#cancer gene therapy market
Explore tagged Tumblr posts
Text
https://zekond.com/read-blog/58813_cancer-gene-therapy-market-size-overview-share-and-forecast-2031.html
The Cancer Gene Therapy Market in 2023 is US$ 2.64 billion, and is expected to reach US$ 14.42 billion by 2031 at a CAGR of 23.6%.
0 notes
Text
#Cancer Gene Therapy Market#Cancer Gene Therapy#Cancer#Therapy#gene#Cancer Gene Therapy Market trends#Cancer Gene Therapy Market size#Cancer Gene Therapy Market growth#Cancer Gene Therapy Market opportunities
0 notes
Text
The Global Cancer Gene Therapy Market was valued at USD 2.80 Billion in 2023 and is estimated to reach USD 15.8 Billion by 2032, growing at a CAGR of 20.45 % from 2023 to 2032.
Cancer is a group of diseases that grow abnormal cells in the human body and can grow in any part of the body. Gene Therapy is a technique to prevent diseases, where the mutated genes are replaced by the specific genes causing the disease or are inactive in this gene. Cancer gene therapy is receiving high importance due to its high success rate during pre-clinical and clinical trials. The research and development on this are trying to replace the use of surgeries and drugs with gene therapy to treat cancer. The ongoing investment in research and development, and acceptance of gene therapy in cancer treatment is propelling this cancer gene therapy market during the forecast period. Biotechnology firms are increasing novel gene therapy vectors that increase levels of protein production or gene expansion, and reducing immunogenicity. According to National Center for Health Statistics, in 2023 there are 19,58,310 cancer cases in the U.S.
Further, the growing geriatric population, advanced product technology, increase in per capita disposable income, and emerging economies are the factors that are creating lucrative opportunities for the growing cancer gene therapy market.
Economic Impact of Covid-19:
The analysis of the COVID-19 recovery trajectory provides an overview of the main strategies that industries are implementing to respond to and recover from the economic crisis. It also focuses on the post-pandemic and pre-pandemic era of the Global Cancer Gene Therapy Market through PEST analysis, SWOT, Quantitative and Qualitative analysis, Attractive analysis, and DROs.
Key Players:
Novartis AG
Pfizer Inc.
Amgen Inc
Johnson & Johnson
AstraZeneca
GlaxoSmithKline plc
Gilead Science, Inc.
Abeona Therapeutics
Altor Bioscience Inc.
Celgene Inc.
Introgen Therapeutics Inc.
BioCancell Inc.
Oncogenex
Shanghai Sunway Biotech
ZioPharm Oncology
Others
The above key players in the Global Cancer Gene Therapy Market can be changed according to the client’s requirements.
Moreover, the key players aim towards expansion, joint ventures, collaboration, mergers, and acquisitions to advance capabilities in gene therapy.
Know More- https://nexbindinsight.com/pharmaceutical-and-healthcare/the-global-cancer-gene-therapy-market-was-valued-at-usd
0 notes
Text
As per the report from nova one advisor, the global cancer gene therapy market is valued at USD 2.45 billion in 2023 and is projected to reach a value of USD 12.75 billion by 2032 at a CAGR (Compound Annual Growth Rate) of 20.1% between 2023 and 2032
0 notes
Text
The Best News of Last Week - March 27, 2023
🐢 - Why did the 90-year-old tortoise become a father? Because he finally came out of his shell!
1. New Mexico governor signs bill ending juvenile life sentences without parole

New Mexico Governor Michelle Lujan Grisham has signed a bill into law that prevents juvenile offenders from receiving life sentences without eligibility for parole. The bill, known as the No Life Sentences for Juveniles Act, allows offenders who committed crimes under the age of 18 and received life sentences to be eligible for parole hearings 15 to 25 years into their sentences.
This legislation also applies to juveniles found guilty of first-degree murder, even if they were tried as adults. The move puts New Mexico in a group of at least 24 other states and Washington, DC, that have enacted similar measures following a 2021 Supreme Court ruling.
2. Promising pill completely eliminates cancer in 18 leukaemia patients

An experimental pill called revumenib has shown promise in curing terminal leukemia patients who were not responding to treatment in a long-awaited clinical trial in the United States. The drug works by inhibiting a specific protein called menin, which is involved in the machinery that gets hijacked by leukemia cells and causes normal blood cells to turn into cancerous ones.
The pill targets the most common mutation in acute myeloid leukemia, a gene called NPM1, and a less common fusion called KMT2A. The US Food and Drug Administration granted revumenib "breakthrough therapy designation" to fast-track its development and regulatory review based on the promising results of the trial.
3. Spain passes law against domestic animal abuse

Spain has passed a new law on animal welfare, accompanied by a reform of the penal code that increases prison sentences for those mistreating animals. The law will make compulsory training for dog owners, and will prohibit them from leaving their dogs alone for more than 24 hours.
