#dr reddy's laboratories share
Text
#dr reddy share price analysis#stock news india#directusinvestments#mohit munjal youtube#youtube shorts#Dr Reddy's Laboratories Limited#dr reddy's laboratories ltd share#dr reddy's laboratories ltd share news#dr reddy's laboratories share#dr reddy's laboratories shares#dr. reddy's laboratories ltd#dr reddy share latest news#dr reddy share news#dr reddy lab share latest news#dr. reddy's laboratories#dr reddy share analysis#Shorts#mohitmunjalyoutube#service
0 notes
Text
Dr. Reddy's Laboratories, a global leader in pharmaceutical manufacturing, is seeking a qualified Clinical Research Associate for its Hyderabad location. This role requires a master's degree in pharmacy or life sciences and 2-5 years of research experience. Join a dynamic team committed to advancing healthcare through innovative clinical trials.
Job Description
Job Summary
We are looking for an experienced Clinical Research Associate to join our team in Hyderabad. This role involves processing, reviewing, and receiving clinical data from therapeutic groups, internal and external investigators, and ensuring accurate and timely data delivery to clinical teams.
Roles & Responsibilities
Facilitate Study Start up activities at Clinical Investigational Site
Conduct Feasibility & assess the data
Conduct site qualification visits to assess suitability of sites for study conduct including review of Investigator qualifications, site staff adequacy, site facilities, patient pool & share feedback to project team
Collection of essential documents including validation records for site equipment from selected sites for regulatory and EC submissions
Identify the training needs for the site to perform adequate conduct of trial
Ensure supply of clinical trial material to sites before study initiation
End to End clinical investigational site management:
Initiate the study at clinical investigational sites
Provide study protocol and related trainings
Perform review of Informed Consent forms and narrative
Perform review of source records, perform SDV
Review of CRF data entry, data queries and coordinate with sites to resolve
Ensure timely completion and review of site visit reports and addressing action items via follow up letters, tracking of action items till closure
IP accountability and reconciliation
Ensure adequate initial supply & re-supply of IP per study plan to clinical trial sites
Provide adequate oversight to IP collection, storage, temperature monitoring including review of log, administration to study subjects
Identification of any temperature excursions and suitability of IP for subject administration
Ensure destruction of expired / used IP on site or return of such IP back to local depot, per study requirement & adequate documentation for same.
Review of completion of logs, filing of relevant shipment etc. documentation in site / pharmacy file.
Site Contracts and Site Payment Coordination
Coordinate for CDA, Clinical trial agreements review and finalization and amendments, as applicable
Ensure that site invoices are being generated as per clinical trial agreement
Review and approval of site invoices and submission to payment processing team
Coordinate for the payment release & confirm for site acknowledgements
Perform ongoing reconciliation of payments against site activities including subject visits conduct etc.
Liaising with safety lab for timely samples receipt, processing and release of reports & identify, resolve any issues / risks around same.
Ensure that the Bio-analytical samples are stored as per storage conditions mentioned in the lab manual including reconciliation and query resolution
Liaising with other vendors and help sites in any query resolutions for vendor related activities
Liaising with internal project teams including but limited to Data Management for EDC issues, data queries and reconciliations, Safety team for SAE related issues, Medical team for any protocol / eligibility related queries etc.
Liaising with Internal & External Stakeholders
Ensuring Compliance to Protocol & Applicable study plans, SOPs, GCP and regulatory requirements
Ensure compliance to Protocol
Ensure compliance to study plans, applicable SOPs and related regulatory requirements
Ensure compliance to ICH GCP
Ensure compliance to good documentation practices including ALCOA-C
Identification of significant deviations to protocol / plans / procedures, escalation to project lead
/ clinical ops lead and propose adequate mitigation plans / CAPA, Review of implementation of CAPA / mitigation plans, identify need for training and provide training / re-training in case of any changes
Support audits, inspections / QC visits, as required per study plans
Trial Master File Review and Maintenance for Inspection Readiness
Ensure that sites are timely updating the documents in Investigator site file
Retrieval of essential documents from sites for In house filing / Central files
Review of onsite and in house files at defined frequency per monitoring / TMF plan for study
Ensure adequacy of TMF for all time inspection readiness
[caption id="attachment_90685" align="aligncenter" width="1200"] Dr. Reddy's Laboratories Hiring Clinical Research Associate Hyderabad[/caption]
Qualifications
Educational Qualification: Master's degree in pharmacy, life sciences, biology, biotechnology, or biochemistry, or a diploma in clinical research.
Work Experience: 2-5 years of research experience.
Technical Skills: Proficiency in clinical trial operations, market research, regulatory guidelines (GCP, ICH), medical terminology, and EDC systems.
Behavioral Skills: Excellent communication, negotiation, and interpersonal skills, strong project management and analytical abilities, and a results-oriented mindset.
How to Apply
Interested candidates can apply via LinkedIn.
0 notes
Text
The Generic Sterile Injectables Market poised for strong growth driven by increasing demand for affordable healthcare
The generic sterile injectables market encompasses pharmaceutical formulations such as vials, ampoules, bottles, syringes and bags, which are administered parenterally into the body for treatments. They offer effective and affordable alternatives to branded sterile injectable drugs across therapeutic areas including oncology, cardiovascular diseases, infectious diseases and autoimmune diseases. The growing prevalence of chronic diseases and increasing healthcare expenditure have boosted the demand for generic sterile injectables globally.
The global generic sterile injectables market is estimated to be valued at US$ 46.33 Bn in 2024 and is expected to exhibit a CAGR of 10% over the forecast period 2024 to 2031.
Key Takeaways
Key players operating in the generic sterile injectables market are Baxter International Inc., AstraZeneca plc, Merck and Co., Inc., Pfizer Inc., Fresenius Kabi, Novartis International AG, Teva Pharmaceuticals, Hikma Pharmaceuticals, Dr. Reddy's Laboratory, Mylan N.V., Sun Pharmaceutical Industries Ltd. The key players dominate the market with their wide array of products in various dosages.
The increasing prevalence of chronic diseases and aging population has amplified the demand for affordable healthcare solutions. The rising healthcare costs have prompted patients and providers to shift towards cost-effective generic injectable drugs from branded equivalents. This has accelerated the growth of the global generic sterile injectables market.
With rising healthcare expenditures, healthcare providers are boosting investments in emerging markets of Asia Pacific, Latin America, Middle East and Africa for expansion of their generic sterile injectables portfolio. Generic Sterile Injectables Market Trends is expected to drive during the forecast period.
Market Key Trends
Increased Research & Development and manufacturing capabilities of emerging players: With growing demand for affordable and effective biologics, emerging players are investing significantly in R&D and expanding their sterile injectables manufacturing infrastructure. This has led to increased competition and entry of more affordable biologics in the market.
Porter’s Analysis
Threat of new entrants: Low barriers to entry make it easy for new companies to enter the market. However, regulations and requirement of high capital to set-up sterile facilities pose challenges.
Bargaining power of buyers: Large group purchasing organizations and hospital networks have significant influence on prices. However, need for essential medicines keeps bargaining power in check.
Bargaining power of suppliers: Few major global players supply key starting materials and APIs. However, potential for forward integration limits suppliers' bargaining power.
Threat of new substitutes: Limited threat as generics have few major therapeutic substitutes. Biosimilars pose a potential long-term threat in certain disease segments.
Competitive rivalry: Intense competition on pricing and new product development. Major players compete by improving quality, reliability of supply and enhancing portfolios. Frequent litigation and regulatory issues also impact competition.
The United States dominates the Generic Sterile Injectables Market Regional Analysis accounting for over 40% revenue share in 2024. Strong payer system, sizable healthcare spending and increasing generic adoption to contain costs drive high growth.
China sterile injectables market is projected to grow at over 12% till 2031, making it the fastest growing regional market. This can be attributed to rising living standards, healthcare reforms focusing on essential medicines and initiatives to expand domestic sterile manufacturing capabilities.
Get more insights on Generic Sterile Injectables Market
Get More Insights—Access the Report in the Language that Resonates with You
French
German
Italian
Russian
Japanese
Chinese
Korean
Portuguese
Alice Mutum is a seasoned senior content editor at Coherent Market Insights, leveraging extensive expertise gained from her previous role as a content writer. With seven years in content development, Alice masterfully employs SEO best practices and cutting-edge digital marketing strategies to craft high-ranking, impactful content. As an editor, she meticulously ensures flawless grammar and punctuation, precise data accuracy, and perfect alignment with audience needs in every research report. Alice's dedication to excellence and her strategic approach to content make her an invaluable asset in the world of market insights.
