#delphinidin
Text
Anthocyanins are the pigments responsible for the blue and purple color in some berries (Figure 21.36).

"Plant Physiology and Development" int'l 6e - Taiz, L., Zeiger, E., Møller, I.M., Murphy, A.
#book quotes#plant physiology and development#nonfiction#textbook#anthocyanin#blueberry#glycoside#malvidin#delphinidin#petunidin#cyanidin#peonidin#purple#blue
0 notes
Text




Rosa (var: "Rapsody in Blue")
The Blue Rose?
There is no such thing as a blue rose. "Rapsody in Blue" is as close as cutting-edge, genetic engineering has so far accomplished. In 2004, researchers created roses that contain delphinidin, the pigment that gives violas and delphiniums that vivid blue color. All the 'blue roses' I've seen look more like fuchsia to me. I regard fuchsia as a shade of purple. What do you think?
#flowers#photographers on tumblr#blue rose#botany#purple#fleurs#flores#fiori#blumen#bloemen#Queens Park New Westminster#Rapsody in Blue#vancouver
134 notes
·
View notes
Text
I've been meaning to make more posts about this, but there's so much i have to say/images to show that this will be a part 1 out of ??? and this one focuses mainly on the first batch of Purple Iris watercolors and the pH mystery they made me unravel through chaotically-organized researching.
Basically, I've been messing around with anthocyanins, a common class of plant pigments that are pH sensitive/can be used as a pH indicator. The first source I've tried has been purple irises, which i've only vaguely been familiar with in the past. The ones I picked were the ones that had begun to shrivel slightly, to the point where they were still a deep purple but picking them they would almost be leaking a purple liquid that stained my hands. I put them in a thing of hot tap water (not boiled, just the hot setting on the faucet), enough to cover the flowers, and let them steep. they began changing the color of the water almost immediately, with the fresher ones not losing their color as quickly as the ones that had begun to wilt on the plant. within 30 minutes i decided it was extracted enough.

This left a strong purple in the water, which i then poured off into three other containers, two of which i would alter the pH of.
The purple is due to delphinidin, a type of anthocyanidin that forms the building blocks of anthocyanins. Note i italicize the word anthocyanidin just so it's easier to tell apart the two.
there are anywhere from 16-31 anthocyanidins depending on what source you find, but they are basically the backbone structure of anthocyanins, of which there are over 600 something. The main thing that turns an anthocyanidin (aglycon) into an anthocyanin (glycoside form) is a sugar attached to it.
Realistically, that distinction isn't useful when simply extracting things from flowers in hot water, but i thought it was a fun fact to note. Anthocyanidins also come in handy for knowing what builds the anthocyanins in your flowers/plant part;
cyanidin (30%), delphinidin (22%), and pelargonidin (18%) make up the base for a good majority of all the anthocyanins in plants (~60% collectively),
peonidin, malvidin, and petunidin being runnerups (20% collectively)
the 20-something remaining anthocyanidins make up the rest
So basically, they all have slightly different colors that are pH reactive, and can provide anything from red to pink to orange to purple to blue. But, for our purposes, if you have a blue/purple flower, that likely means it has some amount of delphinidin-based anthocyanins in it! there can also be more than one anthocyanidin type present in the same plant.
Other well-known sources of anthocyanins are grape skins, red cabbage, red onions, butterfly pea tea, and purple violets. However, they're also very abundant in many many other plants, these are just the common ones i can think of that lots of people are probably familiar with to some degree.
Fun fact, grape skins are actually really well-studied as far as anthocyanins go (i believe they mainly have malvidin-based ones) because they're so important for the coloration of wine!
Anthocyanins as a whole are also studied as a natural source of food dyes, along with other flavonoids such as carotenoids.
As for why it turns colors, this is because of the way the anthocyanin changes structure in different pHs. The short answer is it turns red/pink in low pH (acidic) conditions, purple in slightly acidic/neutral conditions, and blue/green in slightly high pH conditions.
The long answer is something I'll explain in a moment, but for now here's the acid/base colors:


