#hematology analyzer calibration
Explore tagged Tumblr posts
Text
Hematology Analyzer
Labtro Hematology Analyzer offers 60 tests/hour, supports whole/prediluted blood, and analyzes 22 parameters with 3-part WBC differentials. It has a wide linearity range, high accuracy, LCD display. Features include ports,built-in printer,self-checks,clog prevention, and eco-friendly reagents.

0 notes
Text
Automatic Hematology Analyzer LMAHA-A101

Labmate Automatic Hematology Analyzer provides reliable blood analysis with a 60 tests/hr throughput. It features advanced optical and impedance technologies for accurate cell counting and differentiation. With a large touchscreen, automatic calibration, and comprehensive hematology parameters.
0 notes
Text
Exploring the Role of a Certified Medical Laboratory Technician in Modern Healthcare
In today's fast-evolving healthcare industry, the role of allied healthcare professionals has become indispensable. Among these professionals, Certified Medical Laboratory Technicians (CMLTs) play a critical role in diagnosing, monitoring, and treating patients. As the backbone of diagnostic services, these technicians work closely with doctors and medical teams, providing essential data that influences nearly every aspect of patient care.
But what exactly does a CMLT do, and how does this role impact modern healthcare? In this article, we’ll explore the responsibilities of a CMLT, the growing demand for skilled professionals in this field, and how pursuing a CMLT course can lead to a rewarding career in healthcare.
The Role of a Certified Medical Laboratory Technician (CMLT)
A Certified Medical Laboratory Technician is responsible for performing a wide range of tests and procedures that aid in the detection, diagnosis, and treatment of diseases. These professionals handle everything from routine blood tests and tissue analysis to more complex diagnostics like microbial identification and hormone testing.
Key Responsibilities of a CMLT:
Sample Collection and Preparation CMLTs collect blood, urine, and tissue samples from patients. They are trained to follow precise protocols to ensure that samples are collected, labeled, and stored properly to prevent contamination or degradation.
Running Diagnostic Tests Once samples are collected, CMLTs use sophisticated lab equipment to perform diagnostic tests. These may include hematology, immunology, chemistry, and microbiology tests that are crucial for accurate diagnoses.
Analyzing Results After running tests, CMLTs analyze the results and prepare reports. These reports are shared with doctors who rely on the data to make informed decisions about patient care.
Maintaining Equipment Ensuring that lab equipment is properly maintained and calibrated is another important duty. A malfunctioning machine can lead to inaccurate test results, which could have serious consequences for patients.
Collaborating with Medical Teams CMLTs often collaborate with doctors, nurses, and other healthcare professionals, providing them with the necessary data to make decisions on patient treatment plans.
The Impact of CMLTs on Modern Healthcare
Medical Laboratory Technicians play a crucial role in improving patient outcomes. By ensuring that accurate and timely diagnostic information is available, they help doctors detect diseases in their early stages, monitor ongoing treatments, and assess the effectiveness of interventions.
For example, CMLTs are vital in managing chronic conditions such as diabetes, heart disease, and cancer. By analyzing blood sugar levels, cholesterol, and cancer markers, they provide essential data that helps medical teams track a patient’s progress and adjust treatment plans as needed.
In the context of infectious diseases, CMLTs are on the front lines, identifying pathogens and helping to contain outbreaks. During the COVID-19 pandemic, the role of laboratory technicians became even more visible, as they were responsible for conducting PCR tests that were critical in diagnosing and controlling the virus.
The Growing Demand for CMLTs
The demand for Certified Medical Laboratory Technicians is on the rise. According to recent reports, the healthcare industry is facing a shortage of skilled lab technicians, driven by an aging population, an increase in chronic diseases, and the need for more advanced diagnostic services.
As healthcare facilities expand and adopt new technologies, the need for CMLTs with specialized training in modern diagnostic equipment and techniques continues to grow. This makes a career in medical laboratory technology not only stable but also full of growth potential.
Why Pursue a CMLT Course?
For those looking to enter the healthcare field, becoming a Certified Medical Laboratory Technician offers numerous advantages. It is a career that combines hands-on work with cutting-edge technology, providing an opportunity to make a direct impact on patient care. Additionally, it offers a diverse range of work environments, including hospitals, research labs, diagnostic centers, and even forensic laboratories.
Scope College: Your Path to a Career as a Certified Medical Laboratory Technician
If you’re interested in pursuing a career as a CMLT, SCOPE College offers an excellent Certificate Course in Medical Laboratory Technician. This course is designed to provide students with the technical skills and practical knowledge needed to excel in modern laboratories.
At SCOPE College, students receive hands-on training in real-world laboratory settings, learning the latest techniques in diagnostic testing and laboratory management. With a curriculum that covers key areas like hematology, microbiology, and pathology, graduates are well-prepared to enter the workforce and contribute to the future of healthcare.
For more information about the CMLT course at SCOPE College, visit the course page.
Career Opportunities for CMLTs
After completing a CMLT course, graduates have a wide range of career opportunities. Certified Medical Laboratory Technicians can find employment in:
Hospitals Many hospitals rely on in-house labs to handle diagnostic testing. CMLTs working in hospitals play a direct role in patient care by delivering timely and accurate test results.
Diagnostic Centers These facilities specialize in providing laboratory services to outpatient clinics and other healthcare providers. CMLTs in diagnostic centers perform routine tests and may also specialize in certain areas like pathology or immunology.
Research Laboratories For those interested in scientific research, working in a research lab offers the opportunity to be at the forefront of medical discoveries. CMLTs working in research labs help develop new diagnostic techniques, drugs, and treatments.
Forensic Laboratories In forensic science, CMLTs assist in criminal investigations by analyzing biological evidence. This is a unique career path that combines medical knowledge with legal work.
Why Choose SCOPE College for Your CMLT Course?
At SCOPE College, we believe in providing students with the skills and confidence to succeed in the healthcare field. Our CMLT course not only offers comprehensive training in laboratory techniques but also emphasizes the importance of ethical practices, safety protocols, and quality control in the medical laboratory setting.
As part of our commitment to excellence, we offer state-of-the-art laboratory facilities, experienced faculty, and strong industry connections that ensure our graduates are highly employable. Ready to take the next step in your career? Enroll Now in our CMLT course and start your journey toward becoming a vital member of the healthcare team.
To learn more about the opportunities available at SCOPE College, don’t hesitate to contact us today. Our team is ready to assist you with any questions and guide you through the enrollment process.