(LinkedIn: www.linkedin.com/in/alice-mutum-3b247b137 )

#Coherent Market Insights#Generic Sterile Injectables Market#Generic Sterile Injectables#Generic Pharmaceuticals#Sterile Injections#Injectable Medications#Pharmaceutical Industry#Generic Drugs#Injectables#Sterile
0 notes
Text
Bortezomib Market Estimated to Witness High Growth Owing to Rising Adoption of Proteasome Inhibitors

The bortezomib market is primarily driven by high incidence and prevalence of multiple myeloma across the globe. Bortezomib, which is commonly sold under the brand name Velcade among others, is a proteasome inhibitor primarily used for the treatment of multiple myeloma and mantle cell lymphoma.
The global proteasome inhibitor drug market size is valued at approximately US$ 24.54 million in 2024 and is expected to register a CAGR of 4.7% over the forecast period of 2024-2031.
The introduction of novel proteasome inhibitors and their increasing adoption in the treatment of cancer are the major factors anticipated to propel market growth.
Key Takeaways
Key players operating in the bortezomib market are Hikma Pharmaceuticals, Pfizer, Meitheal Pharmaceuticals, Novartis International AG, Bristol Myers Squibb, NATCO Pharma, Teva Pharmaceuticals, Dr. Reddy's Laboratories, Gland Pharma, Shilpa Medicare, Qilu Pharmaceutical, Scion Pharmaceuticals, Farmhispania Group, Coresyn, Chem-Stone (Guangzhou), Hubei Honch Pharmaceutical, Vinkem Labs, Icrom, TAPI Teva, and Chengdu Aslee Biopharmaceuticals.
The introduction of generic versions of Bortezomib Market Demand has led to increased adoption and lowered treatment costs. Moreover, ongoing clinical trials evaluating the efficacy of bortezomib in other cancer indications are expected to expand the eligible patient pool. Technological advancements in proteasome inhibitor development focused on overcoming resistance, reducing toxicity, and novel delivery systems are further anticipated to support market growth.
Market Drivers
The primary factors driving the growth of the global bortezomib market include rising prevalence of multiple myeloma globally, increasing adoption of proteasome inhibitors in treatment regimens, availability of generic versions, and ongoing clinical research evaluating the efficacy of bortezomib in other cancer indications. Additionally, improving healthcare infrastructure and expenditures in emerging economies will further support the market growth during the forecast period.
Current challenges in Bortezomib Market
The Bortezomib Market Size And Trends faces several challenges primarily due to the presence of alternative therapeutic options for treating multiple myeloma (MM). Some of the major challenges include increasing generic competition from drugs like ixazomib and daratumumab which are leading to lower sales of bortezomib drugs. Further, the patents of bortezomib drugs have expired in several regions making them available in generic forms at lower costs. This increasing availability of low-cost generics is a major challenge faced by innovator bortezomib drug companies. Additionally, the adverse side effects associated with bortezomib drugs like neuropathy and thrombocytopenia require close patient monitoring during treatment posing operational challenges. Stringent regulations for drug approval is another regulatory challenge for new market entrants.
SWOT Analysis
Strength: Well-established drug with proven efficacy and safety profile in treating MM. It was the first proteasome inhibitor approved and remains a standard of care.
Weakness: Patent expiry has led to availability of low-cost generics reducing sales of innovator brands. Further, it causes serious side effects like neuropathy requiring cautious use.
Opportunity: Emerging economies with growing cancer burden and healthcare spending present an opportunity. Combination therapies with other anti-MM drugs can boost its use further.
Threats: Increasing competition from newer oral proteasome inhibitors and monoclonal antibody based therapies poses pricing and market share threats. Stringent regulations for approval delays market entry of new players.
Geographical regions with high market concentration
In terms of value, North America accounts for the largest share of over 40% of the global bortezomib market led by the US. This is due to established healthcare infrastructure and higher adoption of innovative therapies. Europe is the second major regional market with a value share of over 30% supported by favourable reimbursement policies. The Asia Pacific region is projected to be the fastest growing market during the forecast period due to rising healthcare expenditure, growing cancer incidence and increasing demand for cancer treatments from middle-income countries like China and India.
Fastest growing geographical region
The Asia Pacific region is poised to exhibit the highest growth rate during the forecast period in the global bortezomib market. This is attributed to rising disposable incomes, growing awareness about cancer treatments, expansion of healthcare facilities and increasing private sector investment in pharmaceutical research in emerging economies like China and India. Large patient pools undergoing cancer treatment in Asia present lucrative opportunities for bortezomib drug makers looking to tap high future growth potential in this region.
Get More Insights On, Bortezomib Market
For More Insights Discover the Report In language that Resonates with you
French, German, Italian, Russian, Chinese, Korean
About Author:
Ravina Pandya, Content Writer, has a strong foothold in the market research industry. She specializes in writing well-researched articles from different industries, including food and beverages, information and technology, healthcare, chemical and materials, etc. (https://www.linkedin.com/in/ravina-pandya-1a3984191)
#Bortezomib Market Demand#Bortezomib Market Size#Bortezomib Market Trends#Bortezomib Market Forcast#Bortezomib#Bortezomib Market
0 notes
Text
Understanding the Dynamics of Pharmaceutical Exports from India
India's pharmaceutical sector is a cornerstone of the country's economy, playing a vital role in global health by supplying a wide array of medications and healthcare products. The Indian pharmaceutical industry, renowned for its extensive production capacity and significant global contributions, has seen remarkable growth in recent years. This article delves into the key highlights of India's pharmaceutical exports, examining major destinations, top products, and leading exporters, as well as providing guidance for newcomers interested in entering the pharma export market.

Key Highlights of Indian Pharmaceutical Exports
Export Growth and Major Destinations
In the fiscal year 2023-24, India's pharmaceutical exports reached an impressive USD 27.9 billion, marking a 9.67% increase from the previous year. This growth is notable given the overall 3% decline in total exports during the same period.
The United States remains the largest market for Indian pharmaceutical products, accounting for over 31% of total exports. Other significant destinations include the United Kingdom, the Netherlands, South Africa, and Brazil.
Top Pharmaceutical Products Exported
Drug Formulations and Biologicals: Representing more than 75% of exports, this category includes finished dosage forms such as tablets, capsules, and injections.
Bulk Drugs and Drug Intermediates: Bulk drugs, which are chemically identical to branded counterparts but offered at lower prices, form a substantial portion of exports.
Ayush and Herbal Products: India exports a variety of herbal and Ayurvedic products, including popular items like Ashwagandha, Turmeric, and Ginger.
Surgical Instruments and General Medications: India exports a wide range of surgical instruments and other general medications, contributing significantly to global health supplies.
Leading Pharmaceutical Exporters
Prominent pharmaceutical companies driving exports from India include Elkos Healthcare Pvt. Ltd., Dr. Reddy’s Laboratories, Aurobindo, Sun Pharmaceutical Industries, Cipla Limited, and Lupin Limited.
Detailed Analysis of Indian Pharmaceutical Exports
Export Statistics and Trends
India's pharmaceutical exports have demonstrated resilience and growth despite broader economic challenges. In 2023-24, pharmaceutical exports surged by approximately 9.67% to USD 27.9 billion. This increase is driven by strong demand in key markets, especially the United States, which remains the largest importer of Indian pharmaceuticals.
Monthly data also shows a robust performance, with exports valued between USD 2-3 billion consistently. The US, UK, and Netherlands are notable for their significant shares in India's pharmaceutical exports.
Major Pharmaceutical Products
Drug Formulations and Biologicals: These products are crucial for global healthcare, making up the majority of India's pharmaceutical exports. They include various forms of medications essential for treating numerous health conditions.
Bulk Drugs and Drug Intermediates: Known for cost-effectiveness, bulk drugs form the largest segment of pharmaceutical exports, providing essential medications at competitive prices.
Ayush and Herbal Products: With a growing global demand for natural and traditional remedies, India has become a significant exporter of Ayush and herbal products, catering to various therapeutic needs.
Surgical Instruments and General Medications: India exports a diverse range of surgical instruments and general medications, with substantial export values to countries like the US and the UK.
