#adaptimmune
Explore tagged Tumblr posts
Text
Engineered T Cells Market — Forecast(2024–2030).

Engineered T Cells Market Overview
Engineered T cells market represents a revolutionary advancement in cellular therapy, leveraging cutting-edge biotechnology to treat various diseases, primarily cancer. Engineered T cells involve modifying a patient’s T cells to enhance their ability to recognize and attack diseased cells, offering new hope for conditions that were previously difficult to treat. This comprehensive market overview provides insights into the current landscape, key players, applications, challenges, and future trends shaping this rapidly evolving sector.
Request Sample
Market Dynamics
1. Market Growth and Drivers
The engineered T cells market is experiencing substantial growth, driven by several factors:
Increasing Cancer Incidence: The global rise in cancer cases is a major driver, as engineered T cell therapies, such as CAR-T (Chimeric Antigen Receptor T-cell) therapy, offer novel treatments for various cancers.
Advancements in Technology: Innovations in genetic engineering, such as CRISPR and advanced gene-editing techniques, are enhancing the efficacy and safety of engineered T cell therapies.
Growing Investment: Significant investments from both public and private sectors in research and development are fueling advancements and commercialization in this field.
2. Technology and Innovation
Two primary technologies dominate the engineered T cells market:
CAR-T Therapy: This involves modifying T cells to express chimeric antigen receptors (CARs) that target specific proteins on cancer cells. Approved CAR-T therapies, such as Kymriah (Novartis) and Yescarta (Kite Pharma), have shown remarkable success in treating hematologic cancers, including leukemia and lymphoma.
TCR Therapy: T-cell receptor (TCR) therapies focus on enhancing T cells to recognize specific cancer antigens presented by MHC (Major Histocompatibility Complex) molecules. TCR therapies are designed to target a broader range of cancers and are currently in various stages of clinical development.
Inquiry Before Buying
Key Players
Several companies are leading the engineered T cells market, each contributing to the development and commercialization of these therapies:
Novartis: A pioneer in CAR-T therapy, Novartis’ Kymriah was one of the first CAR-T therapies to receive FDA approval. The company continues to advance its pipeline of cell therapies and explore new indications.
Gilead Sciences: Through its subsidiary Kite Pharma, Gilead Sciences has developed Yescarta, another leading CAR-T therapy. Gilead is actively involved in expanding its cell therapy portfolio and researching new treatment options.
Bristol-Myers Squibb: With its acquisition of Celgene, Bristol-Myers Squibb has gained access to innovative CAR-T therapies like Breyanzi. The company is also exploring other cell and gene therapies.
Bluebird Bio: Known for its focus on gene therapies, Bluebird Bio is developing both CAR-T and TCR therapies. The company is advancing its investigational therapies through various stages of clinical trials.
Adaptimmune: Specializing in TCR therapies, Adaptimmune is developing innovative treatments targeting specific cancer antigens. The company is actively working on expanding its clinical trials and therapeutic indications.
Schedule a Call
Applications
1. Oncology
The primary application of engineered T cells is in oncology. CAR-T therapies have shown significant efficacy in treating:
Hematologic Cancers: CAR-T therapies like Kymriah and Yescarta have been particularly effective in treating blood cancers, including acute lymphoblastic leukemia (ALL) and diffuse large B-cell lymphoma (DLBCL).
Solid Tumors: Research is ongoing to extend the use of CAR-T therapies to solid tumors, such as breast, lung, and pancreatic cancers. Challenges include identifying suitable target antigens and overcoming the tumor microenvironment’s immunosuppressive effects.
2. Other Diseases
Beyond oncology, engineered T cells are being explored for:
Autoimmune Diseases: There is potential for engineered T cells to target autoreactive T cells involved in autoimmune conditions such as type 1 diabetes and multiple sclerosis.
Infectious Diseases: Research is investigating the use of engineered T cells to target chronic viral infections, including HIV and hepatitis B.
Buy Now
Regulatory Landscape
1. Approval Process
Engineered T cell therapies must undergo rigorous regulatory scrutiny to ensure their safety and efficacy. The approval process typically involves:
Preclinical Studies: Initial research to evaluate the safety and effectiveness of the therapy in animal models.
