#abecma
Explore tagged Tumblr posts
Text
El impulso científico de los hospitales españoles ha logrado que empiece a ser una realidad un escenario inimaginable hace muy poco e inédito en el mundo: que la sanidad pública pueda ser en buena parte autosuficiente en la producción de las revolucionarias terapias CAR-T frente a tipos de cáncer como el mieloma múltiple... estas terapias son cuatro veces más baratas, lo que contribuye a la sostenibilidad del sistema... Frente al mieloma múltiple hay dos terapias comerciales aprobadas [Abecma y Carvycti], pero estas no llegan a muchos enfermos por varias razones: falta de capacidad de producción de las farmacéuticas, estrategias comerciales, elevados precios... El avance ha despertado los recelos de las compañías farmacéuticas que tienen aprobadas estas terapias... “La industria maniobra en Bruselas para hacer valer sus intereses. Las farmacéuticas pretenden, por lo que hemos visto, impedir los CAR-T académicos en las indicaciones en las que existe uno comercial. Esto supondría el fin del ARI-0002h” (Oriol Güell )
#comparacion medicina privada y publica#privatizacion medicina efectos#privatizacion medicina#medicina
0 notes
Text
BMS vs. Janssen: Who Will Prevail in the Race for Multiple Myeloma Treatment Dominance?

The landscape of multiple myeloma treatment has experienced unprecedented growth and innovation in recent years, thanks to advancements in therapeutic options and a deeper understanding of the disease. Among the key players in this evolving market, Bristol Myers Squibb (BMS) and Janssen Pharmaceuticals have emerged as leaders, each vying for a dominant position. This article explores their competitive strategies, product portfolios, and future prospects as they navigate the complexities of the multiple myeloma treatment market throughout this decade.
The Current Landscape of Multiple Myeloma Treatments
Multiple myeloma, characterized by the abnormal growth of plasma cells in the bone marrow, has traditionally been challenging to treat. However, the development of new therapies has significantly improved patient outcomes. The current treatment landscape includes several categories of drugs, including:
Proteasome Inhibitors: These agents, such as bortezomib and carfilzomib, disrupt the degradation of proteins in cancer cells, effectively inducing cell death.
Immunomodulatory Drugs (IMiDs): Drugs like lenalidomide and pomalidomide have shown efficacy in stimulating the immune system to fight cancer cells while simultaneously inhibiting their growth.
Monoclonal Antibodies: Agents such as daratumumab and elotuzumab are designed to specifically target myeloma cells, enhancing the immune response against them.
CAR-T Cell Therapies: Innovative treatments that engineer a patient's T cells to specifically attack cancer cells have gained traction in managing relapsed cases.
Given the increasing prevalence of multiple myeloma, driven by an aging population, the competition among pharmaceutical companies to develop effective therapies has intensified, with BMS and Janssen at the forefront.
Bristol Myers Squibb: Driving Innovation in Oncology
Bristol Myers Squibb has established itself as a formidable force in the oncology space, particularly in multiple myeloma treatment. The company's flagship drug, Revlimid (lenalidomide), remains a standard of care and has significantly improved survival rates for patients. Looking ahead, BMS is dedicated to expanding its treatment offerings through:
Advanced CAR-T Cell Therapies: BMS is pioneering CAR-T therapies, notably Abecma (idecabtagene vicleucel), which targets BCMA (B-cell maturation antigen) and has shown promise in treating patients with relapsed or refractory multiple myeloma.
Innovative Monoclonal Antibodies: The company is actively investigating new monoclonal antibodies to improve treatment outcomes and target various mechanisms of resistance.
Combination Treatment Strategies: BMS is focused on developing combination therapies to enhance efficacy and counteract treatment resistance, positioning itself well for future success.
BMS’s commitment to research and innovation is evident in its collaborations and partnerships that aim to broaden treatment access and improve patient outcomes.
Janssen Pharmaceuticals: A Comprehensive Approach to Treatment
Janssen, a subsidiary of Johnson & Johnson, is a powerful contender in the multiple myeloma treatment arena. The company’s diverse portfolio of therapies has redefined treatment options for patients. Key products include:
Darzalex (daratumumab): This pioneering monoclonal antibody has become a cornerstone therapy for multiple myeloma, providing significant survival benefits and becoming standard practice for many patients.
Ninlaro (ixazomib): An oral proteasome inhibitor that simplifies treatment regimens, enhancing patient adherence and overall satisfaction.
Carvykti (ciltacabtagene autoleucel): A newly approved CAR-T therapy that targets BCMA and offers new hope for patients with limited treatment options.
Janssen's strategic focus on clinical research and real-world evidence ensures that its therapies meet the evolving needs of patients and healthcare providers. The company is committed to exploring combination therapies to optimize treatment outcomes.
Comparative Analysis: Strengths, Weaknesses, and Market Strategies
Pipeline Robustness:
BMS boasts a strong pipeline, particularly in CAR-T cell therapies, positioning it favorably for treating relapsed cases.
Janssen's diverse approach, incorporating monoclonal antibodies and oral therapies, allows it to cater to a broader patient demographic.
Market Penetration and Access:
BMS has made significant strides in securing market access for its therapies, but faces competition from Janssen’s established distribution channels.
Janssen's vast resources enable it to reach a wide patient population, bolstering its competitive advantage.
Clinical Research and Development:
Both companies are heavily invested in clinical trials to expand indications and improve treatment efficacy. Janssen's focus on combination therapies may yield quicker results in enhancing patient outcomes.
Future Outlook: Who Will Lead the Market?
As the decade unfolds, both BMS and Janssen are poised to significantly impact the multiple myeloma treatment landscape. The competition between these two companies will be shaped by several key factors, including:
Advancements in Treatment Paradigms: The introduction of innovative therapies and combination regimens will influence clinician preferences and treatment guidelines.
Patient-Centric Approaches: Companies that effectively communicate the benefits of their therapies and ensure patient access will likely gain a competitive edge.
Regulatory Approvals: Timely approvals for new therapies and indications can drastically alter market dynamics, providing significant competitive advantages.
Conclusion
The rivalry between BMS and Janssen in the multiple myeloma treatment market is intense, with both companies poised to lead through their innovative therapies and extensive pipelines. While BMS focuses on advancing its CAR-T cell offerings, Janssen’s diverse product portfolio positions it well for continued growth. Ultimately, the next decade will be defined by advancements in treatment options, patient accessibility, and the adaptability of these companies to meet the evolving needs of patients and healthcare providers. The competition for dominance in the multiple myeloma treatment market is just beginning, and the outcome will significantly influence the future of cancer care.