Conclusion
Certified Medical Laboratory Technicians are an integral part of modern healthcare. Their work behind the scenes ensures that doctors have the information they need to make life-saving decisions. If you are passionate about healthcare and enjoy working with technology, becoming a CMLT could be the perfect career for you.
With the increasing demand for skilled technicians and the rise of advanced diagnostic techniques, now is the perfect time to pursue a CMLT course. SCOPE College is here to help you achieve your goals, offering comprehensive training and support every step of the way.
Ready to start your career as a Certified Medical Laboratory Technician? Enroll today and make a difference in the future of healthcare!
0 notes
Text
What is a Lab Tech/Lab Scientist?
To put it simply, a lab tech is the person who runs the testing on specimens that are sent down to the lab. Testing is often performed on blood, urine, stool, swabs, and tissue samples.
Lab techs can work in hospitals, clinics, reference labs, blood banks, and research labs.
•chemistry
There are many areas/specialties within the lab including:
•hematology
•immunology
•microbiology
•coagulation
•blood bank
•molecular biology
•cytology
•histology
Although many techs are generalists and can work in many of these areas.
What do lab techs do most of the time?
•perform patient testing
•perform analyzer and equipment maintenence
•run calibrations and quality controls on analyzers
•troubleshooting issues with analyzers and test results
•phlebotomy
•working together with phlebotomists, lab assistants, physicians, and other healthcare personnel to provide quality patient care (teamwork makes the dream work!)
Lab Tech (MLT) vs Lab Scientist (MLS)
Although the term 'lab tech' can be used as a general term to refer to both of these, there is a difference.
A medical lab technician (MLT) requires a two-year degree and a MLT certification.
A medical lab scientist (MLS) requires a four-year degree and a MLS certification.
MLTs and MLSs will generally perform the same job, except an MLS can perform more of the complex testing that may require more involvement.
0 notes
Text
ICHOR Biologics Recruitment ICHOR Biologics Pvt. Ltd. is seeking experienced QC Biologics professionals to join the team. they are offering positions across various sub-departments, requiring qualifications in MSc Biotech/Biochemistry with 1-9 years of experience. This is an excellent opportunity for those looking to advance their careers in a leading biotechnology company. Join us at ICHOR Biologics Pvt. Ltd. and contribute to cutting-edge research and development. About ICHOR Biologics ICHOR Biologics Pvt. Ltd. is at the forefront of biotechnology innovation, dedicated to advancing the field of biologics through rigorous research and development. Our team is committed to improving healthcare outcomes by developing high-quality biologics that meet global standards. We offer a collaborative and supportive work environment where professionals can thrive and make significant contributions to the industry. Positions Available 1. QC Biologics Professionals Department: QC Biologics Sub-Department: Quality Management System (QMS) Qualifications: MSc Biotech/Biochemistry Experience Required: 6 to 9 years Responsibilities: Oversee quality management systems. Ensure compliance with regulatory standards. Conduct audits and implement corrective actions. 2. QC Biologics Professionals - Immuno Chemical Sub-Department: Immuno Chemical Qualifications: MSc Biotech/Biochemistry Experience Required: 1 to 3 years Responsibilities: Perform immunochemical assays. Maintain and calibrate laboratory equipment. Analyze and interpret data accurately. Experience Required: 3 to 6 years Responsibilities: Lead immunochemical testing projects. Train junior staff on immunoassay techniques. Develop and validate new methods. 3. QC Biologics Professionals - Hematology Sub-Department: Hematology Qualifications: MSc Biotech/Biochemistry Experience Required: 3 to 6 years Responsibilities: Conduct hematology tests and analyses. Manage laboratory operations and ensure safety compliance. Collaborate with cross-functional teams for research projects. 4. QC Biologics Professionals - Instrumentation Sub-Department: Instrumentation Qualifications: MSc Biotech/Biochemistry Experience Required: 1 to 3 years Responsibilities: Operate and maintain laboratory instruments. Troubleshoot and resolve technical issues. Ensure accurate data collection and documentation. Experience Required: 3 to 6 years Responsibilities: Oversee instrumentation operations. Develop and optimize protocols for instrument use. Provide technical support and training to staff. 5. QC Biologics Professionals - Stability Sub-Department: Stability Qualifications: MSc Biotech/Biochemistry Experience Required: 1 to 6 years Responsibilities: Monitor and evaluate the stability of biologics. Conduct stability studies and report findings. Ensure compliance with regulatory requirements. 6. QC Biologics Professionals - Physico/Sample Management Sub-Department: Physico/Sample Management Qualifications: MSc Biotech/Biochemistry Experience Required: 1 to 3 years Responsibilities: Manage sample collection and storage. Perform physicochemical analyses. Maintain accurate records and inventory. How to Apply Interested candidates are invited to send their resumes to [email protected]. Please mention the sub-department and relevant experience in your application. [caption id="attachment_80578" align="aligncenter" width="930"] ICHOR Biologics Pvt ltd Recruitment - Job vacancies[/caption]
0 notes
Text
FETMX8MP-C Industrial Computer on Module: High-Performance Drive Automatic Biochemical Analyzer
Biochemical analyzer, abbreviated as bioanalyzer, primarily provides various clinical biochemical, hematological, and immunological testing projects for all levels of hospitals in the field of laboratory medicine.
It is used to test clinical biochemistry indicators such as liver function, kidney function, blood lipids, diabetes, infection, rheumatology, and immunology. It provides important scientific evidence for the diagnosis, treatment, and prevention of diseases, and is an essential monitoring medical equipment in hospitals.

Biochemical analyzers can be divided into fully automatic instruments and semi-automatic instruments, of which semi-automatic instruments are smaller in size, simple in structure, cheaper in price, and have higher application demand in the current market; fully automatic biochemical analyzers have less error in the results due to the analytical process without manual operation, and this type of instrument usually has an automatic report of abnormalities. It can automatically calibrate the working state, reducing the system error.
According to the functional structure, the automatic biochemical analyzer is divided into an optical system, a constant temperature system, sample reaction stirring technology and probe technology, and a computer control part.
With the enhanced performance of the ARM CPU-based, the computer control part of the automatic biochemistry analyzer has also started to switch to ARM Main Control Board to Folinx Embedded provide customers with Cortex-A53 platform master control Solution FETMX8MP-C industrial grade Computer on Module.
