#Water supplier in Kurla
Explore tagged Tumblr posts
Text
Benefits of warehouse for lease - ESR India
ESR is a leader in warehouse solutions, offering warehouse space for rent. We specialize in helping businesses secure their storage needs so that they can focus on what they do best. Our warehouses are large and air-conditioned, offering state of the art security features such as keyless entry, climate-controlled systems, fire detection systems and many more features that create an excellent business environment. Whether you're just getting started or you've been in business for a long time, ESR's warehouse for lease offers a wide range of benefits that help to improve business efficiency and customer service. We provide the space and resources necessary to ensure the success of your business, helping you meet all your business goals with ease.
ESR Warehouse for Lease provides a unique opportunity tolease an empty warehouse space. This efficient and cost-effective way to improve your business is perfect for small businesses looking for an extra space to use without having to invest in a full-fledged business. With ESR Warehouse for Lease, you can take advantage of our advanced technology and lease space that is already set up with required equipment.
The challenges of leasing a warehouse:
Leasing a warehouse is an excellent solution for businesses that require extra space to store their products, machinery, or equipment. However, it is not always an easy process. There are several challenges that businesses face when leasing a warehouse, starting from finding the right location to negotiating lease terms. This article will explore some of the challenges involved in leasing a warehouse and provide practical tips to help you overcome them.
The first challenge that businesses face when looking for a warehouse for lease is finding the right location. You need to find a location that is easily accessible by your employees and customers and has good transport links. Additionally, you should consider factors such as proximity to suppliers, availability of utilities like water and electricity, and zoning regulations. It may take time to find the perfect location that meets all your requirements but don't rush into signing on any space before carrying out due diligence.
The benefits of owning a warehouse:
Owning a warehouse is an investment that can yield substantial long-term benefits for businesses. With the increasing demand for warehouse space, many companies are now considering owning or leasing warehouses to expand their operations. For businesses looking to increase their storage capacity, having access to a warehouse leased from ESR provides several advantages.
Firstly, having a dedicated storage facility can reduce costs associated with third-party warehousing and improve supply chain efficiency. Warehouse owners have greater control over inventory management and can optimize storage space utilization to minimize operational expenses. Additionally, owning a warehouse provides flexibility in terms of customization and expansion options as per the business needs.
Secondly, owning a warehouse offers increased security for valuable assets and goods. ESR's warehouses come equipped with advanced security features such as 24/7 surveillance systems, gated access points, fire safety measures and trained staff who ensure that inventory remains secure at all times.
Conclusion:
In conclusion,ESR is a leader in warehouse solutions that can help businesses secure their storage needs so that they can focus on what they do best. If you're looking for space to store your belongings, be sure to check out ESR!
Contact: [email protected]
Phone Number: +919627233333
Location: A-214 / B –201, Level 2,
The Capital, Plot No C-70, G Block,
Bandra Kurla Complex, Bandra (East)
Mumbai 400051, India
#warehouse for lease#warehouse in mumbai#warehouse for rent#warehouse in chennai#warehouse in punjab#warehouse in pune#factory space for rent
0 notes
Link
Being one of the demanded #Water #supplier in #Kurla catering the massive demand of water. It never compromises on any sort of purifying or manufacturing quality. To know more about us Call: +91-9870274081.

0 notes
Photo

Mumbai's chapati supplier. Chapati is made from whole wheat flour know as Atta, water and salt and cooked in Tava. These ladies roll out about 200 hundred or more chapatis during morning hours and also cook vegetable curries. Such chapati joints have sprung up at nook and corner of the city for supplying chapati charged at reasonabe price between Rs 5 to 7 and had become popular with middle class office goers, bachelors, workers and Students. It provide employment to house wife and young girls. @rajennair #food #chapati #eatable #Indian #gettyimages #gettyreportage #reportage #reportagesspotlight #everydaymumbai #streetphotographyinternational #streetphotography #photography #photojournalism #mumbai (at Kurla East, Chuna Bhatti)
#photography#reportage#streetphotography#eatable#food#photojournalism#reportagesspotlight#everydaymumbai#gettyimages#indian#streetphotographyinternational#chapati#mumbai#gettyreportage
1 note
·
View note
Text
Formulation and Evaluation of Fast Dissolving Tablet of Lamotrigine
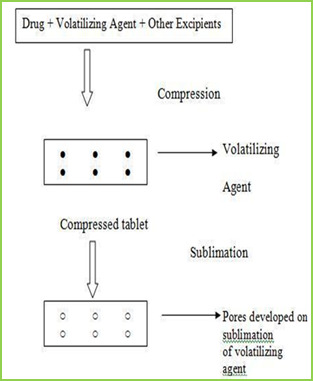
INTRODUCTION Oral routes of drug administration have wide acceptance up to 50-60% of total dosage forms. Solid dosage forms are popular because of ease of administration, accurate dosage, self-medication, pain avoidance and most importantly the patient compliance. The most popular solid dosage forms are being tablets and capsules; one important drawback of this dosage forms for some patients, is the difficulty to swallow. Drinking water plays an important role in the swallowing of oral dosage forms. Often times people experience inconvenience in swallowing conventional dosage forms such as tablet when water is not available, in the case of the motion sickness (ketosis) and sudden episodes of coughing during the common cold, allergic condition and bronchitis. For these reason, tablets that can rapidly dissolve or disintegrate in the oral cavity have attracted a great deal of attention. Or dispersible tablets are not only indicated for people who have swallowing difficulties, but also are ideal for active people4. Fast dissolving tablets areal so called as mouth-dissolving tablets, melt-in mouth tablets, Orodispersible tablets, rapid melts, porous tablets, quick dissolving etc. Fast dissolving tablets are those when put on tongue disintegrate instantaneously releasing the drug which dissolve or disperses in the saliva5. The faster the drug into solution, quicker the absorption and onset of clinical effect. Some drugs are absorbed from the mouth, pharynx and oesophagus as the saliva passes down into the stomach. In such cases, bioavailability of drug is significantly greater than those observed from conventional tablets dosage form. The advantage of mouth dissolving dosage forms are increasingly being recognized in both, industry and academics7. Their growing importance was underlined recently when European pharmacopoeia adopted the term ―Orodispersible tablet‖ as a tablet that to be placed in the mouth where it disperses rapidly before swallowing. According to European pharmacopoeia, the ODT should disperse/disintegrate in less than three minutes. The basic approach in development of FDT is the use of superdisintegrants like cross linked carboxy methyl cellulose (crosscarmellose), sodium starch glycolate (primogel, explotab), polyvinyl pyrollidon (polyplasdone) etc, which provide instantaneous disintegration of tablet after putting on tongue, their by release the drug in saliva. The bioavailability of some drugs may be increased due to absorption of drug in oral cavity and also due to pre gastric absorption of saliva Containing dispersed drugs that pass down into the stomach. More ever, the amount of drug that is subject is to first pass metabolism is reduced as compared to standard tablet. The technologies used form manufacturing fast-dissolving tablets are tablet sublimation. Following conventional techniques are used for preparation of fast dissolving drug delivery system7-9 Sublimation