#multiple myeloma#multiple myeloma Market#multiple myeloma Forecast#multiple myeloma Companies#multiple myeloma Drugs#multiple myeloma Therapies#multiple myeloma Epidemiology#multiple myeloma Pipeline#multiple myeloma Market Size#multiple myeloma Market Trends
0 notes
Text

Chimeric antigen receptor (CAR) T-cell therapy is a technique that uses genetic engineering to modify T cells, a subset of white blood cells, to enable them to recognize and eliminate cancerous cells. Because CAR T-cell therapy modifies T cells' genes to enable them to fight cancer, it is occasionally referred to as a form of cell-based gene therapy.
Even in cases where alternative treatments are no longer effective, this kind of care can help treat specific cancers.

How CAR T-cell therapy work
In CAR T-cell therapies, T cells are taken from the patient's blood and are changed in the lab by adding a gene for a receptor (called a chimeric antigen receptor or CAR), which helps the T cells attach to a specific cancer cell antigen. The CAR T cells are then given back to the patient.
CAR T-cell therapy can take a few weeks to complete.
Leukapheresis is the first procedure used to separate white blood cells (including T cells) from the patient's blood. Patients typically lie in bed or recline in a reclining chair for this operation. Because blood is drawn from one IV line, white blood cells are extracted, and then the blood is reinfused, two IV lines are required. Patients typically lie in bed or recline in a reclining chair for this operation. White blood cells are extracted from the blood through one IV line, and the blood is reinfused into the body through the other, necessitating the use of two IV lines. Central venous catheters, a unique kind of IV line with both IV lines integrated in, are occasionally utilized.
For two to three hours during the process, the patient will need to remain seated or in a prone position. During leukapheresis, blood calcium levels might occasionally fall, which can result in tingling and numbness as well as muscle spasms. The replacement of calcium, which can be either intravenously or orally, can be used to treat this.
Once the white cells are extracted, the T cells are isolated, transferred to a laboratory, and manipulated by introducing the gene corresponding to the particular chimeric antigen receptor (CAR). They are therefore CAR T cells. Then, in the lab, these cells are expanded and multiplied. The production of the vast quantity of CAR T cells required for this treatment can take many weeks.
Once sufficient CAR-T cells have been produced, they will be returned to the patient. The patient may get chemotherapy a few days before to the CAR T-cell infusion in order to assist reduce the quantity of other immune cells. This increases the likelihood that the CAR T cells will become activated to combat the malignancy. Because CAR T cells function best while there are still some cancer cells to assault, this chemotherapy is typically not very strong. The CAR T cells proliferate and have the ability to aid in the destruction of other cancer cells once they attach to cancer cells.
Some of the approved CAR T-cell therapies include:
Tisagenlecleucel, also known as tisa-cel (Kymriah)
Axicabtagene ciloleucel, also known as axi-cel (Yescarta)
Brexucabtagene autoleucel, also known as brexu-cel (Tecartus)
Lisocabtagene maraleucel, also known as liso-cel (Breyanzi)
Idecabtagene vicleucel, also known as ide-cel (Abecma)
Ciltacabtegene autoleucel, also known as cilta-cel (Carvykti)
Market Of CAR-T Cell Therapy
The market for CAR T-cell treatment was estimated to be worth USD 2.75 billion globally in 2022. From 2023 to 2030, it is projected to expand at a compound annual growth rate (CAGR) of 23.32%, peaking at USD 15.97 billion. Increased cancer prevalence, clinical success, regulatory approvals, and expanding indications are some of the factors driving the market. But the medications' side effects and exorbitant costs could also impede expansion.
The pipeline highlights of CAR-T cell therapy are following
In August 2023, the US FDA granted orphan drug designation (ODD) to NXC-201 (Nexcella), a next generation chimeric antigen receptor (CAR) T-cell therapy, for the treatment of patients suffering from multiple myeloma.
In August 2023, Invectys announced that FDA granted a Fast Track Designation to the chimeric antigen receptor (CAR) T-cell therapy, IVS-3001, which is used for the treatment of patients suffering from renal cell carcinoma.
In August 2023, GenScript Biotech entered into a strategic agreement with T-MAXIMUM Biotech with an aim to develop CAR T cell therapy by using GenScript’s CRISPR nucleic acid reagents
In January 2023, CARsgen Therapeutics had announced a collaboration with Huadong Medicine to commercialize zevorcabtagene autoleucel (zevor-cel), CT053, in mainland China. Under the collaboration, Huadong Medicine will have the exclusive right to commercialize CARsgen’s CT053 in mainland China. According to the deal, CARsgen will receive $29.7m (RMB200m) in upfront payment and is also eligible for up to $152.4m (RMB1,025m) in regulatory and commercial milestones.
In December 2022, Arcellx, Inc. announced a global strategic collaboration to co-develop and co-commercialize Arcellx's lead late-stage product candidate, CART-ddBCMA, for the treatment of patients with relapsed or refractory multiple myeloma. Currently in Phase 2 clinical development, CART-ddBCMA is an investigational cell therapy product comprising autologous T cells that have been genetically modified to target multiple myeloma. CART-ddBCMA utilizes Arcellx's novel D-Domain binder. Kite and Arcellx will jointly advance the CART-ddBMCA asset.
In December 2022, CARsgen Therapeutics Holdings Limited announced that at the 2022 American Society of Hematology (the "ASH") Annual Meeting, the Company presented a poster with the results of the phase I/II LUMMICAR STUDY 1 clinical trial of zevorcabtagene autoleucel. Results for the 14 subjects treated in phase I of LUMMICAR STUDY 1 showed a well-tolerated safety profile, plus deep and durable responses with an objective response rate (ORR) of 100% and a complete response/stringent complete response (CR/sCR) rate of 78.6%.
In October 2022, CARsgen Therapeutics Holdings Limited announced that the National Medical Products Administration (NMPA) of China has accepted the New Drug Application (NDA) for zevorcabtagene autoleucel for the treatment of relapsed and/or refractory multiple myeloma (R/R MM). The acceptance of the NDA was based on data from an open-label, single arm Phase I/II clinical trial (LUMMICAR STUDY 1 [Protocol number CT053-MM-01]) in China. Study results showed that zevor-cel has excellent safety and efficacy profiles. Zevor-cel also represents a promising treatment option for patients with high-risk disease.
