#acute ischemic stroke Clinical Trials Market Analysis
Explore tagged Tumblr posts
Text
Discover the latest trends and developments in acute ischemic stroke clinical trials Analysis. Explore insights on market growth, key players, and innovative treatments advancing stroke care in this comprehensive analysis. Acute ischemic stroke (AIS) occurs when blood flow to the brain is obstructed by a clot, leading to significant disability or death if untreated. Recent years have seen substantial progress in AIS clinical trials, with a focus on innovative therapies and faster diagnosis.
Key Players and Innovations in the Market
Key pharmaceutical companies and medical device manufacturers are actively involved in AIS trials, aiming to push the boundaries of stroke treatment. Leading companies include Genentech, Medtronic, and Bayer, each bringing innovative solutions to the market.
Key Innovations:
Endovascular Therapy: Mechanical thrombectomy devices that remove clots directly are becoming more common in trials, especially for severe AIS cases.
Stem Cell Therapy: Although still in the experimental phase, stem cell therapy shows promise in promoting neural repair and regeneration.
AI in Diagnostics: Artificial intelligence and imaging software aid in rapid diagnosis, helping to reduce treatment delays and improve outcomes.
Market Drivers and Restraints
Several factors are driving growth in the AIS clinical trial market, including:
Increased Incidence of Stroke: As populations age, the prevalence of stroke increases, driving demand for more effective treatments.
Advancements in Imaging Technology: Improved diagnostics enable faster and more accurate treatment initiation.
Rising Investment in Neurology: Pharmaceutical companies recognize the demand for stroke treatments, leading to heightened investment in clinical trials.
0 notes
Text
Market Share Strategies: Tenecteplase Drug Market Industry Insights
Market Overview –
The Tenecteplase Drug Market was valued at USD 1.8 billion in 2022. The Tenecteplase Drug Market is expected to increase from USD 1.89 billion in 2023 to USD 2.8 billion by 2032, with a compound annual growth rate (CAGR) of 5.10% over the forecast period (2023-2032).
The Tenecteplase Drug Market is witnessing steady growth, driven by the increasing use of TNK Injection in the treatment of acute myocardial infarction (heart attack). Tenecteplase is a thrombolytic medication that helps dissolve blood clots, restoring blood flow to the heart. The market offers various formulations and dosages to meet clinical needs, supporting improved outcomes for patients with heart attacks.
The tenecteplase drug market is experiencing growth driven by its efficacy and safety profile in the management of acute ischemic stroke and myocardial infarction. Tenecteplase is a recombinant tissue plasminogen activator (rt-PA) used to dissolve blood clots and restore blood flow to occluded arteries, thereby reducing the risk of stroke or heart attack-related disability and death.
Key drivers of market growth include the increasing incidence of cardiovascular diseases, aging populations, and the growing adoption of thrombolytic therapy in emergency care settings.
Moreover, the availability of tenecteplase as a single bolus injection simplifies administration and improves treatment adherence compared to older rt-PA formulations. Additionally, clinical trials evaluating the efficacy of tenecteplase in extended time windows for stroke treatment and in prehospital settings are expanding its potential indications and market opportunities.
However, challenges such as bleeding complications, treatment delays, and healthcare disparities in access to emergency care remain concerns for patients and healthcare providers. Overall, the tenecteplase drug market is poised for further growth as research continues to validate its role in improving outcomes for patients with acute ischemic stroke and myocardial infarction.
Segmentation –
The global tenecteplase drug market is segmented on the basis of application, dosage and end users.
The tenecteplase drug market, by application segmented into myocardial infarction, stroke, deep vein thrombosis. The tenecteplase drug market, by dosage intravenous dosage and intracatheter instillation dosage. Intravenous dosage is sub-segmented into adult and geriatric and intracatheter instillation dosage is sub-segmented into adult, children, and adolescents weighing 30 kg, infants. On the bases of end-user, it is segmented into hospitals and clinics, surgical centers, research centers and other.
Regional Analysis –
The market for tenecteplase, a thrombolytic drug used in the treatment of acute myocardial infarction, exhibits regional variations influenced by factors such as healthcare infrastructure, treatment guidelines, and prevalence of cardiovascular diseases.
North America dominates the market, with the United States accounting for a significant share of tenecteplase usage. Advanced healthcare facilities and high prevalence of cardiovascular diseases contribute to market growth in this region. Europe follows suit, with countries like the UK, Germany, and France adopting tenecteplase as part of standard treatment protocols for myocardial infarction.
In the Asia Pacific region, increasing awareness about cardiovascular health and improving access to healthcare services drive market growth, particularly in countries like China and India. Latin America and the Middle East & Africa regions also show potential for market expansion, albeit with challenges related to healthcare access and affordability. Overall, the regional analysis highlights the importance of evidence-based treatment guidelines and access to quality care in optimizing the use of tenecteplase across different regions.
Key Players –
Tenecteplase drug companies include Boehringer Ingelheim International GmbH, Genentech Inc., Genova Pharmaceutical, Rewine Pharmaceuticals, Hisun USA, Emcure Pharmaceuticals, Merck Ltd., and Crunchbase Inc.
Related Reports –
Anticoagulation
Heart Failure POC and LOC Devices
Cellulite Treatment
Epilepsy Devices
For more information visit at MarketResearchFuture
0 notes
Text
Interventional Neurology Market: Advancements in Neurovascular Treatments Drive Growth
A) Market Overview:
The global Interventional Neurology Market is estimated to be valued at US$2,450.6 million in 2022 and is expected to exhibit a CAGR of 5.3% over the forecast period of 2022-2030. Interventional neurology involves minimally invasive procedures to treat neurological disorders, such as stroke, aneurysms, and arteriovenous malformations. These procedures offer several advantages, including shorter hospital stays, reduced recovery time, and improved patient outcomes. The increasing prevalence of neurological diseases and the need for effective treatment options are driving the demand for interventional neurology products globally.
B) Market Key Trends:
One key trend in the Interventional Neurology Market Size is the rise in demand for neurovascular devices. Neurovascular devices are crucial in the diagnosis and treatment of various neurological conditions. For instance, the demand for clot retrieval devices used in the treatment of acute ischemic strokes is on the rise. These devices help remove blood clots from the brain, restoring blood flow and minimizing long-term damage. The market is witnessing advancements in technologies, such as the development of stent retrievers and aspiration catheters, which enable efficient clot extraction.
An example of supporting this trend is the Solitaire™ Revascularization Device developed by Medtronic. It is a highly effective clot retrieval device that has shown improved outcomes in clinical trials. The device enables rapid restoration of blood flow, minimizing neurological damage and improving patient outcomes. The increasing adoption of such advanced neurovascular devices is significantly contributing to the growth of the market.
C) Porter's Analysis:
- Threat of New Entrants: The high entry barriers due to strict regulatory requirements and the need for substantial investments in research and development act as deterrents for new entrants.
- Bargaining Power of Buyers: The demand for interventional neurology products is increasing, giving buyers the advantage of choice and negotiation. However, the critical nature of these products allows suppliers to maintain a certain level of bargaining power.
- Bargaining Power of Suppliers: The market is dominated by established players with a strong supplier base. This gives suppliers significant bargaining power in terms of pricing and product quality.
- Threat of New Substitutes: The market is witnessing advancements in alternative treatment methods, such as telemedicine-based interventions. However, these substitutes are not yet considered as effective as traditional interventional neurology procedures. - Competitive Rivalry: The market is highly competitive, with key players constantly investing in research and development to introduce innovative products. The presence of established players and high market consolidation contribute to intense competition.
D) Key Takeaways:
Market size: The global Interventional Neurology Market is expected to witness high growth, exhibiting a CAGR of 5.3% over the forecast period. This growth can be attributed to the increasing prevalence of neurological disorders, coupled with advancements in interventional neurology technologies.
Regional analysis: North America is currently the fastest-growing and dominating region in the Interventional Neurology Market. This can be attributed to factors such as the presence of key market players, favorable reimbursement policies, and a high prevalence of neurological disorders in the region.
Key players: Key players operating in the global Interventional Neurology Market include Penumbra, Inc., Medtronic, Stryker, Terumo Corporation, Johnson & Johnson Services, Inc., Boston Scientific Corporation, MicroPort Scientific Corporation, Merit Medical Systems, W. L. Gore & Associates, Inc., Abbott, Cook, and Palex Medical. These companies focus on product innovation, partnerships, and acquisitions to strengthen their market position.
In conclusion, the Interventional Neurology Market is witnessing significant growth due to the increasing demand for neurovascular devices and advancements in treatment options. The market is highly competitive, with key players constantly striving to introduce innovative products. North America is currently the leading market in terms of growth and dominance. With the rise in neurological disorders worldwide, the demand for interventional neurology products is only expected to increase in the coming years.