The Computer on Module is based on NXP i.MX8M Plus processor development, with strong video processing capabilities and, a smooth operation experience, to help users develop powerful performance, beautiful interface devices.
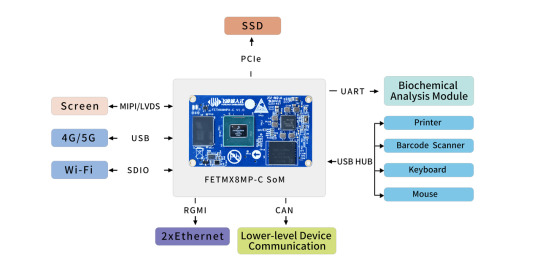
High performance: The CPU adopts 1.6GHz quad-core 64-bit Cortex-A53 architecture with a neural processing unit (NPU), offering a maximum operating speed of up to 2.3 TOPS. It comes with a standard configuration of 4GB DDR4 RAM + 16GB eMMC, providing sufficient hardware resources.
Multiple Display Interfaces: CPU supports MIPI, LVDS and HDMI display natively, with the highest resolution up to 4K;
Powerful Image recognition: Dual hardware ISP, resolution up to 12 MP, input rate up to 375M pixels/second, bringing significant improvement to the image effect;
Abundant high-speed interface resources: 2 Gigabit Ethernet, 2 dual-purpose USB 3.0/2.0, 1 PCIe Gen 3, 2 SDIO 3.0, 2 CAN (including 1 CAN-FD), bringing more possibilities for high-speed signal transmission;
Software equipped with: Linux5.4.70 and Andriod 10 operating system, the driver is perfect, and the system source code is open, giving you more support;
Stable supply: full industrial design, full consideration of Medical application scenarios, 15 years + life cycle, free from your worries.
Originally published at www.forlinx.net.
0 notes
Text
What is a Medical Laboratory Technologist? Roles and Responsibilities
Medical Laboratory Technologists play a crucial role in the healthcare industry, working behind the scenes to perform laboratory tests and analyze patient samples. They provide vital information to healthcare professionals, aiding in the diagnosis, treatment, and prevention of diseases. In this article, we will explore the roles and responsibilities of Medical Laboratory Technologists and shed light on the importance of their contributions to the healthcare field.
Understanding the Medical Laboratory Technologist Role: Medical Laboratory Technologists, also known as Medical Laboratory Scientists or Clinical Laboratory Scientists, are highly skilled professionals who perform a wide range of laboratory tests on various body fluids, tissues, and specimens. They work in medical laboratories, hospitals, research facilities, and other healthcare settings, utilizing advanced laboratory equipment and techniques to generate accurate and reliable test results.
Roles and Responsibilities of Medical Laboratory Technologists:
Specimen Collection and Processing:Medical Laboratory Technologists are responsible for collecting and processing patient samples, such as blood, urine, tissue, and other bodily fluids. They follow strict protocols to ensure proper handling, labeling, and storage of specimens to maintain the integrity of the samples.
2.Laboratory Testing:Medical Laboratory Technologists conduct a diverse array of laboratory tests, including hematology, clinical chemistry, microbiology, immunology, serology, and molecular diagnostics. They use sophisticated instruments and analytical techniques to analyze samples, identify abnormalities, and interpret test results.
3. Quality Control and Assurance: Medical Laboratory Technologists adhere to strict quality control measures to ensure the accuracy and reliability of laboratory test results. They perform routine maintenance and calibration of laboratory equipment, participate in proficiency testing programs, and follow standardized protocols to maintain high standards of quality assurance.
4.Data Analysis and Interpretation: Medical Laboratory Technologists analyze test results, interpret findings, and generate comprehensive reports for healthcare professionals. Their accurate and detailed reports assist physicians in making informed diagnoses, monitoring treatment effectiveness, and developing appropriate patient care plans.
5. Laboratory Safety and Compliance:Medical Laboratory Technologists prioritize safety protocols in the laboratory to protect themselves, colleagues, and patients. They follow strict guidelines for handling hazardous substances, disposing of biohazardous waste, and maintaining a clean and sterile work environment. They also ensure compliance with regulatory standards set by Canadian health authorities and professional organizations.
6. Medical Laboratory Technicians: In addition to Medical Laboratory Technologists, the healthcare field also relies on Medical Laboratory Technicians. Medical Laboratory Technicians work alongside Medical Laboratory Technologists, supporting their work by performing routine laboratory tests and assisting with specimen collection and processing. While Medical Laboratory Technologists require advanced education and training, individuals interested in pursuing a career as a Medical Laboratory Technician can enroll in lab technician courses that provide the necessary knowledge and skills.
7. Anderson College's Medical Laboratory Technologist Program: Anderson College offers a comprehensive Medical Laboratory Technologist program that prepares students for this challenging and rewarding career. The program, available through their website, combines theoretical knowledge with hands-on practical training. Students learn essential laboratory techniques, gain proficiency in various laboratory disciplines, and develop critical thinking and problem-solving skills necessary for success in the field. Graduates of the program are eligible to write the national certification examination conducted by the Canadian Society for Medical Laboratory Science (CSMLS), further demonstrating their competency and commitment to professional excellence.
Medical Laboratory Technologists play a vital role in the healthcare system, contributing to patient care through accurate laboratory testing and analysis. Their expertise, attention to detail, and commitment to quality assurance are invaluable in providing reliable diagnostic information. If you have a passion for science, a strong attention to detail, and a desire to contribute to the healthcare field, consider exploring a career as a Medical Laboratory Technologist. Anderson College's Medical Laboratory Technologist program and lab technician courses can provide you with the necessary knowledge and skills to embark on this fulfilling profession.
For more information about Anderson College's Medical Laboratory Technologist program and lab technician courses, visit our website or book a virtual appointment with an Admissions Advisor.
0 notes
Text
Hematology Analyzers and Reagents Market - Quality Results, Every Time: Hematology Analyzers and Reagents You Can Trust
Newark, New Castle, USA: The “Hematology Analyzers and Reagents Market” provides a value chain analysis of revenue for the anticipated period from 2023 to 2031. The report will include a full and comprehensive analysis of the business operations of all market leaders in this industry, as well as their in-depth market research, historical market development, and information about their market competitors
This latest report researches the industry structure, sales, revenue, price and gross margin. Major producers’ production locations, market shares, industry ranking and profiles are presented. The primary and secondary research is done in order to access up-to-date government regulations, market information and industry data. Data were collected from the Hematology Analyzers and Reagents manufacturers, distributors, end users, industry associations, governments’ industry bureaus, industry publications, industry experts, third party database, and our in-house databases.