In August 2022, Poseida Therapeutics, Inc. announced it has entered into a broad strategic collaboration and license agreement with Roche, focused on developing allogeneic CAR-T therapies directed to hematologic malignancies. The global collaboration covers the research and development of multiple existing and novel "off-the-shelf" cell therapies against targets in multiple myeloma, B-cell lymphomas and other hematologic indications.
Conclusion
CAR T-cell therapy is a rapidly growing segment within the broader immunotherapy market, offering a transformative approach to cancer treatment. The therapy involves modifying a patient's T cells to recognize and destroy cancer cells, providing personalized and highly targeted treatment. The CAR T-cell therapy market is expected to grow significantly due to advancements in biotechnology and increasing approval of CAR T-cell therapies for various cancer types. Ongoing research and development are likely to result in more effective therapies with fewer side effects. The market is becoming increasingly competitive, with several biotech and pharmaceutical companies developing CAR T-cell therapies. Collaborations, mergers, and acquisitions are common as companies seek to strengthen their portfolios and market positions.
Contact Us:
BlueWeave Consulting & Research Pvt. Ltd.
+1 866 658 6826 | +1 425 320 4776 | +44 1865 60 0662
0 notes
Text
Earnings call: 2Seventy Bio sees progress with ABECMA in Q2 2024
0 notes
Text
T Cell Therapy Market Size was valued at USD 3.8 Billion in 2023 and is expected to reach a market size of USD 49.9 Billion by 2032
The T cell therapy market size was valued at USD 3.8 Billion in 2023 and is expected to reach a market size of USD 49.9 Billion by 2032 at a CAGR of 32.9%.
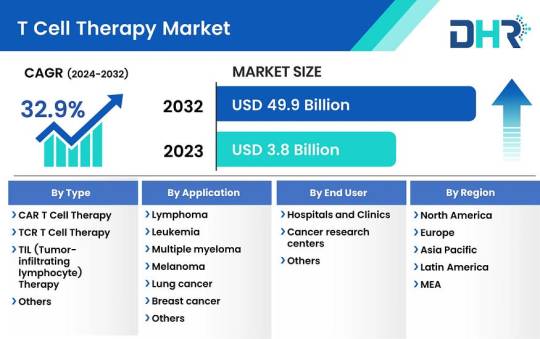
Request Sample Report: https://datahorizzonresearch.com/request-sample-pdf/t-cell-therapy-market-3114
Top Companies are:
· Novartis AG
· Gilead Sciences, Inc.
· Bristol-Myers Squibb Company
· Adaptimmune Therapeutics plc
· Amgen Inc.
· Atara Biotherapeutics, Inc.
· Autolus Therapeutics plc
· bluebird bio, Inc.
· Cellectis S.A.
· Iovance Biotherapeutics, Inc.
· Kite Pharma (a Gilead Company)
· Tmunity Therapeutics, Inc.
Market Segmentations:
By Type-
CAR T cell therapy
TCR T cell therapy
TIL (Tumor-infiltrating lymphocyte) therapy
Others
By Application-
Lymphoma
Leukemia
Multiple myeloma
Melanoma
Lung cancer
Breast cancer
Colorectal cancer
Autoimmune disorders
Infectious diseases
Others
By End User-
Hospitals and Clinics
Cancer research centers
Others
Regional Analysis:
The dominance of the T Cell Therapy market in North America is underpinned by the presence of established biopharmaceutical firms, a robust clinical trial infrastructure, and a favorable regulatory landscape. Among North American countries, the United States stands out as the primary contributor, buoyed by the FDA’s approval of several CAR T cell therapies such as Kymriah, Yescarta, and Abecma for treating hematological malignancies. The U.S. National Library of Medicine’s database reveals an extensive presence of over 1,000 active clinical trials dedicated to evaluating T cell therapies, underscoring the region’s steadfast commitment to research and development in this field.
Key highlights of the report include:
1. The report delivers thorough Market analysis, furnishing valuable insights to guide strategic decision-making.
2. The comprehensive research outlined in the study enhances the depth of your presentations and marketing strategies.
3. By offering crucial insights into key market competitors, the study empowers businesses with a strategic edge.
4. It delivers a precise assessment of evolving market dynamics, ensuring readers stay abreast of the latest industry trends.
5. With meticulous breakdowns of various market niches, the report facilitates informed decision-making processes.
0 notes
Text
Headline: 2seventy Bio Inc Faces Urgent Challenges Throughout First Quarter of 2024 $TSVT #Nasdaq
Company navigates through critical time with strategic decision-making and innovative solutionsWith the recent release of their first quarter 2024 financial results, biotechnology company 2seventy bio, Inc. is showing promising signs of growth and value creation for their shareholders. The company, headquartered in Cambridge, Massachusetts, has undergone a strategic realignment that prioritizes their groundbreaking cancer therapy, Abecma, leading to positive financial outcomes. During the financial first quarter of 2024, 2seventy bio experie
0 notes
Text
FDA Menyetujui Idecabtagene Vicleucel untuk Multiple Myeloma Setelah 2 atau Lebih Jalur Terapi
Majalah Farmasetika – Persetujuan ini memperluas indikasi sebelumnya dari idecabtagene vicleucel (Abecma; Bristol Myers Squibb), yang akan membuat obat tersedia untuk pasien pada jalur sebelumnya. FDA telah menyetujui idecabtagene vicleucel (Abecma; Bristol Myers Squibb) untuk pengobatan multiple myeloma yang kembali atau refrakter pada orang dewasa yang menerima 2 atau lebih jalur terapi…
View On WordPress
0 notes
Link
2seventy says it is refocusing its efforts on CAR-T therapy Abecma as a restructure sees it cut 176 jobs – 40% of its workforce – to help free up $130+ million. Having spun-out of Bluebird bio in 2021, 2seventy took responsibility for codeveloping A #BioTech #science
0 notes
Text
CAR-T Cell Therapy Market – Transforming lives through personalized CAR-T cell therapy for cancer patients

This latest report researches the industry structure, sales, revenue, price and gross margin. Major producers’ production locations, market shares, industry ranking and profiles are presented. The primary and secondary research is done in order to access up-to-date government regulations, market information and industry data. Data were collected from the CAR-T Cell Therapy manufacturers, distributors, end users, industry associations, governments’ industry bureaus, industry publications, industry experts, third party database, and our in-house databases.