0 notes
Text
Global Alteplase Market Size, Share, Trends, Application Analysis and Growth Opportunities Forecast to 2028
Alteplase is a thrombolytic medication which used for the treatment of acute myocardial infarctions and other severe conditions that are caused due to blood clotting. This thrombolytic medication works by breaking up and dissolving the blood clots that can result in artery blockage. It is manufactured by recombinant DNA technology and given by injection into a vein or artery. It is sterile and purified glycoprotein of 527 amino acids. It is also known as Activase and synthesized using the complementary DNA (cDNA) for natural human tissue type plasminogen activator which is obtained from the human melanoma cell line.
Global Alteplase Market, By Product Type (Powder, Solution, Others), Dosage (2 mg, 50 mg, 100 mg), Application (Acute Ischemic Stroke, Acute Myocardial Infarction, Acute Massive Pulmonary Embolism, Others), End-Users (Clinic, Hospital, Others), Distribution Channel (Hospital Pharmacy, Retail Pharmacy, Online Pharmacy), Country (U.S., Canada, Mexico, Brazil, Argentina, Peru, Rest of South America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Hungary, Lithuania, Austria, Ireland, Norway, Poland, Rest of Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Vietnam, Rest of Asia-Pacific, Saudi Arabia, U.A.E, Egypt, Israel, Kuwait, South Africa, Rest of Middle East and Africa) Industry Trends and Forecast to 2028.
Get Sample Copy Of This Report @ https://www.databridgemarketresearch.com/request-a-sample/?dbmr=global-alteplase-market
The major players covered in the global alteplase market report are Boehringer Ingelheim International GmbH, Kyowa Kirin Co., Ltd., Genentech, Inc., Mitsubishi Tanabe Pharma Corporation, F. Hoffmann-La Roche Ltd., Microbix Biosystems, medac GmbH, MOCHIDA PHARMACEUTICAL CO., LTD., Taj Pharma (India) Ltd., and Sedico Co., among other domestic and global players. Market share data is available for Global, North America, Europe, Asia-Pacific (APAC), Middle East and Africa (MEA), and South America separately. DBMR analysts understand competitive strengths and provide competitive analysis for each competitor separately.
The study objectives of this report are :
To define, describe and forecast the market by type, application and region.
To analyze the global and key regions market potential and advantage, opportunity and challenge, restraints and risks.
To strategically analyze each submarket with respect to individual growth trend and their contribution to the market
To analyze competitive developments such as expansions, agreements, new product launches, and acquisitions in the market
To strategically profile the key players and comprehensively analyze their growth strategies.
To analyze and study the global alteplase sales, value, status (2021-2021) and forecast (2021-2028);
To analyze the top players in North America, Europe, China, Japan, Southeast Asia and India, to study the sales, value and market share of top players in these regions.
Focuses on the key alteplase players, to study the sales, value, market share and development plans in future.
Focuses on the global key manufacturers, to define, describe and analyze the market competition landscape, SWOT analysis.
Alteplase Market Scope and Market Size
The alteplase market is segmented on the basis of product type, dosage, application, end-users, and distribution channel. The growth amongst these segments will help you analyze meager growth segments in the industries and provide the users with valuable market overview and market insights to help them in making strategic decisions for identification of core market applications.
· On the basis of product type, the alteplase market is segmented into powder, solution, and others.
· On the basis of dosage, the alteplase market is segmented into 2 mg, 50 mg and 100 mg.
· On the basis of application, the alteplase market is segmented into acute ischemic stroke, acute myocardial infarction, acute massive pulmonary embolism and others.
· On the basis of end-users, the alteplase market is segmented into clinic, hospital and others.
· The alteplase market is also segmented on the basis of distribution channel into hospital pharmacy, retail pharmacy and online pharmacy.
Customization Available: Global Alteplase Market
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Factbook) or can assist you in creating presentations from the data sets available in the report.
0 notes
Text
Neuropathic Pain Market – Insights
Neuropathic pain is a complex, chronic pain state that usually is accompanied by tissue injury. It may lead to damage, dysfunction, or injury of nerve fibers, thereby leading to misfiring of signals to other pain centers. The impact of a nerve fiber injury includes a change in nerve function both at the site of injury and areas around the injury.
The global neuropathic pain market is estimated to account for US$ 6,313.4 Mn in terms of value in 2019 and is expected to reach US$ 9,862.3 Mn by the end of 2027.
Global Neuropathic Pain Market: Drivers
High prevalence of cancer is expected to boost growth of the global neuropathic pain market over the forecast period. For instance, according to the World Health Organization, around 18.1 million new cases and 9.6 million deaths were registered due to cancer worldwide in 2018.
Moreover, increasing geriatric population is also expected to aid in growth of the market. For instance, according to the World Health Organization, geriatric population is expected to reach 2 billion by 2050, up from 900 million in 2015.
North America region held dominant position in the global neuropathic pain market in 2019, accounting for 35.9% share in terms of value, followed by Europe.
Global Neuropathic Pain Market: Restraints
Incomplete pain relief is expected to hinder growth of the global neuropathic pain market. For instance, use of strong opioids such as oxycodone in the treatment of neuropathic pain often leads to low reduction in pain.
Moreover, side-effects of medications are also expected to limit growth of the market. Use of opioids and steroids may lead to complications such as heart attack, kidney failure, and damage in lungs.
Global Neuropathic Pain Market: Opportunities
High prevalence of neuropathic-like pain in psoriatic arthritis is expected to offer lucrative growth opportunities for players in the market. For instance, in July 2019, researchers from Cardiff University, U.K., reported that neuropathic-like pain as evidence of abnormal pain processing is common in patients with psoriatic arthritis.
Moreover, R&D in neuropathic pain is also expected to aid in growth of the market. For instance, in March 2020, researchers from Heidelberg University, Germany, reported assessment of the phytochemical composition and the possible prophylactic effects of an aqueous ethanol extract of Haematoxylon campechianum flowers on peripheral neuropathic pain in a chronic constriction injury rat model.

Anticonvulsants segment in the global neuropathic pain market was valued at US$ 2,548.1 Mn in 2019 and is expected to reach US$ 4,310.3 Mn by 2027 at a CAGR of 6.4% during the forecast period.
Market Trends/Key Takeaways
Use of flavonoids can be effective in the treatment of neuropathic pain due to spared nerve injury, spinal nerve ligation, partial sciatic nerve injury, diabetes-induced neuropathy, chemotherapy-induced neuropathy, and chronic constriction injury.
Several studies have demonstrated that electroacupuncture can be effective in decreasing neuropathic pain. For instance, in January 2020, researchers from Huazhong Agricultural University, China, demonstrated that Synaptotagmin 1 (Syt-1), a synaptic vesicle protein for regulating exocytosis of neurotransmitters, impairs neuropathic pain and that electroacupuncture relieves neuropathic pain through down-regulating spinal Syt-1.
Request the sample copy of here:
https://www.coherentmarketinsights.com/insight/request-sample/3656
Download the PDF Brochure here:
https://www.coherentmarketinsights.com/insight/request-pdf/3656
Global Neuropathic Pain Market: Competitive Landscape
Major players operating in the global neuropathic pain market include, Pfizer, Inc., Johnson & Johnson Services, Inc., Sanofi S.A., Eily Lily and Company, GlaxoSmithKline PLC, Biogen Idec., Bristol-Myers Squibb, Baxter Healthcare Corporation, and Depomed, Inc.
Global Neuropathic Pain Market: Key Developments
Major players in the market are focused on approval and launch of new products in order to expand their product portfolio. For instance, in March 2020, Senzer Pharmaceuticals secured the Investigational New Drug application and data package for its ongoing FDA registration program from its former US strategic partner in order to get approval for its cannabinoid respiratory device for the treatment of side effects induced by anti-cancer treatments, specifically nausea, vomiting, and neuropathic pain.
Major players in the market are also focused on conducting clinical trials to expand their product portfolio. For instance, in February 2020, NoNO Inc., a privately-held biotechnology company, reported that novel peptide, nerinetide, without prior administration of alteplase, demonstrated medically important improvements in patients with acute ischemic stroke, in a multicenter, randomized, study.
Buy now the market research report here:
https://www.coherentmarketinsights.com/insight/buy-now/3651
About Us:
Coherent Market Insights is a global market intelligence and consulting organization focused on assisting our plethora of clients achieve transformational growth by helping them make critical business decisions.
What we provide:
Customized Market Research Services
Industry Analysis Services
Business Consulting Services
Market Intelligence Services
Long term Engagement Model
Country Specific Analysis
Explore CMI Services here
Contact Us:
Mr. Shah
Coherent Market Insights Pvt. Ltd.
Address: 1001 4th ave, #3200 Seattle, WA 98154, U.S.
Phone: +1-206-701-6702
Email: [email protected]
Source: https://www.coherentmarketinsights.com/market-insight/neuropathic-pain-market-3656
0 notes
Text
Ischemia Reperfusion Injury Therapeutics Market Analysis (2020-2027)
Ischemic injury occurs due to the insufficient blood supply to the tissue. According to the National Institutes of Health, the burden of cardiovascular diseases (CVDs) such as stroke and myocardial infarction is increasing rapidly at a global level. This would, in turn, increase the number of patients with ischemia-reperfusion injury. The condition lowers the level of ATP production and thus inactivates ion-exchange channels. Ischemia reperfusion injury is a critical medical condition that poses a major therapeutic challenge for physicians. Key players in the market are involved in research and development of novel drugs and treatment which is expected to escalate the growth of the global ischemia reperfusion injury therapeutics market.