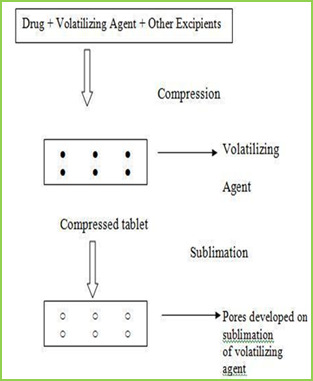
The slow dissolution of the compressed tablet containing even highly water-soluble ingredients is due to the low porosity of the tablets. Inert solid ingredients that volatilize readily (e.g. urea, ammonium carbonate, ammonium bicarbonate, hexa Methylene tetramine, camphor etc.) were added to the other tablet ingredients and the mixture is compressed into tablets. The volatile materials were then removed via sublimation, which generates porous structures. Additionally, several solvents (e.g. cyclohexane, benzene) can be also used as pore forming agents, MATERIAL & METHODS Table no-1 Sr no. Name of Ingredient Supplier 1 Lamotrigine Abottpharmapvt. Ltd., Goa 2 Sodium StarchGlycollate Yash Scientific Enterprises, Pune 3 Crosscarmellose Sodium Kurla Complex, Mumbai 4 β-Cyclodextrin Ozone international, Mumbai 5 Aerosil Yash Scientific Enterprises, Pune 6 Camphor Yash Scientific Enterprises, Pune 7 Directly Compressible Lactose Yash Scientific Enterprises, Pune Method Preformulation Study Organoleptic Characteristics Physico-chemical Characterization 1 Bulk Density 2 Tapped Density 3 Carr‘s index 4 Hausner‘s Ratio 5 Angle of Repose Calibration curve of Drug Formulation & Evaluation of Tablet Hardness Disintegration Time Thickness Friability Wetting Time Drug Content Weight Variation 8. Invitro Drug Release (Dissolution Study) Formulation procedure of tablet (direct compression) In process of direct compression techniques, the all ingredients were accurately weighed and passed through sieve no.40 then mixed together and then compressed using 6 mm flat punch on Cemach R&D Tablet press 10 station compression machine. Hardness of the tablet was maintained at 3-3.5 Kg/cm2. Tablet weight was maintained at 170 to 180 mg. All the product and process variables like mixing time and hardness were kept as practically constant. Table no-2 Sr No. Name of ingredients F1 (mg) F2 (mg) F3 (mg) F4 (mg) F5 (mg) F6 (mg) 1 Lamotrigine 25 25 25 25 25 25 2 β-cyclodextrin 25 25 25 25 25 25 3 Sodium starch 21 26.25 31.5 - - - glycolate 4 Crosscarmellose - - - 21 26.25 31.5 sodium 5 Direct compressible 10.7 5.45 0.2 10.7 5.45 0.2 6 Camphor 7 7 7 7 7 7 8 Total weight 90 90 90 90 90 90 RESULTS AND DISCUSSION In this study fast dissolving tablet of Lamotrigine were prepared by direct compression. Method and effect of different superdisintegrating and sublimating agent camphor on in vitro release were evaluated. Organoleptic Characteristics Organoleptic characteristics like colour, odour, and taste were studied. The Lamotrigine complies with specifications. The results are illustrated in table Table No.3 Organoleptic properties of Lamotrigine Sr. No Properties Specification Lamotrigine 1 Appearance White White 2 Description Crystalline Crystalline 3 Odour Odourless Odourless 4 Taste Bitter Bitter Physical characterization The powder bed was evaluated for the blend property like Bulk density, Tapped density, Carr‘s index, Hausner‘s ratio and Angle of repose. Batch code Bulk density (gm/ml) ± SD Tapped density (gm/ml) ± SD Carr’s index % ± SD Hausner’s ratio % ± SD Angle of repose (0) ± SD F1 0.6032±0.03 0.6912 ± 0.01 14.25 ± 0.20 1.1124 ± 0.02 20.07 ± 0.54 F2 0.6133 ± 0.05 0.6999 ± 0.02 14.09 ± 0.39 1.1358 ± 0.07 19.45 ± 0.85 F3 0.6258 ± 0.01 0.7134 ± 0.06 15.00 ± 0.13 1.1425 ± 0.06 19.39 ± 0.29 F4 0.6078 ± 0.07 0.7088 ± 0.09 15.04 ± 0.75 1.1298 ± 0.04 20.14 ± 0.17 F5 0.6125 ± 0.02 0.7032 ± 0.05 14.58 ± 0.09 1.1340 ± 0.03 20.73 ± 0.65 F6 0.6289 ± 0.08 0.7155 ± 0.04 14.99 ± 0.67 1.1536 ± 0.01 20.10 ± 0.44 Calibration curve of Drug Stock solution of 100 µg/ml was prepared in 0.1 ml N HCL, from which dilution were made to obtain 2, 4, 6, 8, 10 µg/ml solution. Absorbance of these solutions when measured at ƛmax 267 nm and the results are given Table. Table No 5 Calibration curve of lamotrigine in 0.1 N HCL Sr. No. Concentration (µg/ml) Absorbance at 267 nm ±SD 1 0 0 ± 00 2 2 0.1927± 0.00015 3 4 0.2360± 0.00023 4 6 0.2924± 0.00011 5 8 0.3207± 0.00046 6 10 0.3913± 0.00078 Calibration curve
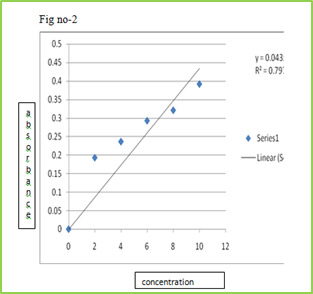
Figure 2: Standard calibration curve of Lamotrigine in 0.1 N HCl Evaluation of compression characteristics of formulations Tablets of all batches were evaluated for weight variation, hardness, thickness and friability results were tabulated in Table. Tablet No.6 Post compression properties of tablets F1 to F6 Batch code Weight variation (mg) ± SD Hardness (kg/cm2) ± SD Thickness (mm) ± SD Friability ± SD% F1 0.090 ± 0.02 12.25 ± 0.28 5.02 ± 0.03 0.63 ± 0.02 F2 0.090 ± 0.01 13.20 ± 0.90 5.05 ± 0.08 0.52 ± 0.02 F3 0.090 ± 0.03 13.00 ± 0.26 5.06 ± 0.02 0.73 ± 0.01 F4 0.090 ± 0.02 13.10 ± 0.13 5.07 ± 0.05 0.89 ± 0.03 F5 0.090 ± 0.03 13.23 ± 0.58 5.03 ± 0.04 0.75 ± 0.04 F6 0.090 ± 0.01 13.05 ± 0.10 5.04 ± 0.01 0.45 ± 0.03 Evaluation of various Parameters of Tablets The tablets were evaluated for disintegration time, wetting time, and drug content. Results obtained were given in Tablet. Table No.7 other post compression parameters of tablets F1 to F6 Batch code Disintegration time (s)± SD Wetting Time (s) ± SD Drug content ± SD F1 58.05 ± 0.07 62.37 ± 0.54 95.49 ± 1.11 F2 53.14 ± 0.04 59.48 ± 0.34 96.76 ± 0.92 F3 45.38 ± 0.03 56.35 ± 0.12 98.48 ± 1.07 F4 15.20 ± 0.10 57.01 ± 0.89 97.68 ± 1.15 F5 17.02 ± 0.07 55.42 ± 0.45 95.21 ± 1.01 F6 08.20 ± 0.03 53.32 ± 0.75 101.23 ± 1.05
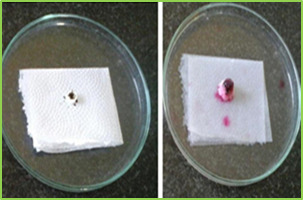
Figure 3: wetting time of fast dissolving tablet of Lamotriginre (Tablet wetting initial, Tablet wetting after 53.32 sec) In vitro Drug Release (Dissolution Study) Dissolution test were carried out using USP Type dissolution test apparatus at 37 ± 0.50C and rpm speed. 900 ml of 0.1 N HCl was used as dissolution medium. Two tablets from each tablets were tested individually in 0.1 N HCl with sample withdraw 5 ml. Collected samples were analysed at ƛ max 267 nm using 0.1 N HCl as blank. The percentage drug release was found to formulation F1 to F6 are given in following tables. Table No.7 In vitro drug release data of formulation F1 (n=2) Time (min) Absorbance (267nm) Concentration (µg/ml) Cumulative drug release Percentage CDR (%) 0 0 0 0 0 1 0.0415 1.22 0.048 4.8 2 0.1737 5.18 0.20 20.43 5 0.3995 11.75 0.47 47.00 15 0.5805 17.07 0.68 68.28 30 0.8298 24.40 0.97 97.60 Table No.8 In vitro drug release data of formulation F2 (n=2) Time (min) Absorbance (267nm) Concentration (µg/ml) Cumulative drug release Percentage CDR (%) 0 0 0 0 0 1 0.0761 2.23 0.08 8.9 2 0.1908 5.61 0.22 22.44 5 0.38.37 11.28 0.45 45.12 15 0.5638 16.58 0.66 66.32 30 0.8344 24.54 0.98 98.16 Table No.9 In vitro drug release data of formulation F3 (n=2) Time (min) Absorb-ance (267nm) Concn (µg/ml) Cumulative drug release Percentage CDR (%) 0 0 0 0 0 1 0.0625 1.83 0.07 7.35 2 0.2248 6.61 0.26 26.44 5 0.4158 12.22 0.48 48.88 15 0.5960 17.52 0.70 70.08 30 0.8495 24.98 0.99 99.92 Table No.10 In vitro drug release data of formulation F4 (n=2) Time (min) Absor-bance (267nm) Concn (µg/ml) Cumulative drug release Percentage CDR (%) 0 0 0 0 0 1 0.0305 0.89 0.03 3.5 2 0.2348 6.90 0.27 27.62 5 0.4009 11.79 0.47 47.16 15 0.6010 17.67 0.70 70.68 30 0.8489 24.96 0.99 99.84 Table No.11 in vitro drug release data of formulation F5 (n=2) Time (min) Absorbance (267nm) Concen (µg/ml) Cumulative drug release Percentage CDR (%) 0 0 0 0 0 1 0.0238 0.7 0.02 2.8 2 0.1983 5.83 0.23 23.32 5 0.3785 11.13 0.44 44.52 15 0.5969 17.55 0.70 70.20 30 0.8239 24.23 0.96 96.92 Table No.12 In vitro drug release data of formulation F6 (n=2) Time (min) Absorb-ance (267nm) Concn (µg/ml) Cumulative drug release Percentage CDR (%) 0 0 0 0 0 1 0.0843 2.47 0.09 9.9 2 0.2248 6.61 0.26 26.44 5 0.4475 13.16 0.52 52.64 15 0.6308 18.55 0.74 74.20 30 0.8399 24.70 0.98 98.81
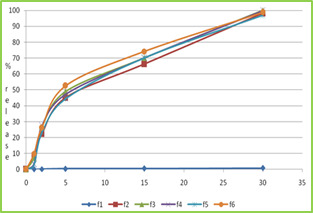