Get a Free Sample PDF of the Report – https://www.growthplusreports.com/inquiry/request-sample/cart-cell-therapy-market/8902
Key Players in the CAR-T Cell Therapy Market: –
Novartis AG Bristol-Myers Squibb Company Amgen Inc. Gilead Sciences Inc. (Kite Pharma) Johnson & Johnson Eli Lilly and Company Sorrento Therapeutics Inc. ACROBiosystems Celyad Oncology Sangamo Therapeutics Inc. Servier Laboratories Noile-Immune Biotech Inc. Miltenyi Biotec
Market Segmentation:
GLOBAL CAR-T CELL THERAPY MARKET – ANALYSIS & FORECAST, BY DRUG TYPE
Kymriah
Yescarta
Abecma
Breyanzi
Tecartus
Carvykti
Others
GLOBAL CAR-T CELL THERAPY MARKET – ANALYSIS & FORECAST, BY INDICATION
Diffuse Large B-Cell Lymphoma
Acute Lymphoblastic Leukemia
Multiple Myeloma
Chronic Lymphocytic Leukemia
Follicular Lymphoma
Mantle Cell Lymphoma
Others
Market segment by Region/Country including: –
-North America (United States, Canada and Mexico) -Europe (Germany, UK, France, Italy, Russia and Spain etc.) -Asia-Pacific (China, Japan, Korea, India, Australia and Southeast Asia etc.) -South America (Brazil, Argentina and Colombia etc.) -Middle East and Africa (South Africa, UAE and Saudi Arabia etc.)
This report also includes a discussion of the major players across each regional CAR-T Cell Therapy market. Further, it explains the major drivers and regional dynamics of the global CAR-T Cell Therapy market and current trends within the industry.
Request for customization in Report: https://www.growthplusreports.com/inquiry/customization/cart-cell-therapy-market/8902
Key Benefits for Industry Participants and Stakeholders One can find in-depth research data and industry trends of the CAR-T Cell Therapy Market Research. The report offers details on potential investment opportunities, including those that are local and sector-specific that may benefit stakeholders and members of the industry. One can gain a thorough grasp of market dynamics by looking at prices as well as the activities of producers and consumers. With the use of market research, which will assist in discovering and visualizing new market participants as well as their portfolios, will be better able to make decisions and create an efficient counter strategy to maximize market advantage.
COVID 19 Impact Analysis
The CAR-T Cell Therapy Market Research Reports include a thorough discussion of the coronavirus’s effects in addition to the major market trends. When considering the impact of the COVID-19 on the industry, insights, analysis, projections, and predictions are given in the report study.
Given the breadth of the pandemic’s disruption, it is evident that the current depression is fundamentally different from previous recessions. Due to the sudden drop in demand and growing unemployment, the business climate will alter. In this uncomfortable environment, businesses may carve new roads by embracing novel ideas like ”advance toward localization, cash conservation, supply chain resilience, and innovation.”
CAR-T Cell Therapy Market TOC: https://www.growthplusreports.com/report/toc/cart-cell-therapy-market/8902
the market share and rank (in volume and value), competitor ecosystem, new product development, expansion, and acquisition.
This report stays updated with novel technology integration, features, and the latest developments in the market
This report helps stakeholders to understand the COVID-19 and Russia-Ukraine War Influence on the CAR-T Cell Therapy industry.
This report helps stakeholders to gain insights into which regions to target globally
This report helps stakeholders to gain insights into the end-user perception concerning the adoption of CAR-T Cell Therapy .
This report helps stakeholders to identify some of the key players in the market and understand their valuable contribution.
QUICK BUY: https://www.growthplusreports.com/checkout-8902
Browse Latest Healthcare Reports:
Aptamers Market
Influenza Diagnostics Market
Depression Apps Market
Leukapheresis Market
Mastopexy Market
Catheter Introducer Sheaths Market
Stuttering Therapeutics Market
Fibrous Dysplasia Market
Stoma Care Market
Contact Us:
Manan Sethi Director, Market Insights Email: [email protected] Phone no: +1 888 550 5009 Web: https://www.growthplusreports.com/
About Us Growth Plus Reports is part of GRG Health, a global healthcare knowledge service company. We are proud members of EPhMRA (European Pharmaceutical Marketing Research Association). Growth Plus portfolio of services draws on our core capabilities of secondary & primary research, market modelling & forecasting, benchmarking, analysis and strategy formulation to help clients create scalable, ground-breaking solutions that prepare them for future growth and success. We were awarded by the prestigious CEO Magazine as “Most Innovative Healthcare Market Research Company in 2020.”
0 notes
Text
Global Europe CAR-T Cell Therapy Market Size, Trends, and Report
The Europe CAR-T Cell Therapy Market was valued at USD 296.21 Mn in 2020 which expected to reach USD 1,904.40 Mn by 2027 at a CAGR 30.45% from 2020-2027.
The CAR-T Cell Therapy market is a conceptual examination of all commercial activities related to CAR-T Cell Therapy, either directly or indirectly. As a result, a new investor can learn about CAR-T Cell Therapy firms, their important products, their basic strategy, key CAR-T Cell Therapy market trends, and more.
Get a Sample Copy of this Report@ https://qualiketresearch.com/request-sample/Europe-CAR-T-Cell-Therapy-Market/request-sample
Market Drivers
Increasing approvals for CAR-T cell therapy products.
Increase in awareness about the new approach to treat cancer leads to increase in demand for the CAR-T cell therapy products. Thus, the key players in market are engaged in developing new products & thereby drive the growth of Europe CAR-T Cell Market.
For Instance, in June 2020, Kite Pharma, received approval to implement a variation to the Yescarta (axicabtagene ciloleucel) Marketing Authorization from the European Medicine Agency for end-to-end manufacturing. With this approval, Kite’s European manufacturing facility, designed & dedicated to the manufacture of individualized cell therapies, is now fully operational.
Market Restraints
CAR T therapies often come with unique drug development challenges. Some potential challenges associated with CAR T development is likely to hamper the growth of the Europe CAR-T Cell Therapy market. The potential challenges include limited guidance, manufacturing and distribution logistics, products safety etc.
Moreover, high cost involved in research & development for CAR-T cell therapies & lack of expertise as well as inadequate knowledge about CAR-T cell therapies are the major factors among others acting as restraints, and will further challenge the market in the forecast period.