Get a Free Sample PDF of the Report – https://www.growthplusreports.com/inquiry/request-sample/hematology-analyzers-and-reagents-market/8127
Key Players in the Hematology Analyzers and Reagents Market : –
Sysmex Corporation
Siemens Healthineers AG
Abbott Laboratories
Bio-Rad Laboratories, Inc.
Nihon Kohden Corporation
ERBA Diagnostics Mannheim GmbH
Sysmex Corporation
Beckman Coulter, Inc.
Boule Diagnostics AB
F. Hoffmann-La Roche AG
Ortho Clinical Diagnostics
Horiba Ltd.
Market Segmentation:
GLOBAL HEMATOLOGY ANALYZERS and REAGENTS MARKET - ANALYSIS & FORECAST, BY PRODUCTS AND SERVICES
Instruments
5 Part and 6 Part Fully Automated Hematology Analyzers
3-part Fully Automated Hematology Analyzers
Point-of-care Testing Hematology Analyzers
Semi-automated Hematology Analyzers
Reagents and Consumables
Hematology Reagents
Slide Stainers/Makers
Controls and Calibrators
Consumables
Services
GLOBAL HEMATOLOGY ANALYZERS AND REAGENTS MARKET - ANALYSIS & FORECAST, BY APPLICATION
Hemorrhagic Conditions
Infection-related Conditions
Immune System-related Conditions
Blood Cancer
Anemia
Others
GLOBAL HEMATOLOGY ANALYZERS AND REAGENTS MARKET - ANALYSIS & FORECAST, BY USAGE TYPE
Standalone Analyzers
Point-of-care Analyzers
GLOBAL HEMATOLOGY ANALYZERS AND REAGENTS MARKET - ANALYSIS & FORECAST, BY END-USER
Commercial Service Providers
Hospital Laboratories
Government Reference Laboratories
Research and Academic Institutes
Market segment by Region/Country including : –
-North America (United States, Canada and Mexico) -Europe (Germany, UK, France, Italy, Russia and Spain etc.) -Asia-Pacific (China, Japan, Korea, India, Australia and Southeast Asia etc.) -South America (Brazil, Argentina and Colombia etc.) -Middle East and Africa (South Africa, UAE and Saudi Arabia etc.)
This report also includes a discussion of the major players across each regional Hematology Analyzers and Reagents market. Further, it explains the major drivers and regional dynamics of the global Hematology Analyzers and Reagents market and current trends within the industry.
Request for customization in Report: https://www.growthplusreports.com/inquiry/customization/hematology-analyzers-and-reagents-market/8127
Key Benefits for Industry Participants and Stakeholders One can find in-depth research data and industry trends of the Hematology Analyzers and Reagents Market Research. The report offers details on potential investment opportunities, including those that are local and sector-specific that may benefit stakeholders and members of the industry. One can gain a thorough grasp of market dynamics by looking at prices as well as the activities of producers and consumers. With the use of market research, which will assist in discovering and visualizing new market participants as well as their portfolios, will be better able to make decisions and create an efficient counter strategy to maximize market advantage.
COVID 19 Impact Analysis
The Hematology Analyzers and Reagents Market Research Reports include a thorough discussion of the coronavirus’s effects in addition to the major market trends. When considering the impact of the COVID-19 on the industry, insights, analysis, projections, and predictions are given in the report study.
Given the breadth of the pandemic’s disruption, it is evident that the current depression is fundamentally different from previous recessions. Due to the sudden drop in demand and growing unemployment, the business climate will alter. In this uncomfortable environment, businesses may carve new roads by embracing novel ideas like ”advance toward localization, cash conservation, supply chain resilience, and innovation.”
Hematology Analyzers and Reagents Market TOC: https://www.growthplusreports.com/report/toc/hematology-analyzers-and-reagents-market/8127
the market share and rank (in volume and value), competitor ecosystem, new product development, expansion, and acquisition.
This report stays updated with novel technology integration, features, and the latest developments in the market
This report helps stakeholders to understand the COVID-19 and Russia-Ukraine War Influence on the Hematology Analyzers and Reagents industry.
This report helps stakeholders to gain insights into which regions to target globally
This report helps stakeholders to gain insights into the end-user perception concerning the adoption of Hematology Analyzers and Reagents .
This report helps stakeholders to identify some of the key players in the market and understand their valuable contribution.
QUICK BUY: https://www.growthplusreports.com/checkout-8127
Contact Us:
Manan Sethi Director, Market Insights Email: [email protected] Phone no: +1 888 550 5009 Web: https://www.growthplusreports.com/
About Us
Growth Plus Reports is part of GRG Health, a global healthcare knowledge service company. We are proud members of EPhMRA (European Pharmaceutical Marketing Research Association).
Growth Plus portfolio of services draws on our core capabilities of secondary & primary research, market modelling & forecasting, benchmarking, analysis and strategy formulation to help clients create scalable, ground-breaking solutions that prepare them for future growth and success.
We were awarded by the prestigious CEO Magazine as "Most Innovative Healthcare Market Research Company in 2020."
0 notes
Text
The HemoCue® Hb 201+ System
Calibrated against the International Council for Standardization in Hematology (ICSH) reference method, the HemoCue® Hb 201+ System provides lab-quality results in CLIA-waived settings. Each point on the diagram represents an actual hemoglobin test result. The closer to the line, the more closely related the result is to the “gold standard” reference method. For more info, please visit: Hematology Analyzer & Systems
0 notes
Text
The sum Will be the Earnings of a Medical Laboratory Pc proficient?
That they investigate framework substance natural materials in a very lab setting for you to track down parasitic organic entities, microorganisms and furthermore different microbes which may be creating your individual's illness. Lab tests are for the most part achieved all through toxicology, hematology, organic chemistry and science, microbial science, alongside immunology in order to build up alongside deal with individuals. Medical services lab specialists work in an assortment of wellbeing highlights including specialist's workplaces, places of business including experts, examination research centers, prosperity medical clinics, veterinary facility labs, medical care investigation labs, alongside biotechnology firms.