(on the left, i altered it with vinegar and it became a bright magenta color; on the right, i altered it with baking soda and it became a sea green/blue cyan that refused to show up accurately on camera). That's one thing I've noticed, and others have too, is that when working with pigments (especially natural ones) the color accuracy of the camera often just completely fails. there's only so many colors a digital camera can capture!
Here's a slightly more accurate color due to different lighting, note how it's more a malachite green than a pure blue. off the bat this was interesting becuase I wasn't expecting as green of a liquid as i got.

Anyways, the first thing i did with them was use them as-is, no alterations past the addition of the respective vinegar and baking soda. I painted with them just as one would paint with watercolors, and interestingly enough, when i put them onto paper, they began to change from their pink/purple/malachite colors to a teal/indigo/emerald set instead.
This seems to be the result of something in the paper itself, likely calcium carbonate (which i only recently learned is added to "buffer" paper against acidic substances; the cellulose in paper is more stable long term when there's no acid present, and the calcium carbonate neutralizes any acids applied to a degree).
It's still interesting that even though the acids are neutralized, they give a unique color when compared to the basic paint.
I also tried soaking some of the same paper in vinegar water, which got rid of that buffer and let me paint with the pinks intact, but that's for another post.
Also, note that i said "neutral" for the middle, this is just what i wrote down for the tap water sample; in actuality the tap water is actually a bit closer to pH 6 instead of a true neutral 7, which i only found out after i had gotten this far. So whenever i say "neutral," i mean "tap water that's slightly acidic"
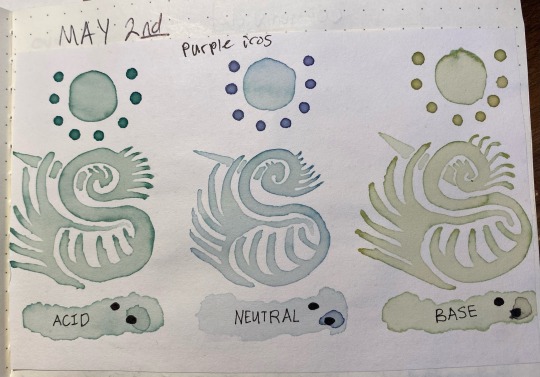
Here's something interesting that happened, though. Overnight i left the jars on my desk, and while the acid and neutral colors were the same when I came back ~24 hours later, the basic had degraded into a murky brown. this was interesting since that meant the instability was pH-dependent.
So, i made another color swatch with the acid/neutral/base, and used that as a comparison to look at how it had changed. Surprisingly, it painted out a yellowy-green instead of a murky gray-brown
here's the murky water that the once-malachite-green turned into:

I also poured off a bit into another container, and shifted it back into a low pH with a bit of vinegar to see if it would still change color. Surprisingly, it turned a slight pink, like pink lemonade, which means there were still anthocyanins in there but they were likely a lot less concentrated than they used to be.
Here's the pink, with a few leftover bubbles from the baking soda/vinegar reaction:

