#hydrochemistry
Explore tagged Tumblr posts
Text
I have my first test tomorrow and it's Hydrochemistry, wish me luck
#i suck at chemistry#thankfully my boyfriend is a chemist and he's helping me study but that doesn't mean I won't fail#😬
2 notes
·
View notes
Text
Five steps of Wikipedia for Monday, 15th January 2024
Welcome, bem-vindo, tervetuloa, mirë se vjen 🤗 Five steps of Wikipedia from "OctaDist" to "Acetic acid". 🪜👣

Start page 👣🏁: OctaDist "OctaDist is computer software for crystallography and inorganic chemistry program. It is mainly used for computing distortion parameters of coordination complex such as spin crossover complex (SCO), magnetic metal complex and metal–organic framework (MOF). The program is developed and maintained in..."

Image licensed under CC BY-SA 4.0? by Rangsiman1993
Step 1️⃣ 👣: APBS (software) "APBS (previously also Advanced Poisson-Boltzmann Solver) is a free and open-source software for solving the equations of continuum electrostatics intended primarily for the large biomolecular systems. It is available under the BSD license. PDB2PQR prepares the protein structure files from Protein..."
Step 2️⃣ 👣: AMBER "Assisted Model Building with Energy Refinement (AMBER) is a family of force fields for molecular dynamics of biomolecules originally developed by Peter Kollman's group at the University of California, San Francisco. AMBER is also the name for the molecular dynamics software package that simulates..."

Image by Bensaccount at English Wikipedia
Step 3️⃣ 👣: Aqion "Aqion is a hydrochemistry software tool. It bridges the gap between scientific software (such like PhreeqC) and the calculation/handling of "simple" water-related tasks in daily routine practice. The software aqion is free for private users, education and companies...."
Step 4️⃣ 👣: Acid "An acid is a molecule or ion capable of either donating a proton (i.e. hydrogen ion, H+), known as a Brønsted–Lowry acid, or forming a covalent bond with an electron pair, known as a Lewis acid.The first category of acids are the proton donors, or Brønsted–Lowry acids. In the special case of aqueous..."

Image by Chemicalinterest
Step 5️⃣ 👣: Acetic acid "Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula CH3COOH (also written as CH3CO2H, C2H4O2, or HC2H3O2). Vinegar is at least 4% acetic acid by volume, making acetic acid the main component of vinegar apart from water. It..."

Image licensed under CC BY-SA 3.0? by W. Oelen
0 notes
Text
nothing but love for writing a mail to city officials like:
from: [email protected] sent: 1st june 2023 to: [email protected] title: small request from your citizen
your honour, i am but a mere peasant; in desperate need for information regarding the topic of hydrochemistry surrounding the city and its rivers - would it be thinkable of the Exalted One to maybe send me an EXCEL-sheet containing the necessary information? in your undying duty, loyal citizen #8375289
-------
from: [email protected] sent: 31st june 2023 to: [email protected] title: re: small request from your citizen
>hey you, >dunno have you checked your mum's butthole? >sent from my iphone
1 note
·
View note
Text



15/11/2018
14:50
Petnica is one of my favorite places on earth. It is a science research center for young people who want to know more about science, art, humans... I’m currently here for my hydrochemistry curse and I’m so sad because I have to leave tomorrow 😣 Petnica is full of amazing people and I met a lot of new friends here. It’s really interesting and I feel so lucky to have a place like this in my country
1 note
·
View note
Link
In this present study, 34 Hydrochemical data of groundwater samples in Annaba – El-Tarf areas were examined with an aim to assess the Scaling and corrosion ten…
0 notes
Text
Juniper Publishers- Open Access Journal of Environmental Sciences & Natural Resources
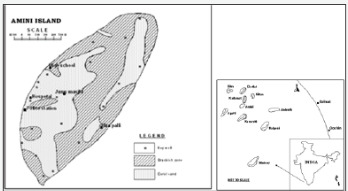
Hydrogeochemical Processes of Evolution Of Ground Water In A Small Tropical Coral Island Of Amini, Union Territory Of Lakshadweep, India
Authored by Joji VS
Abstract
In the small tropical island of Amini ground water occurs under phreatic condition and is seen as a thin lens floating over the saline water. The coral sands and coral limestone act as principal aquifers. The depth of the wells varies from 1.6 to 5.5 mbgl and depth to the water table 1.20 to 4.80 mbgl. The ground water is generally alkaline and EC varies from 465 to 999 micromhos /cm at 25o C. The ground water is under Na+- SO42- type and shallow to deep meteoric percolation types and generally alkaline in nature. The factors affecting the quality of ground water are rainfall, tides, ground water recharge and draft, human and animal wastes, oil spills and fertilizers. Water samples collected from different parts of the island during pre-monsoon and post monsoon seasons. The water sample results of chemical analysis indicate that water type ranges from Ca-HCO3 (recharge type) to Ca-Mg-Cl type (reverse ion exchange water type). The hydrochemistry is mainly controlled by evaporation, partly influenced by water-rock interaction and aquifer materials. The evaporation process played major role in the evolution of water chemistry. The ground water in the study area is generally suitable irrigation for all types of soil.
Keywords: Atoll; Fresh water lens; Chloro alkali indices; Base exchange indices and Irrigation
Introduction
The Lakshadweep islands (LD islands) are a group of tiny coral islands, located in the Arabian Sea, about 400 km from the main land (southern tip of the Indian peninsula). They spread over a distance of 300 km, consists of 36 coral islands and a number of sunken banks, open coral reef and sand banks. These islands are typically a chain of low islands surrounding a shallow lagoon, consisting of large recent sediments on top of older coral limestone. Amini Island has a delicate ecosystem with very limited fresh water resources. Though the island receives high rainfall, lack of surface storage and the limited ground water storage capacity, where fresh water is occurring as a small lens floating over saline water, makes fresh water a precious commodity. High porosity of the aquifers allow mixing of freshwater with sea water and due to high population density, waste water gets mixed with the fresh water in the aquifer, make the management of the limited fresh water resources multifaceted.
The purpose of the study is to assess the evolution of ground water resources of the island and to know the hydro geological characteristics. The Small Island hydro geological and hydro chemical studies were carried out by many authors at international and regional levels. These include Nura Umar Kura et al. 2013 on Evaluation of Factors Influencing the Groundwater Chemistry in a Small Tropical Island of Malaysia [1], applied factor analysis tool to the hydro chemical data set of Manukan Island in order to extract the principal factors corresponding to the different sources of variation in the hydrochemistry, Belkhiria et al. 2012 studied geochemical evolution of groundwater in an alluvial aquifer in the case of El Eulma aquifer, East Algeria, [2] used application of multivariate statistical techniques in the assessment of groundwater quality in seawater intrusion area in Bafra Plain, Turkey [3] carried out assessment of groundwater quality for Veppanthattai taluk, Perambalur district, Tamil Nadu using Remote Sensing and GIS, [4] on fresh water - salt water relation, [5] Appraisal of groundwater resources in an island condition and many others. The present study is an attempt to highlight hydro geochemical processes of evolution groundwater in a small tropical coral island of Amini, Union Territory of Lakshadweep, India.