Top Export Destinations
India’s pharmaceutical products reach over 200 countries, reflecting the sector’s global reach and significance. Key regions include:
Middle East and Africa: These regions are major importers of generic medications and bulk drugs.
North America: The US is the largest market, reflecting the high demand for both generic and specialty medications.
Europe and Latin America: The UK, Netherlands, and Brazil are notable importers, with increasing demand for diverse pharmaceutical products.
Entering the Pharma Export Market
For those considering entering the pharmaceutical export market, it is crucial to understand the dynamics and requirements of this sector. Here are key steps to get started:
Research and Planning: Conduct thorough research on market needs, regulations, and potential export destinations. Identify high-demand products and target markets that align with your business goals.
Compliance and Certification: Ensure compliance with international quality standards and obtain necessary certifications. This includes Good Manufacturing Practices (GMP) and other relevant certifications.
Building Partnerships: Establish relationships with key players in the pharmaceutical industry, including exporters and importers. Networking and forming strategic partnerships can provide valuable insights and opportunities.
Utilizing Resources: Platforms like Eximpedia offer updated data and insights on pharmaceutical exports, helping you navigate the market and connect with potential partners.
Conclusion
India’s pharmaceutical sector continues to thrive as a global leader in medication production and export. With a diverse range of products and strong export growth, the Indian pharmaceutical industry remains a significant player in the global market. For newcomers to the pharma export business, understanding market dynamics, regulatory requirements, and leveraging resources like Eximpedia can enhance your chances of success in this competitive field. For further information and updates, consider connecting with Eximpedia for comprehensive data and insights into the pharmaceutical export landscape.
#eximpedia#pharmaceutical export from India#pharma export#export of pharmaceuticals from india#india pharma export#export of pharma products from india#export pharmaceutical products from india#import export pharmaceuticals in india#pharmaceutical exporters#pharma export company in india
0 notes
Text
Tizanidine HCl Market Growth Opportunities
The Tizanidine Hydrochloride (HCl) market has seen significant growth over recent years, driven by increasing demand for effective muscle relaxants and the rising prevalence of conditions like multiple sclerosis, spinal cord injuries, and other muscle-related disorders. As healthcare systems globally advance and awareness regarding muscle relaxation therapies improves, the Tizanidine HCl market is set for robust growth.
Market Size and Share Analysis
Tizanidine hcl Market Size was estimated at 1.9 (USD Billion) in 2023. The Tizanidine Hcl Market Industry is expected to grow from 2.1(USD Billion) in 2024 to 4.5 (USD Billion) by 2032. The tizanidine hcl Market CAGR (growth rate) is expected to be around 10.02% during the forecast period (2024 - 2032). North America holds the largest market share, followed by Europe and Asia-Pacific, due to the high prevalence of muscle-related disorders and advanced healthcare infrastructure.
In terms of market segmentation, the oral tablet form of Tizanidine HCl dominates the market due to its ease of use and high efficacy. The rising demand for generic drugs and the entry of new market players are also contributing to market growth. However, the market faces challenges such as side effects associated with Tizanidine HCl, including drowsiness, dry mouth, and dizziness, which may restrain its widespread adoption.
Industry Trends Shaping the Tizanidine HCl Market
Increasing Prevalence of Muscle Disorders: The growing incidence of conditions such as multiple sclerosis, back pain, and spinal cord injuries is driving the demand for muscle relaxants like Tizanidine HCl. As these conditions become more common, especially in aging populations, the need for effective treatments is rising.
Shift Towards Generic Drugs: As patents for branded Tizanidine HCl drugs expire, the market is witnessing a shift towards more affordable generic versions. This trend is expected to increase market accessibility, particularly in emerging economies where healthcare budgets are constrained.
Rising Awareness and Accessibility: Increased awareness about muscle relaxants and improved accessibility to healthcare services are contributing to the market's growth. Public health campaigns and educational programs are helping to spread knowledge about the benefits of Tizanidine HCl in managing muscle spasticity.
Technological Advancements in Drug Delivery: Innovations in drug delivery systems are enhancing the efficacy and patient compliance of Tizanidine HCl. New formulations, such as extended-release tablets, are being developed to provide longer-lasting effects and reduce the frequency of dosing.
Expansion in Emerging Markets: Emerging markets in Asia-Pacific, Latin America, and the Middle East are witnessing increased demand for Tizanidine HCl due to improving healthcare infrastructure and rising disposable incomes. These regions present significant growth opportunities for market players.
Key Players in the Tizanidine HCl Market
Several pharmaceutical companies are actively involved in the production and distribution of Tizanidine HCl, contributing to the market's competitive landscape. Some of the key players include: Dr. Reddy's Laboratories ,Glenmark Pharmaceuticals ,Sun Pharmaceutical Industries ,Lupin ,Torrent Pharmaceuticals ,Cipla ,Aurobindo Pharma ,Mylan ,Teva Pharmaceutical Industries, Sandoz, Apotex, Hikma Pharmaceuticals, Cadila Healthcare, Zydus Cadila.
Challenges and Opportunities in the Tizanidine HCl Market
While the Tizanidine HCl market is poised for growth, it also faces several challenges. The potential side effects of the drug, including drowsiness, dry mouth, and dizziness, may limit its widespread adoption. Additionally, the availability of alternative treatments for muscle spasticity, such as baclofen and diazepam, could pose competition.
However, opportunities abound for market players. The growing demand for generic drugs presents a significant opportunity for pharmaceutical companies to expand their market share. Additionally, advancements in drug delivery systems, such as extended-release formulations, offer the potential to improve patient compliance and enhance treatment outcomes.
Regional Analysis: A Global Perspective
The Tizanidine HCl market is segmented into several regions, each with unique growth dynamics:
North America: Dominating the global market, North America benefits from a high prevalence of muscle-related disorders and advanced healthcare infrastructure. The presence of key market players in this region further boosts market growth.
Europe: Europe is another significant market for Tizanidine HCl, driven by the region's aging population and high incidence of conditions like multiple sclerosis. The market in Europe is also supported by strong healthcare systems and regulatory frameworks.
Asia-Pacific: The Asia-Pacific region is expected to witness the highest growth rate during the forecast period. This growth is attributed to improving healthcare infrastructure, rising awareness about muscle relaxants, and increasing disposable incomes in emerging economies.
Latin America and Middle East & Africa: These regions are also experiencing growing demand for Tizanidine HCl due to expanding healthcare access and rising prevalence of muscle disorders. However, market growth in these regions may be limited by economic constraints and lack of awareness.
Conclusion
The Tizanidine HCl market is on a growth trajectory, driven by the rising prevalence of muscle-related disorders, increasing demand for generic drugs, and technological advancements in drug delivery. While challenges such as side effects and competition from alternative treatments exist, the market presents significant opportunities for growth, particularly in emerging markets.
As the global healthcare landscape continues to evolve, the Tizanidine HCl market is expected to expand, offering new avenues for pharmaceutical companies to innovate and capture market share. With continued investment in research and development, the market is poised to achieve steady growth, benefiting patients worldwide who rely on effective muscle relaxation therapies.
0 notes
Text
Exploring Growth Opportunities in the Indian Share Market
The Indian share market presents a plethora of growth opportunities for investors, driven by the country's robust economic fundamentals, expanding consumer base, and dynamic entrepreneurial landscape. With the right strategies and insights, investors can tap into the potential offered by this vibrant market. This article explores key sectors and factors that make the share market India a fertile ground for growth opportunities.
Booming Sectors
1. Technology and IT Services
India's technology and IT services sector has been a significant growth driver for the economy. Home to global IT giants like TCS, Infosys, and Wipro, the sector benefits from a skilled workforce and a robust outsourcing model. The increasing digitalization and adoption of emerging technologies such as artificial intelligence, blockchain, and cloud computing provide ample growth opportunities. Investors can look to capitalize on the sustained demand for IT services and the sector's innovation-driven growth.
2. Pharmaceuticals and Healthcare
The pharmaceutical and healthcare sector in India is another promising area for investors. With a growing population, rising income levels, and increasing health awareness, the demand for healthcare services and pharmaceutical products is on the rise. India is also a major player in the global generic drug market, offering cost-effective solutions to global healthcare challenges. Companies like Sun Pharma, Dr. Reddy's Laboratories, and Cipla are well-positioned to benefit from both domestic and international markets.