Here's the results of painting with these:
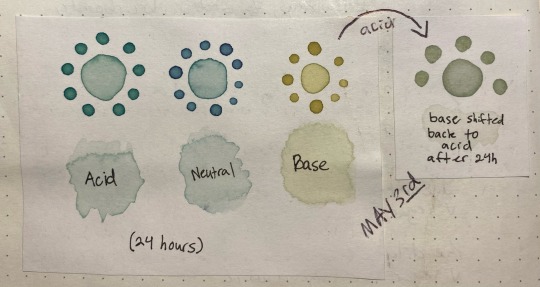
the acid was basically unchanged after sitting in a jar for 24 hours, the neutral had lost a bit of its purple color but was still about the same, and the base was now a lot yellower/tanner with a bit of green still showing through. The shifted sample was a pale stormy gray that ended up taking on a green color as it dried, as though following the trend of pink shifting to a bluer color but on a much more muted scale.
Now obviously, i wanted to figure out what caused this, so i dug around both on wikipedia and other sites but found myself eventually reading into scientific papers on the topic, at which point it became very clear that i would need to learn like, organic chemistry and such to be able to say for sure what was happening.
I did eventually manage to figure out a few things despite the dense terminology; for one thing, anthocyanins are more unstable than other plant pigments such as carotenoids. there are plenty of things that can affect their stability, including the pH of the substance they're stored in. Any higher than pH 7 (basic pHs) and theyll begin to degrade. This explains why the high pH sample lost its blue/green color, and why there was very little left to be shifted back to a pink color.
I also found out that the pH color shift isn't as simple as it seemed. Rather, there are multiple chemical forms of anthocyanins.
At the lowest pHs, basically all of them are in the "flavylium cation" state, which basically means it's positively charged and this is what gives a red color
still at a low pH (2-4), there's anothe chemical form that appears, the "quinoidal" structure that gives a blue color. Note that the red cation is still present, just no longer the only form
the more the pH rises, the more forms start to coexist, with some of those forms being colorless (one of which is a "colorless carbinol"
so, between 4 and 6 there are the cations (red) quinoidal (blue) carbinol (colorless) and something called a chalcone that gives a pale yellow
and then past that, I'm unsure, but of course that's around when the anthocyanins begin to degrade
There are also a lot more than these that i've encountered in various contexts but these seem to be the basic ones.
Do note that i do not fully understand these terms (flavylium, quinoidal, carbinol, chalcone, etc.) and have only recently begun to actually try to learn what they mean and the context surrounding them as i only had a class of basic high school chemistry under my belt prior to this. The main paper i combed over to try to find info on it seems to be behind a paywall but the DOI is:
doi.org/10.1016/j.foodchem.2008.09.001
for anyone curious and able to access it, whether through legit means or what have you.
That being said, to me, the takeaway here seems to be that there's a yellow form that appears around the time that other color forms begin to disappear, and as those degrade it makes sense that the resulting degraded forms also contribute to a murky color. This helps explain why it changed color in the jar and also retained a bit of yellow and green.
This also explains why the blue form seems to also be slightly green, it's got the blue quinoidal chemical form as well as the yellow chalcones.
There are also interesting things of note that I will get into at a later date, such as the texture/reflectivity of the way it dries, the differences in extraction ease between this and purple violets, the addition of a genuinely neutral/pH 7 sample later, a sample from a plant that doesnt seem to have delphinidins, and sample the seems to genuinely sparkle??? Much more of interest to come soon!
#art#traditional art#anthocyanins#watercolors#purple irises#painting#science#natural dyes#pigments#dyes#pH indicators
67 notes
·
View notes
Text
I went on quite an etymology adventure today.
So as you can see, my blog name is delphinidin, which is the chemical that makes flowers and fruits blue or blue-ish. I figured the word was from “delphinium,” the blue flower, and I wanted to know if the word delphinium came from Delphi, as in Greece, as in, The Oracle Of.
So I looked up delphinium. Etymonline.com, my usual port of call, was no help in this case, so I used wikipedia:
“The genus name Delphinium derives from the Ancient Greek word δελφίνιον (delphínion) which means "dolphin", a name used in De Materia Medica for some kind of larkspur. Pedanius Dioscorides said the plant got its name because of its dolphin-shaped flowers.”