Study area
Amini Island is the sixth largest of the inhabited islands of the UT of Lakshadweep, with an area of 2.50 Km2 and is elliptical in shape. Amini island is located between Kadmath and Kavarati (in the N-S direction) and between Agathi and Androth (in the E-W direction) in a NE-SW alignment, about 11 km south of Kadmath island. It is about 58 km SW of Kiltan Island. It is 294 km from Mangalore, 324 km from Kozhikode and 407 km from Kochi. It is located between north latitudes 110 07' 00" and 11008'00" and east longitude 720 44' 00" and 720 45' 00". The climate of Amini is similar to the climatic conditions of Kerala. March to May is the hottest period of the year. The temperature ranges from 25oC to 35oC and humidity ranging from 70 -76 per cent for most of the year. The average rainfall received is 1600 mm a year. Monsoon prevails here from 15th May to 15th September. During the monsoon time, boats are not allowed outside the lagoon because of the violent sea. The presence of the reef maintains calm at the lagoon.
The location map of LD islands including Amini Island is compiled (Figure 1) and various salient features of Amini are compiled (Table 1). The coral island is the work of minute sea organisms called coral polyps and they congregate in large colonies. When the organisms die, their skeletons, which are made of limestone, form big clusters, some of which rise above the water. Charles Darwin first described the different types of coral reef after his voyage by HMS Beagle among the Galapagos Isles in Pacific Ocean (Subsidence theory for the origin of coral In oceanic island fresh ground water occurs as a lens floating over saline water. The hydro dynamic balance of fresh and saline water determines the shape and movement of interface and may be controlled by some of the following factors viz. water table fluctuation due to diurnal tides, seasonal fluctuation of water table due to recharge or draft, dispersion and molecular diffusion. Due to these factors there is an alternate up and down movement of the interface.
Materials and Methods
The base map of Amini and various layers were prepared by using Map Info 6.5 techniques and in the ground water resource of Amini has been computed based on the methodology recommended by the GEC 1997. The recharge to ground water lens = rain fall - interception - evapotranspiration and Ground water utilisation = Evapotranspiration + mixing + pumping + outflow, for water balance study monthly water budgeting or weekly water budgeting gives appropriate value of recharge. The main consumer of ground water is coconut palms because one coconut tree consumes 40 lpd and density of coconut trees is 25 000 - 35000 sq. km but draft through plant is slow, steady and spread uniformly.
The various hydro geological parameters collected during the field study and water level data observed during low and high tide. The pre-monsoon groundwater samples collected from shallow aquifers (dug wells) in polyethylene bottles and analysed for pH, EC, F-, Cl-, NO3-, HCO3-, SO42-, Ca2+, Mg2+, Na+, and K+ as per standard procedures (APHA, 1995) and the in-situ measurements of EC and pH were carried out by using EC and pH meters. The total dissolved solids were estimated by ionic calculation methods. The F-, Cl- and NO3- ions were determined by ion selective electrode; HCO3- by potentiometric titration; SO42- by modified titration method after [6,7]; Ca2+ and Mg2+ in absorption mode while Na+ and K+ in emission mode of the atomic absorption spectrophotometer. The analytical results were tested for accuracy by calculating the Normalized Inorganic Charge Balance [8]. The analytical precision was such that the ion charge balance was little above ±5% for the samples. The quality of the analysis was ensured by standardization using blank, spike, and duplicate samples.
Results and Discussion
The major factors influence the hydrological characteristics of the island are climate, humidity, temperature, evapotranspiration, physiography, hydro geological aspects, soil, vegetation, population geomorphology, aquifer nature and human interference. The major hydrochemistry is discussed below.
Climate, humidity, temperature, evapotranspiration, physiography, hydro geological aspects, soil, vegetation, population, geomorphology, aquifer nature and human interference
The climate of a small island is one of the major influences on the availability of naturally occurring freshwater resources [9]. The rainfall distribution, quantity and its spatial and temporal variations and the evapotranspiration play an important role on the availability of the freshwater resources. The climate of Amini is similar to the climatic conditions of Kerala. March to May is the hottest period of the year. The temperature ranges from 25oC to 35oC and humidity ranging from 70 -76 per cent for most of the year. The average rainfall received is 1600 mm a year. Monsoon prevails here from 15th May to 15th September. The monsoon period raises temperature to the mercury level between 2730 degrees. During the monsoon time, boats are not allowed outside the lagoon because of the violent sea. The presence of the reef maintains calm at the lagoon. The evapotranspiration is very high and most of the months except in high rainfall season it exceeds the rainfall making the water surplus on the negative side.
The entire LD islands lay on the northern edge of the 2500 km long North-South aligned submarine Lakshadweep- Chagos ridge. The island has coarse sandy soil of high porosity and permeability resulting in little or no surface runoff. The vegetation of LD islands consists of coconut trees, bushes and grasses. The Amini Island is a typical atoll and height of the land above msl is about 1-2 m and the coral sands and the coral limestone act as principal aquifers. The Ghyben-Herzberg relation determines the depth of the interface between fresh water and sea water. The water level data of monitoring wells in Amini Island reveals that depth of the wells ranges from 1.6 to 5.5 mbgl and the DTW ranges between 1.2 to 4.8 mbgl whereas diurnal fluctuation in water level due to tides is in the range of 0 to 80 cms. The climate water balance method of recharge estimation widely used for estimating the recharge on small islands Falkland 1992. Human activities influence both the availability of freshwater and water quality.
Hydro geochemical processes
The hydro geochemical processes of ground water and its evolution have been examined. The groundwater of different geological horizons can be classified depending upon their ionic strength of select anions and [10] categorized groundwater based on the meq/l content of Cl- , SO42- , and HCO3- . The water is Normal chloride type if Cl- is <15 meq/l, Normal sulphate type if SO42- is <6 meq/l and Normal bicarbonate type if HCO3- varies between 2 and 7 meq/l. Distribution of groundwater samples based on the Soltan's classification has indicated that majority of the samples are of Normal chloride type, followed by Normal bicarbonate type (values rounded off) and concentration of salts in natural waters depend on the geology, environment, and movement of water [11,12].