Figure no-4 dissolution profile of formulation F1 to F6 CONCLUSION The results obtained so far encouraged as to derive following conclusion, Fast dissolving tablet of Lamotrigine was formulated by using various superdisintegrants like Crosscarmellose sodium and Sodium starch glycolate in different proportions by sublimating agent like camphor. The values of pre-compression parameters of all formulation showed good flow properties and compressibility, so these can be used for tablet manufacture. The disintegration time for all formulations was considered to be within the acceptable limit. It observed that when sublimating agent like camphor was used disintegration time of tablet is decreased. Wetting time studies showed that wetting time was rapid in formulations containing camphor followed by CCS and SSG. It was found that as the concentration of CCS and SSG was increases, then wetting was reduces. The post compression parameters of all formulations were determined and the values were found to be within IP limits. In-vitro disintegration of F3 gives rapid disintegrating time and wetting time. As result of this study, it may be concluded inclusion the complexation techniques may be useful to enhance solubility and dissolution rate. The concept of formulating high porous fast dissolving tablets of Lamotrigine inclusion complexes using superdisintegrants by sublimation technique offers a suitable and practical approach in serving desired objectives of faster disintegration and dissolution characteristics. REFERENCES Lachman L, Lieberman HA, Kanig JL. The theory and practice of industrial pharmacy. Third Edition, Varghese Publication House, Bombay, India 1987: 296‐ Amin, A. F., Shah, T. J., Bhadani, M. N., & Patel, M. M. (2005). Emerging trends in orally disintegrating tablets. Prakash Goudanavar et al. (2011). Development and characterization of lamotrigineorodispersible tablets Inclusion complex with hydroxypropyl β cyclodextrin‖ International Journal of Pharmacy and Pharmaceutical Sciences, 3(3), 208-214 Seager, H. (1998). Drug‐delivery products and the Zydis fast‐dissolving dosage form. Journal of pharmacy and pharmacology, 50(4), 375-382. https://doi.org/10.1111/j.2042-7158.1998.tb06876.x, PMid:9625481 Remon, J. P., & Corveleyn, S. (2000). S. Patent No. 6,010,719. Washington, DC: U.S. Patent and Trademark Office. Masaki, K., Intrabuccaly disintegrating preparation and production thereof, US Patent No.5, 466, 464, 1995. Pebley, W.S., Jager, N.E., Thompson, S.J., Rapidly disintegrating tablets, US Patent No.5, 298, 261, 1994. Amrutkar, P. P., Patil, S. B., Todarwal, A. N., Wagh, M. A., Kothawade, P. D., & Surawase, R. K. (2010). Design and evaluation of taste masked chewable dispersible tablet of lamotrigine by melt granulation. International Journal of Drug Delivery, 2(2). https://doi.org/10.5138/ijdd.2010.0975.0215.02028 Allen Jr, L. V., & Wang, B. (1996). S. Patent No. 5,587,180. Washington, DC: U.S. Patent and Trademark Office. Biradar, S. S., Bhagavati, S. T., & Kuppasad, I. J. (2006). Fast dissolving drug delivery systems: a brief overview. The internet journal of pharmacology, 4(2), 26-30. Zade, P. S., Kawtikwar, P. S., & Sakarkar, D. M. (2009). Formulation, evaluation and optimization of fast dissolving tablet containing tizanidine hydrochloride. Int J Pharm Tech Res, 1(1), 34-42. Sukhavasi, S., & Kishore, V. S. (2012). Formulation and evaluation of fast dissolving tablets of amlodipine besylate by using hibiscus rosa-sinensis mucilage and modified gum karaya. International Journal of Pharmaceutical Sciences and Research, 3(10), 3975. Patil, C., & Das, S. (2009). Effect of various superdisintegrants on the drug release profile and disintegration time of Lamotrigine orally disintegrating tablets. African journal of pharmacy and pharmacology, 5(1), 76-82. https://doi.org/10.5897/AJPP10.279 Swamy, P. V., Areefulla, S. H., Shirs, S. B., Smitha, G., & Prashanth, B. (2007). Orodispersible tablets of meloxicam using disintegrant blends for improved efficacy. Indian journal of pharmaceutical sciences, 69(6), 836. https://doi.org/10.4103/0250-474X.39448 Shah S. D. (2010). M.Pharm thesis, Formulation and evaluation ofsublingual tablet of Sumatriptan succinate, Saurashtra University. Read the full article
0 notes
Text
Formulation and Evaluation of Fast Dissolving Tablet of Lamotrigine
INTRODUCTION Oral routes of drug administration have wide acceptance up to 50-60% of total dosage forms. Solid dosage forms are popular because of ease of administration, accurate dosage, self-medication, pain avoidance and most importantly the patient compliance. The most popular solid dosage forms are being tablets and capsules; one important drawback of this dosage forms for some patients, is the difficulty to swallow. Drinking water plays an important role in the swallowing of oral dosage forms. Often times people experience inconvenience in swallowing conventional dosage forms such as tablet when water is not available, in the case of the motion sickness (ketosis) and sudden episodes of coughing during the common cold, allergic condition and bronchitis. For these reason, tablets that can rapidly dissolve or disintegrate in the oral cavity have attracted a great deal of attention. Or dispersible tablets are not only indicated for people who have swallowing difficulties, but also are ideal for active people4. Fast dissolving tablets areal so called as mouth-dissolving tablets, melt-in mouth tablets, Orodispersible tablets, rapid melts, porous tablets, quick dissolving etc. Fast dissolving tablets are those when put on tongue disintegrate instantaneously releasing the drug which dissolve or disperses in the saliva5. The faster the drug into solution, quicker the absorption and onset of clinical effect. Some drugs are absorbed from the mouth, pharynx and oesophagus as the saliva passes down into the stomach. In such cases, bioavailability of drug is significantly greater than those observed from conventional tablets dosage form. The advantage of mouth dissolving dosage forms are increasingly being recognized in both, industry and academics7. Their growing importance was underlined recently when European pharmacopoeia adopted the term ―Orodispersible tablet‖ as a tablet that to be placed in the mouth where it disperses rapidly before swallowing. According to European pharmacopoeia, the ODT should disperse/disintegrate in less than three minutes. The basic approach in development of FDT is the use of superdisintegrants like cross linked carboxy methyl cellulose (crosscarmellose), sodium starch glycolate (primogel, explotab), polyvinyl pyrollidon (polyplasdone) etc, which provide instantaneous disintegration of tablet after putting on tongue, their by release the drug in saliva. The bioavailability of some drugs may be increased due to absorption of drug in oral cavity and also due to pre gastric absorption of saliva Containing dispersed drugs that pass down into the stomach. More ever, the amount of drug that is subject is to first pass metabolism is reduced as compared to standard tablet. The technologies used form manufacturing fast-dissolving tablets are tablet sublimation. Following conventional techniques are used for preparation of fast dissolving drug delivery system7-9 Sublimation