3. Renewable Energy
The renewable energy sector is gaining significant traction in India, supported by government initiatives aimed at reducing carbon emissions and promoting sustainable energy sources. The country's commitment to expanding solar and wind energy capacities offers substantial growth prospects for investors. Companies involved in renewable energy projects, such as Adani Green Energy and Tata Power, are poised to benefit from this transition towards greener energy solutions.
4. E-commerce and Consumer Goods
India's burgeoning middle class and increasing internet penetration have fueled the growth of the e-commerce sector. Companies like Reliance Retail, Flipkart, and Amazon India are expanding their market reach, catering to the evolving consumer preferences. Additionally, the fast-moving consumer goods (FMCG) sector continues to thrive, with companies like Hindustan Unilever and Nestlé India seeing consistent demand for their products. The combination of digital transformation and consumer-driven growth makes this sector attractive for long-term investments.
Favorable Demographics
India's demographic dividend is a critical factor contributing to the growth opportunities in the share market. With a large and youthful population, the country boasts a significant workforce that drives economic activity and consumption. This demographic advantage supports sustained demand across various sectors, creating a conducive environment for businesses to flourish and expand.
Government Initiatives
The Indian government has launched several initiatives aimed at fostering economic growth and creating a business-friendly environment. Programs like "Make in India," "Digital India," and "Startup India" encourage innovation, entrepreneurship, and investment across sectors. These initiatives provide a supportive framework for companies to grow and thrive, offering investors numerous opportunities to benefit from the resulting economic expansion.
1. Make in India
"Make in India" aims to transform India into a global manufacturing hub by encouraging both domestic and foreign investments in the manufacturing sector. The initiative focuses on enhancing infrastructure, reducing regulatory complexities, and providing incentives for businesses. As a result, sectors like automotive, electronics, and textiles are witnessing increased investment and growth potential.
2. Digital India
"Digital India" seeks to promote digital infrastructure, enhance digital literacy, and deliver government services digitally. This initiative has accelerated the adoption of digital technologies, benefiting IT services, e-commerce, and fintech sectors. Companies that leverage digital solutions are well-positioned to capitalize on the opportunities created by this initiative.
3. Startup India
"Startup India" aims to foster innovation and support the growth of startups through funding, mentorship, and regulatory support. The initiative has led to the emergence of a vibrant startup ecosystem in India, spanning sectors like technology, healthcare, and fintech. Investors can explore opportunities in the burgeoning startup landscape, particularly in companies that demonstrate strong growth potential and innovative solutions.
Infrastructure Development
India's focus on infrastructure development is another key driver of growth in the share market. Massive investments in roads, railways, ports, and urban infrastructure are expected to boost economic activity and improve connectivity. Companies involved in infrastructure projects, such as Larsen & Toubro and IRB Infrastructure, stand to benefit from the government's push for infrastructural improvements. These developments not only enhance the business environment but also create multiplier effects across various sectors.
Conclusion
The Indian share market offers a wealth of growth opportunities across multiple sectors, supported by favorable demographics, government initiatives, and infrastructure development. By identifying key trends and sectors poised for growth, investors can strategically position themselves to benefit from India's economic expansion. As the country continues to evolve and adapt to global economic changes, the Indian share market remains a dynamic and promising landscape for growth-oriented investors.
0 notes
Text
Antiepileptic Drug Market Trends, Analysis and Forecast
The global antiepileptic drug (AED) market is projected to grow at a CAGR of around 2% from 2021 to 2027. This growth is fueled by the continuous development of new drugs and increasing research efforts to produce more specific and effective treatments for epilepsy. According to the World Health Organization (WHO), epilepsy affects approximately 50 million people worldwide, making it one of the most prevalent neurological disorders globally.
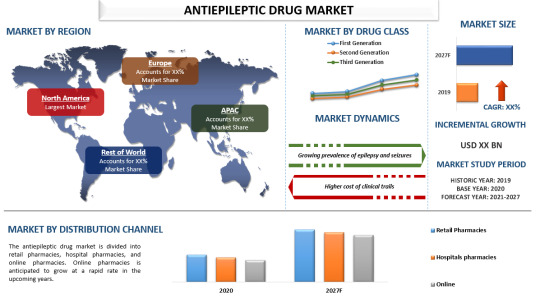
Key Market Drivers:
1. Increasing Prevalence of Epilepsy: With around 50 million people affected globally, the demand for effective AEDs is substantial. The increasing incidences of other neurological disorders and prenatal injuries also contribute to the rising number of epilepsy cases.
2. Advancements in Drug Development: Ongoing research and development efforts aim to create more effective and targeted AEDs. This innovation is crucial for enhancing patient outcomes and minimizing the side effects of existing treatments.
3. Impact of COVID-19: The pandemic has had a mixed impact on the market. While the financial stability of the private healthcare sector was challenged, leading to delays in clinical trials and research, the ongoing need for effective epilepsy treatments remains unchanged, driving demand in the long term.
For a comprehensive analysis of the market drivers, visit https://univdatos.com/report/antiepileptic-drug-market/
Market Segmentation:
1. By Drug Class:
- First Generation: Established treatments with a long history of use.
- Second Generation: Held a significant market share in 2020 due to their efficacy in slowing brain impulses and controlling seizures.
- Third Generation: Represents the latest advancements in AEDs, focusing on improved efficacy and reduced side effects.
2. By Distribution Channel:
- Retail Pharmacies: Traditional brick-and-mortar outlets.
- Hospital Pharmacies: Integral to in-hospital treatment and ongoing patient care.
- Online Pharmacies: Expected to witness significant growth due to the increasing penetration of e-pharmacy platforms and rising awareness of their benefits.
Regional Insights:
- North America: Dominates the market due to the high prevalence of epilepsy, with about 1.2% of the U.S. population having active epilepsy. The region's developed healthcare infrastructure and the presence of major pharmaceutical companies contribute to its leading market position.
- Europe: Significant market share due to advanced healthcare systems and substantial investments in healthcare research and development.
- Asia-Pacific: Rapidly growing market driven by increasing healthcare access, rising awareness, and improving economic conditions.
Major Market Players:
Key players in the global AED market include Novartis AG, GlaxoSmithKline Plc, Johnson & Johnson Service, Inc., Teva Pharmaceutical Industries Ltd., Pfizer Inc., Zogenix, Dr. Reddy’s Laboratories Ltd., Alkem Labs, SK Biopharmaceuticals, and Eisai Co. These companies are engaged in various strategic initiatives such as mergers, acquisitions, and partnerships to develop new and advanced antiepileptic drugs.
For a sample report, visit https://univdatos.com/get-a-free-sample-form-php/?product_id=22772
Conclusion:
The global antiepileptic drug market is set to experience steady growth over the forecast period, driven by the increasing prevalence of epilepsy, advancements in drug development, and the expansion of online pharmacy platforms. Despite challenges posed by the COVID-19 pandemic, the ongoing demand for effective AEDs ensures continued market growth.
Contact Us:
UnivDatos Market Insights
Email - [email protected]
Contact Number - +1 9782263411
Website -www.univdatos.com
#Antiepileptic Drug Market#Antiepileptic Drug Market Size#Antiepileptic Drug Market Growth#Antiepileptic Drug Market Forecast
0 notes
Text
Losartan Potassium Market Size, Share, Analysis, Growth, Key Players, Trend and Forecast to 2034

The global volume of the Losartan Potassium market was approximately 24 thousand tonnes in 2023, with an expected growth rate of 4.8% per annum throughout the forecast period until 2034.
Introduction:
Losartan Potassium, a widely prescribed medication renowned for its efficacy in managing hypertension and reducing the risk of cardiovascular events, occupies a significant position within South Africa's healthcare landscape. As the country grapples with the growing burden of cardiovascular diseases, the Losartan Potassium Market emerges as a critical component in promoting cardiovascular health. This article delves into the intricacies of the Losartan Potassium Market in South Africa, elucidating its dynamics, therapeutic applications, and impact on public health.
Healthcare Landscape and Demand:
South Africa faces a considerable prevalence of hypertension and related cardiovascular conditions, attributed to factors such as urbanization, lifestyle changes, and genetic predisposition. Losartan Potassium, with its potent antihypertensive properties and renoprotective effects, plays a pivotal role in managing hypertension and reducing the risk of cardiovascular morbidity and mortality. The increasing demand for effective antihypertensive medications underscores the significance of Losartan Potassium in South Africa's healthcare system.