Impact of COVID-19
Many businesses have seen their operations & financial performance suffer as a result of the COVID–19 pandemic and a slowing of global research activity. The negative impact is mostly due to the closure of academic & research institutes, as well as testing laboratories. As a result, the clinical trials conducted for the car T cell therapy has been delayed.
COVID-19 is posing a significant threat to health of vulnerable patients, like immunocompromised patients. CAR-T-cell therapy recipients are at high risk of poor COVID-19 due to their severely immunocompromised state, caused by prior lymphodepleting immunochemotherapy & CAR-T-cell therapy related side effects like B-cell depletion, hypogammaglobulinemia, and cytopenias.
Market Segmentation
The Europe CAR-T Cell Therapy Market is segmented into Type, Application, End-user and Growing System. By Type such as Abecma, Breyanzi , Kymriah, Tecartus, Yescarta. Further, market is segmented into By Application such as Cancer, Lymphoma, Others. By End-user such as Hospitals, Specialty Clinics, Others.
Country Analysis
Europe CAR-T Cell Therapy Market is segmented into fifteen regions such as Germany, France, UK, Turkey, Switzerland, Norway, Sweden, Spain, Denmark, Finland, Iceland, Poland, Luxembourg, Netherlands, and Belgium. Germany dominated the market and accounted for the largest revenue share of 15.10% in 2020. The region is expected to continue its dominance over the forecast period. UK is expected to grow at significant growth rate, as end-user such as Hospitals and Specialty Clinics segment are growing in the country. Due to rapid advances in the healthcare infrastructure and disposable income, the market
Get Discount on this Report@ https://qualiketresearch.com/request-sample/Europe-CAR-T-Cell-Therapy-Market/ask-for-discount
Key Players
Various key players are listed in this report such as Mustang Bio Inc, Calgene Corporation, Bluebird Bio Inc., Kite Pharma, Inc, CARsgen Therapeutics, Ltd., Legend Biotech, Immune Therapeutics, Pfizer Inc, Bellicum Pharmaceuticals, Inc, Sorrento Therapeutics, Inc, Novartis.
Market Taxonomy
By Type
Abecma
Breyanzi
Kymriah
Tecartus
Yescarta
By Application
Cancer
Lymphoma
Others
By End-user
Hospitals
Specialty Clinics
Others
By Country
Germany
France
UK
Turkey
Switzerland
Norway
Sweden
Spain
Denmark
Finland
Iceland
Poland
Luxembourg
Netherlands
Belgium
Key Questions Addressed by the Report
What are the Key Opportunities in Europe CAR-T Cell Therapy Market?
What will be the growth rate from 2020 to 2027?
Which segment/region will have highest growth?
What are the factors that will impact/drive the Market?
What is the competitive Landscape in the Industry?
What is the role of key players in the value chain?
Browse Full Report https://qualiketresearch.com/reports-details/Europe-CAR-T-Cell-Therapy-Market
0 notes
Photo

2021 drug approvals: In a year dominated by COVID, biopharma managed to deliver 55 new drugs #fdaapproval #MSL #FSTP #BoardcertifiedMSL #BCMSLcert “Each year, many scientists and other experts working in the biopharma industry see years of their work culminate in FDA approvals. With the COVID-19 crisis stretching into its third year, the 2021 batch of new drug approvals clearly demonstrates that the sector has learned how to operate in a pandemic. All told, the industry nabbed 55 FDA approvals in 2021. They include the controversial accelerated approval for Biogen's Alzheimer's drug Aduhelm, Pfizer's record-shattering COVID-19 vaccine, Comirnaty, and many others. Not all 2021 approvals commanded as many headlines as Aduhelm and Comirnaty. Aside from those notable companies, also represented in 2021's crop of new approvals were Amgen, Bristol Myers Squibb, GlaxoSmithKline, Johnson & Johnson, Merck and Regeneron. And among the dozens of new drugs green-lit last year, companies such as BridgeBio, ChemoCentryx and Apellis won their first-ever FDA approvals, setting them up to make their commercial debuts. RELATED: FDA missing more approval target dates as COVID slows inspections Yet, some applications did face pandemic challenges. The FDA struggled to keep up with its inspection duties during the pandemic, leading to regulatory delays for certain drugmakers. In November, the agency reported that 55 new drug applications were facing inspection delays. Avadel Pharmaceuticals and UCB were among those to experience inspection-related delays in 2021. “ 2021 drug approvals: In a year dominated by COVID, biopharma managed to deliver 55 new drugs 1. #Verquvo 2. #Cabenuva 3. #Lupkynis 4. #Tepmetko 5. #Ukoniq 6. #Breyanzi 7. #Evkeeza 8. #Cosela 9. Amondys 45 10. #Pepaxto 11. #Nulibry 12. #Azstarys 13. #Fotivda 14. #Ponvory 15.#Zegalogue 16. #Abecma 17. #Qelbree 18. #Nextstellis 19. #Jemperli 20. #Zynlonta 21. #Empaveli 22. #Rybrevant 23. #Lybalvi 24. #Truseltiq 25. #Lumakras … https://lnkd.in/gwV7JXXe https://www.instagram.com/p/CYVaOqVpos3/?utm_medium=tumblr
#fdaapproval#msl#fstp#boardcertifiedmsl#bcmslcert#verquvo#cabenuva#lupkynis#tepmetko#ukoniq#breyanzi#evkeeza#cosela#pepaxto#nulibry#azstarys#fotivda#ponvory#zegalogue#abecma#qelbree#nextstellis#jemperli#zynlonta#empaveli#rybrevant#lybalvi#truseltiq#lumakras
0 notes
Text
Multiple Myeloma: Advancements and Future Outlook in Treatment

Multiple Myeloma, the second most common form of blood cancer following non-Hodgkin lymphoma, is characterized by the proliferation of malignant plasma cells in the bone marrow. These abnormal cells outnumber healthy blood cells, severely compromising the body's ability to function and fight infections. Early diagnosis and appropriate treatment are critical to managing the disease. Multiple Myeloma presents in various forms, including symptomatic (active disease), asymptomatic (smoldering myeloma), solitary plasmacytoma, and rare plasma cell leukemia, each requiring tailored diagnostic and therapeutic strategies.
Rising Prevalence and Demographic Trends
The prevalence of Multiple Myeloma is rising, especially in the elderly population. The incidence rate is highest among older adults, with over half of the cases diagnosed in males. This demographic shift is influencing the Multiple Myeloma drug, with a greater focus on therapies suited to the aging population.