The base exchange indices, r1(r1 = Na+ - Cl-/ SO42- meq/ l) and r2 (r2 = K+ + Na+ - Cl- SO42- meq/l) after [13] could be applied for the further classification of groundwater. The groundwater can be grouped as Na+ - HCO3- type if r1 > 1 and Na+- SO4- type with r1 < 1; r2 < 1- groundwater is of deep meteoric percolation type and >1, shallow meteoric percolation type. The groundwater of the area comes Na+- SO4 - type type and deep meteoric percolation type except a few one which is deep meteoric percolation type, chemical analysis data of ground water and other details are compiled (Tables 2 & 3). Hydro chemical evolution study based on Na+ / Cl- molar ratio Na+ / Cl- molar ratio will be 1 if halite dissolution is responsible for sodium dominance in groundwater and >1 if Na+ is released from silicate weathering process [14]. The Na+ / Cl- molar ratio is <1 in many samples of the season, indicating that halite dissolution was the primary process responsible for the release of Na+ into the groundwater.
Hydro chemical facies
The groundwater is further evaluated to determine its facies by plotting the percentages of select chemical constituents in Modified Piper diagram [15]. The plots for the PRS season indicated distribution within the fields 5 and 6 of the Chadha's diagram (Figures 2a & 2b) and are characterized by alkaline earths and weak acidic anions exceed both alkali metals and strong acidic anions, respectively i.e (Ca+Mg)+ (CO3+HCO3)> (Na +K) +( Cl+SO4) and the sample at Ammini - Sidiqui Palli with alkaline earths exceed alkali metals and strong acidic anions exceed weak acidic anions ie (Ca+Mg)>(Na+K)>(Cl+SO4)>(CO3+HCO3). All the water samples of PSM are falling under field 5. All the samples except Ammini - Sidiqui Palli falling under Sub-field I and are Ca-HCO3 Type / recharge type water but that of Ammini - Sidiqui Palli falling under sub-field II Sub-field II and is Ca-Mg-Cl type, reverse ion exchange type. The plots also suggest that among cations Ca+ and Na+ and anions HCO3- and Cl - dominate the ionic concentration in groundwater.
Hydro geochemical evaluation
The high sodium content among cations in the groundwater for the period could be due to halite dissolution which was further enhanced by evaporation and/or evapotranspiration processes. The Na+ /Cl- molar ratio will be 1 if halite dissolution is responsible for sodium dominance in groundwater and >1 if Na+ is released from silicate weathering process Meybeck 1987. The Na+ / Cl- molar ratio is >1 in the samples of water can only evolve to brine rich in NaCl if it encounters highly soluble chloride minerals, typically associated with evaporative deposits / evaporates [16]. As all the groundwater samples of the season with Na+ /Cl- molar ratio less than one or nearer to one, halite dissolution is responsible for sodium dominance in groundwater of the small coral island of Amini, Union Territory of Lakshadweep.
Evolution of groundwater
[17] plots, in which TDS vs Na+ /(Na+ +Ca2+ ) for cations and TDS vs Cl- /(Cl- + HCO3 - ) for anion were plotted to know evolution process of the groundwater and the influence of host rock on ground water chemistry. It is revealed that the samples, occupied the evaporation dominance field. The rock water interaction played minimum role in the evolution of water chemistry, which was dominated by evaporation process during PRM and PSM. The geological location is one of the most important factors affecting the groundwater quality [18].
Chloro alkali indices
The role of aquifer material in the evolution of groundwater chemical composition has been examined by determining the chloro alkali indices for cations (CAI-1) and anions (CAI-2). The CAI-1 [Cl- - (Na+ + K+ )]Cl- and CAI-2 [Cl- - (Na+ + K+ )/(SO42- + HCO3- + CO3 - + NO- )], developed by [19], relate the ion exchange process between ground water and aquifer material. The CAI-1 and CAI-2 are negative in the samples indicating the ion exchange between Na+ -K+ in water and Ca2+ -Mg2+ in rocks [20]. It is imperative to understand the modifications in water chemistry during its movement and residency time for better evaluation of the hydrochemistry of any area more so when different geological formations are involved in a watershed or river basin [21]. As CAI-1 and CAI-2 are negative in the samples of PSM indicating the ion exchange predominance in the study area during post-monsoon and positive values during PRM shown lesser role of ion exchange during pre-monsoon.
Irrigation Suitability: The irrigation suitability of ground water has been attempted based on the study of electrical conductivity (EC), Sodium Adsorption Ratio (SAR), Percent Sodium (% Na), Permeability index (PI), Kelley's Index (KI), Soluble Sodium Percentage (SSP) and Magnesium Ratio (MR) methodologies (Table 4) and Wilcox classification of irrigation water and U S Salinity diagram for irrigation Water, methodology and analytical results are compiled (Table 5).
Electrical conductivity: The EC is a measure of salinity hazard to crops and classified into five major types, as per Raghunath 1987 and that the samples in the study area are under excellent, good, permissible and doubtful categories.
Sodium Absorption Ratio, SAR: The sodium alkali hazard or Sodium Absorption Ratio (SAR) of water is an indicator of sodium hazard in irrigation water Gholami and Srikantaswamy 2009. As per Richard 1954, the computed SAR values show that all the samples are excellent.
Percent Sodium (% Na): The % Na is used to assess the ground water quality, because a higher level of sodium in irrigation water may increase the exchange of sodium content of irrigated soil and affect soil permeability, structure and create toxic condition for plants [22,23] and Todd 1980. Based on the relative proportions of cation concentration, samples come under excellent to good categories and can be used for irrigation on almost all types of soil.
Permeability index, PI: Doneen 1964 has classified the irrigation water quality into three classes based on permeability- class I, II and III and all the samples come under Class II and suitable for irrigation in all types of soil.
Kelley's Index, KI: Kelley 1940 and Paliwal 1967 proposed the suitability of irrigation water quality based on the sodium concentration against calcium and magnesium. The water is suitable for irrigation if KI value is <1; water with KI value of >1 is considered as of poor quality for irrigation and >2 KI makes the water unsuitable for irrigation. Both cation exchange and reverse ion exchange are encouraged by aquifer materials and land use practices, in waterlogged area, marshy/swampy land, creek, mud/tidal flat represented by Montmorillonite clays, which lead to the release of Na or Ca into groundwater and adsorption of Ca or Na, respectively Alison et al. 1992. In all samples KI values are below 1 indicating the water in the study area is suitable for irrigation.
Soluble Sodium Percentage, SSP: Water with less than or equal to 50 SSP value is of good quality and more than 50 is not suitable for irrigation as permeability will be very low [24-26]. In the study area all the water samples with SSP values less than 50.
Magnesium Ratio MR: Water with less than or equal to 50 MR value is of good quality and >50 is considered unsuitable for irrigation Lloyd and Heathcote et al. 1985. In the study area majority of the water samples except one water sample with MR values less than 50.