Okay, I can see it.
So then I looked up the etymology of Delphi:
“Delphi shares the same root with the Greek word for womb, δελφύς delphys.“
Oookay. I mean, that makes sense, as far as it goes: Delphi is the site of the omphalos, the navel of the earth. But now I had another question: are the words “dolphin” and “womb” connected in Greek??
Aaaaaand.... Yes. Yes, they are:
“Etymologically speaking, the word ‘dolphin’ comes from the ancient Greek delphis, itself related to the Greek delphus, or womb, so the animal’s name more or less means ‘a ‘fish’ with a womb.’ “
So that was a real ride.
72 notes
·
View notes
Note
it’s fantasy can’t you just assume that naturally some rose are blue??? there’s so much subtle magic in asoiaf this can be apart of that
well yes that would obviously be the RATIONAL response but no absolutely not. it raises too many questions about the wider presence of blue pigmentation in westerosi phytochemistry actually i just remembered that shade of the evening has blue leaves so you know what yeah. we got delphinidin in everything out here why not. i still think they should be hellebore though maybe george could send me his address so i can walk him through my botanical symbolism passions
17 notes
·
View notes
Text
Another drawing of my rain world oc, Sailing Lilies Blue

Delphinidin Voyager
4 notes
·
View notes
Text
Hibiscus Corno Aspersum 🌺
Delphinidin Corno Aspersum 🌹
6 notes
·
View notes
Text
0 notes
Text
Glucoberry
Author
Albert Mathew
Published
February 10, 2023
Word count
553
What is GlucoBerry?
GlucoBerry is a revolutionary breakthrough formula designed to support healthy blood sugar levels by maintaining a proper sticky SG2 protein level in the body. It’s the most common and straightforward method to obtain the required Delphinidin, which instructs your system to make The appropriate amount of…
0 notes
Link
What's New and Beneficial About Blueberries A recent study on frozen versus fresh blueberries suggest that while the frozen version may still provide us with great nutrient benefits, there may be some important nutritional advantages related to consump…
0 notes
Text
Hyaluronidase inhibitor delphinidin inhibits cancer metastasis
http://dlvr.it/T8x9DN
0 notes
Text
Discover GlucoBerry: Your Natural Solution for Balanced Blood Sugar Levels
Unlock the power of delphinidin with GlucoBerry, a unique supplement designed to support healthy blood sugar levels. Learn how this antioxidant-rich formula can enhance your well-being safely and conveniently. Consult with your healthcare provider to see if GlucoBerry is right for you!
visit now:- http://glucoberry.colibrip.com/