The rising number of tissue damage cases and the increasing awareness about ischemia reperfusion injury is expected to drive growth of the global ischemia reperfusion injury therapeutics market during the forecast period. Moreover, the increasing investments by key players in research & development of novel treatment for ischemia reperfusion are estimated to boost the market growth. For instance, in November 2018, Revive Therapeutics received the Orphan Drug designation for Cannabidiol from the U.S. Food and Drug Administration (FDA) to prevent ischemia reperfusion injury resulting from organ transplantation.
Global Ischemia Reperfusion Injury Therapeutics Market - Impact of Coronavirus (Covid-19) Pandemic
The global ischemia reperfusion therapeutics market growth has been affected by the COVID-19 pandemic as it has become difficult to conduct research in the current situation. Thus, several market players are focusing on strategies which can help patients with ischemic reperfusion injury to remain agile during the disruption of healthcare services. Moreover, several government organizations across the globe are implementing policies and regulations to deal with the current crisis.
The all-inclusive version of the report will include the impact of COVID-19 and the probable changes in the future outlook of the industry, by taking into account the technological, social, political, and economical parameters.
The ischemia reperfusion injury therapeutics market is estimated to be valued at US$ 1,582.33 million in 2020 and is expected to rise at a CAGR of 5.4% during the forecast period (2020-2027).
Figure 1: Global Ischemia Reperfusion Injury Therapeutics Market Share (%) Analysis, By Distribution Channel, 2020

An increase in the number of mergers & acquisitions, product launches, and approvals from drug regulatory authorities are expected to propel the growth of the global ischemia reperfusion injury therapeutics market.
Key players in the market are involved in mergers & acquisitions to develop and launch advanced drugs and treatment in the market. The drug launches and approvals from drug regulatory authorities are expected to facilitate the market growth and create lucrative growth opportunities for players operating in the market during the forecast period.
For instance, in November 2019, Faraday Pharmaceuticals announced positive results from phase II clinical trials of FDY-5301 for ischemia reperfusion injury treatment following a STEMI heart attack. FDY-5301 is a formulated, patented, elemental reducing agent that contains sodium iodide. It destroys the hydrogen peroxide that is naturally generated as a response to acute ischemia reperfusion injury and also contributes to loss of muscle function and mass.
In January 2020, Revive Therapeutics Ltd., a cannabis life sciences company, provided an update regarding its clinical development plan for treatment of liver disorders. The aim of the company is to expand its product pipeline by leveraging its FDA orphan drug designation for Cannabidiol in the prevention of ischemia reperfusion injury from organ transplantation.

Global Ischemia Reperfusion Injury Therapeutics Market – Regional Analysis
The market in North America accounted for majority of the share in the global ischemia reperfusion therapeutics market in 2019 owing to the increasing focus of key players on research & development and obtaining FDA approvals. For instance, in May 2019, Abiomed received the U.S. FDA approval for its STEM DTU trial of delayed ischemic reperfusion injury with impella CP. The trial will test the hypothesis that unloading the left ventricle for 30 minutes before ischemia reperfusion injury will reduce myocardial damage from a heart attack and lead to a reduction in future heart failure-related events.
Furthermore, Asia Pacific is expected to witness significant CAGR during the forecast period owing to research and development and collaborative agreements among key industry players. For instance, in May 2016, NeuroVive Asia and Sanofi entered a collaboration agreement to develop and commercialize CicloMulsion in South Korea for treatment of ischemia reperfusion injury in cardiovascular disease (CVDs). Under this agreement, NeuroVive Asia received upfront payment and royalty on future sales in the country.
Figure 2: Global Ischemia Reperfusion Injury Therapeutics Market Value (US$ Mn) & Y-o-Y Growth (%), 2017-2027

Global Ischemia Reperfusion Injury Therapeutics Market - Competitive Landscape
Key players operating in the global ischemia reperfusion injury therapeutics market are Omeros Corporation, Nyken B.V., Opsona Therapeutics Limited, Pharming Group N.V., Orexo AB, PledPharma AB, Proteo, Inc., Prolong Pharmaceuticals, Prothix BV, Zealand Pharma A/S, Stealth BioTherapeutics Inc., Amyndas Pharmaceuticals LLC, Antipodean Pharmaceuticals, Inc., Angion Biomedica Corp., Bayer AG, Bolder Biotechnology, Inc., Biomedica Management Corporation, Curatis Pharma GmbH, Erimos Pharmaceuticals, LLC, Ensemble Therapeutics Corporation, and Gilead Sciences, Inc.
Request sample copy here :
https://www.coherentmarketinsights.com/insight/request-sample/4105
Request PDF brochure here:
https://www.coherentmarketinsights.com/insight/request-pdf/4105
About Us:
Coherent Market Insights is a global market intelligence and consulting organization focused on assisting our plethora of clients achieve transformational growth by helping them make critical business decisions.
What we provide:
· Customized Market Research Services
· Industry Analysis Services
· Business Consulting Services
· Market Intelligence Services
· Long term Engagement Model
· Country Specific Analysis
Contact Us:
Mr. Shah
Coherent Market Insights Pvt. Ltd.
Address: 1001 4th ave, #3200 Seattle, WA 98154, U.S.
Phone: +1-206-701-6702
Email : [email protected]
Reference/Source: https://www.coherentmarketinsights.com/
#CoherentMarketInsights#MarketAnalysis#Healthcare#smallembeddedelectronicdevices#sensors#IngestibleSmartPillsMarketAnalysis#gastrointestinaltract#cameras#patches#trackers
0 notes
Text
REPERFUSION TREATMENT MARKET ANALYSIS(2020-2027)
Reperfusion Treatment Market, by Treatment Type (Therapeutic Hypothermia, Cyclosporin, Stem Cell Therapy, Hydrogen Sulphide Treatment, and Others), by Injury Type (Heart Injury, Kidney Injury, Intestine Injury, and Others), by Distribution Channel (Hospital Pharmacies, Retail Pharmacies, and Others), and by Region (North America, Latin America, Europe, Asia Pacific, Middle East, and Africa) - Size, Share, Outlook, and Opportunity Analysis, 2020 - 2027
Reperfusion injury is caused due to the damage in the tissue, which occurs due to the lack of blood supply. Examples of reperfusion injury include brain damage after stroke and many others, where reperfusion therapy leads to flow of blood in the tissue which results in inflammation and oxidative damage due to oxidative stress. Reperfusion injury can be treated by therapeutic hypothermia, hydrogen sulphide treatment, cyclosporins, stem cell therapy, and others. Furthermore, delay in reperfusion therapy results in oxidative damage.
Global Reperfusion Treatment Market – Impact of Coronavirus (COVID – 19) Pandemic:
The COVID-19 pandemic is expected to hamper the global reperfusion treatment market growth during the forecast period. The COVID-19 pandemic and resulting lockdowns in various countries across the globe have impacted the financial status of businesses in all sectors. The private healthcare sector has been impacted majorly due to the COVID-19 pandemic. Many clinical trials have been suspended during the pandemic. In order to restart the clinical trials, the U.S. Food and Drug Administration (FDA) released guidelines during the COVID-19 public health emergency in March 2020. The guidelines were further updated on July 02, 2020. The guidelines include general considerations to assist sponsors and researchers, which ensure the safety of trial participants, and compliance with good clinical practice (GCP) for the duration of the COVID-19 public health emergency. The appendix of the guidelines also provide answers to some general questions, which the U.S. Food and Drug Administration (FDA) received from various sponsors and researchers about conducting clinical trials during the COVID-19 public health emergency. The above guidelines are also applicable for conducting the clinical trials for testing the safety and efficacy of the drugs for the reperfusion injury. Thus, the COVID – 19 pandemic is expected to decrease the growth of the reperfusion treatment market over the forecast period.
The global reperfusion treatment market is estimated to be valued at US$ 1,293.6 million in 2020 and is expected to exhibit a CAGR of 4.5% during the forecast period (2020-2027).
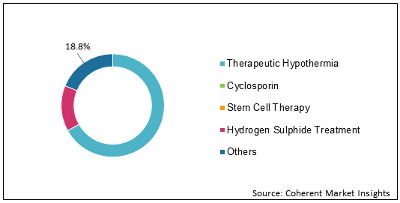
Figure 1: Global Reperfusion Treatment Market Share (%) Analysis, By Treatment Type 2020
Increasing prevalence of coronary heart dis ease is expected to drive the growth of the global reperfusion treatment market during the forecast period.
The rising incidence of coronary artery disease (CAD) or ischemic heart disease (IHD) is a major factor which is expected to drive the market growth. The CAD or IHD is caused due to the buildup of cholesterol and fatty deposits on the inner walls of the arteries, which may lead to the reduction of blood flow to the heart cells. This condition may lead to ischemia, myocardial infraction or sudden cardiac arrest. Moreover, medicines approved from the regulatory authorities are not available in the market for the treatment of ischemia/reperfusion injury. According to the National Center for Biotechnology Information (NCBI), 2020, in 2017, globally, around 126 million people suffered from ischemic heart disease (1,655 per 100,000), which constituted to 1.72% of the total world population.