It also mandates the sterilisation of cats, with exceptions for farms, and increases the penalties for mistreatment of animals to up to two years in prison, or three years in the event of aggravating circumstances.
4. Bravery medals for women who raced into 'rough, crazy' surf to save drowning girls
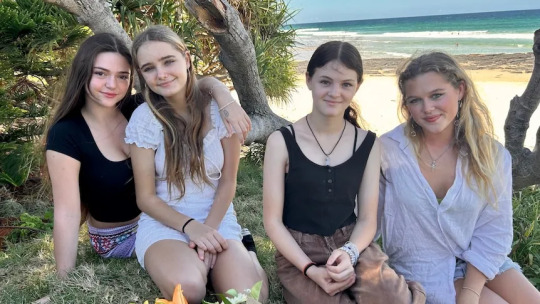
Elyse Partridge (far left) and Bella Broadley (far right) raced into dangerous surf to save Chloe and Violet from drowning.(ABC North Coast: Hannah Ross)
Bella Broadley and Elyse Partridge saved two 11-year-old girls from drowning at Angels Beach near Ballina, an unpatrolled beach in Australia. The younger girls, Chloe and Violet, became trapped in a rip and overwhelmed by waves and the current. Bella and Elyse jumped into action, using an esky lid as a flotation device to help them swim to the girls. Elyse helped Chloe back to shore while Bella swam further out to help Violet.
Elyse and Bella were on Wednesday named on the Governor General's Australian Bravery Decorations Honours List, which recognised 66 Australians for acts of bravery.
5. Almost every cat featured in viral Tik Tok posted by Kansas City animal shelter adopted
Let's find homes for the rest
youtube
6. A 90-year-old tortoise named Mr. Pickles just became a father of 3. It's a big 'dill'

These critically endangered tortoises are native to Madagascar and have seen their numbers decline due to over-collection for illegal sales on the black market. Captive breeding programs have helped produce new radiated tortoises, but the species still faces extinction in the wild.
That's why the arrival of these hatchlings, born to 90-year-old Mr. Pickles and his 53-year-old partner Mrs. Pickles, is such great news. Mr. Pickles is considered the most genetically valuable radiated tortoise in the Association of Zoos and Aquariums' Species Survival Plan, and the births represent a significant contribution to the survival of the species.
7. EU strikes ‘ground-breaking’ deal to cut maritime emissions

The European Parliament and EU ministers have agreed on a new law to cut emissions in the maritime sector. The law aims to reduce ship emissions by 2% as of 2025 and 80% as of 2050, covering greenhouse gas, methane, and nitrous oxide emissions.
The European Commission will review the law in 2028 and will decide whether to place carbon-cutting requirements on smaller ships. The agreement will also require containerships and passenger ships docking at major EU ports to plug into the on-shore power supply as of 2030. Penalties collected from those that fail to meet the targets will be allocated to projects focused on decarbonising the maritime sector.
- - - -
That's it for this week :)
This newsletter will always be free. If you liked this post you can support me with a small kofi donation:
Buy me a coffee ❤️
Also don’t forget to share this post with your friends.
467 notes
·
View notes
Text


Biotechnology and the future of humanity
Animals Are Commodities Too
Under slavery human individuals are owned, are property. Under capitalism workers aren’t owned but they have to sell their labour/time/creativity because capitalists own everything (land, the means of production, transport and communication etc) that would enable people to live outside of wage labour and the market place. Now, instead of individuals owning non-human animals as part of their subsistence, corporations are claiming the right to ‘own’ whole species of animals. This process of patenting life can be traced back to the 1980 US Supreme Court ruling, which stated that a GM bacterium (modified to digest oil) could be patented. Not just that one bacterium of course but the whole, created species. In 1985 the US Patent and Trademark Office ruled that GM plants, seeds and plant tissues could be patented. Now the corporations can demand royalties and licence payments every time farmers use those plants or seeds. Monsanto holds a patent on (i.e. owns and rents out) all GM cotton and soya. Patents have been granted on biological characteristics of plants as well. For example, a patent has been issued to Sungene for a variety of sunflower that has a high oleic acid content. But the patent covers the characteristic as well as the genes that code for it, so any plant breeder who achieves the same result by traditional methods could be sued.
In 1987 animals joined the biotech market place when a Harvard biologist patented ‘oncomouse’, a GM organism (mouse) predisposed to develop cancer for use in medical ���research’. By 1997 40 GM ‘species’ of animal had been patented, including turkey, nematodes, mice and rabbits. Hundreds of other patents are pending on pigs, cows, fish, sheep and monkeys among others. In 1976 a leukaemia patient named John Moore had his cancerous spleen removed under surgery at the University of California. Without his knowledge or consent some of the cells from his spleen were cultured and found to produce a protein which could be used in the manufacture of anti-cancer drugs. The estimated value of this cell-line to the pharmaceutical industry is $3 billion. In 1984 the California Supreme Court ruled that he was not entitled to any of these profits.