Click Here : https://www.chemanalyst.com/industry-report/losartan-potassium-market-4184
Therapeutic Applications:
Losartan Potassium serves as a cornerstone therapy in the management of hypertension, diabetic nephropathy, and heart failure, among other cardiovascular conditions. As an angiotensin II receptor blocker (ARB), it effectively lowers blood pressure, improves cardiac function, and mitigates end-organ damage associated with hypertension. In South Africa, where cardiovascular diseases impose a significant public health burden, Losartan Potassium contributes to improved patient outcomes and reduced healthcare costs.
Pharmaceutical Industry Dynamics:
The pharmaceutical industry in South Africa is characterized by a mix of domestic production and importation, ensuring the availability of essential medications to meet healthcare needs. Losartan Potassium formulations, both locally manufactured generics and imported brands, cater to the demands of healthcare providers and patients across the country. Stringent regulatory oversight and adherence to quality standards uphold the safety and efficacy of Losartan Potassium products in the market.
Market Opportunities and Growth Drivers:
The Losartan Potassium Market in South Africa presents significant opportunities for pharmaceutical manufacturers, distributors, and healthcare providers. With the increasing prevalence of hypertension and related cardiovascular conditions, the demand for Losartan Potassium is expected to rise. Moreover, initiatives aimed at promoting cardiovascular health awareness, improving access to essential medications, and implementing evidence-based treatment guidelines drive market growth and foster innovation in Losartan Potassium formulations.
Click Here : https://www.chemanalyst.com/industry-report/losartan-potassium-market-4184
Challenges and Strategies:
Despite its promising outlook, the Losartan Potassium Market in South Africa faces challenges such as pricing pressures, generic competition, and medication adherence issues. However, strategic initiatives focused on patient education, physician engagement, and healthcare system strengthening can mitigate these challenges and enhance the uptake of Losartan Potassium therapy. Collaboration among stakeholders, including government agencies, healthcare professionals, and patient advocacy groups, is essential to address barriers to access and optimize the use of Losartan Potassium in clinical practice.
Major players in the Global Losartan Potassium market are Dr. Reddy's Laboratories, Merck & Co. Inc., Pfizer Inc., Teva Pharmaceutical Industries Ltd., Sanofi S.A., Novartis International AG, Sun Pharmaceuticals Industries Ltd., Aurobindo Pharma Ltd., Torrent Pharmaceutical Ltd., Lupin Ltd., and Others.
Emerging Trends and Future Outlook:
As the healthcare landscape evolves, several emerging trends are poised to shape the trajectory of the Losartan Potassium Market in South Africa. These include innovations in drug delivery systems, personalized medicine approaches, and the integration of digital health technologies. Moreover, initiatives aimed at addressing social determinants of health, promoting lifestyle modifications, and implementing multidisciplinary care models are expected to improve cardiovascular outcomes and drive market expansion in the long term.
Conclusion:
The Losartan Potassium Market in South Africa represents a critical component in the management of hypertension and cardiovascular diseases. With its proven efficacy, safety profile, and therapeutic benefits, Losartan Potassium contributes to improved patient outcomes and reduced healthcare burden in the country. By capitalizing on market opportunities, addressing challenges, and fostering collaboration among stakeholders, South Africa can optimize the use of Losartan Potassium therapy, promoting cardiovascular health and well-being across the population.
0 notes
Text
Biosimilar Market Size, Share, Key Growth Drivers, Trends, Challenges and Competitive Landscape
Data Bridge Market research has recently issued comprehensive industry research on Global Biosimilar Market which includes growth analysis, regional marketing, challenges, opportunities, and drivers analysed in the report.
Besides, Biosimilar market report studies market growth opportunities and restraining factors. The geographical division of this market analysis report offers data that gives an idea of the revenue of the companies and sales figures of the market growth. The market report also contains the drivers and restraints for the Biosimilar market that are obtained with the help of SWOT analysis, and also shows all the recent developments, product launches, joint ventures, mergers and acquisitions by the several key players and brands with their systemic company profiles, that are driving the market.
Access Full 350 Pages PDF Report @
Data Bridge Market Research analyses that the global biosimilar market which was USD 37,270.20 million in 2022, is expected to reach USD 343,520.90 million by 2030, and is expected to undergo a CAGR of 32.00% during the forecast period 2023-2030. “Oncology” dominates the product segment of the global biosimilar market owing to the owing to the increase in number of research studies, increasing research and development activities by key players focused on oncology and advancements in healthcare technologies. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
TABLE OF CONTENTS
Part 01: Executive Summary
Part 02: Scope of the Report
Part 03: Research Methodology
Part 04: Market Landscape
Part 05: Pipeline Analysis
Part 06: Market Sizing
Part 07: Five Forces Analysis
Part 08: Market Segmentation
Part 09: Customer Landscape
Part 10: Regional Landscape
Part 11: Decision Framework
Part 12: Drivers and Challenges
Part 13: Market Trends
Part 14: Vendor Landscape
Part 15: Vendor Analysis
Part 16: Appendix
Key Questions Answered with this Study
1) What makes Biosimilar Market feasible for long term investment?
2) Know value chain areas where players can create value?
3) Teritorry that may see steep rise in CAGR & Y-O-Y growth?
4) What geographic region would have better demand for product/services?
5) What opportunity emerging territory would offer to established and new entrants in Biosimilar Market?
6) Risk side analysis connected with service providers?
7) How influencing factors driving the demand of Biosimilarin next few years?
8) What is the impact analysis of various factors in the Global Biosimilar Market growth?
9) What strategies of big players help them acquire share in mature market?
10) How Technology and Customer-Centric Innovation is bringing big Change in Biosimilar Market?
Some of the major players operating in the global biosimilar market are:
Novartis AG (Switzerland)
Orion Pharma AB (Sweden)
Pfizer Inc. (U.S.)
Samsung Bioepis. (South Korea)
Coherus BioSciences, Inc. (U.S.)
Amgen Inc. (U.S.)
Eli Lilly and Company (U.S.)
Takeda Pharmaceutical Company Limited. (Japan)
Bristol-Myers Squibb Company (U.S.)
Merck KGaA (Germany)
Teva Pharmaceutical Industries Ltd. (U.S.)
Biocon. (India)
Bayer AG (Germany)
AbbVie Inc. (U.S.)
Allergan (Ireland)
Dr. Reddy’s Laboratories Ltd. (India)
Boehringer Ingelheim International GmbH. (Germany)
Biogen (U.S.)
Browse Trending Reports:
Prenatal Testing And New Born Screening Market
Rapid Oral Fluid Screening Device Market
North America Eclinical Solutions Market
Medical Tuning Fork Market
North America Cbct Dental Imaging Market
Myopia Treatment Market
Induced Pluripotent Stem Cells Market
Pneumococcal Vaccine Market
Dupuytrens Disease Market
Mitogen Activated Protein Kinase Mapk Inhibitors Therapeutics Market
Neonatal Seizures Drugs Market
Chikungunya Treatment Market
Sickle Cell Disease Treatment Market
About Data Bridge Market Research:
Data Bridge set forth itself as an unconventional and neoteric Market research and consulting firm with unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process.
Contact Us:
Data Bridge Market Research
US: +1 888 387 2818
UK: +44 208 089 1725
Hong Kong: +852 8192 7475
Email: [email protected]
#Biosimilar Market Size#Key Growth Drivers#Challenges and Competitive Landscape#market report#market share#market size#market trends#market research#market analysis#marketresearch#markettrends#Biosimilar Market
0 notes
Text
Dr. Reddy’s Laboratories, a leading multinational pharmaceutical company, is seeking a dedicated Pharmacovigilance Associate to join their team in Hyderabad. This role offers a unique opportunity to contribute to the company's mission of accelerating access to affordable and innovative medicines.
Job Details
Position: Pharmacovigilance Associate
Location: Hyderabad, Telangana
Qualification: Bachelor's degree in Pharmacy
Experience: 3-4 years in drug safety
About Dr. Reddy’s Laboratories
Founded in 1984, Dr. Reddy’s Laboratories has grown from a modest investment and 20 employees to a global presence with research and development centers, manufacturing facilities, and a commercial footprint in 66 countries. The company is driven by the purpose of making good health accessible to everyone, emphasizing access, affordability, and innovation.