Investments and expansion of production facility by market players are expected to boost growth of the global reperfusion treatment market during the forecast period.
Market players are focusing on facility expansions in order to strengthen their product portfolio. For instance, on March 9, 2020, Pharming Group NV received the Food and Drug Administration (U.S. FDA) approval for its new production facility in the Netherlands for the production of the starting material required for manufacturing of RUCONEST. RUCONEST is a C1-esterase inhibitor, which is plasma free and is proven to help treat hereditary angioedema (HAE) attacks. Furthermore, on January 21, 2020, Pharming Group NV received the European Medicines Agency (EMA) approval for the production facility for RUCONEST in Europe.
Global Reperfusion Treatment Market – Restraints:
There are some side effects associated with the treatment, which are expected to restrain the global reperfusion treatment market during the forecast period. Ischemia reperfusion causes the mediator to infiltrate other tissues, which leads to Multiple Organ Dysfunction Syndrome (MODS). For instance, according to an article published in the International Institute of Anticancer Research in 2019, Multiple Organ Dysfunction Syndrome (MODS) was the leading cause of mortality globally and the incidence of MODS ranged from 25-40%. Furthermore, according to the Critical Care Nephrology Journal 2019, the pediatric multiple organ dysfunction syndrome (MODS) epidemiology ranges from 10% to 50% of the children admitted to the pediatric intensive care unit.
Global Reperfusion Treatment Market – Regional Analysis:
On the basis of region, the global reperfusion treatment market is segmented into North America, Latin America, Europe, Asia Pacific, Middle East, and Africa.
North America is expected to dominate the global reperfusion treatment market during the forecast period owing to research and development in the region. For instance, in November 2019, Faraday Pharmaceuticals announced positive results from phase II clinical trials of FDY-5301 for ischemia reperfusion injury treatment, following a STEMI heart attack. FDY-5301 is a formulated, patented, elemental reducing agent that contains sodium iodide. It destroys the hydrogen peroxide that is naturally generated as a response to acute ischemia reperfusion injury and also contributes to loss of muscle function and mass.
Europe is an emerging reperfusion treatment market owing to the funding provided for research and development by regulatory authorities. For instance, in February 2019, Balmes Transplantation SAS received around US$ 605,597 million from the European Regional Development Fund (ERDF) for its research program REMEDIRA for developing combinations of repurposed drugs against kidney ischemia-reperfusion injury (IRI).
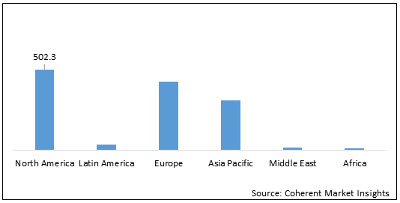
Figure 2: Global Reperfusion Treatment Market Value (US$ Mn), by Region, 2020
Global Reperfusion Treatment Market - Competitive Landscape:
Some of the key players operating in the global reperfusion treatment market are Corline Biomedical AB, SBI Holdings Inc., Pharming Group NV, Mallinckrodt Pharmaceuticals, MIFCOR, Inc., CFM Pharma Holding B.V., Balmes Transplantation SAS, Revive Therapeutics Ltd., Faraday Pharmaceuticals, Radikal Therapeutics Inc., Amyndas Pharmaceuticals, Perfusion.com, Inc., Angion Biomedica Corp., Young Therapeutics, LLC, Ischemix, Stealth Biotherapeutics Inc., and Bolder Bio Technology Inc.
Request sample report here:
https://www.coherentmarketinsights.com/insight/request-sample/4248
Download PDF brochure here:
https://www.coherentmarketinsights.com/insight/request-pdf/4248
About Us:
Coherent Market Insights is a global market intelligence and consulting organization focused on assisting our plethora of clients achieve transformational growth by helping them make critical business decisions.
What we provide:
• Customized Market Research Services
• Industry Analysis Services
• Business Consulting Services
• Market Intelligence Services
• Long term Engagement Model
• Country Specific Analysis
Contact Us:
Mr. Shah
Coherent Market Insights Pvt. Ltd.
Address: 1001 4th ave, #3200 Seattle, WA 98154, U.S.
Phone: +1-206-701-6702
Email: [email protected]
Source: https://www.coherentmarketinsights.com/market-insight/reperfusion-treatment-market-4248
0 notes
Text
Report: Neurovascular Devices Market Analysis by Top Companies, Size, Share and Trends
Neurovascular Devices Market Highlights
Neurovascular devices encompass a range of devices which are used for neurovascular management. The rise of neurovascular devices such as brain aneurysms, carotid artery disease, and others are likely to warrant advanced devices which can prevent the possibility of strokes and other diseases. The global neurovascular devices market report by Market Research Future (MRFR) comprises a comprehensive analysis of the current state and future predictions for the period of 2018 to 2023 (forecast period).
Get Free Sample Copy @ https://www.marketresearchfuture.com/sample_request/5544
Neurovascular Devices Market Scope
The global neurovascular devices market is expected to grow at a CAGR of 8.8% during forecast period. It is driven by the rise of neurovascular diseases and development of minimally invasive procedures. According to the Brain Aneurysm Foundation, cerebral aneurysms are accountable for close to a million deaths annually. Progress in neurovascular management and innovations in microsurgical devices and treatments can drive market growth in a positive direction.
Investments by government organizations for management of neurological conditions can bode well for the market. This is exemplified by the use of funds amounting to USD 6.6 billion by the NHS in the U.K. Clinical trials being conducted for new methods and procedures can encourage market growth. Endovascular treatments are gaining traction with many patients opting it as a method for acute ischemic stroke.
However, high procedural costs and lack of skilled physicians can dampen the market growth prospects.
Neurovascular Devices Market Segmentation
The global neurovascular devices market is segmented by product, therapeutic application, and end-user.
On the basis of the product, the market is segmented into, aneurysm coiling & embolization devices, neurothrombectomy devices, cerebral balloon angioplasty and stenting systems, and support device. Aneurysm coiling & embolization devices are sub-segmented into liquid embolic, embolic coils, and flow diversion devices. Embolic coils, a part of aneurysm coiling & embolization devices segment, are further segmented into detachable coils and pushable coils. Similarly, neurothrombectomy devices segment is sub-segmented into suction and aspiration devices and retrieval systems. The support device segment is sub-segmented into microcatheters and microguidewires. The cerebral balloon angioplasty and stenting systems segment is sub-segmented into carotid artery stents and embolic protection systems. Embolic protection systems are further divided into distal filter devices and balloon occlusion devices.
By therapeutic application, the global neurovascular devices market is categorized into hemorrhagic stroke and ischemic strokes.
By end-users, the market is segmented into hospitals & clinics and ambulatory surgical units.
Neurovascular Devices Market Regional Analysis
The Americas, Asia Pacific (APAC), Europe, and the Middle East & Africa are regions considered for measuring the value and volume of the global neurovascular devices market.
The Americas are touted to dominate the global neurovascular devices market owing to high prevalence of neurovascular diseases. According to a study by the Methodist DeBakey Cardiovascular Journal in 2014, close to 1 million strokes occur annually due to intracranial atherosclerotic disease. The large healthcare expenditure of nations in the region and presence of major players can further regional market growth.
APAC is predicted to exhibit a stellar growth rate during the forecast period owing to the growth of economies of China and India. Demand for minimally invasive surgeries by the geriatric populace can fuel the demand in the global neurovascular devices market.
Neurovascular Devices Market Competitive Outlook
Evasc, OxfordEndovascular, Gynesonics, Delaware Corporation, TERUMO CORPORATION, Merit Medical Systems, Inc., Secant Group, LLC, Rapid Medical, Neuravi, Medikit co., ltd., Stryker, Abbott, MicroPort Scientific Corporation, W. L. Gore & Associates, Inc., Medtronic, Penumbra, Inc., Johnson & Johnson Services, Inc., Blockade Medical, LLC., and Sensome are major players of the global neurovascular devices market. Product launches, acquisitions, and distribution agreements are strategies being implemented by players to sustain their position in the market. In 2020, Arterio Medical submitted the results of the clinical trial of Endura Embolization System in bifurcation systems. The results demonstrated the low relapse of coils and provide immediate cerebral aneurysm occlusion.
Read Complete Report With Toc @ https://www.marketresearchfuture.com/reports/neurovascular-devices-market-5544
Contact:
Market Research Future
Office No. 528, Amanora Chambers
Magarpatta Road, Hadapsar,
Pune - 411028
Maharashtra, India
+1 646 845 9312
Email: [email protected] .
0 notes
Text
Hypoxia Market Insights, Epidemiology and Market Forecast 2030
Hypoxia is a state in which oxygen is not available in sufficient amounts at tissue level to maintain adequate homeostasis; this can result from inadequate oxygen delivery to the tissues either due to low blood supply or low oxygen content in blood (hypoxemia).