A US company called Biocyte holds a patent on (owns) all umbilical cord cells. Systemix Inc has a patent on (owns) all human bone marrow stem cells, these being the progenitors of all cells in the blood. The worldwide market for cell lines and tissue cultures was estimated to be worth $426.7 million to the corporations in 1996. Not only cells but also fragments of DNA can be patented (owned) in this way. Incyte, for example, has applied for patents on 1.2 million fragments of human DNA. The logic of this is that ‘genes for’ particular diseases such as cystic fibrosis, diabetes, various cancers etc could become the property of pharmaceutical companies who could then make huge profits on tests for such genes and genebased therapies. There is no space here to get into a lengthy criticism of the reductionist idea that individual genes simply map onto well-defined physical traits underlying the whole theory and practice of GM. It’s enough to say that research into patenting (owning), for example, a supposed’ breast cancer gene’ is of little benefit to humanity if it is true, as some scientists have estimated, that 90% of breast cancers are unrelated to genetics but are triggered by environmental pollution, diet and lifestyle factors. So what’s new? Capitalism, indeed class-society in general, always seizes the living and turns it into profit and power, declares ownership where previously there was only life: from the enclosure of the commons to the seizing of millions of human beings from Africa to be slaves to the current looting of tropical biodiversity for use in the biotech labs.
#classism#ecology#climate crisis#anarchism#resistance#community building#practical anarchy#practical anarchism#anarchist society#practical#revolution#daily posts#communism#anti capitalist#anti capitalism#late stage capitalism#organization#grassroots#grass roots#anarchists#libraries#leftism#social issues#economy#economics#climate change#climate#anarchy works#environmentalism#environment
5 notes
·
View notes
Text
Gene Editing Market Forecast: High-Tech Therapies and Agriculture Lead $12.9B Growth

The gene editing market is one of the most revolutionary industries in modern science, transforming healthcare, agriculture, and biotechnology. Valued at USD 5.0 billion in 2023, it is projected to skyrocket to USD 12.9 billion by 2030, reflecting a remarkable compound annual growth rate (CAGR) of 14.5%. This exponential growth highlights the increasing demand for innovative solutions in genetics, driven by technological advancements and expanding applications across industries.
What is Gene Editing?
Gene editing, or genome editing, involves making precise changes to an organism's DNA to correct, modify, or improve genetic outcomes. Technologies like CRISPR-Cas9, TALENs, and zinc finger nucleases allow scientists to edit genes efficiently and accurately. This technology has profound applications, ranging from curing genetic disorders to enhancing crop yields in agriculture.
Download Sample Report @ https://intentmarketresearch.com/request-sample/gene-editing-market-3120.html
Factors Driving the Market Growth
1. Increasing Prevalence of Genetic Disorders With a rise in genetic and rare diseases, the demand for gene editing as a therapeutic solution has surged. CRISPR-based therapies hold potential for treating conditions like cystic fibrosis, sickle cell anemia, and Duchenne muscular dystrophy.
2. Expanding Application in Agriculture Gene editing is reshaping agriculture by enabling the development of pest-resistant, drought-tolerant, and high-yield crops. With climate change affecting global food supplies, these innovations are crucial for sustainable farming.
3. Government Funding and Investments Governments worldwide are pouring resources into genetic research. Supportive policies and funding boost innovation and ensure accessibility to cutting-edge technologies in developing economies.
4. Rise of Biotech Startups The surge of startups focused on gene editing underscores the market’s potential. These companies innovate faster, catering to niche areas like personalized medicine and genome-based agriculture.
Challenges in the Gene Editing Market
Despite the immense promise, several challenges persist:
Ethical Concerns: Editing human genes raises ethical questions regarding designer babies and unforeseen consequences.
Regulatory Barriers: Stringent regulations in many countries slow down the development and approval processes.
High Costs: The technology is expensive, making accessibility a significant issue, especially in underdeveloped regions.
Key Technologies Leading the Charge
1. CRISPR-Cas9 CRISPR is the poster child of gene editing, celebrated for its precision, speed, and versatility. Researchers leverage CRISPR for everything from drug discovery to disease eradication.
2. TALENs and Zinc Finger Nucleases These earlier technologies laid the foundation for the current success in gene editing. While they lack the simplicity of CRISPR, they still hold relevance in specific applications.
3. Emerging Tools Novel platforms like Prime Editing and Base Editing are rapidly evolving, offering enhanced specificity and fewer errors, pushing the industry into new frontiers.
Access Full Report @ https://intentmarketresearch.com/latest-reports/gene-editing-market-3120.html
Prominent Applications of Gene Editing
1. Healthcare Innovations Gene therapies are a game-changer in medicine, targeting previously untreatable diseases. For instance, CAR-T cell therapy uses gene editing to modify T-cells to fight cancer effectively.