Key Responsibilities
The Pharmacovigilance Associate will be responsible for supporting the Pharmacovigilance (PV) Quality Management System and associated training. Specific duties include:
Drafting procedural documents including Standard Operating Procedures, Work Instructions and Guidance Documents to capture PV processes.
Participate in the review, formatting and routing for review of procedural documents authored by other team members.
Assist in the development of training materials for procedural documents as a mechanism for testing and documenting understanding.
Support the development of training curricula for the global PV Team and affiliates, as applicable.
Develop/deliver adverse event reporting training for non-PV internal teams and drug safety vendors on routine as well as annual basis.
Perform and compile request for information (RFI) with PV data, adverse event collection, case processing, signal and risk management, clinical studies, PV safety concern/related issues, etc. The compilation of information to process PADERS.
PADERS submission and publishing for all DRL products & acquired products.
Regulatory correspondences by updating the PV shared drive labeling updates and send over to other PV stakeholders.
Ensure provision of strategic information and complete PV Due Diligence for potential product acquisitions/divestitures and creation/modification of Safety Data Exchange Agreements.
North America Product List for PV oversight including Master list, external & in-licensing products.
[caption id="attachment_84749" align="aligncenter" width="1200"] Pharmacovigilance Associate Jobs in Hyderabad at Dr. Reddy’s Laboratories[/caption]
Additional Responsibilities
Receive and independently triage adverse events received from other manufacturers via regular mail and/or dedicated mailbox and forward promptly for case processing.
Responsible for compliance with executed Pharmacovigilance Safety Data Exchange Agreements (SDEA) and routine reconciliations.
Support finance team in collating and processing multiple invoices from Safety as well as risk management program vendors.
Maintains document security and integrity by filing the Pharmacovigilance documents in a secure room or sending indexed files to offshore archival.
With minimal assistance, should be able to prioritize multiple projects, able to suggest solutions to complex issues, and delivers complete and accurate information within deadlines.
Support North America and Global PV team with audits/inspection readiness.
Perform all duties in accordance with Dr. Reddy’s business principles, corporate directives, SOPs and government and industry guidelines.
Perform other relevant tasks as requested by management
Qualifications and Skills
Educational Qualification: Bachelor's degree in Pharmacy
Experience: 3-4 years of drug safety experience in the pharmaceutical industry with knowledge of global pharmacovigilance regulations.
Technical Skills:
Working knowledge of ICH-GCP guidelines and global regulations for PV, including FDA, Health Canada and EU
Experience in drafting SOPs and Work Instructions and assisting in the preparation of training materials and e.
g. quizzes to test SOP understanding
With minimal assistance be able to take necessary decisions by applying FDA, HC, ICH-GCP and GPVP standard regulations/guidance.
Proficient in MS Office: Word, Excel, & PowerPoint
Behavioral Skills:
Excellent communication skills for liaising with external stakeholders and internal teams.
Benefits and Work Culture
Dr. Reddy’s Laboratories offers competitive benefits, including personalized learning programs, relocation support, family support (maternity and paternity benefits), learning and development opportunities, and comprehensive medical and life coverage.
The company fosters a culture of empathy and dynamism, with a focus on helping patients lead healthier lives. Employees are supported by an enabling environment that promotes individual ability while fostering teamwork and shared success.
Apply Now
If you meet the qualifications and are passionate about contributing to a company committed to improving global health, apply online at Dr. Reddy’s Careers.
0 notes
Text
Amebiasis Drugs Market Projected to Show Strong Growth

dvance Market Analytics added research publication document on Worldwide Amebiasis Drugs Market breaking major business segments and highlighting wider level geographies to get deep dive analysis on market data. The study is a perfect balance bridging both qualitative and quantitative information of Worldwide Amebiasis Drugs market. The study provides valuable market size data for historical (Volume** & Value) from 2018 to 2022 which is estimated and forecasted till 2028*. Some are the key & emerging players that are part of coverage and have being profiled are Dr. Reddy's Laboratories (India), Mission Pharmacal (United States), Aceto Corporation (United States), Mylan Pharmaceuticals (United States), Impax Laboratories (United States), Pfizer (United States), Sanofi (France), Lupin Pharmaceuticals (India), Glenmark Pharmaceuticals (India), Sun Pharmaceutical (India), Heritage Pharmaceuticals (United States).
Get free access to Sample Report in PDF Version along with Graphs and Figures @ https://www.advancemarketanalytics.com/sample-report/159161-global-amebiasis-drugs-market
Amebicide is a kind of anti-infective drug. Amebicides are agents that kill or destroy amebae. Every amebicide features a totally different mode of action. Amebicides are used for the destruction of parasitic species of amebae in humans or animals. Amebicides are used for the treatment of various infectious diseases like amebiasis. Amebiasis is an infection that's caused by Entamoeba histolytica. Entamoeba histolytica infects the internal organ tract of humans or animals. Amebicide medicine is given orally or intravenously. The absorption of an amebicide drug is complete and rapid. Due to speedy absorption through the gastrointestinal tract, these medicines are less effective against parasites within the lumen. Amebicide medicine is distributed to all tissues and body fluids like milk, saliva, and cerebrospinal fluid. An amebicide drug is metabolized by oxidization within the liver by a mixed-function enzyme, followed by glucuronidation. The drug is excreted within the urine as an unchanged drug and matter. Amebicide medicine will cause sure adverse effects such as oral thrush, diarrhea, metallic taste, dry mouth, vomiting, or nausea.
Keep yourself up-to-date with latest market trends and changing dynamics due to COVID Impact and Economic Slowdown globally. Maintain a competitive edge by sizing up with available business opportunity in Amebiasis Drugs Market various segments and emerging territory.
Influencing Market Trend
The Rising Level of Awareness
Better Treatment Facilities
Market Drivers
Increasing Prevalence of Parasitic Infections
The Increasing Pool of Patients Suffering from Amebic Infections
Opportunities:
Continued Launch of Effective Drugs
Challenges:
Presence Of Unfavourable Reimbursement Policies
Have Any Questions Regarding Global Amebiasis Drugs Market Report, Ask Our Experts@ https://www.advancemarketanalytics.com/enquiry-before-buy/159161-global-amebiasis-drugs-market
Analysis by Type (Branded Drugs, Generic Drugs), Infection Type (Bone Infection, Skin Infection, Ent Infections, Gastrointestinal Infection, Surgical Site Infections, Bloodstream Infections, Others), Distribution Channel (Online Pharmacies, Retail Pharmacies, Hospital Pharmacies), Site of Action (Luminal Amebicides, Systemic Amebicides, Mixed (Luminal and Systemic) Amebicides)
Competitive landscape highlighting important parameters that players are gaining along with the Market Development/evolution
• % Market Share, Segment Revenue, Swot Analysis for each profiled company [Dr. Reddy's Laboratories (India), Mission Pharmacal (United States), Aceto Corporation (United States), Mylan Pharmaceuticals (United States), Impax Laboratories (United States), Pfizer (United States), Sanofi (France), Lupin Pharmaceuticals (India), Glenmark Pharmaceuticals (India), Sun Pharmaceutical (India), Heritage Pharmaceuticals (United States)]
• Business overview and Product/Service classification
• Product/Service Matrix [Players by Product/Service comparative analysis]
• Recent Developments (Technology advancement, Product Launch or Expansion plan, Manufacturing and R&D etc)
• Consumption, Capacity & Production by Players
The regional analysis of Global Amebiasis Drugs Market is considered for the key regions such as Asia Pacific, North America, Europe, Latin America and Rest of the World. North America is the leading region across the world. Whereas, owing to rising no. of research activities in countries such as China, India, and Japan, Asia Pacific region is also expected to exhibit higher growth rate the forecast period 2023-2028.
Table of Content
Chapter One: Industry Overview
Chapter Two: Major Segmentation (Classification, Application and etc.) Analysis
Chapter Three: Production Market Analysis
Chapter Four: Sales Market Analysis
Chapter Five: Consumption Market Analysis
Chapter Six: Production, Sales and Consumption Market Comparison Analysis
Chapter Seven: Major Manufacturers Production and Sales Market Comparison Analysis
Chapter Eight: Competition Analysis by Players
Chapter Nine: Marketing Channel Analysis
Chapter Ten: New Project Investment Feasibility Analysis
Chapter Eleven: Manufacturing Cost Analysis
Chapter Twelve: Industrial Chain, Sourcing Strategy and Downstream Buyers
Read Executive Summary and Detailed Index of full Research Study @ https://www.advancemarketanalytics.com/reports/159161-global-amebiasis-drugs-market
Highlights of the Report
• The future prospects of the global Amebiasis Drugs market during the forecast period 2023-2028 are given in the report.