Clinical Trials: Phases I through III trials to assess safety, efficacy, and optimal dosing in humans. Successful trials are crucial for obtaining regulatory approval.
Regulatory Review: Submission of clinical trial data to regulatory agencies such as the FDA (U.S. Food and Drug Administration) and EMA (European Medicines Agency) for review and approval.
2. Challenges
Cost: Engineered T cell therapies are expensive to develop and administer, posing challenges for widespread adoption and accessibility.
Manufacturing Complexity: The process of modifying and expanding T cells is complex and requires specialized facilities and expertise.
Side Effects: Potential side effects, such as cytokine release syndrome (CRS) and neurotoxicity, need to be carefully managed and mitigated.
Future Trends
1. Innovations in Technology
Future developments in the engineered T cells market are expected to include:
Next-Generation CAR-T Therapies: Improvements in CAR design, such as dual-targeting CARs and armored CARs, aim to enhance efficacy and reduce side effects.
Combination Therapies: Combining engineered T cells with other modalities, such as immune checkpoint inhibitors, may improve treatment outcomes and address limitations.
2. Personalized Medicine
The shift towards personalized medicine will likely drive market growth. Tailoring therapies to individual patients’ genetic and tumor profiles can enhance treatment efficacy and minimize adverse effects.
3. Global Expansion
As research advances and manufacturing capabilities improve, engineered T cell therapies are expected to become more widely available across different regions, including emerging markets. Collaborative efforts between pharmaceutical companies and research institutions will play a key role in expanding access to these therapies.
The global market size for engineered T cells was $20.21 billion in 2022, and is expected to grow to $348.9 billion by 2032.
For More Information click Here
1 note
·
View note
Text
US FDA approves Adaptimmune's gene therapy for rare type of cancer
0 notes
Text
Adaptimmune Therapeutics Plc faces significant losses in first quarter of 2024 $ADAP #spx #Nasdaq
company recorded first quarter of 2024 operating shortfall of $-49.261 millionThe recent financial results of Adaptimmune Therapeutics Plc have sent shockwaves through the market, revealing a disastrous start to the year. The company disclosed its January to March 31, 2024 numbers, and the figures are far from encouraging. During this period, Adaptimmune experienced a staggering decline in revenue, which plummeted by a significant -85.453%, falling to a meager $7.02 million. This drastic reduction has raised numerous concerns among investors,
0 notes
Text

Canada Car T-Cell Therapy Market Report 2022 to 2030
Canada Car T-Cell Therapy Market valued at $232 Mn in 2022, projected to reach $393 Mn by 2030 with a 6.8% CAGR. The growing occurrence and prevalence of cancer worldwide, driven by factors like population aging, unhealthy lifestyles, and environmental influences, contribute to an expanding pool of patients who may potentially benefit from CAR T-Cell therapy, thereby expanding the market for this treatment. The top pharmaceutical entities presently operating in the market are Gilead Sciences (Kite Pharma), Novartis, Bristol Myers Squibb, Celgene, Johnson & Johnson, Cellectis, Atara Biotherapeutics, Adaptimmune Therapeutics, Poseida Therapeutics and UCAR-T.
#canada#market analysis#market forecast#market research#market insights#market report#healthcare#pharmaceutical
0 notes
Text
T Cell Therapy Market Size was valued at USD 3.8 Billion in 2023 and is expected to reach a market size of USD 49.9 Billion by 2032
The T cell therapy market size was valued at USD 3.8 Billion in 2023 and is expected to reach a market size of USD 49.9 Billion by 2032 at a CAGR of 32.9%.
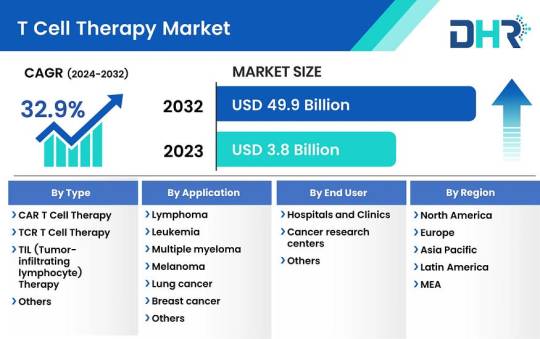
Request Sample Report: https://datahorizzonresearch.com/request-sample-pdf/t-cell-therapy-market-3114
Top Companies are:
· Novartis AG
· Gilead Sciences, Inc.