0 notes
Text
Butterfly pea flower tea

Click to get yours
Butterfly pea flower tea, also known as blue tea, is a delightful herbal infusion made from the vibrant blue flowers of the butterfly pea plant (Clitoria ternatea).
Antioxidant Powerhouse:
Butterfly pea flowers are rich in antioxidants, particularly ternatins. These compounds help fight inflammation and may even prevent cancer cell growth.
Other antioxidants present include kaempferol, known for its cancer-fighting properties, p-coumaric acid (anti-inflammatory, antimicrobial, and antiviral), and delphinidin-3,5-glucoside, which boosts immune function and fights cancer cells.
Color :
When steeped in boiling water, the tea turns a brilliant blue color due to the presence of anthocyanins.
Add lemon or any acidic ingredient, and the tea transforms into a bright violet hue.The change in pH level causes this magical color shift.
Health Benefits:
Skin and Hair Health: The antioxidants in butterfly pea flower may promote healthy skin and hair.
Weight Loss: Some studies suggest it could aid in weight loss.
Blood Sugar Control: It may help regulate blood sugar levels.
Eye Health: Antioxidants support vision health.
Digestive Health: Butterfly pea tea has been used traditionally for digestive benefits.
Brain Health: Antioxidants play a role in cognitive well-being.
Steep the dried butterfly pea flowers in hot water, and enjoy the mesmerizing transformation as you add lemon or lime.
0 notes
Note
Hi, I tried to make paint from grape Hyacinths a few days ago and it didn't really work. I just stumbled across your blog and you really look like you know what you're doing! Do you have any advice?
Sure, I'd be happy to help! I'm new to a lot of this myself but I have found a good bit of info that I'm glad to share
To help specifically, I'd need a bit more info on what you did or didn't do, but you're also on the right track as grape hyacinths do have anthocyanins and it seems like they're around the same type (delphinidin-based) that I've been using
Were you able to get some color, or did it not show up at all? Did you put them in cold/warm/hot water, or did you add something to the water right off the bat?
When extracting the flower from the purple irises, i just had to put the flowers in hot tap water (not boiled) and they dropped a bunch of pigment quickly
However, i also tried doing the same thing with purple violets, and they didn't change the color of the water at all. I had to simmer them by putting their glass into a larger water bath to get the pigment to drop (and even then it was lighter than the purple iris). So if you're having trouble getting the pigment out of them in the first place, lightly simmering them could get them to release their colors
I'd also say to not full-on boil the flowers, as anthocyanins are more unstable than other pigments. I only used the simmering once i realized the violets didn't give any color from a gentler extraction. If you overheat them the chemical structure will degrade quicker and turn yellow/brown. They will also degrade in high (basic) pHs but this takes longer
If it's something else like the colors not being like expected, do let me know and i might be able to help!
10 notes
·
View notes
Text
IJMS, Vol. 24, Pages 16462: Association Analysis of Transcriptome and Targeted Metabolites Identifies Key Genes Involved in Iris germanica Anthocyanin Biosynthesis
The anthocyanin biosynthetic pathway is the main pathway regulating floral coloration in Iris germanica, a well-known ornamental plant. We investigated the transcriptome profiles and targeted metabolites to elucidate the relationship between genes and metabolites in anthocyanin biosynthesis in the bitone flower cultivar ‘Clarence’, which has a deep blue outer perianth and nearly white inner perianth. In this study, delphinidin-, pelargonidin-, and cyanidin-based anthocyanins were detected in the flowers. The content of delphinidin-based anthocyanins increased with the development of the flower. At full bloom (stage 3), delphinidin-based anthocyanins accounted for most of the total anthocyanin metabolites, whereas the content of pelargonidin- and cyanidin-based anthocyanins was relatively low. Based on functional annotations, a number of novel genes in the anthocyanin pathway were identified, which included early biosynthetic genes IgCHS, IgCHI, and IgF3H and late biosynthetic genes Ig F3′5′H, IgANS, and IgDFR. The expression of key structural genes encoding enzymes, such as IgF3H, Ig F3′5′H, IgANS, and IgDFR, was significantly upregulated in the outer perianth compared to the inner perianth. In addition, most structural genes exhibited their highest expression at the half-color stage rather than at the full-bloom stage, which indicates that these genes function ahead of anthocyanins synthesis. Moreover, transcription factors (TFs) of plant R2R3-myeloblastosis (R2R3-MYB) related to the regulation of anthocyanin biosynthesis were identified. Among 56 R2R3-MYB genes, 2 members belonged to subgroup 4, with them regulating the expression of late biosynthetic genes in the anthocyanin biosynthetic pathway, and 4 members belonged to subgroup 7, with them regulating the expression of early biosynthetic genes in the anthocyanin biosynthetic pathway. Quantitative real-time PCR (qRT-PCR) analysis was used to validate the data of #RNA sequencing (#RNA-Seq). The relative expression profiles of most candidate genes were consistent with the FPKM of #RNA-seq. This study identified the key structural genes encoding enzymes and TFs that affect anthocyanin biosynthesis, which provides a basis and reference for the regulation of plant anthocyanin biosynthesis in I. germanica. https://www.mdpi.com/1422-0067/24/22/16462?utm_source=dlvr.it&utm_medium=tumblr