The slow dissolution of the compressed tablet containing even highly water-soluble ingredients is due to the low porosity of the tablets. Inert solid ingredients that volatilize readily (e.g. urea, ammonium carbonate, ammonium bicarbonate, hexa Methylene tetramine, camphor etc.) were added to the other tablet ingredients and the mixture is compressed into tablets. The volatile materials were then removed via sublimation, which generates porous structures. Additionally, several solvents (e.g. cyclohexane, benzene) can be also used as pore forming agents, MATERIAL & METHODS Table no-1 Sr no. Name of Ingredient Supplier 1 Lamotrigine Abottpharmapvt. Ltd., Goa 2 Sodium StarchGlycollate Yash Scientific Enterprises, Pune 3 Crosscarmellose Sodium Kurla Complex, Mumbai 4 β-Cyclodextrin Ozone international, Mumbai 5 Aerosil Yash Scientific Enterprises, Pune 6 Camphor Yash Scientific Enterprises, Pune 7 Directly Compressible Lactose Yash Scientific Enterprises, Pune Method Preformulation Study Organoleptic Characteristics Physico-chemical Characterization 1 Bulk Density 2 Tapped Density 3 Carr‘s index 4 Hausner‘s Ratio 5 Angle of Repose Calibration curve of Drug Formulation & Evaluation of Tablet Hardness Disintegration Time Thickness Friability Wetting Time Drug Content Weight Variation 8. Invitro Drug Release (Dissolution Study) Formulation procedure of tablet (direct compression) In process of direct compression techniques, the all ingredients were accurately weighed and passed through sieve no.40 then mixed together and then compressed using 6 mm flat punch on Cemach R&D Tablet press 10 station compression machine. Hardness of the tablet was maintained at 3-3.5 Kg/cm2. Tablet weight was maintained at 170 to 180 mg. All the product and process variables like mixing time and hardness were kept as practically constant. Table no-2 Sr No. Name of ingredients F1 (mg) F2 (mg) F3 (mg) F4 (mg) F5 (mg) F6 (mg) 1 Lamotrigine 25 25 25 25 25 25 2 β-cyclodextrin 25 25 25 25 25 25 3 Sodium starch 21 26.25 31.5 - - - glycolate 4 Crosscarmellose - - - 21 26.25 31.5 sodium 5 Direct compressible 10.7 5.45 0.2 10.7 5.45 0.2 6 Camphor 7 7 7 7 7 7 8 Total weight 90 90 90 90 90 90 RESULTS AND DISCUSSION In this study fast dissolving tablet of Lamotrigine were prepared by direct compression. Method and effect of different superdisintegrating and sublimating agent camphor on in vitro release were evaluated. Organoleptic Characteristics Organoleptic characteristics like colour, odour, and taste were studied. The Lamotrigine complies with specifications. The results are illustrated in table Table No.3 Organoleptic properties of Lamotrigine Sr. No Properties Specification Lamotrigine 1 Appearance White White 2 Description Crystalline Crystalline 3 Odour Odourless Odourless 4 Taste Bitter Bitter Physical characterization The powder bed was evaluated for the blend property like Bulk density, Tapped density, Carr‘s index, Hausner‘s ratio and Angle of repose. Batch code Bulk density (gm/ml) ± SD Tapped density (gm/ml) ± SD Carr’s index % ± SD Hausner’s ratio % ± SD Angle of repose (0) ± SD F1 0.6032±0.03 0.6912 ± 0.01 14.25 ± 0.20 1.1124 ± 0.02 20.07 ± 0.54 F2 0.6133 ± 0.05 0.6999 ± 0.02 14.09 ± 0.39 1.1358 ± 0.07 19.45 ± 0.85 F3 0.6258 ± 0.01 0.7134 ± 0.06 15.00 ± 0.13 1.1425 ± 0.06 19.39 ± 0.29 F4 0.6078 ± 0.07 0.7088 ± 0.09 15.04 ± 0.75 1.1298 ± 0.04 20.14 ± 0.17 F5 0.6125 ± 0.02 0.7032 ± 0.05 14.58 ± 0.09 1.1340 ± 0.03 20.73 ± 0.65 F6 0.6289 ± 0.08 0.7155 ± 0.04 14.99 ± 0.67 1.1536 ± 0.01 20.10 ± 0.44 Calibration curve of Drug Stock solution of 100 µg/ml was prepared in 0.1 ml N HCL, from which dilution were made to obtain 2, 4, 6, 8, 10 µg/ml solution. Absorbance of these solutions when measured at ƛmax 267 nm and the results are given Table. Table No 5 Calibration curve of lamotrigine in 0.1 N HCL Sr. No. Concentration (µg/ml) Absorbance at 267 nm ±SD 1 0 0 ± 00 2 2 0.1927± 0.00015 3 4 0.2360± 0.00023 4 6 0.2924± 0.00011 5 8 0.3207± 0.00046 6 10 0.3913± 0.00078 Calibration curve
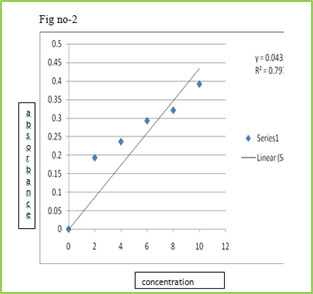
Figure 2: Standard calibration curve of Lamotrigine in 0.1 N HCl Evaluation of compression characteristics of formulations Tablets of all batches were evaluated for weight variation, hardness, thickness and friability results were tabulated in Table. Tablet No.6 Post compression properties of tablets F1 to F6 Batch code Weight variation (mg) ± SD Hardness (kg/cm2) ± SD Thickness (mm) ± SD Friability ± SD% F1 0.090 ± 0.02 12.25 ± 0.28 5.02 ± 0.03 0.63 ± 0.02 F2 0.090 ± 0.01 13.20 ± 0.90 5.05 ± 0.08 0.52 ± 0.02 F3 0.090 ± 0.03 13.00 ± 0.26 5.06 ± 0.02 0.73 ± 0.01 F4 0.090 ± 0.02 13.10 ± 0.13 5.07 ± 0.05 0.89 ± 0.03 F5 0.090 ± 0.03 13.23 ± 0.58 5.03 ± 0.04 0.75 ± 0.04 F6 0.090 ± 0.01 13.05 ± 0.10 5.04 ± 0.01 0.45 ± 0.03 Evaluation of various Parameters of Tablets The tablets were evaluated for disintegration time, wetting time, and drug content. Results obtained were given in Tablet. Table No.7 other post compression parameters of tablets F1 to F6 Batch code Disintegration time (s)± SD Wetting Time (s) ± SD Drug content ± SD F1 58.05 ± 0.07 62.37 ± 0.54 95.49 ± 1.11 F2 53.14 ± 0.04 59.48 ± 0.34 96.76 ± 0.92 F3 45.38 ± 0.03 56.35 ± 0.12 98.48 ± 1.07 F4 15.20 ± 0.10 57.01 ± 0.89 97.68 ± 1.15 F5 17.02 ± 0.07 55.42 ± 0.45 95.21 ± 1.01 F6 08.20 ± 0.03 53.32 ± 0.75 101.23 ± 1.05
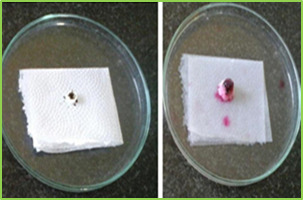
Figure 3: wetting time of fast dissolving tablet of Lamotriginre (Tablet wetting initial, Tablet wetting after 53.32 sec) In vitro Drug Release (Dissolution Study) Dissolution test were carried out using USP Type dissolution test apparatus at 37 ± 0.50C and rpm speed. 900 ml of 0.1 N HCl was used as dissolution medium. Two tablets from each tablets were tested individually in 0.1 N HCl with sample withdraw 5 ml. Collected samples were analysed at ƛ max 267 nm using 0.1 N HCl as blank. The percentage drug release was found to formulation F1 to F6 are given in following tables. Table No.7 In vitro drug release data of formulation F1 (n=2) Time (min) Absorbance (267nm) Concentration (µg/ml) Cumulative drug release Percentage CDR (%) 0 0 0 0 0 1 0.0415 1.22 0.048 4.8 2 0.1737 5.18 0.20 20.43 5 0.3995 11.75 0.47 47.00 15 0.5805 17.07 0.68 68.28 30 0.8298 24.40 0.97 97.60 Table No.8 In vitro drug release data of formulation F2 (n=2) Time (min) Absorbance (267nm) Concentration (µg/ml) Cumulative drug release Percentage CDR (%) 0 0 0 0 0 1 0.0761 2.23 0.08 8.9 2 0.1908 5.61 0.22 22.44 5 0.38.37 11.28 0.45 45.12 15 0.5638 16.58 0.66 66.32 30 0.8344 24.54 0.98 98.16 Table No.9 In vitro drug release data of formulation F3 (n=2) Time (min) Absorb-ance (267nm) Concn (µg/ml) Cumulative drug release Percentage CDR (%) 0 0 0 0 0 1 0.0625 1.83 0.07 7.35 2 0.2248 6.61 0.26 26.44 5 0.4158 12.22 0.48 48.88 15 0.5960 17.52 0.70 70.08 30 0.8495 24.98 0.99 99.92 Table No.10 In vitro drug release data of formulation F4 (n=2) Time (min) Absor-bance (267nm) Concn (µg/ml) Cumulative drug release Percentage CDR (%) 0 0 0 0 0 1 0.0305 0.89 0.03 3.5 2 0.2348 6.90 0.27 27.62 5 0.4009 11.79 0.47 47.16 15 0.6010 17.67 0.70 70.68 30 0.8489 24.96 0.99 99.84 Table No.11 in vitro drug release data of formulation F5 (n=2) Time (min) Absorbance (267nm) Concen (µg/ml) Cumulative drug release Percentage CDR (%) 0 0 0 0 0 1 0.0238 0.7 0.02 2.8 2 0.1983 5.83 0.23 23.32 5 0.3785 11.13 0.44 44.52 15 0.5969 17.55 0.70 70.20 30 0.8239 24.23 0.96 96.92 Table No.12 In vitro drug release data of formulation F6 (n=2) Time (min) Absorb-ance (267nm) Concn (µg/ml) Cumulative drug release Percentage CDR (%) 0 0 0 0 0 1 0.0843 2.47 0.09 9.9 2 0.2248 6.61 0.26 26.44 5 0.4475 13.16 0.52 52.64 15 0.6308 18.55 0.74 74.20 30 0.8399 24.70 0.98 98.81
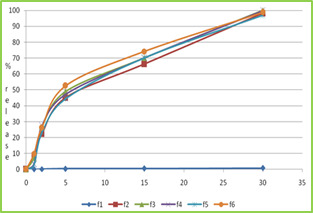
Figure no-4 dissolution profile of formulation F1 to F6 CONCLUSION The results obtained so far encouraged as to derive following conclusion, Fast dissolving tablet of Lamotrigine was formulated by using various superdisintegrants like Crosscarmellose sodium and Sodium starch glycolate in different proportions by sublimating agent like camphor. The values of pre-compression parameters of all formulation showed good flow properties and compressibility, so these can be used for tablet manufacture. The disintegration time for all formulations was considered to be within the acceptable limit. It observed that when sublimating agent like camphor was used disintegration time of tablet is decreased. Wetting time studies showed that wetting time was rapid in formulations containing camphor followed by CCS and SSG. It was found that as the concentration of CCS and SSG was increases, then wetting was reduces. The post compression parameters of all formulations were determined and the values were found to be within IP limits. In-vitro disintegration of F3 gives rapid disintegrating time and wetting time. As result of this study, it may be concluded inclusion the complexation techniques may be useful to enhance solubility and dissolution rate. The concept of formulating high porous fast dissolving tablets of Lamotrigine inclusion complexes using superdisintegrants by sublimation technique offers a suitable and practical approach in serving desired objectives of faster disintegration and dissolution characteristics. REFERENCES Lachman