Current Treatment Options and Therapeutic Innovations
Historically, Multiple Myeloma treatment has centered around chemotherapy, corticosteroids, and stem cell transplantation. However, the treatment landscape has evolved with the introduction of new therapies such as Proteasome Inhibitors, Immunomodulating Agents, Histone Deacetylase (HDAC) Inhibitors, Monoclonal Antibodies, and groundbreaking CAR-T cell therapies.
Recent advancements include:
BLENREP (2020), an anti-BCMA immunoconjugate.
NINLARO (2015), the first oral proteasome inhibitor.
EMPLICITI (2015), which enhances immune response.
TECVAYLI (2022), a bispecific antibody redirecting T-cells.
DARZALEX (2015) and TALVEY (2023), targeting CD38 and refractory cases, respectively.
These therapies have extended survival rates and improved the quality of life for patients, making them dependent on continuous treatment.
Advances in Relapsed/Refractory Treatment
The treatment landscape for relapsed or refractory Multiple Myeloma is rapidly evolving, with FDA approvals of CAR-T therapies like ABECMA and CARVYKTI. Companies like Pfizer, Johnson & Johnson, and GSK are competing in this market, with their innovative therapies shaping the future of treatment. Despite challenges like the high cost of CAR-T therapies, ongoing improvements may enhance their accessibility.
Dominant Players and Market Trends
Bristol Myers Squibb (BMS) and Janssen (a subsidiary of Johnson & Johnson) dominate the Multiple Myeloma treatment market with their robust portfolios. Janssen's DARZALEX and TECVAYLI, along with BMS's CAR-T therapy ABECMA, are setting the standard in treatment innovation.
Moreover, the expiration of patents on drugs like REVLIMID has led to generic competition, but companies like BMS are focusing on new therapies such as CELMoDs (Iberdomide and Mezigdomide) to maintain their market presence.
Future Outlook
The Multiple Myeloma treatment market, valued at USD 21,300 million in 2023, is poised for significant growth through 2034. Factors such as rising incidence rates, adoption of newer treatments, and a strong pipeline of emerging therapies will drive this growth. Investment in research and development will be critical in introducing more effective treatments, particularly for a growing elderly population.
Conclusion
Multiple Myeloma treatment is undergoing a transformative period, with new therapies improving patient outcomes and expanding options for relapsed or refractory cases. As the market continues to grow, ongoing innovation and investment will be key to addressing the needs of patients and advancing treatment paradigms.
#Multiple myeloma#Multiple myeloma Market#Multiple myeloma Forecast#Multiple myeloma Companies#Multiple myeloma Drugs#Multiple myeloma Therapies#Multiple myeloma Epidemiology#Multiple myeloma Pipeline#Multiple myeloma Market Size#Multiple myeloma Market Trends
0 notes
Text
BMS reports data that could give its cell therapy an edge over rival J&J product
BMS reports data that could give its cell therapy an edge over rival J&J product
Bristol Myers Squibb (BMS) drug Abecma was the first FDA-approved CAR T-cell therapy for multiple myeloma, but like other drugs in its class, the treatment’s serious side effect risks limit its use to the most advanced cases. The pharmaceutical giant now has data from a pivotal study showing that Abecma beat the standard of care, preliminary results that could support moving the cell therapy up…

View On WordPress
0 notes
Text
BMS reports data that could give its cell therapy an edge over rival J&J product
BMS reports data that could give its cell therapy an edge over rival J&J product
Bristol Myers Squibb (BMS) drug Abecma was the first FDA-approved CAR T-cell therapy for multiple myeloma, but like other drugs in its class, the treatment’s serious side effect risks limit its use to the most advanced cases. The pharmaceutical giant now has data from a pivotal study showing that Abecma beat the standard of care, preliminary results that could support moving the cell therapy up…

View On WordPress
0 notes
Text

Pfizer Presents First Data from Planned Interim Analysis of Pivotal Phase II MagnetisMM-3 Trial of BCMA-CD3 Bispecific Antibody Elranatamab (PF-06863135)
Mechanism of Action: Binds BCMA on myeloma cells and CD3 T cells, thereby bringing together T cells and activating them in the vicinity of the myeloma cell resulting in cell kill.
Main Content- Elranatamab is a BCMA x CD3-targeted investigational therapy currently being developed to treat multiple myeloma. The agent has been granted fast track designation by the United States Food and Drug Administration (FDA). Pfizer presented findings of Elranatamab’s Phase II MagnetisMM-3 study (NCT04649359) at ASCO 2022. MagnetisMM-3 is a Phase II open-label, multicenter, single-arm trial examining the safety and efficacy of elranatamab monotherapy in patients with relapsed/refractory multiple myeloma.
Initial efficacy findings of elranatamab showed a 60.6% objective response rate after a median follow-up of 3.71 months. At the time of the data cut-off, 89.5% of objective responders were ongoing with no evidence of progression or death.
The safety and effectiveness of 94 patients who had received at least one dose of elranatamab (Cohort A – BCMA- naïve) as of the data cutoff on March 23, 2022, were studied in this interim analysis. In patients with triple-class refractory Multiple Myeloma, the data revealed that 76 mg of elranatamab weekly (QW) might have a tolerable safety profile. Hematologic adverse events such as anemia, neutropenia, thrombocytopenia, lymphopenia, and cytokine release syndrome (CRS) were the most prevalent treatment-emergent adverse events. All CRS were Grade I (40.0%) or II (40.0%) in the 90 individuals who underwent the 2-step-up priming regimen (18.9%). In addition, ICANS (immune effector cell-associated neurotoxicity syndrome) affected 2.2% of patients, all of whom were in Grade 2 or below.
“Bispecific antibodies hold promise as the next breakthrough in treating multiple myeloma. These data represent an important step forward as we look to bring elranatamab as a potential innovative new treatment to people living with relapsed or refractory multiple myeloma, where there is a high need”-Expert Opinion.
“We are encouraged by these early efficacy and safety results, which suggest elranatamab may have a manageable safety profile coupled with early promising clinical responses. We look forward to the final analysis from MagnetisMM-3, which is expected later this year.” -Expert Opinion.