The ordinary morning of a medical care lab pc expert will start by essentially beginning alongside calibrating research instruments. At the entire hours, their obligations fuse storing up alongside gaining body, construction, pee, and furthermore other framework substance organic materials through individuals. That they should make these sort of natural materials relating to plan appraisal by involving a magnifying lens too as electronic analyzer, that can supply benefits knowing pretty much any irregularities just as illnesses. The eventual outcomes will be put right PC or PC relating to significantly more examination utilizing value diagrams, stock outline, stages, and numerous others. Various natural materials should forever be situated under controlled conditions relating to possible utilize. All through prep relating to via the post office, that they clear your examination instruments towards the end in the first part of the day.
Your control of a medical services lab pc expert may be satisfying in addition to requesting also. Someone should be equipped for work coupled adequately utilizing different other clinical stars, which incorporate individuals alongside experts. Another Health care lab pc proficient must be impacted person alongside thorough thinking about that you'll track down the top ascribes on this work Most information got should consent to your information security make a move alongside impacted individual tact train.
Training and getting the hang of, Coaching alongside Qualifications
A task up-and-comer need to first utilize an optional school level in order to begin another medical care lab pc proficient programming. People are by and large and afterward compelled to exhaustive basically the Associate's sum, just like a 2-year training and learning programming provided by a neighborhood advanced education, geek establishment, proficient organization, just as college or school. Also there are 1-year capability bundles offered by means of specialist's workplaces for individuals at this point all through comparable work regions, including nursing jobs.EN1276
Medical services lab specialists are for the most part bound inside assignments they may direct, like they won't lead refined symptomatic tests since they can't contain exactly the same kind of serious schooling and learning just like a medical services lab technologist. Pursuing considerably more training and figuring out how to become medical care lab technologist can be consequently enthusiastically prescribed relating to lab specialists.
Training bundles aim in supporting understudies get the required ability alongside information they should type in your workforce. People experience active train all through obvious lab changes.
Medical services lab specialists tend not to call for pretty much any capabilities for most cases be that as it may, many cases alongside business bosses may require another declaration in front of an individual might prepare. Licensed specialists most frequently have more noteworthy business gives alongside bigger profit. Medical services lab specialists that should be certify need to extensive your endorsed training and learning programming in front of that they produce your authorizing assessment. Continuing with training and learning advances are fundamental yearly to deal with capabilities.
1 note
·
View note
Text
Hematology Diagnostics Market Overview , Trends , Share
Hematology Diagnostics Market Overview
The global hematology diagnostics market trends is profiled in detail in the latest research report from Market Research Future (MRFR). The global hematology diagnostics market’s historical growth trajectory is studied in detail in the report. The market’s development over the historical review period is analyzed in detail in the report. The leading drivers and restraints on the global hematology diagnostics market are also studied in detail in the report. The major drivers and restraints affecting the global hematology diagnostics market are studied with a view to providing readers with a detailed overview of what’s causing the market to rise and what is holding the market back. This provides the readers with a complete overview of the market’s major drivers and restraints. The leading players operating in the global hematology diagnostics market are also profiled in the report to give readers an accurate idea of the competitive landscape of the market.
Hematology diagnostics are diagnostics related to blood disorders. The growing prevalence of hematological conditions is the major driver for the global hematology diagnostics market. In addition to the growing prevalence, the growing awareness about hematological conditions is also a major driver for the global hematology diagnostics market. The increasing prevalence and awareness about hematological diseases have led to a growing demand for hematological testing, leading to a growing demand from the hematology diagnostics market. The increasing prevalence of leukemia, lymphoma and other blood disorders is likely to be a major driver for the hematology diagnostics market. The rising prevalence of genetic blood disorders is also a major driver for the global hematology diagnostics market.
Get Premium Research Report, Inclusive of COVID-19 Impact Analysis, Find more information @ https://www.marketresearchfuture.com/reports/hematology-diagnostics-market-6262
The rising volume of blood donation is also likely to be a major driver for the global hematology diagnostics market over the forecast period. While blood donations are a highly valuable resource and a public good, they need to be checked to eliminate the chances of disease transmission. This has driven up the demand for hematology diagnostics. The increasing demand for automated hematology analyzers is also likely to be a major driver for the global hematology diagnostics market over the forecast period. Automated hematology analyzers are likely to remain a major part of the global hematology diagnostics market over the forecast period due to their convenience and ease of operation. On the other hand, the high cost of hematology diagnostics and lack of proper insurance coverage for hematology diagnostics are the major restraints on the global hematology diagnostics market over the forecast period.
Hematology Diagnostics Market Competitive Leaderboard:
Leading players in the global hematology diagnostics market include Sysmex, Danaher, Nihon Kohden, Siemens, Abbott Laboratories, Boule Diagnostics, HORIBA, Bio-Rad Laboratories, BioSystems, Diatron, Drew Scientific, EKF Diagnostics, Mindray, Ortho Clinical Diagnostics, and Roche. The development of automated hematology analyzers is likely to remain a key strategy for players in the global hematology diagnostics market, as automated hematology analyzers are likely to rise in demand over the forecast period.
Hematology Diagnostics Market Segmentation:
The global hematology diagnostics market is segmented on the basis of product type, test type, end use, and region.
By product type, the global hematology diagnostics market is segmented into hematology analyzers, flow cytometers, consumables, and others. The hematology analyzers segment is further sub-segmented into fully automated and semi-automated hematology analyzers, while the consumables segment is further sub-segmented into reagents, stains, and controls and calibrators. The fully automated hematology analyzers segment is expected to be a rapidly growing segment of the global hematology diagnostics market over the forecast period.
By test type, the global hematology diagnostics market is segmented into anemia, hemoglobinopathy, leukemia/lymphoma phenotyping, hematology pathophysiology, complete blood count, and others.
By end user, the global hematology diagnostics market is segmented into hospitals and clinics, ambulatory surgical centers, diagnostic laboratories, academic institutes, and others. The hospitals and clinics segment holds the largest share in the global hematology diagnostics market over the forecast period.
Hematology Diagnostics Market Regional Analysis:
The regional analysis of the hematology diagnostics market states that the North American region dominates the market due to the mounting incidence of blood diseases. The presence of the primary market players within the region is influencing the market growth. Factors such as growing healthcare expenditure, technological improvements within the population are driving the market development in this region. The European region is the subsequent market contender in the global hematology diagnostics market due to the growing hematology research within this region. The growing hematology research and government initiatives within this region are influencing the development of the hematology diagnostic market in this region.