• The major developmental strategies integrated by the leading players to sustain a competitive market position in the market are included in the report.
• The emerging technologies that are driving the growth of the market are highlighted in the report.
• The market value of the segments that are leading the market and the sub-segments are mentioned in the report.
• The report studies the leading manufacturers and other players entering the global Amebiasis Drugs market.
Thanks for reading this article; you can also get individual chapter wise section or region wise report version like North America, Middle East, Africa, Europe or LATAM, Southeast Asia.
Contact US :
Craig Francis (PR & Marketing Manager)
AMA Research & Media LLP
Unit No. 429, Parsonage Road Edison, NJ
New Jersey USA – 08837
Phone: +1 201 565 3262, +44 161 818 8166
[email protected]
#Global Amebiasis Drugs Market#Amebiasis Drugs Market Demand#Amebiasis Drugs Market Trends#Amebiasis Drugs Market Analysis#Amebiasis Drugs Market Growth#Amebiasis Drugs Market Share#Amebiasis Drugs Market Forecast#Amebiasis Drugs Market Challenges
0 notes
Text
Highly Potent API Market: Global Industry Analysis | Bis Research

The global highly potent API market consists of innovative high-potency APIs and generic high-potency APIs used for therapeutic areas, which include oncology, immunology, hormonal disorders, infectious diseases, and others. Highly potent API market is projected to reach $84.20 billion by 2033 from $ 27.44 billion in 2023, growing at a CAGR of 11.86% during the forecast period 2023-2033.
Understanding Highly Potent API:
Highly Potent APIs are substances that exhibit pharmacological activity at very low concentrations. These compounds play a crucial role in the development of medications for various therapeutic areas, including oncology, hormonal disorders, and autoimmune diseases. The unique characteristics of HPAPIs make them challenging to handle, requiring specialized facilities and stringent safety measures throughout the manufacturing process.
A comprehensive analysis of the Highly Potent API market would be incomplete without exploring regional dynamics. The report presents a detailed assessment of market trends, opportunities, and challenges in key regions, providing stakeholders with a strategic understanding of the global landscape.
Empower Your Strategies: Receive Your Sample Report and Conquer Highly Potent API Market
These actively provide the following
Increased Therapeutic Efficacy
Cost Efficiency
Advanced Drug Delivery Systems
Expanded Treatment Options
Stringent Regulatory Standards
Key Market Players are as follows
Almac Group
Asymchem Inc.
Axplora Group GmbH
BASF Pharma Solutions
CARBOGEN AMCIS
CordenPharma International
Curia Global, Inc.
Dr. Reddy’s Laboratories Ltd.
Lonza
Visit the vertical page and grab better understanding of the report @ Precision Market
Key Market Trends and Drivers:
The Highly Potent API market report highlights several trends and drivers shaping the industry:
Oncology Dominance: A significant portion of HPAPIs is dedicated to oncology drugs, reflecting the industry's emphasis on developing targeted therapies for cancer treatment.
Contract Manufacturing Organizations (CMOs): The outsourcing of API manufacturing to CMOs has become a prevalent practice among pharmaceutical companies.
Regulatory Challenges: Stringent regulations govern the production and handling of HPAPIs due to their potent nature. Compliance with regulatory standards, such as containment measures and documentation, is a key concern for industry players.
Technological Advancements: Innovations in manufacturing technologies, such as isolators and closed systems, contribute to improved safety and efficiency in handling highly potent compounds.
Market Segmentation:
The Highly Potent API market report provides a detailed segmentation analysis, categorizing the market based on:
Type: Segmentation by API type includes innovative HPAPIs and generic HPAPIs, offering insights into the market share and growth potential of each segment.
Therapeutic Area: The report examines the demand for HPAPIs across therapeutic areas such as oncology, hormonal disorders, and autoimmune diseases, unraveling the specific drivers within each category.
End-User: The end-user segmentation covers pharmaceutical companies, CMOs, and research institutions, shedding light on the varied demand and preferences within the industry.
Conclusion
The Highly Potent API market report serves as an invaluable resource for pharmaceutical industry professionals, investors, and policymakers seeking to navigate the evolving landscape of potent drug development. As the demand for targeted and potent therapies continues to grow, staying informed about market trends, regulatory developments, and technological advancements is essential for making informed business decisions in this dynamic sector.
#Highly Potent API Market#Highly Potent API Market Report#Highly Potent API Market Industry#Highly Potent API Market Trends#Highly Potent Key Players
0 notes
Text
The Myelodysplastic Syndrome Treatment Market will grow at highest pace owing to increasing drug approvals

Myelodysplastic syndrome (MDS) treatment involves drugs that help improve blood cell production or control symptoms. The main drugs used are erythropoiesis-stimulating agents, granulocyte colony-stimulating factors, immunomodulatory drugs, and hypomethylating agents. Erythropoiesis-stimulating agents help stimulate the bone marrow to produce more red blood cells and reduce the need for blood transfusions. Granulocyte colony-stimulating factors help increase white blood cell counts and fight infections. Immunomodulatory drugs work by modifying the patient's immune response. Hypomethylating agents help restore normal cell growth and differentiation by modifying DNA. The growing need to treat persistent cytopenias and reduce transfusion dependency in MDS patients is projected to drive the demand for these drugs over the forecast period. The Global Myelodysplastic Syndrome Treatment Market is estimated to be valued at US$ 3,265.6 mn in 2024 and is expected to exhibit a CAGR of 9.3% over the forecast period 2023 to 2030.
Key Takeaways
Key players operating in the Myelodysplastic Syndrome Treatment market are Celgene Corporation, Otsuka Pharmaceutical Co., Ltd. Teva Pharmaceutical Industries Ltd., Sun Pharmaceutical Industries Limited, Dr. Reddy's Laboratories Ltd., Mylan NV, Cipla Limited, Acceleron Pharma, Inc., Aprea Therapeutics, FibroGen Inc., Onconova Therapeutics Inc., and Geron. Celgene Corporation holds a leading market share due to its key brands such as Revlimid and Vidaza. The increasing diagnosis of MDS cases globally due to growing geriatric population is a major factor driving the demand for treatment drugs. Technological advancements in gene therapy and new drug candidates in clinical trials are expected to present new opportunities for market growth over the forecast period.
Market Trends
Rising Adoption of Gene Therapy: Gene therapy has gained significance in recent years due to its ability to target the root cause of the disease. Companies are investigating gene therapies that target mutations associated with MDS. For instance, Onconova Therapeutics is developing a RAS-targeted viral therapeutic ON 123300 for MDS patients.
Increasing Combination Therapies: Combining drugs with different mechanisms of action is being explored to increase treatment efficacy. Some clinical trials are evaluating hypomethylating agents in combination with immunomodulatory drugs or cytotoxic chemotherapy to improve outcomes in MDS patients. Such combination regimens are expected to gain adoption if successful.
Market Opportunities
Emerging Oral Formulations: The introduction of orally administered drugs offers more convenience and improves patient compliance compared to intravenous treatment. Companies are developing oral formulations of drugs currently available as injections. For example, Acceleron Pharma's controversial Sotatercept is in late-stage trials as an oral therapy for MDS.
Personalized Medicine Approach: With advances in genomics and molecular diagnostics, tailored treatment approaches based on patient-specific biomarkers and genetics are an opportunity. Predicting response to specific therapies could improve clinical benefit in select patient subgroups. Several firms are exploring companion diagnostics.
0 notes
Text
Oral Thin Films Market Set to Reach US$ 7.1 Billion by 2031, Indicates Transparency Market Research Study
The global Oral Thin Films market is estimated to attain a valuation of US$ 7.1 Bn by the end of 2031, states a study by Transparency Market Research (TMR). Besides, the report notes that the market is prognosticated to expand at a CAGR of 9.3% during the forecast period, 2022-2031.
The key objective of the TMR report is to offer a complete assessment of the global market including major leading stakeholders of the Oral Thin Films industry. The current and historical status of the market together with forecasted market size and trends are demonstrated in the assessment in simple manner. In addition, the report delivers data on the volume, share, revenue, production, and sales in the market.
