there's any abnormalities or a source of the bleed and I'm also going to get the results of the CT scan. I'm not really feeling anxious, but that'll most likely change tomorrow. At least it's at 1:10 and not just right off the bat. I always like a little time to get used to the idea of something.
#Grateful#Gratitude Journal#Personal Growth#Self-Love#Health Journey#Weight Loss Motivation#Blood Sugar Management#Diabetes Awareness#Personal Challenge#Poetry Challenge#Overcoming Fear#Daily Affirmations#Self-Improvement#Mental Health#Positive Thinking#Faith and Spirituality#Coping with Anxiety#Health Checkup#Urology Appointment#Body Positivity
0 notes