DelveInsight's "Hypoxia - Market Insights, Epidemiology, and Market Forecast-2030" report delivers an in-depth understanding of the Hypoxia , historical and forecasted epidemiology as well as the Hypoxia market trends in the United States, EU5 (Germany, Spain, Italy, France, and United Kingdom) and Japan.

The Hypoxia market report provides current treatment practices, emerging drugs, Hypoxia market share of the individual therapies, current and forecasted Hypoxia market Size from 2017 to 2030 segmented by seven major markets. The Report also covers current Hypoxia treatment practice/algorithm, market drivers, market barriers and unmet medical needs to curate best of the opportunities and assesses the underlying potential of the market.
Geography Covered
· The United States
· EU5 (Germany, France, Italy, Spain, and the United Kingdom)
· Japan
Study Period: 2017-2030
Hypoxia Disease Understanding and Treatment Algorithm
Hypoxia can vary in intensity from mild to severe and can present in acute, chronic, or acute and chronic forms. The response to hypoxia is variable; while some tissues can tolerate some forms of for a longer duration, other tissues are severely damaged by low oxygen levels. Hypoxia/ ischemia
Cerebral hypoxia refers to a situation in which there is a decrease of oxygen supply to the brain even though there is adequate blood flow, it affects the largest parts of the brain, called the cerebral hemispheres.
The DelveInsight Hypoxia market report gives a thorough understanding of the Hypoxia by including details such as disease definition, symptoms, causes, pathophysiology, diagnosis and treatment.
Diagnosis
This segment of the report covers the detailed diagnostic methods or tests for Hypoxia.
Treatment
It covers the details of conventional and current medical therapies available in the Hypoxia market for the treatment of the condition. It also provides Hypoxia treatment algorithms and guidelines in the United States, Europe, and Japan.
Request for sample pages: https://www.delveinsight.com/sample-request/hypoxia-market
Hypoxia Epidemiology
As per a study:
• Stroke is the third leading cause of death in the United States. More than 140,000 people die each year from stroke in the United States.
• Every year, more than 795,000 people in the United States have a stroke. About 610,000 of these are first or new strokes.
• About 87% of all strokes are ischemic strokes, in which blood flow to the brain is blocked
The Hypoxia epidemiology division provide insights about historical and current Hypoxia patient pool and forecasted trend for every seven major countries. It helps to recognize the causes of current and forecasted trends by exploring numerous studies and views of key opinion leaders. This part of the DelveInsight report also provides the diagnosed patient pool and their trends along with assumptions undertaken.
Key Findings
The disease epidemiology covered in the report provides historical as well as forecasted Hypoxia epidemiology scenario in the 7MM covering the United States, EU5 countries (Germany, Spain, Italy, France, and the United Kingdom), and Japan from 2017 to 2030.
Country Wise- Hypoxia Epidemiology
The epidemiology segment also provides the Hypoxia epidemiology data and findings across the United States, EU5 (Germany, France, Italy, Spain, and the United Kingdom), and Japan.
Hypoxia Drug Chapters
The current pipeline for Hypoxia has many significant products. The dynamics of Hypoxia market is anticipated to change in the coming years owing to the improvement in the diagnosis methodologies, incremental healthcare spending across the world, and also due to the expected launch of many therapies during the forecast period.
Drug chapter segment of the Hypoxia report encloses the detailed analysis of Hypoxia marketed drugs and late stage (Phase-III and Phase-II) pipeline drugs. It also helps to understand the Hypoxia clinical trial details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug and the latest news and press releases.
Marketed Drugs
The report provides the details of the marketed product available for Hypoxia treatment.
Hypoxia Emerging Drugs
The report provides the details of the emerging therapies under the late and mid-stage of development for Hypoxia treatment.
Request access to free sample pages: https://www.delveinsight.com/sample-request/hypoxia-market
Hypoxia Market Outlook
Treatment of vascular brain disorders has great impact clinically, financially, and socially throughout the world. Prevention of their occurrence through lifestyle adjustments and clinical antihypertensive treatment is an essential part of the therapy to reduce stroke probability and vascular malfunction-related gradual decline in memory.
Treatment depends on the underlying cause of the hypoxia, the most important treatment for cerebral hypoxia involves removing the source of the oxygen deprivation, along with extensive physical, occupational, or speech therapy to teach brain how to work around any damaged areas. Such therapy can be challenging and emotionally draining, but the more committed treatment approaches.
Management of hypoxia falls under 3 categories: maintaining patent airways, increasing the oxygen content of the inspired air, and improving the diffusion capacity
Some other treatments include drugs to prevent future hypoxia episodes; this may include the use of blood thinners, antibiotics to treat infections that caused or resulted from the hypoxia, surgery to remove any blockages or to discover the source of the blockage. Also, it is supported by the use of assistive gear, such as a wheelchair, psychotherapy and basic life-support systems (mechanical ventilation to secure the airway; fluids, blood products, or medications to support blood pressure and heart rate; and medications to suppress seizures).
To meet the current unmet needs of the Hypoxia market, companies like Diffusion Pharmaceuticals, Biogen and many others are developing therapies for the treatment of this indication.
The factors that shall expedite the growth of Hypoxia market include increasing awareness about available treatments during the forecast period (2019–2030). Overall, the increasing Incidence, disease awareness, and promising emerging pipeline therapies will propel the market size forward during the forecast period. A better understanding of disease pathogenesis will also contribute to the development of novel therapeutics for Hypoxia.
The Hypoxia market outlook of the report helps to build the detailed comprehension of the historic, current, and forecasted Hypoxia market trends by analyzing the impact of current therapies on the market, unmet needs, drivers and barriers and demand of better technology.
This segment gives a thorough detail of Hypoxia market trend of each marketed drug and late-stage pipeline therapy by evaluating their impact based on annual cost of therapy, inclusion and exclusion criteria's, mechanism of action, compliance rate, growing need of the market, increasing patient pool, covered patient segment, expected launch year, competition with other therapies, brand value, their impact on the market and view of the key opinion leaders. The calculated market data are presented with relevant tables and graphs to give a clear view of the market at first sight.
According to DelveInsight, Hypoxia market in 7MM is expected to change in the study period 2017-2030.
Key Findings
This section includes a glimpse of the Hypoxia market in 7MM.
The United States Market Outlook
This section provides the total Hypoxia market size and market size by therapies in the United States.
EU-5 Countries: Market Outlook
The total Hypoxia market size and market size by therapies in Germany, France, Italy, Spain, and the United Kingdom is provided in this section.
Japan Market Outlook
The total Hypoxia market size and market size by therapies in Japan is also mentioned.
Download sample pages of the report @ https://www.delveinsight.com/sample-request/hypoxia-market
View a detailed report on Hypoxia: https://www.delveinsight.com/report-store/hypoxia-market
#hypoxia market#hypoxia market share#hypoxia market size#hypoxia market trends#hypoxia market research reports#hypoxia epidemiology#hypoxia key companies
0 notes
Text
Neuropathic Pain Market Size, Share, Outlook, and Opportunity Analysis, 2020 - 2027
Neuropathic pain is a complex, chronic pain state that usually is accompanied by tissue injury. It may lead to damage, dysfunction, or injury of nerve fibers, thereby leading to misfiring of signals to other pain centers. The impact of a nerve fiber injury includes a change in nerve function both at the site of injury and areas around the injury.
The global neuropathic pain market is estimated to account for US$ 6,313.4 Mn in terms of value in 2019 and is expected to reach US$ 9,862.3 Mn by the end of 2027.
Browse Summary of the Research Report- https://www.coherentmarketinsights.com/market-insight/neuropathic-pain-market-3656
High prevalence of cancer is expected to boost growth of the global neuropathic pain market over the forecast period. For instance, according to the World Health Organization, around 18.1 million new cases and 9.6 million deaths were registered due to cancer worldwide in 2018. Moreover, increasing geriatric population is also expected to aid in growth of the market. For instance, according to the World Health Organization, geriatric population is expected to reach 2 billion by 2050, up from 900 million in 2015.
North America region held dominant position in the global neuropathic pain market in 2019, accounting for 35.9% share in terms of value, followed by Europe.Incomplete pain relief is expected to hinder growth of the global neuropathic pain market. For instance, use of strong opioids such as oxycodone in the treatment of neuropathic pain often leads to low reduction in pain.
Moreover, side-effects of medications are also expected to limit growth of the market. Use of opioids and steroids may lead to complications such as heart attack, kidney failure, and damage in lungs.
High prevalence of neuropathic-like pain in psoriatic arthritis is expected to offer lucrative growth opportunities for players in the market. For instance, in July 2019, researchers from Cardiff University, U.K., reported that neuropathic-like pain as evidence of abnormal pain processing is common in patients with psoriatic arthritis. Moreover, R&D in neuropathic pain is also expected to aid in growth of the market. For instance, in March 2020, researchers from Heidelberg University, Germany, reported assessment of the phytochemical composition and the possible prophylactic effects of an aqueous ethanol extract of Haematoxylon campechianum flowers on peripheral neuropathic pain in a chronic constriction injury rat model.