· Bristol-Myers Squibb Company
· Adaptimmune Therapeutics plc
· Amgen Inc.
· Atara Biotherapeutics, Inc.
· Autolus Therapeutics plc
· bluebird bio, Inc.
· Cellectis S.A.
· Iovance Biotherapeutics, Inc.
· Kite Pharma (a Gilead Company)
· Tmunity Therapeutics, Inc.
Market Segmentations:
By Type-
CAR T cell therapy
TCR T cell therapy
TIL (Tumor-infiltrating lymphocyte) therapy
Others
By Application-
Lymphoma
Leukemia
Multiple myeloma
Melanoma
Lung cancer
Breast cancer
Colorectal cancer
Autoimmune disorders
Infectious diseases
Others
By End User-
Hospitals and Clinics
Cancer research centers
Others
Regional Analysis:
The dominance of the T Cell Therapy market in North America is underpinned by the presence of established biopharmaceutical firms, a robust clinical trial infrastructure, and a favorable regulatory landscape. Among North American countries, the United States stands out as the primary contributor, buoyed by the FDA’s approval of several CAR T cell therapies such as Kymriah, Yescarta, and Abecma for treating hematological malignancies. The U.S. National Library of Medicine’s database reveals an extensive presence of over 1,000 active clinical trials dedicated to evaluating T cell therapies, underscoring the region’s steadfast commitment to research and development in this field.
Key highlights of the report include:
1. The report delivers thorough Market analysis, furnishing valuable insights to guide strategic decision-making.
2. The comprehensive research outlined in the study enhances the depth of your presentations and marketing strategies.
3. By offering crucial insights into key market competitors, the study empowers businesses with a strategic edge.
4. It delivers a precise assessment of evolving market dynamics, ensuring readers stay abreast of the latest industry trends.
5. With meticulous breakdowns of various market niches, the report facilitates informed decision-making processes.
0 notes
Text

𝐄𝐱𝐩𝐥𝐨𝐫𝐢𝐧𝐠 𝐭𝐡𝐞 𝐅𝐫𝐨𝐧𝐭𝐢𝐞𝐫 𝐨𝐟 𝐂𝐚𝐧𝐜𝐞𝐫 𝐆𝐞𝐧𝐞 𝐓𝐡𝐞𝐫𝐚𝐩𝐲 !
𝐑𝐞𝐪𝐮𝐞𝐬𝐭 𝐚 𝐅𝐑𝐄𝐄 𝐬𝐚𝐦𝐩𝐥𝐞 : https://www.nextmsc.com/cancer-gene-therapy-market/request-sample?utm_source=sanyukta-27-march-24&utm_medium=sanyukta-tumblr&utm_campaign=sanyukta-cancer-gene-market
The landscape of cancer treatment is evolving, and gene therapy is emerging as a promising frontier in the fight against this relentless disease. With advancements in molecular biology and genetic engineering, researchers are harnessing the power of genes to target and treat cancer in ways previously unimaginable.
𝐔𝐧𝐝𝐞𝐫𝐬𝐭𝐚𝐧𝐝𝐢𝐧𝐠 𝐭𝐡𝐞 𝐌𝐞𝐜𝐡𝐚𝐧𝐢𝐬𝐦𝐬: Gene therapy involves the modification of a patient's genetic material to either repair or replace faulty genes, or to bolster the body's ability to fight cancer cells. This approach holds immense potential for personalized medicine, as treatments can be tailored to an individual's specific genetic makeup and cancer subtype.
𝐏𝐫𝐨𝐦𝐢𝐬𝐢𝐧𝐠 𝐈𝐧𝐧𝐨𝐯𝐚𝐭𝐢𝐨𝐧𝐬: In recent years, there have been groundbreaking breakthroughs in cancer gene therapy. From CAR-T cell therapy, which involves modifying a patient's own immune cells to recognize and destroy cancer cells, to oncolytic viruses that selectively infect and kill tumor cells while sparing healthy tissue, the possibilities are expanding rapidly.