L, Lieberman HA, Kanig JL. The theory and practice of industrial pharmacy. Third Edition, Varghese Publication House, Bombay, India 1987: 296‐ Amin, A. F., Shah, T. J., Bhadani, M. N., & Patel, M. M. (2005). Emerging trends in orally disintegrating tablets. Prakash Goudanavar et al. (2011). Development and characterization of lamotrigineorodispersible tablets Inclusion complex with hydroxypropyl β cyclodextrin‖ International Journal of Pharmacy and Pharmaceutical Sciences, 3(3), 208-214 Seager, H. (1998). Drug‐delivery products and the Zydis fast‐dissolving dosage form. Journal of pharmacy and pharmacology, 50(4), 375-382. https://doi.org/10.1111/j.2042-7158.1998.tb06876.x, PMid:9625481 Remon, J. P., & Corveleyn, S. (2000). S. Patent No. 6,010,719. Washington, DC: U.S. Patent and Trademark Office. Masaki, K., Intrabuccaly disintegrating preparation and production thereof, US Patent No.5, 466, 464, 1995. Pebley, W.S., Jager, N.E., Thompson, S.J., Rapidly disintegrating tablets, US Patent No.5, 298, 261, 1994. Amrutkar, P. P., Patil, S. B., Todarwal, A. N., Wagh, M. A., Kothawade, P. D., & Surawase, R. K. (2010). Design and evaluation of taste masked chewable dispersible tablet of lamotrigine by melt granulation. International Journal of Drug Delivery, 2(2). https://doi.org/10.5138/ijdd.2010.0975.0215.02028 Allen Jr, L. V., & Wang, B. (1996). S. Patent No. 5,587,180. Washington, DC: U.S. Patent and Trademark Office. Biradar, S. S., Bhagavati, S. T., & Kuppasad, I. J. (2006). Fast dissolving drug delivery systems: a brief overview. The internet journal of pharmacology, 4(2), 26-30. Zade, P. S., Kawtikwar, P. S., & Sakarkar, D. M. (2009). Formulation, evaluation and optimization of fast dissolving tablet containing tizanidine hydrochloride. Int J Pharm Tech Res, 1(1), 34-42. Sukhavasi, S., & Kishore, V. S. (2012). Formulation and evaluation of fast dissolving tablets of amlodipine besylate by using hibiscus rosa-sinensis mucilage and modified gum karaya. International Journal of Pharmaceutical Sciences and Research, 3(10), 3975. Patil, C., & Das, S. (2009). Effect of various superdisintegrants on the drug release profile and disintegration time of Lamotrigine orally disintegrating tablets. African journal of pharmacy and pharmacology, 5(1), 76-82. https://doi.org/10.5897/AJPP10.279 Swamy, P. V., Areefulla, S. H., Shirs, S. B., Smitha, G., & Prashanth, B. (2007). Orodispersible tablets of meloxicam using disintegrant blends for improved efficacy. Indian journal of pharmaceutical sciences, 69(6), 836. https://doi.org/10.4103/0250-474X.39448 Shah S. D. (2010). M.Pharm thesis, Formulation and evaluation ofsublingual tablet of Sumatriptan succinate, Saurashtra University. Read the full article
0 notes
Text
Formulation and Evaluation of Fast Dissolving Tablet of Lamotrigine
INTRODUCTION Oral routes of drug administration have wide acceptance up to 50-60% of total dosage forms. Solid dosage forms are popular because of ease of administration, accurate dosage, self-medication, pain avoidance and most importantly the patient compliance. The most popular solid dosage forms are being tablets and capsules; one important drawback of this dosage forms for some patients, is the difficulty to swallow. Drinking water plays an important role in the swallowing of oral dosage forms. Often times people experience inconvenience in swallowing conventional dosage forms such as tablet when water is not available, in the case of the motion sickness (ketosis) and sudden episodes of coughing during the common cold, allergic condition and bronchitis. For these reason, tablets that can rapidly dissolve or disintegrate in the oral cavity have attracted a great deal of attention. Or dispersible tablets are not only indicated for people who have swallowing difficulties, but also are ideal for active people4. Fast dissolving tablets areal so called as mouth-dissolving tablets, melt-in mouth tablets, Orodispersible tablets, rapid melts, porous tablets, quick dissolving etc. Fast dissolving tablets are those when put on tongue disintegrate instantaneously releasing the drug which dissolve or disperses in the saliva5. The faster the drug into solution, quicker the absorption and onset of clinical effect. Some drugs are absorbed from the mouth, pharynx and oesophagus as the saliva passes down into the stomach. In such cases, bioavailability of drug is significantly greater than those observed from conventional tablets dosage form. The advantage of mouth dissolving dosage forms are increasingly being recognized in both, industry and academics7. Their growing importance was underlined recently when European pharmacopoeia adopted the term ―Orodispersible tablet‖ as a tablet that to be placed in the mouth where it disperses rapidly before swallowing. According to European pharmacopoeia, the ODT should disperse/disintegrate in less than three minutes. The basic approach in development of FDT is the use of superdisintegrants like cross linked carboxy methyl cellulose (crosscarmellose), sodium starch glycolate (primogel, explotab), polyvinyl pyrollidon (polyplasdone) etc, which provide instantaneous disintegration of tablet after putting on tongue, their by release the drug in saliva. The bioavailability of some drugs may be increased due to absorption of drug in oral cavity and also due to pre gastric absorption of saliva Containing dispersed drugs that pass down into the stomach. More ever, the amount of drug that is subject is to first pass metabolism is reduced as compared to standard tablet. The technologies used form manufacturing fast-dissolving tablets are tablet sublimation. Following conventional techniques are used for preparation of fast dissolving drug delivery system7-9 Sublimation

The slow dissolution of the compressed tablet containing even highly water-soluble ingredients is due to the low porosity of the tablets. Inert solid ingredients that volatilize readily (e.g. urea, ammonium carbonate, ammonium bicarbonate, hexa Methylene tetramine, camphor etc.) were added to the other tablet ingredients and the mixture is compressed into tablets. The volatile materials were then removed via sublimation, which generates porous structures. Additionally, several solvents (e.g. cyclohexane, benzene) can be also used as pore forming agents, MATERIAL & METHODS Table no-1 Sr no. Name of Ingredient Supplier 1 Lamotrigine Abottpharmapvt. Ltd., Goa 2 Sodium StarchGlycollate Yash Scientific Enterprises, Pune 3 Crosscarmellose Sodium Kurla Complex, Mumbai 4 β-Cyclodextrin Ozone international, Mumbai 5 Aerosil Yash Scientific Enterprises, Pune 6 Camphor Yash Scientific Enterprises, Pune 7 Directly Compressible Lactose Yash Scientific Enterprises, Pune Method Preformulation Study Organoleptic Characteristics Physico-chemical Characterization 1 Bulk Density 2 Tapped Density 3 Carr‘s index 4 Hausner‘s Ratio 5 Angle of Repose Calibration curve of Drug Formulation & Evaluation of Tablet Hardness Disintegration Time Thickness Friability Wetting Time Drug Content Weight Variation 8. Invitro Drug Release (Dissolution Study) Formulation procedure of tablet (direct compression) In process of direct compression techniques, the all ingredients were accurately weighed and passed through sieve no.40 then mixed together and then compressed using 6 mm flat punch on Cemach R&D Tablet press 10 station compression machine. Hardness of the tablet was maintained at 3-3.5 Kg/cm2. Tablet weight was maintained at 170 to 180 mg. All the product and process variables like mixing time and hardness were kept as practically constant. Table no-2 Sr No. Name of ingredients F1 (mg) F2 (mg) F3 (mg) F4 (mg) F5 (mg) F6 (mg) 1 Lamotrigine 25 25 25 25 25 25 2 β-cyclodextrin 25 25 25 25 25 25 3 Sodium starch 21 26.25 31.5 - - - glycolate 4 Crosscarmellose - - - 21 26.25 31.5 sodium 5 Direct compressible 10.7 5.45 0.2 10.7 5.45 0.2 6 Camphor 7 7 7 7 7 7 8 Total weight 90 90 90 90 90 90 RESULTS AND DISCUSSION In this study fast dissolving tablet of Lamotrigine were prepared by direct compression. Method and effect of different superdisintegrating and sublimating agent camphor on in vitro release