2. Agricultural Advancements From creating crops resilient to extreme weather to improving livestock health, gene editing addresses critical challenges in food production.
3. Industrial Biotechnology Biotech companies employ gene editing to engineer microorganisms that produce biofuels, biodegradable plastics, and other sustainable materials, revolutionizing multiple industries.
Future Prospects of the Gene Editing Market
As gene editing becomes increasingly mainstream, the industry is poised for groundbreaking advancements. Key areas of future growth include the development of off-the-shelf gene therapies, breakthroughs in combating aging at a genetic level, and expanding the role of genetics in precision medicine.
FAQs
1. What is driving the growth of the gene editing market? The growth is fueled by advancements in technology, rising prevalence of genetic disorders, agricultural needs, and increased investments in biotech research.
2. What is CRISPR-Cas9? CRISPR-Cas9 is a groundbreaking gene-editing tool known for its precision and efficiency, allowing scientists to edit DNA effectively.
3. What are some ethical concerns about gene editing? Concerns include the creation of designer babies, potential unintended consequences, and accessibility disparities.
4. Which industries benefit most from gene editing? Gene editing significantly impacts healthcare, agriculture, and industrial biotechnology.
5. What challenges does the gene editing market face? Ethical issues, regulatory barriers, and high costs are major challenges hindering widespread adoption.
About Us
Intent Market Research (IMR) is dedicated to delivering distinctive market insights, focusing on the sustainable and inclusive growth of our clients. We provide in-depth market research reports and consulting services, empowering businesses to make informed, data-driven decisions.
Our market intelligence reports are grounded in factual and relevant insights across various industries, including chemicals & materials, healthcare, food & beverage, automotive & transportation, energy & power, packaging, industrial equipment, building & construction, aerospace & defense, and semiconductor & electronics, among others.
We adopt a highly collaborative approach, partnering closely with clients to drive transformative changes that benefit all stakeholders. With a strong commitment to innovation, we aim to help businesses expand, build sustainable advantages, and create meaningful, positive impacts.
Contact Us
US: +1 463-583-2713
0 notes
Text
Plasmid DNA Manufacturing Market: A Deep Dive into Industry Innovations and Growth Projections
According to a new report by UnivDatos Market Insights, The Plasmid DNA Manufacturing Market was valued at USD 521.54 million in 2023 and is expected to grow at a CAGR of 20.11% during the forecast period (2024-2032). This growth is due to several factors mainly, the need for better treatment solutions for diseases that are on the rise including cancer and infectious diseases. The expansion of combination therapy, improved plasmid DNA-based therapeutics, and the increasing manufacturing facilities will also drive the market further. Furthermore, the growing collaboration activities between pharmaceutical companies and plasmid DNA manufacturers are fuelling the growth of the plasmid DNA manufacturing market. North America remains the largest buyer of the equipment, while Asia-Pacific is expected to show the highest growth over the forecast period as a result of a growing healthcare expenditure and a larger population of patients.
Request To Download Sample of This Strategic Report - https://univdatos.com/get-a-free-sample-form-php/?product_id=69125&utm_source=LinkSJ&utm_medium=Snehal&utm_campaign=Snehal&utm_id=snehal
Increasing Demand for Gene Therapies
Gene therapies are on the verge of tremendous growth and are a leading contributing factor to the growth of the plasmid DNA (pDNA) manufacturing market. This upsurge is attributed to higher incidences of genetic diseases, coupled with the shift to more patient-centered treatment options. Gene therapies transpose the genes as plasmid DNA into the patient’s cells to help cure diseases that were once considered to be incurable. However, this trend has exploded in the past decade, and the COVID-19 pandemic has provided examples of using pDNA in RNA vaccines and other pharmaceuticals such as RNA therapeutics to prove the efficiency of this approach in terms of reaction to crises.
As more plasmid DNA-based therapies move through the development pipeline and are anticipated to enter the market soon, the demand for high-quality plasmid DNA has become urgent. This demand is not exclusive to conventional gene therapies but also covers advanced products like DNA vaccines and regenerative medicines which ensures a steady and sustainable market for the producers and suppliers of plasmid DNA. More companies are enhancing their product portfolio of pDNA by investing and expanding their manufacturing facilities.
For instance, in October 2024, Ferring Pharmaceuticals announced the opening of a state-of-the-art global manufacturing hub in Finland for the drug substance of its intravesical non-replicating gene therapy Adstiladrin® (nadofaragene firadenovec-vncg). This represents a significant milestone in Ferring’s capabilities and capacity to meet the current and expected growth in demand for this gene therapy for people with non-muscle invasive bladder cancer (NMIBC).