𝐌𝐚𝐫𝐤𝐞𝐭 𝐎𝐮𝐭𝐥𝐨𝐨𝐤: The global cancer gene therapy market is poised for substantial growth, with projections indicating a compound annual growth rate (CAGR) of over 33% from 2024-2030. Factors driving this growth include increasing prevalence of cancer, growing investments in research and development, and regulatory approvals for novel gene therapy treatments.
𝐂𝐨𝐥𝐥𝐚𝐛𝐨𝐫𝐚𝐭𝐢𝐨𝐧 𝐚𝐧𝐝 𝐈𝐧𝐧𝐨𝐯𝐚𝐭𝐢𝐨𝐧: As we navigate this dynamic landscape, collaboration between researchers, clinicians, biotech companies, and regulatory agencies is crucial. By pooling our expertise and resources, we can accelerate the development and commercialization of innovative gene therapies, ultimately improving outcomes for cancer patients worldwide.
𝐌𝐚𝐣𝐨𝐫 𝐌𝐚𝐫𝐤𝐞𝐭 𝐏𝐥𝐚𝐲𝐞𝐫𝐬: Comprehensive competitive analysis and profiles of the major market players such as Bluebird bio, Inc., Merck, Adaptimmune, GlaxoSmithKline, BioCancell, Shenzhen SiBiono GeneTech, SynerGene Therapeutics, Celgene, Shanghai Sunway Biotech, OncoGenex Pharmaceuticals, and others are provided in the cancer gene therapy market report.
Together, let's continue to push the boundaries of science and innovation to transform the future of cancer treatment.
#cancergenetherapy#innovation#lifesciences#cancerresearch#biotechnologyanddiagnotics#healthcare#marketresearch#markettrends#businessinsights
0 notes
Text
Synovial Sarcoma Market Poised for Phenomenal Expansion During the Forecast Period (2023-32) – Estimates DelveInsight | Adaptimmune, Advenchen, Bayer, Eli Lilly, Epizyme, GSK, Ipsen, Takara Bio
http://dlvr.it/T2flwq
0 notes
Text
🌎 Highwoods Properties Downgraded, Salesforce Upgraded, Extra Space Storage Upgraded and more...
Ratings changes for Micron Technology, Micron Technology, Amazon.com, Cipher Mining, vTv Therapeutics, Adaptimmune Therapeutics, argenx and more…Upgrade to MarketBeat All Access to get early delivery, personalized stock alerts, portfolio monitoring tools, and more. Start Your Free Trial. Unsubscribe. VIEW MY PORTFOLIO December 21st, 2023 The A.I. Trend Has These 7 Stocks Set To Soar…

View On WordPress
0 notes
Text
CAR-T Pipeline Drugs Analysis Report, 2023: FDA Approvals, Clinical Trials, Therapies, by DelveInsight | AbbVie Inc., Adaptimmune Therapeutics PLC., Amgen, Inc., Atara Biotherapeutics, Inc., Aurora
http://dlvr.it/Srn5JD
0 notes
Text
CAR-T Pipeline Drugs Analysis Report, 2023: FDA Approvals, Clinical Trials, Therapies, by DelveInsight | AbbVie Inc., Adaptimmune Therapeutics PLC., Amgen, Inc., Atara Biotherapeutics, Inc., Aurora
http://dlvr.it/Srmls1
0 notes
Text
CAR-T Pipeline Drugs Analysis Report, 2023: FDA Approvals, Clinical Trials, Therapies, by DelveInsight | AbbVie Inc., Adaptimmune Therapeutics PLC., Amgen, Inc., Atara Biotherapeutics, Inc., Aurora
http://dlvr.it/SrmlMH
0 notes
Text
CAR-T Pipeline Drugs Analysis Report, 2023: FDA Approvals, Clinical Trials, Therapies, by DelveInsight | AbbVie Inc., Adaptimmune Therapeutics PLC., Amgen, Inc., Atara Biotherapeutics, Inc., Aurora
http://dlvr.it/Srmkgy
0 notes
Text
Cancer Gene Therapy Market 2022 New Technological Development Projecting Massive Growth till 2032
[300+ Pages Report] As per Future Market Insights’ latest revised industry analysis, the global cancer gene therapy market is projected to expand at a 10.1% CAGR over the forecast period, with the market size reaching US$ 5.3 Bn in 2032.