were evaluated. Organoleptic Characteristics Organoleptic characteristics like colour, odour, and taste were studied. The Lamotrigine complies with specifications. The results are illustrated in table Table No.3 Organoleptic properties of Lamotrigine Sr. No Properties Specification Lamotrigine 1 Appearance White White 2 Description Crystalline Crystalline 3 Odour Odourless Odourless 4 Taste Bitter Bitter Physical characterization The powder bed was evaluated for the blend property like Bulk density, Tapped density, Carr‘s index, Hausner‘s ratio and Angle of repose. Batch code Bulk density (gm/ml) ± SD Tapped density (gm/ml) ± SD Carr’s index % ± SD Hausner’s ratio % ± SD Angle of repose (0) ± SD F1 0.6032±0.03 0.6912 ± 0.01 14.25 ± 0.20 1.1124 ± 0.02 20.07 ± 0.54 F2 0.6133 ± 0.05 0.6999 ± 0.02 14.09 ± 0.39 1.1358 ± 0.07 19.45 ± 0.85 F3 0.6258 ± 0.01 0.7134 ± 0.06 15.00 ± 0.13 1.1425 ± 0.06 19.39 ± 0.29 F4 0.6078 ± 0.07 0.7088 ± 0.09 15.04 ± 0.75 1.1298 ± 0.04 20.14 ± 0.17 F5 0.6125 ± 0.02 0.7032 ± 0.05 14.58 ± 0.09 1.1340 ± 0.03 20.73 ± 0.65 F6 0.6289 ± 0.08 0.7155 ± 0.04 14.99 ± 0.67 1.1536 ± 0.01 20.10 ± 0.44 Calibration curve of Drug Stock solution of 100 µg/ml was prepared in 0.1 ml N HCL, from which dilution were made to obtain 2, 4, 6, 8, 10 µg/ml solution. Absorbance of these solutions when measured at ƛmax 267 nm and the results are given Table. Table No 5 Calibration curve of lamotrigine in 0.1 N HCL Sr. No. Concentration (µg/ml) Absorbance at 267 nm ±SD 1 0 0 ± 00 2 2 0.1927± 0.00015 3 4 0.2360± 0.00023 4 6 0.2924± 0.00011 5 8 0.3207± 0.00046 6 10 0.3913± 0.00078 Calibration curve

Figure 2: Standard calibration curve of Lamotrigine in 0.1 N HCl Evaluation of compression characteristics of formulations Tablets of all batches were evaluated for weight variation, hardness, thickness and friability results were tabulated in Table. Tablet No.6 Post compression properties of tablets F1 to F6 Batch code Weight variation (mg) ± SD Hardness (kg/cm2) ± SD Thickness (mm) ± SD Friability ± SD% F1 0.090 ± 0.02 12.25 ± 0.28 5.02 ± 0.03 0.63 ± 0.02 F2 0.090 ± 0.01 13.20 ± 0.90 5.05 ± 0.08 0.52 ± 0.02 F3 0.090 ± 0.03 13.00 ± 0.26 5.06 ± 0.02 0.73 ± 0.01 F4 0.090 ± 0.02 13.10 ± 0.13 5.07 ± 0.05 0.89 ± 0.03 F5 0.090 ± 0.03 13.23 ± 0.58 5.03 ± 0.04 0.75 ± 0.04 F6 0.090 ± 0.01 13.05 ± 0.10 5.04 ± 0.01 0.45 ± 0.03 Evaluation of various Parameters of Tablets The tablets were evaluated for disintegration time, wetting time, and drug content. Results obtained were given in Tablet. Table No.7 other post compression parameters of tablets F1 to F6 Batch code Disintegration time (s)± SD Wetting Time (s) ± SD Drug content ± SD F1 58.05 ± 0.07 62.37 ± 0.54 95.49 ± 1.11 F2 53.14 ± 0.04 59.48 ± 0.34 96.76 ± 0.92 F3 45.38 ± 0.03 56.35 ± 0.12 98.48 ± 1.07 F4 15.20 ± 0.10 57.01 ± 0.89 97.68 ± 1.15 F5 17.02 ± 0.07 55.42 ± 0.45 95.21 ± 1.01 F6 08.20 ± 0.03 53.32 ± 0.75 101.23 ± 1.05

Figure 3: wetting time of fast dissolving tablet of Lamotriginre (Tablet wetting initial, Tablet wetting after 53.32 sec) In vitro Drug Release (Dissolution Study) Dissolution test were carried out using USP Type dissolution test apparatus at 37 ± 0.50C and rpm speed. 900 ml of 0.1 N HCl was used as dissolution medium. Two tablets from each tablets were tested individually in 0.1 N HCl with sample withdraw 5 ml. Collected samples were analysed at ƛ max 267 nm using 0.1 N HCl as blank. The percentage drug release was found to formulation F1 to F6 are given in following tables. Table No.7 In vitro drug release data of formulation F1 (n=2) Time (min) Absorbance (267nm) Concentration (µg/ml) Cumulative drug release Percentage CDR (%) 0 0 0 0 0 1 0.0415 1.22 0.048 4.8 2 0.1737 5.18 0.20 20.43 5 0.3995 11.75 0.47 47.00 15 0.5805 17.07 0.68 68.28 30 0.8298 24.40 0.97 97.60 Table No.8 In vitro drug release data of formulation F2 (n=2) Time (min) Absorbance (267nm) Concentration (µg/ml) Cumulative drug release Percentage CDR (%) 0 0 0 0 0 1 0.0761 2.23 0.08 8.9 2 0.1908 5.61 0.22 22.44 5 0.38.37 11.28 0.45 45.12 15 0.5638 16.58 0.66 66.32 30 0.8344 24.54 0.98 98.16 Table No.9 In vitro drug release data of formulation F3 (n=2) Time (min) Absorb-ance (267nm) Concn (µg/ml) Cumulative drug release Percentage CDR (%) 0 0 0 0 0 1 0.0625 1.83 0.07 7.35 2 0.2248 6.61 0.26 26.44 5 0.4158 12.22 0.48 48.88 15 0.5960 17.52 0.70 70.08 30 0.8495 24.98 0.99 99.92 Table No.10 In vitro drug release data of formulation F4 (n=2) Time (min) Absor-bance (267nm) Concn (µg/ml) Cumulative drug release Percentage CDR (%) 0 0 0 0 0 1 0.0305 0.89 0.03 3.5 2 0.2348 6.90 0.27 27.62 5 0.4009 11.79 0.47 47.16 15 0.6010 17.67 0.70 70.68 30 0.8489 24.96 0.99 99.84 Table No.11 in vitro drug release data of formulation F5 (n=2) Time (min) Absorbance (267nm) Concen (µg/ml) Cumulative drug release Percentage CDR (%) 0 0 0 0 0 1 0.0238 0.7 0.02 2.8 2 0.1983 5.83 0.23 23.32 5 0.3785 11.13 0.44 44.52 15 0.5969 17.55 0.70 70.20 30 0.8239 24.23 0.96 96.92 Table No.12 In vitro drug release data of formulation F6 (n=2) Time (min) Absorb-ance (267nm) Concn (µg/ml) Cumulative drug release Percentage CDR (%) 0 0 0 0 0 1 0.0843 2.47 0.09 9.9 2 0.2248 6.61 0.26 26.44 5 0.4475 13.16 0.52 52.64 15 0.6308 18.55 0.74 74.20 30 0.8399 24.70 0.98 98.81

Figure no-4 dissolution profile of formulation F1 to F6 CONCLUSION The results obtained so far encouraged as to derive following conclusion, Fast dissolving tablet of Lamotrigine was formulated by using various superdisintegrants like Crosscarmellose sodium and Sodium starch glycolate in different proportions by sublimating agent like camphor. The values of pre-compression parameters of all formulation showed good flow properties and compressibility, so these can be used for tablet manufacture. The disintegration time for all formulations was considered to be within the acceptable limit. It observed that when sublimating agent like camphor was used disintegration time of tablet is decreased. Wetting time studies showed that wetting time was rapid in formulations containing camphor followed by CCS and SSG. It was found that as the concentration of CCS and SSG was increases, then wetting was reduces. The post compression parameters of all formulations were determined and the values were found to be within IP limits. In-vitro disintegration of F3 gives rapid disintegrating time and wetting time. As result of this study, it may be concluded inclusion the complexation techniques may be useful to enhance solubility and dissolution rate. The concept of formulating high porous fast dissolving tablets of Lamotrigine inclusion complexes using superdisintegrants by sublimation technique offers a suitable and practical approach in serving desired objectives of faster disintegration and dissolution characteristics. REFERENCES Lachman L, Lieberman HA, Kanig JL. The theory and practice of industrial pharmacy. Third Edition, Varghese Publication House, Bombay, India 1987: 296‐ Amin, A. F., Shah, T. J., Bhadani, M. N., & Patel, M. M. (2005). Emerging trends in orally disintegrating tablets. Prakash Goudanavar et al. (2011). Development and characterization of lamotrigineorodispersible tablets Inclusion complex with hydroxypropyl β cyclodextrin‖ International Journal of Pharmacy and Pharmaceutical Sciences, 3(3), 208-214 Seager, H. (1998). Drug‐delivery products and the Zydis fast‐dissolving dosage form. Journal of pharmacy and pharmacology, 50(4), 375-382. https://doi.org/10.1111/j.2042-7158.1998.tb06876.x, PMid:9625481 Remon, J. P., & Corveleyn, S. (2000). S. Patent No. 6,010,719. Washington, DC: U.S. Patent and Trademark Office. Masaki, K., Intrabuccaly disintegrating preparation and production thereof, US Patent No.5, 466, 464, 1995. Pebley, W.S., Jager, N.E., Thompson, S.J., Rapidly disintegrating tablets, US Patent No.5, 298, 261, 1994. Amrutkar, P. P., Patil, S. B., Todarwal, A. N., Wagh, M. A., Kothawade, P. D., & Surawase, R. K. (2010). Design and evaluation of taste masked chewable dispersible tablet of lamotrigine by melt granulation. International Journal of Drug Delivery, 2(2). https://doi.org/10.5138/ijdd.2010.0975.0215.02028 Allen Jr, L. V., & Wang, B. (1996). S. Patent