Conclusion
Hydrogeochemical processes of evolution of groundwater in in the small coral island ofAmini, Union Territory ofLakshadweep, India has been examined. The Amini island is of coral origin (a typical atoll) and ground water occurs under phreatic condition floats as thin lens over saline water and is abstracted mainly by open dug wells. The DWT in the island varies from 1.20 to 4.80 mbgl and depth of the wells varies from less than two meters to about 5.5 mbgl and is controlled by tides. The ground water in the island is generally alkaline ranges from 465 to 999 micromhos / cm at 25 o C. The factors affecting the quality are rainfall, tides, ground water recharge and draft, human and animal wastes, oil spills and fertilizers. The groundwater samples of different areas are of Na- SO4 Type and deep meteoric percolation type. The ground water in the area is mainly Ca-HCO3 type / recharge type water and Ca-Mg-Cl type (reverse ion exchange water type. The hydrochemistry is mainly controlled by evaporation, followed by water-rock interaction and aquifer material.
For more articles in Open Access Journal of Environmental Sciences & Natural Resources please click on: https://juniperpublishers.com/ijesnr/index.php
#Juniper Publishers Indexing Sites List#Juniper Publishers Review#Environmental Chemistry#Oceanology#Microbial Ecology
0 notes
Photo

Support Letter Write a 1200 words support letter to a faculty supervisor , explaining why you should be granted Masters program admission to study environment and sustainability , focusing on ” Catchment hydrochemistry; Ecosystem bio-geochemistry; Climate change; Acidification; Mineral weathering; Biogenic greenhouse gas emissions ”. "Looking for a Similar Assignment? Get Expert Help at an Amazing Discount!"
0 notes
Text
The market prospect of processing granular potash fertilizer is good
The most basic variety of potassium fertilizer is potassium chloride, which is rich in resources, simple in processing technology and high in potassium content. It is the most widely used potassium fertilizer and the basic potassium source of potassium sulfate and potassium nitrate, accounting for about 90% of the total. Potassium sulfate is mainly suitable for use in chlorine resistant crops, which can improve the yield and quality of crops. As an important raw material for the production of compound fertilizer, potassium nitrate is a physiological neutral fertilizer. Therefore, using fertilizer granulator machine to make potash fertilizer into granules is very promising.

Potassium fertilizer manufacturing process
The production of potassium sulfate mainly includes double decomposition and production of potassium sulfate with alunite.
1. The raw material of the double decomposition method is to control the concentration and temperature according to the difference of the solubility of the salt produced by the reaction of sulfate such as mirabilite or ammonium sulfate with potassium chloride at different temperatures, to carry out crystallization separation, so as to obtain potassium sulfate.
2. There are reduction pyrolysis, hydrochemistry and ammonia leaching methods to produce potassium sulfate from alunite. The crude potassium product obtained by reduction pyrolysis has high purity, but the consumption of caustic soda and steam is large, and the economic benefit is poor. The hydrochemistry process has the advantages of short process, low investment, low energy consumption, high yield, small pollution and simple special equipment. It is an advanced alunite processing method at present. The process of ammonia leaching is more complicated, but the potassium sulfate and ammonium sulfate produced by the reaction can not be separated into NPK fertilizer. Therefore, from the perspective of fertilizer, it is more advantageous to produce potassium sulfate by ammonia leaching.
Potash fertilizer manufacturing process meets the needs of the market. Extrusion granulation is the most viable method of making granular potassium fertilizer. At present, the most advanced extrusion granulation technology is the use of double roller fertilizer granulator, which has the characteristics of low energy consumption and low investment. In the granulation process, there is no need to add additional binder. The size range of raw materials used is large, the capacity range of double roller fertilizer granulator is wide, and the economic benefit is good.
0 notes
Text
Temporal and Spatial Variability in Water and Sediment Characteristics of Abule Agege, Abule Eledu, Ogbe, Creeks Adjoining Lagos Lagoon, Nigeria | Chapter 06 | Current Perspectives to Environment and Climate Change Vol. 3
Lagos lagoon is known to contain a vast number of anthropogenic stressors that resulted from the influx of human activities due to the increase in human population, industries and incursion of contaminants from adjoining thus making the ecosystem highly contaminated. The degree of this contamination can be affected by the seasonal variations in time and space. The spatial and temporal variations in the hydrochemistry and sediments characteristics of three (3) Lagos lagoon’s creeks were investigated for six months (June, 2016 to November, 2016). Sub-surface water and sediments were collected with a 1 dm3 water sampler and Van-veen grab, respectively and analyzed. Water temperature, pH, dissolved oxygen and conductivity of the water samples and pH, nutrients (nitrate and phosphate), total organic matter (TOM) and total organic content (TOC), alkalinity, acidity and particle size of the sediment samples were analyzed. The physico-chemical parameters in the water and sediment from the sampled creeks showed none significant differences (P>0.05). The study showed an increasing level of parameters’ rates analyzed, indicating increased contaminants in Abule Eledu and Ogbe creeks. Water temperature maintained a relatively uniform temperature with dissolved oxygen values range of 1.6 to 3.1 mg/L. Conductivity was higher in June to August while high prevalence of nutrients was observed in October and November. Abule Agege and Abule Eledu recorded TOM and TOC that were above 15 mg/kg in June to August while alkalinity and acidity were high in October (6.63 mg/kg) and November (7.72 mg/kg) in the study creeks. The sediment particles size of the creeks ranged from clay, muddy and sandy substratum signifying that they were macro benthic specific. The increase of the parameters’ concentration indicates that the three creeks are highly impacted by anthropogenic stressors, dependent on the source of pollution occurring at the sites as well as controlled by seasonal variations. Continuous monitoring and concerted efforts are needed to be done to prevent future heavy metal pollution, total degradation thereby formulating appropriate protective and conservation measures in the water’s quality of the Lagos lagoon’s creeks.
Author(s) Details
A. P. Onyena Department of Marine Environment and Pollution Control, Faculty of Marine Environmental Management, Nigeria Maritime University, Okerenkoko, Delta State, Nigeria.
C. A. Okoro Department of Marine Sciences, Faculty of Science, University of Lagos, Lagos, Nigeria.
View Book : http://bp.bookpi.org/index.php/bpi/catalog/book/131
0 notes
Text
Hydrochemistry with special to fluoride contamination in groundwater of the Bongo District, Upper East Region, Ghana.