Anticonvulsants segment in the global neuropathic pain market was valued at US$ 2,548.1 Mn in 2019 and is expected to reach US$ 4,310.3 Mn by 2027 at a CAGR of 6.4% during the forecast period. Use of flavonoids can be effective in the treatment of neuropathic pain due to spared nerve injury, spinal nerve ligation, partial sciatic nerve injury, diabetes-induced neuropathy, chemotherapy-induced neuropathy, and chronic constriction injury.
Several studies have demonstrated that electroacupuncture can be effective in decreasing neuropathic pain. For instance, in January 2020, researchers from Huazhong Agricultural University, China, demonstrated that Synaptotagmin 1 (Syt-1), a synaptic vesicle protein for regulating exocytosis of neurotransmitters, impairs neuropathic pain and that electroacupuncture relieves neuropathic pain through down-regulating spinal Syt-1.
Major players operating in the global neuropathic pain market include, Pfizer, Inc., Johnson & Johnson Services, Inc., Sanofi S.A., Eily Lily and Company, GlaxoSmithKline PLC, Biogen Idec., Bristol-Myers Squibb, Baxter Healthcare Corporation, and Depomed, Inc.
Major players in the market are focused on approval and launch of new products in order to expand their product portfolio. For instance, in March 2020, Senzer Pharmaceuticals secured the Investigational New Drug application and data package for its ongoing FDA registration program from its former US strategic partner in order to get approval for its cannabinoid respiratory device for the treatment of side effects induced by anti-cancer treatments, specifically nausea, vomiting, and neuropathic pain.
Major players in the market are also focused on conducting clinical trials to expand their product portfolio. For instance, in February 2020, NoNO Inc., a privately-held biotechnology company, reported that novel peptide, nerinetide, without prior administration of alteplase, demonstrated medically important improvements in patients with acute ischemic stroke, in a multicenter, randomized, study.
Request A Sample Copy - https://www.coherentmarketinsights.com/insight/request-sample/3656
Contact Us:
Mr. Shah Coherent Market Insights 1001 4th Ave, #3200 Seattle, WA 98154 Tel: +1-206-701-6702 Email: [email protected] *****************************************************************************
0 notes
Text
Citicoline Market to Witness Robust Expansion Throughout the Forecast Period 2018-2028
Growing popularity and increasing investments in eSports is helping the market go mainstream which, in turn, is opening an assortment of opportunities for various industries. Players participating in eSports tournaments are seeking cognitive enhancing supplements which can help them concentrate, plot strategies, and improve working memory. Additionally, a ban on the use of other nootropics such as Ritalin and Adderall in eSports leagues is creating demand for ‘permissible’ supplements. Citicoline’s negligible toxicity and its organic nature is making it an appealing prospect for eSports players. With regulations allowing the use of citicoline in functional food and supplements, manufacturers are increasingly focusing towards including the compound in their products to capitalize on the bolstering demand for cognitive-enhancing supplements in eSports.
Request for the Report Summary: https://www.factmr.com/report/2868/citicoline-market The adverse and often fatal impact of ischemic stroke on human health, coupled with increasing prevalence is bolstering demand for effective treatment of the condition. According to WHO, stroke is the second leading cause of death in the world and the third leading cause of disabilities in adults. Additionally, the lack of a standard procedure of treatment for different ischemic stroke patients is influencing healthcare researchers to focus on the development of acute therapy for all patients. According to Fact.MR’s study, citicoline is being viewed as a potential treatment option by researchers and healthcare providers. These factors are vital to citicoline market growth and are expected propel proliferation during the forecast period. The study opines that citicoline demand is also likely to be influenced by its role in treatment of brain injuries. Studies suggest treatment of patients with citicoline has been beneficial in reducing headaches, dizziness, tinnitus, and in substantially relieving motor, cognitive, and mental symptoms. These developments are further driving researchers towards testing its potential use in treatment for pervasive developmental disorders such as atypical autism, Asperger Syndrome, and autism. Intensifying clinical trials and research and developmental activities in the area are expected to open new and lucrative opportunities for companies in the citicoline market.
Request for the Sample of the Report: https://www.factmr.com/connectus/sample?flag=S&rep_id=2868 The growth in the geriatric population around the world is expected to continue with the Population Reference Bureau (PRB) estimating the number of people aged over 65 to account for 16 percent of the world population by 2050. Citicoline finds widespread usage in the production of supplements for enhancing cognition in the elderly. In addition, intensifying clinical trials towards improving citicoline efficacy through its synergy with other chemicals is another vital factor estimated to drive market growth. Fact.MR opines that advancements in tablet manufacturing will bolster production of highly effective supplements, with sales of citicoline tablets estimated to reach nearly US$ 235 million in 2019. The Fact.MR report tracks the citicoline market for the period 2018-2028. According to the report, the citicoline market is projected to grow at 8.5% CAGR through 2028.
Request for Report Methodology: https://www.factmr.com/connectus/sample?flag=RM&rep_id=2868
About Us Fact.MR’s methodology is robust and comprehensive. We employ a range of tools and assets to develop an all-encompassing coverage of a range of industries. We compile data points at local, country, regional, and global level – our approach to capturing the finest nuances, without losing sight of the bigger picture helps us in developing accurate and reliable forecasts and estimates. Fact.MR has a standard set of guidelines and standards that help maintain a level of consistency across all of our research offerings. The standardization includes step-by-step documentation of the methodologies and guidelines on the sources that are to be used for incorporation of objective and accurate data. The standardization also involves use of industry-wide analytical tools, and rigorous quality checks to validate market forecasts and sizes. Our unwavering focus on standardization ensures that clients receive the same quality of research and analysis that Fact.MR is known for. Contact Us 11140 Rockville Pike Suite 400 Rockville, MD 20852 United States E: [email protected] Read the Blog: http://www.dailyhealthtribune.com
0 notes
Text
Aptamers Market Growth, Market Insights and Forecast to 2025
The global aptamers market accounted for US$ 110 Mn in 2018 and burgeoning over the forthcoming years. Growing investment in research and development, increasing usage of aptamers in drug delivery and diagnostics, low-cost and high-efficiency as compared to antibodies and technological advancements for aptamers are some of the key factors favoring the market growth. However, factors such as lack of trained professionals and low market acceptance are the major factors hindering the market growth.
Global aptamers market segmented on the basis of aptamers type, application, end user and region.
Request for Sample Report @ https://www.precisionbusinessinsights.com/request-sample?product_id=16635
Nucleic acid aptamers dominate the Global Aptamers Market
Based on aptamers type, global aptamers market is segmented into Nucleic acid aptamers, RNA-based, XNA-based, DNA-based and Peptide aptamers. Nucleic acid aptamers held considerable market growth during estimated period. Nucleic acid aptamers are a class of high affinity nucleic acid ligands. They work as “chemical antibodies” due to their high affinity and specificity. Nucleic acid aptamers are produced from nucleic acid random-sequence using a systematic evolution of ligands by exponential enrichment (SELEX) technology.
North America Leads the Global Aptamers market
PBI’s global aptamers market report analyses the market in different regions such as North America, Europe, Asia Pacific, Latin America, and Middle East and Africa. According to regional analysis. North America accounted for larger revenue share in global aptamers market with considerable CAGR. The growth in this region can be attributed to rising funding & investment to the development of aptamers and technological advancements. Also, Asia Pacific held significant market share during estimated period owing to increasing R&D activities and positive government regulations in emerging countries like India and China.
Strategic Expansion are the Key Strategies Adopted by Market Players
Global aptamers market further reveals that the key players increasingly adopting strategies such as launch of newer products, frequent product approvals, and long term alliance to improve market revenue share and gaining significant geographic presence across the region. For Instance, In May 2017, AptaTargets raised € 2.7 Mn funding from Caixa Capital Risc for the development of clinical trials for ApTOLL, a drug used in the treatment of acute ischemic stroke.
Key player’s profiles in the report are NeoVentures Biotechnology, Inc. (Canada), Aptagen, LLC (U.S.), Aptamer Solutions Ltd. (U.K.), SomaLogic, Inc. (U.S.), AM Biotechnologies, LLC (U.S.), TriLink BioTechnologies, Inc. (U.S.), Vivonics, Inc. (U.S.), and Apta Targets (Spain).
Precision Business Insights (PBI) in its report titled “Global Aptamers Market: Market Estimation, Dynamics, Regional Share, Trends, Competitor Analysis 2014-2018 and Forecast 2019-2025” assesses the market performance over seven years forecast period over 2019-2025. The report analyses the market value forecast and provides the strategic insights into the market driving factors, challenges that are hindering the market revenue growth over forecast period. Moreover, the report also includes the total revenue and volume for the market.