Gene therapy is a field of medicine that aims to cure or greatly improve the treatment of diseases that have few or no treatment options. Advanced-stage cancer and hematological illnesses make up a large share of gene therapy candidates. In addition to this, gene therapy is frequently used to treat rare or inherited diseases.
While gene therapy research is still in its early stages, firms are increasingly investing in the technology. A handful of products have recently received approval outside of Canada or are in the final stages of clinical trials.
Gene therapy is currently extremely expensive. Multi-stakeholder discussions about pricing and reimbursement management for these goods are required. To provide accessibility and quality of care, specialized manufacturing facilities, care centers, and doctors skilled to undertake specific procedures for such therapies are required.
The growth in the cancer gene therapy market is reliant on increasing awareness about health, the growing incidence of cancer, and the latest advancements in cancer gene therapy.
“Rising awareness regarding cancer gene therapy across emerging economies, along with favorable healthcare reimbursement plans in various countries will create opportunities for growth in the market over the forecast period,” says an FMI analyst.
Key Players :
Merck KGaA
Novartis AG
AstraZeneca Plc.
BIOCAD
Crinetics Pharmaceuticals, Inc.
EffRx Pharmaceuticals S.A.
Euroscreen S.A.
Vicore Pharma AB
Amgen
Bristol-Myers Squibb
Cell Genesys Inc.
Adaptimmune Therapeutics plc.
Achieve Life Science Inc.
BioCanCell Ltd.
Genelux Corporation
Advantagene Inc.,
GenVec Inc.
GlaxoSmithKline PLC
Amgen Inc.
For More Information: https://www.futuremarketinsights.com/reports/cancer-gene-therapy-market
Key Takeaways:
Based on therapy, the oncolytic virotherapy segment accounted for about 3% of the total market share in 2021.
In terms of indication, sales in the breast cancer segment are forecast to grow at a CAGR of 7% in the forecast period.
By service provider, demand in the hospitals segment will grow at a 9.3% CAGR through 2032.
The U.S. will dominate the North America cancer gene therapy market over the forecast period.
China will emerge as a lucrative pocket, accounting for 32.3% of the East Asia cancer gene therapy market share over the assessment period.
Demand in Germany is expected to increase at a 11.9% CAGR over the assessment period.
Competition Landscape
Acquisitions, partnerships, geographical recognition, and product launches are the key strategies adopted by leading players to increase the consumer base. For instance:
In March 2022, Novartis and Carisma Therapeutics agreed to collaborate on the development of HER 2 targeted CAR-M cell therapy.
A2 Biotherapeutics and Merck announced a collaboration in December 2020 to develop allogeneic cell therapy for solid tumor cancers.
Want More Insights
Future Market Insights brings a comprehensive research report on forecasted revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2017 to 2032. The global cancer gene therapy market is segmented in detail to cover every aspect of the market and present a complete market intelligence approach to the reader. The study provides compelling insights on the cancer gene therapy market segment based on therapy – (gene induced immunotherapy, oncolytic virotherapy, gene transfer, and others), by indication (breast cancer, ovarian cancer, liver cancer, pancreatic cancer, lung cancer, and prostate cancer), by service provider (hospitals, clinical research laboratory, and oncology institutes), across seven major regions.
About Healthcare Division at Future Market Insights
Future Market Insights facilitates corporates, government, investors, and associated audiences in the healthcare sector to identify and accentuate vital aspects applicable to product strategy, regulatory landscape, technology evolution, and other crucial issues to achieve sustainable success. Our unique approach to gathering market intelligence equips you in devising innovation-driven trajectories for your business.