https://link.springer.com/epdf/10.1007/s40899-019-00335-0?author_access_token=EWzbZSsUMlpXPPnWJ0bqV_e4RwlQNchNByi7wbcMAY7J9SvuCHx9_ygPnY9DOncv7fpKWOOtyxXFt5jjG8sxfqi_uCPtvhC09WkTewrgu8qK7NNzOQxW1vc2CvVRPfUARnGUORvbAunTK54Ie0NwEQ%3D%3D
View On WordPress
0 notes
Quote
CSIR-NIO Recruitment 2019 CSIR-NIO Recruitment 2019: Council of Scientific & Industrial Research – National Institute of Oceanography(CSIR-NIO) published an advertisement for the recruitment of 27 candidates for the post of Project Assistant (I/ II/ III) to fill up this vacant posts in Mumbai, Goa, Kochi & Vishakhapatnam. CSIR-NIO arranged a walk-in interview on 12th, 13th, 14th, 17th, 19th & 20th June 2019. Interested eligible candidates may attend an interview at the address mentioned below further necessary details regarding vacancy is mentioned below for more details read the full advertisement or visit the official website. CSIR – राष्ट्रीय ओशनोग्राफी संस्था नि प्रसिद्ध केलेल्या जाहिराती नुसार येथे प्रकल्प सहाय्यक पदाच्या एकूण 27 रिक्त जागांसाठी अर्ज मागविण्यात येत आहेत. इच्छुक आणि पात्र उमेदवारांनी 12, 13, 14, 17, 19 आणि 20 जून 2019 तारखेला मुलाखती करिता हजर राहावे. National Institute of Oceanography Mumbai Vacancy 2019 Details: Name of Recruitment Board: CSIR – National Institute of Oceanography (CSIR – NIO) Name of Vacant Post: Project Assistant (I/ II/ III) Number of Vacant Post: 27 vacancies Selection Method: Walk-in Interview Official Website: www.nio.org Job Location: Mumbai, Goa, Kochi & Vishakhapatnam Application Address: Goa: CSIR-NIO Dona Paula, Goa – 403004 Mumbai: CSIR-NIO Regional Center, Lokhandwala Road 4 Bungalows, Andheri(West), Mumbai-400053 Kochi: CSIR-NIO Regional Center, Dr. Salim Ali Road, Post Box No. 1913, Kochi- 682018 For Visakhapatnam: CSIR-NIO Regional Center, 176, Lawsons Bay Colony, Visakhapatnam – 530017 Interview Dates: 12th, 13th, 14th, 17th, 19th & 20th June 2019 Qualification Requirement for CSIR-NIO Project Assistant Bharti 2019: Vacancy number Post Essential Qualifications Date & Time of interview No of positions Stipend(Rs.pm) MLP1803/1929-19 PA-II B.E / B. Tech in Electronics and Communications / Instrumentation / Electrical and Electronics / Mechatronics or M.Sc. Electronics / Instrumentation / Embedded Systems / Mobile Computing / 12-Jun-2019 10:00 AM 1 25000 GAP3156/1926-19 PA-II B.E / B.Tech ( Electrical & Electronics / Electronics & Communications / Computer / IT / Instrumentation) 13-Jun-2019 10:00 AM 1 25000 GAP3156/1927-19 PA-I Three years diploma in Electronics / Computer / IT Or B.Sc Physics / Electronics/ Computer Science / IT 13-Jun-2019 10:00 AM 1 15000 GAP3223/1931-19 PA-II B.E / B.Tech ( Electrical & Electronics / Electronics & Communications / Computer / IT / Instrumentation ) 13-Jun-2019 10:00 AM 1 25000 GAP3147/1938-19 PA-II BE / BTech Electronics & Communications / Computers / Information Technology / Instrumentation 14-Jun-2019 10:00 AM 1 25000 GAP3201/1936-19 PA-II M.Sc Marine Sciences / Physical Oceanography / Atmospheric sciences 14-Jun-2019 10:00 AM 1 25000 GAP3201/1937-19 PA-II B.E / B.Tech Electronics & Communications / Computers / Information Technology / Instrumentation 14-Jun-2019 10:00 AM 1 25000 GAP3201/1941-19 PA-I B.Sc in Mathematics / Physics / Chemsitry / Computer Science or BCA 14-Jun-2019 10:00 AM 2 15000 GAP3297/1939-19 PA-II M.Sc. Microbiology 17-Jun-2019 10:00 AM 1 25000 GAP3179/1928-19 PA-II B.E / B. Tech Electronics / Electronics and Communications / Instrumentation / Computers / IT or M.Sc. Electronics / Computer Science 19-Jun-2019 10:00 AM 1 25000 SSP3272/1932-19 PA-I Three years Diploma in Information Technology / Electronics & Communication Or B.Sc Computer science / Information Technology 12-Jun-2019 10:00 AM 1 15000 GAP3293/1947-19 PA-I Three Years Diploma in Instrumentation / Electronics or B.Sc in Electronics / Physics / Statistics 12-Jun-2019 10:00 AM 1 15000 GAP3168/1945-19 PA-II M.C.A or B.E / B.Tech in Computer Science or IT 12-Jun-2019 10:00 AM 1 25000 SSP3103/1925-19 PA-II M.Sc Marine Biology / Biological Oceanography / Zoology / Marine Science / Biological Oceanography and Biodiversity / Ocean Science and Technology 13-Jun-2019 10:00 AM 4 25000 GAP3296/1930-19 PA-II M. Sc Hydrochemistry / Marine Chemistry / Chemical Oceanography / Marine Science with specialization in Chemical Oceanography 20-Jun-2019 10:00 AM 2 25000 GAP3195/1933-19 RA-II(Project) Ph.D. in Science with minimum 2 years research experience post PhD 13-Jun-2019 10:00 AM 1 38000 GAP3195/1934-19 PA-II M.Sc. in Marine Chemistry /Applied Chemistry / Analytical Chemistry / Organic Chemistry / Inorganic Chemistry / Hydro Chemistry 13-Jun-2019 10:00 AM 3 25000 GAP3195/1935-19 PA-I B.Sc Microbiology / Botany / Zoology 13-Jun-2019 10:00 AM 1 15000 GAP3195/1944-19 PA-II M.Sc Instrumentation 13-Jun-2019 02:00 PM 2 25000 How To Apply For CSIR-NIO Recruitment 2019: To apply for this vacancy candidates need to prepare an application including all the necessary details Attach self-attested copies of testimonial documents and certificates Attend interview on given date and time mentioned above at the given address At the time of the interview bring original copies of testimonial documents and certificates Important Links for CSIR-NIO Recruitment 2019: Official Website Full Advertisement
http://www.mahaparikshagov.in/2019/05/csir-nio-recruitment-2019-wwwnioorg.html
#CSIR-NIO Recruitment 2019 www.nio.orgSEO#Local SEO training http://www.mahaparikshagov.in/2019/05/c
0 notes
Text
Oceanography & Hydrochemistry in the Great Australian Bight
Dispatch: Welcome on board RV Investigator, Australia’s blue water research vessel. We’re currently sailing from Hobart to Fremantle as part of the 2019 Collaborative Australian Postgraduate Sea Training Alliance Network (CAPSTAN) voyage, a hands-on training experience for marine science students.
The post Oceanography & Hydrochemistry in the Great Australian Bight appeared first on AGU Blogosphere.