For More Informative Information, Please Visit Us- https://univdatos.com/get-a-free-sample-form-php/?product_id=69125&utm_source=LinkSJ&utm_medium=Snehal&utm_campaign=Snehal&utm_id=snehal
According to the report, the Asia-Pacific region is expected to be the fastest-growing region in the forecast period
The Asia-Pacific (APAC) plasmid DNA manufacturing market is expected to experience impressive growth over the forecast period due to its increasing use in the production of gene therapy, vaccines, and regenerative medicine. Some of the major drivers that have been observed are an increase in investment in biotechnology, improvement in manufacturing technologies, and favorable government policies toward enhancing the healthcare system. Japan and South Korea are at the forefront of such efforts through the emphasis on developing new therapies and cooperation between the public and private sectors in terms of increasing investments in R&D.
For instance, in March 2023, Teijin Limited, TFBS Bioscience Inc., and Mediridge Co., Ltd. announced that they have agreed to supply viral vectors and plasmids for cell and gene therapy products in Japan, that aim to contribute to the provision of innovative medical treatments in this country.
0 notes
Text
https://tannda.net/read-blog/74731_cancer-gene-therapy-market-size-overview-share-and-forecast-2031.html
The Cancer Gene Therapy Market in 2023 is US$ 2.64 billion, and is expected to reach US$ 14.42 billion by 2031 at a CAGR of 23.6%.
0 notes
Text
#Cancer Gene Therapy Market#Cancer Gene Therapy#Cancer Gene#cancer#gene#tehrapy#therapy#Cancer Gene Therapy Market trends#Cancer Gene Therapy Market size#Cancer Gene Therapy Market growth#Cancer Gene Therapy Market oppportunities#Cancer Gene Therapy Market analysis
0 notes
Text
0 notes
Text
Gene Therapy Market Report: Growth Drivers, Challenges, and Projections
The global gene therapy market is expected to reach USD 18.20 billion by 2030, registering a CAGR of 18.88% from 2024 to 2030, according to a new report by Grand View Research, Inc. The development of the market is owing to an increase in the number of gene therapy-based discoveries, increasing investment in this sector, and rising approval of gene therapy products. According to the WHO, 10 to 20 new cell and gene therapies are expected to be approved each year by 2025.
Continuous developments in recombinant DNA technology are anticipated to enhance the efficiency of gene therapy in the coming years. Hence, ongoing progresses in recombinant DNA technology are anticipated to expand the number of ongoing clinical trials for gene therapy. Primarily, these advancements are taking place in the context of various gene-editing tools and expression systems to augment the R&D for products. The advent of CRISPR/Cas9 nuclease, ZFN, and TALEN allows easy & precise genome editing. As a result, in recent times, the gene-editing space has witnessed a substantial number of research activities, which, in turn, is expected to influence the growth of the gene therapy market.
The growth of the gene therapy market is expected to be majorly benefitted from the increasing prevalence of cancer. The ongoing increase in cancer patients and related death per year emphasizes the essential for the development of robust treatment solutions. In 2020, there were around 18.1 million new cases of cancer worldwide. 9.3 million of these cases involved men, while 8.8 million involved women. Continuing developments in tumor genetic studies have delivered substantial information about cancer-related molecular signatures, which in turn, is expected to support ongoing clinical trials for cancer therapeutics.
With rising demand for robust disease treatment therapies, companies have focused their efforts to accelerate R&D for effective genetic therapies that target the cause of disease at a genomic level. . Furthermore, the U.S. FDA provides constant support for innovations in this sector via a number of policies with regard to product manufacturing. In January 2020, the agency released six final guidelines on the manufacturing and clinical development of safe and efficient products.
Furthermore, facility expansion for cell and gene therapies is one of the major factors driving the gene therapy market growth. Several in-house facilities and CDMOs for gene therapy manufacturing have begun investing to enhance their production capacity, which, in turn, is anticipated to create lucrative opportunities for market players. For instance, in April 2022, the FDA approved commercial licensure approval to Novartis for its Durham, N.C. site. This approval permits the 170,000 square-foot facility to make, test, and issue commercial Zolgensma, as well as manufacture therapy products for current & upcoming clinical trials.
Gene Therapy Market Report Highlights
The AAV segment shows a significant revenue contribution of 22% in 2023. Several biopharma companies are offering their viral vector platform for the development of AAV-based gene therapy product.
By indication, the spinal muscular atrophy (SMA) segment dominated the market in 2023 with a share of 46.8%. Although SMA is a rare disorder, it is one of the most common fatal inherited diseases of infancy.
The Beta-Thalassemia Major/SCD segment is anticipated to register the fastest CAGR of 38.3% over the forecast period. Gene therapy for SCD and β-thalassemia is based on transplantation of gene-modified hematopoietic stem cells.
North America dominated the market in 2023 with the largest revenue share of 65.2% in 2023. This region is expected to become the largest routine manufacturer of gene therapy in terms of the number of approvals and revenue generated during the forecast period.