By Therapy:
Gene Induced Immunotherapy
Oncolytic Virotherapy
Gene Transfer
Others
By Indication:
Breast Cancer
Ovarian Cancer
Liver Cancer
Pancreatic Cancer
Lung Cancer
Prostate Cancer
By Service Provider:
Hospitals
Clinical Research Laboratory
Oncology institutes
0 notes
Text

Adaptimmune Therapeutics Reports a Slight Per-Share Shortfall of $0.03 during Q3 2023 Earnings Season https://csimarket.com/stocks/news.php?code=ADAP&date=2023-11-09211950&utm_source=dlvr.it&utm_medium=tumblr
0 notes
Link
The stock for stock transaction will see Adaptimmune shareholders own 75% of the combined company, which will target solid tumors. The deal, which is expected to close in the second quarter of 2023, sees Adaptimmune and TCR² Therapeutics merge to #BioTech #science
0 notes
Text
Fourth-generation chimeric antigen receptor T Cells for hepatocellular carcinoma
Results for multiple hepatocellular carcinoma (HCC) clinical trials were presented at the 2021 American Society of Clinical Oncology (ASCO) virtual yearly meeting held June 4-8 June. CARsgen Therapeutics presented data from a Stage I trial (NCT03980288) of its fourth-generation fanciful antigen receptor T (Vehicle T) cells targeting glypican-3 (GPC3). Fourth-generation Vehicle T cells are additionally designed to secrete a transgenic cytokine upon Vehicle motioning in the targeted tumor tissue. As a result, in addition to the direct antitumor attack, they can trigger T cells to eliminate antigen-negative malignant growth cells at the target site. GPC3 is a cell-Cells Expressing T Cell Surface Glycoprotein CD5 Drugs Development Market that is profoundly communicated in HCC and is thus being evaluated as a target for HCC immunotherapy. CARsgen's original pipeline therapy for cutting edge HCC stands out for being a first-in-class approach with an extraordinary system of action that targets vigorously pretreated progressed HCC patients.
In this study, CARSgen's fourth-generation Vehicle GPC3 T cells in combination with the tyrosine kinase inhibitors (TKIs) Nexavar (sorafenib) or Stivarga (regorafenib) demonstrated sensible safety and potential antitumor activity for hepatitis B infection (HBV)- related metastatic HCC patients who have been recently treated with systemic therapy. Safety results showed a shortfall of portion limiting toxicity, treatment-related serious unfavorable events (AEs), AE-related death or study withdrawal, and neurotoxicity. Three out of the six patients in the study experienced grade 3 cytokine discharge condition (CRS), a typical AE found because of Vehicle T cell therapy. Fundamental viability results uncovered the objective reaction rate and infectious prevention rate were 16.7% and half, respectively. The middle movement free endurance was 4.2 months. The middle pinnacle Vehicle GPC3 duplicate number in patients' fringe blood on day 7 after treatment was 5,067 duplicates for each μg genomic DNA and Vehicle GPC3 duplicates were still detectable on day 28 at levels running between 113 and 2,071 duplicates for every μg genomic DNA. Prior Stage I trials for the second-generation variant of this therapeutic agent (NCT02395250 and NCT03146234) likewise reported clinical benefit and tolerability.
KOLs interviewed by GlobalData noted that Vehicle T cells or adoptive cellular therapy is an arising region that might change HCC treatment practice from here on out. The majority of late-stage pipeline drugs for cutting edge HCC are for use in the first-line setting, which has the highest number of treated cases. Therefore, subsequent lines of therapy are being neglected by drug designers, despite high unmet need. Notwithstanding, CARsgen Therapeutics conducted the study of their Vehicle T cell therapy on patients who advanced on at least two lines of systemic therapy, having tried at least one TKI joined with anti-modified death-1 (PD-1)/customized death ligand 1 (PD-L1) immunotherapy or FOLFOX4 (5-fluorouracil, leucovorin, and oxaliplatin) chemotherapy. A comparative strategy of zeroing in on subsequent lines of therapy was utilized by other organizations studying adoptive cellular therapy for HCC in the beginning phase development including Immunotech Biopharm, Immunicum Stomach muscle, Aha Therapeutics, Adaptimmune Therapeutics, Fate Therapeutics, and Origincell Therapeutics. Therefore, investigation of vigorously pretreated progressed HCC patients is by all accounts a general trend with Vehicle T cell designers in HCC.
The HCC clinical pipeline is packed with me-too drugs targeting patients in the first-line setting. CARsgen's Vehicle GPC3 T cell therapy is a first-in-class targeted drug tending to cutting edge HCC patients who have advanced on or created resistance to past therapy — a patient population with little to no treatment options. GlobalData expects that assuming effective in later stage trials, CARsgen's therapy will be advantageous to underserved patients in late-stage progressed HCC.
Cell and Quality Therapy Inclusion on Clinical Trials Field supported by Cytiva.
Editorial content is independently created and keeps the highest guidelines of journalistic integrity. Topic supports are not associated with the creation of editorial content.
0 notes