Oceanography & Hydrochemistry in the Great Australian Bight published first on https://triviaqaweb.tumblr.com/
0 notes
Text
The effects of antecedent moisture conditions on the relationship of hydrology to hydrochemistry in a small fo
http://dlvr.it/Pr1RN1
0 notes
Text
Juniper Publishers- Open Access Journal of Environmental Sciences & Natural Resources

Characterization, Classification and Evaluation of Groundwater in and around the Open Cast Mining of Clay Deposits of Thonnakkal, South India
Authored by Joji VS
Abstract
Characterization, classification, and evaluation of groundwater in and around the open cast mining of clay deposits of Thonnakkal, South India have been studied. The impact studied include changes happened to the qualitative and quantitative aspects related to the ground water. It is found that changes occurred in depth to the water level (decline of water level), lowering pH of ground water in the area, high sulfate and trace metals in the ground water. The groundwater of the area comes under Na+-HCO3- type and shallow meteoric percolation type and the scatter diagram of Ca2+ +Mg2+ vs HCO3 - + SO42- revealed that majority of samples in the season and different geological terrain fall below the equiline, indicating that silicate weathering was the primary process involved in the evolution of groundwater. The rock -water interaction played a major role in the evolution of water chemistry, which was partly by an evaporation process. The qualitative analysis of ground-water samples found that many samples are unfit for both domestic and irrigational purposes.
Keywords: Hydrochemistry; pH; Electrical conductivity; Suitability; Calcium concentration; Total hardness
Introduction
Population explosion coupled with industrialization over the past many years has increased a tremendous pressure on our natural resources resulting in rapid exploitation of our mineral resources. Local residents of the study area alleging that the mining continues to contaminate water resources and leads to an increase in the number of cancer patients in the locality. Detailed hydrogeological survey was carried out to evaluate the ground water scenario of the clay mining areas located in Azhoor, Mangalapuram and Andoorkonam Panchayaths of Thiruvananthapuram district to know the general ground water scenario of ground water reservoirs both quantitative and qualitative aspects viz. pre and post monsoon depth to water level and its fluctuation, flow direction, quality deterioration of sub-surface water, EIA of open cast clay mining on ground water conditions.
Mining hydrogeological studies were carried out by many authors viz. Stoertz et al. [1] on long-term water quality trends at a sealed, partially flooded underground mine, Alexander [2] on environmental impact of deep opencast limestone mine in Laegerdorf, Northern Germany, Suraj et al. [3] on GIS approaches for sustainable mining of tile and brick clay, Parappukara Panchayath, Thrissur district, Kerala, Anon [4] on environmental impact of clay mining for tile and brick industries in Thrissur District, Kerala using Remote Sensing and GIS and many others. The purpose of this investigation is to examine the various changes occurred in the hydrogeological ecosystem in and around Thonnakkal, Thiruvananthapuram District, South India due to of open cast mining of clay deposits.
Study Area
The Thonnakkal belt of the Kaolinised china clay is situated at Melthonnackal (8o38”05”:76°51’22”), Azhoor (8°38’31”:76°49’29”), Pallippuram (8°35’30”: 76° 51’30”), Chilampil (8°37’00”:76°50’00”) and Sasthavattam (8°38’45”:76°49’30”) areas. The English Indian Clays Ltd., Thiruvananthapuram operates a China clay mine since 1966 around Thiruvananthapuram, where the processing plant produces several grades of refined Kaolin, Metakaolin and Calcined Kaolin (Clays) for the paper, paint, rubber, plastic, fiberglass, cement and ultra-marine industries and the plant has a capacity of 190, 000 metric tons per annum and is the largest in South East Asia. The area lies between 76o 43' 21” and 77 o 12' North latitudes and 8 o 32' East and 8 o 53’34” longitudes and falls in parts of Survey of India Toposheet D/14 (Figure 1). Physiographically the study area falls mainly in the lowland (elevation between msl and 7.6 magl) and midland (elevation between 7.6 and 76 magl) areas of Trivandrum district of Kerala. The main drainages in the study area are the Vamanapuram River and its tributaries. The coastal alluvial soil, riverine alluvial soil and lateritic soil are the soils of the study area. The land use of the area includes open scrub, mixed crops, rubber plantations, paddy fields and water bodies. The study area is generally occupied by Precambrian crystalline rock overlain unconformably by a thick sequence of the Warkali Formation of Miocene age. The regional geological setting of the area is compiled (Table 1).
Materials and Methods
The Survey of India Topographical Map (Scale 1:50,000 of 1967-1968), Geo-coded imageries of Indian Remote Sensing Satellite (IRS -IC LISS III False Color Composite - FCC of 7.02.1997 of 1:50,000 scale) and Aerial photographs (of 1990 of 1:15,000 scale) are used for the preparation of base map of the study area. Major ground control points (GCPs) were identified and verified during field truth, which resulted in modifying the units and their boundaries. Map Info 6.5 has been used in GIS studies. The scanned maps were digitized and edited using Map Info 6.5. During editing the segment checking like intersection, self-overlap and dead-end corrections were carried out. Projection and polygonization of units followed the editing. After polygonization, annotations were given for different polygons and the maps were subjected for further analysis.
There were 10 groundwater samples collected from open dug wells. The pre monsoon samples (First week of May, 2014) were collected in polyethylene bottles in 2014 and analysed for pH, EC, F-, Cl-, NO3-, HCO3-, SO42-, Ca2+, Mg2+, Na+, and K+ as per standard procedures (APHA, 1995) and the in situ measurement of electrical conductivity and pH carried out by using EC and pH meters. The total dissolved solids were estimated by ionic calculation methods. The F-, Cl- and NO3- ions were determined by ion selective electrode; HCO3- by potentiometric titration; SO42- by modified titration method after Fritz and Yamamura (1955) and Haartz et al. [5] Ca2+ and Mg2+ in absorption mode while Na+ and K+ in emission mode of the atomic absorption spectrophotometer. Chemical standards and blanks were run and replicate analysis of each sample was done for each parameter and variations were ±5 - 10%. The analytical results are shown as Tables 2a & 2b.
Results and Discussion
Aquifer Systems
Groundwater occurs in all the geological formations ranging in age from Archean to the Recent and water bearing formations in the area are crystalline formations, Laterite, Tertiaries and Alluvium. The crystalline formations consist of Khondalites and Gneisses. The water bearing properties of crystalline formations which lack primary porosity depend on the extent of development of secondary inter granular porosity either through weathering or fracturing. Groundwater generally occurs under phreatic conditions in the weathered mantle and under semi confined conditions in the fissured and fractured zones at deeper levels. The thickness of weathered zone ranges less than 1m to more than 15m and the depth to the water level from 1015 mbgl.
Major portion of the study area is covered by laterites, the thickness of which increases from East to West. This includes the Tertiary laterites and the laterite occurring as highly weathered product of crystallines capping it. In crystalline formations the water bearing properties depend on the extent of development of secondary porosity either through weathering or fracturing. These aquifers are highly heterogeneous in nature due to variation in lithology and texture even within short distance. The in situ laterite development by the weathering of Khondalites in the study area is rich in clay content due to higher amount of feldspathic minerals in the parent rock. These aquifers mainly occur along the valleys and topographic lows and possess high porosity. The depth of the well in laterite ranges from 8 to 27 mbgl and depth to the water level is more than 10 m. By the onset of monsoon, the water level becomes shallow due to the porous nature of the aquifer. The thickness of weathered zones ranges from less than 1 m to more than 10 m. The yield of the well ranges from 0.5 to 6 m3/day and the recovery co-efficient ranges from 0.00096 to 0.025/min.