Europe is estimated to be the fastest-growing regional segment from 2024 to 2030. This is attributed to its large population with unmet medical needs and increasing demand for novel technologies in the treatment of rare but increasingly prevalent diseases.
Gene Therapy Market Segmentation
Grand View Research has segmented the global gene therapy market report based on indication, vector type, route of administration, and region:
Gene Therapy Indication Outlook (Revenue, USD Million, 2018 - 2030)
Large B-Cell Lymphoma
Multiple Myeloma
Spinal Muscular Atrophy (SMA)
Acute Lymphoblastic Leukemia (ALL)
Melanoma (lesions)
Inherited Retinal Disease
Beta-Thalassemia Major/SCD
Others
Gene Therapy Vector Type Outlook (Revenue, USD Million, 2018 - 2030)
Lentivirus
RetroVirus & gamma RetroVirus
AAV
Modified Herpes Simplex Virus
Adenovirus
Others
Gene Therapy Route of Administration Outlook (Revenue, USD Million, 2018 - 2030)
Intravenous
Others
Gene Therapy Regional Outlook (Revenue, USD Million, 2018 - 2030)
North America
US
Canada
Mexico
Europe
UK
Germany
Switzerland
Asia Pacific
Japan
China
South Korea
Australia
Rest of the world
Order a free sample PDF of the Gene Therapy Market Intelligence Study, published by Grand View Research.
0 notes
Text
The 15th Digital Pharmaceutical Innovations Exhibition & Congress: Pharma Marketing and Business Strategies
Introduction
The world of healthcare is evolving at an unprecedented pace, driven by groundbreaking innovations in pharmaceuticals and technology. One of the most anticipated events in this transformative journey is the 15th Digital Pharmaceutical Innovations Exhibition & Congress, set to take place from May 14-16, 2025, in San Francisco, USA, and virtually. This premier gathering of global experts, industry leaders, and visionaries promises to showcase the latest advancements in drug development, medical devices, and digital health solutions. Whether you're a researcher, healthcare professional, or industry innovator, this event offers a unique opportunity to engage with cutting-edge ideas and explore how digital transformation is reshaping the future of healthcare.
Why Attend This Conference?
Attending the 15th Digital Pharmaceutical Innovations Exhibition & Congress offers numerous benefits for professionals looking to stay ahead in the rapidly evolving pharmaceutical industry. Here's why you should mark your calendar for this must-attend event:
Cutting-Edge Insights: Discover the latest trends, technologies, and innovations driving the future of pharmaceutical sciences, including advances in drug delivery, nanomedicine, and personalized medicine.
Networking Opportunities: Connect with thought leaders, potential collaborators, and industry professionals from around the world to exchange ideas, build relationships, and foster new partnerships.
Interactive Sessions: Participate in engaging workshops, panel discussions, and live demonstrations that offer hands-on experiences with emerging technologies and breakthrough solutions.
Global Perspective: Whether you're attending in-person or virtually, the congress brings together experts from across the globe, providing a diverse range of perspectives on critical issues and solutions in the pharmaceutical industry.
Career Advancement: Stay at the forefront of innovation, gain valuable knowledge, and enhance your professional development with the latest scientific research, industry trends, and cutting-edge solutions in healthcare.
Conference Tracks
This year’s event features ten specialized tracks designed to provide deep insights into critical areas of pharmaceutical innovation:
Pharmaceutical: Explore the latest developments in pharmaceutical research, formulation, manufacturing, and regulation, focusing on drug efficacy, safety, and compliance.
Drug Discovery and Development: Gain insights into methodologies and technologies driving drug discovery and development, from identifying drug candidates to clinical trials and market readiness.
Bioavailability and Bioequivalence: Learn about advancements in optimizing drug formulations to ensure maximum therapeutic effect, regulatory compliance, and minimal side effects.
Innovations in Pharma: Witness groundbreaking innovations, from novel drug delivery systems to transformative technologies revolutionizing medicine development and distribution.
Nanotechnology in Drug Delivery: Discover how nanomaterials enhance drug precision and effectiveness, enabling targeted treatments with reduced side effects.
Advanced Drug Delivery Systems: Dive into innovative strategies like sustained, controlled, and targeted drug delivery systems aimed at improving efficiency and patient outcomes.
Biopharmaceuticals and Biosimilars: Explore the growing field of biologic drugs and the role of biosimilars in making life-saving treatments more accessible.
Gene and Cell Therapy: Learn about cutting-edge technologies enabling treatments for genetic disorders and cancers, offering new hope for previously untreatable conditions.
Preventive Medicine: Examine pharmaceutical interventions, vaccines, and lifestyle strategies aimed at reducing chronic disease prevalence and improving public health.
Health Promotion: Explore public health initiatives and pharmaceutical solutions designed to encourage healthier behaviors and improve health outcomes.