Detailed Segmentation
By Aptamers Type
o Nucleic acid aptamers
o RNA-based
o XNA-based
o DNA-based
o Peptide aptamers
By Application
o Research & Development
o Therapeutic development
o Diagnostics
By End User
o Pharmaceutical companies
o Biopharmaceutical companies
o Research and academic institutes
o Others
By Geography
o North America
· U.S
· Canada
o Europe
· Germany
· France
· U.K
· Italy
· Spain
· Russia
· Poland
· Rest of Europe
o Asia-Pacific
· Japan
· China
· India
· Australia & New Zealand
· ASEAN (Includes Indonesia, Thailand, Vietnam, Philippines, Malaysia, and Others)
· South Korea
· Rest of Asia-Pacific
o Latin America
· Brazil
· Mexico
· Argentina
· Rest of Latin America
o Middle East and Africa (MEA)
· Gulf Cooperation Council (GCC) Countries
· Israel
· South Africa
· Rest of MEA
For more information: https://www.precisionbusinessinsights.com/market-reports/global-aptamers-market/
About Us:
Precision Business Insights is one of the leading market research and business consulting firm, which follow a holistic approach to solve needs of the clients. We adopt and implement proven research methodologies to achieve better results. We help our clients by providing actionable insights and strategies to make better decisions. We provide consulting, syndicated and customized market research services based on our client needs.
Contact Us:
Kemp House,
152 – 160 City Road,
London EC1V 2NX
Email: [email protected]
Toll Free (US): +1-866-598-1553
Website @ https://www.precisionbusinessinsights.com
0 notes
Text
Neurovascular Devices Market - Predicted to Accelerate the Growth by 2017-2025
Global Neurovascular Devices Market: Snapshot
Over the past decade, neurointerventional techniques have gained increased significance in the management of acute ischemic strokes. Endovascular and microsurgical devices are fast gaining traction as minimally invasive techniques for the management of brain aneurysms. In recent years, advances in balloon- and stent- assisted coiling in endovascular therapies have expanded the options for surgeons and patients in the treatment of aneurysms. The rising morbidity and mortality of various types of strokes, particularly acute ischemic strokes, world over has propelled the demand for endovascular mechanical treatment devices. Endovascular thrombectomy has come to be associated with a higher rate of recanalization and has significantly enhanced clinical outcomes for the management of acute ischemic strokes.
Broad advances in neurovascular-related tools and techniques are further expected to accentuate the market in the coming years. Rising expenditure on improving healthcare infrastructure in various developing nations and favorable reimbursement policies in several developed nations are crucial factors expected to boost the neurovascular devices market in the forthcoming years. In addition, the rising number of clinical trials investigating the viability and efficacy of various neurointerventional techniques for the management of acute ischemic strokes bodes well for the market growth. Furthermore, recent advances in stent retrievers have led to better clinical outcomes in endovascular stroke treatment. In recent years, these stents have shown great promise in decreasing the time to recanalization advancing the treatment of acute ischemic strokes.
Request Sample Copy of the Report @
https://www.tmrresearch.com/sample/sample?flag=B&rep_id=1364
Global Neurovascular Devices Market: Overview
The global market for neurovascular devices is projected to witness robust growth in the next few years. With the expanding pool of patients, healthcare providers and prominent players operating in the global neurovascular devices market are focusing on developing new and effective devices in the near future. The promising opportunities in the emerging economies are projected to fuel the growth of the global neurovascular devices market in the coming few years.
Global Neurovascular Devices Market: Key Trends
The rising number of ischemic strokes and brain aneurysm is one of the prominent factors expected to encourage the growth of the global neurovascular devices market in the next few years. In addition, a substantial rise in the demand for surgical processes with minimum invasion and technological developments in this field are anticipated to fuel the growth of the overall market in the coming years.
On the other hand, the rising need for introducing more effective and low cost therapeutics and the increasing concerns for leading players for commercialization of products are some of the factors predicted to restrict the growth of the global neurovascular devices market in the next few years. Nonetheless, the rising number of initiatives imposed by governments in order to expand and modernize healthcare infrastructure are estimated to supplement the growth of the global neurovascular devices market in the forecast period.
Request TOC of the Report @
https://www.tmrresearch.com/sample/sample?flag=T&rep_id=1364
Global Neurovascular Devices Market: Market Potential
Technological advancements and the introduction of new products are predicted to create promising opportunities for the key players. In addition, these players are making efforts to create an awareness among patients concerning the availability and other benefits of these devices, which is likely to accelerate the growth of the overall market in the coming years.
Furthermore, the key end users of neurovascular devices such as ambulatory services, hospitals, and clinics are promoting the use of neurovascular devices. This will encourage the development of the market across the forecast period.
Global Neurovascular Devices Market: Regional Outlook
According to the research study, North America is expected to witness substantial growth in the next few years and maintain its leading position in the global neurovascular devices market. In this region, the U.S. is predicted to account for a massive share and is considered as a key contributor, due to the rising prevalence rate of brain aneurysm. The high growth of this region can be attributed to the rising number of technological advancements, favorable medical reimbursements, and high-tech healthcare infrastructure.
On the other hand, Latin America and Asia Pacific are projected to register a progressive growth rate throughout the forecast period. However, the lack of awareness among patients regarding the availability of neurovascular devices is anticipated to inhibit the growth of the overall market in the coming years. Nonetheless, the advent of new devices and technologies and the increasing efforts by leading players are projected to accelerate the growth of the market in the near future.
Read Comprehensive Overview of Report @
https://www.tmrresearch.com/neurovascular-devices-market
Global Neurovascular Devices Market: Competitive Analysis
The global neurovascular devices market is a consolidated market with a few number of players operating in it. These players are making notable efforts in order to create a niche for themselves and expand their presence across the globe. Some of the leading players operating in the neurovascular devices market across the globe are Johnson & Johnson (Depuy), Medtronic, Stryker, Terumo, and Penumbra.
Furthermore, the leading players in the global neurovascular devices are focusing on new product development, mergers and acquisitions, and innovations. These aspects are expected to contribute substantially towards the development of the overall market in the coming years. Moreover, the rising competition among players is predicted to encourage the growth of the overall market in the forecast period.
About TMR Research
TMR Research is a premier provider of customized market research and consulting services to business entities keen on succeeding in today’s supercharged economic climate. Armed with an experienced, dedicated, and dynamic team of analysts, we are redefining the way our clients’ conduct business by providing them with authoritative and trusted research studies in tune with the latest methodologies and market trends.
Contact:
TMR Research,
3739 Balboa St # 1097,
San Francisco, CA 94121
United States
Tel: +1-415-520-1050
Email: [email protected]
0 notes
Text
Acute Ischemic Stroke Diagnosis and Treatment Market to achieve US$ 1.9 bn by 2020
A recent report on global acute ischemic stroke diagnosis and treatment market has been analyzed by Transparency Market Research (TMR). The acute ischemic stroke diagnosis and treatment market is expected to increase during the forecast period with the increasing incidence of strokes, technically advanced techniques, and successful results through treatment surgeries. Technological advancement and constant efforts made by leading companies are providing improved treatments for people suffering from ischemic stroke. The companies contributing significantly in the acute ischemic stroke diagnosis and treatment market are Siemens Healthcare, Stryker Corporation, Abbott Laboratories, Philips Healthcare, GE Healthcare, Hitachi, Ltd., Covidien plc, Johnson & Johnson, and Penumbra, Inc.
As per the analysis done by TMR, the global acute ischemic stroke diagnosis and treatment market is estimated to achieve US$ 1.9 bn by the end of the forecast period. The availability of advanced medical equipment and developing treatment facilities have backed the dominance of North America followed by Europe. Increasing number of the geriatric population mainly in North America and Europe have expanded the need for acute ischemic stroke diagnosis and treatment in these regions. The market for acute ischemic stroke diagnosis and treatment market segmented into six categories based on diagnosis type, among which CTs is expected to dominate the overall market.
Request to View Sample of Report -
https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=1799
Ischemic stroke occurs when the supply of the blood is insufficient due to which brain cell dies and brain functions inappropriately. Blockage of the blood vessel or bleeding can be some of the causes of ischemic stroke. Technological advancements in surgical devices to treat acute ischemic stroke have fueled the demand for acute ischemic stroke diagnosis and treatment market. Factors such as increasing geriatric population, higher spending on healthcare facilities and use of minimally invasive procedures have also boosted the demand for acute ischemic stroke diagnosis and treatment market globally. Additionally, the growing consumption of tobacco, high blood cholesterol, obesity are some of the significant factors increasing ischemic strokes. However, excessive use of medication for the treatment of ischemic stroke is projected to hamper the global market growth of surgery for an acute stroke.
Launch of novel therapeutics and recent innovations aimed at improving treatment devices are boosting the market for acute ischemic stroke diagnosis and treatment market. Additionally, government initiatives have sustained the growth of the market mainly in developed economies. The advancement in treatment technology of acute ischemic stroke has introduced desmoteplase; a next generation thrombolytic will boost the market in coming years. Desmoteplase is a major contribution to treat acute ischemic stroke. Furthermore, the presence of e-prescription, e-medical, and telemedicine will also raise the demand for acute ischemic stroke diagnosis and treatment during the forecast period.