Groundwater occurs under phreatic condition in the shallow zone in Tertiary aquifers. Of the three tertiary formations (Warkali, Quilon and Vaikom), the Warkali beds are encountered in this area. Thick deposits of kaolin having an overall thickness of 25 to 45 m are encountered at Melthonnakkal and Pallipuram forming part of the Warkali Formation belonging to the Tertiary sequence in southern Kerala. The sedimentary clays are composed mainly of kaolinite, quartz and gibbsite. These sedimentary kaolins (hydrated aluminous silicate, Al2O3. SiO2.2H2O) are considered to have been formed by intense tropical weathering of the khondalites, and subsequently transported and deposited with high organic input into lakes near the weathering crust over the basement rock. Besides, the surficial parts of the sedimentary deposits are extensively lateralised with the formation of Goethite and Hematite by tropical weathering processes.
The thickness of the clay bed varies from 25 to 45 mbgl and is encountered in almost all the bore wells drilled in the area. The aquifer properties of clay include Grain size (< 0.002), Porosity (33-60), Specific Yield (2 to 5%) and Permeability (10-9 to 10-6 K cm/s). These properties of clay suggest that it is a poor aquifer and by large an aquitard with very low groundwater potential. The field survey in the area reveals that groundwater occurs under phreatic conditions in these Tertiary sediments. The premonsoon depth to water level ranges from 3.1 mbgl to 27.75 m bgl.
Maps depicting the depth to water level in the study area during April 2010 (Premonsoon) and November 2010 (Post monsoon) based on the water level data of the key wells established and the observation wells of CGWB are shown in Figures 2 & 3 respectively. During April 2010, the depth to water levels in wells of the study area ranged from 3.1 mbgl to 27.75 mbgl and the depth of well ranges from 6.0 to 29 mbgl. The deepest water levels were observed in Mangalapuram, Sasthanagar, Chilambil, Sasthavattom, Pallipuram, Karamoode areas. During November 2010, the depth to water levels in wells of the study area ranged from 3.04 to 25.9 mbgl. The deepest water levels were observed in Mangalapuram, Sasthanagar, Chilambil, Sasthavattom, Pallipuram, Karamoode areas. The yield of wells ranges from 0.5m3/day to 10m3/day and the recovery co-efficient ranges from .00056 to 0.0087/min.
Alluvium is the most potential phreatic aquifer in the study area and is extensively developed by dug wells and filter point wells. The depth to water level in this formation ranges from 1.89 to 3.04 m. The depth of wells ranges from 3.66 to 7.55 mbgl. The yield of the shallow dug wells ranges from 15 to 50 m3/day and the recovery co-efficient varies between 0.0093 and 0.094/ min.
Water Table Elevation
The elevation of ground water table during the period ranged from 7.75 above msl to 63.44 above msl. The flow lines from east to west indicate that the general hydrogeological regime is undisturbed except around the clay mines. A ground water trough has been formed around the clay mines indicating a reversal of hydraulic gradient. The deeper water level in the vicinity of the clay mines is the localized effect of the mines. The general hydraulic gradient is towards west in general. The closely spaced contours around the clay mines is indicative of a potential recharge zone underlying clay formation which is an aquitard inhibits any sort of recharge except for the construction of injection wells to penetrate the weathered zone encountered at a depth below 40 metres. The hydrogeological map and water table elevation contour maps of the area have been prepared (Figures 4 & 5).
Analysis of Hydrograph
An analysis of depth to the water level has been carried out to understand the influence of lithology on water level. The hydrograph of the well at Korani tapping the Laterites show a decline trend in the premonsoon and post monsoon periods (Figure 6).
Hydrochemistry and Hydro Geochemical processes of Ground Water
The groundwater samples of different geological terrains can be classified on the basis of ionic strength of Cl- , SO42-, and HCO3- and the water is Normal Chloride type if Cl- is <15 meq/l, Normal Sulphate type if SO42- is <6 meq/l and Normal Bicarbonate type if HCO3- varies between 2 and 7 meq/l. The ground water samples of the study area are of Normal Chloride type and Normal Sulphate type and concentration of salts in natural waters depends on the geology, environment, and movement of water Raghunath [6], Gopinath Seralathan [7](Tables 3 & 4).
The Base Exchange indices, r1 (r1 = Na+ - Cl-/ SO42- meq/ l) and meteoric genesis index (r2) (r2 = K+ + Na+ - Cl- SO42-, meq/l) can be used for classifying groundwater Soltan [8]. The groundwater can be grouped as Na+ - HCO3- type if r1 > 1 and Na+- SO4 - type with r1 < 1; r2 < 1- groundwater is of deep meteoric percolation type and >1, shallow meteoric percolation type. The groundwater samples of the Thonnakkal area comes under Na+-HCO3- type and shallow meteoric percolation type except one which is deep meteoric percolation type and the details are plotted (Figures 7 & 8).
Red -Good correlation (r=> 0.6)
Yellow - Poor correlation (r = x003;0)
Hydro geochemical Evaluation of ground water
The Na+ / Cl- molar ratio will be 1 if halite dissolution is responsible for sodium dominance in groundwater and >1 if Na+ is released from silicate weathering process Meybeck [8,9]. As the Na+ / Cl- molar ratio is <1 in all samples of the season except at sample location No.2 (Abandoned Quarry), reveals that halite dissolution was the primary process responsible for the release of Na+ into the groundwater.
The study of Ca2+ / Mg2+ ratio reveals Ca2+ - Mg2+ contribution in the groundwater. The Ca2+ / Mg2+ ratio of 1 indicates dissolution of dolomite and of >2 an effect of silicate minerals May & Loucks [10]. Majority of the samples with Ca2+ / Mg2+ ratio between 1 and <2, indicating dolomite dissolution responsible for Ca2+ - Mg2+ contribution. The scatter diagram of Ca2+ + Mg2+ vs. HCO3- + SO42- (Figure 9) shows that majority of samples fall below the equiline, indicating that silicate weathering played primary role in the evolution of groundwater Datta & Tyagi [11]. If bicarbonate and sulphate are dominating than calcium and magnesium, it reflects silicate weathering was dominating and, therefore, was responsible for the increase in the concentration of HCO3- in groundwater, Elango et al. [12], Elango & Kannan [13].