Conclusion
The 15th Digital Pharmaceutical Innovations Exhibition & Congress is not just an event—it’s a unique opportunity to be at the forefront of the pharmaceutical industry’s most exciting transformations. From novel drug delivery systems and cutting-edge technologies to personalized medicine and gene therapies, the congress offers a comprehensive view of the innovations shaping the future of healthcare. Don’t miss out on this invaluable chance to connect, learn, and collaborate with industry leaders.
Secure your spot today and be part of this landmark event in 2025. We look forward to seeing you there!
How to Register
Ready to be part of this transformative event? Register now for the 15th Digital Pharmaceutical Innovations Exhibition & Congress and secure your spot to connect with the leaders and innovators of the pharmaceutical world.
👉 Register here: -https://pharmacy.utilitarianconferences.com/registration
More Information
To learn more about the 15th Digital Pharmaceutical Innovations Exhibition & Congress, visit our website or connect with us on social media:
Website: https://utilitarianconferences.com/
Twitter: @UCGConferences
Facebook: Utilitarian Conferences Gathering
Trending Hashtags
#PharmaceuticalInnovation #DrugDiscovery #PharmaTech #PharmaResearch #AdvancedTherapeutics #Biopharmaceuticals #DrugDevelopment #Nanomedicine #GeneTherapy #CellTherapy #SmartDrugDelivery #Bioavailability #Biosimilars #PreventiveMedicine #HealthPromotion
0 notes
Text
How Pharmacometrics In New Jersey – Princeton Can Improve Drug Development
When it comes to Pharmacometrics, it is the quantitative analysis of drug efficiency and safety. By combining data science, statistical modeling, and clinical expertise, Pharmacometrics in New Jersey–Princeton plays a key role in the modern-day drug development process.
Pharmacometrics In New Jersey – Princeton- Why It Is Important In Drug Development?
The pharmaceutical industry often faces pressure to develop effective drugs quickly and cost-efficiently. Pharmacometrics addresses these challenges in different ways:
Enhancing Drug Efficacy and Safety:
Pharmacometric models offer information about the optimal dosage and administration schedules for new drugs. It helps researchers to identify potential side effects and minimize risks.
Reducing Development Time:
Pharmacometric tools allow for predicting the performance of a drug in different patient populations. It boosts the decision-making process.
Personalizing Medicine:
It supports the development of precision medicine by tailoring treatments to individual patients. This improves therapeutic outcomes and lessens the probability of adverse reactions.
Key Applications of Pharmacometrics:
Dose-Response Modeling:
Pharmacometrics enables researchers to model the relationship between drug concentration and therapeutic effect, ensuring optimal dosing strategies.
Clinical Trial Design:
It helps developers to recognize the most competent study designs, reducing the number of participants required and minimizing trial costs.
Regulatory Submissions:
Regulatory agencies, such as the FDA rely on pharmacometric data to evaluate new drug applications. Companies benefit from local expertise in preparing submissions that meet strict regulatory requirements.
Post-Market Surveillance:
Even after a drug is approved, pharmacometrics helps in monitoring its safety and efficiency. Real-world data analysis helps identify any long-term risks or benefits.
Success Stories: Pharmacometrics in Action:
Accelerating Cancer Therapies:
By using pharmacometric models, many Pharmaceutical companies develop targeted cancer treatments. It improves survival rates.
Advancing Rare Disease Treatments:
Pharmacometrics comes with sufficient patient data, allowing the development of therapies for conditions previously deemed untreatable.
Combating Antimicrobial Resistance:
Pharmacometrics is instrumental in designing effective antibiotics that combat resistant strains. It ensures public health safety.
How To Leverage Pharmacometrics For Your Drug Development Needs?
Many pharmaceutical companies and researchers are looking to harness the power of pharmacometrics. They can-
Partner with Local Experts:
By partnering with Princeton-based pharmacometricians and research institutions, they can access advanced tools and methodologies. It offers a competitive edge in drug development.
Invest in Training:
It ensures that your team stays up-to-date with the latest advancements.
Embrace Innovation:
Adopting emerging technologies, positions your company at the forefront of pharmaceutical research. Stay informed about industry trends to remain competitive.
Conclusion:
Pharmacometrics in New Jersey–Princeton is driving innovation in drug development, enabling faster, safer, and more effective therapies.
Galina Bernstein has provided preclinical, clinical pharmacology, Pharmacometrics New Jersey – Princeton and analytical support for multiple clinical programs for over 300 studies including over 30 studies for the pediatric population. She was involved in a large number of studies for rare diseases as well as advanced therapy medicinal products (ATMP) such as gene therapy and many more. Learn more at www.azuredeltaconsulting.com
#clinical pharmacology boston-cambridge#pharmacometrics new jersey - princeton#regulatory strategy boston-cambridge#fda new york - new york city#research triangle park#modeling and simulation m&s north carolina#clinical study protocol san diego#clinical study report san diego
0 notes
Text
The Glioblastoma Treatment Drugs Market is projected to grow from USD 2665 million in 2024 to an estimated USD 5589.2 million by 2032, with a compound annual growth rate (CAGR) of 9.7% from 2024 to 2032. Glioblastoma multiforme (GBM) is the most aggressive and common type of primary brain tumor in adults. Known for its rapid progression and resistance to standard therapies, GBM presents a significant challenge to healthcare professionals and researchers worldwide. The growing prevalence of glioblastoma, coupled with advancements in oncology, has spurred significant developments in the glioblastoma treatment drugs market.