The market for acute ischemic stroke diagnosis and treatment market can be hindered with long and strenuous clinical trials, which includes long testing hours after drug intake and ill-managed selection of patients for trails.
Request to View Brochure of Report -
https://www.transparencymarketresearch.com/sample/sample.php?flag=B&rep_id=1799
0 notes
Text
Global Ischemic Cerebral Stroke market report provides an in-depth overview of Product Specification, technology, product type and production analysis considering major factors such as Revenue, Cost, Gross and Gross Margin
Global Ischemic Cerebral Stroke market report provides an in-depth overview of Product Specification, technology, product type and production analysis considering major factors such as Revenue, Cost, Gross and Gross Margin.
The global Ischemic Cerebral Stroke market report also contains the drivers and restrains for the market that are derived from SOWT analysis, and also shows what all the recent developments, product launches, joint ventures, merges and accusations by the several key players and brands that are driving the market are by systemic company profiles.

The Global Ischemic Cerebral Stroke Market is expected to reach USD 42.17 billion by 2025, from USD 22.78 billion in 2017 growing at a CAGR of 8.0% during the forecast period of 2018 to 2025. The upcoming market report contains data for historic years 2016, the base year of calculation is 2017 and the forecast period is 2018 to 2025.
Download PDF Sample Copy of Report@ https://databridgemarketresearch.com/request-a-sample/?dbmr=global-ischemic-cerebral-stroke-market
Competitive Analysis:
The global ischemic cerebral stroke market is highly fragmented and the major players have used various strategies such as new product launches, expansions, agreements, joint ventures, partnerships, acquisitions, and others to increase their footprints in this market. The report includes market shares of global continuous glucose monitoring market for global, Europe, North America, Asia Pacific and South America.
For instance, Stempeutics Research Pvt.,Ltd.(Bangalore) developed a drug Stempeucel which is expected to show efficient results in the forecast period leading to the growth of ischemic cerebral stroke market. Currently, Stempeucel is under phase II clinical trials for treatment of ischemic cerebral stroke.
Global Ischemic Cerebral Stroke Market By Drug Type ( Anticoagulation Therapy, Revascularization, Reperfusion, Antiplatelet, Neuroprotective), End User (Hospitals, Clinics, Palliative Care, Ambulatory Surgery Centers),Geography (North America, South America, Europe, Asia-Pacific, Middle East and Africa) – Industry Trends and Forecast to 2025
Reasons to Purchase this Report · Current and future of Global Ischemic Cerebral Stroke Market outlook in the developed and emerging markets · The segment that is expected to dominate the market as well as the segment which holds highest CAGR in the forecast period. · Regions/countries that are expected to witness the fastest growth rates during the forecast period · The latest developments, market shares, and strategies that are employed by the major market players Customization of the Report · The report includes the complete segmentation displayed above across all above mentioned countries
Major Market Competitors/Players:
Some of the major players operating in the global ischemic cerebral stroke market are:-
Abbott Laboratories,
Medtronic plc,
Boston Scientific Corporation,
Cordis Corporation,
Koninklijke Philips N.V. ,
GE Healthcare,
Stryker Corporation,
Genentech, Inc.,
Merck & Co., Inc.,
Bayer AG ,
Boehringer Ingelheim ,
Sanofi,
Covidien plc ,
Philips Healthcare,
Johnson & Johnson,
Penumbra, Inc.,
GE Healthcare,
Siemens Healthcare,
Hitachi, Ltd ,
Biogen,
Daiichi Sankyo ,
Pfizer Inc.,
ThromboGenics NV,
Vernalis plc,
Neurotec Pharma SL.
To Avail 10% Discount On This Report Mail Us on :- [email protected]
Market Definition:
Ischemic cerebral stroke is the disease in which blood vessels supplies to brain are blocked due to the formation of blood clots which is a cause loss of neurologic function. The symptoms of ischemic cerebral stroke are abrupt onset of hemiparesis, quadriparesis and monoparesis, monocular visual loss, diplopia, visual field deficits, hemisensory deficits, dysarthria, facial droop, vertigo, ataxia, nystagmus, aphasia and decrease in the level of consciousness. According to the American Heart Association, in 2016, approximately 795,000 people suffered from stroke in U.S. Stroke is the major cause of approximately 130,000 people death in a year. According to the World Health Organization, 15 million people suffers stroke worldwide each year. Of these, 5 million die and another 5 million are permanently disabled. High blood pressure contributes to more than 12.7 million strokes worldwide wherein Europe averages approximately 650,000 stroke deaths occurred annually. In the ischemic stroke treatment drug type segment, warfarin is the highest revenue generating class of drug. Due to the increasing incidence of ischemic cerebral stoke disease worldwide drives the ischemic cerebral stroke market.
Major Market Drivers and Restraints:
Increase in the number of cerebral stroke cases.
High prices for AIS therapy.
Increase prevalence of acute ischemic strokes.
Limited availability of efficient drugs is restricting the market growth.
Market Segmentation:
The global ischemic stroke market is segmented based on drug type, testing, end user, geography.
Based on drug type the market is segmented into anticoagulation therapy, revascularization, reperfusion, antiplatelet, neuroprotective. On the basis of anticoagulation therapy, the market is classified into
Based on geography, the market report covers data points for 28 countries across multiple geographies namely North America & South America, Europe, Asia-Pacific and, Middle East & Africa. Some of the major countries covered in this report are U.S., Canada, Germany, France, U.K., Netherlands, Switzerland, Turkey, Russia, China, India, South Korea, Japan, Australia, Singapore, Saudi Arabia, South Africa and, Brazil among others
Key Developments in the Market:
In 2015, Silk Road Medical, Inc.(U.S) got the FDA 510(K) approval for Anti-Stroke Enroute Device.
In 2015, NeuroVive Pharmaceutical AB(Sweden), entered into collaboration with BBB therapeutics, for development of the NVP014 drug candidate used in the treatment of ischemic strokes.
Inquire Regarding This Report @ https://databridgemarketresearch.com/inquire-before-buying/?dbmr=global-ischemic-cerebral-stroke-market
Note: If you have any special requirements, please let us know and we will offer you the report as you want.
About Data Bridge Market Research:
Data Bridge Market Research set forth itself as an unconventional and neoteric Market research and consulting firm with unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process.
Contact:
Data Bridge Market Research
Tel: +1-888-387-2818
Email: [email protected]
#Ischemic Cerebral Stroke market#Ischemic Cerebral Stroke market Share#Ischemic Cerebral Stroke market Trends#Ischemic Cerebral Stroke market Forecast
0 notes
Text
Acute Ischemic Stroke Global Clinical Trials Review, H2, 2018 published on
http://www.sandlerresearch.org/acute-ischemic-stroke-global-clinical-trials-review-h2-2018.html
Acute Ischemic Stroke Global Clinical Trials Review, H2, 2018
Acute Ischemic Stroke Global Clinical Trials Review, H2, 2018
Summary
GlobalData’s clinical trial report, “Acute Ischemic Stroke Global Clinical Trials Review, H2, 2018” provides an overview of Acute Ischemic Stroke clinical trials scenario. This report provides top line data relating to the clinical trials on Acute Ischemic Stroke. Report includes an overview of trial numbers and their average enrollment in top countries conducted across the globe. The report offers coverage of disease clinical trials by region, country (G7 & E7), phase, trial status, end points status and sponsor type. Report also provides prominent drugs for in-progress trials (based on number of ongoing trials). GlobalData Clinical Trial Reports are generated using GlobalData’s proprietary database – Pharma eTrack Clinical trials database. Clinical trials are collated from 80+ different clinical trial registries, conferences, journals, news etc across the globe. Clinical trials database undergoes periodic update by dynamic process.
The report enhances the decision making capabilities and helps to create an effective counter strategies to gain competitive advantage.
*Note: Certain sections in the report may be removed or altered based on the availability and relevance of data for the indicated disease.
Scope
– The report provides a snapshot of the global clinical trials landscape – Report provides top level data related to the clinical trials by Region, Country (G7 & E7), Trial Status, Trial Phase, Sponsor Type and End point status – The report reviews top companies involved and enlists all trials (Trial title, Phase, and Status) pertaining to the company – The report provides all the unaccomplished trials (Terminated, Suspended and Withdrawn) with reason for unaccomplishment – The Report provides enrollment trends for the past five years – Report provides latest news for the past three months
*Note: Certain sections in the report may be removed or altered based on the availability and relevance of data for the indicated disease.
Reasons to buy
– Assists in formulating key business strategies with regards to investment – Helps in identifying prominent locations for conducting clinical trials which saves time and cost – Provides top level analysis of Global Clinical Trials Market which helps in identifying key business opportunities – Supports understanding of trials count and enrollment trends by country in global therapeutics market – Aids in interpreting the success rates of clinical trials by providing a comparative scenario of completed and uncompleted (terminated, suspended or withdrawn) trials – Facilitates clinical trial assessment of the indication on a global, regional and country level
*Note: Certain sections in the report may be removed or altered based on the availability and relevance of data for the indicated disease.
0 notes