No. 5,587,180. Washington, DC: U.S. Patent and Trademark Office. Biradar, S. S., Bhagavati, S. T., & Kuppasad, I. J. (2006). Fast dissolving drug delivery systems: a brief overview. The internet journal of pharmacology, 4(2), 26-30. Zade, P. S., Kawtikwar, P. S., & Sakarkar, D. M. (2009). Formulation, evaluation and optimization of fast dissolving tablet containing tizanidine hydrochloride. Int J Pharm Tech Res, 1(1), 34-42. Sukhavasi, S., & Kishore, V. S. (2012). Formulation and evaluation of fast dissolving tablets of amlodipine besylate by using hibiscus rosa-sinensis mucilage and modified gum karaya. International Journal of Pharmaceutical Sciences and Research, 3(10), 3975. Patil, C., & Das, S. (2009). Effect of various superdisintegrants on the drug release profile and disintegration time of Lamotrigine orally disintegrating tablets. African journal of pharmacy and pharmacology, 5(1), 76-82. https://doi.org/10.5897/AJPP10.279 Swamy, P. V., Areefulla, S. H., Shirs, S. B., Smitha, G., & Prashanth, B. (2007). Orodispersible tablets of meloxicam using disintegrant blends for improved efficacy. Indian journal of pharmaceutical sciences, 69(6), 836. https://doi.org/10.4103/0250-474X.39448 Shah S. D. (2010). M.Pharm thesis, Formulation and evaluation ofsublingual tablet of Sumatriptan succinate, Saurashtra University. Read the full article
0 notes
Text
Formulation and Evaluation of Fast Dissolving Tablet of Lamotrigine
INTRODUCTION Oral routes of drug administration have wide acceptance up to 50-60% of total dosage forms. Solid dosage forms are popular because of ease of administration, accurate dosage, self-medication, pain avoidance and most importantly the patient compliance. The most popular solid dosage forms are being tablets and capsules; one important drawback of this dosage forms for some patients, is the difficulty to swallow. Drinking water plays an important role in the swallowing of oral dosage forms. Often times people experience inconvenience in swallowing conventional dosage forms such as tablet when water is not available, in the case of the motion sickness (ketosis) and sudden episodes of coughing during the common cold, allergic condition and bronchitis. For these reason, tablets that can rapidly dissolve or disintegrate in the oral cavity have attracted a great deal of attention. Or dispersible tablets are not only indicated for people who have swallowing difficulties, but also are ideal for active people4. Fast dissolving tablets areal so called as mouth-dissolving tablets, melt-in mouth tablets, Orodispersible tablets, rapid melts, porous tablets, quick dissolving etc. Fast dissolving tablets are those when put on tongue disintegrate instantaneously releasing the drug which dissolve or disperses in the saliva5. The faster the drug into solution, quicker the absorption and onset of clinical effect. Some drugs are absorbed from the mouth, pharynx and oesophagus as the saliva passes down into the stomach. In such cases, bioavailability of drug is significantly greater than those observed from conventional tablets dosage form. The advantage of mouth dissolving dosage forms are increasingly being recognized in both, industry and academics7. Their growing importance was underlined recently when European pharmacopoeia adopted the term ―Orodispersible tablet‖ as a tablet that to be placed in the mouth where it disperses rapidly before swallowing. According to European pharmacopoeia, the ODT should disperse/disintegrate in less than three minutes. The basic approach in development of FDT is the use of superdisintegrants like cross linked carboxy methyl cellulose (crosscarmellose), sodium starch glycolate (primogel, explotab), polyvinyl pyrollidon (polyplasdone) etc, which provide instantaneous disintegration of tablet after putting on tongue, their by release the drug in saliva. The bioavailability of some drugs may be increased due to absorption of drug in oral cavity and also due to pre gastric absorption of saliva Containing dispersed drugs that pass down into the stomach. More ever, the amount of drug that is subject is to first pass metabolism is reduced as compared to standard tablet. The technologies used form manufacturing fast-dissolving tablets are tablet sublimation. Following conventional techniques are used for preparation of fast dissolving drug delivery system7-9 Sublimation

The slow dissolution of the compressed tablet containing even highly water-soluble ingredients is due to the low porosity of the tablets. Inert solid ingredients that volatilize readily (e.g. urea, ammonium carbonate, ammonium bicarbonate, hexa Methylene tetramine, camphor etc.) were added to the other tablet ingredients and the mixture is compressed into tablets. The volatile materials were then removed via sublimation, which generates porous structures. Additionally, several solvents (e.g. cyclohexane, benzene) can be also used as pore forming agents, MATERIAL & METHODS Table no-1 Sr no. Name of Ingredient Supplier 1 Lamotrigine Abottpharmapvt. Ltd., Goa 2 Sodium StarchGlycollate Yash Scientific Enterprises, Pune 3 Crosscarmellose Sodium Kurla Complex, Mumbai 4 β-Cyclodextrin Ozone international, Mumbai 5 Aerosil Yash Scientific Enterprises, Pune 6 Camphor Yash Scientific Enterprises, Pune 7 Directly Compressible Lactose Yash Scientific Enterprises, Pune Method Preformulation Study Organoleptic Characteristics Physico-chemical Characterization 1 Bulk Density 2 Tapped Density 3 Carr‘s index 4 Hausner‘s Ratio 5 Angle of Repose Calibration curve of Drug Formulation & Evaluation of Tablet Hardness Disintegration Time Thickness Friability Wetting Time Drug Content Weight Variation 8. Invitro Drug Release (Dissolution Study) Formulation procedure of tablet (direct compression) In process of direct compression techniques, the all ingredients were accurately weighed and passed through sieve no.40 then mixed together and then compressed using 6 mm flat punch on Cemach R&D Tablet press 10 station compression machine. Hardness of the tablet was maintained at 3-3.5 Kg/cm2. Tablet weight was maintained at 170 to 180 mg. All the product and process variables like mixing time and hardness were kept as practically constant. Table no-2 Sr No. Name of ingredients F1 (mg) F2 (mg) F3 (mg) F4 (mg) F5 (mg) F6 (mg) 1 Lamotrigine 25 25 25 25 25 25 2 β-cyclodextrin 25 25 25 25 25 25 3 Sodium starch 21 26.25 31.5 - - - glycolate 4 Crosscarmellose - - - 21 26.25 31.5 sodium 5 Direct compressible 10.7 5.45 0.2 10.7 5.45 0.2 6 Camphor 7 7 7 7 7 7 8 Total weight 90 90 90 90 90 90 RESULTS AND DISCUSSION In this study fast dissolving tablet of Lamotrigine were prepared by direct compression. Method and effect of different superdisintegrating and sublimating agent camphor on in vitro release were evaluated. Organoleptic Characteristics Organoleptic characteristics like colour, odour, and taste were studied. The Lamotrigine complies with specifications. The results are illustrated in table Table No.3 Organoleptic properties of Lamotrigine Sr. No Properties Specification Lamotrigine 1 Appearance White White 2 Description Crystalline Crystalline 3 Odour Odourless Odourless 4 Taste Bitter Bitter Physical characterization The powder bed was evaluated for the blend property like Bulk density, Tapped density, Carr‘s index, Hausner‘s ratio and Angle of repose. Batch code Bulk density (gm/ml) ± SD Tapped density (gm/ml) ± SD Carr’s index % ± SD Hausner’s ratio % ± SD Angle of repose (0) ± SD F1 0.6032±0.03 0.6912 ± 0.01 14.25 ± 0.20 1.1124 ± 0.02 20.07 ± 0.54 F2 0.6133 ± 0.05 0.6999 ± 0.02 14.09 ± 0.39 1.1358 ± 0.07 19.45 ± 0.85 F3 0.6258 ± 0.01 0.7134 ± 0.06 15.00 ± 0.13 1.1425 ± 0.06 19.39 ± 0.29 F4 0.6078 ± 0.07 0.7088 ± 0.09 15.04 ± 0.75 1.1298 ± 0.04 20.14 ± 0.17 F5 0.6125 ± 0.02 0.7032 ± 0.05 14.58 ± 0.09 1.1340 ± 0.03 20.73 ± 0.65 F6 0.6289 ± 0.08 0.7155 ± 0.04 14.99 ± 0.67 1.1536 ± 0.01 20.10 ± 0.44 Calibration curve of Drug Stock solution of 100 µg/ml was prepared in 0.1 ml N HCL, from which dilution were made to obtain 2, 4, 6, 8, 10 µg/ml solution. Absorbance of these solutions when measured at ƛmax 267 nm and the results are given Table. Table No 5 Calibration curve of lamotrigine in 0.1 N HCL Sr. No. Concentration (µg/ml) Absorbance at 267 nm ±SD 1 0 0 ± 00 2 2 0.1927± 0.00015 3 4 0.2360± 0.00023 4 6 0.2924± 0.00011 5 8 0.3207± 0.00046 6 10 0.3913± 0.00078 Calibration curve