Browse the full report at https://www.credenceresearch.com/report/glioblastoma-treatment-drugs-market
Market Overview
The glioblastoma treatment drugs market is experiencing steady growth, driven by the increasing incidence of GBM and rising investments in cancer research. According to recent statistics, the global glioblastoma treatment market was valued at approximately USD 2.1 billion in 2022 and is projected to grow at a compound annual growth rate (CAGR) of around 8% during the forecast period of 2023–2030.
Current Treatment Options
The primary treatment for glioblastoma typically involves surgical resection, followed by radiation therapy and chemotherapy. Temozolomide (TMZ), an oral alkylating agent, remains the gold standard chemotherapy drug for GBM treatment. However, the recurrence of glioblastoma post-treatment remains a critical concern.
To address these challenges, several new drugs and therapeutic approaches are entering the market, including:
Targeted Therapies Drugs targeting specific molecular pathways, such as EGFR (epidermal growth factor receptor) inhibitors, have shown promise. Agents like bevacizumab, an anti-VEGF (vascular endothelial growth factor) monoclonal antibody, are being used to control tumor angiogenesis and prolong progression-free survival.
Immunotherapies Immunotherapy has emerged as a game-changer in oncology. Immune checkpoint inhibitors, cancer vaccines, and adoptive T-cell therapies are being explored to harness the body’s immune system to fight glioblastoma. Clinical trials of drugs like nivolumab are underway, aiming to improve patient outcomes.
Gene and Cell-Based Therapies Advances in gene editing and cell-based therapies have paved the way for personalized medicine in glioblastoma treatment. Oncolytic viral therapies, which use genetically modified viruses to target cancer cells, are gaining traction in research and development pipelines.
Challenges in the Market
Despite significant progress, the glioblastoma treatment drugs market faces several hurdles:
High Costs: Advanced therapies often come with a hefty price tag, limiting access for many patients.
Complex Biology of GBM: The heterogeneity and adaptability of glioblastoma tumors make them highly resistant to treatment.
Regulatory Barriers: Stringent approval processes for new drugs can delay market entry.
Future Outlook
The future of the glioblastoma treatment drugs market looks promising, driven by ongoing research and technological advancements. The integration of artificial intelligence and big data analytics in drug discovery is expected to expedite the development of effective treatments. Additionally, combination therapies that incorporate multiple approaches—such as chemotherapy, immunotherapy, and gene therapy—are likely to emerge as the new standard of care.
Government initiatives to support cancer research and increasing funding from private organizations further bolster the market’s growth potential. However, addressing the affordability and accessibility of these advanced treatments remains crucial to ensuring better outcomes for patients worldwide.
Key Player Analysis:
Amgen, Inc.
Amneal Pharmaceuticals
Arbor Pharmaceuticals, LLC
AstraZeneca PLC
Hoffmann-La Roche Ltd.
Gene Therapy
GlaxoSmithKline plc (GSK)
Glioma Steam Cell Targeting
Johnson & Johnson
Karyopharm Therapeutics, Inc.
Kinase Inhibitor
Merck & Co., Inc.
MiRNA Targeting
Novartis AG
Pfizer Inc.
Roche Holding AG
Sanofi S.A.
Sun Pharmaceutical Industries Ltd.
Teva Pharmaceutical Industries Ltd.
Segmentation:
By Treatment
Surgery
Radiation Therapy
Chemotherapy
Targeted Therapy
Tumor Treating Field (TTF) Therapy
Immunotherapy
By Drug Class:
Antineoplastic
VEGF/VEGFR Inhibitors
Alkylating Agents
Miscellaneous Antineoplastic
By Distribution Channel:
Hospitals
Cancer Research Organizations
Long Term Care Centers
Diagnostic Centers
By Region:
North America
U.S.
Canada
Mexico
Europe
Germany
France
U.K.
Italy
Spain
Rest of Europe
Asia Pacific
China
Japan
India
South Korea
South-east Asia
Rest of Asia Pacific
Latin America
Brazil
Argentina
Rest of Latin America
Middle East & Africa
GCC Countries
South Africa
Rest of the Middle East and Africa
Browse the full report at https://www.credenceresearch.com/report/glioblastoma-treatment-drugs-market
Contact:
Credence Research
Please contact us at +91 6232 49 3207
Email: [email protected]
0 notes