The Asia Pacific region was recognized as the quickest rising region for the hematology diagnostics market globally in 2017. The market is projected to observe considerable development due to the intensifying commonness of blood disorders and genetic diseases. The predisposition of individuals in the region to genetic disorders due to marriages to blood relatives or marrying within the same community, ethnicity, caste, etc. The Middle Eastern and African region controls the smallest stake in the hematology diagnostics market globally owing to the incidence of strict government policies and deprived economies. Though, the market is projected to observe growth owing to increased investments by private market players in the Middle East. Moreover, the increasing incidence of genetic blood disorders and expanding government initiatives to develop the healthcare sector within the region is expected to contribute to the market’s growth.
0 notes
Photo

Dr. Kent Hoskins
Study finds racial disparities in breast cancer prognosis testing
Black women have higher recurrence and mortality rates than non-Hispanic white women for certain types of breast cancer, according to a University of Illinois Chicago researcher’s study published recently in JAMA Oncology.
Dr. Kent Hoskins, associate professor in the UIC College of Medicine’s division of hematology/oncology, and co-leader of the Breast Cancer Research group in the University of Illinois Cancer Center, published the study, “Association of race/ethnicity and the 21-gene Recurrence Score with breast cancer-specific mortality among US women” in the Jan. 21 online issue.
Hoskins and the research team sought to discover if breast cancer-specific mortality among women with estrogen receptor-positive, axillary node-negative breast cancer differs by race within risk categories defined by the Oncotype Recurrence Score, or RS, which is a genomic test that analyzes the activity of a group of genes that can affect how a cancer is likely to behave and respond to treatment. They also wanted to find out if the prognostic accuracy of the RS differs by race.
“Using data from the national SEER registry that included more than 70,000 patients across the U.S., we found there was a much higher mortality rate for African American women with the most common subtype of breast cancer event when they are diagnosed at an early stage,” Hoskins said.
Women with hormone-dependent breast cancer typically have a favorable prognosis, but Hoskins found that even after adjusting for age at diagnosis, tumor stage and treatment, there is still a significant mortality gap between Black and non-Hispanic white women with axillary node-negative, hormone-dependent tumors that have a comparable RS.
The research also found that Black women are more likely to have a high-risk RS, indicating that Black women disproportionately develop biologically aggressive tumors. According to Hoskins, the underlying cause of this is unknown, but this is an active area of research among UI Cancer Center investigators. The Oncotype test also had lower prognostic accuracy in Black women, indicating that genomic tumor tests used to identify candidates for chemotherapy may require re-calibration in populations with greater racial and ethnic diversity, according to Hoskins’ research.
The paper’s findings are based on a retrospective, population-based cohort study database that contained data on women 18 years and older who were diagnosed with first primary stages I-III, estrogen receptor-positive breast cancer between 2004-2015.
“This study highlights a widespread problem that may contribute to health disparities,” Hoskins said. “Research to develop and validate new medical tests frequently have inadequate representation of individuals from racial/ethnic minority groups. Because of this, new tests may be less accurate in individuals who belong to minority groups.”
Because the RS is used by oncologists to make informed treatment decisions on the need for chemotherapy, Hoskins’ study points out that the Oncotype RS model needs to be recalibrated to reflect racial differences.
The same lack of adequate representation of individuals from racial/ethnic minority groups plagues clinical trials of new cancer treatments, according to Hoskins.
“Our study is just one more example of how exclusion of minority patients from research can lead to inequities in health outcomes,” he said.
While the study concluded that there is a disparity in mortality among Black women with early-stage breast cancer, further research is needed to understand the role of tumor biology and of structural violence in this disparity. Structural violence refers to systematic ways in which social structures harm or otherwise disadvantage individuals. Hoskins said the next step is to look at population health data and molecular data to determine the mechanisms driving the differences.
“We really think there is an intersection between social determinants of health and tumor biology and that is what we are trying to understand with follow-up studies,” Hoskins said.
In addition to Hoskins, the research team included Dr. Oana Danciu and Gregory Calip, who are both UI Cancer Center members, and Dr. Naomi Ko of Boston University.
#breast cancer#breast cancer disparities#breast cancer testing#breast cancer prognosis#social determinants of health#Dr. Kent Hoskins#JAMA Oncology
0 notes
Text
Global In-Vitro Diagnostics (IVD) Market Key Geographies, Key Players and Target Audience 2020-2026
Summary - A new market study, titled “Global In-Vitro Diagnostics (IVD) Market: Industry Analysis & Outlook (2017-2021)”has been featured on WiseGuyReports.
Diagnosis is the identification of the nature and cause of a certain phenomenon. It involves two different types of testing method: in vivo and in vitro diagnostics. In-vitro diagnostics (IVDs) tests are defined as tests executed outside a patient’s body which put analytical instruments, devices, reagents, calibrators and systems to use for checking of health status or diagnosing diseases. Purpose for doing the IVD test is to prevent, effectively cure and mitigate any kind of risk and for post-treatment intensive care diseases and infections. IVD products are precisely designed and produced for collecting samples derived from the human body and scrutinizing them outside the human body.
Also Read: https://www.newsmaker.com.au/news/378806/global-invitro-diagnostics-ivd-market-2020-analysis-opportunities-and-forecast-to-2024#.X1jEYClR3IU
The global IVD market is segmented on the basis of product, technology used and test location. On the basis of product, this market can be divided into three sub segments; reagents, analytical instruments and accessory products. By technologies employed, it can be segmented into various sub segments; immunochemistry, SMBG, POCT, molecular diagnostics, clinical chemistry, microbiology, hematology and others. Further, depending upon test location, the market can be divided into three sub segments; labs, POC and self-test.
Growth of the global IVD market is driven by several factors including rapid occurrence of infectious diseases, increasing diabetic patient base, and surge in incidences of chronic ailments. Further, growing geriatric population, rising GDP per capita and increasing healthcare expenditure per capita will act as a catalyst for market growth. Owing to the suitable demographical changes and rising disposable income coupled with improving basic infrastructure, developing regions such as Asia-Pacific and Latin America are expected to experience robust growth. However, the market growth prospects could be hindered by lack of customer adoption, lack of proper facilities and infrastructure and strict government regulations.