Evolution of Groundwater and Hydro Chemical Facies
(# Concentration, meq/l)
The evolution process of the groundwater tested by plotting TDS vs. Na+ /(Na+ +Ca2+ ) for cations and TDS vs. Cl- /(Cl- + HCO3- ) for anion Gibbs [14] and revealed that rock - water interaction played significant role in the hydrochemistry, which was partly influenced by evaporation process (Figures10a & 10b). It is also to be noted that geological location is another factor controlling quality variation Beck et al. [15]. The evolution of groundwater chemical composition further tested by calculating chloroalkali indices for cations (CAI-1- Cl- - (Na+ + K+) Cl-) and anions (CAI-2 - Cl- - (Na+ + K+)/(SO42- + HCO3- + CO3- + NO-) after Schoeller [16]. The chloroalkali indices are negative indicating the predominance of ion exchange between Na+ -K+ in water and Ca2+ -Mg2+ in rocks McIntosh & Walter [17]. A close perusal of hydro chemical facies and water type study Chadha (1999) is compiled (Table 5).
Statistical analysis - correlation coefficient
The relationship between two variables is the correlation coefficient which shows how one variable predicts the other. Associated with correlation coefficient r, the multiple correlations, which are the percentages of variance in the dependent variable, explained collectively by all of the independent variables. A high correlation coefficient (near 1 or -1) means a good relationship between two variables, and a correlation coefficient around zero means no relationship. Positive values of r indicate a positive relationship while negative values indicate an inverse relationship and are compiled (Table 6). The correlation coefficient is a measure of the extent to which two measurement variables vary together. Unlike the covariance, the correlation coefficient is scaled so that its value is independent of the units in which the two measurement variables are expressed. The value of any correlation coefficient must be between -1 and +1 inclusive. The parameters having good and poor correlations have been attempted.
Suitability of ground water for Irrigation purposes
The irrigation suitability of ground water has been tested by using various methods (Table 5), methodology and analytical results are compiled (Tables 6 & 7). The electrical conductivity (EC) is a measure of salinity hazard to crops and there are of five major types for irrigational purposes (Raghunath (1987) and the samples in the area varies from excellent (50%) to good categories. The Sodium Absorption Ratio, SAR is an indicator of sodium hazard in irrigation water Gholami & Srikantaswamy [18] and all the water samples are excellent category Richard [19]. The per cent sodium (% Na) in irrigation water may increase the exchange of sodium content and affect soil permeability, structure and create toxic condition for plants Bangar et al. [20], Durfer & Backer [21] and Todd [22]. The % Na study revealed that samples come under permissible (70%), doubtful (20%) and other unsuitable categories and cannot be used for irrigation on almost all types of soil. On the basis of permeability index, PI Doneen [23] irrigation water quality classes include class I, II and III and all the samples come under Class II and suitable for irrigation in all types of soil.
The water is suitable for irrigation if Kelley's Index, KI value is <1; water with KI value of >1 is considered as of poor quality for irrigation and >2 KI makes the water unsuitable for irrigation Kelley [24]. In the study area about 70% KI values are above 1, and the water is poor quality for irrigation and 30% unsuitable for irrigation in all types of soil. Water with less than or equal to 50 Soluble Sodium Percentage, SSP value is of good quality and more than 50 is not suitable for irrigation as permeability will be very low. In the study area 60% of the water samples with SSP values more than 50. As per Lloyd and Heathcoat [25] water with less than or equal to 50 Magnesium ratio, MR value is of good quality and >50 is considered unsuitable for irrigation and except two water samples others with MR value more than 50 and are unsuitable for irrigation.
Variations in pH, Sulphate and Heavy Metal concentrations
The open cast mining in the area resulted rapid circulation of oxygen and water into the deep crust, where high concentrations of sulphides (Pyrite) exist. Thus, sulphides undergo the reaction.
2FeS2 + 2H2O + 7O2 = 2Fe2+ + 4SO42- + 4H+
This reaction developed low pH, high sulphate and trace metal concentrations. The chemical analysis of the ground water samples collected in the study area show low pH side indicating a mine environment similar to the above mentioned category [26-29]. The Pyrite present in the deep clay horizon gets exposed resulting in its oxidation and the end products being sulphate and hydrogen ions. The pyrite is present in the depth range of 35 to 40 mbgl. Hence ground water from deep wells puncturing this horizon generally has low pH value. The chemical data shows that the ground water collected from Mangalapuram, Chilambil, Sasthanagar and Valikonam have low pH [30-33]. It was also observed that the depth of these wells were also very deep (> 25 mbgl). The pH contours are depicted (Figure 11).
The general chemical reactions involved are
2FeS2 + 2H2O + 7O2 = 2Fe2+ + 4SO42- + 4H+
4Fe2+ + O2+ 4H+= 4Fe3+ + 2H2O
4Fe3+ + 12H2O = 4Fe (OH)3 + 12H+
FeS2 + 14Fe3+ + 8H2O = 15Fe2+ + 2SO42- + 16H+
The reactions indicate release of H+ which lowers the pH of the ground water of the area and produces sulphate ions. The field truth verification in the open cast mining areas of clay mining revealed the marked changes in the hydrogeological, agricultural and socio-economic environments of the area. The various impact include decline of depth to the water table in wells or changes in hydro geologic conditions in areas adjacent to the mining sites, thixotropism resulted land subsidence, sliding / slumping of walls of mines and well collapse, reversal of hydraulic gradient, inducing increased flow of ground water from the aquifer into the mines, causing deepening of water levels, drying up of shallow wells and reduction in the sustainability of ground water abstraction structures. Water quality deterioration resulted in the lowering of pH values and many areas water unsuitable for cultivation, reclamation and conversion of these wetlands to non-agricultural purposes has resulted transformation of this ecosystem, changes in landscape, land stability and soil loss especially areas of Mangalapuram, Andoorkonam, Azhoor and Sasthavattom and atmospheric pollution in the area.
Conclusion
Characterization, classification and evaluation of groundwater in and around the Open cast mining of clay deposits of Thonnakkal, South India have been examined. The general hydrogeological scenario has been identified during the study The opencast mining of clay deposits resulted lowering of depth to the water level, topographic changes and general changes in biodiversity. The groundwater of the area comes under Na+- HCO3- type and shallow meteoric percolation type except a few one which is deep meteoric percolation type. The rock water interaction played major role in the evolution of water chemistry, which was partly by evaporation process. The mining resulted low pH, high sulphate and trace metal concentrations in the ground water in and around the Thonnakkal clay belt. It is seen that the water quality is degrading very fast in the study area.
For more Open Access Journals in Juniper Publishers please click on: https://juniperpublishers.business.site/ For more articles in Open Access Journal of Environmental Sciences & Natural Resources please click on: https://juniperpublishers.com/ijesnr/ To know more about Open Access Journals Publishers To read more…Fulltext please click on: https://juniperpublishers.com/ijesnr/IJESNR.MS.ID.555630.php
#Juniper Publishers group#Juniper Publishers Review#Environmental Chemistry#Human Ecology#Mineralogy#Soil Science#Waste Management and Disposal'
0 notes