Conclusion: The antibody-drug conjugate (Blenrep) and the FDA-approved CAR T-cell therapies (Abecma and Carvykti) are the current BCMA-directed choices for heavily pretreated Multiple Myeloma patients. In the registrational directed Phase II MagnetisMM-3 trial, Pfizer's BCMA x CD3 bispecific antibody elranatamab demonstrated encouraging preliminary effectiveness and tolerability. Before this, the company disclosed results from the phase I MagnetisMM-1 study (NCT03269136), which demonstrated that when elranatamab was administered in dosages up to 1000 µg/kg, the drug had a tolerable safety profile with no dose-limiting toxicities (DLT’s) when presented at the 2021 ASCO Annual Meeting. It is worth noting that the FDA slapped a partial clinical hold on the drug last year owing to peripheral neuropathy cases. But now, the hold has been lifted.
Along with Pfizer, many companies, including J&J, Roche, Regeneron Pharmaceuticals, and others, are investigating bispecific antibodies in Multiple Myeloma. Some of the companies are investigating non-BCMA targets, including FcRH5 and GPRC5D. J&J holds the lead among many bispecific antibodies being explored for Multiple Myeloma. Janssen's teclistamab is the first BCMA x CD3 bispecific to be filed to the FDA (filing in December 2021) and EMA (filing in January 2022) for approval in Multiple Myeloma. While teclistamab appears to be on track to become the first BCMA-targeting bispecific antibody to hit the market, Pfizer's elranatamab is closely following J&J's footsteps. In the registration MagnetisMM-3 trial, elranatamab was linked with an ORR of 60.6 percent after a median follow-up of just under four months; on the other hand, teclistamab's MajesTEC-1 study gave an ORR of 63%. Pfizer’s therapy has a competitive edge in terms of safety profile. The MagnetisMM-3 data show a 60% rate of CRS, compared to roughly 72% in the teclistamab trial. Because bispecific antibodies are off-the-shelf, readily available medicines, they are anticipated to become widely employed in the relapsed/refractory arena. In this area, ADC and CAR T-cell therapies are already available, but the inclusion of bispecific antibodies may provide patients with more options (different treatments will be appropriate for different patients).
Companies- Oncopeptides, GlaxoSmithKline, Bluebird Bio, Janssen Pharmaceutical, Legend Biotech, AbbVie, Roche (Genentech), Bristol Myers Squibb, Regeneron Pharmaceuticals, Pfizer, Takeda, Amgen, SpringWorks Therapeutics, Arcellx, Gracell Biotechnologies, Oricell, Poseida Therapeutics, Precision Biosciences, CRISPR Therapeutics AG, Collectis SA, Allogene Therapeutics, Fortis Therapeutics, Novartis, I-Mab/MorphoSys, Cartesian Therapeutics, CASI Pharmaceuticals, LAVA Therapeutics, and others.
To Get a Detailed analysis of ASCO Conference 2022 Abstracts, Visit: ASCO 2022 Detailed Coverage | ASCO 2022 Conference | ASCO Conference 2022 | ASCO Abstract 2022
Some of the Latest ASCO Abstract 2022 Launched:-
· Can Breyanzi be a hit CAR-T in second-line treatment after failing in the first-line setting for the patients with R/R Large B-cell lymphoma (LBCL)?
· Teclistamab, a soon-to-be-approved bispecific antibody, demonstrates its promise in Multiple Myeloma during ASCO 2022.
· Merus’ Zenocutuzumab, a HER2-HER3 Bispecific Antibody, Successfully Targets NRG1 Fusions in Lung and Pancreatic Cancer
Get in touch with our Business Expert- Business Consulting Services | Biotech Consulting
Reactive Airway Disease Market
Reactive Airway Disease Market report delivers an in-depth understanding of the historical and forecasted epidemiology as well as the market trends in the 7MM
Coronary Microvascular Dysfunction CMD Market
DelveInsight’s Coronary Microvascular Dysfunction Market Insights, Epidemiology, & Market Forecast 2032 report delivers an in-depth understanding of the CMD epidemiology & CMD market trends
Familial Lipoprotein Lipase Deficiency Pipeline
Familial Lipoprotein Lipase Deficiency Pipeline Insights, 2022 report by DelveInsight outlays comprehensive insights of present clinical development scenario and growth prospects across.
Pediatric Growth Hormone Deficiency PGHD Market
Pediatric Growth Hormone Deficiency Market Insights, Epidemiology, and Market Forecast report delivers an understanding of the Pediatric Growth Hormone Deficiency forecasted epidemiology
Shigellosis Market
DelveInsight’s Shigellosis Market Insights, Epidemiology, and Market Forecast-2032″ report delivers an in-depth understanding of the Shigellosis, historical and forecasted epidemiology
About Us
DelveInsight is a Business Consulting and Market research company, providing expert business solutions for the life science vertical and offering quintessential advisory services in the areas of R&D, Strategy Formulation, Operations, Competitive Intelligence, Competitive Landscaping, and Mergers & Acquisitions.
Contact Us
Yash
0 notes
Text
Impact of COVID-19 on CAR T-cell Therapy Market
CAR T-cell Therapy Market: Introduction
According to the report, the global CAR T-cell therapy market was valued at US$ 1.1 Bn in 2020 and is projected to expand at a CAGR of 30.6% from 2021 to 2031. CAR T-cell therapy is a recently approved treatment option for various types of cancer. CAR T cell therapy is provided by removing or harvesting T cells from a patient with cancer, transfecting the cells with CAR genes that are directed against the patient’s tumor type, expanding the modified T cell population, and reinfusing the cells back into the patient. Currently, Kymriah, Yescarta, Tecartus, Breyanzi, and ABECMA are the CAR T-cell therapy approved products for acute lymphocytic leukemia, diffuse large B-cell lymphoma, mantle cell lymphoma, follicular lymphoma, multiple myeloma, and others.
Request a PDF Brochure - https://www.transparencymarketresearch.com/sample/sample.php?flag=B&rep_id=83242
The global CAR T-cell therapy market is driven by increase in incidence of cancer and strong product pipeline. North America dominated the global CAR T-cell therapy market in 2020 and the trend is anticipated to continue during the forecast period. Highly structured healthcare industry, early adoption of new products, high prevalence rate of cancer, and presence of major players are expected to propel the CAR T-cell therapy market in North America. Asia Pacific is likely to be a highly lucrative market for CAR T-cell therapy during the forecast period. The market in the region is projected to expand at a high CAGR from 2021 to 2031.