Figure 2: Standard calibration curve of Lamotrigine in 0.1 N HCl Evaluation of compression characteristics of formulations Tablets of all batches were evaluated for weight variation, hardness, thickness and friability results were tabulated in Table. Tablet No.6 Post compression properties of tablets F1 to F6 Batch code Weight variation (mg) ± SD Hardness (kg/cm2) ± SD Thickness (mm) ± SD Friability ± SD% F1 0.090 ± 0.02 12.25 ± 0.28 5.02 ± 0.03 0.63 ± 0.02 F2 0.090 ± 0.01 13.20 ± 0.90 5.05 ± 0.08 0.52 ± 0.02 F3 0.090 ± 0.03 13.00 ± 0.26 5.06 ± 0.02 0.73 ± 0.01 F4 0.090 ± 0.02 13.10 ± 0.13 5.07 ± 0.05 0.89 ± 0.03 F5 0.090 ± 0.03 13.23 ± 0.58 5.03 ± 0.04 0.75 ± 0.04 F6 0.090 ± 0.01 13.05 ± 0.10 5.04 ± 0.01 0.45 ± 0.03 Evaluation of various Parameters of Tablets The tablets were evaluated for disintegration time, wetting time, and drug content. Results obtained were given in Tablet. Table No.7 other post compression parameters of tablets F1 to F6 Batch code Disintegration time (s)± SD Wetting Time (s) ± SD Drug content ± SD F1 58.05 ± 0.07 62.37 ± 0.54 95.49 ± 1.11 F2 53.14 ± 0.04 59.48 ± 0.34 96.76 ± 0.92 F3 45.38 ± 0.03 56.35 ± 0.12 98.48 ± 1.07 F4 15.20 ± 0.10 57.01 ± 0.89 97.68 ± 1.15 F5 17.02 ± 0.07 55.42 ± 0.45 95.21 ± 1.01 F6 08.20 ± 0.03 53.32 ± 0.75 101.23 ± 1.05

Figure 3: wetting time of fast dissolving tablet of Lamotriginre (Tablet wetting initial, Tablet wetting after 53.32 sec) In vitro Drug Release (Dissolution Study) Dissolution test were carried out using USP Type dissolution test apparatus at 37 ± 0.50C and rpm speed. 900 ml of 0.1 N HCl was used as dissolution medium. Two tablets from each tablets were tested individually in 0.1 N HCl with sample withdraw 5 ml. Collected samples were analysed at ƛ max 267 nm using 0.1 N HCl as blank. The percentage drug release was found to formulation F1 to F6 are given in following tables. Table No.7 In vitro drug release data of formulation F1 (n=2) Time (min) Absorbance (267nm) Concentration (µg/ml) Cumulative drug release Percentage CDR (%) 0 0 0 0 0 1 0.0415 1.22 0.048 4.8 2 0.1737 5.18 0.20 20.43 5 0.3995 11.75 0.47 47.00 15 0.5805 17.07 0.68 68.28 30 0.8298 24.40 0.97 97.60 Table No.8 In vitro drug release data of formulation F2 (n=2) Time (min) Absorbance (267nm) Concentration (µg/ml) Cumulative drug release Percentage CDR (%) 0 0 0 0 0 1 0.0761 2.23 0.08 8.9 2 0.1908 5.61 0.22 22.44 5 0.38.37 11.28 0.45 45.12 15 0.5638 16.58 0.66 66.32 30 0.8344 24.54 0.98 98.16 Table No.9 In vitro drug release data of formulation F3 (n=2) Time (min) Absorb-ance (267nm) Concn (µg/ml) Cumulative drug release Percentage CDR (%) 0 0 0 0 0 1 0.0625 1.83 0.07 7.35 2 0.2248 6.61 0.26 26.44 5 0.4158 12.22 0.48 48.88 15 0.5960 17.52 0.70 70.08 30 0.8495 24.98 0.99 99.92 Table No.10 In vitro drug release data of formulation F4 (n=2) Time (min) Absor-bance (267nm) Concn (µg/ml) Cumulative drug release Percentage CDR (%) 0 0 0 0 0 1 0.0305 0.89 0.03 3.5 2 0.2348 6.90 0.27 27.62 5 0.4009 11.79 0.47 47.16 15 0.6010 17.67 0.70 70.68 30 0.8489 24.96 0.99 99.84 Table No.11 in vitro drug release data of formulation F5 (n=2) Time (min) Absorbance (267nm) Concen (µg/ml) Cumulative drug release Percentage CDR (%) 0 0 0 0 0 1 0.0238 0.7 0.02 2.8 2 0.1983 5.83 0.23 23.32 5 0.3785 11.13 0.44 44.52 15 0.5969 17.55 0.70 70.20 30 0.8239 24.23 0.96 96.92 Table No.12 In vitro drug release data of formulation F6 (n=2) Time (min) Absorb-ance (267nm) Concn (µg/ml) Cumulative drug release Percentage CDR (%) 0 0 0 0 0 1 0.0843 2.47 0.09 9.9 2 0.2248 6.61 0.26 26.44 5 0.4475 13.16 0.52 52.64 15 0.6308 18.55 0.74 74.20 30 0.8399 24.70 0.98 98.81

Figure no-4 dissolution profile of formulation F1 to F6 CONCLUSION The results obtained so far encouraged as to derive following conclusion, Fast dissolving tablet of Lamotrigine was formulated by using various superdisintegrants like Crosscarmellose sodium and Sodium starch glycolate in different proportions by sublimating agent like camphor. The values of pre-compression parameters of all formulation showed good flow properties and compressibility, so these can be used for tablet manufacture. The disintegration time for all formulations was considered to be within the acceptable limit. It observed that when sublimating agent like camphor was used disintegration time of tablet is decreased. Wetting time studies showed that wetting time was rapid in formulations containing camphor followed by CCS and SSG. It was found that as the concentration of CCS and SSG was increases, then wetting was reduces. The post compression parameters of all formulations were determined and the values were found to be within IP limits. In-vitro disintegration of F3 gives rapid disintegrating time and wetting time. As result of this study, it may be concluded inclusion the complexation techniques may be useful to enhance solubility and dissolution rate. The concept of formulating high porous fast dissolving tablets of Lamotrigine inclusion complexes using superdisintegrants by sublimation technique offers a suitable and practical approach in serving desired objectives of faster disintegration and dissolution characteristics. REFERENCES Lachman L, Lieberman HA, Kanig JL. The theory and practice of industrial pharmacy. Third Edition, Varghese Publication House, Bombay, India 1987: 296‐ Amin, A. F., Shah, T. J., Bhadani, M. N., & Patel, M. M. (2005). Emerging trends in orally disintegrating tablets. Prakash Goudanavar et al. (2011). Development and characterization of lamotrigineorodispersible tablets Inclusion complex with hydroxypropyl β cyclodextrin‖ International Journal of Pharmacy and Pharmaceutical Sciences, 3(3), 208-214 Seager, H. (1998). Drug‐delivery products and the Zydis fast‐dissolving dosage form. Journal of pharmacy and pharmacology, 50(4), 375-382. https://doi.org/10.1111/j.2042-7158.1998.tb06876.x, PMid:9625481 Remon, J. P., & Corveleyn, S. (2000). S. Patent No. 6,010,719. Washington, DC: U.S. Patent and Trademark Office. Masaki, K., Intrabuccaly disintegrating preparation and production thereof, US Patent No.5, 466, 464, 1995. Pebley, W.S., Jager, N.E., Thompson, S.J., Rapidly disintegrating tablets, US Patent No.5, 298, 261, 1994. Amrutkar, P. P., Patil, S. B., Todarwal, A. N., Wagh, M. A., Kothawade, P. D., & Surawase, R. K. (2010). Design and evaluation of taste masked chewable dispersible tablet of lamotrigine by melt granulation. International Journal of Drug Delivery, 2(2). https://doi.org/10.5138/ijdd.2010.0975.0215.02028 Allen Jr, L. V., & Wang, B. (1996). S. Patent No. 5,587,180. Washington, DC: U.S. Patent and Trademark Office. Biradar, S. S., Bhagavati, S. T., & Kuppasad, I. J. (2006). Fast dissolving drug delivery systems: a brief overview. The internet journal of pharmacology, 4(2), 26-30. Zade, P. S., Kawtikwar, P. S., & Sakarkar, D. M. (2009). Formulation, evaluation and optimization of fast dissolving tablet containing tizanidine hydrochloride. Int J Pharm Tech Res, 1(1), 34-42. Sukhavasi, S., & Kishore, V. S. (2012). Formulation and evaluation of fast dissolving tablets of amlodipine besylate by using hibiscus rosa-sinensis mucilage and modified gum karaya. International Journal of Pharmaceutical Sciences and Research, 3(10), 3975. Patil, C., & Das, S. (2009). Effect of various superdisintegrants on the drug release profile and disintegration time of Lamotrigine orally disintegrating tablets. African journal of pharmacy and pharmacology, 5(1), 76-82. https://doi.org/10.5897/AJPP10.279 Swamy, P. V., Areefulla, S. H., Shirs, S. B., Smitha, G., & Prashanth, B. (2007). Orodispersible tablets of meloxicam using disintegrant blends for improved efficacy. Indian journal of pharmaceutical sciences, 69(6), 836. https://doi.org/10.4103/0250-474X.39448 Shah S. D. (2010). M.Pharm thesis, Formulation and evaluation ofsublingual tablet of Sumatriptan succinate, Saurashtra University. Read the full article
0 notes