Request for a sample of this research report at (Use Corporate Mail Id for Quick Response) - https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=42869
The report by TMR is the end-product of a study performed using different methodologies including the PESTEL, PORTER, and SWOT analysis. The study with the help of these models shed light on the key financial considerations that players in the Oral Thin Films market need to focus on identifying competition and formulate their marketing strategies for both consumer and industrial markets. The report leverages a wide spectrum of research methods including surveys, interviews, and social media listening to analyze consumer behaviors in its entirety.
Oral Thin Films Market: Industry Trends and Value Chain
The study on the Oral Thin Films market presents a granular assessment of the macroeconomic and microeconomic factors that have shaped the industry dynamics. An in-depth focus on industry value chain help companies find out effective and pertinent trends that define customer value creation in the market. The analysis presents a data-driven and industry-validated frameworks for understanding the role of government regulations and financial and monetary policies. The analysts offer a deep-dive into the how these factors will shape the value delivery network for companies and firms operating in the market.
Buy this Premium Research Report | Immediate Delivery Available at - https://www.transparencymarketresearch.com/checkout.php?rep_id=42869<ype=S
Oral Thin Films Market: Branding Strategies and Competitive Strategies
Some of the key questions scrutinized in the study are:
What are some of the recent brand building activities of key players undertaken to create customer value in the Oral Thin Films market?
Which companies are expanding litany of products with the aim to diversify product portfolio?
Which companies have drifted away from their core competencies and how have those impacted the strategic landscape of the Oral Thin Films market?
Which companies have expanded their horizons by engaging in long-term societal considerations?
Which firms have bucked the pandemic trend and what frameworks they adopted to stay resilient?
What are the marketing programs for some of the recent product launches?
The list of key players operating in the Oral Thin Films market includes following names:
Aquestive Therapeutics, Inc., C.L. Pharm, Cure Pharmaceutical, Dr. Reddy's Laboratories, Indivior plc, IntelGenx Corp., Kyu Kyu Pharmaceutical Co., Ltd., DK Livkon Pvt. Ltd., NAL Pharma, Seoul Pharma Co., Ltd., Shilpa Therapeutics Pvt. Ltd., Sunovion Pharmaceuticals, Inc., and ZIM Laboratories Limited are key entities operating in this industry.
Request for customization of this research report at - https://www.transparencymarketresearch.com/sample/sample.php?flag=CR&rep_id=42869
Oral Thin Films Market: Assessment of Avenues and Revenue Potential in Key Geographies
Some of the key aspects that the study analyzes and sheds light are:
Which regions are witnessing rise in investments in the supply chain networks?
Which countries seems to have benefitted from recent import and export policies?
Which regions have witnessed decline in consumer demand due to economic and political upheavals?
Which are some the key geographies that are likely to emerge as lucrative markets?
Which regions are expected to lose shares due to pricing pressures?
Which regions leading players are expected to expand their footprints in the near future?
What are some the sustainability trends impacting the logistics and supply chain dynamics in the Oral Thin Films market?
What are some of the demographic and economic environments that create new demand in developing economies?
How are changing government regulations shaping business strategies and practices?
About Us Transparency Market Research
Transparency Market Research, a global market research company registered at Wilmington, Delaware, United States, provides custom research and consulting services. The firm scrutinizes factors shaping the dynamics of demand in various markets. The insights and perspectives on the markets evaluate opportunities in various segments. The opportunities in the segments based on source, application, demographics, sales channel, and end-use are analysed, which will determine growth in the markets over the next decade.
Our exclusive blend of quantitative forecasting and trends analysis provides forward-looking insights for thousands of decision-makers, made possible by experienced teams of Analysts, Researchers, and Consultants. The proprietary data sources and various tools & techniques we use always reflect the latest trends and information. With a broad research and analysis capability, Transparency Market Research employs rigorous primary and secondary research techniques in all of its business reports.
Contact Us
Nikhil Sawlani
Transparency Market Research Inc.
CORPORATE HEADQUARTER DOWNTOWN,
1000 N. West Street,
Suite 1200, Wilmington, Delaware 19801 USA
Tel: +1-518-618-1030
USA – Canada Toll Free: 866-552-3453
0 notes
Text
Chronic Obstructive Pulmonary Disease Market Insights, Demand and Growth Opportunity
Market Insights
Chronic obstructive pulmonary diseases, more commonly referred to as COPD has been increasing rapidly in incidence due to various factors. Various types of COPD exist, and increasing demand for treatment and early diagnosis of COPD have significantly increased the market's growth in recent years. The Chronic Obstructive Pulmonary Disease (COPD) Market share was valued at USD 1.82 billion in 2021 and is expected to expand from USD 3.07 billion in 2022 to USD 29.33 billion by 2030, with a compound yearly growth rate (CAGR) of 4.50% during the forecast period (2022 - 2030).
COPD most commonly includes diseases such as emphysema and chronic bronchitis among others. The overall increasing prevalence of chronic diseases combined with the demand for effective treatment of chronic diseases are expected to encourage market growth. Rising levels of smoking among adults has been found to be a major factor driving the growth of the COPD market. Most types of COPD occur due to external factors which obstruct proper pulmonary function. Rising pollution levels and the increasing prevalence of asthma are some other factors driving the growth of the market. COPD market is likely to grow consistently as there are no true cures for COPD. However, treatment can minimize symptoms and reduce further damage to the pulmonary system. COPD primarily afflicts adults in the 50 and above age demographic. The expanding geriatric population across the globe is also driving market growth.
The increasing efficiency in healthcare systems across the globe and rising healthcare investments are expected to assist growth. Moreover, increasing R&D activities toward helping reduce COPD as well advancement of medical technology is expected to provide market opportunities in the coming years.
Market Segmentation
MRFR's segmentation of the global chronic obstructive pulmonary disease market insights includes type, diagnosis, treatment, and region. Types of COPD are segmented to include chronic bronchitis and emphysema. Diagnosis of COPD includes spirometry, diagnostic tests, and others. Diagnostic tests have been segmented further into chest X-ray and complete blood count.
Treatment of chronic obstructive pulmonary disease has been segmented into lung transplant, oxygen therapy, drug therapy, vaccination, surgery, and others. The drug therapy segment in the COPD market has been sub-segmented to include inhaled steroids, combination inhalers, oral steroids, and others.
Key geographies that have been included in the analysis of the global COPD market include the Americas, Europe, Asia Pacific, and the Middle East and Africa
Regional Analysis
The Americas have been observed to have captured the largest share of the COPD market. The North American segment in the region leads growth and is expected to retain its position. The presence of a developed healthcare system, combined with the growing demand for advanced treatments have increased R&D activities for the same. The Americas region is followed by Europe which has several key players making efforts toward the development of COPD treatments.
The Asia Pacific market is slated to grow at the most rapid pace. The region has a high pollution rate and an immense patient pool which impacts the COPD market considerably. Increasing incidences of COPD diseases in the region, and the healthcare reforms taking place are expected to make the region a leading regional segment in the years to come.
Key Players
Dr. Reddy’s Laboratories Ltd, Abbott, GSK, F. Hoffmann-La Roche Ltd, Novartis AG, Pfizer Inc, Merck, AstraZeneca, and others are among the leading competitors in the global chronic obstructive pulmonary disease market. These players have been studied in MRFR's report extensively for their competitive strategies.
About Market Research Future:
Market Research Future (MRFR) is a global market research company that takes pride in its services, offering a complete and accurate analysis with regard to diverse markets and consumers worldwide. Market Research Future has the distinguished objective of providing the optimal quality research and granular research to clients. Our market research studies by products, services, technologies, applications, end users, and market players for global, regional, and country level market segments, enable our clients to see more, know more, and do more, which help answer your most important questions.
Contact Us:
Market Research Future (Part of Wantstats Research and Media Private Limited)
99 Hudson Street, 5Th Floor
New York, NY 10013
United States of America
+1 628 258 0071 (US)
+44 2035 002 764 (UK)
Email: [email protected]
Website: https://www.marketresearchfuture.com
#Chronic Obstructive Pulmonary Disease Market#Chronic Obstructive Pulmonary Disease Market Size#Chronic Obstructive Pulmonary Disease Market Share#Chronic Obstructive Pulmonary Disease Market Growth#Chronic Obstructive Pulmonary Disease Market Analysis#Chronic Obstructive Pulmonary Disease Market Report#Chronic Obstructive Pulmonary Disease Market Research
0 notes