0 notes
Text
What You Need To Learn About The Benefits Of Blood Pressure Supplements
Blood pressure levels are an important health concern However, it is possible to lower the diastolic as well as systolic blood pressure through regular exercise, adhering to a healthy diet low in sodium, managing stress and losing weight. Certain medications can aid in managing blood pressure naturally.
Folic acid is an B vitamin that is commonly given during pregnancy to protect women from birth defects. It could also assist in decreasing high blood pressure.
Omega-3 Fatty acids
A staggering one out of three people suffer from high blood pressure. And a new study suggests that omega-3 fatty acids may help. Researchers reviewed 71 published clinical trials that looked at the effects of taking supplements with fish oil or meals high in omega-3 fatty acids, particularly docosahexaenoic acids (DHA) and eicosapentaenoic acid (EPA). Researchers discovered that individuals who took in DHA or EPA often experienced a reduction in both systolic (high blood pressure) as well as diastolic (low blood pressure).
Scientists believe that consumption of omega-3 fatty acids decreases blood pressure through directly affecting large-conductance calcium dependent potassium channels in vascular muscles. They regulate the control of secretion as well as cell function.
Aside from the cardiovascular benefits, omega-3 fatty acids are known for their role in brain health, fetal development, the health of your eyes, weight loss and much more. Similar to all nutritional supplements, it's best to talk to your doctor or health professional to review the most recent research findings and get advice regarding the most effective way to incorporate omega-3s into your food regimen. In https://www.dziennikwschodni.pl/artykuly-sponsorowane/ultra-cardio-x-kapsulki-na-nadcisnienie-opinie-sklad-cena-kapsulek,n,1000321864.html, you can easily understand hypertension.
Green Tea
The leafy leaves from Camellia sinensis are used make green tea. As it's not treated as oolong or black, green tea contains more antioxidants as well as polyphenols. Green tea can be used to manage a range of health conditions, including hypertension and liver disease. Also, it has been proven to lower the chance of pancreatic cancer.
A study of a large scale found that people who drank the majority of green tea were twice less likely to be diagnosed with pancreatic cancer when compared to non-drinkers. More research will be needed to determine if this decreases stomach cancer and other types of cancers too.
Green tea is a source of caffeine which can increase blood pressure if consumed in excess. Consult your physician before making it a regular part of your diet, especially in the case of medications that affect blood pressure, such as beta-blockers. The caffeine in coffee can affect the effectiveness of some drugs.
Hibiscus Tea
Hibiscus is a great source of vitamins, minerals and nutrients. Additionally, it has diuretic properties which encourage urination. It can also assist in reducing blood pressure. It also has a flavonoids called delphinidin and cyanidin-3-sambubioside which have been shown to relax the tension of blood vessels, decreasing blood pressure.
The tropical leaf of this plant is full of antioxidants and polyphenols which have been proven to be efficient in the fight against cancer. In test-tube studies, hibiscus extract was able to slow down the growth of mouth cancer cells and prostate cancer.
For best results, drink three cups of hibiscus tea per daily. For the drink make sure to cover one tablespoon of dried hibiscus with 8 ounces of boiling water and let it sit for around 10 minutes. After straining out the petals, you can drink it cold or warm. The drink can be sweetened by adding a teaspoon of honey, or add a dash of lime to enhance the flavor.
Vitamin D
Vitamin D is a group of secosteroids, which have fat soluble properties. Vitamin D regulates calcium levels and blood pressure levels in the body. Deficiency in vitamin D is linked with high blood pressure along with high cholesterol levels and other health problems.
Researchers have discovered that lower levels of vitamin D have been linked with hypertension, heart disease and different conditions. However, the reason of this has not been identified. Low vitamin D is also related to a higher risk of developing rickets. This is a disease that is preventable with frequent exposure to sunlight, as well as vitamin D-enriched foods.
According to several research investigations, it has been found that vitamin D can reduce systolic and diastolic pressure. You should consult with your physician prior to deciding to consume vitamin D supplements since it can interfere with certain medication. It may reduce the effectiveness of blood pressure medicines like diuretics containing thiazide (Hygroton, Lozol) and verapamil (Cardizem, Tiazac). Long-term use of stimulant laxatives may also reduce the absorption of vitamin D.
0 notes