Request for Analysis of COVID19 Impact on CAR T-cell Therapy Market- https://www.transparencymarketresearch.com/sample/sample.php?flag=covid19&rep_id=83242
Increase in Incidence of Cancer and Strong Product Pipeline to Drive Global Market
Increase in incidence of cancer and strong product pipeline are anticipated to boost the growth of the global CAR T-cell therapy market. According to the WHO, one in five people develop cancer during their lifetime, and one in eight men and one in 11 women die from the disease. These new estimates suggest that more than 50 million people are living within five years of a past cancer diagnosis. According to the WHO, in 2020, around 544,352 new cases of non-Hodgkin lymphoma were recorded globally.
Strong product pipeline is a major factor driving the demand for CAR T-cell therapy products. For instance, Autolus Therapeutics plc has one innovative product, AUTO1, in phase I trial for treatment of adult acute lymphoblastic leukemia. AUTO1 contains obecabtagene autoleucel, which is a CD19 CAR T cell investigational therapy designed to overcome the limitations in clinical activity and safety compared to current CD19 CAR T cell therapies.
Request for Custom Research - https://www.transparencymarketresearch.com/sample/sample.php?flag=CR&rep_id=83242
Axicabtagene Ciloleucel to Dominate CAR T-cell Therapy Market
In terms of product type, the global CAR T-cell therapy market has been classified into axicabtagene ciloleucel, tisagenlecleucel, brexucabtagene autoleucel, lisocabtagene maraleucel, idecabtagene vicleucel, and others. The axicabtagene ciloleucel segment dominated the global CAR T-cell therapy market in 2020 and the trend is expected to continue during the forecast period. Yescarta is a product, which contains axicabtagene ciloleucel. Increase in demand for Yescarta in the treatment of diffuse large B-cell lymphoma and follicular lymphoma is likely to boost the growth of the segment during the forecast period. In October 2017, Kite Pharma, Inc. received the U.S. FDA approval for Yescarta for adult patients with relapsed or refractory follicular lymphoma (FL) after two or more lines of systemic therapy.
Tisagenlecleucel was the second largest segment in terms of market share in 2020. Increase in demand for Kymriah for treatment of acute lymphoblastic lymphoma and product approval in different countries are likely to drive the segment.
Enquiry before Buying CAR T-cell Therapy Market Report - https://www.transparencymarketresearch.com/sample/sample.php?flag=EB&rep_id=83242
Diffuse Large B-cell Lymphoma to be Highly Lucrative
Based on indication, the global CAR T-cell therapy market has been categorized into acute lymphocytic leukemia, diffuse large B-cell lymphoma, mantle cell lymphoma, follicular lymphoma, multiple myeloma, and others. The diffuse large B-cell lymphoma segment dominated the CAR T-cell therapy market due to increase in incidence of diffuse large B-cell lymphoma. Diffuse large B-cell lymphoma is the most common type of non-Hodgkin lymphoma. Increase in number of cases of non-Hodgkin lymphoma is likely to propel the segment during the forecast period. According to WHO, globally, around 544,352 new non-Hodgkin lymphoma cases were recorded in 2020.
Hospitals to Emerge as Significant End-user
In terms of end-user, the global CAR T-cell therapy market has been divided into hospitals and cancer treatment centers. The hospitals segment led the global CAR T-cell therapy market in terms of revenue in 2020 and the trend is projected to continue during the forecast period. The hospitalization for cancer treatment is anticipated to boost the growth of the hospitals segment. Moreover, increase in use of CAR T-Cell therapy in the treatment of cancers contributes to the segment's large market share. The segment is driven by surge in number of cancer patients who visit hospital for treatment of cancer.
Buy now CAR T-cell Therapy Market Report - https://www.transparencymarketresearch.com/checkout.php?rep_id=83242<ype=S
North America to Dominate CAR T-cell Therapy Market
In terms of region, the global CAR T-cell therapy market has been segmented into North America, Europe, Asia Pacific, Latin America, and Middle East & Africa. North America dominated the global CAR T-cell therapy market in 2020, followed by Europe. North America’s large market share can be ascribed to technologically advanced research and treatment platforms, increase in number of cancer patients, new product launches, and presence of major players. The CAR T-cell therapy market in Asia Pacific is anticipated to expand at a high CAGR from 2021 to 2031. This can be ascribed to the presence of developing countries with commercial hubs, expansion of business organizations, rise in awareness about CAR T-cell therapy, improvement in healthcare infrastructure, and increase in investments by companies.
Competition Landscape
The global CAR T-cell therapy market is consolidated due to limited approved products. Key players operating in the global market include Pfizer, Inc., Novartis AG, Bristol-Myers Squibb, Amgen, Inc., Sorrento Therapeutics, Inc., Johnson & Johnson Services, Inc., Gilead Sciences, Inc., Merck & Co., Inc., and bluebird bio, Inc.
More Trending Reports by Transparency Market Research:
D-dimer Testing Market D-dimer tests are used for diagnosing conditions related broadly to deep vein thrombosis (DVT), pulmonary embolism, and disseminated intravascular coagulation (DIC). Among all the applications, the demand for the test for diagnosing DVT is prominent.
Pyrogen Testing Market North America dominated the global pyrogen testing market in 2018and the trend is anticipated to continue during the forecast period. New drug approvals by the Food and Drug Administration (FDA) and increase in demand for pyrogen testing for medical devices are expected to drive the pyrogen testing market in North America.
Conserving Devices Market While nasal cannulae for supplementary supply of oxygen is reliable and effective, it is very wasteful. To address this, oxygen conserving methodologies have emerged to improve the efficiency of oxygen delivery for patients requiring oxygen therapy. This is because oxygen conserving devices enable a high degree of portability, improve the efficiency of oxygen delivery, and lighten the load for these patients.
About Us Section:
Transparency Market Research is a global market intelligence company, providing global business information reports and services. Our exclusive blend of quantitative forecasting and trends analysis provides forward-looking insight for thousands of decision makers. Our experienced team of Analysts, Researchers, and Consultants, use proprietary data sources and various tools and techniques to gather, and analyse information. Now avail flexible Research Subscriptions, and access Research multi-format through downloadable databooks, infographics, charts, interactive playbook for data visualization and full reports through MarketNgage, the unified market intelligence engine. Sign Up for a 7 day free trial!
Contact Us
Mr. Rohit Bhisey Transparency Market Research, 90 State Street, Suite 700, Albany, NY 12207 Tel: +1-518-618-1030 USA – Canada Toll Free: 866-552-3453 Email: [email protected]
Website: https://www.transparencymarketresearch.com/
0 notes