The report “Global In-Vitro Diagnostics (IVD) Market: Industry Analysis & Outlook (2017-2021)” analyzes the development of this market, with focus on the North America, EMEA, Asia-Pacific and Latin America markets. The major trends, growth drivers as well as issues faced by the market are discussed in detail in this report. The four major players: Siemens AG., Roche Holdings AG., Abbott Laboratories and Danaher Corp. are being profiled along with their key financials and strategies for growth.
FOR MORE DETAILS: https://www.wiseguyreports.com/reports/2379202-global-in-vitro-diagnostics-ivd-market-industry-analysis-outlook-2017-2021
About Us:
Wise Guy Reports is part of the Wise Guy Research Consultants Pvt. Ltd. and offers premium progressive statistical surveying, market research reports, analysis & forecast data for industries and governments around the globe.
Contact Us:
NORAH TRENT
Ph: +162-825-80070 (US)
Ph: +44 203 500 2763 (UK)
0 notes
Text
North America In Vitro Diagnostics Market size Rear Excessive Growth during 2025
[116 pages report] This market research report includes a detailed segmentation of the North America in vitro diagnostics market – By Technology Types (Clinical Chemistry, Molecular Diagnostics, Immunoassay, Clinical Microbiology, Coagulation, Hematology, and Others), By Applications (Oncology, Infectious Disease, Diabetes, Cardiology, Nephrology, Autoimmune Disease, and Others), By End-users (Hospitals, Laboratories, Home Care, Academic & Research Institutes, and Others), and By Country (US and Others).
Get PDF Sample Copy of this Report to understand the structure of the complete report: (Including Full TOC, List of Tables & Figures, Chart) @ https://www.trendsmarketresearch.com/report/sample/9818
Overview of the In Vitro Diagnostics Market Research
Infoholic’s market research report predicts that the North America In Vitro Diagnostics market will grow at a CAGR of 5.17% during the forecast period 2019–2025. The market has witnessed steady growth in the past few years, and the advancements in technology with the introduction of innovative products have increased the adoption of in vitro diagnostics products in the market. The market is fueled by the increasing incidence of lifestyle and chronic diseases, rising adoption of point-of-care testing (POCT), the upsurge in the biomarker-based tests, and growing significance of companion diagnostics.
The market continues to grow, and in vitro diagnostics is one of the most widely used techniques for screening, diagnosis, treatment, and monitoring purposes. The field is getting revolutionized with the advancement in technology. Vendors are focusing on new product launches, approvals, and targeting toward end-user’s perspective. The market generates revenue from the key players operating in this field, and few of them include Abbott Laboratories, F. Hoffmann-La Roche, Thermo Fisher Scientific, Danaher Corporation, and Sysmex Corporation.
According to Infoholic Research analysis, the US accounted for the largest share of the North America in vitro diagnostics market in 2018 and “others” segment is expected to grow a high CAGR during the forecast period. According to the estimation of the National Cancer Institute, in the US, around 1.6 million new cases of cancer were diagnosed, and 595,690 people have died due to cancer in 2016. In 2016, Canada had an estimated 202,400 new cases of cancer and nearly 78,000 deaths according to the Canadian Cancer Society. Lifestyle-related diseases such as diabetes and heart diseases are becoming a major threat to the population across the world with 425 million people suffering from diabetes. Cardiovascular disease (CVD) accounts for approximately 800,000 deaths in the US and on an average, one person dies from CVD every 40 seconds in the US. Favorable reimbursement policies, the presence of dominant market vendors in the country, increased awareness among patients, availability of government funds, and increasing adoption of molecular diagnostics make the US a dominant shareholder in the market.
In Vitro Diagnostics Market by Technology Types
Clinical Chemistry
Molecular Diagnostics
Immunoassay
Clinical Microbiology
Coagulation
Hematology
Others
In 2018, the clinical chemistry segment occupied the largest share, and molecular diagnostics segment is expected to grow at a high CAGR during the forecast period. Clinical chemistry includes metabolic panel, electrolyte panel, liver panel, lipid profile, renal profile, thyroid function panel, and specialty chemistry tests. Increased technological advancements such as next-generation sequencing & polymerase chain reaction and growing number of strategic deals are likely to propel the molecular diagnostics segment during the forecast period.
North America In Vitro Diagnostics Market by Applications
Oncology
Infectious Disease
Diabetes
Cardiology
Nephrology
Autoimmune Disease
Others
The infectious disease segment occupied a major market share in 2018, and the oncology segment is predicted to hold a significant share during the forecast period. Increase in the prevalence of the disease such as tuberculosis & pneumonia and growing healthcare awareness among the population make the segment a dominant shareholder.
North America In Vitro Diagnostics Market by End-users
Hospitals
Laboratories
Home Care
Academic & Research Institutes
Others
Hospitals occupied a significant market share in 2018, and hospitals and laboratory segments are expected to dominate the market for the next few years.
In Vitro Diagnostics Market by Country
US
Others
The US occupied a significant market share in 2018, and the “others” segment is expected to grow at a high CAGR during the forecast period. The increased technological advancement and higher GDP of the nation make “others”, that includes Canada and Mexico, the fastest growing segment during the forecast period.
In Vitro Diagnostics Market Research Competitive Analysis – The in vitro diagnostics market has massive growth opportunities in the North American region. The advancements of technology will increase competition among vendors. The diagnostics and biotechnology continuously focus on the market due to an increase in lifestyle and chronic diseases. This has resulted in approvals and collaborations related to in-vitro diagnosis in recent years. For instance, Roche launched VENTANA pan-TRK (EPR17341) Assay to detect tropomyosin receptor kinase (TRK) to diagnose multiple solid tumor types at the end of 2018. FDA has approved F1CDx test to identify genetic alterations in tumors in December 2017. The genomic test can identify cancer-related alterations in 324 genes in any type of solid tumor. FDA approved Ortho Clinical Diagnostics VITROS Immunodiagnostic Products HIV Combo Reagent Pack and Calibrator for use on the VITROS 5600 Integrated System in 2018. This 4th generation test can detect both HIV-1 and HIV-2 antibodies as well as the p24 antigen, which provides a shorter diagnostic window compared to 3rd generation assays. In late 2018, Precision for Medicine acquired ApoCell, a next-generation lab specializing in the identification and analysis of biomarkers. In addition, other leading players are focusing on hugely investing in R&D activities to develop new products to attain the maximum share in the market.
Key Vendors
Abbott Laboratories
Hoffmann-La Roche (Roche)
Thermo Fisher Scientific Inc.
