#chloester
Explore tagged Tumblr posts
Text
Liquid State
Intermolecular forces in liquids are collectively called van der Waals forces. These forces are essentially electrical in nature and result from the attraction of charges of opposite sign.
The principal kinds of intermolecular attractions are: dipole-dipole attractions, London forces, and hydrogen bonding.
Dipole-dipole attractions exist between molecules that are polar. This requires the presence of polar bonds and an unsymmetrical molecule. These molecules have a permanent separation of positive and negative charge. For example HCl, H atom is always slightly positive and the Cl atom is always slightly negative, the H atom in one molecule is attracted to the Cl in a neighbor.
London forces exist in nonpolar molecules. They result from temporary charge imbalances. Temporary charges exist because the electrons in a molecule or ion move randomly in the structure. The nucleus of one atom attracts electrons form the neighboring atom. At the same time, the electrons in one particle repel the electrons in the neighbor and create a short lived charge imbalance.
Hydrogen bonding is unique and requires two conditions: Covalent bonds between an H atom and either F, O, or N atom, and interaction of the H atom in this kind of polar bond with a lone pair of electrons on a nearby atom with F, O, or N. Hydrogen bonding can increase boiling point(ehh) and is responsible for the expansion of water when it freezes.
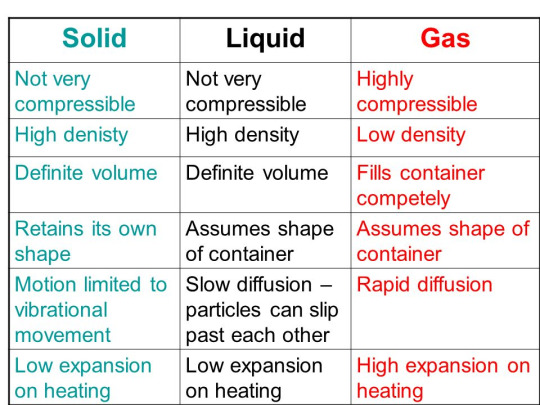
Structural differences between solids, liquids and gases:
Liquid crystals are an intermediate stage between solids and liquids. Some organic solids having long rod-like molecules which do not melt to give the liquid substance directly, they pass through the liquid crystal state first.
In a liquid, the molecules have a random arrangement and are able to move past each other. In a solid crystal, molecules have an ordered arrangement and are in fixed positions. In a liquid crystals, however, molecules are arranged parallel to each other and can flow like a liquid. Thus the liquid crystals have the fluidity of a liquid and the optical properties of a solid.
Liquid crystals are classified into three types: Nematic, Smectic, and Chloesteric.
Nematic liquid crystals have molecules parallel to each other, but they are free to slide or roll individually.
Smectic liquid crystals also have molecules parallel to each other, but they are arranged in compact layers. The layers can slide past each other.
Chloesteric: molecules are again parallel and arranged in layers, but molecules in successive layers are slightly rotated with respect to the layers above and below so as to form a spiral structure.
Applications of LCDs:
Aircraft cockpit displays
Calculator screens
Displays images in digital cameras
Television
Computer Monitors
Phone screens
Digital watches
Number Displays
0 notes
Photo

Me and the #chloester #quads #gettingitin #chloe #offroad #offroading #hungryvalley
0 notes
Note
💌💙
CC. Chlo. Chloester.
I love plenty things about you, trust, but I really love your big ole brain. I'm all but willing to let you keep the braincells between the two of us, thanks for letting me borrow them from time to time tho 😊
I love listening to you talk about stuff even if I don't understand completely what you're talkin about and I love that it doesn't take much for you to make me smile 💛
Mutuals send in a 💌and I'll tell you somethin that I love about ya :3
0 notes
Photo

My baby trying to complete the look of me! #thuglife says all she needs is #tattoos #jeans sagging #cigarettes in her back pocket #peirced ears she called these #accessories #tinydowndown lol #comedy #comedian #mylilbambeetle #chloe #chloester
#cigarettes#peirced#comedian#thuglife#tattoos#chloester#tinydowndown#comedy#chloe#accessories#mylilbambeetle#jeans
0 notes