Danaher Corporation
Sysmex Corporation
bioMerieux S.A.
Becton Dickinson and Company
Bio-Rad Laboratories
Qiagen N.V.
Siemens Healthineers
Ortho Clinical Diagnostics (Carlyle Group)
Key Competitive Facts
The market is highly competitive with all the players competing to gain market shares. Intense competition, rapid advancements in technology, frequent changes in government policies, and the prices are key factors that confront the market.
The requirement of high initial investment, implementation, and maintenance cost in the market are also limiting the entry of new players.
Pre-Book Right New for Exclusive Analyst Support @ https://www.trendsmarketresearch.com/report/analyst/9818
Benefits – The report provides complete details about the usage and adoption rate of in vitro diagnostic products. Thus, the key stakeholders can know about the major trends, drivers, investments, vertical player’s initiatives, and government initiatives towards the tests in the upcoming years along with details of the pureplay companies entering the market. Moreover, the report provides details about the major challenges that are going to impact the market growth. Additionally, the report gives complete details about the key business opportunities to key stakeholders in order to expand their business and capture the revenue in specific verticals, and to analyze before investing or expanding the business in this market.
Key Takeaways
Understanding the potential market opportunity with precise market size and forecast data.
A detailed market analysis focusing on the growth of the in vitro diagnostics
Factors influencing the growth of the in vitro diagnostics
In-depth competitive analysis of dominant and pure-play vendors.
Key insights related to major segments of the in vitro diagnostics
Latest market trend analysis impacting the buying behavior of the consumers.
0 notes
Photo

Alifax Test1 THL Automatischer ESR Analysator Marke Alifax Typ Test 1 THL Seriennummer 1302 Jahr 2006 Leistung 0,25 kW Elektrischer Anschluss 230 V Gesamtabmessung L 550 mm Gesamtabmessung B 500 mm Gesamtabmessung H 580 mm Beschreibung inkl. Dokumentation Vollautomatischer Analysator für die Bestimmung des ESR Direktes Laden vom Original-Zellblut-Zählergestell AUTOMATISCHES WASCHSYSTEM 175 μl EDTA-Blutprobe pro Test Nur 800 μl Probe in der Röhre angefordert Kapazität bis zu 60 Proben Direkte Beladung von CBC-Regalen Durchsatz bis zu 150 Proben/Stunde Interner Strichcode-Leser Bidirektionale Verbindung zum LIS Alifax ESR analyzers are the only ones capable to provide results in 20 seconds by measuring red blood cells aggregation overcoming the variables and limitations of the sedimentation method also listed in the CLSI document. Results in 20 seconds related to red cells aggregation First result available after 5 minutes from analysis start No reagents required Results expressed in mm/h High correlation with the Westergren method No influence of low hematocrit levels Use of the same CBC tubes 175 μl EDTA blood sample per test Only 800 μl sample requested in the tube Capacity up to 60 samples Throughput up to 180 samples/hour Latex Calibration & Controls Smart cards Thermostated at 37°C Mixing cycles in accordance with CLSI requirements Internal bar code reader Connection to LIS Simplified needle replacement Thermal printer Complete integration in the hematology laboratory Test 1 - THL-BLC-SDL-YDL-MDL-XDL Technical Features Power supply: 115-260 VAC 10%, 50/60 Hz Power consumption: 150 VA max. Operative temperature: from +10 to +30 °C Size: 510x560x600 mm Weight: 45 Kg In/ out specifications: two RS232 serial ports Bar code reader:internal Gebrauchter funktionsfähiger Zustand. Altersbedingter Verschleiß. Herkunft: Krankenhaus Hinweis: Bei Fragen können Sie uns anhand den Daten im eBay Impressum kontaktieren. Ihr Med-Tec24 Team. Note: If you have any questions, you can contact us using the data in the eBay imprint. Your Med-Tec24 team. (hier: Würzburg) https://www.instagram.com/p/B_xGcgOKlDN/?igshid=dhmek17bblvf
0 notes
Text
Hematology Analyzers for Clinical Laboratories

Hematology analyzers are indispensable for many laboratory procedures and applications and assist in the diagnosis and treatment of various diseases. The instrument helps in the analysis of blood cells and count, platelet enumeration, and much more. Leading lab equipment neogen indonesia suppliers offer various models for clinical laboratories as well as professional guidance to help them choose the right one for their needs.
Advanced Features
A quality hematology analyzer comes with many advanced features:
Capability to store months of patient data in diverse forms Reduced operational errors Results within seconds/improved turnaround time Real-time QC information Simple operation Automated quality assurance functions Time saving capabilities Quick and accurate results Automatic cleaning modes User-friendly software interface Throughput capacity of at least 50 samples per hour Minimal maintenance Improved detection capabilities Advanced data management systems thermogravimetric analyzer tga Point-of-care testing (POCT) and near patient testing (NPT) using portable benchtop hematology analyzers has become necessary in many medical fields. As a result leading manufacturers have come out with compact and user-friendly instruments suitable for use in these settings.
Popular Hematology Analyzer Models
Beckman Coulter, Abbott Diagnostics, Horiba ABX, Siemens, QBC Diagnostics and Sysmex Corporation are among the leading manufacturers of quality analyzers. Popular models include the Beckman Coulter AcT Diff 2, Abbott Cell Dyn 1800, ABX Micros 60, QBC STAR Tubes, Capillary Tubes, Siemens Advia 2120 and Sysmex K-1000 Hematology Analyzer.
Budget constraints cause most laboratories to refrain from investing in costly, new lab equipment. Established lab equipment suppliers offer them solutions such as the option to purchase refurbished instruments or convenient reagent rental programs to deal with this. They are purchased, refurbished to work like new, recertified and put up for sale at a price much lower than the original. Clinical labs that opt for a reagent rental plan can acquire the equipment without having to pay for it upfront. Payment needs to be made in monthly installments. This Cost per Agreement (CPT) plan indicates a minimum number of tests per month that the laboratory has to pay.
Locate a Reliable Lab Equipment Store
The challenge lies in finding a reliable supplier. Look for a dealer that offers new and recertified hematology analyzers for clinical laboratories at competitive prices as well as customer-friendly purchase options. A reliable online store would also ensure a timely supply of the specially formulated reagents, controls and calibrators necessary to ensure accurate and prompt test results. It is also important that the supplier you choose can provide timely and efficient technical support.
0 notes